Laparoscopic Myomectomy
Ceana Nezhat, MD, Kimberly Kho, MD, Herbert Goldfarb, MD, Daniel Seidman, MD
INTRODUCTION
Benign diseases of the uterus are found commonly in gynecologic patients and account for most laparotomies and hysterectomies. Myomas are the most common uterine neoplasm, affecting approximately 20 to 25% of women of reproductive age.1,2 They can develop in any area where there are smooth muscle cells of mullerian origin, such as the fallopian tubes, uterine corpus, and cervix. They arise from the benign transformation and proliferation of smooth muscle cells. Increased estrogen stimulation alone or in concert with growth hormone or human placental lactogen are the major growth regulators.
Progesterone appears to inhibit the growth of myomas but under certain conditions may promote their growth.3
SYMPTOMS
The severity and type of symptoms associated with uterine leiomyomas are dependent on their number, size, and location. Common symptoms are abnormal uterine bleeding, abdominal pressure, urinary frequency, and constipation. Although they are seldom the only cause of infertility, data from several studies shows a link between myomas, fetal wastage, and premature delivery.1 Indications for treatment are summarized in Table 1. Factors such as the size, number, and location of the myomas influence the choice of the operation.
PREOPERATIVE EVALUATION AND TREATMENT
In patients with leiomyomas who complain of menorrhagia, the hematocrit is used to assess the degree of anemia. For anemic patients, preoperative suppressive therapies such as danazol or gonadotropic-releasing hormone agonists (GnRH), may enable restoration of a normal hematocrit, decrease the size of myomas,4 and reduce the need for transfusion.1 However, studies that examined the effects of GnRH agonists on intraoperative blood loss have revealed conflicting results.5,6 GnRH agonists have negative side effects as well such as the development of a pseudo-menopausal hypoestrogenic state, and are associated with a possible increased risk of myoma recurrence.4 In addition, studies have also shown that agonist therapy can soften myomas, obscuring the cleavage plane between the fibroid and the pseudocapsule, making the surgery more difficult with increased bleeding.7
The presence of large broad ligament myomas may necessitate the performance of an intravenous pyelogram to search for ureteral obstruction. Periodic pelvic and ultrasound examinations aid in monitoring the growth rate of asymptomatic myomas. Submucous myomas can be detected by pelvic ultrasound, hysterosonogram, or hysteroscopy. Since small interstitial myomas palpated during laparotomy can be missed at laparoscopy, a vaginal ultrasound should be done preoperatively, and can also be performed intraoperatively to aid in myoma identification and localization.4,5
Depending on the myoma’s size and location, preoperative autologous blood donation may be suggested. Patients are counseled regarding the potential for intra- and post- operative bleeding and the possible need for a laparotomy as well as blood transfusion. Although myomectomy may rarely result in hysterectomy, patients should be informed of this possibility.
LAPAROSCOPIC MYOMECTOMY
Women who have large intramural fibroids should be managed laparoscopically only if the surgeon is capable of meticulous repair of the uterus which can be difficult. Nezhat et al. reported the results of myomectomies performed on 137 women from whom 196 myomas were removed.8 The fibroids ranged in size from 2 to 14 cm. The operative time ranged from 50 to 160 minutes (mean: 116 minutes). Estimated blood loss was between 10 and 600 mL, and two women received blood transfusions because of intraoperative blood loss. The hospital stays ranged from 7 to 48 hours, with a mean of 19.6 hours.
In 114 women undergoing laparoscopic myomectomy (LM) who desired future pregnancy, the average number of myomas removed was 3.0 +/- 2.9 and the mean size was 5.9 +/- 3.0 cm.9 In 52.4% of the cases, the most deeply infiltrating myoma was intramural, in 42.9% subserosal, and in 4.7% pedunculated. Thirty-one pregnancies occurred in 29 women. Of the 26 that could be followed, 5 had vaginal deliveries at term. Cesarean sections were done for 14 patients: 9 at term, 1 at 26 weeks, and 4 at unknown gestational ages. Six women miscarried in the first trimester, and one had an ectopic pregnancy. No spontaneous uterine ruptures were noted. Compared with women with ectopic pregnancies, miscarriages, and preterm deliveries, those who delivered at term were younger (33.1 +/- 1.9 versus 36.6 +/- 4.8 years, p < 0.005) and had fewer myomas at surgery (1.9 +/- 2.0 versus 4.8 +/- 3.0, p < 0.05). Those who had intramural myomas were most likely to develop complications during pregnancy. In another study, 28 infertile patients with at least one uterine leiomyoma of > 5cm in diameter underwent laparoscopic myomectomy.10 The average size of the myomas removed was 6 cm (range: 4 to 13.3 cm). The postoperative intrauterine pregnancy rate was 64.3% (n = 18), including one of two patients who underwent concomitant hysteroscopic myomectomy.
Four patients had spontaneous abortions, and 14 delivered viable term neonates. Six patients had a vaginal delivery without complications, and 8 had a cesarean delivery.
TECHNIQUE1
Essential instruments to perform a laparoscopic myomectomy include cutting devices such as the CO2 laser, unipolar electrode, or harmonic scalpel, a bipolar Kleppinger forceps, and clawed grasping forceps. Newer sealing devices using bipolar radiofrequency can be used for pedunculated myomas. In addition, dilute vasopressin (Pitressin: 1 Ampule of 20 IU in 60-100 mL lactated Ringer’s solution) decreases the amount of blood loss.
Dilute Pitressin in increments of 3-5 mL is injected into the base of the myoma stalk at its junction with the uterine fundus until blanching is visualized. Pedunculated leiomyomas are removed by coagulating and cutting the stalk. Bleeding areas are coagulated with bipolar forceps (Figure 1) and suturing of the serosa if necessary. For intramural myomas, the technique of myomectomy is different (Figures 2A, 2B, 2C, 2D, and 2E). Dilute vasopressin is injected into multiple sites between the myometrium and fibroid capsule. An incision is made on the serosa overlying the leiomyoma, using the CO2 laser (superpulse or ultrapulse mode), a monopolar electrode, or harmonic scalpel. The incision is extended until it reaches the level of the fibroid capsule. The myometrium retracts as the incision is made, exposing the tumor. Two grasping, toothed forceps hold the edges of the myometrium, and the suction-irrigator is used as a blunt probe to shell out the leiomyoma from its capsule. A myoma screw or laparoscopic single toothed tenaculum is inserted into the leiomyoma to apply traction while the suction-irrigator is used as a blunt dissector. Vessels are electrocoagulated before being cut.
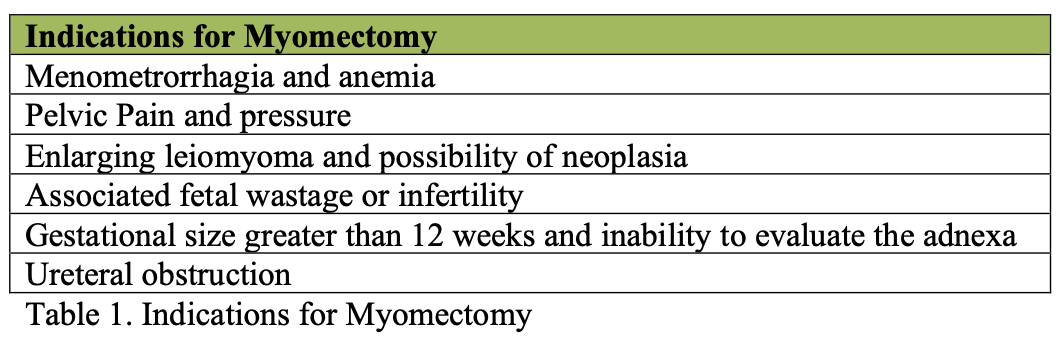
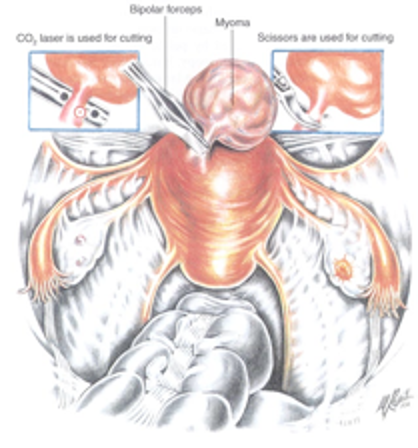 Figure 1. A pedunculated myoma is seen and its pedicle is electrocoagulated with a bipolar forceps. After this maneuver, the myoma can be cut with the CO2 laser (left inset) or the electrosurgical scissors (right inset). Reproduced with permission from Nezhat et al. for Operative Gynecologic Laparoscopy: Prinicples and Techniques.
Figure 1. A pedunculated myoma is seen and its pedicle is electrocoagulated with a bipolar forceps. After this maneuver, the myoma can be cut with the CO2 laser (left inset) or the electrosurgical scissors (right inset). Reproduced with permission from Nezhat et al. for Operative Gynecologic Laparoscopy: Prinicples and Techniques.
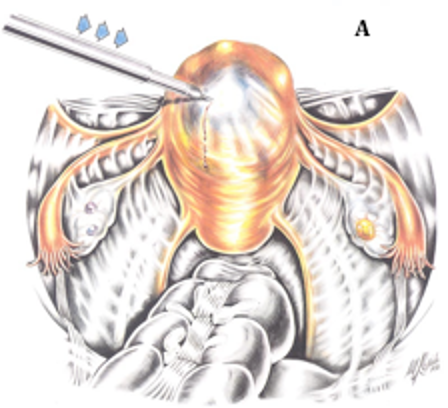 Figure 2A. Dilute vasopressin is injected into several sites between the myometrium and the myoma. It is essential to be certain that this injection is not intravascular, and the anesthesiologist should be alert of the possibility of acute hypotension. Reproduced with permission from Nezhat et al. from Operative Gynecologic Laparoscopy: Principles and Techniques.
