Complications of Laparoscopic Gynecologic Surgery
Bulent Berker, MD, Salih Taskin, MD, Elif Aylin Taskin, MD
INTRODUCTION
Approximately 30 years after its introduction, the use of laparoscopy in gynecology has expanded from diagnosis and tubal sterilization to more sophisticated operations and is even being used for the management of malignancies. Despite rapidly improving technical equipment and surgical skill, complication rates and preventable injuries demonstrate a continuous pattern. The actual incidence of complications possibly exceeds reported rates. Because levels of operative laparoscopy, study populations, and definitions of complications vary in different series, it is difficult to determine the exact incidence of complications. Also there may be bias in reporting, especially of minor complications. Delayed recognition and intervention adds to morbidity and mortality.
Reported overall complication rates range from 0.2% and 10.3%.1 Major laparoscopic procedures are associated with a higher rate of complications compared with minor procedures, 0.6% to 18% and 0.06% to 7.0%, respectively.1
The majority of complications occur during entry of instruments into the abdomen used to create pneumoperitoneum. Therefore, open-entry and closed-entry have commonly been compared. The incidence of many complications decreases with open-entry, and intraoperative diagnosis of complications becomes more probable, decreasing the mortality resulting from delayed diagnosis. Some authors advocate routine use of open- entry.1 Other reasons for complications are thermal and energy source injuries, operative manipulations, and suturing, and the presence of CO2 out of the peritoneum. Because some complications result from more than one cause, clear classification ofcomplications is challenging. Complications are summarized in Table 1 and are presented in this chapter.
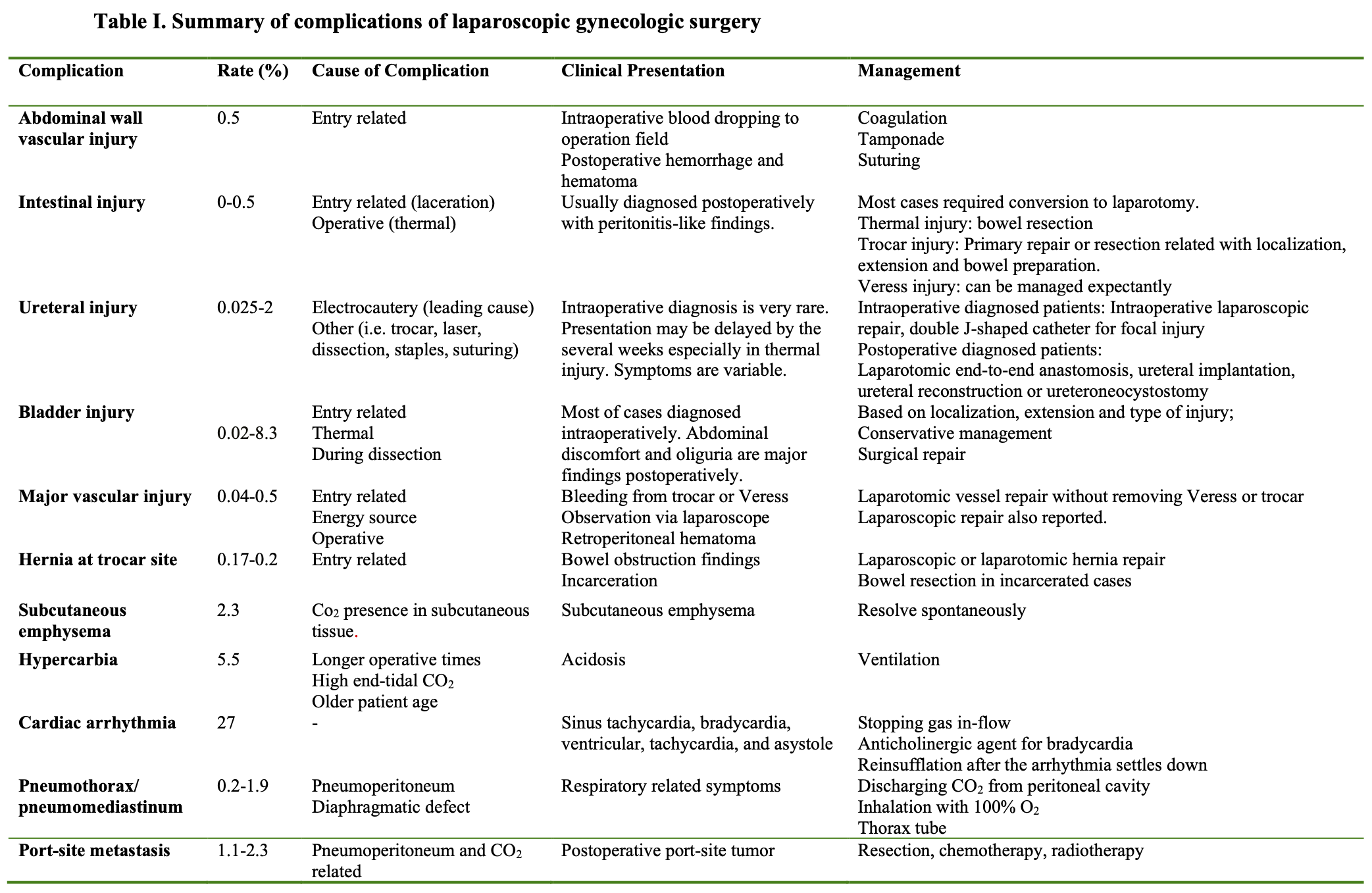
INTESTINAL INJURY
The incidence of bowel injury is reported to be 0% to 0.5%.1 Approximately half of bowel injuries occur during entry, and the rest occur during the operation (Figure 1). Entry-related bowel injuries are mostly lacerations, and intraoperative injuries are mostly thermal. Most bowel injuries are not diagnosed during surgery. Delayed intervention may be life-threatening. Therefore, this complication is reported to be the most common cause of laparoscopy-related mortality.2 Soderstrom reviewed 66 cases of missed intestinal perforation and concluded that if the diagnosis is delayed more than 72 hours, the mortality rate significantly increases.3 In a review,1 less than half of 363 bowel injury cases were diagnosed intraoperatively. Rates of small and large bowel injuries were similar, 46.9% and 40.2%, respectively. The mean time from operation to diagnosis was 4.4 days, but recognition of thermal injury may lag behind 10 days.
