Complications of Laparoscopic and Robotic Pelvic Lymph Node Dissection
David S. Wang and Howard N. Winfield
INTRODUCTION
Laparoscopic pelvic lymph node dissection (LPLND) for the staging of prostate cancer was introduced in 1991 by Schuessler and associates.1 LPLND rapidly became the first widely accepted laparoscopic procedure embraced by urologists and in the early 1990s was the most commonly performed urologic laparoscopic procedure. LPLND was performed in many patients with prostate cancer prior to definitive therapy. The presence or absence of metastatic pelvic lymph node involvement was crucial in determining the extent and nature of prostate cancer treatment. In addition to patients with prostate cancer, LPLND has also been useful in select patients with other urologic malignancies such as bladder, penile, and urethral cancer.
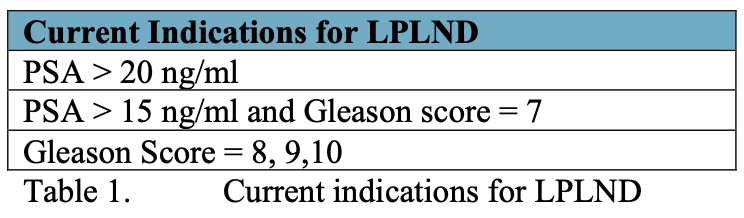
Today, most patients present with earlier stages of prostate cancer due to routine use of prostate specific antigen and improved public awareness. As such, fewer patients require LPLND prior to definitive therapy for prostate cancer. Yet, LPLND remains an important urologic procedure for select patients with prostate cancer. Current indications for performing LPLND are listed in Table 1.
Robotic assisted laparoscopic radical prostatectomy (RALRP) has emerged as an accepted treatment modality for clinically localized prostate cancer. At present, most laparoscopic or robotic pelvic lymph node dissections are performed at the time of RALRP, due to increased ability to determine pre-operative extra-prostatic extension of prostate cancer. On occasion, robot assisted pelvic lymph node dissection (PLND) may be performed as an isolated procedure based on surgeon preference. Thus, this chapter will review complications of LPLND performed as a distinct procedure and also at the time of RALRP.
KEY PROCEDURAL STEPS
Whether performed at the time of RALRP or as a separate procedure, the key steps of performing LPLND or robotic PLND are identical. Moreover, the risks and types of complications arising from LPLND or robotic PLND are similar.
Pre-operatively, patients receive a single dose of a broad-spectrum antibiotic and are typed and screened for blood products. If the pelvic lymph node dissection is being performed at the time of RALRP, a mechanical bowel preparation is required. The patient is positioned supine with the arms tucked. Pneumatic compression stockings are placed. A Foley catheter is placed in the bladder and an orogastric tube in the stomach prior to establishing access and port placement. A transperitoneal or extraperitoneal approach may be performed. Typical port placement for a transperitoneal approach is shown in Figure 1. Port placement for RALRP differs from that of isolated LPLND and is presented in another chapter in this text.
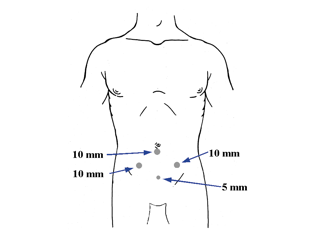 Figure 1. Port placement for transperitoneal laparoscopic pelvic lymph node dissection.
Figure 1. Port placement for transperitoneal laparoscopic pelvic lymph node dissection.
After initial access is obtained, it is important to identify key landmarks to ensure full removal of nodal tissue and minimize injury to surrounding structures. If PLND is performed at the time of RALRP, the lymph node dissection is most commonly performed at the start of the procedure. Alternatively, extended pelvic lymph node dissection may be more easily performed after the prostate is fully removed. If performed at the start of RALRP, robotic or LPLND is performed following bladder mobilization though alternatively may be performed prior to seminal vesical dissection if posterior dissection is performed initially.
The obliterated umbilical ligament is identified medially and the testicular vessels are identified laterally. An incision is made in the posterior peritoneum just lateral to the obliterated umbilical ligament, carried up to the pubic bone, and extended downward and medial to the external iliac artery pulsations. The vas deferens is identified and transected. The external iliac vein is identified, the lymphatic tissue is dissected away from the vein, and the pelvic sidewall and obturator nerve are identified. The borders of the nodal packet are the pelvic side wall laterally, the external iliac vein anteriorly, the obturator nerve and vessels posteriorly, the pubic bone inferiorly, and the obliterated umbilical ligament medially. The nodal pack is dissected superiorly toward the bifurcation of the iliac vessels and subsequently transected. The nodal packet is removed, hemostasis ensured, and port sites closed in the usual fashion.
