Laparoscopic Lymphadenectomy
Shaghayegh M. DeNoble, MD, Douglas N. Brown, MD, and Farr Nezhat, MD
INTRODUCTION
Lymphadenectomy has traditionally been performed using large incisions during laparotomy. Since the initial report by Dargent and colleagues in the late 1980s, laparoscopic lymphadenectomy has been utilized in the management of gynecologic and urologic malignancies as well as in some lymphomas.1 After Dargent’s description of the first pelvic lymphadenectomy performed laparoscopically, Nezhat et al. described the first para-aortic lymphadenectomy performed laparoscopically for cancer of the uterine cervix.2 Many reports since have described the safety and effectiveness of laparoscopic lymphadenectomy for gynecologic and urologic malignancies, as well as for some lymphomas. Over recent years, there has been an expanding literature available regarding outcomes and complications of laparoscopic lymphadenectomy. Current reports, however, often reflect the developing skill set of the pioneering laparoscopists and the variable facility support for such advanced laparoscopic procedures.
LAPAROSCOPIC PELVIC AND PARA-AORTIC LYMPHADENECTOMY
Pelvic lymphadenectomy performed either laparoscopically or by open laparotomy begins with identification of the ureter, transection of the round ligament, and opening of the posterior peritoneum and the pelvic sidewall. The common iliac and external iliac lymph nodes are dissected proximally from the common iliac artery bifurcation and distally to the deep circumflex vein. Attention is taken to avoid the genitofemoral nerve that lies on the medial psoas muscle during this dissection. The paravesical space is developed between the obliterated hypogastric artery and the external iliac vessels. The obturator space is then opened to identify the obturator nerve and vessels. The obturator vessels are located dorsal to the obturator nerve in the majority of patients; however, an aberrant obturator vein can be found in up to 10% of cases and can be a ready target for an unanticipated vascular injury. Once the landmarks of the obturator space are identified, the lymph nodes are grasped just under the external iliac vein and traction is applied medially. The nodal chain is separated from the obturator nerve and vessels and dissected cephalad to the hypogastric artery. Finally, the hypogastric lymph nodes are removed up to the common iliac artery with close attention to avoid the hypogastric vein. For the para-aortic dissection, the peritoneum is then incised over the sacral promontory or over the right common iliac artery with the right ureter in view. This incision is extended up to the inferior mesenteric artery or up to the left renal vein. The lymph node packages are retrieved along the common iliac vessels, the aorta, and the vena cava with careful attention to the ovarian and renal vessels and the inferior mesenteric artery.3,4
EXTRAPERITONEAL ENDOSCOPIC PARA-AORTIC LYMPHADENECTOMY (RETROPERITONEOSCOPY)
An alternative to the transperitoneal laparoscopic lymphadenectomy is the extraperitoneal endoscopic para-aortic lymphadenectomy, which was first described by Vasilev in 1996 and later by Dargent.5,6 In 2000, the first large series utilizing this surgical technique was reported by Querleu and Dargent.7 This approach has been applied to the lymph node assessment of nonseminomatous germ cell tumor of the testis, which allows for a nerve- sparing approach as described by LeBlanc in 2001.8 It has also been found useful for resection of para-aortic lymph node recurrence of gynecologic cancers.9,10 Extraperitoneal endoscopic lymphadenectomy combines the benefits of laparoscopy with those of an extraperitoneal dissection. Compared with the laparoscopic transperitoneal approach, the advantages include operative feasibility, decreased risk of direct bowel injury and bowel adhesion formation, which can result in a reduced incidence of postradiation enteritis.11 The technique includes two steps. First, a traditional transumbilical diagnostic laparoscopy is performed. If the laparoscopic findings indicate no evidence of peritoneal metastasis and that a lymph node dissection is still indicated, then an endoscopic extraperitoneal lumbo-aortic lymphadenectomy is undertaken. The extraperitoneal approach to the aorta is obtained through a 15-mm incision made at McBurney’s point or at the same location on the contralateral side. Once the fascia parietalis is opened, the surgeon introduces his or her forefinger into the preperitoneal space and starts developing the extraperitoneal space under the guidance of the transumbilical laparoscope. A trocar with a pneumostatic device is introduced through the iliac incision to facilitate the extraperitoneal insufflation, creating a retropneumoperitoneum at 10 mmHg to 11 mmHg pressure while the gas is expelled from the peritoneal cavity. Additionally, two operative trocars are used. A 10-mm trocar is placed midway between the iliac crest and the costal margin at the midaxillary line, and a 5-mm trocar is placed anteriorly under the subcostal margin.
Complications specific to extraperitoneal endoscopic lymphadenectomy include the potential for creating defects in the peritoneum during dissection requiring the conversion to a laparoscopic transperitoneal approach. An alternative solution, however, is to reduce any remaining pneumoperitoneum and thereby facilitate further extraperitoneal dissection. In cases of larger peritoneal defects, clips or sutures may be applied to reapproximate the peritoneal edges and thereby maintain the extraperitoneal insufflation. In early experiences with this technique, the formation of symptomatic lymphoceles comprised the majority of postoperative complications. The rate has been shown in several studies to vary from 3.8-21%.6,7,12,13,14,15 This complication has been reduced by creating a peritoneal window in the paracolic gutter upon completion of the procedure.12
At the Advanced Minimally Invasive Surgery in Oncology meeting in New York, May 2003, E. LeBlanc presented his results with this procedure to date. In 144 cases (133 with advanced cervical cancer, 9 with adnexal cancer and 2 with endometrial cancer), the number of nodes retrieved was 19±12 with an estimated blood loss <100 mL and an average operative time of 156 minutes (±46). Notably, 42% of these patients had had prior abdominal surgery. Five failures of this procedure were reported: 1 due to obesity, 1 due to inadequate pneumoperitoneum, and 3 due to extensive lymph node metastases.
Lymphedema was reported in 3 cases. Lymphoceles were reported in 23 cases; however, only 2 cases of lymphocele were found among the 31 cases that included creation of a peritoneal window in the paracolic gutter at the completion of the procedure. Two hematomas and one blood transfusion were reported in LeBlanc’s series.
In 2004, Mehra et al performed laparoscopic extraperitoneal para-aortic lymphadenectomy in 32 women with cervical, ovarian and endometrial cancers.13 The mean nodal yield was 12 nodes (range 5-22), operating time was 80 minutes (range 40- 200), and length of hospital stay was 2 days (range 1-5). There was failure in one patient as a result of a peritoneal tear with subsequent leakage of CO2 gas. Two major complications were reported: umbilical hernia through the port site and pulmonary embolism. Late complications included 1 lymphocele, 1 pelvic abscess and 1 thigh cellulitis.
ADVANTAGES OF LAPAROSCOPY FOR LYMPHADENECTOMY
Technically, a laparoscopic approach has many benefits specific to pelvic and para-aortic lymphadenectomy. The laparoscope provides a 7- to 10-fold magnification on the operative dissection, which facilitates the identification of small tributary vessels.
Further, the pressure of the pneumoperitoneum assists in developing the pelvic spaces and decreases venous oozing, which maintains a clean operative dissection and especially good visualization of the vasculature and nodal bundles. The distribution of the lymph chains is shown in Figure 1. The anatomical relationships are demonstrated in laparoscopic images as shown in Figure 2.
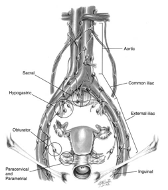 Figure 1. Distribution of pelvic and para-aortic lymph node chains. Illustration for Figure 1 reproduced with permission from Nezhat CR, et al. Operative Gynecologic Laparoscopy: Principles and Techniques. 2nd ed. New York, NY: McGraw Hill; 2000.
Figure 1. Distribution of pelvic and para-aortic lymph node chains. Illustration for Figure 1 reproduced with permission from Nezhat CR, et al. Operative Gynecologic Laparoscopy: Principles and Techniques. 2nd ed. New York, NY: McGraw Hill; 2000.
