Preventing and Managing Complications in Infertility Surgery
Mettler, L., Alkatout, I. and Al-Dulaimi, Z.S.
History
At present laparoscopy is the most frequently performed gynaecologic procedure in the world. The development of laparoscopy has decreased the number of laparotomies constantly and has also reduced the costs. Postoperative morbidity has been reduced and patient recovery is faster due to laparoscopy. The worldwide need for permanent sterilization methods has been one of the stimulators of the development of endoscopic surgery.
Most important, therefore, has been progress, in particular the ability to perform haemostasis through the use of electrocoagulation and ultrasound. These were the most important achievements for the performance of surgical procedures through the laparoscope, besides the development of the rod lens system, CO2 insufflation, light sources and HDV (high definition television cameras).
Attempts at the visualization of the uterine cavity preceded the development of peritoneoscopy.
Development of Hysteroscopy
As early as 1000 A.D. Abulkasim used a mirror to reflect light into the vaginal vault. In 1853, Desormeaux inspected the uterine cavity with an early endoscope and reported the first hysteroscopy.1 He also identified polyps in the uterus as the cause of postmenopausal bleedings. He described several models of hysteroscopic instrumentation and published material on the technique of hysteroscopy. In 1869, Pantaleoni described hysteroscopy in an English journal.2 In 1807, Bozzini constructed the light guide for the uterine cavity.3
The development of effective distending media and clear visualization of the uterine cavity were the next steps forward.
In 1901, Kelling performed the first endoscopic procedure in the stomach of a dog.4 In 1925, Rubin combined the cystoscope with CO2 insufflation of the uterine cavity. In 1928, Gauss used water to distend the uterine cavity and to flush the blood.
Since the lens systems were in the beginning rather inferior, inadequate light and image transmission occurred frequently. Vulmiere in 1952 presented a rigid one piece mineral glass guide which could illuminate the cavity and magnify the image when in direct contact to the object.5
The clinical utility of hysteroscopy was increased by the development of high molecular weight dextran as a distension medium. In 1971 Lindemann used CO2 for uterine distension.6 In 1963, an optical trocar was used and was perfected in France in 1973 for clinical use. Contact hysteroscopy optics were combined with modern panoramic hysteroscopy into a single instrument and introduced by Hamou in 1981.7 Baggish introduced a focusing panoramic hysteroscope with a four-channel operating sheath particularly useful for laser in 1983.8 In 2007, Bettochi presented a one-piece diagnostic hysteroscope.9
Development of Laparoscopy
In 1807, Bozzini visualized the urethral orifice with candlelight and a simple tube.3 This led to the development of the first urethroscope and cystoscope in 1843 by Desormeaux who used mirrors to reflect the light from a kerosene lamp. Nitze added a lens system to the endoscopic tube to magnify the viewed area. After the invention of the light bulb, Newman developed a cystoscope using a small bulb at the distal end. Kelling reported the first peritoneoscopy on dogs. He used a needle and a cystoscope designed by Nitze.
The first reported observation of the human peritoneal cavity with an optical instrument was by Jacobaeus in 1910 using a trocar and cannula to induce a pneumoperitoneum in women.10 He introduced a Nitze cysoscope through the same cannula to achieve a pelviscopy, laparoscopy or peritoneoscopy. The first pneumoperitoneum was created by using air, only later was the use of CO2 introduced. The main reason was the possibility of gas embolism. It has been demonstrated that the injection of 200cc CO2 per minute into the veins is not lethal, while injection of the same amount of air causes immediate death. This is explained by the great solubility of CO2 in the plasma. Another reason is the rapid resorption by the tissue.
In 1929, a 45° lens system and the use of a second puncture were introduced by Kalk.11 Later, biopsy instrumentation and cauterization of intraabdominal adhesions as well as a single puncture operating laparoscope were introduced.
In 1937, Hope used Ruddock’s laparoscope for the diagnosis of ectopic pregnancies.12
The first gynaecologist to use laparoscopy clinically on a wide basis was Palmer in 1946.13 He was responsible for the development of chromopertubation.
The introduction of fiber optics in 1953 by Hopkins made another huge step forward in the performance of surgical procedures with laparoscopy.14 In the early 1960’s, Hopkins also started to design the rod lens system that is used in most endoscopes today.
Frangenheim and Palmer continued to develop electrocoagulation for sterilization.15 Hemostasis via electrocoagulation was a major achievement in laparoscopy since it is impossible to perform this surgery without that achievement.
In 1964, Semm introduced several advancements in pelviscopic techniques and instrumentation.16 He introduced the use of an automatic insufflator to maintain pneumoperitoneum. He was the first who presented a complete pelviscopy instrumentation, which has since been modified.
In the 1970’s, laparoscopy was used to recover the first oocyte for IVF. This method was used until the introduction of the transvaginal retrieval of the oocyte in 1980’s. Until that time, it was an integral part of IVF.
In 1973, Hulka introduced clips for tubal ligation and Rioux developed bipolar coagulation for tubal sterilization.17,18
The first textbook and atlas of laparoscopy was published by Korbsch in 1927 and later in 1976 by Semm and others.19-25
Laparoscopic Anatomy
The knowledge of abdominal and pelvic anatomy is the most important factor to avoid and handle complications during laparoscopy. Understanding the abdominal wall and the location of the retroperitoneal vessels is extremely important for the blind placement of the primary umbilical trocar. The knowledge of the abdominal wall vasculature is important for the placement of secondary trocars. Even the simplest cases of endometriosis and adhesiolysis require an understanding of the retroperitoneal structures.