Figure 2A. Dilute vasopressin is injected into several sites between the myometrium and the myoma. It is essential to be certain that this injection is not intravascular, and the anesthesiologist should be alert of the possibility of acute hypotension. Reproduced with permission from Nezhat et al. from Operative Gynecologic Laparoscopy: Principles and Techniques.
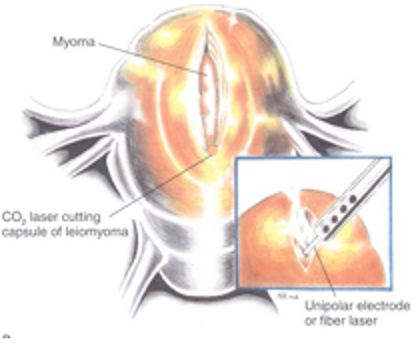 Figure 2B. An incision has been made into the myometrium over the myoma, using a CO2 laser, fiber laser, or monopolar electroduce (inset). The incision extends until it reaches the myoma; the myometrium retracts as the tumor becomes visible. Reproduced with permission from Nezhat et al. from Operative Gynecologic Laparoscopy: Principles and Techniques.
Figure 2B. An incision has been made into the myometrium over the myoma, using a CO2 laser, fiber laser, or monopolar electroduce (inset). The incision extends until it reaches the myoma; the myometrium retracts as the tumor becomes visible. Reproduced with permission from Nezhat et al. from Operative Gynecologic Laparoscopy: Principles and Techniques.
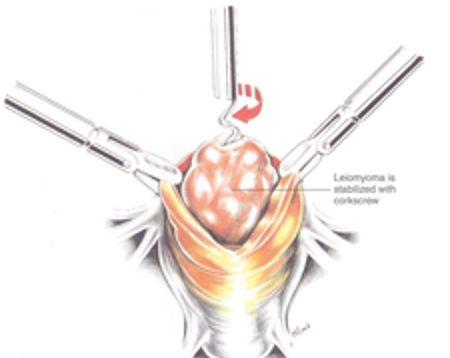 Figure 2C. Two grasping toothed forceps hold the edges of the myometrium, and a myoma screw is inserted into the myoma to apply traction. Reproduced with permission from Nezhat et al. from Operative Gynecologic Laparoscopy: Principles and Techniques.
Figure 2C. Two grasping toothed forceps hold the edges of the myometrium, and a myoma screw is inserted into the myoma to apply traction. Reproduced with permission from Nezhat et al. from Operative Gynecologic Laparoscopy: Principles and Techniques.
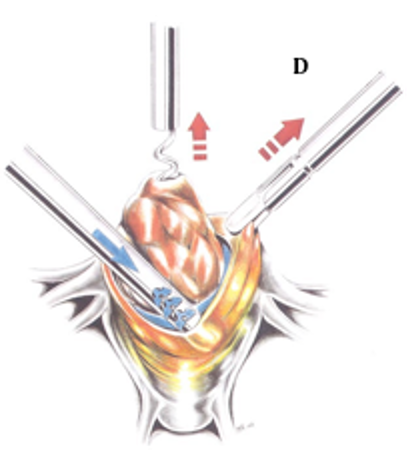 Figure 2D. The suction irrigator, acting as a blunt dissector, is used as a probe to aid in the enucleation of the tumor. Reproduced with permission from Nezhat et al. from Operative Gynecologic Laparoscopy: Principles and Techniques.
Figure 2D. The suction irrigator, acting as a blunt dissector, is used as a probe to aid in the enucleation of the tumor. Reproduced with permission from Nezhat et al. from Operative Gynecologic Laparoscopy: Principles and Techniques.
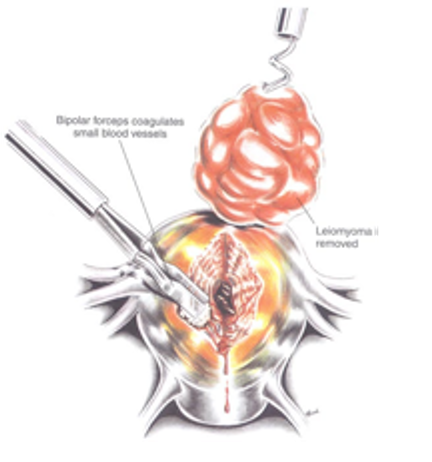 Figure 2E. Myometrial vessels are electrocoagulated as the myoma is removed. After the enucleation process, hemostasis is obtained with the bipolar electrode is the exposed myometrial surfaces are irrigated. Reproduced with permission from Nezhat et al. from Operative Gynecologic Laparoscopy: Principles and Techniques.
Figure 2E. Myometrial vessels are electrocoagulated as the myoma is removed. After the enucleation process, hemostasis is obtained with the bipolar electrode is the exposed myometrial surfaces are irrigated. Reproduced with permission from Nezhat et al. from Operative Gynecologic Laparoscopy: Principles and Techniques.
After complete removal of the myoma, the uterine defect is irrigated. Bleeding points are identified and controlled with electrocoagulation. Care is taken to minimize the use of energy and tissue desiccation so as to decrease tissue necrosis. The edges of the uterine defect are approximated by suturing.
If the defect is deep or large, the myometrium and serosa are approximated by using 4-0 polydioxanone and 1-0 polyglactin sutures. While two layers of sutures can be applied, this maneuver may be difficult to perform. The repair of the serosal and subserosal layers and can be accomplished in one layer. For deeper myometrial defects we use 1-0 and 2-0 polyglactin in layers for closure or the myometrium and polydioxane for the serosa. The sutures are applied in 1 cm increments, using intra or extracorporeal knot tying (Figures 3A, 3B, and 3C). Recently, the use of bidirectional barbed suture, Quill, (Angiotech, Vancouver British Columbia) has been proposed to assist with approximation of myometrial defects. Proposed benefits include more evenly distributed wound tension and obviation of the need for knot tying.11
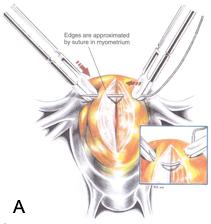 Figure 3A. If the myometrial defect is large or deep, the edges are approximated with 4-0 polydioxanone or 1-0 polyglactin suture on a straight or curved needle (inset). The suture is brought through the superficial myometrium and serosa in a one-layer closure. Reproduced with permission from Nezhat et al. from Operative Gynecologic Laparoscopy: Principles and Techniques.
Figure 3A. If the myometrial defect is large or deep, the edges are approximated with 4-0 polydioxanone or 1-0 polyglactin suture on a straight or curved needle (inset). The suture is brought through the superficial myometrium and serosa in a one-layer closure. Reproduced with permission from Nezhat et al. from Operative Gynecologic Laparoscopy: Principles and Techniques.
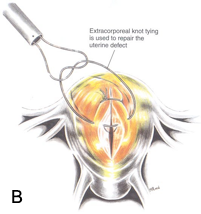 Figure 3B. The sutures are applied in 1-cm increments by using extracorporeal or intracorporeal knot-tying techniques.
Figure 3B. The sutures are applied in 1-cm increments by using extracorporeal or intracorporeal knot-tying techniques.
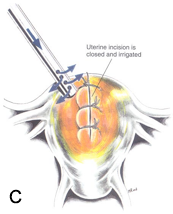 Figure 3C. After the approximation of the uterine wound, the surface is irrigated with warm lactated Ringer’s solution to locate any oozing that might require electrocoagulation. Reproduced with permission from Nezhat et al. from Operative Gynecologic Laparoscopy: Principles and Techniques.
Figure 3C. After the approximation of the uterine wound, the surface is irrigated with warm lactated Ringer’s solution to locate any oozing that might require electrocoagulation. Reproduced with permission from Nezhat et al. from Operative Gynecologic Laparoscopy: Principles and Techniques.
Although suturing remains one of the most skill-dependant parts of the procedure, in the past decade there has been significant advancement in instrumentation with the development of various types of needle holders ( i.e. Koh suturing set by Storz), suturing devices such as Endostitch (Covidien), and facilitators such as Laparotie (Ethicon, Somerville, NJ ). Also the introduction of pelvic trainers and courses on suturing has made it easier to teach and learn how to perform laparoscopic suturing.
Suturing is limited because of the high incidence of postoperative adhesions, especially when sutures are placed in the serosal layer.8,12 In order to decrease the risk of adhesion development and to improve uterine repair integrity, intraoperative planning is advised to minimize the number of hysterotomies. One uterine incision is advised for the removal of multiple leiomyomas. Though microsurgical closure of the myometrial defect can be difficult, the goal should be good approximation without strangulation.
After repair, the uterine surface is irrigated with warmed lactated Ringer’s solution and an anti-adhesive barrier such as Interceed (Ethicon, Somerville, NJ) is applied over the suture line (Figure 4). If Interceed is used, excellent hemostasis must be obtained at the site of application to ensure optimal results.13 Spraying 10,000 IU of thrombin over the Interceed may contribute to the maintenance of hemostasis. Use of additional hemostatic agents such as a gelatin-thrombin matrix have been reported to be useful in achieving hemostasis and can be applied easily to the suture line during laparoscopy.14
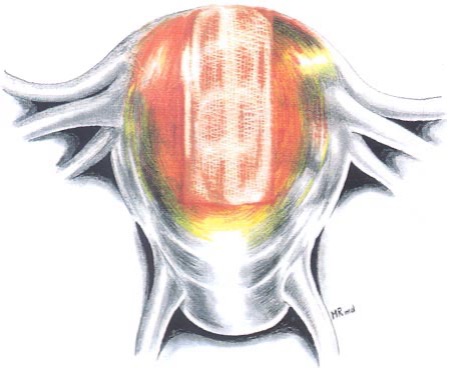 Figure 4. The myometrial defect is covered with Interceed. Reproduced with permission from Nezhat et al. from Operative Gynecologic Laparoscopy: Principles and Techniques.