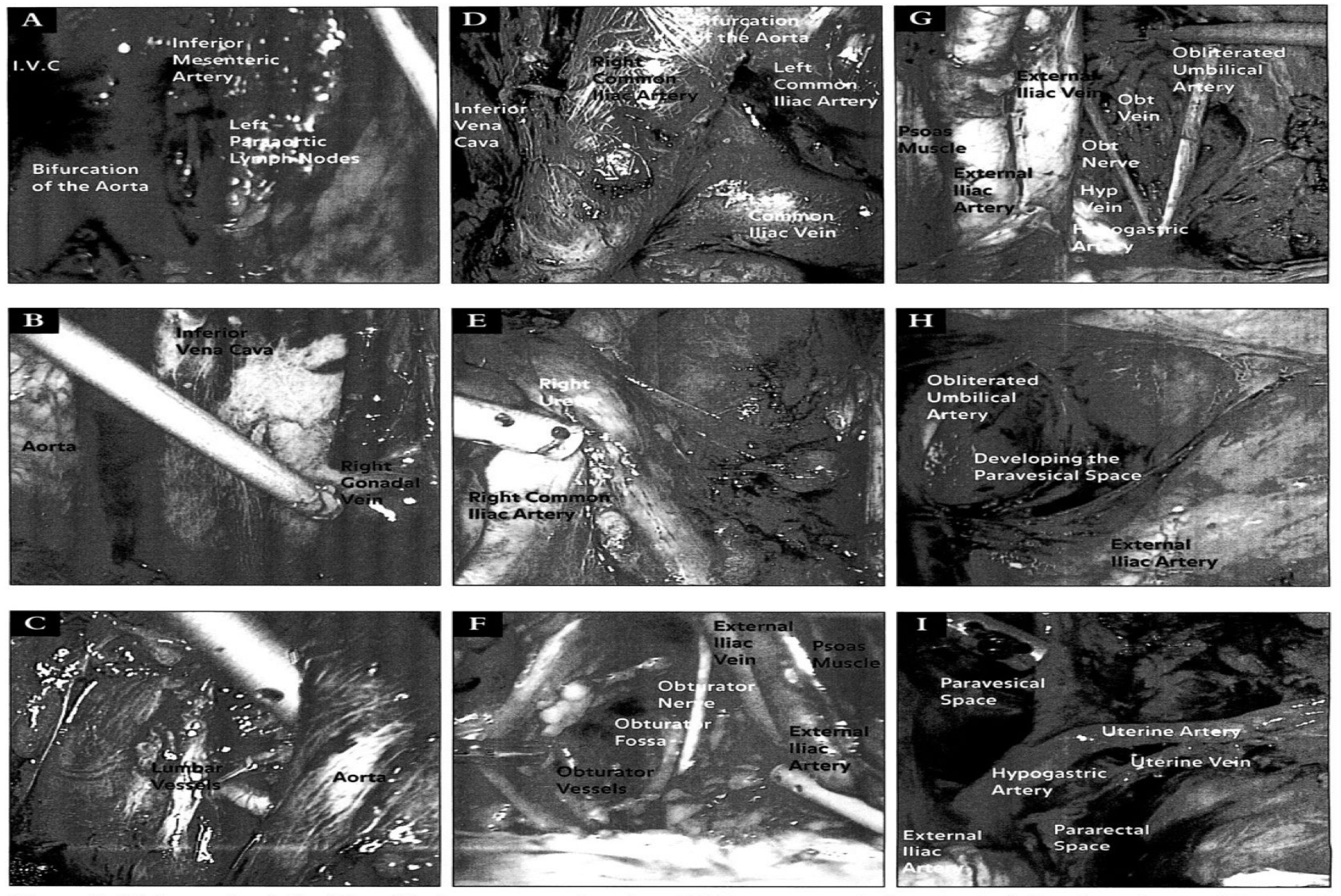 Figure 2. (A) Anatomic relationships of the lower para-aortic region. (B) Upper para-aortic dissection including the right gonadal vein and the inferior vena cava. (C) Anatomic relationships of the lumbar vessels to the aorta. (D) Bifurcation of the aorta and its relationship to the venous system. (E) Anatomic relationship of the right ureter to the major pelvic vasculature. (F) Anatomic relationships during a right pelvic lymphadenectomy including landmarks of the obturator fossa. (G) Anatomic relationships during a left pelvic lymphadenectomy. (H) Development of the right paravesical space during a laparoscopic lymphadenectomy. (I) Anatomic relationships of the left pararectal and paravesical space as shown laparoscopically.
Figure 2. (A) Anatomic relationships of the lower para-aortic region. (B) Upper para-aortic dissection including the right gonadal vein and the inferior vena cava. (C) Anatomic relationships of the lumbar vessels to the aorta. (D) Bifurcation of the aorta and its relationship to the venous system. (E) Anatomic relationship of the right ureter to the major pelvic vasculature. (F) Anatomic relationships during a right pelvic lymphadenectomy including landmarks of the obturator fossa. (G) Anatomic relationships during a left pelvic lymphadenectomy. (H) Development of the right paravesical space during a laparoscopic lymphadenectomy. (I) Anatomic relationships of the left pararectal and paravesical space as shown laparoscopically.
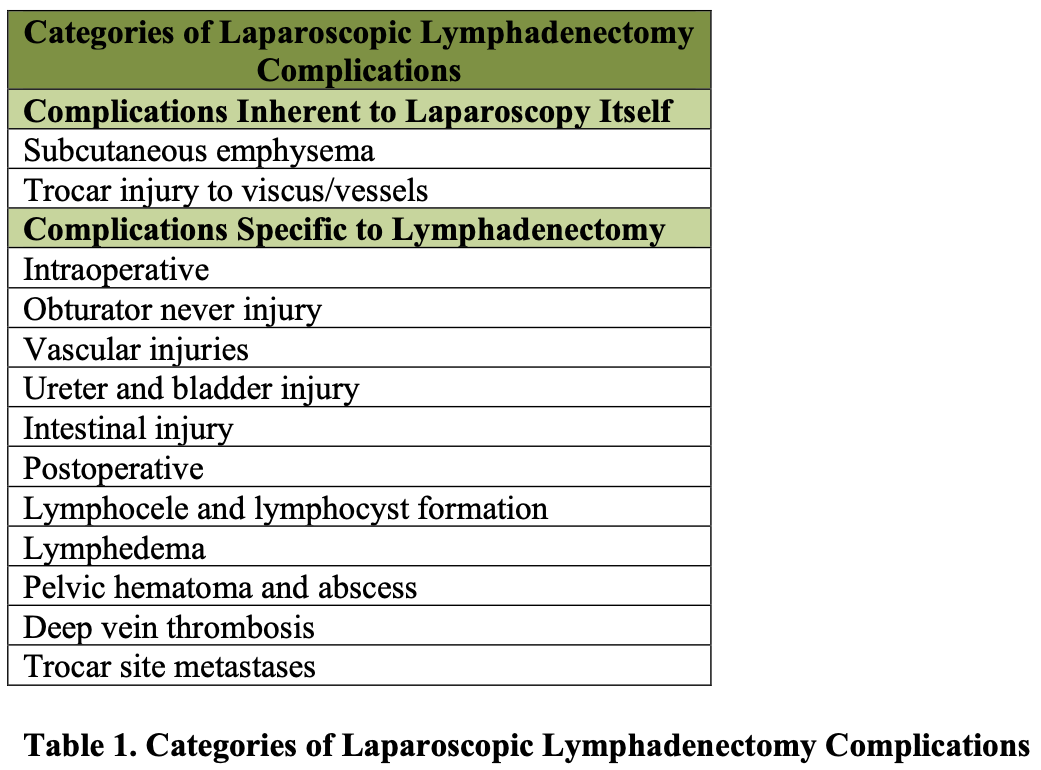
Complications of laparoscopic lymphadenectomy can be divided into 2 general categories. As listed in Table 1, these complications include those that are inherent to laparoscopy itself, regardless of the specific procedure being performed. Such complications include the development of subcutaneous emphysema or trocar injuries. The second group of complications includes those that are inherent to the procedure of lymphadenectomy and can occur regardless of the method by which it is performed.
Obturator nerve injury, deep venous thrombosis, and postoperative lymphocele are all possible complications of a pelvic lymphadenectomy.
Adequate exposure is critical to optimize surgical dissection and to reduce complications. This basic surgical tenet for open abdominal surgery is also a necessary component of effective laparoscopic technique. For a laparoscopic lymphadenectomy, adequate exposure requires a thorough preoperative bowel preparation, steep Trendelenburg patient positioning, and an adequate pneumoperitoneum. These steps are essential to effectively sweep the bowel away from the operative field and to reduce the opportunity for bowel injury. Bowel decompression with an orogastric or nasogastric tube and bladder decompression with a Foley catheter should be performed at the start of the procedure. The patient should be placed in the lithotomy position with the thighs no higher than the level of the anterior abdominal wall. This allows free range of motion of the lower quadrant laparoscopic instruments without interference from the legs. Full mobility of the lower quadrant instruments allows for optimal traction and countertraction of tissue planes for dissection.