The base of the umbilicus is the thinnest part of the anterior abdominal wall in all patients. The placement of the Veress needle and the trocar should be at a 45° angle in thin patients. In overweight patients, the angle of insertion should be increased to 60° to decrease the preperitoneal placement. In obese patients, the placement angle should be 75-80° to avoid preperitoneal placement. With obese patients, the distance is shorter in the base of the umbilicus. For the placement of secondary trocars, it is advisable to use the smallest trocars available to avoid vessel injuries. The wide anatomic variation of the vessels is one of the risks that cannot be predicted. When larger ports are required, the dilatation of a 5 mm incision is possible. Secondary ports must be placed under vision.
Open laparoscopy, as propagated by Hasson, is also an interesting approach and has an identical complication rate as laparoscopy.26
Complications at Laparoscopy
Complications occur during surgical procedures and should be recognized and dealt with immediately.
Patient’s Position
Prolonged procedures under general anaesthesia can lead to nerve injuries especially to the brachial plexus, ulnar, femoral and common peroneal nerves.
If the shoulder rest is improperly placed, it causes the compression of the brachial plexus. Therefore, it should be positioned laterally on the acromioclavicular joint. The prevention of ulnar and brachial plexus injuries is achieved by placing the right arm extended and abducted at an angle less than 90°. The elbow joint is pronated and tucked loosely. The left arm should be in a sling close to the body; it should also be extended and pronated. If the surgery does not require a steep Trendelenburg position, it is better to avoid shoulder rests.
The legs should be placed in stirrups with attention to the common peroneal and femoral nerve. The common peroneal nerve passes very close to the head of the fibula and should not be in contact with the stirrups.
The femoral nerve can be stretched when the legs are placed at an extreme external rotation and abduction. Postoperative numbness usually resolves within the first 3-5 postoperative days. Any symptoms that persist beyond five days require a neurological evaluation. A patient with joint problems is better placed on the OR-table when conscious.
Trocar Related Injuries
Trocar related injuries are the most common and one of the most serious injuries. They are potentially preventable.
The bladder should be emptied before starting the settlement as the dome of the bladder lies a few centimeters below the symphysis. In patients with a previous laparotomy, the dome of the bladder might be extended cephalad even when the bladder is catherized. It is therefore recommended to place the suprapubic trocar above the scar.
The three major areas of intraoperative complications are: bowel, vascular and urological injuries. They all can be life-threatening if they are left unrecognized.
In an average patient, the distance between anterior abdominal wall and retroperitoneal vessels is normally 3-4 cm. It can be increased to 8-14 cm by creating a pneumoperitoneum.
Usually, the pneumoperitoneum is created by using a Veress needle through a subumbilical incision. The injection of a few cc of water to distant the bowel and afterwards aspiration with a syringe before connecting to the CO2 source is useful to avoid gas embolism.23,24 The intraabdominal pressure should be at 12 mmHg. For primary entry, the trocar screwing motion gives better control than direct thrusting. If severe adhesions are expected, it is recommended to insert the trocar after opening the abdominal layers of the incision. The secondary trocars are placed under vision lateral to the inferior epigastric and suprapubic vessels.
Avoid the Trendelenburg position during primary trocar insertion as it can make the angle of insertion more perpendicular. Preset the intraabdominal pressure to 20 mmHg for the primary entry; then continue with an intraabdominal pressure of 15 – 16 mmHg.
Gastrointestinal Injuries
The occurrence of injuries to the bowel can lead to significant consequences. Delayed diagnosis of bowel injuries leads to sepsis and mortality. The combined Dutch and ISGE survey reported 14 cases of delayed diagnosis with a mortality of 21%.
Mechanical injury is caused by trocar insertion or as a result of operative trauma. If adhesions are expected, an enema and decompression of the stomach can help to recognise the injuries more easily. Injuries of the small bowel may occur during dissection of adhesions.
Thermal injuries may be difficult to recognize at the time they occur.
Morcellator-related injuries occur. The rotating blade should be visible throughout the procedure.
Bladder and Ureteric Injuries
Urological complications can be due to thermal or mechanical injuries.
Patients with a previous caesarean section and those with endometriosis have an increased likelihood of these complications. The presence of gas and/or blood in the urinary drainage bag allows early recognition.
Endometriosis is a major factor for increased serious complications. Most occur in the distal portion of the ureter.
Fertiloscopy = Transvaginal Hydro Laparoscopy digital
A pelvic examination is obligatory prior to the procedure. Failure or complications can be avoided if the examination is performed. If adhesions of the Douglas pouch are expected, fertiloscopy should not be performed.
Fertiloscopy is a diagnostic procedure and can be done under local anaesthesia. It is a procedure using the assessment of culdoscopy and hysteroscopy. The first step is hydropelviscopy with the dye test and the second step hysteroscopy with the endometrial biopsy. Within the procedure, an assessment is made of the ovaries, endometriotic disease of the ovaries and the pelvis, tubal infertility and uterine fibroids. With salpingoscopy the state of health of the fimbriae is assessed. The state of tubal mucosa is determined. With microsalpingoscopy the staining of the nuclei of the tubal cells by methylene blue dye determines the functional capacity of the tube: the greater the degree of staining the lesser the functional state of the mucosa.
If laparoscopy has to be performed, the patient will have to undergo a further operative procedure in general anaesthesia.
Myomectomy
Uterine fibroids are benign muscle tumors that are found in at least 20% of women over 30 years of age. Most women are asymptomatic. Submucous myomas or large intramural myomas may be associated with recurrent pregnancy loss and infertility.
In 1976, Semm and Mettler et al. reported the first utilization of endocoagulation, ultrasonic energy for laparoscopic myomectomy.27,28 One of the advantages is that there is no electrical energy used at the point of delivery. Therefore, there is no risk of electrical injuries to adjacent structures. The superficial depth of penetration results in fewer complications. Myomectomy via this technique is safe in patients interested in achieving pregnancy. Blood loss is the most disturbing and limiting factor of the procedure. It can be reduced by infiltration of a suprarenine solution (1:100 diluted vasopressin or octapressin, a derivative of vasopressin) into the myometrium surrounding the myoma. The incision should be vertical or horizontal. The closure of the myoma bed should be done in two or three layers. This reduces the bleeding into the myometrium. It is very important for infertility patients. Suturing in two layers gives more anatomical closure and added strength to the myoma bed which can endure the stress of pregnancy. However, the less number of knots the fewer the adhesions.