Figure 4. The myometrial defect is covered with Interceed. Reproduced with permission from Nezhat et al. from Operative Gynecologic Laparoscopy: Principles and Techniques.
Intraligamentous and broad ligament myomas require careful observation of the course of the ureters and large blood vessels. Depending on the location of the myoma, an incision is made on the anterior or posterior leaf of the broad ligament. The myoma is removed employing the techniques described above for its subserosal and intramural counterparts. Throughout the procedure, the location of the ureters should be noted. Hemostasis is obtained through the careful use of sutures, clips, or bipolar forceps. None of the available lasers, regardless of the power setting or focus of the beam, adequately can coagulate bleeding myometrial vessels. A bipolar forceps or argon beam coagulator has been used for this purpose. Care should be taken to minimize use of energy to decrease the chances of tissue necrosis. Hemostatic suturing is preferred when ever is possible.
The broad ligament and peritoneum are not closed but allowed to heal spontaneously. Drains are used infrequently.
Removal of myomas from the abdominal cavity is a time-consuming procedure, and no methods or instruments are ideally suited for this purpose.
- A claw-toothed forceps is inserted through a 10-mm sleeve for a myoma < 5 cm.
- A long Kocher clamp is inserted through one of the ancillary trocar incisions. The midline incision, which must often be extended, is preferred to avoid injury to the inferior epigastric arteries. This technique is quick and easily performed.
- Larger myomas can be removed through a posterior colpotomy15 (Figures 5 and 6). Potential risks are infectious morbidity and the risk of bowel and ureteral injury; in women with concurrent posterior cul-de-sac abnormalities, colpotomy is not safe unless anatomy is restored with treatment of endometriosis, adhesions, or any other pathology.
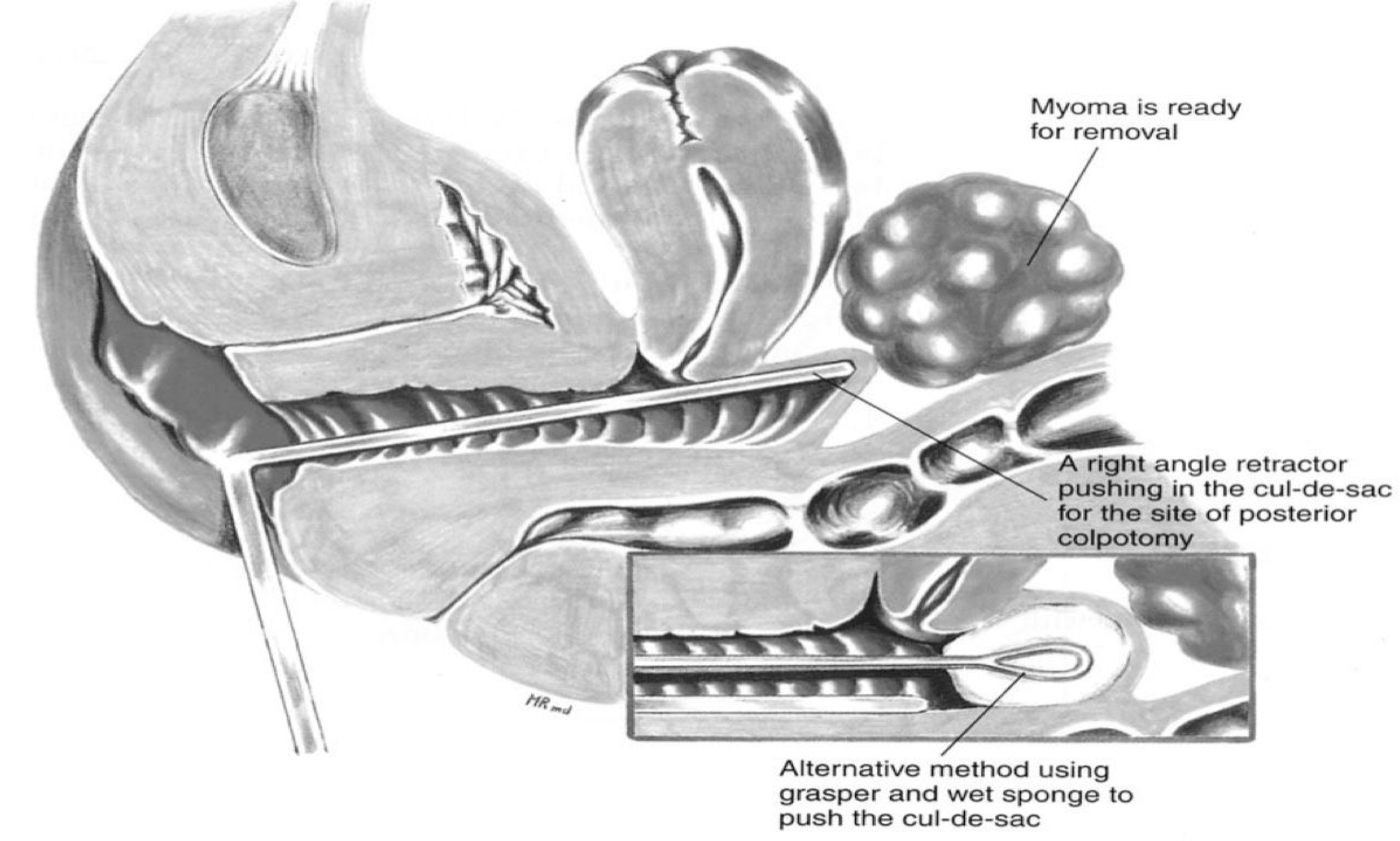 Figure 5. Some myomas are removed through the posterior colpotomy. The right-angle retractor pushes the vagina towards the myoma, indicating the site for the vaginal incision. The inset shows the use of a sponge on a sponge stick to accomplish the same purpose.
Figure 5. Some myomas are removed through the posterior colpotomy. The right-angle retractor pushes the vagina towards the myoma, indicating the site for the vaginal incision. The inset shows the use of a sponge on a sponge stick to accomplish the same purpose.
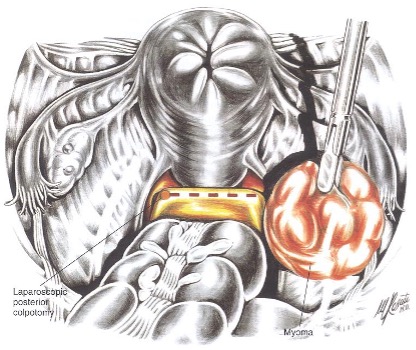 Figure 6. This illustration shows the laparoscopic view of the proposed incisional site. The edges of the uterine incision have been approximated without suturing. Reproduced with permission from Nezhat et al. from Operative Gynecologic Laparoscopy: Principles and Techniques.
Figure 6. This illustration shows the laparoscopic view of the proposed incisional site. The edges of the uterine incision have been approximated without suturing. Reproduced with permission from Nezhat et al. from Operative Gynecologic Laparoscopy: Principles and Techniques.
Improvements in electronic morcellators have made this task easier. Examples include electric morcellators from Karl Storz (Tuttlingen, Germany), Gynecare (Ethicon, Summerville, NJ) and SEMM/Wisap (Wisap, Muchen, Germany), which come in 12, 15, 20 and 25 mm diameters with variable speeds. Gyrus ACMI (Southborough, MA) has recently introduced a nonelectric morcellator that utilizes bipolar technology. Smoke can be a negative factor if not evacuated properly. Though expedient, use of these devices require larger incisions and increase the risk of collateral abdominal wall vascular injury, especially if placed laterally. Also, if not appropriately closed, there is an increased risk of incisional hernias at the port sites where the morcellators are introduced due to the increased and oftentimes forceful manipulation at these sites. Extreme care should be taken when using electric morcellators as inadvertent visceral and vascular injuries resulting in deaths have been reported.16 With large or calcified myomas, the morcellator blade may grow dull necessitating the use of multiple devices. This leads to additional financial and ecological costs especially when multiple disposable instruments are used.
Myoma extraction can be cumbersome in addition to time consuming. Large myomas can be removed through a posterior colpotomy without additional risk of adhesion formation. Though improvements in the design of electronic morcellators have made their use easier and faster, the morcellator remains a bulky and expensive device. Additionally, the process of morcellation produces fragments of tissue which may be left behind and go on to seed the peritoneal cavity. This tissue can go on to implant ectopically anywhere in the abdomen and produce parasitic myomas, masses, adenomyosis, and potentially malignancy. In the literature, an increasing number of case reports of parasitic myomas have been described in relation to laparoscopic uterine morcellation.17-19 Kho and Nezhat reported a large series of parasitic myomas in which several large, symptomatic myomas were found after laparoscopic myomectomy.20 Myomas were found in various locations including the retroperitoneum and on the bladder, appendix, and liver surfaces.
Furthermore, though rare, isolated cases of endometriosis, adenomyosis, and potential dissemination of myomalignant cells with morcellation have been reported.21-23
Patients who attempt a pregnancy after a laparoscopic myomectomy risk uterine rupture in a subsequent pregnancy6 because of the difficulties of adequately closing all layers laparoscopically,8,12 which results in inadequate myometrial approximation and poor healing. Uterine rupture following myomectomy is still a rare phenomenon however, accounting for about 2% of all pregnancy-related uterine ruptures.13 Uteroperitoneal fistulas can also occur postoperatively.