COMPLICATIONS
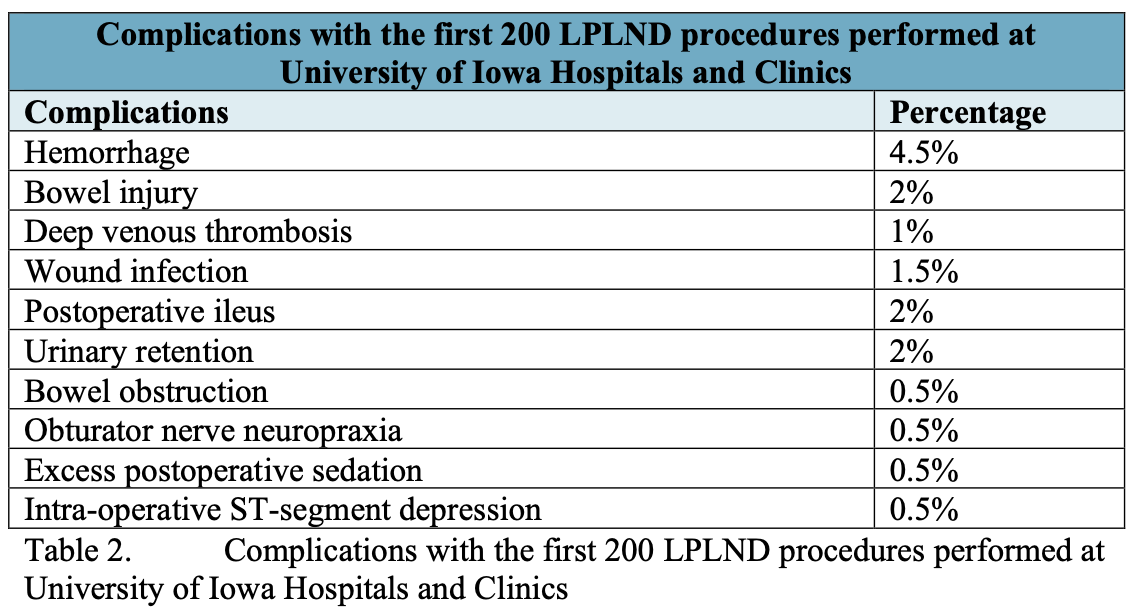
Fortunately, complications following robotic or LPLND are rare and include those common to all laparoscopic procedures, such as bowel injury and major vascular injury, as well as those unique to LPLND. In early series, the complication rate for LPLND was as high as 15%.2 However, increased experience with LPLND, robotic PLND, and other laparoscopic and robotic procedures has decreased this complication rate considerably. A summary of complications of the first 200 LPLND procedures performed at the University of Iowa is presented in Table 2.
Vascular Injury
Minor vascular injuries can occur due to transection of an abdominal wall vessel during port placement. Injuries to the inferior epigastric artery can be avoided by placing ports sufficiently lateral to the border of the rectus muscles. In addition, transmitting light through the anterior abdominal wall and inspection of the peritoneal surface can minimize the chance of injury. An injured or transected abdominal vessel is best controlled by percutaneous suture placement as would be performed for port closure.
Fortunately, injuries to the external iliac artery or vein are exceedingly rare. More often, smaller accessory vessels (such as accessory obturator vessels) can be inadvertently transected. The obliterated umbilical artery is patent in some patients and care should be taken to avoid injury. Hemorrhage resulting from transection of accessory vessels is usually easily controlled with the judicious use of bipolar electrocautery or cautious use of clips. Careful inspection of the obturator fossa under low insufflation pressure should be conducted at the conclusion of the case.
Major vascular injuries, whether occurring during initial access or during intra-operative dissection, can necessitate conversion to an open procedure. While minor injuries to the external iliac vein can generally be managed with compression, hemostatic agents, and gentle bipolar electrocautery, the majority of major vascular injuries must be repaired formally either laparoscopically, robotically, or in an open fashion. Prevention of major vascular injuries is paramount, and hence extreme care should be taken when dissecting near the external iliac artery and vein. In particular, when performing robotic PLND, the surgeon must be exceedingly cautious when performing dissection near the major vessels, especially given the lack of tactile feedback. Moreover, the assistant surgeon (bedside assistant) must take care when exchanging instruments to avoid inadvertent injury to major vessels.
Laparoscopic or robotic repair of major vascular injuries during LPLND can be challenging to perform in the face of severe hemorrhage. When these occur, the surgeon may attempt to repair these injuries with laparoscopy or robotic assistance. In general direct compression of the injury (by either the assistant surgeon or by the fourth arm) is necessary to try and slow the hemorrhage so that some visualization of the injured area is possible. Next, suture repair must be performed; the use of self-locking suture clips, such as Lapra Ty clips, may greatly facilitate such repair. Often, if one stitch is placed properly at or near the location of the injury, then further tension by the single stitch will significantly reduce hemorrhage to the point where repair is possible. If hemorrhage is severe and/or injury is not feasible, then immediate open conversion is required.