When bowel is entered via Veress needle or trocar, bowel content or gas passage may be observed, or may be controlled with a syringe if suspected. A laparoscope will show bowel lumen in such a case. If intraoperatively unrecognized, patients present with nonspecific complaints not exactly pointing to peritonitis, such as mild abdominal pain, fever, diarrhea, and abdominal distension, further delaying the diagnosis.4 Late consequences may be generalized peritonitis, abscess formation, and septic shock.
Between 52.4% and 90.0% of intestinal injuries are repaired laparotomically.5,6 Injury caused by the Veress needle may be managed expectantly. However, trocar injuries deserve laparotomic repair according to the size and location of the injury. Primary closure in 2 layers is adequate in small bowel injuries. If the large bowel is involved, the treatment options include primary repair, colostomy, or segmental resection. Resection is mandatory in thermal injuries. Intraoperative lacerations may be repaired laparoscopically according to the size of the lesion, surgeon experience, and preoperative bowel preparation.7
URINARY TRACT INJURY
While some authors report a higher incidence of urinary system injury in laparoscopy than in laparotomy in their reviews,8,9 some others demonstrate a lack of such an increase in their series.10,11 Brummer et al12 also reported that this incidence decreased from 1.4% to 0.7% between 2000 and 2005. Of all urinary injuries, 64.7% occurred with laparoscopic- assisted vaginal hysterectomy, 18.0% during operations for endometriosis, and 12.3% during diagnostic or sterilization procedures.13
Ureteral Injury
Pelvic surgery is the most common cause of iatrogenic ureteral injury. It is becoming more common as a result of the increasing number of laparoscopic hysterectomies and retroperitoneal laparoscopic procedures that are being performed. The majority of patients with ureteral injuries have no identifiable predisposing risk factors. Estimated incidence of ureteral injury during laparoscopic hysterectomy is 2.6 to 35 times more common (0.2% to 6.0%) than in abdominal hysterectomy.14 Ureteral injury accounted for 4.3% to 7% of the total laparoscopy complications.15,16 Overall ureteral injuries were identified with incidence rates ranging from 0.025% to 2%.16,17
Electrocautery (unipolar or bipolar) was identified as the leading cause of laparoscopic ureteral injury. Injuries with loop suturing, trocars, laser devices, staples, and sharp dissection were also described. In particular, the 3 following locations carry the risk for ureteral injury during laparoscopic surgeries: at the pelvic brim, where the ureters lie beneath the insertions of the infundibulopelvic ligaments; deep ovarian fossa where the ureter passes; and the ureteral canal.18 In 44 patients with a known injury site, the most common site of injury was near the infundibulopelvic ligament (29.5%), followed by injury near the uterosacral ligaments (18.1%) and uterine artery (9.9%), and below the uterine vessels (4.5%).19 Injuries may include transection, ligation, avulsion, crush injury, devascularization, resection, fulguration, and perforation. In a review,17 20% of ureteral injuries occurred during laparoscopic-assisted vaginal hysterectomy, 11% during oophorectomy, 10% during laparoscopic pelvic lymphadenectomy, 7% during laparoscopic sterilization, 7% during excision of endometriosis, 6% during endometriosis ablation, and 4% during each of the following: drainage of lymphoceles, electrocoagulation, and laparoscopic adhesiolysis.