LAPAROSCOPICALLY ASSISTED MYOMECTOMY
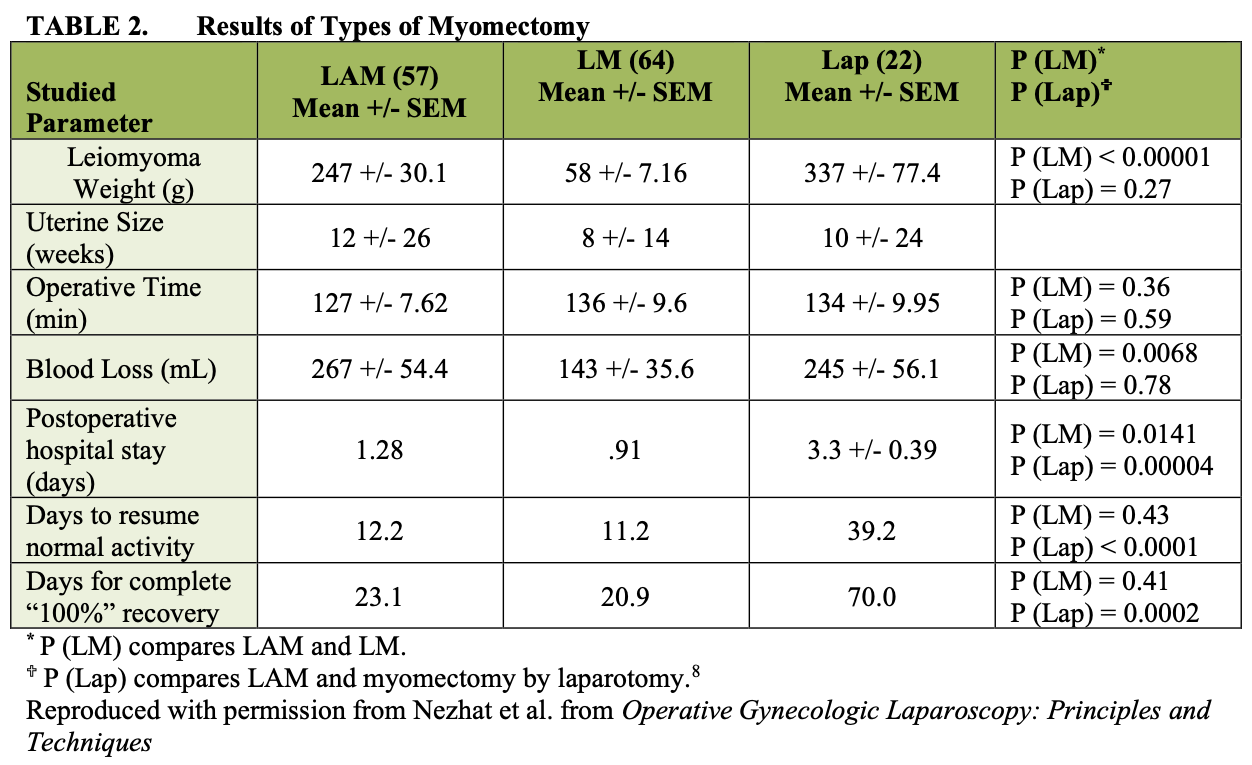
Laparoscopically assisted myomectomy (LAM) is a safe alternative to LM. It is less difficult and requires less time to complete. These considerations are summarized in Table 2. The decision to perform LAM usually is made in the operating room after diagnostic laparoscopy and treatment of other pelvic abnormalities are completed. Cases for which LAM may be superior to LM include a myoma greater than 8 cm, many myomas requiring extensive morcellation, and a deep, large, intramural myoma that requires uterine repair in multiple layers.
The hybrid approach first employs laparoscopy to map pathology, perform adhesiolysis, and treat endometriosis or other pathologies. Then the myomectomy is performed via a 3- 4 cm mini-laparotomy; this allows for full palpation of the myometrium and also facilitates easier, faster, and more secure closure of the hysterotomy. Conventional uterine suturing in two or three layers reduces the potential for uterine dehiscence, fistulas, and adhesions. Morcellation is faster with diminished risk of seeding tissue. By allowing for simultaneous laparoscopic treatment of associated abdominopelvic pathology, such as endometriosis, the patient benefits from a more complete treatment than myomectomy by laparotomy alone. Postoperative pain and recovery are similar to LM, and recent comparative studies have demonstrated the uterine incision is smaller with LAM.
Three major objectives of LAM are reduction of blood loss, prevention of postoperative adhesions, and maintenance of myometrial integrity. LAM, with “open” morcellation and conventional suturing, reduces the duration of the operation and the need for more extensive laparoscopic experience. This approach may enable more gynecologists to apply a minimally invasive technique.
Intraoperatively, injections of dilute vasopressin into the myoma help reduce blood loss. Vertical uterine incisions bleed less than do transverse incisions, and pneumoperitoneum seems to decrease intraoperative bleeding.1
TECHNIQUE
In patients with multiple myomas, the most prominent myoma is injected at its base with 3 to 7 mL of diluted vasopressin. A vertical incision is made over the uterine serosa onto the surface of the myoma and extended until the capsule of the leiomyoma is reached. A corkscrew manipulator or single tooth grasper is inserted into the leiomyoma and used to elevate the uterus toward the midline suprapubic puncture site. With the trocar and manipulator attached to the myoma, this midline 5-mm puncture is enlarged to a 2-4 cm transverse skin incision. After the fascia is incised in a transverse fashion, the rectus muscle is divided using a monopolar electrode. If the inferior epigastric vessels are found, they are coagulated. This approach provides excellent access to the abdominal cavity. There are numerous would retractors, such as Alexis (Applied Medical, Rancho Santa Margarita, CA), etc., which facilitate exposure through small incisions.
The peritoneum is entered transversely, and the leiomyoma can now be visualized. It is brought to the mini-laparotomy incision by using the corkscrew manipulator to raise the uterus. The corkscrew manipulator is then replaced with two Lahey tenacula. The myoma is shelled and morcellated sequentially, and after its complete removal, the uterine wall defect shows through the incision. If uterine size and mobility allow it to be exteriorized, this is done in order to complete the repair.
When multiple leiomyomas are found, as many as possible are removed through one uterine incision. When other myomas are located which cannot be removed via the same uterine incision, the abdominal 2-4 cm opening is approximated temporarily with two or three Allis clamps or an inflated latex glove to assist in reestablishing the pneumoperitoneum. The laparoscope is reintroduced, and the remaining myomas are identified and brought to the level of the abdominal incision. They are removed under laparoscopic control. If possible, the uterus is exteriorized, and the myometrium is closed in layers with 0 and 2-0 polydioxanone or Quill SRS (Angiotech, Vancouver, BC, Canada) sutures. The serosa is closed microsurgically. The uterus is palpated to ensure that no small intramural myomas remain and it is then returned to the peritoneal cavity.
The fascia is closed with 1-0 polyglactin suture, and the skin is closed in a subcuticular fashion. The laparoscope is re-introduced to evaluate hemostasis. The pelvis is reexamined to detect and treat endometriosis and adhesions that may have been obscured previously by myomas. Copious irrigation is used, blood clots are removed, and an anti- adhesion barrier may be applied over the uterus to help decrease adhesion formation.
COMPARISON OF RESULTS OF LAM AND LM
We performed a retrospective chart review of 143 charts from our practice. Those patients who had myomectomy by laparotomy (15.3%), laparoscopic myomectomy (44.7%), or laparoscopically assisted myomectomy (39.8%) were included (Tables 2 and 3).8 The 22 myomectomies by laparotomy were done before the development of LAM. Since LAM replaced myomectomy by laparotomy and patient selection criteria were comparable, the myoma weights of the two groups were similar.
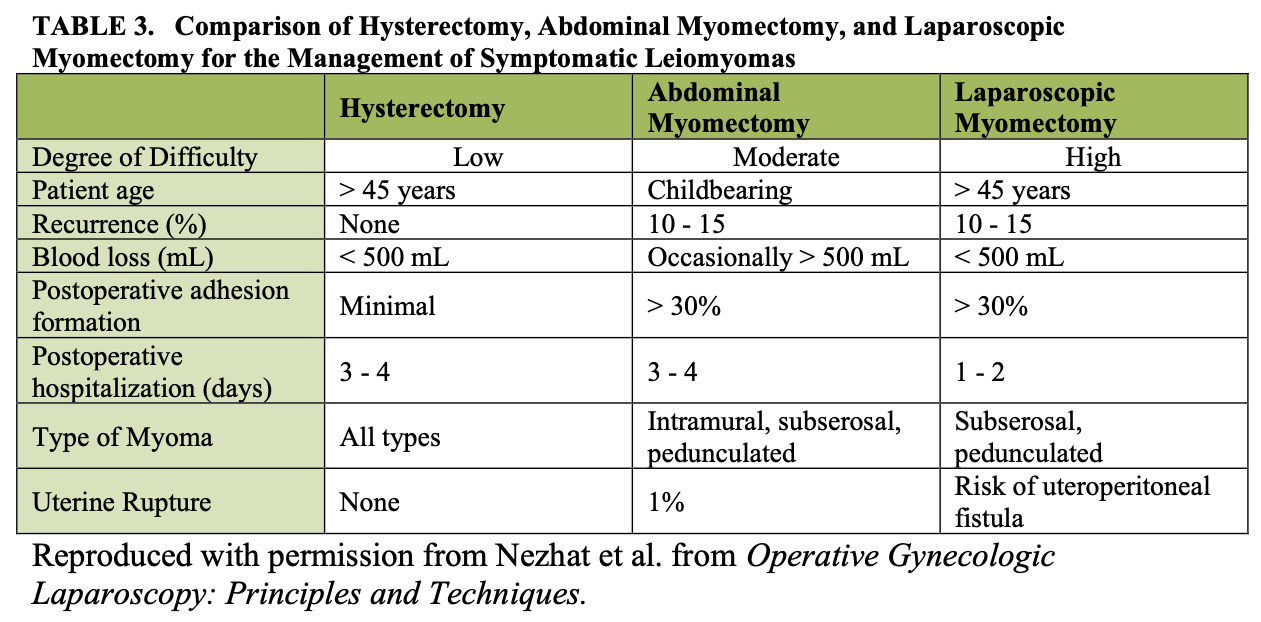
Mean operating times were the same for LM and LAM despite larger myomas, difficult locations of intramural myomas, and adjunctive laparoscopy in the latter group. The leiomyoma weights were greater in the LAM group than in the LM group (p< 0.05).