If the uterine cavity was opened during the procedure, the cavity has to be sutured. A normal delivery should be carefully discussed with the obstetrician who may opt for a caesarean section.
Adhesiolysis
For the fertility procedure, laparoscopic adhesiolysis includes salpingo-ovariolysis, often necessary prior to fimbrioplasty or salpingostomy. One of the advantages of laparoscopic adhesiolysis is the closed internal environment which avoids the drying of the peritoneum and therefore the recurrence of adhesions. Pelvic adhesions under the ovaries can easily be detached. The usage of ultrasonic devices produces less carbonisation and bleeding which leads to less macrophage activation and adhesion formation. The results of the pure adhesiolysis depend on the adhesion stage. In cases of slight to moderate adhesions, pregnancy rates are around 60%, in severe cases 20%.
Management of ectopic pregnancies
The incidence of ectopic pregnancies has increased since the 1970’s. Early diagnosis and therapy have reduced the morbidity and mortality. Transvaginal ultrasound and the measurement of ß-hCG values give us a high diagnostic capability. If an ectopic pregnancy is diagnosed early, it can be treated before tubal destruction or haemorrhage occurs. Patients of childbearing age who present with pelvic pain and abnormal vaginal bleeding should be screened with a urine ß-hCG test. The treatment is either salpingotomy or salpingectomy depending on the individual case. If the test is positive and the uterine cavity has no fetus, proceed rapidly to laparoscopic salpingotomy or salpingectomy, depending on the situation of the individual patient.
In a hemodynamically unstable and symptomatic patient with a positive urine pregnancy test, a free uterine cavity and intraabdominal fluid the laparoscopy should be immediately performed.
Ectopic pregnancy
A pregnancy implanted other than in the uterine cavity is an ectopic pregnancy. The most common location is the fallopian tube with 95%. Other locations can be the cervix, ovary, bowel or other structures.
Frequencies of implantation sites are: 78% ampulla, 12% isthmus, 5% fimbriae, 2% corneal, interstitial or intramural, 3% abdominal, cervical or ovarian.
The classic triad of abdominal pain, vaginal bleeding and adnexal mass is present in less than half of these patients and represents today mostly the advanced cases. Early diagnosis is important to lessen serious morbidity.
The marker ß-hCG is very sensitive and can detect the presence of pregnancy as early as a week after implantation. A serial measurement over a 48-hour interval with a rise of less than 66% is highly suggestive of either an abnormal intrauterine pregnancy or ectopic pregnancy.
Even in laparoscopy the presence of ectopic pregnancies could be undetected in 3-4 %. This is mostly in cases at an early stage of pregnancy.
Treatment:
In the past, salpingectomy was the standard management. Today, because of early diagnosis, technical advances have opened new pathways. It is often possible to detect the pregnancies unruptured and thereby mostly preserve the tube and increase the chance of subsequent intrauterine pregnancy. The therapy selected depends on the patient’s desire for future pregnancies and her gynecological and surgical history.
Options:
1. Chemotherapy with methotrexate
- When the woman desires a pregnancy in the future
- Sac size is ≤ 3 cm
- Peak hCG ≤ 15,000 mUI/ml
- No active bleeding
- No cardiac activity in TVS
Contraindications:
- Poor patient compliance
- Active hepatic or renal disease
- Active peptic ulcer disease
- Leucocyte ≤ 3000
- Thrombocytes ≤ 100000
Success rate of single dose regime 85%.
Success rate of variable dose regime 93%.
2. Laparoscopy is the gold standard in the surgical management
- Advantages over laparotomy:
- Decreased adhesion formations and improved fertility
- Shorter recovery period
- Less postoperative pain.
- Laparoscopic Approach
The size and location of the pregnancy can be evaluated. Ruptured tubal pregnancies can be treated if the bleeding has stopped or can be arrested adequately. Once the bleeding has been controlled, blood clots and the products of conception can be removed.
Unruptured tubal pregnancies should be treated by salpingotomy. The identification and mobilization of the tube should be followed by making an anitmesenteric linear incision on the surface just above the pregnancy. The pregnancy usually protrudes through the incision. It might be mobilized gently using aqua dissection. Some of the products of conception can adhere to the implantation site. The tissue should be coagulated before removal.
The incision should better be left unsutured to heal by secondary intention. The risk of subsequent ectopic pregnancy is slightly higher than in the methotrexate group. The chance of subsequent intrauterine pregnancy is 60 %, 16 % are repeated ectopics.
Segmental resection is performed for ruptured tubes or isthmic pregnancy.
Salpingectomy can be performed if the family planning has been completed, in cases of a ruptured ectopic pregnancy and tube, in badly damaged tubes and when the contra lateral tube is healthy.
Tubal Recanalization
The first microsurgical reversals of sterilization were done by Gomel and Winston, 1975 – 1977.29,30
25-30% of sub fertile women have tubal damage. There are also women who seek the reversal of sterilization. This can be achieved by laparoscopic microsurgery.