These injuries are frequently undetected, but often the failure to recognize such anatomic disruption is because a surgeon does not suspect a ureteral injury. Depending on the type of injury, patients may present in the early postoperative period (first 3 days), or presentation of the thermal or laceration injuries may be delayed by several days or weeks. Only 9% of ureteral injuries were diagnosed intraoperatively, 70% postoperatively, and in 21% the time of ureteral injury was not specified.17 Hurt et al20 and Li et al21 discuss 5 methods for identifying ureteral injury: (1) retrograde ureteral dye injection; (2) 5mL to 10mL intravenous indigo carmine injection; (3) intraoperative ureteral catheterization; (4) intravenous excretory urography; and (5) dissection of the ureter. A coagulated or ligated ureter may not demonstrate an intraperitoneal urine leak when tested intraoperatively. The lack of a ureteral jet at cystoscopic examination will indicate the problem clearly in such a case.
Postoperative presentation is variable and nonspecific, such as acute pelvic pain, nausea, vomiting, malaise, leakage of fluid via the trocar sites, abdominal distension and an inflammatory reaction in the serum (elevated CRP and leucocytosis), elevated creatinine level, costovertebral angle tenderness, ileus, fever, flank pain, and ileus peritonitis.22 X- ray of the abdomen may reveal a ground-glass appearance indicative of fluid collection.21 A computed tomography scan revealed the presence of urine in the peritoneal cavity after intravenous contrast medium injection.10 Ligation of the ureter, for example, with stapling or stricture resulting from thermal injury may lead to obstructive uropathy and superimposed pyonephrosis. The diagnosis should be confirmed by an intravenous urogram.21
Laparoscopic repair is frequently used in cases recognized intraoperatively, while the laparotomic approach is performed in patients diagnosed postoperatively. Focal ureteral injuries can be treated using a double J-shaped catheter allowing for spontaneous healing. However, more extensive damage may require laparotomy to perform an end-to-end anastomosis or ureteral implantation. In delayed recognition of ureteral injury, initial treatment with ureteral stenting may not be useful, and early open repair (ureteral reconstruction, ureteroneocystostomy) for these injuries is advocated.23
To minimize ureteral injury, use of sutures should be preferred instead of staplers or electrocautery in close proximity to ureters. Visualization of ureters during the operation, use of ureteral catheters, and creating hydroprotection by injection of saline to parietal peritoneum are the other protective measures.