The Gynecologic Oncology Group (GOG) recently reported the largest randomized trial in endometrial cancer thus far, the LAP-2 study.16 Patients with clinical stage I to IIa uterine cancer were randomly assigned to laparotomy or laparoscopy for staging. The rate of conversion to laparotomy was 25.8%. There was no difference in the rate of intraoperative complications, and fewer moderate to severe postoperative events and shorter hospital stay in the laparoscopy group. There was no difference in the percentage of patients who had pelvic lymphadenectomy (99% and 98%), but 92% of patients in the laparoscopy group and 96% in the laparotomy had both pelvic and paraaortic lymphadenectomy. The median number of pelvic lymph nodes retrieved in the pelvic and paraaortic regions was similar for both groups.
As discussed in the following sections, potential complications of laparoscopic pelvic and para-aortic lymphadenectomy can be categorized by systems including vascular, gastrointestinal, genitourinary, and neurologic events.
ROBOTIC-ASSISTED LAPAROSCOPY FOR LYMPHADENECTOMY
The advantages of computer-enhanced technology (robotics), including 3-dimensional image, high magnification, tremor filtration, 7-degrees of movement, ergonomics, and shorter learning curve, have leant to the popularity and wide spread use of the robot in various surgical fields.17 The advantages of the robotic system are especially useful in radical surgical procedures including oncologic staging and lymphadenectomies. In robotic-assisted laparoscopy, traditional laparoscopy is first implemented to evaluate for feasibility of the procedure and the extent of disease. A 12-mm trocar for camera placement is next placed, either at the umbilicus for pelvic lymphadenectomy (Figure 3), or above the umbilicus if para-aortic lymphadenectomy will also be performed (Figure 4). Two 8-mm trocars for attachment to the robotic arms are placed bilaterally, 1-2cm below and 8-10cm lateral to the camera. An ancillary port is placed in the left or right upper quadrant, for use by an assistant. Monopolar electrosurgical scissors and bipolar forceps are used (Figure 5), and lymphadenectomy is performed as mentioned earlier in this chapter.
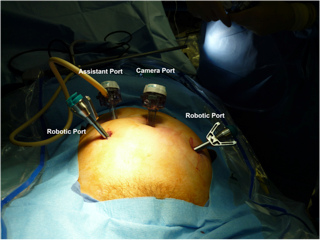 Figure 3. Trocar placement for robotic-assisted pelvic lymphadenectomy.
Figure 3. Trocar placement for robotic-assisted pelvic lymphadenectomy.
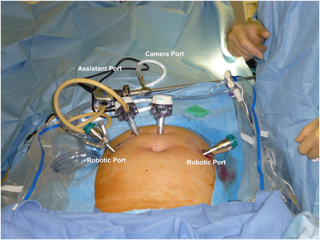 Figure 4. Trocar placement for robotic-assisted para-aortic lymphadenectomy.
Figure 4. Trocar placement for robotic-assisted para-aortic lymphadenectomy.
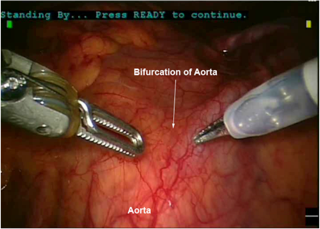 Figure 5. Bipolar forceps (left) and monopolar electrosurgical scissors (right) in robotic-assisted laparoscopic lymphadenectomy.
Figure 5. Bipolar forceps (left) and monopolar electrosurgical scissors (right) in robotic-assisted laparoscopic lymphadenectomy.
Nezhat et al. compared robotic-assisted laparoscopy to conventional laparoscopy for radical hysterectomy in patients with early-stage cervical cancer.18 There were no statistical differences in pelvic lymph node retrieval (24.7 vs. 31 nodes), operative time, estimated blood loss, hospital stay, or complications. There were no conversions to laparotomy. Another study by Magrina et al. compared radical hysterectomy performed by robotic-assisted laparoscopy, conventional laparoscopy, and laparotomy.19 The robotic and laparoscopic groups were similar in regards to blood loss and length of hospital stay, and reduced as compared to laparotomy. Mean operative times were similar for robotics and laparotomy, and shorter as compared to laparoscopy. There were no differences in the mean number of lymph nodes removed or complications between the three groups.
Drawbacks to the use of robotics include lack of tensile feeback and the size and cost of the system. These factors, as well as the benefits of robotic surgery already mentioned, must be weighed by the surgeon in deciding between robotic-assisted laparoscopy and traditional laparoscopy.
VASCULAR INJURIES
Vascular injuries related to lymphadenectomy are potentially life-threatening complications. Perhaps the most common vascular injury of laparoscopy in general, and laparoscopic lymphadenectomy in particular, is injury to the inferior epigastric and other superficial anterior abdominal wall vessels (Figure 6). Kavoussi et al. in a series of laparoscopic lymphadenectomies reported that 7 out of 9 vascular complications occurred as a result of trocar injury to the anterior abdominal wall vasculature.20 Vascular injuries can also occur as a result of Veress needle insertion. A systematic literature review of 696,502 cases from 55 articles by Azevedo et al. showed a total of 1,575 injuries (0.23%) caused by insertion of the Veress needle.21 Of these, 98 (6.2%) were vascular injuries, 42 of which were major vascular injuries including the aorta and the common iliac arteries.
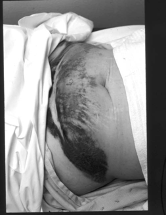 Figure 6. Extensive postoperative ecchymosis extending from the suprapubic port site.
Figure 6. Extensive postoperative ecchymosis extending from the suprapubic port site.
Potential vascular compromise specific to pelvic lymphadenectomy include injury to the obturator, internal iliac, external iliac, or the common iliac vessels. In the male, the testicular artery and vein should be carefully identified during a laparoscopic lymphadenectomy to maintain their integrity. Vessels at risk for injury during a para- aortic lymphadenectomy include the aorta and vena cava, as well as the common iliac, inferior mesenteric, lumbar, gonadal, and renal vessels.
Fortunately, injury to major pelvic vessels during laparoscopic pelvic lymphadenectomy is an uncommon occurrence. In Kavoussi’s series, 7 of 9 vascular injuries resulted from trocar injuries to the abdominal wall vessels as reported by the combined experience of 8 institutions performing laparoscopic pelvic lymphadenectomy for early prostatic carcinoma. In the remaining cases, one patient sustained injury to the obturator vein and the other case involved interruption of the external iliac artery.
In Childers’ experience with more than 300 pelvic lymphadenectomies for gynecologic malignancy, injury to major pelvic vessels was a rare event.22,23 Overall, the vessel most likely to be injured is an aberrant obturator vein emerging from the obturator canal and crossing the nodal bundle to empty into the external iliac vein. In Childers’ series, a 5- mm laceration in the external iliac vein was sustained at the time of lymphadenectomy during a second-look laparoscopic procedure. The patient had undergone a previous lymph node sampling at the time of cytoreductive surgery for ovarian cancer, and the external iliac vein was injured while the retroperitoneal space was being opened. The laceration was repaired laparoscopically using a surgical clip. The patient did not require transfusion or experience any postoperative complications. Three other patients had vena cava injuries complicating their laparoscopic lymphadenectomies. The first required open laparotomy for vascular repair and was transfused with 4 units of packed red blood cells. The patient went on to develop a deep venous thrombosis. The remaining 2 cases were managed laparoscopically with endoscopic vascular clip applicators (Figure 7).24 Neither of these 2 patients received a transfusion or had any other postoperative complications.
Experience with external iliac and vena cava injuries has revealed that bleeding in the presence of adequate pneumoperitoneum (ie, 15 mmHg), even from large veins, is relatively limited. In our experience, the most common vascular injury during para-aortic lymphadenectomy is injury to the tributary vessels. This can be best controlled by bipolar electrocautery or by applying surgical clips.
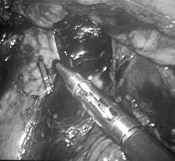 Figure 7. Endoscopic clip applied to the lower vena cava following a small venotomy created during a laparoscopic para-aortic lymphadenectomy.
Figure 7. Endoscopic clip applied to the lower vena cava following a small venotomy created during a laparoscopic para-aortic lymphadenectomy.
More recently, Querleu and Leblanc in their series of 1000 gynecologic patients who underwent laparoscopic pelvic and/or para-aortic lymphadenectomies reported a significant vascular injury rate of 1.1.25 Four injuries occurred in the external and internal veins, and the obturator and superior vesicle arteries. Six injuries were to the vena cava, aorta, common iliac, ovarian, and left renal arteries. There was also transection of the inferior mesenteric artery in one patient. All of the vascular injuries in this series were controlled laparoscopically using bipolar cautery or clips, and there were no reoperations.