Advantages of laparoscopic tubal anastomosis:
- Magnification, tissue handling, hemostasis, lavage
- Avoids laparotomy and tissue trauma associated with packing and retractors
- Minimal tissue handling and trauma
- Minimal adhesions
- Cosmetically better
- Faster recovery
Preoperative work up
- Rule out male factor with semen analysis
- Day 3 FSH level to know the ovarian reserve
- Hysterosalpingography to know the length and condition of the proximal tube
Indications
- Reversal of tubal sterilization
- Mid-tubal block secondary to various pathologies
- Tubal occlusion
- Salpingitis isthmica nodosa
Absolute Contraindications
- Decreased ovarian reserve or ovarian failure
- Extensive tubal damage
- Hydrosalpinx with a diameter ≥ 3 cm
- Inadequate proximal or distal tubal segment for reanastomosis
- Projected tubal length less than 3 cm after reconstruction
- Extensive adhesions
- Abnormal uterine cavity
- Any contraindications for pregnancy or surgery
- Severe male factor
Before proceeding to laparoscopic microsurgery the inside of the tube must be evaluated to confirm that the anastomosis is going to be fruitful.
Salpingoscopy allows direct inspection of tubal mucosa in the ampullary part.
The major factor is the degree of the damage of tubal mucosa.
The Brosens classification is useful for evaluation.31
Surgical steps
- Transcervical chromo pertubation to know the exact site of the block.
- Excision of the pathological segment, the incision should not extend beyond the mesosalpinx.
- Mesosalpinx should be sutured first.
- End-to end anastomosis in two layers
- First the mucosal-muscularis layer
Keep the knot outside of the lumen. The stitches must be taken from the outer to the inner side on the proximal end and vice versa on the distal end.
- Second layer the sero-muscularis layer
Types of anastomosis:
- Isthmo-isthmic anastomosis
- Isthmo-ampullary anastomosis
- Ampullo-ampullary anastomosis
- Tubo-cornual anastomosis
Laparoscopic Fimbrioplasty and salpingostomy are usually associated with adhesiolysis.
Steps for adhesiolysis
- Adhesions should be cut under tension
- Beginning in midline towards adnexa
- Lysis should be done layer wise
- Simple adhesions first, without previous coagulation, just cutting in the right layer
- Important anatomical landmark in difficult adhesiolysis is the round ligament insertion
- Injuries of the peritoneum should be avoided
- Aim is to free fallopian tubes and ovaries thereby maintaining their relationship and restoring pelvic anatomy
Laparoscopic Fimbrioplasty
Treating phimosis and agglutination is the principle to restore the original anatomy of the tubal opening.
- Incision at the antimesenteric border to remove fibrotic bands and expose the fimbria when it is encapsulated
- Putting a small incision at the center of agglutination when folds are stuck. After that, dilatation of the opening by closed atraumatic forceps and opening it in different positions
- In tubal phimosis, atraumatic forceps should be introduced inside the infundibulum. The adhesions can be seen by opening the jaws. Incision of these is now possible.
Hemostasis is to be established at the end of the operation.
Small oozers are controlled by submersing the infundibular portion of the tube in warm (37°C) normal Ringer’s lactate for a few minutes. Any residual oozers should be treated by fine coagulation. Damage of the mucosa should be avoided. Severe phimosis is to be treated by salpingostomy.
Laparoscopic Salpingostomy
- Cuff salpingostomy for thin wall hydrodalpinx
- Flap salpingostomy for thick wall hydrosalpinx
1. Cuff Salpingostomy
In three parts
- Opening the tube by incision after injection of methylene blue via cervix and uterus to see the end of the tube. The central whitish fibrotic area is now the exposed end to be incised.
- Everting the mucosa by gradually exerting pressure in opposing direction
- Stabilizing the eversion by coagulation, vaporisation, cutting and suturing
2. Flapsalpingostomy-cutting
Benign Ovarian Neoplasmas = ovarian cystectomies
Benign ovarian masses occur in all stages of a woman’s life, from childhood to menopause. Germ cell tumors are most common in childhood. During the reproductive age functional cysts are predominant. Thirty percent of the ovarian masses in menopause and beyond are borderline or malignant.
The majority of ovarian tumors are benign and most of them occur in the reproductive age group.
Preoperative screening
- Clinical history
- Bimanual pelvic examination
- Vaginal ultrasound and color Doppler of ovarian mass-if suspicious
- Tumor markers (CA 125, CEA)
Maggino et al. proposed the following criteria for classification of ovarian masses:32
Class I: Probably benign
- Dimension < 5 cm
- Clear thin wall
- Non echogenous content
- No septa
- No free fluid in pouch of Douglas
Class II: Borderline malignant mass
- Dimension from 5-10cm
- Clear, smooth, thick wall and/or hyperechogenic liquid content or solid homogenous content and/or
- More than three thin septa and/or thick but regular septa and/ or
- Absence of endocystic projections and/or
- Absence of free peritoneal fluid
Class III: Malignant mass
- None of the above features are observed.
- The diagnosis of malignant tumors by laparoscopy can easily follow Maggino’s criteria.
Surgical Procedures
Cystectomy is the choice of surgery for women of reproductive age with the purpose of preserving fertility.
Three methods are available:
- Intact cyst removal
- Aspiration and excision of the cyst wall
- Inspiration and ablation of the cyst wall
Complete removal with minimal trauma to the residual ovarian tissue provides a specimen for histology and minimizes the chances of recurrence.
Aspiration and ablation do not destroy the entire cyst wall but increase the chance of recurrence and damage the underlying cortex by heat. Excision is therefore preferred. The excision of cysts larger than 10 cm in diameter may sometimes be difficult because the cyst wall is thinned out.
If a tumor other than an endometrioma is suspected, peritoneal washings should always be done before cystectomy.
For ovarian tumors that lie within the body of the ovary, an incision should be made directly over the cyst. It should be made parallel to the long axis of the ovary and as far as possible posterior taking care not to incise the cyst wall but only the cortex. The posterior placement minimizes the possibility of adhesions to the bowel, uterus or tube. Spreading the tips of the scissors will create a plane while the edge of the cortex can be gasped using a biopsy forceps. The cyst can be aspirated and opened or enucleated once an adequate dissection plane is created circumferentially. Injection of normal saline between the cortex and cyst capsule creates a tissue plane and reduces bleeding. The cyst is opened for inspection once it is drained. If solid areas are found at laparoscopy, removal for frozen section is carried out. After excision the base of the cyst should be coagulated. Generally, the bleeding is self-limiting and the ovary heals without the need for suturing.