Bladder Injury
The most common type of urinary injury during laparoscopy is bladder perforation with an incidence of 0.02% to 8.3%.24 Most injuries occur during hysterectomy operations. Pillet et al25 reported the incidence of bladder injury during laparoscopic hysterectomy to range between 1%. Endometriosis, previous surgery, an inexperienced surgeon, over distended bladder, and pelvic adhesions are proposed risk factors for bladder injury.
Bladder injury may occur during insertion of trocars, especially suprapubic ones, dissection of the bladder during gynecologic operations, or as a result of thermal energy. Of bladder injuries, 90% have been reported to occur at the bladder dome, and the rest at the base.24 Of these injuries, 33% were sharp electrocautery dissections, 21% were blunt, 30% were via laser, and 15% by scissors.24 Five percent of thermal injuries may lead to fistula formation.26 More than half of trocar-related injuries occur via 5-mm and the rest via 10-mm trocars.
Only 9.2% of bladder injuries were not recognized intraoperatively, in contrast to ureteral injuries; therefore, morbidity is lower.19 In suspected cases, filling the bladder with methylene blue dye to observe dye leakage will help with diagnosis. Patients with unrecognized cases presented with abdominal discomfort and oliguria as a cardinal sign. According to type, size, and localization of injury, conservative or surgical management via laparoscopic, laparotomic, or vaginal approaches may be considered.
To prevent bladder injures: (1) perform a very careful dissection of the vesico-vaginal pouch and use uterine cannulation; (2) fill the bladder with a methylene blue dye solution to visualize its limits in case of difficult dissection, such as previous surgery (cesarean delivery, endometriosis surgery, conization, and others.); (3) perform very careful and restricted use of bipolar coagulation in the vesico-vaginal space for hemostasis; and (4) follow the safety rules of introduction, in particular avoiding the Pfannenstiel scar.
Abdominal Wall Vascular Injury
Injuries involving the inferior epigastric vessel are the most common type of vascular complication. The incidence of abdominal wall bleeding is 0.3% to 0.5%.1,27 Epigastric and less commonly muscular vessels may be the origin of bleeding. These injuries usually occur in relation to the positioning of accessory ports, used principally to allow the insertion of the hand instruments necessary for dissecting and manipulating tissue. Blood dripping into the operative field may indicate intraoperative bleeding, but postoperatively diagnosed massive bleeding is also possible. Postoperative hematoma and abscess according to infection of the hematoma may be other consequences.
The most rapid and practical technique for hemostasis is coagulation. If bleeding is not controlled in this way, tamponade and suturing may be used. Suturing may be either laparoscopic or thru an enlarged incision at the trocar site. Minilaparotomy may also be used. The site of bleeding should be re-evaluated under low pneumoperitoneal pressure after coagulation, tamponade, and suturing. Transillumination of the anterior abdominal wall, insertion of the trocar lateral to the sheath of the rectus muscle, and use of smaller trocars in lateral ports may help avoid vascular injury. It is good practice to inspect all secondary trocar sites for active bleeding before the laparoscope is finally withdrawn.
HERNIA AT THE SITE OF THE ABDOMINAL WALL TROCAR
It is believed that up to one-third of all trocar injuries cause incisional hernia formation. It is a preventable complication with an incidence of 0.17% to 0.2%.28,29 Generally, extraumbilical and >5-mm trocar sites are prone to herniation; however, Nezhat et al29 have reported 5 hernia cases (out of 11) to occur at 5-mm trocar sites. Intestines, colon, and omentum may be involved. Signs of intestinal obstruction, increased bowel sounds, diarrhea, nausea, and vomiting may indicate a hernia.
Although repairing the hernia is enough in most of these cases, 19% require intestinal resection secondary to incarceration.30 Hernias are repaired laparotomically or less commonly, laparoscopically. Trocar sites >10mm should be sutured for prevention.
Nezhat et al29 recommend repairing fascia at 5-mm trocar sites if enlarged during operative manipulations. To minimize the risk of herniation, secondary trocars should be removed under supervision before the primary one, valves should be kept closed to prevent a sucking effect, and 5-mm trocars should be preferred.
