Complications of Laparoscopic and Robotic Surgery in Pediatrics
Gaayana Raju, Michael Ost
INTRODUCTION
Minimally invasive surgery has proven to be safe and effective in the treatment of multiple pediatric urologic diseases. Laparoscopy was introduced to pediatric urology as a diagnostic tool in the 1970’s and has since significantly evolved.1 More recently, robotic surgery is being applied to the pediatric urologic population to assess the potential utility.2
Benefits of minimally invasive surgery include decreased postoperative pain, decreases blood loss, and improved cosmesis.3 With any surgical procedure there are always inherent risks. Laparoscopy and robotic surgery posses a unique set of potential complications compared to open surgery. The incorporation of these newer technologies into practice requires an understanding of the potential hazards. From the experience of others, knowledge has been gained as to how to identify and appropriately manage complications. Early recognition is essential to minimize the morbidity that may be endured by the patient.
PATIENT POSITIONING
Laparoscopic and robotic surgery often requires the patient to be in a static position longer than may be expected with open procedures. Patients are often rotated laterally or placed in trendelenburg position to assist in moving bowels out of the visual field. These extreme positions place additional pressure on various points on the patient. It is imperative to ensure that there is appropriate and sufficient padding to avoid any temporary or permanent paresthesia and pressure injuries. Once the patient is positioned, a test roll should be conducted to confirm the patient is secured to the table with appropriate padding in areas that may experience increased pressures.
ACCESS
Various techniques are used to gain access to the abdominal cavity.4 These include the Veress needle, the Hassan cannula, or the Bailez technique with or without radially dilating trocars.5,6 The layers of the pediatric abdomen are thinner and easily penetrable with different access systems. The peritoneum more readily separates from the anterior abdominal wall when compared to the adult abdomen (Figure 1).
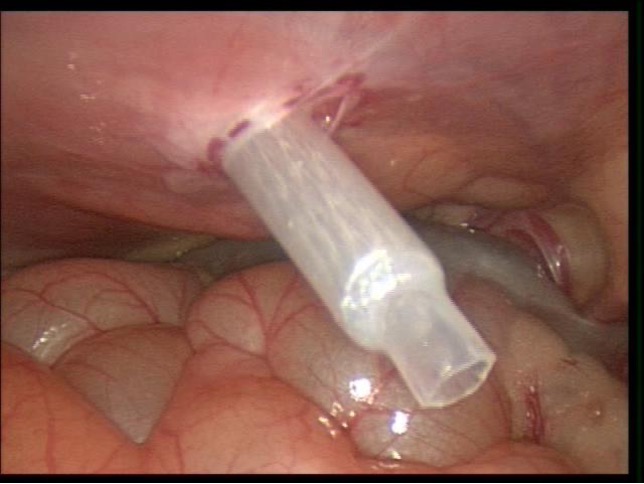 Figure 1. Trocar sheath placed through abdominal wall demonstrating compliance of peritoneum in pediatric abdomen
Figure 1. Trocar sheath placed through abdominal wall demonstrating compliance of peritoneum in pediatric abdomen
The Veress needle uses blind placement of a 2 mm needle into the abdominal cavity. Appropriate placement of the Veress needle is confirmed by flushing the Veress with normal saline. If the Veress is in proper intraperitoneal position, it will flush easily with no return on aspiration. The saline drop test is done by placing a drop of normal saline on the top of the Veress needle that should fall in with elevation of the abdominal wall.