The mean estimated blood loss of the LAM and laparotomy groups was not different. In contrast, blood loss among the LM patients was significantly lower, and this may be attributed to the smaller leiomyomas. In comparing the hospitalization time of LAM and LM patients, that of the LAM group appeared to be longer (p(LM) = 0.014). This may be explained by the initial reluctance of some physicians to discharge LAM patients on the day of the operation or on postoperative day 1. After the initial 10 to 15 operations, all patients underwent LAM on an outpatient basis. In fact, when the initial 15 cases are removed from the LAM group, the mean hospital stay drops to 1.06 days. This period is not statistically different from that of the LM group.
The comparison of postoperative recovery times shows important distinctions between the LAM and LM groups. Here, despite differences in size and location of myomas removed, recovery times can be compared because of the different types of incisions; the time elapsed before patients resumed work or regular activity was similar (p > 0.05).
However, introducing a 4 cm mini-laparotomy incision in the LAM group did appear to prolong (p > 0.05) the subjectively perceived time for the women to achieve 100% recovery. Hospitalization was much longer for the patients who underwent myomectomy by laparotomy (p < 0.05) compared with both the LAM and the LM groups.
Previous studies underscored the need to decrease the operative time of LM.6,26 While myomas less than 8 cm are managed laparoscopically, those which are larger or intramural in location require prolonged morcellation and laparoscopic suturing of the uterine defect. The largest reported myomas removed by laparoscopy were 15 to 16cm,8,26 while another group reported that 10 cm was its limit.27 Both laparoscopic morcellation and myometrial suturing are difficult and prolong operations.
Second-look laparoscopies of post-myomectomy patients who had pedunculated and superficial subserosal myomas removed without sutures showed complete uterine healing. In contrast, intramural myomas were associated with granulation tissue and indentation of the uterus proportional to the size of the leiomyoma excised unless sutures were used. Use of sutures is associated with more adhesion formation.8
Most patients are observed in an outpatient unit and discharged within 23 hours of the operation, although some can leave the hospital on the afternoon or evening of the procedure. For women of childbearing age who are planning for future pregnancy and require a myomectomy for an intramural tumor one needs to ensure proper closure of the myometrial incision. A cesarean delivery is safest for such patients. The laparoscopic approach is more utilized for pedunculated or subserosal myomas.
ROBOTIC MYOMECTOMY AND ROBOTICALLY ASSISTED MYOMECTOMY
Though studies have shown decreased morbidity associated with laparoscopic myomectomy compared to abdominal myomectomy, the vast majority of myomectomies are still performed as an open procedure.28 Advances in robotic surgery have increased the popularity of this method of minimally invasive surgery for various reasons. Shorter learning curves, a three dimensional vision system, wristed instrumentation, and ergonomic positioning are all advantages to straight laparoscopy.29 Because of the intricate steps required during laparoscopic myomectomy including myoma enucleation and multilayer myometrial closure, straight laparoscopy can be difficult even in the hands of experienced surgeons. Because of its inherent advantages and principles similar to open surgery, robotic technology has the potential to facilitate utilization of a less invasive approach to the surgical management of myomas, thereby increasing this option for patients.
Advincula et al. presented a retrospective case-matched study of robot-assisted laparoscopic myomectomy compared to abdominal myomectomy and found that though costs and operative times were increased in the robotic group, patients experienced less blood loss and had fewer complications than in the laparotomy group.30 Furthermore, length of stay was significantly reduced in the robotic cohort (mean 1.5 days compared with 3.6 days).
In another study, Nezhat et al. compared robot-assisted laparoscopic myomectomies to conventional laparoscopic myomectomy in a case-matched analysis.31 The groups were matched by age, body mass index, parity, previous abdominopelvic surgery, and size, number, and location of the myomas. Though mean surgical time for the robotic myomectomy was longer: 234 minutes (range 140–455) compared with 203 minutes (range 95–330) for laparoscopic myomectomy, blood loss, length of stay, and postoperative complications were not significantly different.
While further long-term randomized studies and cost analyses need to be performed, a main advantage of robot-assisted laparoscopic myomectomy is that it may offer more gynecologists a minimally invasive modality with which they are comfortable over straight laparoscopy.
LAPAROSCOPIC MYOMECTOMY AND POST-OPERATIVE ADHESIONS
While myomectomy is often performed to preserve fertility, post-operative adhesions can jeopardize this goal. Single, vertical, anterior, and midline uterine incisions cause fewer adhesions.1 Although sutures predispose patients to adhesions, they often are necessary to close the uterine defect.32 While several adhesion barriers are available or under development, none of them are completely effective in preventing postoperative adhesion formation.13,33
A prospective randomized, blinded multicenter study evaluated 127 women undergoing uterine myomectomy with at least one posterior uterine incision > 1 cm in length.
Patients were randomized to Seprafilm (HAL-F) Bioresorbable Membrane (Genzyme Corporation, Cambridge, MA) or to no treatment.34 At second-look laparoscopy, the incidence of adhesion formation, measured as the mean number of sites adherent to the uterine surface, was significantly lower in the treated group, as were the mean uterine adhesion severity scores and mean area of adhesions. In another study, 50 premenopausal, nonpregnant women who underwent laparoscopic myomectomy were randomized to the control group (25 patients) with surgery alone, or to the treatment group (25 patients) who had their uterine incisions covered with Interceed (Ethicon, Somerville, NJ).35 At second look laparoscopy 12 to 14 weeks later, 12%(3) of the women in the control group were adhesion free, compared with 60%(15) of those treated with Interceed (p<0.05). In a similar study, 45 patients undergoing myomectomy by either laparoscopy or laparotomy were randomized to treatment with SprayGel (Confluent Surgical, Waltham, MA) or to no treatment. Although the SprayGel group began with a higher incidence of pre-existing adhesions, 28% were adhesion free compared to 8% in the no treatment group.36 Recently, Mettler et al. published data demonstrating Coseal (Angiotech Pharmaceuticals, Inc. Vancouver, Canada), a hydrogel matrix sealant, may be effective in adhesion reduction during myomectomy.37 In 71 women undergoing both abdominal and laparoscopic myomectomy, Coseal was placed on all suture lines. Patients underwent second look laparoscopy at 8-10 weeks and were found to have a significantly lower risk of adhesion formation.
MYOLYSIS
Laparoscopic myolysis or leiomyoma coagulation can be used as an alternative to laparoscopic myomectomy for the treatment of intramural or subserosal leiomyomas. The procedure was developed in Germany in 1986 and was first performed in the United States by Goldfarb in 1990.38,39 Myolysis is designed to destroy the blood supply and subsequently cause stromal death of symptomatic myomas in order to achieve a decrease in their size. A neodymium:yttrium-aluminum-garnet (ND:YAG) laser bare fiber was initially used to perform the procedure,39 although additional modalities such as the monopolar coagulation needle,40 bipolar coagulation needle,39,41 or the hyperthermia electrode (diathermy),42 can also be utilized.
Patients with symptomatic intramural or subserosal leiomyomas who do not desire future pregnancy can be considered for myolysis. The decision to offer myolysis to patients who wish to maintain their fertility is controversial. Although there have been several reported successful pregnancies following myolysis,43 multiple uterine ruptures have also been described. Vilos et al.44 reported that 2 of 3 patients who conceived within 3 months after undergoing myolysis developed a third trimester uterine rupture. The replacement of uterine myomas and myometrium with scar tissue may decrease the strength of the uterine wall, although studies examining the resultant tensile strength have not been performed.43
Myolysis should also not be performed if a suspicion of uterine malignancy exists. Myolysis would not afford the opportunity to obtain a tissue diagnosis and is an inadequate treatment of uterine malignancies.
Preoperatively, GnRH agonists have been used for 2 to 3 months prior to therapy to decrease myoma size, with 30% to 50% reductions in myoma volume having been reported.44,45 Intraoperatively, dilute vasopressin can be injected into the uterine serosa overlying the myoma and into the myoma itself prior to the start of the procedure.43 The instrument to be used for myolysis is then introduced via a 5 mm trocar and multiple punctures are made through the uterine serosa into the myoma towards its base at 5 to 10 mm increments. At the completion of the procedure, the myoma should appear blanched and cyanotic. An anti-adhesion barrier can then be placed over the operative site.