In cases of endometrioma, an adhesiolysis is usually performed prior to the cystectomy because endometriomas are generally involved in dense adhesions to the pelvic sidewall or sigmoid. Endometriomas will drain during dissection of the ovary from surrounding structures. The side where the cyst is attached to the pelvic side is usually thinned out in comparison to the other ovarian cortex covering the endometrioma. To enucleate the cyst, the border between the ovarian cortex and cyst wall has to be visualized. They are then gently teased apart using two biopsy forceps. Dissection has to be performed circumferentially. Twisting the cyst wall over a biopsy forceps can be helpful. The hair curling movement is advised. Another treatment proposed by the Belgian School is to open the cyst, coagulate the borders and let the endometrioma dry out. This practice is based on the theory of how an endometrioma is formed; however, enucleation is the preferred method. In cases of dermoid cysts, a complete enucleation without spillage is necessary. Oophorectomy or salpingo-oophorectomy is only performed in postmenopausal women.
Hysteroscopy
Hysteroscopy is considered the gold standard for the evaluation of endometrial cavity in infertile patients. It is the most reliable method for determining the nature of intracavitary abnormalities and is also a very effective treatment modality.
Hysteroscopy gives an assessment of size, shape and regularity of the endometrial cavity. It allows direct visualization of proximal tubal ostia. A diagnostic hysteroscopy determines the status of the endometrium in terms of presence or absence of intrauterine pathology, endometrial hyperplasia, vascularity and endometritis. Diagnostic accuracy compared with histological diagnosis showed a sensitivity of 98%, specificity of 95%, positive predictive value of 96% and negative predictive value of 98%.
Hysteroscopy allows the evaluation of the cervical mucosal lining and also the channel for embryo transfer. It is highly sensitive for diagnosis of submucous fibroids or endometrial polyps.
Fibroids of the uterus are the most common solid pelvic tumours in females and occur among 20-40% of women in the reproductive age group. They can grow in various locations in the uterus.
Depending upon hysteroscopically observed findings submucous myomas are classified into the following types:
ESGE Classification of submucous myoma
- Type 0 No intramural extension
- Type 1 < 50% intramural extension
- Type 2 > 50% intramural extension
This classification is part of the preoperative assessment and plays an important role in determining the operative possibility by hysteroscopic myomectomy.
Congenital anomalies of the female reproductive tract are detected in 1-2 per 1000 women. In cases of infertility the incidence is 5-10%. Uterine anomalies are also associated with recurrent pregnancy losses and failure of IVF treatment.
Uterine anomalies listed by the AFS (American Fertility Society) are: aplasia, unicornuate uterus, bicornuate uterus, septate uterus, arcuate uterus and DES related anomalies. Hysteroscopy alone can diagnose only septate uterus. For diagnosis of the remaining anomalies a combined procedure of hysteroscopy and laparoscopy is used.
Hysteroscopic Myomectomy:
Before surgery the location and the size are to be determined.
Leiomyoma of the uterus are the most common solid pelvic tumors found in women and are estimated to occur in 20-40% of women with increased frequency during the late reproductive years. The incidence of myomas in infertile women without any obvious cause of infertility is estimated to be between 1-2.4%. Myomas may cause dysfunctional uterine contraction that may interfere with sperm migration, ovum transport and nidation. Myomas may also be associated with implantation failure or gestation discontinuation due to focal endometrial vascular disturbance, endometrial inflammation, secretion of vasoactive substances or an enhanced endometrial androgen environment. Myomas are known to be associated with infertility and the causal relationship in this regard appears to be more evident for submucous myomas. Operative hysteroscopy is the gold standard for treatment of submucous myoma. Neuwirth and Amin were the first to report the use of a resectoscope for removal of submucous fibroids.33
Preoperative reduction by treatment with GnRH Agonists helps by:
- Reducing the vascularity of the fibroid
- Reducing size of the fibroid
- Reducing operative time
- Reduction of symptoms and anemia
Surgical Technique
Intracavitory fibroids: Removal by using a resectoscope or bipolar electrodes.
Fibroids with < 50% intramural extension: GnRHa for 6-8 weeks is helpful. This allows vascular transformation and consequent volume reduction. In some centres there is no pretreatment with GnRHa because cervical dilatation is more difficult.
Fibroids with > 50% intramural extension: a two-step operative hysteroscopy after pretreatment with GnRHa is proposed:
First step: Resection of the salient portion of myoma protruding in the cavity.
The intramural portion is devascularized by laser fiber into the myoma to a depth of 5-10 mm depending on the depth of remaining intramural portion. The distance between the deepest portion of myoma and the uterine serosa is evaluated by sonography. The end point of laser coagulation is identifiable by the observation of distinct craters with brown borders on all fibroid areas. This technique is called transhysteroscopic myolysis. After the first operative procedure the patient is given another 8 weeks of GnRHa therapy. At second look hysteroscopy the hysteroscopic view shows a white avascular fibroid remnant protruding in the lumen, as if the shrinkage of uterus induced by GnRHa therapy had virtually expelled the residual necrotic fibroid. This is to be resected and the second step is completed.
Multiple submucous myomas: Each myoma is either separated from the surrounding myometrium or totally coagulated. Postoperative estrogen therapy is indicated after resection of multiple myomas, especially if the myomas are on the opposing wall as there is a risk of formation of adhesion between the two raw surfaces.
Uterine septum resection
Uterine malformations can be present in patients with normal fertility, with infertility, or with recurrent pregnancy loss. The estimated incidence in recurrent pregnancy loss is about 2% in the general population.