Increased insufflation pressures and asymmetric abdominal wall expansion indicate that the needle may have stopped short of the peritoneal cavity, causing insufflation of the preperitoneal space. Another indication of incorrect placement of the Veress needle is elevated insufflation pressures with low volumes.7
The open Hassan technique uses direct visualization and incision of the fascial layers to gain access to the abdomen.8 The open technique is thought to be safer although the Veress needle has also resulted in low complication rates.4,7,9 A retrospective review identified a complication rate of 2% with the Veress technique and 0.8% with the open technique, with no statistical difference.10
Passing any trocar through the abdominal wall may cause significant injury to the abdominal wall vessels. This may result in formation of a hematoma or cause a persistent drip from the trocar into the abdominal cavity. Direct visualization and transillumination of the anterior abdominal wall allows visualization and subsequent avoidance of vessels (Figure 2). If injury does occur, the vessel may be coagulated directly through another port. If the bleeding is significant and difficult to stop, fascial sutures may be placed in a figure of eight fashion to occlude the vessel. Other methods include placement of a foley catheter through the site, inflating the balloon and putting upward traction on the foley to tamponade the bleeding.11
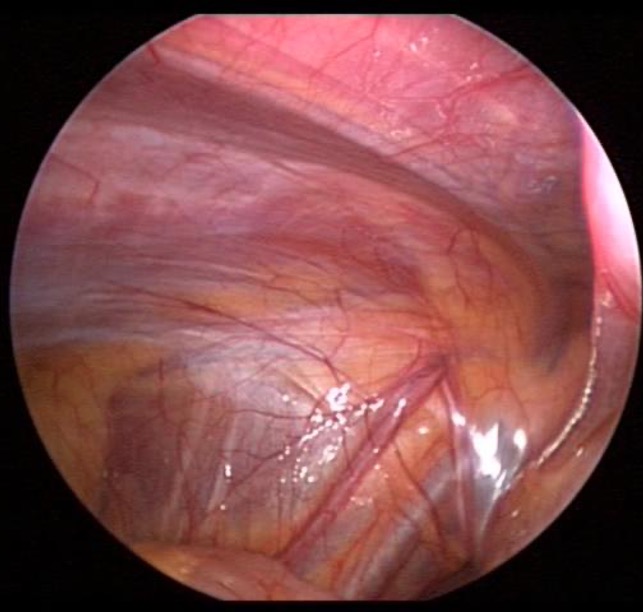 Figure 2. Intra abdominal view of iliac and epigastric vessels
Figure 2. Intra abdominal view of iliac and epigastric vessels
The Veress needle or trocar may be inadvertently placed into the bowel, nearby vascular structures, or other abdominal viscera. If placed into the bowel, the injury may be addressed laparoscopically or by a laparotomy depending on the extent of injury.12 Similarly, vascular injuries can either be managed with expeditious control and intracorporeal suturing, or speedy open conversion and immediate vascular control.13 If the injury seems severe in nature requiring immediate open exploration, the misplaced trocar should be left in position to potentially tamponade the injury as well to expedite identifying the location and guide the surgeon to the site of injury. Bladder perforation with trocar placement is always a risk, especially if the bladder is not decompressed.
Various trocar arrangements also put the bladder at increased risk such as scrotal trocars that are sometimes used during a laparoscopic orchidopexy (Figure 3).
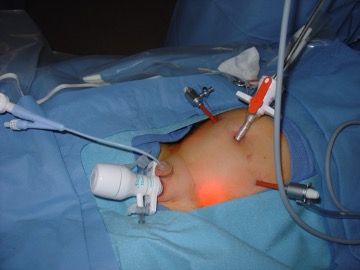 Figure 3. Scrotal trocar placed during laparoscopic orchidopexy.
Figure 3. Scrotal trocar placed during laparoscopic orchidopexy.
CONSEQUENCES OF PNEUMOPERITONEUM
The pneumoperitoneum affects normal physiology. Ventilation is more challenging secondary to the pressures within the abdomen. It is always best to use the minimum insufflation pressures required to achieve an adequate surgical working space. Children have less pulmonary reserve when compared to adults and are not able to tolerate decreases in oxygen saturation as well.14 Ventilatory difficulties should be communicated to the surgeon and the pneumoperitoneum should be decreased, or completely released.
Most healthy children tolerate the pneumoperitoneum without difficulty. Children with compromised cardiovascular or respiratory status should be appropriately selected to exclude those that would have trouble tolerating an insufflated abdomen.
During insufflation, and during the course of the procedure it is imperative to be aware of the potential for a gas embolus. Although CO2 is highly soluble in blood, a gas embolus may still occur. The risk is increased if there is an open vessel or high intraperitoneal pressures. The signs include sudden cardiovascular collapse, mill wheel murmur, decreased oxygen saturation, arrhythmia, and hypotension.15 Treatment is emergent, requiring immediate release of pneumoperitoneum, hyperventilating with 100% oxygen, placement of patient in left lateral decubitus position, and placement of central line into the right atrium with attempted aspiration of the embolus.16 Clear communication between the surgeon and anesthetist is paramount.
Urine output is minimal as the insufflation pressures compress the renal parenchyma and renal vasculature. This is an important point to note as fluid management should not depend on urine output.
INTRAABDOMINAL DISSECTION
Direct cautery injury can occur to any of the intraabdominal organs if the entire activated part of the heated element is not in full visualization. In addition, if there is a break in the integrity of the insulation of the instrument thermal injury may occur. It is also difficult to appreciate the amount of heat the instruments transmit to surrounding structures while activated and care should be taken to minimize the amount of time any heating element is activated.