In our series of over 100 laparoscopic lymphadenectomies for gynecologic malignancy, we reported an overall complication rate of 13% and vascular injury rate of 2%.26 Two of the complications were intraoperative and directly related to the lymphadenectomy portions of the cases. There was injury to a branch of the inferior mesenteric arteryduring staging of a patient with stage IIB cervical cancer. Hemostasis was achieved using bipolar cautery. There was also an injury to the right hypogastric vein during pelvic lymphadenectomy, controlled using hemostatic clips. All complications were managed laparoscopically.
A theoretical, but nonetheless important, concern, particularly with damage to large vascular structures, is the possibility of an air embolus. Air embolus is especially a concern if the lymphadenectomy is performed with an argon beam coagulator because the argon gas is not readily absorbed into the circulation. The diagnosis of gas embolism is made via auscultation with a conventional or transthoracic stethoscope. A “wheel-mill” murmur is presumptive evidence of air entrapment in the right heart. Management consists of placement of a central line into the right ventricle and subsequent aspiration of the embolized gas. Although such a complication is yet to be reported for laparoscopic lymphadenectomy, CO2 embolism has been reported with other laparoscopic procedures.
Current reports confirm the low incidence of vascular injury during laparoscopic lymphadenectomy, as summarized in Table 2.27-30 A review of eleven series of laparoscopic pelvic and para-aortic lymphadenectomy showed a 1-10% rate of major vascular injuries.26 Further, the 2002 study by Scribner et al. demonstrates the technical feasibility and low complication rate of laparoscopic lymphadenectomy in obese endometrial cancer patients.31
MANAGEMENT OF VASCULAR INJURIES
Laparoscopic management of vascular injuries varies according to the type (artery or vein) and the size of the vessel injured. The modalities utilized in managing these vascular injuries include pressure, monopolar and bipolar electricity, clips, and sutures. Depending on the caliber of the injured vessel, any one of these modalities may achieve hemostasis satisfactorily. When a vessel is injured, it is important to control the bleeding as quickly as possible. This usually can be accomplished by using a laparoscopic grasper to occlude the bleeding vessel or by applying gentle pressure with a suction irrigator cannula. If visualization is obscured, or if the vessel is a large vein where grasping may result in further laceration, pressure should be applied. Laparoscopic instruments, lymph node pads, 4-inch x 4-inch gauze, or mini-laparotomy pads can be placed to achieve immediate control. To be prepared for any bleeding, a mini-laparotomy pad can be placed through a 10-mm to 12-mm port prior to beginning the lymph node dissection.
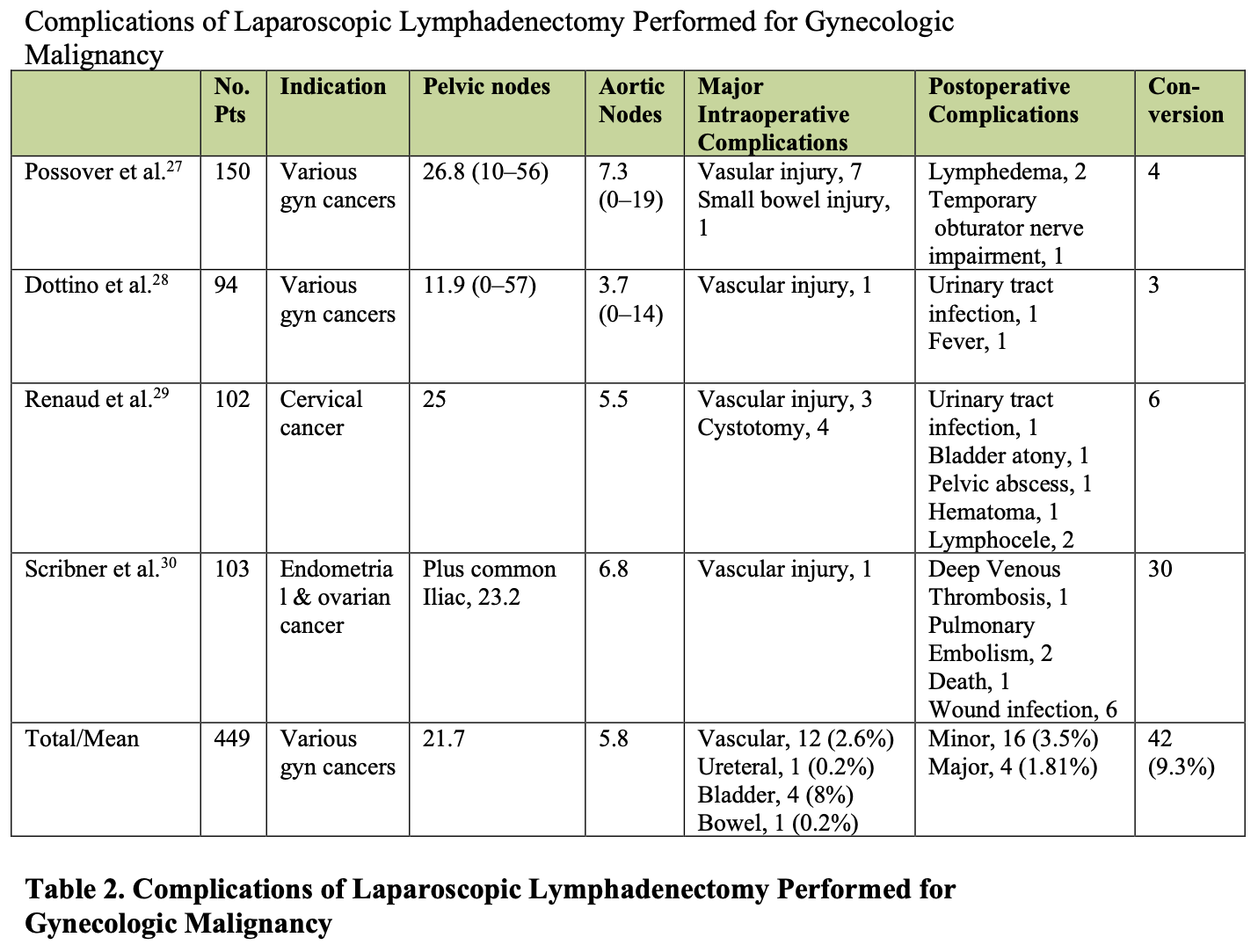
Small venous bleeders can be controlled with pressure alone. A laparotomy pad can be packed into the retroperitoneal space while the procedure is continued in another part of the surgical field. Later in the operative procedure, the packing can be removed and the area inspected for hemostasis. Placing pads into the abdomen laparoscopically can be a helpful technique; however, it also produces a new potential complication–retained laparotomy pads. Laparotomy rings, such as those used when a laparotomy is performed, cannot be used laparoscopically, so the surgeon relies primarily on the operative pad count. If the count is incorrect, or if the surgeon wants to include an additional safety measure, a postoperative x-ray can be obtained. Figure 8 illustrates a radiograph of the abdomen demonstrating 2 retained retroperitoneal laparotomy pads that were placed during a laparoscopic para-aortic lymphadenectomy.
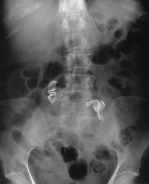 Figure 8. Radiograph demonstrating the radiolucent tags of a retained mini-laparotomy pad. These pads were initially placed following a laparoscopic bilateral low paraaortic lymphadenectomy.
Figure 8. Radiograph demonstrating the radiolucent tags of a retained mini-laparotomy pad. These pads were initially placed following a laparoscopic bilateral low paraaortic lymphadenectomy.
Arterial bleeders should be quickly controlled because the blood loss can be rapid. Care should be taken to avoid arterial pumping of blood onto the laparoscope. When this occurs, visualization of the operative field is obscured, and the laparoscope must be removed for cleansing, which expends valuable time and increases blood loss. Another common pitfall in controlling vascular injuries occurs during the aspiration of pooled blood. While the pooled blood is being suctioned, the pneumoperitoneum frequently is suctioned as well if the suction tip is not completely submerged. This can result in poor exposure of the operative site and can create a cascade of time-consuming events with inadequate pneumoperitoneum, bowel falling out of the upper abdomen, and further reduced visualization of the vessel that is requiring repair.