The uterine septum is due to a lack of reabsorption of an original septum that results from fusion of the two Müllerian ducts in the mid portion to form the uterus. The remaining septum is usually avascular and composed of fibrotic tissue. If implantation occurs on this site, the blastocyst does not have sufficient nutrition and is eventually aborted.
Furthermore, the compromised distending ability of a hemiuterus can cause irritability and premature labour.
The uterine septum may be of different widths and lengths involving only the corporal portion or extending also into the cervix. Occasionally it results in septation of the vagina.
Three methods can be used to divide the uterine septum: resectoscope, scissors or laser.
To avoid complications, it is necessary to have knowledge of the anatomical uterine landmarks. Systematic, delicate and shallow cuts should be performed in order to observe at all times the symmetry of the uterine cavity. The use of a resectoscope is useful in cases with broad septa where the scissors may be more difficult to use. The use of a resectoscope provides continuous washing of the uterine cavity and keeps bleeding to a minimum.
If fiberoptic lasers are used, the same precautions should be taken as with electrosurgery to avoid invading the fundal myometrial wall.
Adhesiolysis
We performed a selective pubmed/medline search using “adhesions”, “laparoscopy” and “prevention of adhesions” as key words. Of all known methods barriers appeared to be the most effective technology. In the forefront, however, stand the meticulous surgical technique and the aim to traumatize as little as necessary. Any peritoneal damage leadsto an acute inflammatory response and to fibrous adhesions which may provoke bowel obstruction, chronic pelvic pain, dyspareunia, infertility and a higher complication rate for subsequent surgeries. In laparoscopy and hysteroscopy the use of heated and moist gas definitely causes fewer adhesions. A continuous suction and irrigation at endoscopic procedures is also advisable.
The first generation of barriers consisted of meshes, such as “Interceed”. Later viscous solutions, such as “Intergel” and “tissue col” were propagated. In recent years sprayable liquids, such as polyethylene glycols = PEGs (SprayShield and Coseal) which polymerize to hydrogels with addition of colorants and without colour, revealed 65–70% reduced adhesion formation compared to the use of saline solution and Ringer’s lactate. Evaluated were the extent of adhesions, the severity and the tenacity.
Hydroflotation with liters of icodextrin solution (4%) for rinsing and instillation at the end of surgery resulted in a significant adhesion reduction on the surgical site. “Hya corp endogel“ is a product on hyaluronate basis that has gained recent attraction.
A combination of a site-specific spray or gel together with hydroflotation, possibly assisted by an anti-inflammatory medication, seems promising.
Prevention of Complications
As in any kind of surgery, robotic, laparoscopic or conventional, the best prevention consists of a careful analysis of the medical history of the patient, the planning of the surgery, the evaluation of available imaging reports and careful, gentle and precise surgery. Infertility surgery deals with the male and female reproductive tract, testicles, ovaries, tubes and uterus, the organs and pathways of gametes. The precursor destruction of gametes is irreversible.
In laparoscopic and hysteroscopic surgery of the female, access complications as well as intra- and postsurgical complications are well differentiated and have been discussed in this chapter and by other authors.34-36 Any complication arising during the surgery has to be treated immediately. Postoperative complications, such as unrecognized bleeding, infections, and dehiscence of wound round up the picture and also have to be considered. We all know that complications should not arise and feel very unhappy if they do; however, early recognition is still the best key for a good outcome.
Summary
Infertility surgery performed by laparoscopy and hysteroscopy is subject to surgical risks, such as access lacerations, intraoperative traumas and infections, but it is usually performed without complications.
In this chapter we deal with the development of laparoscopic and hysteroscopic complications with the following subtitles: patient’s position, trocar related injuries, surgical lacerations as bowel and vascular lesions as well as gastrointestinal and bladder- ureter injuries. We look into laparoscopic procedures, such as fertiloscopy, myomectomy, adhesiolysis, management of ectopic pregnancies, tubal recanalization, fimbrioplasty, salpingostomy and ovarian cystectomies as well as hysteroscopic procedures, such as myomectomy, septum resection and adhesiolysis.
Figure Legend
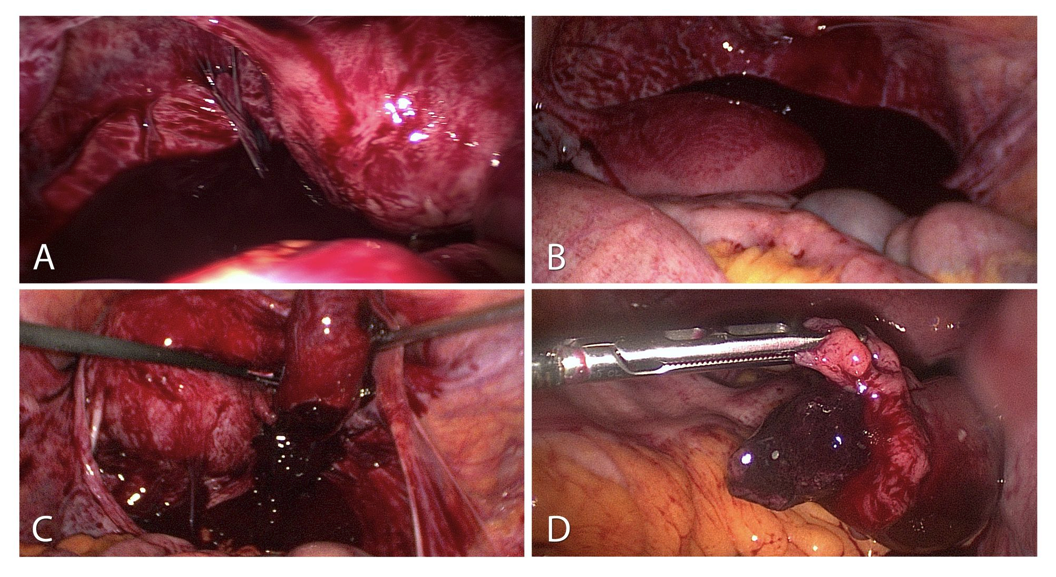 Figure 1. Ectopic pregnancy:
Figure 1. Ectopic pregnancy:
A-Haemoperitoneum of a ruptured tubal pregnancy. Sight after inserting the optic trocar.