Postoperatively, the symptomatic improvements and decrease in myoma size following myolysis have been promising. Phillips et al.43 reported on 167 patients who underwent myolysis, 52 of whom also had endomyometrial ablations with or without submucous myoma resection due to chronic menorrhagia. Patients were discharged, on average, within 5 hours of the procedure and had mild abdominal cramps that resolved in less than 15 hours. 96% of patients who underwent a concomitant hysteroscopic procedure achieved satisfactory results consisting of amenorrhea, hypomenorrhea, or eumenorrhea, within 6 months of surgery. Total leiomyoma volume decreased from 198 (+/- 13.9) after leuprolide therapy to 26 (+/- 2.4) 3 to 6 months postoperatively while total uterine volume decreased from 291 (+/- 35.7) to 121 (+/- 7.8) during the same time period. Eight patients (4.8%) required repeat surgeries with 5 of the 8 choosing to undergo a hysterectomy. There were no cases of myoma regeneration, although myoma development and growth in areas where myolysis was not performed, did occur.
Goldfarb et al. reported on 300 patients who underwent myolysis, with 150 performed with the Nd:YAG laser and 150 with bipolar coagulation.41,45,46 Leiomyoma size decreased by 30% to 50% beyond the effect of leuprolide 6 months postoperatively.
Nearly all patients had satisfactory relief of their pelvic symptoms, although 6 women developed myoma degeneration. Goldfarb also combined the use of myolysis and endometrial ablation or resection to treat women with leiomyoma and profuse vaginal bleeding, and compared them to women undergoing ablation alone.47 Combined therapy reduced the subsequent surgery rate in these patients who were followed for up to 10 years from 38% in the ablation group to 12% in the combined therapy group.
Adhesion development following myolysis is quite prevalent and is thought to occur due to the extensive serosal damage that occurs during the procedure. During second look laparoscopies, Nisolle et al.48,49 reported dense fibrous adhesions in 8 of 15 patients who underwent myolysis with the Nd: YAG laser, while Phillips et al.43 reported adhesion development in 5 of 19 patients, in a study in which the myomas were treated by bipolar coagulation. In 1995 Goldfarb developed the bipolar circumferential technique in order to spare the uterine serosa and decrease adhesion formation.45 More studies need to be performed in this area to determine its effect on adhesion prevention.
Newer techniques such as cryomyolysis and laser-induced thermotherapy are also being studied.50 In a small study, Zreik et al. performed laparoscopic cryomyolysis in 14 patients with symptomatic leiomyoma with a 6% decrease in post-GnRH myoma size noted.51 Cryomyolysis, therefore, maintained the reduction in myoma size obtained from GnRH use after its discontinuation, while the size of normal uterine tissue returned to pretreatment levels. Keshavarzi et al. reported on a new modality of high-intensity focused ultrasound (HIFU) in a rat model.52 A 69% reduction in myoma volume was noted 5 weeks following treatment. HIFU is currently used in Europe, Japan, and China as a treatment for patients with liver cancer, breast cancer, bone tumors, soft tissue sarcoma, and benign prostatic hypertrophy.
UTERINE ARTERY EMBOLIZATION
Uterine artery embolization (UAE) was described in obstetrics and gynecology initially for the management of severe, uncontrollable uterine bleeding. It involves the catheterization of both uterine arteries and the instillation of microparticles to cause occlusion thereby disrupting the blood supply to the myomas and subsequently leading to devascularization and infarction.53 It has been used as a primary treatment for symptomatic myomas since 1995, with an estimated 200,000 UAE’s performed.54 Pron et al. reported on a series of over 530 patients who underwent embolization.52,55,56 The procedure length was about 1 hour and hospital stay averaged 1.3 nights. There were 30 (5.3%) procedural complications, 2 of which involved serious allergic reactions.
Embolization did result in significant improvements in patient symptomatology.53,55,56 Menorrhagia, dysmenorrhea, and urinary frequency/urgency decreased by 83%, 77%, and 86% respectively. Median uterine volume decreased by 35% and dominant fibroid volume by 42%. 91% of patients expressed satisfaction with the procedure. Similar results have been reported in other smaller studies.57-61 Although the treatment of large myomas by embolization is somewhat controversial, Katsumori et al. reported no increase in complications when comparing embolization in two groups: women with one or more myomas greater than 10 cm and a second group with myomas less than 10 cm.62
In another study by Marret et al. the rate of myoma recurrence was examined in 85 patients after embolization.63 One case of myoma progression was noted along with seven cases of new leiomyomas accounting for about a 10% recurrence rate, with a mean time to recurrence of 27.4 months. Other prospective studies with follow-up periods of 5- 7 years demonstrate 72-73% symptom control and high rates of overall satisfaction with the procedure.54
Randomized controlled clinical trials directly comparing UAE to surgical treatments have been few due to difficulty with recruitment and randomization to very different therapies. The EMMY trial examined the post procedure experiences of 177 women in The Netherlands who underwent either hysterectomy or UAE. At 24 months, both groups of women had similar results for health related quality of life, bulk related symptoms, and pain. The authors note that though UAE is a good alternative to hysterectomy, for those patients desiring cessation of menorrhagia, hysterectomy should be recommended.64 Another RCT, the REST trial, compared UAE with open surgery (hysterectomy and myomectomy) in 157 women. In this study 22% of women who underwent UFE required hospitalization or a secondary intervention (re-embolization or hysterectomy) within 2 years of their procedure. Also, symptoms at 1 and 12 months post-procedure were significantly better in the surgery arm of the study.65
Embolization can result in other postoperative complications that must be discussed with patients. General side effects that are to be expected from UFE include vaginal discharge, transient vasomotor symptoms, cramping, constipation, and spontaneous fibroid tissue expulsion.54 Pain is a commonly reported patient complaint following embolization. In the study by Pron et al. patients were given perioperative anti-inflammatory medications and narcotics with intraprocedural pain being reported by 30% of patients.53,55,56 Postprocedural pain, however, was reported by 92% of patients and was the most common indication for hospital stay greater than 1 night (18%), return visits to the hospital (10% ), readmissions (3%), and accounted for 32 of 44 postprocedural complications.
Most commonly, complications are related to the necrosis and transcervical expulsion of fibroids which can occur up to several months postoperatively.66 This occurs in approximately 2.5% patients and may require surgical intervention.67,68 In addition to the risks associated with angiography, Murgo et al. reported vaginal discharge with possible expulsion of fibroids in 5% of patients, transient or permanent amenorrhea in 4-5% and extensive necrosis with possible perforation and infection in 1-2% of patients.61 The most serious complications that can occur are uterine infection and possible bowel infection, necrosis and/or obstruction that can result from necrosis of subserosal or pedunculated myomas and subsequent adhesion formation.69,70 Such complications may require emergency laparotomy, hysterectomy, and possible bowel resection. However, these complications may present with symptoms which are similar to those found in “postembolization syndrome.” This syndrome includes a constellation of symptoms including pelvic pain, fever, leukocytosis, nausea, vomiting, and malaise, and may be difficult to differentiate from other much more serious conditions.70
As the number of uterine artery embolizations performed increases, the number of procedure-related complications also increases. There are numerous reports of post- embolization complications requiring hysterectomy.61,70-72 The rate of hysterectomy after UAE has been reported between 0.25%-1.6% and are thought to be due to infection, pain, and bleeding (Spies) Uterine necrosis is a rare but severe complication which necessitates hysterectomy. There have also been post-embolization deaths reported, usually as a result of overwhelming sepsis.73-75 Preprocedure, patients should be counseled regarding the risk of emergency hysterectomy in the case of severe complications.
The effect of uterine artery embolization on future fertility and ovarian reserve must also be discussed. Goldberg et al. reported on 50 published cases of pregnancy following uterine artery embolization.76 Malpresentation occurred in 17% of patients, small for gestational age infants in 7%, preterm delivery in 28%, cesarean delivery in 58%, and postpartum hemorrhage in 13% of patients. Confounding variables inherent in this study’s patient population may account for the high pregnancy complication rate. Honda et al.77 described the development of intrauterine adhesions in 4 of 7 patients who wished to conceive following embolization. In a randomized controlled trial comparing reproductive outcomes after UAE myomectomy, Mara, et al found that though the two procedures were similarly safe and effective in reduction of symptoms, myomectomy resulted in significantly improved reproductive outcomes.78 At a mean follow-up period of 24.9 months, women undergoing myomectomy had significantly more successful deliveries and fewer pregnancy losses. In fact, the study demonstrated a 60% spontaneous abortion rate in the UAE group, compared to 23% after myomectomy. The available studies suggest that though women are able to conceive after UAE, myomectomy appears to have superior reproductive outcomes in at least the first 2 years aftertreatment.54
Studies on the effects of uterine artery embolization on ovarian reserve have provided conflicting results. Ahmad et al. reported no change in ovarian function in 32 patients with a mean age of 34.79 In a study by Messina et al. however, 3 patients developed ovarian failure out of 26 that underwent embolization.58 Amato and Roberts described a case of a 49 year old female that experienced transient ovarian failure following embolization.80 Within 3 months of her surgery, the patient’s FSH level increased from 8.2 to 140.1 mIU/mL and she developed amenorrhea and hot flashes. Four months later, her menses resumed and her FSH level decreased to 2.1 mIU/mL. Another study reported a 14% ovarian failure rate in a group of 66 patients who underwent embolization.81 The complication occurred in 9 of 21 (43%) of women older than age 45 and in none (0 of 45) younger than age 45. Larger scale studies need to be performed to determine the effect of uterine artery embolization on ovarian reserve in women of different age groups.