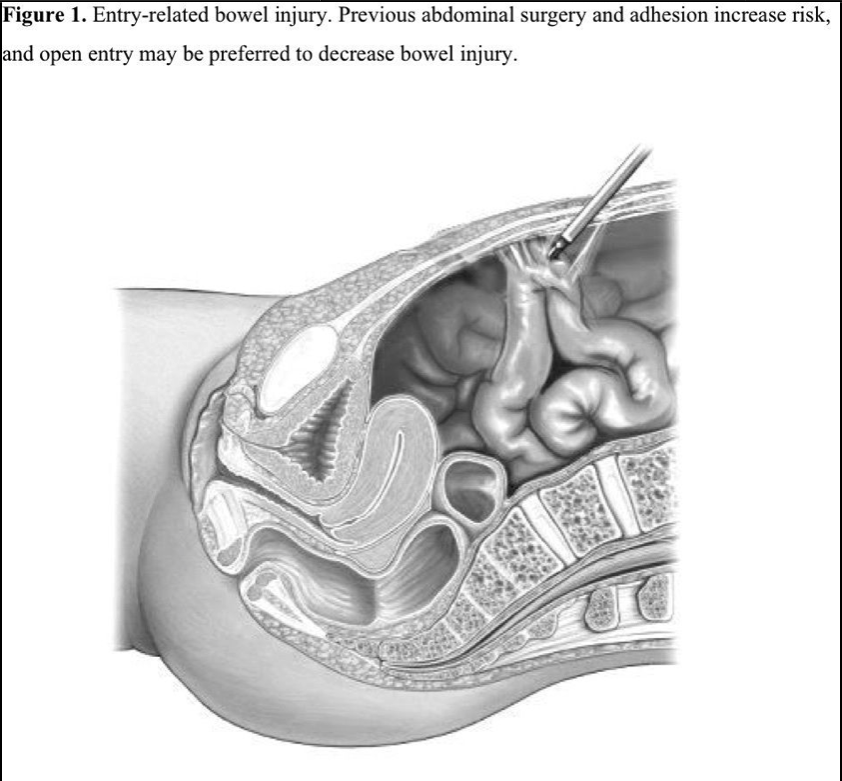
MAJOR VASCULAR INJURY
Retroperitoneal major vascular injuries are mortal complications and may be related to the Veress needle, trocar, or energy source. Of the cases reported, approximately 20% of the major vascular injuries resulted in death. The incidence is estimated to range from 0.04% to 0.5%.31,32 Most injuries were entry related and independent of the complexity of the surgery.33 In this situation, either the aorta, vena cava, or, more commonly, the common iliac vessel has been traumatized (Figure 2).
Bleeding from the Veress and trocars at the entry site may be an alert. Also intraabdominal or retroperitoneal visualization (generally as a hematoma) with a laparoscope is possible. Therefore, visualization of the peritoneal cavity immediately after entry prevents any delay in diagnosis. Shock may develop directly due to abundant blood loss. Patients with anatomic detorsions and retroperitoneal manipulations are more prone to energy source injuries. Major vascular injuries may rarely remain unrecognized intraoperatively.34
The removal of the Veress needle or trocars in case of a vascular injury may result in abundant bleeding and the inability to locate the site of bleeding because of a retroperitoneal hematoma. Through a midline incision, access to the bleeding site should be achieved before these instruments are removed, and injury should be repaired.
PNEUMOPERITONEUM RELATED COMPLICATIONS
Hypercarbia and therefore acidosis develop due to absorption of CO2 in prolonged operations with an incidence of 5.5%.35
Subcutaneous emphysema, pneumomediastinum, and pneumothorax may result from preperitoneal insufflation or leakage of CO2 around the cannula sites. Prolonged operations, higher maximum measured end-tidal CO2, a greater number of surgical ports, and older patient age increase the risk.
Subcutaneous emphysema means the subcutaneous presence of CO2. Its incidence is 2.3%.35 Generally, it has no significant clinical outcome and resolves spontaneously. Subcutaneous emphysema may be extensive involving the extremities, the neck the mediastinum, and even the pericardium, and can result in hypercapnia and cardiovascular collapse.