B-Severe haemoperitoneum with 1200 ml blood in the pouch of Douglas.
C-Protuding ectopic pregnancy of the fimbrial end with the option of extirpation without incising the tube.
D-Tubal pregnancy already protruding from the fimbrial end into the abdominal cavity.
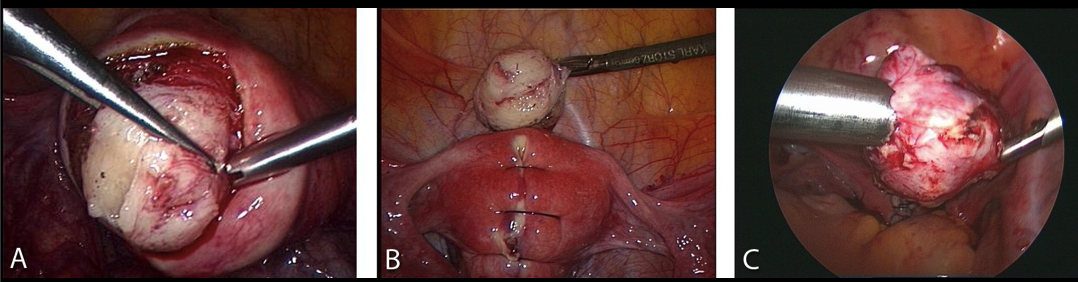 Figure 2. Myomectomy:
Figure 2. Myomectomy:
A-Intraoperative sight of the myoma and its surrounding vascularized capsula.
B-Reconstruction of the uterine wall after excision of the tumor.
C-Removing the myoma by morcellation.
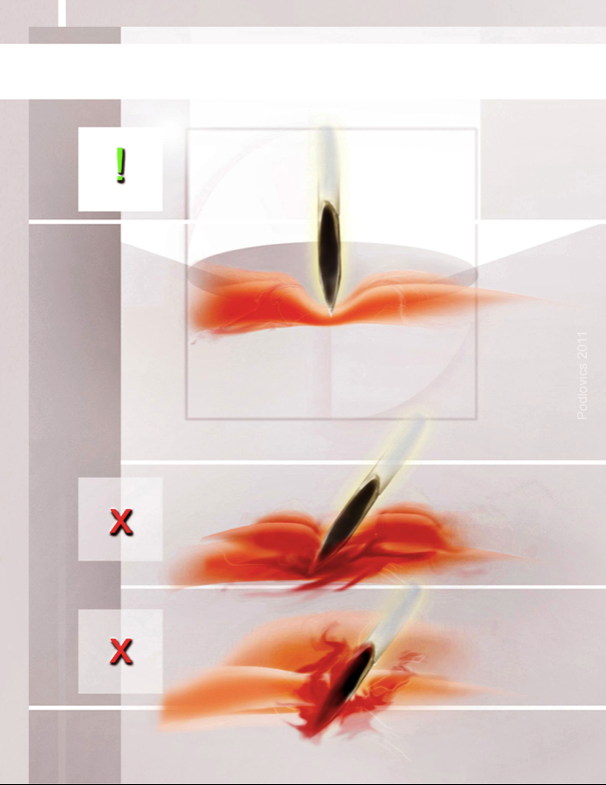 Figure 3. Access complications:
Figure 3. Access complications:
Trocar insertion technique
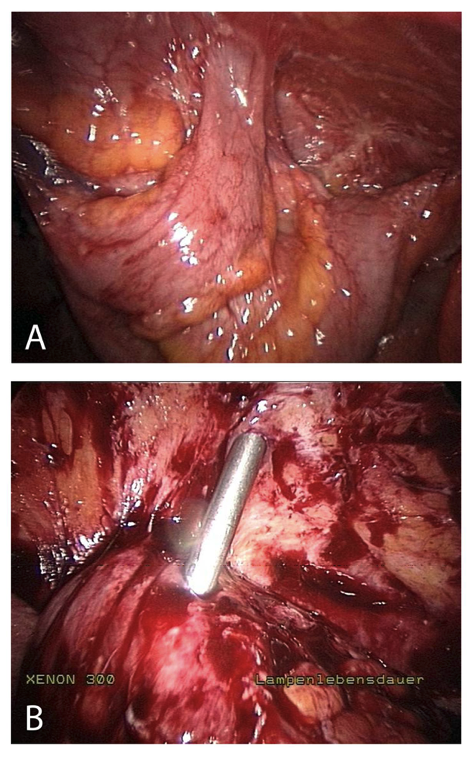 Figure 4. Insertion complication in abdomen with extensive adhesions
Figure 4. Insertion complication in abdomen with extensive adhesions
References
- Desormeaux AJ. De I’Endoscope et de ses Applications au Diagnostic et au Traitement de Affections de Furetre et de la Vessie. Paris: Bailliere 1865a, 1853.
- On endoscopic examination of the cavity of the womb. Med Press Circ 1869; 14. July, Canst. Jahresb. III: 582.
- Bozzini P. Der Lichtleiter oder die Beschreibung einer einfachen Vorrichtung und ihrer Anwendung zur Erleuchtung innerer Höhlen und Zwischenräume des lebenden animalischen Körpers. Weimar: Landes Industrie Comptoir 1807.
- Kelling G. Über Oesophagoskopie, Gastroskopie und Coelioskopie. Münch Med Wschr 1902; 49: 21-4.