To achieve hemostasis, monopolar electricity or ultrasonic Harmonic coagulation can be used for small arterial and venous bleeders. The bipolar electrocautery is especially useful to control venous and arterial bleeders of a larger caliber. The bipolar method can be used to coapt vessels or can be applied along the length of the instrument to provide surface cauterization over a friable area. Bipolar electrocautery, however, is an unacceptable form of vascular injury control in vessels that should not be sacrificed or vessels of extremely large caliber, such as the iliac and the vena cava. In these situations, clips or suturing techniques should be used. Laparoscopically applied clips can control large venous bleeders adequately. Prefabricated slipknots can be used to control moderate-sized arterial bleeders. In these instances, a grasper is placed through the prefabricated loop and affixed to the injured vessel. The loop is slipped over the grasper and onto the vessel. Suturing techniques utilizing needles may also be used, and in experienced hands suturing can be performed laparoscopically. With improvements in technology and instrumentation, such as robotics, laparoscopic suturing is more practical. Utilizing a 4-0 proline suture, we have been successful in robotically repairing common iliac vein and small aortic injuries that have occurred during para-aortic lymphadenectomy, without sequelae. Otherwise, laparotomy may be needed, and consideration should be given to intraoperative consultation with a vascular surgeon.
GASTROINTESTINAL INJURIES
Bowel injury is a potential complication of any laparoscopic procedure. Bowel can be easily damaged at trocar and Veress needle insertion, adhesiolysis, or thermal injury during dissection (Figure 9). In their series of 1000 gynecologic patients who underwent laparoscopic pelvic and/or para-aortic lymphadenectomies, Querleu and Leblanc reported a bowel injury rate of 0.4%.25 Prevention of this complication begins with appropriate patient selection and preoperative preparation. This involves a complete mechanical bowel preparation and the intraoperative placement of an orogastric or nasogastric tube during all laparoscopic procedures. The net effect is to keep the bowel flat and empty, resulting in easier “packing” of the bowel into the upper abdomen.
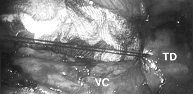 Figure 9. Laparoscopic photograph from the umbilical port. The surgeon is placing an imbricating silk suture on the transverse duodenum to oversew a superficial cautery injury during a paraaortic lymphadenectomy. TD = transverse duodenum;VC = vena cava
Figure 9. Laparoscopic photograph from the umbilical port. The surgeon is placing an imbricating silk suture on the transverse duodenum to oversew a superficial cautery injury during a paraaortic lymphadenectomy. TD = transverse duodenum;VC = vena cava
Delayed bowel morbidity can occur throughout the postoperative period. Bowel herniation can result as a consequence of absent or inadequate fascial closure of the trocar defects (Figure 10). Nezhat et al. reported 11 incisional hernias in 10 patients (0.2%) in their retrospective case review of 5300 patients who underwent operative laparoscopy.32 The omentum was herniated in 7 cases, and bowel in 4 cases. Five of the 11 herniations were through 5-mm trocar sites, indicating the importance of closing the 5-mm ports if there has been extensive manipulation. Of the 10 patients, 6 required laparoscopy to retract the entrapped omentum or bowel, and one patient required bowel resection. While not reported, bowel obstruction can occur as a result of post-laparoscopic adhesion formation. We have managed a case of partial small bowel obstruction occurring after a laparoscopic lymphadenectomy for endometrial cancer that resolved with conservative management.
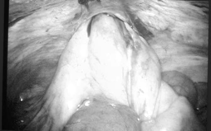
 Figure 10. Laparoscopic and mini-laparotomy of small bowel herniation complicated by extensive ischemia. Multiple loops of small bowel exteriorized through a mini-laparotomy incision over the herniation site (right lower quadrant port).
Figure 10. Laparoscopic and mini-laparotomy of small bowel herniation complicated by extensive ischemia. Multiple loops of small bowel exteriorized through a mini-laparotomy incision over the herniation site (right lower quadrant port).
MANAGEMENT OF GASTROINTESTINAL INJURIES
Laparoscopic management of gastrointestinal injuries has been covered in previous chapters. Consideration of laparoscopic hernia reduction is not unreasonable, depending on the clinical situation, and has been accomplished and reported in the literature.33 Large and small bowel injuries can be repaired intracorporeally or extracorporeally through a slightly enlarged port site. These techniques are discussed in detail in earlier chapters.
GENITOURINARY INJURY
Laparoscopic injury to the urinary tract is well described in the literature. Cystotomy during trocar insertion, adhesiolysis, or dissection with endoscopic scissors with or without electrocautery has been delineated as a complication of laparoscopic lymphadenectomy, although not specific to this procedure.20,32,34 Cystotomy can occur during insertion of the suprapubic trocar, development of the bladder flap and vesicovaginal space in a laparoscopic radical hysterectomy, or when opening the paravesical and pararectal spaces if the obliterated umbilical artery is not retracted medially. Meticulous surgical technique and decompression of the bladder with a Foley catheter are the cornerstones to preventing this type of injury. Ureterovaginal and vesicovaginal fistulae as well as injury to a patent urachus are other potential complications.
Specific to the laparoscopic lymphadenectomy is the possibility of a ureteral injury. Pelvic lymphadenectomy places the ureter at risk for sharp, crush, or thermal injury. This has been described in the urologic literature.20,34 The lumbar portion of the ureter is at risk during para-aortic lymphadenectomy, especially on the left side. This can take place if the lateral dissection overlying the psoas muscle is carried out above the ureter instead of in the correct surgical plane. A portion of the ureter can thereby be included with the nodal bundle for resection. Such an injury, if recognized intraoperatively, can be managed laparoscopically. A transureteral stent should be placed, the ureteral defect oversewn, and a retroperitoneal drain placed laparoscopically. Although this is an area of current investigation, there is no proven role for prophylactic ureteral stent placement prior to laparoscopic lymphadenectomy.
NEUROLOGIC INJURY
Operative nerve injury can complicate any surgical procedure in the pelvis. There are, however, concerns that are particular to lymphadenectomy. Genitofemoral nerve injury is most likely to occur during removal of the lateral pelvic lymph nodes. Such an injury results in medial thigh numbness but is otherwise of limited clinical consequence. It is arguably the most common injury encountered by the gynecologic oncologist. Injury of the obturator nerve is a more worrisome, though extremely rare, complication of laparoscopic lymph node dissection.20,34,35 We have not experienced this complication since the early 1990s when first performing laparoscopic lymphadenectomies. This complication only occurs if the obturator nerve is not reliably identified prior to resecting the obturator lymph node package. Theoretically, the femoral nerve, which lies within the body of the psoas muscle in the pelvis, is also at risk during pelvic lymphadenectomy.
This is particularly true if the nerve is superficial in the belly of the muscle and is exposed to extensive electrocautery during the dissection. Though not a direct operative injury, the ulnar nerve can also be traumatized if not properly padded in the course of tucking the arms for the operative procedure. Other peripheral neuropathies can also occur secondary to poor patient positioning for operative laparoscopy.
ABDOMINAL WALL METASTASES AND PERITONEAL TUMOR DISSEMINATION
Abdominal wall metastases, also called port- or wound-site metastases, have been reported after both laparoscopy and laparotomy. The incidence of these complications is 1% after laparotomy and 1% to 3% after laparoscopy.36-40The most likely mechanism for port-site metastases seems to be by direct contamination during the surgical procedure.
Port sites can be contaminated during tumor extraction or during the removal of contaminated ports at the completion of the procedure. Chu et al. described recurrence at port sites in a patient who underwent laparoscopic staging for cervical cancer.41 Similar port site recurrence was described by Wang et al.42 Although port-site or wound-site recurrences may be disfiguring and difficult to treat, no prospective evidence exists indicating that port-site metastases worsen prognosis.36,39
In animal studies, tumor dissemination has been associated with CO2 pneumoperitoneum.43 Voltz et al. reported diffuse intraperitoneal tumor spread after laparoscopy in a nude-mice model.44 Canis et al. used immunocompetent rats injected intraperitoneally with rat ovarian carcinoma cells.45 In the high-pressure pneumoperitoneum group (10 mmHg), tumor was disseminated more diffusely than in the low-pressure pneumoperitoneum group (4 m Hg). Wound metastases, however, were larger and more frequent after laparotomy than following laparoscopy. As noted by Canis, one has to be cautious when drawing clinical parallels because of animal studies.43 Important variables include the type of animal system utilized, the choice of cell line for inoculation, postprocedure immune status, and the pressure of the pneumoperitoneum sustained. Each of these variables can greatly influence results, and they reflect the challenges to accurately reproduce clinical scenarios in animal model systems.
A few clinical reports, however, have also raised concerns regarding possible intraperitoneal tumor dissemination during laparoscopy. Canis et al. reported a case of pelvic dissemination found at a restaging procedure 3 weeks after initial adenectomy for well-differentiated serous adenocarcinoma of the ovary.46 Although one may argue that these findings are indicative of tumor biology more than a consequence of surgical approach, it is prudent to utilize surgical measures that prevent tumor dissemination.
Removal of all specimens using an endobag and removal of all ports once the pneumoperitoneum is reduced is recommended to prevent possible contamination of the port sites. Table 3 summarizes the surgical rules to prevent abdominal wall metastases and dissemination of disease.