Preperitoneal emphysema results from preperitoneal insufflation, especially in obese patients when the Veress needle cannot reach the peritoneal cavity. The Veress needle can be inserted at a 90° angle in obese patients. However, this application may increase the risk of other complications. Alternatively, open-entry may be preferred. Some authors advocate direct trocar entry that may prevent preperitoneal emphysema without increasing othercomplications.
Pneumomediastinum results from migration of preperitoneal gas to the mediastinum. In the presence of a congenital diaphragmatic defect or intraoperative diaphragm injury, pneumomediastinum and pneumothorax may occur. The incidence is 0.2% to 1.9% for pneumothorax/pneumomediastinum.35,36 Inhalation of 100% O2 and application of a thorax tube after discharging CO2 from the peritoneal cavity must be done.
Pneumopericardium has been reported to occur in association with pneumomediastinum and subcutaneous emphysema.36
Persistence of Pneumoperitoneum
Because CO2 is more rapidly absorbed than air, less intraabdominal air persistence is observed postoperatively than in laparotomic operations. It is even less in obese patients than in lean patients. Generally, it does not persist after the seventh day. Therefore, when there is a lack of clinical suspicion and increments in serial X-rays, this finding does not point to an organ perforation.
GAS EMBOLISM
This is a rare but mortal complication. It results from introduction of CO2 through the Veress needle into the large veins. Its earliest sign is a drop in end-tidal carbon dioxide concentration, due to diminished blood flow to the lungs. Indicative features include sudden circulatory collapse, cyanosis, and raised jugular venous pressure. Blood coming from the Veress needle is an alert, and the needle should not be removed.21 It necessitates cardiopulmonary resuscitation. The patient lies on her left side. Aspiration of intracardiac gas may be tried. To prevent room-air embolism, which is more dangerous than CO2, the air in the insufflation tube should be flushed out.
POSTOPERATIVE SHOULDER PAIN
Secondary to irritation of the diaphragm, shoulder pain is felt through phrenic nerves. Also a stretched falciform ligament due to the Trendelenburg position adds to the shoulder pain. Careful discharge of intraperitoneal gas after the operation and informing the patient is necessary.
VULVAR EDEMA
This occurs especially in patients in whom adhesion barrier solution is used; therefore, its leakage to the vulva is thought to be the cause. The method of the leakage could be either a patent canal of Nuck, as supported by the Adhesion Study Group,37 or a fistulous tract originating in a lower trocar puncture wound and dissecting downward subcutaneously by the force of gravity, as supported by others.38
COMPLICATIONS RELATED TO ANESTHESIA AND PATIENT POSITION
Nerve Injury
Transient nerve injuries may occur during any procedure with incorrect positioning, affecting brachial plexus, common peroneal nerve, and also the saphenous nerve.
Cardiac Arrhythmia
Similar to what happens during other operations, cardiac arrhythmias including bradycardia, sinus tachycardia, ventricular tachycardia, and arrest related to anesthesia have been reported also in laparoscopy. In one series, cardiac arrest occurred in 1 (0.02%) patient and respiratory arrest in another (0.02%).39
OTHER COMPLICATIONS
Venous Thrombosis
Prolonged operation time and the restricting effect of intraperitoneal pressure on venous return may lead to venous embolism.
Port-Site Metastasis
The rate of port-site metastases in patients with gynecological malignancies is 1.1% to 2.3%.40,41 Contrary to general belief, this rate is similar to the rate of wound metastasis seen in laparotomic gynecologic malignancy operations.42 Risk factors proposed for port- site metastasis are:
(1) Aggressive disease;
(2) CO2 compared with other insufflating agents is associated with significantly increasedtumor growth;
(3) Pneumoperitoneum increases risk compared with gasless laparoscopy;
(4) High efflux of gas from the abdominal cavity through the space around the trocars;
(5) Decreased influence of the local immune system during laparoscopy.