The cause of ovarian failure in these patients is thought to arise from non-target embolization where the embolizing medium migrates through anastomotic channels into neighboring vessels. There have been several case reports of histologic diagnosis of embolization microspheres or polyvinyl alcohol particles in the ovarian vessels.82,83 Other nearby structures have also been affected by non-target embolization. Yeagley et al. reported a case of labial necrosis immediately following uterine artery embolization.84 The patient developed a 3 cm tender, necrotic-appearing area on the right labia minora in the first few days following embolization. She was treated conservatively with antibiotics and analgesics and complete resolution of the lesion was observed within 4 weeks of the procedure. Another case of unilateral deep buttock pain following embolization has also been reported.85 There are also 3 case reports of bladder necrosis, with resultant fistula formation in 2 of the 3 cases, following uterine artery embolization.86,88 In one of these cases, operative findings during the subsequent hysterectomy and partial bladder resection revealed a 3 cm vesicouterine fistula with a necrotic myoma within the bladder itself.88 These 3 cases may have resulted from non-target embolization of the superior, middle, and inferior vesical arteries or from direct contact of the bladder wall with the necrotic myoma. In another case report by de Blok et al., evidence of non-target embolization was discovered during autopsy in a patient who died from sepsis following uterine artery embolization.73 Pathologic examination revealed the presence of microspheres in the parametria and vagina, which led to the development of a necrotic vaginal wall and cervix.
Similar to myolysis, uterine artery embolization should not be performed if a pelvic malignancy is suspected. A tissue diagnosis is not obtained, and proper treatment is not provided. Spies et al. reported a case of a patient who was diagnosed with leiomyosarcoma during a myomectomy performed 18 months after a successful embolization.67 Payne et al. reported another case of a Jehovah’s Witness who underwent embolization after declining surgical therapy for symptomatic leiomyomas and an asymptomatic adnexal mass presumed to be a dermoid cyst.60 Postoperatively the patient decompensated requiring surgery, at which time she was diagnosed with a stage 1-C adenocarcinoma arising within a mature cystic teratoma.
More recently, advances in other minimally invasive techniques provide increasing options for women with symptomatic myomas. Similar in principle to myolysis, MRI- guided focused ultrasound is a technique of delivering intensely focused ultrasound to reduce fibroid volume. This technology is USFDA approved for use in premenopausal women with symptomatic fibroids who do not desire future fertility. Long term studies are needed, but adverse events of re-hospitalization, skin burns, skin ulceration, and a case of sciatic nerve palsy were reported by Stewart et al.89
In conclusion, myolysis, uterine artery embolization, and newer techniques such as MR- guided focused ultrasound (MRgFUS), provide viable alternatives to hysterectomy in select patients. Recovery time and return to activities of daily living can be shorter with these procedures. Though “minimally invasive” however, they do carry the risk of serious postoperative complications which should be discussed with patients when providing them options for management of fibroids.
References
- Buttram VC, Reiter RC. Uterine leiomyomata: Etiology, symptomatology, and management. Fertil Steril 1981; 36:433.
- Vollenhoven BJ, Lawrence AS, Healy DL. Uterine fibroids: A clinical review. Br J Obstet Gynaecol 1990;97:285.
- Cramer SF, Robertson AL, Ziatas NP, et al. Growth potential of human uterine leiomyomas: Some in vitro observations and their implications. Obstet Gynecol 1990;97:393.
- Freidman AJ, Rein NS, Harrison-Atlas D, et al. A randomized, placebo-controlled, blind study evaluating leuprolide acetate depot treatment before myomectomy. Fertil Steril 1989; 52:728.
- Lumsden MA, West CP, Baird DT. Goserelin therapy before surgery for uterine fibroids. Lancet 1987; 1:36.
- Fedele L, Vercellmi P, Bianchi S, et al. Treatment with GnRH agonists before myomectomy and the risk of short-term myoma recurrence. Br J Obstet Gynaecol 1990; 97:393.
- Beyth Y. Gonadotropin-releasing hormone analog treatment should not precede conservative myomectomy [Letter]. Fertil Steril 1990; 53:187-88.
- Nezhat C, Nezhat F, Silfen SL, et al. Laparoscopic myomectomy. Int J Fertil 1991;36:275.
- Roemisch M, Nezhat FR, Nezhat A. Pregnancy after laparscopic myomectomy. J Am Assoc Gynecol Laparosc 1996; 3:S42.
- Nezhat C, Nezhat F, Nezhat CH, eds. Nezhat’s Operative Gynecologic Laparoscopy and Hysteroscopy. 3rd edition. New York: Cambridge University Press,
- Greenberg JA, Einarsson JI. The use of bidirectional barbed suture in laparoscopic myomectomy and total laparoscopic hysterectomy. J Minim Invasive Gynecol. 2008 Sep- Oct;15(5):621-3.
- Davis GD, Hruby PH. Transabdominal laser colpotomy. J Reprod Med. 1989; 34:438.
- Harris WJ. Uterine dehiscence following laparoscopic myomectomy. Obstet Gynecol. 1992; 80:545.
- Linsky CB, Diamond MP, Dizerega GS. Effect of blood on the efficacy of barrier adhesion reduction in the rabbit uterine horn model. 1988; 11:273.
- Raga F, Sanz-Cortes M, Bonilla F, Casañ EM, Bonilla-Musoles F. Reducing blood loss at myomectomy with use of a gelatin-thrombin matrix hemostatic sealant. Fertil Steril. 2009 Jul;92(1):356-60.
- Milad M, Sokol E. Laparoscopic Morcellator-Related Injuries. JAAGL.10(3): 383- 385, 2003.
- Moon HS, Koo JS, Park SH, Park GS, Choi JG, Kim SG. Parasitic leiomyoma in the abdominal wall after laparoscopic myomectomy. Fertil Steril. 2008;90:1201.e1–2. External Link Resolver Library Holdings [ContextLink].
- Takeda A, Mori M, Sakai K, Mitsui T, Nakamura H. Parasitic peritoneal leiomyomatosis diagnosed 6 years after laparoscopic myomectomy with electric tissue morcellation: report of a case and review of the literature. J Minim Invasive Gynecol. 2007;14:770–5. External Link Resolver [Context Link]
- Paul PG, Koshy AK. Multiple peritoneal parasitic myomas after laparoscopic myomectomy and morcellation. Fertil Steril. 2006;85:492–3. External Link Resolver Library Holdings [Context Link]
- Kho K, Nezhat C. Parasitic Myomas. Obstet Gynecol. 2009 Sep;114(3):611-5.
- Hilger WS, Magrina JF. Removal of pelvic leiomyomata and endometriosis five years after supracervical hysterectomy. Obstet Gynecol. 2006 Sep;108(3 Pt 2):772-4.
- Donnez O, Squifflet J, Leconte I, Jadoul P, Donnez J. Posthysterectomy pelvic adenomyotic masses observed in 8 cases out of a series of 1405 laparoscopic subtotal hysterectomies. J Minim Invasive Gynecol. 2007 Mar-Apr;14(2):156-60.
- Schneider A. Recurrence of unclassifiable uterine cancer after modified laparoscopic hysterectomy with morcellation. Am J Obstet Gynecol. 1997 Aug;177(2):478-9.
- Nezhat CR, Nezhat F, Bess O, et al. Laparoscopically assisted myomectomy: A report of a new technique in 57 cases. Int J Fertil Menopausal Stud. 1994, 39(1):39.
- Georgakopoulous PA, Bersis G. Sigmoidouterine rupture in pregnancy after multiple myomectomy. Int Surg. 1981; 66:367.
- Hasson HM, Rotman C, Rana N, et al. Laparoscopic myomectomy. Obstet Gynecol. 1992; 80:884.
- Dubuisson JB, Lecuru F, Herve F, et al. Myomectomy by laparoscopy. Fertil Steril. 1991; 56:827.
- Mais V, Ajossa S, Guerriero S, Mascia M, Solla E, Melis GB. Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996;174:654–8.
- Visco AG, Advincula AP. Robotic gynecologic surgery. Obstet Gynecol. 2008 Dec;112(6):1369-84
- Advincula AP, Xu X, Goudeau S 4th, Ransom SB. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol. 2007;14:698–705.
- Nezhat C, Lavie O, Hsu S, Watson J, Barnett O, Lemyre M. Robotic-assisted laparoscopic myomectomy compared with standard laparoscopic myomectomy-a retrospective matched control study. Fertil Steril. 2008 Mar 28.
- Operative Laparoscopy Study Group. Postoperative adhesion development after operative laparoscopy: Evaluation at early second-look procedures. Fertil Steril 1991; 55:700.
- Bayers SP, Jansen D. Gore-Tex surgical membrane. In: DeCherney A, Diamond MP, eds. Treatment of Post-Surgical Adhesions. Wiley-Liss, 1990.
- Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm Membrane (HAL-F): A blinded, prospective, randomized, multicenter clinical Seprafilm Adhesion Study Group. Fertil Steril. 1996; 66:904.
- Melis GB, Ajossa S, Piras B, et al. A randomized trial to evaluate the prevention of de novo adhesion formation after laparoscopic myomectomy using oxidized regenerated cellulose (Interceed) Barrier. J Am Assoc Gynecol Laparosc. 1995; 2:S31.
- Mettler L, Audebert A, Lehmann-Willenbrock E, Jacobs VR, Schive K. New adhesion prevention concept in gynecologic surgery. 2003; 7(3):207-09.
- Metter L, Hucke J, Bojahr B, Tinneberg HR, Leyland B, Avelar R. A safety and efficacy study of a resorbable hydrogel for reduction of post-operative adhesions following myomectomy. Hum Reprod. 2008.23(5):1093-1100.
- Gallinat A, Leuken RP. Current trends in the therapy of myomata. In: Leuken RP, Gallinat A, eds. Endoscopic Surgery in Gynecology. Berlin, Demeter Verlag, 1993:69-71.
- Goldfarb HA. Nd:YAG laser laparoscopic coagulation of symptomatic myomas. J Reprod Med. 1992; 36:636-38.
- Wood C, Maher P, Hill D. Myoma reduction by electrocautery. Gynaicol Endosc. 1994; 3:163-65.