We have evaluated the incidence of port-site metastases in patients undergoing laparoscopic procedures for gynecological cancers. The overall incidence of port-site metastases per procedure and per port placed was 2.3% (2/87) and 2.4% (8/330), respectively.41 Twenty of the procedures were performed for recurrent ovarian or primary peritoneal cancer, 4 of which had ascites at the time of the procedure. Interestingly, no port-site metastases (0/16) occurred in the absence of ascites; however, 50% (2/4) of patients with ascites developed port-site metastases (P<0.035). At our institution, laparoscopy is routinely used in the management of early and advanced ovarian cancers, and we have not had any detectable trocar metastases. We use all the precautions to avoid incisional contamination, and close all trocar sites. We also start chemotherapy as soon as possible.
OTHER COMPLICATIONS
Various other injuries and complications can result as a consequence of the laparoscopic lymphadenectomy. Similar to open laparotomy, both lymphoceles and lymphedema have been reported to occur following laparoscopic lymphadenectomy.20,34,35 In Childers’ experience with more than 300 pelvic lymphadenectomies, 2 symptomatic lymphoceles have occurred.22,23 As might be expected with any operative procedure, infectious complications have also been reported following laparoscopic lymphadenectomy, and these include infected pelvic hematoma, clostridium difficile infection, and wound complications.20,34,47 Likewise, retained foreign bodies and equipment failure can preclude a safe and effective surgical exercise (Figure 8).
Thromboembolic events can complicate any major operative procedure in the pelvis, especially in the cancer patient. Two separate series of laparoscopic lymphadenectomies performed for urologic malignancies reported 3 instances of deep venous thrombosis (1.5%) and no pulmonary emboli among 203 procedures.35,47 Pomel et al. reported a case of lower extremity thrombophlebitis and a subsequent pulmonary embolism following a staging laparoscopy for ovarian carcinoma.48 In another series of 40 patients who underwent bilateral pelvic and para-aortic lymphadenectomy for endometrial and ovarian cancer, 35 patients were completed laparoscopically, 2 of which (5.7%) developed deep vein thrombosis during the postoperative period.49
COMPLICATIONS REQUIRING LAPAROTOMY
Complications that can require conversion to laparotomy include damage to the ureter, bladder, bowel, or vascular injury. Kavoussi et al. reported a 4% (13/372) incidence of laparotomy with laparoscopic pelvic lymphadenectomy.20 Complications recognized at initial laparoscopic surgery occurred in 7 patients. Six individuals required secondary laparotomy. Reasons for laparotomy included transection of the ureter (2 patients), cystotomy (2 patients), bowel injuries or obstruction (4 patients), vascular injury (4 patients), and wound dehiscence (1 patient). Burney et al. reported a laparotomy rate of 8% (4/54) for patients undergoing laparoscopic pelvic lymphadenectomy for urologic indications.34 One of 4 patients required conversion laparotomy at the time of the initial laparoscopic procedure. Indications for laparotomy included ureteral damage (1 patient), small bowel obstruction (2 patients), and mesenteric hematoma (1 patient).
In patients undergoing laparoscopic pelvic or para-aortic lymphadenectomy, or both, for endometrial cancer, laparotomy was required in 10% (3/29) of patients as reported by Boitke et al.50 Two patients underwent secondary operations because of small bowel obstruction, and another patient had a vascular injury to a small branch of the aorta recognized intraoperatively and required minilaparotomy but no transfusion at the time of the primary surgery. Both patients with small bowel obstructions were related to herniations through trocar sites, both of which were 10 mm. No complications were observed to directly result from the pelvic lymphadenectomy, although 2 of 2 patients undergoing para-aortic lymphadenectomy had major complications. An additional patient required percutaneous nephrostomy 3 weeks postoperatively because of a leak in the left lumbar ureter that was attributed to thermal injury from monopolar current during a left paraaortic lymphadenectomy.
Other authors report a lower incidence of laparotomy consequent to laparoscopic lymphadenectomy. None of the 39 patients who underwent laparoscopic lymphadenectomy for cervical cancer in the series by Querleu et al. required laparotomy.51 Pomel et al. reported on 10 cases of pelvic and para-aortic laparoscopic lymphadenectomies performed for early stage carcinoma of the ovary.48
They described one laparotomy at a second surgery, for postoperative hemoperitoneum. Childers et al. have reported a 1.7% (1/60) laparotomy rate for patients undergoing para- aortic lymphadenectomy for cervical, endometrial, and ovarian carcinoma.22 A single patient required laparotomy for vena caval injury that occurred during a right para-aortic lymphadenectomy. She required 4 units of blood, and subsequently developed a deep venous thrombosis following surgery. Table 2 summarizes the incidence of conversion laparotomy in 449 cases from 4 large series to be 9.3%.27-30
ADEQUACY OF NODE RETRIEVAL
Lymphadenectomy is primarily performed to evaluate for micrometastasis in the setting of a malignancy. It is therefore essential that the lymph node dissection achieve adequate node retrieval despite the operative approach, either by laparotomy or by laparoscopy.
The gynecologic oncology group (GOG) protocol 9207 examined laparoscopic para- aortic lymph node sampling and therapeutic pelvic lymphadenectomy in women with stage IA, IB, and IIA cervical cancer. In 69 patients, across 7 institutions, the average lymph node retrieval was up to 70 (mean of 32) for pelvic nodes and up to 37 (mean of 12) for para-aortic nodes. The complication rate was 10% for major vascular injury and 1.4% for ureteral injury. The study thereby concluded that a laparoscopic approach is a feasible alternative for laparoscopic lymphadenectomy.52 More recently, Boggess et al. compared the outcomes in women who underwent staging for endometrial cancer by laparotomy, laparoscopy, or robotic-assisted laparoscopy.51 There were an average of 23.1 and 32.9 nodes retrieved by laparoscopy and robotic techniques, respectively, and 14.9 by laparotomy. In our series of over 100 laparoscopic lymphadenectomies for gynecologic malignancy, we reported that technical feasibility is improved with the use of the Harmonic scalpel.26 Median nodal yield was 22 nodes, with up to 80 lymph nodes retrieved and no conversions to laparotomy necessary. Interestingly, no lymphoceles have occurred, which is potentially due to effective sealing of small lymphatic channels with this instrumentation. In another series at our institution, we compared 50 patients who underwent laparotomy and 47 who underwent laparoscopy for endometrial cancer.53 Nodal yield was greater in the laparoscopy group for pelvic and para-aortic nodes. Mean pelvic nodal yield was 19.3 versus 11.7 nodes, and mean para-aortic nodal yield was 14.2 versus 3.5 nodes. Further, it is our experience that the nodal count is directly related to the surgical goals at the time of the procedure rather than a technical limitation of laparoscopic dissection. Preoperatively, cases are selected for full lymphadenectomy or for a lymph node sampling based on the indication for lymph node retrieval.