Injury to Viscous Structures
Bowel injuries can occur during initial access, as with other laparoscopic procedures. In addition, during LPLND or RALRP the sigmoid colon must be occasionally reflected medially to provide access to the left obturator fossa. Caution should be exercised when handling the sigmoid colon and any injury must be repaired intraoperatively. Rectal injury generally occurs during RALRP and not during LPLND. In general, minor serosal tears of the large and small bowel can be managed with simple interrupted stitches to reapproximate the serosa. If a full thickness bowel injury occurs, then the decision must be made to repair the injury or to perform a bowel resection with primary anastamosis. Intraoperative surgical consultation is required for such major bowel injuries. Caution must be made to avoid thermal injuries of the bowel, particularly when lysis of adhesions from prior surgery is performed. Prevention of and management of bowel injuries during laparoscopic urologic surgery is discussed in another chapter in this text.
Ureteral injury during LPLND is exceedingly rare, with an incidence of 0.5% in one series.2 If identified intra-operatively, repair or reimplant should be immediately performed. To prevent ureteral injury, the distal aspect of the nodal dissection should be kept lateral to the obliterated umbilical artery, allowing the medial border of the nodal packet to easily separate from surrounding tissues. Bladder injury during LPLND is also rare, with an incidence of 0.8%.2 Bladder injury can occur either during trocar placement or during the nodal dissection, and should be suspected if hematuria or pneumaturia occurs intraoperatively. Injury can also occur during bladder mobilization during RALRP, though this is uncommon in the absence of prior pelvic surgery. Repair should be performed intraoperatively by laparoscopic or robotic technique or by open technique if extensive. Maintaining the nodal dissection lateral to the obliterated umbilical ligament can reduce the risk of a bladder injury. If recognized postoperatively, a conservative trial of catheter drainage can be attempted, but laparoscopic or open repair should be performed if bladder leakage persists.
Lymphocele Formation
Lymphocele formation following LPLND is rare and occurs primarily when the extraperitoneal route has been utilized, with an incidence of <1.5%.2 It also appears to be more common in patients who have had previous external beam radiation for localized prostate cancer. Lymphoceles can be managed with percutaneous drainage and sclerotherapy or alternatively, with laparoscopic lymphocele drainage and cyst marsupiliazation. Following transperitoneal laparoscopic pelvic lymph node dissection, a peritoneal window is created, and this in general prevents lymphocele formation.
Obturator Nerve Injury
Obturator nerve injury can occur either with complete transection of the obturator nerve or from inadvertent thermal injury. Monopolar cautery can transmit heat to adjacent tissue, including the obturator nerve. Partial nerve injuries resulting from inadvertent injury to the obturator nerve usually resolve within weeks. Transection of the obturator nerve, albeit exceedingly rare, should be managed by immediate nerve re-anastomosis. Inadvertent clipping of the obturator nerve is rare, as the use of clips during LPLND or robotic PLND is uncommon. The surgeon should identify the obturator nerve before using any clips to avoid inadvertent clipping of the nerve.
Obturator nerve injury from inadvertent coagulation is more common using monopolar cautery. Mild hemorrhage, which occurs during LPLND dissection, should be controlled alternatively with bipolar coagulation. Dissection of the proximal extent of the nodal packet can be challenging because of the bulk of the packet and also the inability to fully visualize the obturator nerve. The obturator nerve should be fully visualized at all times during the later stages of the node dissection, and monopolar or bipolar cautery must be used sparingly in close proximity to the nerve. In addition, if performing robotic assisted LPLND, particularly on the newer DaVinci SI robot, extreme caution must be made to ensure that the correct pedal (bipolar or monopolar) mode is selected to avoid inadvertent coagulation of the obturator nerve, especially when using a bipolar instrument in one hand and a monopolar instrument in the other.
Other Complications
Deep venous thrombosis is a potential complication of any pelvic surgery, not exclusive to LPLND. All patients undergoing LPLND should have lower extremity venous compression devices in place pre-operatively, intra-operatively, and postoperatively until ambulatory.
Urinary retention has been rarely noted following LPLND and nearly always resolves with temporary catheter drainage. Other complications not necessarily unique to LPLND include prolonged ileus, small bowel obstruction, pelvic hematoma or abscess, wound infection, and port site hernia.
CONCLUSION
Once the most common urologic laparoscopic procedure performed, LPLND remains an important procedure that offers a minimally invasive means of obtaining accurate staging information for select patients with urologic malignancies. Today, LPLND is most commonly performed concurrently at the time of RALRP. The complication rate for LPLND is low, and should decrease further once the learning curve for the surgeon is overcome.
References
- Schuessler WW, Vancaillie TG, Reich H, et al. Transperitoneal endosurgical lymphadenectomy in patients with localized prostate cancer. J Urol 1991; 145:988-991.
- Kavoussi LR, Sosa E, Chandhoke P, et al. Complications of laparoscopic pelvic lymph node dissection. J Urol 1993; 149(2):322-325.