The risk of port-site metastases is highest in patients with recurrence of ovarian or primary peritoneal malignancies with ascites.41 The lavage of port sites with cytotoxic agents (heparin, taurolidine, combination heparin and taurolidine, 5-fluorouracil, doxorubicin, Povidine—iodine solution, and methotrexate) can be suggested as a preventive measure.
References
- Magrina JF. Complications of laparoscopic surgery. Clin Obstet Gynecol. 2002;45:469-480.
- Peterson HB, DeStefano F, Rubin GL, et al. Deaths attributable to tubal sterilization in the United States, 1977 to 1981. Am J Obstet Gynecol. 1983;146:131-136.
- Soderstrom RM. Bowel injury ligation after laparoscopy. J Am Assoc Gynecol Laparosc. 1993;1:74-77.
- Bishoff JT, Allaf ME, Kirkels W, et al. Laparoscopic bowel injury: Incidence and clinical presentation. J Urol. 1999;161:887-890.
- Chapron C, Querleu D, Mage G, et al. Complications of gynecologic laparoscopy. Multicentric study of 7,604 laparoscopies. J Gynecol Obstet Biol Reprod. 1992;21:207- 213.
- Jansen FW, Kapiteyn K, Trimbos-Kemper T, et al. Complications of laparoscopy: A prospective multicenter observational study. Br J Obstet Gynaecol. 1997;104:595-600.
- Reich H, McGlynn F, Budin R. Laparoscopic repair of full-thickness bowel injury. J Laparoendoscopic Surg. 1991;1:119-122.
- Johnson N, Barlow D, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2006;CD003677.
- Garry R, Fountain J, Mason S, et al. The eVALuate study: two parallel randomized trials, one comparing laparoscopic with abdominal hysterectomy, the other comparing laparoscopic with vaginal hysterectomy. BMJ. 2004;328:1229-1236.
- Donnez O, Jadoul P, Squifflet J, et al. A series of 3190 laparoscopic hysterectomies for benign disease from 1990 to 2006: evaluation of complications compared with vaginal and abdominal procedures. BJOG. 2009;116:492-500.
- Karaman Y, Bingol B, Günenç Z. Prevention of complications in laparoscopic hysterectomy: experience with 1120 cases performed by a single surgeon. J Minim Invasive Gynecol. 2007;14:78-84.
- Brummer TH, Seppälä TT, Härkki PS. National learning curve for laparoscopic hysterectomy and trends in hysterectomy in Finland 2000-2005. Hum Reprod. 2008;23:840-845.
- Hasson HM, Rotman C, Rana N, et al. Open laparoscopy: 29-year experience. Obstet Gynecol. 2000;96:763-766.
- Tanaka Y, Asada H, Kuji N, et al. Ureteral catheter placement for prevention of ureteral injury during laparoscopic hysterectomy. J Obstet Gynaecol Res. 2008;34:67-72.
- Saidi MH, Vancaillie TG, White AJ, et al. Complications and cost of multipuncture laparoscopy: a review of 264 cases. Endosc. 1994;3:85-90.
- Härkki-Siren P, Sjoberg J, Kurki T. Major complications of laparoscopy: A follow- up Finnish study. Obstet Gynecol. 1999;94:94-98.
- Ostrzenski A, Radolinski B, Ostrzenska KM. A review of laparoscopic ureteral injury in pelvic surgery. Obstet Gynecol Surv. 2003;58:794-799.
- Gordon AG, Lewis BV. Complications of laparoscopy. In: Gordon AG, Lewis BV, DeCherney AH, eds. Atlas of Gynecologic Endoscopy. New York: Mosby-Wolfe; 1995:136-142.
- Hasson HM, Parker WH. Prevention and management of urinary tract injury in laparoscopic surgery. J Am Assoc Gynecol Laparosc. 1998;5:99-114.
- Hurt WG, Jones CM III. Intraoperative ureteral injuries and urinary diversion. In: Nichols DH, ed. Gynecologic and Obstetric Surgery. Baltimore: Mosby; 1993:900-910.