- Fourestier M., Gladu A., Vulmiere J. Improvements in medical endoscoopy with spezial reference to bronchoscopy. Presse Med. 1952 Oct 1; 60 (61):1292-4.
- Lindemann HJ. Eine neue Untersuchungsmethode für die Hysteroskopie. Endosc 1971; 3:194-201.
- Hamou J. Microhysteroscopy. J Reprod Med 1981; 26:375-82.
- Baggish MS, Barbot J. Contact hysteroscopy. Clin Obstet Gynecol. 1983 Jun, 26 (2):219-41.
- Bettochi S., Ceci O, Nappi L, Pontrelli G, Pinto L, Vicino M. Office hysteroscopic metroplasty: three “diagnostic criteria” to differentiate between septate and bicornuate uteri. J Minim Invasive Gynecol. 2007 May-Jun; 14 (3):324-8.
- Jacobaeus HC. Über die Möglichkeit, die Zystoskopie bei Untersuchung seriöser Höhlungen anzuwenden. Münch Med Wschr 1910; 57:2090-2.
- Litynski G., Schaeff B, Paolucci V. The 100 th birthday of Heinz Kalk. A breakthrough in laparoscopy. Z Gastroenterol. 1995 Oct; 33 (10):594-7.
- Modlin IM, Kidd M, Lye KD. From the lumen to the laparoscope. Arch Surg. 2004 Oct; 139 (10):1110-26.
- Palmer R. La coelioscopie gynecologique. Rapport du Prof. Mocquot. Acad De Chir 1946; 72:363-8.
- Hopkins HH. On the diffraction theory of optical images. Proc Roy Soc A 1953; 217:408-15.
- Frangenheim H. Die Bedeutung der Laparoskopie für die gynäkologische Diagnostik. Fortschr Med 1958; 76:451-62.
- Semm K. Zur Technik der Eileiterdurchblasung. Z Geburtsh Gynäk (Beilageheft) 1964; 162:48-53.
- Hulka JF, Fishburne JI, Mercer JP, Omran KF. Laparoscopic sterilization with a spring clip: a report of the first fifty cases. Am J Obstet Gynecol 1973; 116:715-8.
- Rioux JE, Cloutier D. Tubal sterilization using laparoscopy: presentation of a new biopolar instrument. Vie Med Can Fr. 1973 Aug; 2 (8):760-5.
- Korbsch, R: Lehrbuch und Atlas der Laparoskopie und Thorakoskopie. Lehmann, München 1927.
- Semm K. Pelviskopie und Hysteroskopie. Farbatlas und Lehrbuch. Stuttgart, New York: Schattauer 1976 c.
- Semm K. Operationslehre für endoskopische Abdominal-Chirurgie. Operative Pelviskopie – Operative Laparoskopie. F.K. Schattauer Verlag, Stuttgart, New York. 1984.
- Semm K. Operative Manual for Endoscopic Abdominal Surgery, Yearbook Medical Publishers, Inc. Chicago-London-Boca Raton, 1987.
- Mettler L, Semm K, Gebhardt IH, Schollmeyer TH, Schollmeyer M, Meyer P, Ternamian A. Endoskopische Abdominalchirurgie in der Gynäkologie. Schattauer, Stuttgart, New York, 2002.
- Mettler Manual for Laparoscopic and Hysteroscopic Gynecological Surgery. Jaypee Brothers Medical Publishers (P) LTD New Delhi, 2006.
- Mettler Manual of new hysterectomy techniques. Jaypee Brothers Medical Publishers (P) LTD New Delhi, 2007.
- Hasson HM. A modified instrument and method for laparoscopy. Am J Obstet Gynecol 1971; HO: 886-7.
- Semm K. Endocoagulation: a new field of endoscopic surgery. J Reprod Med 1976 a; 16:195 – 203.
- Mettler L, Giesel H, Semm K. Treatment of female infertility due to tubal obstruction by operative laparoscopy. Fertil Steril 1979; 32:384-8.
- Gomel V., McComb PF. Microsurgery for tubal infertility. J Reprod Med. 2006 Mar, 51 (3):177-84.
- Winston R.M.L. Microsurgical Tubocornul Anastomosis for reversal of sterilisation. The Lancet, Volume 309, Issue 8006, Pages 284 – 285, 5 February 1977.
- Brosens I, Gordts S, Campo R, Rombauts Transvaginal access heralds the end of Standard diagnostic laparoscopy in infertility. Hum Reprod 1998; 13:1762-3.
- Maggino T, Gadducci A, D’ Addario V, Pecorelli S, Lissoni A, Stella M, Romagnolo C, Federghini M, Zucca S, Trio D, Trovo’ S. Prospective multicenter study on CA125 in postmenopausal pelvic masses. Gynecol Oncol 1994; 54: 117- 123.
- Neuwirth RS, Amin HK. Excision of submucous fibroids with hysteroscopic control. Am J Obstet Gynecol 1976; 126: 95-99.
- Jansen, Frank Willem, Vredevoogd, Corla B, Van Ulzen, Karin, Hermans, Jo, Trimbos, J, Trimbos-Kemper, Trudy C.M. Complications of Hysteroscopy: A Prospective, Multicenter Study. Obstetrics & Gynecology: August 2000. Volume 96. Issue 2, p 266-270.
- Jean-Bernard Dubuisson, Charles Chapron, Lionel Levy. Difficulties and Complications of Laparoscopic Myomectomy. Journal of Gynecologic Surgery. Fall 1996, 12 (3): 159 – 165. Published in Volume: 12, Issue 3: February 2, 2009.
- Mettler L., Sammur, W., Alkatout, I., Schollmeyer, T. Imaging in gynecologic surgery. Women’s Health, March 2011, Vol. 7, No. 2, p. 239-250.