- Goldfarb HA. Removing uterine fibroids laparoscopically. Contem Obstet Gynecol. 1994; 39:50-72.
- Chapman R. Treatment of uterine myomas by interstitial hyperthermia. Gynaecol Endosc. 1993; 2:227-34.
- Phillips DR, Milim SJ, Nathanson HG, Haselkorn JS. Experience with laparoscopic coagulation and concomitant operative hysteroscopy. J Am Assoc Gynecol Laparosc. 1997; 4(4):425-32.
- Vilos GA, Daly LJ, Tse BM. Pregnancy outcome after laparoscopic electromyolysis. J Am Assoc Gynecol Láparosc. 1998; 5(3):289-92.
- Goldfarb HA. Bipolar laparoscopic needles for myoma coagulation. J Am Assoc Gynecol Laparosc. 1995; 2:175-79.
- Goldfarb HA. Laparoscopic coagulation of myoma (myolysis). In: Hutchins FL, Greenberg MD, eds. Obstetric and Gynecology Clinics of North America: Uterine Fibroids. Philadelphia, WB Saunders, 1995:807-19.
- Goldfarb HA. Combining myoma coagulation with endometrial ablation/resection reduces subsequent surgery rates. 1999; 3(4):253-60.
- Nisolle M, Smets M, Gillerot S, et al. Laparoscopic myolysis with the Nd:YAG laser. J Gynecol Surg. 1993; 9:95-99.
- Nisolle M, Smets M, Gillerot, et al. Laparoscopic myolysis with the Nd:YAG In: Donnez J, Nisolle M, eds. An Atlas of Laser Operative Laparoscopy and Hysteroscopy. Carnforth, UK, Parthenon Publishing, 1994:187-93.
- Chapman R. New therapeutic technique for treatment of uterine leiomyomas using laser-induced interstitial thermotherapy (LITT) by a minimally invasive method. Laser Surg Med. 1998; 22(3):171-78.
- Zreik TG, Rutherford TJ, Palter SF, Troiano RN, Williams E, Brown JM, Olive DL. Cryomyolysis, a new procedure for the conservative treatment of uterine fibroids. J Assoc Gynecol Laparosc. 1998; 5(1):33-38.
- Keshavarzi A, Vaezy S, Noble ML, Paun MK, Fujimoto VY. Treatment of uterine fibroid tumors in an in situ rat model using high-intensity focused ultrasound. Fertil Steril. 2003; 80(S2):761-67.
- Pron G, Bennet J, Common A, Sniderman K, Asch M, et al. Technical results and effects of operator experience on uterine artery embolization for fibroids: the Ontario Fibroid Embolization Trial. J Vasc Interv Radiol. 2003; 14(5):545-54.
- Bradley LD. Uterine fibroid embolization: a viable alternative to hysterectomy. AJOG. 2009 August. 127-135.
- Pron G, Mocarski E, Bennet J, Vilos G, Common A, et al. Tolerance, hospital stay, and recovery after uterine artery embolization for fibroids: the Ontario Uterine Fibroid Embolization Trial. J Vasc Interv Radiol. 2003;14(10):1243-50.
- Pron G, Bennet J, Common A, Wall J, Asch M, et al. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79(1):120-27.
- Klein A, Schwartz ML. Uterine artery embolization for the treatment of uterine fibroids: an outpatient procedure. Am J Obstet Gynecol. 2001; 184(7):1556-60.
- Messina ML, Bozzini N, Halbe HW, Pinotti JA. Uterine artery embolization for the treatment of uterine leiomyomata. Int J Gynaecol Obstet. 2002; 79(1):11-16.
- Badawy SZ, Etman A, Singh M, Murphy K, Mayelli T, et al. Uterine artery embolization: the role in obstetrics and gynecology. Clin Imaging. 2001; 25(4):288-95.
- Helmberger TK, Jakobs TF, Reiser MF. Embolization of uterine fibroids. Abdom Imaging. 2004 Mar-Apr;29(2):267-77.
- Murgo S, Simon P, Golzarian J. Embolization of uterine fibroids. Rev Med Brux. 2002; 23(5):435-42.
- Katsumori T, Nakajima K, Mihara T. Is a large fibroid a high-risk factor for uterine artery embolization? AJR Am J Roentgenol. 2003; 181(5):1309-14.
- Marret H, Alonso AM, Cottier JP, Tranquart F, Herbreteau D, et al. Leiomyoma recurrence after uterine artery embolization. J Vasc Interv Radiol. 2003; 14(11):1395-99.
- Hehenkamp WJ, Volkers NA, Birnie E, Reekers JA, Ankum WM. Symptomatic uterine fibroids: treatment with uterine artery embolization or hysterectomy–results from the randomized clinical Embolisation versus Hysterectomy (EMMY) Trial. Radiology. 2008 Mar;246(3):823-32.
- Edwards RD, Moss JG, Lumsden MA, Wu O, Murray LS, Twaddle S, Murray GD; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine-artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007 Jan 25;356(4):360-70.
- Laverge F, D’Angelo A, Davies NJ, Wood A, Amso NN. Spontaneous expulsion of three large fibroids after uterine artery embolization. Fertil Steril. 2003; 80(2):450-52.
- Spies JB, Spector A, Roth AR, Baker CM, Mauro L, Murphy-Skynarz K. Complications after uterine artery embolization for leiomyomas. Obstet Gynecol. 2002; 100(Suppl 5, Pt 1):873-80.
- Park AJ, Bohrer JC, Bradley LD, et al. Incidence and risk factors for surgical intervention after uterine artery embolization. AJOG. 2008; 199:671.e1-6.
- Al-Fozan H, Tulandi T. Factors affecting early surgical intervention after uterine artery embolization. Obstet Gynecol Surv. 2002; 57(12):810-15.
- Payne JF, Haney AF. Serious complications of uterine artery embolization for conservative treatments of fibroids. Fertil Steril. 2003; 79(1):128-31.
- Pron G, Mocarski E, Cohen M, Colgan T, Bennett J, et al. Hysterectomy for complications after uterine artery embolization for leiomyoma: results of a Canadian multicenter clinical trial. J Am Assoc Gynecol Laparos.c 2003; 10(1):99-106.
- Shashoua AR, Stringer NH, Pearlman JB, Behmaram B, Stringer EA. Ischemic uterine rupture and hysterectomy 3 months after uterine artery embolization. J Am Assoc Gynecol Laparosc. 2002; 9(2):217-20.
- de Blok S, de Vries C, Prinssen HM, Blaauwgeers HL, Jorna-Meijer LB. Fatal sepsis after uterine artery embolization with microspheres. J Vasc Interv Radiol. 2003; 14(6):779-83.
- Vashisht A, Studd J, Carey A, Burn P. Fatal septicaemia after fibroid embolisation. Lancet. 1999; 354(9175):307-08.
- Vashisht A, Studd JW, Carey AH, McCall J, Burn PR, et al. Fibroid embolisation: a technique not without significant complications. 2000; 107(9):1166-70.
- Goldberg J, Pereira L, Berghella V. Pregnancy after uterine artery embolization. Obstet Gynecol. 2002; 100(5 Pt 1):869-72.
- Honda I, Sato T, Adachi H, Kobayashi Y, Shimada K, et al. Uterine artery embolization for leiomyoma: complications and effects on fertility. Nippon Igaku Hoshasen Gakkai Zasshi. 2003; 63(6):294-302.
- Mara M, Maskova J, Fucikova Z, Kuzel D, Belsan T, Sosna O. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008 Jan-Feb;31(1):73-85.
- Ahmad A, Qadan L, Hassan N, Najarian K. Uterine artery embolization treatment of uterine fibroids: effect on ovarian function in younger women. J Vasc Interv Radiol. 2002; 13(10):1017-20.
- Amato P, Roberts AC. Transient ovarian failure: a complication of uterine artery embolization. Fertil Steril. 2001; 75(2):438-39.
- Chrisman HB, Saker MB, Ryu RK, Nemcek AA, Gerbie MV, et al. The impact of uterine fibroid embolization on resumption of menses and ovarian function. J Vasc Interv Radiol. 2000; 11:699-703.
- Payne JF, Robboy SJ, Haney AF. Embolic microspheres within ovarian arterial vasculature after uterine artery embolization. Obstet Gynecol. 2002; 100(5 Part 1):883- 86.
- Goodwin SC, Walker WJ. Uterine artery embolization for the treatment of uterine fibroids. Curr Opin Obstet Gynecol. 1998; 10:315-320.
- Yeagley TJ, Goldberg J, Klein TA, Bonn J. Labial necrosis after uterine artery embolization for leiomyomata. Obstet Gynecol. 2002; 100(5 Part 1):881-82.
- Hutchins FL Jr, Worthington-Kirsch R. Embolotherapy for myoma-induced menorrhagia. Obstet Gynecol Clin North Am. 2000; 27:397-405.
- Sultana CJ, Goldberg J, Aizenman L, Chon J. Vesicouterine fistula after uterine artery embolization: a case report. Am J Obstet Gynecol. 2002; 187(6):1726-27.
- Huang LY, Cheng YF, Huang CC, Chang SY, Kung FT. Incomplete vaginal expulsion of pyoadenomyoma with sepsis and focal bladder necrosis after uterine artery embolization for symptomatic adenomyosis: case report. Hum Reprod. 2003; 18(1):167- 71.
- El-Shalakany AH, Nasr El-Din MH, Wafa GA, Azzam ME, El-Dorry A. Massive vault necrosis with bladder fistula after uterine artery embolization. 2003; 110(2):215-16.
- Stewart EA, Rabinovici J, Tempany CM, Inbar Y, Regan L, Gostuot B, et al. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril. 2006;85:22-29.