- Li TC, Saravelos H, Richmond M, et al. Complications of laparoscopic pelvic surgery: recognition, management and prevention. Hum Reprod Update. 1997;3:505-515.
- Chan JK, Morrow J, Manetta A. Prevention of ureteral injuries in gynecologic surgery. Am J Obstet Gynecol. 2003;188:1273-1277.
- Oh BR, Kwon DD, Park KS, et al. Late presentation of ureteral injury after laparoscopic surgery. Obstet Gynecol. 2000;95:337-339.
- Ostrzenski A, Ostrzenska KM. Bladder injury during laparoscopic surgery. Obstet Gynecol Surv.1998;53:175-180.
- Pillet MCL, Leonard F, Chopin N. Incidence and risk factors of bladder injuries during laparoscopic hysterectomy indicated for benign uterine pathologies: a 14.5 years experience in a continuous series of 1501 procedures. Hum Reprod. 2009;24:842-849.
- Thompson JD. Vesicovaginal fistulas. In: Thompson JD, Rock JA, eds. Te Linde’s Operative Gynecology. 7th ed. Philadelphia: JB Lippincott; 1992:788.
- Zaki H, Penketh R, Newton J. Gynaecological laparoscopy audit: Birmingham experience. Gynaecol Endosc. 1995;4:251-257.
- Kadar N, Reich H, Liu CY, et al. Incisional hernias after major laparoscopic gynecologic procedures. Am J Obstet Gynecol. 1993;168:1493-1495.
- Nezhat C, Childers J, Nezhat F, et al. Major retroperitoneal vascular injury during laparoscopic surgery. Hum Reprod. 1997;12:480-483.
- Boike GM, Miller CE, Spirtos NM, et al. Incisional bowel herniations after operative laparoscopy: A series of nineteen cases and review of the literature. Am J Obstet Gynecol. 1995;172:1726-1731.
- Munro MG. Laparoscopic access: complications, technologies, and techniques Curr Opin Obstet Gynecol. 2002;14:365-374.
- Aksu T, Coskun F. Complications of gynaecological laparoscopy—a retrospective analysis of 3572 cases from a single institute. J Obstet Gynaecol. 2004;24: 813-816.
- Vilos GA, Ternamian A, Dempster J, et al. Laparoscopic entry: a review of techniques, technologies, and complications. The Society of Obstetricians and Gynaecologists of Canada. J Obstet Gynaecol Can. 2007;29:433-465.
- Leron E, Piura B, Ohana E, Mazor M. Delayed recognition of major vascular injury during laparoscopy. Eur J Obstet Gynecol Reprod Biol. 1998 Jul;79(1):91-93.
- Murdock CM, Wolff AJ, Van Geem T. Risk factors for hypercarbia, subcutaneous emphysema, pneumothorax, and pneumomediastinum during laparoscopy. Obstet Gynecol. 2000;95:704-709.
- Kalhan SB, Reaney JA, Collins RL. Pneumomediastinum and subcutaneous emphysema during laparoscopy. Cleve Clin J Med. 1990;57:639-642.
- Adhesion Study Group. Reduction of postoperative pelvic adhesions with intraperitoneal 32% dextran 70: a prospective, randomized clinical trial. Fertil Steril. 1983;40:612-619.
- Pados G, Vavilis D, Pantazis K, et al. Unilateral vulvar edema after operative laparoscopy: a case report and literature review. Fertil Steril. 2005;83:471-473.
- Gupta SP. Experience in 4500 cases of laparoscopic sterilization. Int Surg. 1993;78:76-78.
- Childers JM, Aqua KA, Surwit EA, et al. Abdominal-wall tumor implantation after laparoscopy for malignant conditions. Obstet Gynecol. 1994;84:765-769.
- Nagarsheth NP, Rahaman J, Cohen CJ, et al. The incidence of port-site metastases in gynecologic cancers. JSLS. 2004;8:133-139.
- Ramirez PT, Frumovitz M, Wolf JK, et al. Laparoscopic port-site metastases in patients with gynecological malignancies. Int J Gynecol Cancer. 2004;14:1070-1077.