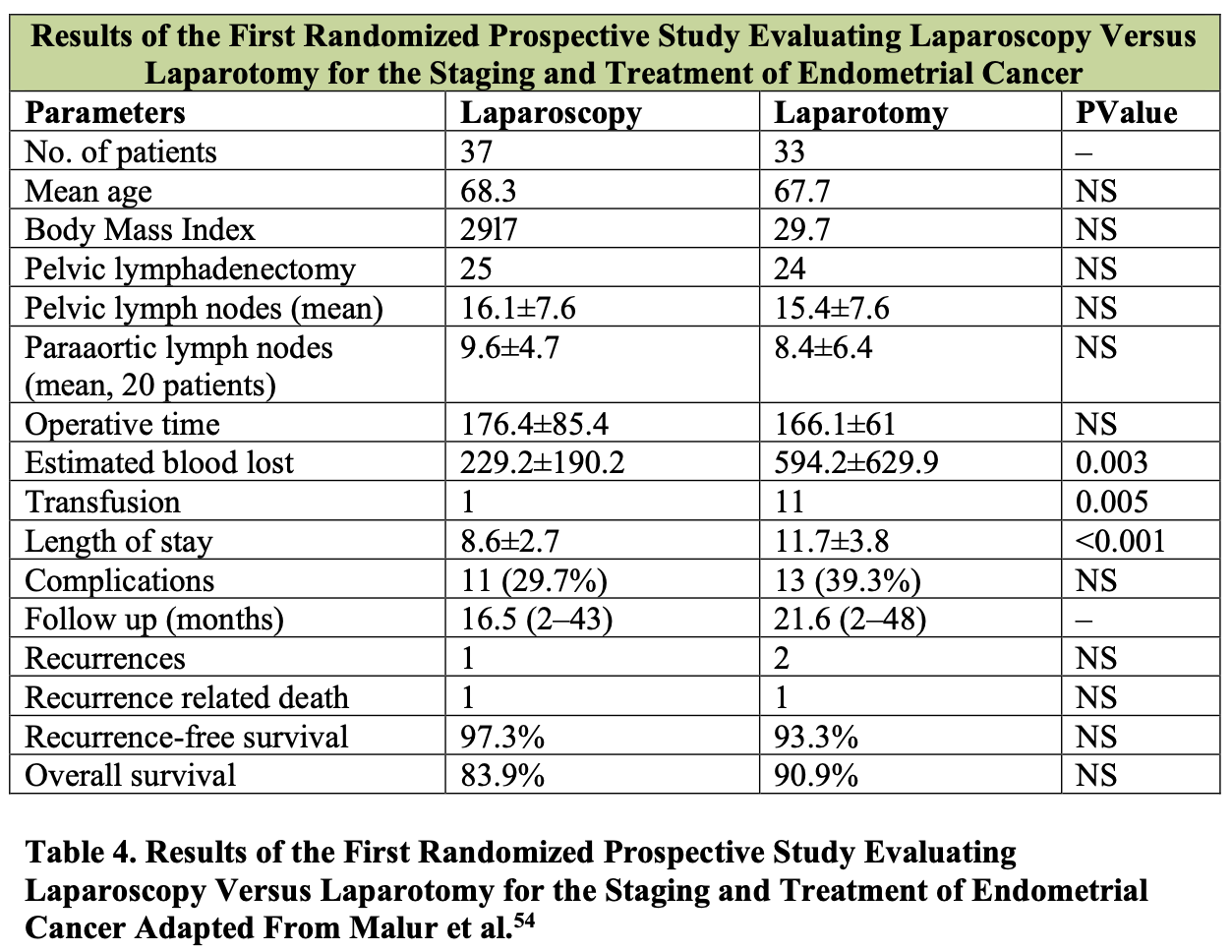
Patient outcomes and survival data support the utility of laparoscopic lymphadenectomy for gynecologic malignancy. Malur et al. have reported the first prospective randomized study comparing a laparoscopic approach to open laparotomy for the staging and treatment of endometrial cancer (Table 4).54 Notably, the operative time and the number of pelvic and para-aortic lymph nodes harvested was independent of the surgical approach; however, the estimated blood loss, transfusion requirements, and length of hospital stay were significantly less for those patients who underwent a laparoscopic procedure. The complication rate, recurrence rate, and overall survival for endometrial cancer were not statistically different between the 2 groups. This study confirms previous reports, including that of Eltabbakh et al., which reported a similar overall 5-year survival in 186 stage I endometrial cancer patients treated either with laparotomy or by laparoscopy.55 The GOG LAP-2 study, the largest randomized trial in endometrial cancer thus far, showed no difference in the rate of intraoperative complications, and fewer moderate to severe postoperative events and shorter hospital stay in the laparoscopy group as compared to the laparotomy group.16There was no difference in the percentage of patients who had pelvic lymphadenectomy (99% and 98%), but 92% of patients in the laparoscopy group and 96% in the laparotomy had both pelvic and paraaortic lymphadenectomy. The median number of pelvic lymph nodes retrieved in the pelvic and paraaortic regions was similar for both groups. Outcomes of laparoscopic lymphadenectomy for other gynecologic malignancies are still maturing; however, the technical feasibility and adequacy of lymph node retrieval documented to date suggest a role for laparoscopic lymphadenectomy in other gynecologic disease sites.
THE LEARNING CURVE
Most surgeons report a decrease in the number and severity of complications and a reduced operative time as experience is gained with the laparoscopic lymphadenectomy procedure.2,24,27,30,51 Eighty-eight percent (14/16) of aborted laparoscopic lymphadenectomies reported by Kavoussi et al. occurred during the initial dissections at each contributing institution.20 Lang et al. reported a significantly higher complication rate for the first 50 laparoscopic lymphadenectomies (14%) as compared to the next 50 such surgeries (4%).35 In fact, 5 of the 9 total complications occurred among the first 20 patients. The adequacy of the procedure also increases with experience. Fowler et al. and Rukstalis et al. reported a clear improvement in the adequacy of the dissection as estimated by the percentage of lymph nodes removed with increasing laparoscopic experience.56,47 Childers et al’s experience with laparoscopically assisted staging for endometrial carcinoma demonstrated a significant decrease in operating time with increasing experience.23 Notably, the major complication rate was unaffected although the rate of conversion to laparotomy decreased significantly. With time, laparoscopic lymphadenectomy becomes a safer and more time efficient procedure.
CONCLUSION
Laparoscopic lymphadenectomy is an evolving technique that plays an increasingly important role in the management of gynecologic malignancies. Pelvic and para-aortic laparoscopic lymphadenectomy appears to be a safe, adequate, and feasible procedure with a low complication rate. The risks include those traditionally attributed to laparoscopy, as well as those inherent to open lymphadenectomy. The use of simple, preventive measures allows the patient to benefit from this technique while diminishing the likelihood of complication.
References
- Dargent D, Salvat J. L’envahissement ganglionnaire pelvien: place de la pelviscopie retroperitoneale. Medsi. Paris, France: McGraw Hill; 1989.
- Nezhat CR, Burrell MO, Nezhat FR, Benigno BB,Welander CE. Laparoscopic radical hysterectomy with paraaortic and pelvic node dissection. Am J Obstet Gynecol. 1992;166(3):864-865.
- Nezhat F, Cohen C. Laparoscopy. In: Smith JR, DelPriore G, Curtin J, Monaghan JM, eds. An Atlas of Gynecologic Oncology. Malden, MA: Blackwell Science, Inc; 2001.
- Melendez T, Childers JM, Nour M, Harrigill K, Surwit EA. Laparoscopic staging of endometrial cancer: the learning experience. JSLS. 1997;1:45-49.
- Vasilev SA, McGonigle KF. Extraperitoneal laparoscopic paraaortic lymph node dissection. Gynecol Oncol. 1996;61(3):315-320.
- Dargent D, Ansquer Y, Mathevet P. Technical development and results of left extraperitoneal laparoscopic paraaortic lymphadenectomy for cervical cancer. Gynecol Oncol. 2000;77(1):87-92.
- Querleu D, Dargent D, Ansquer Y, Leblanc E, Narducci F. Extraperitoneal endosurgical aortic and common iliac dissection in the staging of bulky or advanced cervical carcinomas. Cancer. 2000;88(8):1883-1891.
- LeBlanc E, Caty A, Dargent D, Querleu D, Mazeman E. Extraperitoneal laparoscopic para-aortic lymph node dissection for early stage nonseminomatous germ cell tumors of the testis with introduction of a nerve sparing technique: description and results. J Urol. 2001;165(1):89-92.
- Sanjuan A, Illa M, Torne A, Martinez-Roman S, et al. Extraperitoneal laparoscopic para-aortic lymphadenectomy as a diagnostic procedure for lymph node recurrence of gynaecologic cancers. Acta Obstet Gynecol Scand. 2007;86:491-5.
- Gil-Moreno A, Franco-Camps S, Diaz-Feijoo B, Perez-Benavente A, et al. Usefulness of extraperitoneal laparoscopic paraaortic lymphadenectomy for lymph node recurrence in gynecologic malignancy. Acta Obstet Gynecol Scand. 2008;87:723-30.
- Occelli B, Narducci F, Lanvin D, Querlel D, et al. De novo adhesions with extraperitoneal endosurgical para-aortic lymphadenectomy versus transperitoneal laparoscopic para-aortic lymphadenectomy: a randomized experimental study. Am J Obstet Gynecol. 2000;183:529-33.
- Leblanc E, Narducci F, Frumovitz M, Lesoin A, et al. Therapeutic value of pretherapeutic extraperitoneal laparoscopic staging of locally advanced cervical carcinoma. Gynecol Oncol. 2007;105:304-11.
- Mehra G, Weekes A, Jacobs I, Visvanathan D, et al. Laparoscopic extraperitoneal paraaortic lymphadenectomy: a study of its applications in gynecologic malignancies. Gynecol Oncol. 2004;93:189-93.
- Vergote F, Amant P, Bertelooot P, Van Gramberen M. Laparoscopic lower para- aortic staging lymphadenectomy in stage 1B2, II and III cervical cancer. Int J Gynecol Cancer. 2002;12:22-26.
- Sonoda Y, Leblanc E, Querleu D. Prospective evaluation of surgical staging of advanced cervical cancer via a laparoscopic extraperitoneal approach. Gynecol Oncol. 2003;91:326-31.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009 Nov10;27(32):5331-6.