The pediatric abdomen does not have the same volume to accommodate wide movements as compared to adults. Any instrument, especially sharp instruments may penetrate an organ. Attention must be devoted to instrument movement, as with a limited amount of working space, there is less margin of error available when maneuvering.
POST OPERATIVE COMPLICATIONS
Unrecognized bowel injuries may present several days post-operatively. Patients may complain of abdominal pain that is localized to the port site. Leukopenia and a fever may accompany the symptoms as well.17 Imaging should be done to assess for any injury.
A retrospective review demonstrated no association with port size or location and subsequent development of a hernia.18 Most will close the fascia of 5 mm. There has been no minimum port size established that does not require fascial closure, however many feel that is it unnecessary to close ports 3 mm and smaller. Although a port site hernia is a rare occurrence in children, fascial closure of all incisions may be advisable.
SUMMARY
Minimally invasive surgery is being incorporated at rapid rate into pediatric urology. The ideal way to manage complications is to prevent them. This is best achieved by knowledge of potential hazards. Prompt recognition and expedient management help minimize the consequences assumed by the patient following any complication.
References
- Cortesi N, et al. Diagnosis of bilateral abdominal cryptorchidism by laparoscopy. Endoscopy. 1976;8(1):33-34.
- Casale P. Robotic Pediatric Urology. Expert Rev Med Devices. 2008 Jan; 5(1):59-64.
- Zitsman JL. Current concepts in minimal access surgery for children. Pediatrics. 2003 Jun; 111(6 Pt 1):1239-52.
- Passerotti CC, Nguyen HT, Retik AB, Peters CA. Patterns and predictors of laparoscopic complications in pediatric urology: the role of ongoing surgical volume and access techniques. J Urol. 2008 Aug;180(2):681-5.
- Franc-Guimond J, Kryger J, Gonzalez R. Experience with the Bailez technique for laparoscopic access in children. J Urol. 2003 Sep;170(3):936-8.
- Docimo SG. Re: Experience with the Bailez technique for laparoscopic access in children. J Urol. 2004 Feb;171(2 Pt 1):806.
- Yanke BV, Horowitz M. Safety of the Veress needle in pediatric laparoscopy. J Endourol. 2007 Jul:21(7):695-7.
- Esposito C, Ascione G, Garipoli V, De Bernardo G, Esposito G. Complications of pediatric laparoscopy surgery. Surg Endosc. 1997 Jun;11(6):655-7.
- Cost NG, Lee J, Snodgrass WT, et al. Hernia after pediatric urological laparoscopy. J Urol. 2010 Mar;183(3):1163-7.
- Passerotti CC, Nguyen HT, Retik AB, Peters CA. Patterns and Predictors of Laparoscopic Complications in Pediatric Urology: The Role of Ongoing Surgical Volume and Access Techniques. J Urol. 2008 Aug;180: 681-685.
- Pemberton RJ, Tolley DA, van Velthoven RF. Prevention and management of complications in urological laparoscopic port site placement. Eur Urol. 2006 Nov;50(5):958-68.
- Van der Voort M, Heijnsdijk EA, Gouma DJ. Bowel injury as a complication of laparoscopy. Br J Surg. 2004 Oct;91(10):1253-8.
- Fruhwirth J, Koch G, Mischinger HJ, Werkgartner G, Tesch NP. Vascular complications in minimally invasive surgery. Surg Laparosc Endosc. 1997 Jun;7(3):251- 4.
- Tobias JD. Anaesthesia for minimally invasive surgery in children. Best Pract Res Clin Anaesthesiol. 2002 Mar;16(1):115-30.
- Cottin V, Delafosse B, Viale JP. Gas embolism during laparoscopy: a report of seven cases in patients with previous abdominal surgical history. Surg Endosc. 1996 Feb;10(2):166-9.
- Kudsi OY, Jones SA, Brenn BR. Carbon dioxide embolism in a 3-week-old neonate during laparoscopic pyloromyotomy: a case report. J Pediatr Surg. 2009 Apr;44(4):842- 5.
- Peters CA. Complications in pediatric urologic laparoscopy: results of a survey. J Urol. 1996 Mar:155(3):1070-3.
- Bishoff JT, Allaf ME, Kirkels W, Moore RG, Kavoussi LR, Schroder F. Laparoscopic bowel injury: incidence and clinical presentation. J Urol. 1999 Mar;161(3):887-90.