- Nezhat C, Nezhat F, Nezhat C. Nezhat’s Operative Gynecologic Laparoscopy and Hysteroscopy. 2008, 3rd New York; Camridge University Press.
- Nezhat FR, Datta MS, Liu C, Chuang L, Zakashansky K. Robotic radical hysterectomy versus total laparoscopic radical hysterectomy with pelvic lymphadenectomy for treatment of early cervical cancer. JSLS. 2008;12:227-237.
- Magrina JF, Kho RM, Weaver AL, et al. Robotic radical hysterectomy: comparison with laparoscopy and laparotomy. Gynecol Oncol. 2008;109:86-91.
- Kavoussi LR, Sosa E, Chandhoke P, et al. Complications of laparoscopic pelvic lymph node dissection. J Urol. 1993;149:322- 325.
- Azevedo J, Azevedo O, Miyahira S, Miguel G et al. Injuries caused by Veress needle insertion for creation of pneuomoperitoneum: a systematic literature review. Surg Endosc. 2009;23:1428-32.
- Childers JM, Hatch KD, Surwit EA. Laparoscopic para-aortic lymphadenectomy in gynecologic malignancies. Obstet Gynecol. 1993;82:741-747.
- Childers JM, Lang JF, Surwit EA, et al. Laparoscopic staging of ovarian carcinoma. Gynecol Oncol. 1995;59:25-33.
- Nezhat C, Childers J, Nezhat F, Nezhat CH, Seidman DS. Major retroperitoneal vascular injury during laparoscopic surgery. Hum Reprod. 1997;7(1):56-59.
- Querleu D, Leblanc E, Cartron G, Narducci F. Audit of preoperative and early complications of laparoscopic lymph node dissection in 1000 gynecologic cancer patients. Am J OBstet Gynecol. 2006 Nov;195(5):1287-92.
- Nezhat F, Yadav J, Rahaman J, Gretz H 3rd, Gardner GJ, Cohen CJ. Laparoscopic lymphadenectomy for gynecologic malignancies using ultrasonically activated shears: analysis of first 100 cases. Gynecol Oncol. 2005 Jun;97(3):813-9.
- Possover M, Krause N, Plaul K,Kuhne-Heid R, Schneider A. Laparoscopic para- aortic and pelvic lymphadenectomy: experience with 150 patients and review of the literature. Gynecol Oncol. 1998;71(1):19-28.
- Dottino PR,Tobias DH, Beddoe A, Golden AL, Cohen CJ. Laparoscopic lymphadenectomy for gynecologic malignancies. Gynecol Oncol. 1999;73(3):383-388.
- Renaud MC, Plante M, Roy M. Combined laparoscopic and vaginal radical surgery in cervical cancer. Gynecol Oncol. 2000;79(1):59-63.
- Scribner DR,Walker JL, Johnson GA, McMeekin SD, Gold MA, Mannel RS. Laparoscopic pelvic and paraaortic lymph node dissection: analysis of the first 100 cases. Gynecol Oncol. 2001;82: 498-503.
- Scribner DR,Walker JL, Johnson GA, McMeekin DS, Gold MA, Mannel RS. Laparoscopic pelvic and paraaortic lymph node dissection in the obese. Gynecol Oncol. 2002;84:426-430.
- Nezhat C, Nezhat F, Seidman DS, Nezhat C. Incisional hernias after operative laparoscopy. J Laparoendosc Adv Surg Tech A. 1997 Apr;7(2):111-5.
- Nezhat C, Nezhat F, Ambroze W, Pennington E. Laparoscopic repair of small bowel and colon. Surg Endosc. 1993;7:88-89.
- Burney TL, Campbell EC, Naslund MJ. Complications of staging laparoscopic pelvic lymphadenectomy. Surg Lap Endosc. 1993;3:184-190.
- Lang GS, Ruckle HC, Hadley HR, et al. One hundred consecutive laparoscopic lymph node dissections: comparing complications of the first 50 cases to the second 50 cases. Urology. 1994;44:221-225.
- Kruitwagen RFPM, Swinkels BM, Keyser KGG, et al. Incidence and effect on survival of abdominal wall metastases at trocar puncture sites following laparoscopy or paracentesis in women with ovarian cancer. Gynecol Oncol. 1996;60:233-237.
- Colon Cancer Laparoscopic or Open Resection Study Group, Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Pahlman L, Cuesta MA, Msika S, Morino M, Lacy A, Bonjer HJ. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomized clinical trial. Lancet Oncol. 2009 Jan;10(1):44-52.
- Zivanovic O, Sonoda Y, Diaz JP, Levine DA, Brown CL, Chi DS, Barakat RR, Abu- Rustum NR. The rate of port-site metastases after 2251 laparoscopic procedures in women with underlying malignant disease. Gynecol Oncol. 2008 Dec;111(3):431-7.
- Vergote I, Marquette S, Amant F, Berteloot P, Neven P. Port-site metastases after open laparoscopy: a study in 173 patients with advanced ovarian carcinoma. Int K Gynecol Caner. 2005;15(5):776-779.
- Nagasheth N, Rahaman J, Cohen JC, Gretz H, Nezhat F. The incidence of port-site metastases in gynecologic cancers. JSLS. 2004;8(2):133-9.
- Chu KK, Chang SD, Chen FP, et al. Laparoscopic surgical staging in cervical cancer: preliminary experience among Chinese. Gynecol Oncol. 1997;64:49-53.
- Wang PH,Yuan CC, Chao KC, et al. Squamous cell carcinoma of the cervix after laparoscopic surgery: a case report. J Repro Med. 1997;42:801-804.
- Canis M, Botchorishvilli R, Wattiez A, et al. Cancer and laparoscopy experimental studies: a review. Eur J Obstet Gynecol Reprod Biol. 2000;91:1-9.
- Voltz J, Paolucci V, Schaeff B, et al. Laparoscopic surgery: the effects of insufflation gas on tumour-induced lethality in nude mice. Am J Obstet Gynecol. 1998;178:793-795.
- Canis M, Botchorishvilli R,Wattiez A, et al.Tumor growth and dissemination after laparotomy and CO2 pneumoperitoneum: a rat ovarian cancer model. Obstet Gynecol. 1998;92(1): 104-108.
- Canis M, Mage G, Pouly JL, et al. Laparoscopic diagnosis of adnexal cystic masses: a 12-year experience with long-tern followup. Obstet Gynecol. 1994;83:707-712.
- Rukstalis DB, Gerber GS,Vogelzang NJ, et al. Laparoscopic pelvic lymph node dissection: a review of 103 consecutive cases. J Urol. 1994;151:670-674.
- Pomel C, Provencher D, Dauplat J, et al. Laparoscopic staging of early ovarian cancer. Gynecol Oncol. 1995;58:301-306.
- Spirtos NM, Schaerth JB, Spirtos TW, et al. Laparoscopic bilateral pelvic and para- aortic lymph node sampling: An evolving technique. Am J Obstet Gynecol. 1995;173:105-111.
- Boitke GM, Lurain JR, Burk JJ.A comparison of laparoscopic management of endometrial cancer with traditional laparotomy. Presented at: Annual Meeting of the Society of Gynecologic Oncologists; February 6-9,1994; Orlando, FL.
- Querleu D, Le Blanc E, Castelain B. Laparoscopic pelvic lymphadenectomy in the staging of early carcinoma of the cervix. Am J Obstet Gynecol. 1991;164:579-581.
- Schlaerth JB, Spirtos NM, Carson LF, Boike G, Adamec T, Stonebraker B. Laparoscopic retroperitoneal lymphadenectomy followed by immediate laparotomy in women with cervical cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2002;85:81-88.
- Nezhat F, DeNoble S, Anderson L. Consistent outcomes of laparoscopic intraperitoneal paraaortic lymphadenectomy support incorporation into fellowship training program curricula. Abstract, presented at 17thAnnual Meeting and Endo Expo of the Society of Laparoscopic Surgeons. Chicago. Sept 17-20, 2008.
- Malur S, Possover M, Michels W, Schneider A. Laparoscopic-assisted vaginal versus abdominal surgery in patients with endometrial cancer. A prospective randomized trial. Gynecol Oncol. 2001;80:239-244.
- Eltabbakh GH, Shamonki MI, Moody JM, Garafano LL. Hysterectomy for obese women with endometrial cancer: laparoscopy or laparotomy? Gynecol Oncol. 2000;78(3):329-335.
- Fowler JM, Carter J, Carlson J, et al. Lymph-node yield from laparoscopic lymphadenectomy in cervical cancer: a comparative study. Gynecol Oncol. 1993;51:187- 192.
