Laparoscopic Management of Common Bile Duct Stones
Joseph B. Petelin, MD, FACS
INTRODUCTION
Prior to the introduction of laparoscopic cholecystectomy choledocholithiasis was documented in approximately 9-16% of those patients who presented for open cholecystectomy.1,2 The incidence of common bile duct (CBD) stones remains around 10% today.3 Definitive treatment of these patients includes cholecystectomy and clearance of the ductal system. In 1890, nearly eight years after Langenbuch performed the first “open” cholecystectomy, Courvoisier showed that indeed the CBD could be cleared at the time of cholecystectomy.4 Around one hundred years later, laparoscopic cholecystectomy (LC) became the standard of care for treatment of symptomatic gallbladder disease. Within a few years several laparoscopic techniques proved successful in the treatment of choledocholithiasis at the time of laparoscopic cholecystectomy.
Historically, surgeons were expected to clear the common ductal system during cholecystectomy in approximately 90% of patients who harbored common bile duct (CBD) stones. The literature prior to 1990 documented a 5 to 10 % failure rate of common bile duct exploration.5,6,7 However, since the advent of laparoscopic cholecystectomy, many surgeons have more or less abrogated their intraoperative responsibility for doing a complete job–i.e. returning the biliary tract to its normal healthy status without ductal calculi. Most surgeons today do not even attempt laparoscopic common bile duct exploration (LCBDE). Instead, they rely on alternative interventional methods (ERCP, dissolution agents, and lithotripsy), to handle these problems.8-10 While these other techniques are certainly useful in managing complicated biliary tract problems, they are not without cost, morbidity, mortality, and significant life- style disruption. These issues become of paramount importance in today’s cost-conscious society. It seems logical then, for surgeons to reconsider our approach to CBD pathology. As Perissat indicated in 1994, the laparoscopic solution—a single-stage treatment, proves to be the safest, most cost-effective, and efficient approach in returning the patient to his or her former life style with the least financial or social disruption.11-16
Laparoscopic choledocholithotomy may involve a number of technical maneuvers. These include: administration of glucagon, dilatation of the distal common bile duct, balloon catheter manipulation, basket manipulation–with or without fluoroscopic guidance, and choledochoscopic manipulations.17-26 All of these techniques presuppose that intraoperative imaging has been performed, whether or not preoperative ductal evaluation (chemical, radiographic, or endoscopic (ERC)) has been used to evaluate or treat the common duct pathology prior to that time.
This chapter explores the techniques and technology currently available for safe laparoscopic treatment of common duct pathology. The general approach to these patients has solidified during the past two decades, and the effectiveness of several techniques has been documented. Proper application of these techniques should decrease the likelihood of complications.
LAPAROSCOPIC COMMON BILE DUCT EXPLORATION
Patient Management Overview
Common bile duct stones may be found preoperatively, intraoperatively, or post- operatively.
In the first situation, the clinician must decide whether to attempt ductal treatment, i.e. endoscopic retrograde cholangiography and extraction with or without sphincterotomy (ERC +/- S), before operation or to proceed directly with laparoscopic cholecystectomy (LC) and laparoscopic common duct exploration (LCDE).17 ERC +/- S is successful in clearing the common duct in 70% to > 90% of cases.10,24,27-41 LCDE is successful in clearing the duct in 70% to > 90% of cases as well.18,19,42-64 In the early 1990s however, when preoperative ERCP +/- S was used quite liberally for patients suspected of having CBD stones, a normal exam was documented in 40-60% of cases.10,24,27-41 So, almost half of these procedures were unnecessary. Moreover, these patients were exposed to the added morbidity and mortality for ERC +/- S, which has been reported as high as 5-19% and 1.3%, respectively.65,66 Consequently, when CBD stones are found preoperatively, choosing the clearance method should be based on the local availability of expert endoscopists capable of a high degree success with ERC +/- S, the availability of laparoscopic and choledochoscopic equipment, the surgeon’s own expertise in laparoscopic surgery, and the general condition of the patient.6,8,27,67,68
Common bile duct stones discovered intra-operatively allow three options: the surgeon either proceeds with LCDE, converts the case to “open” common duct exploration, or leaves the stones in place for subsequent ERC +/- S.6,17,23 Although any of these alternatives is acceptable, the latter two are more costly and both are associated with increased morbidity. Therefore it would seem wise in most situations, if the surgeon is properly trained, to attempt LCDE unless the patient’s condition warrants termination of the anesthetic as soon as possible. If LCDE is unsuccessful or not attempted, then the decision regarding conversion to “open” common duct exploration versus post-operative ERC +/- S will depend on the local availability of expert endoscopists. If expert endoscopists are not readily available, then conversion to open CBDE would be more likely.
When CBD stones are found post-operatively, ERC +/- S is usually the best initial treatment. If this is unsuccessful or unavailable, then either LCBDE or open CBD exploration may be necessary. In the rare cases where the CBD is widely dilated and/or packed with stones or harbors an impacted distal stone, biliary bypass with either choledocho-duodenostomy or choledocho-jejunostomy may be necessary. These may be performed either open or laparoscopically. These considerations are illustrated in Figure 1.
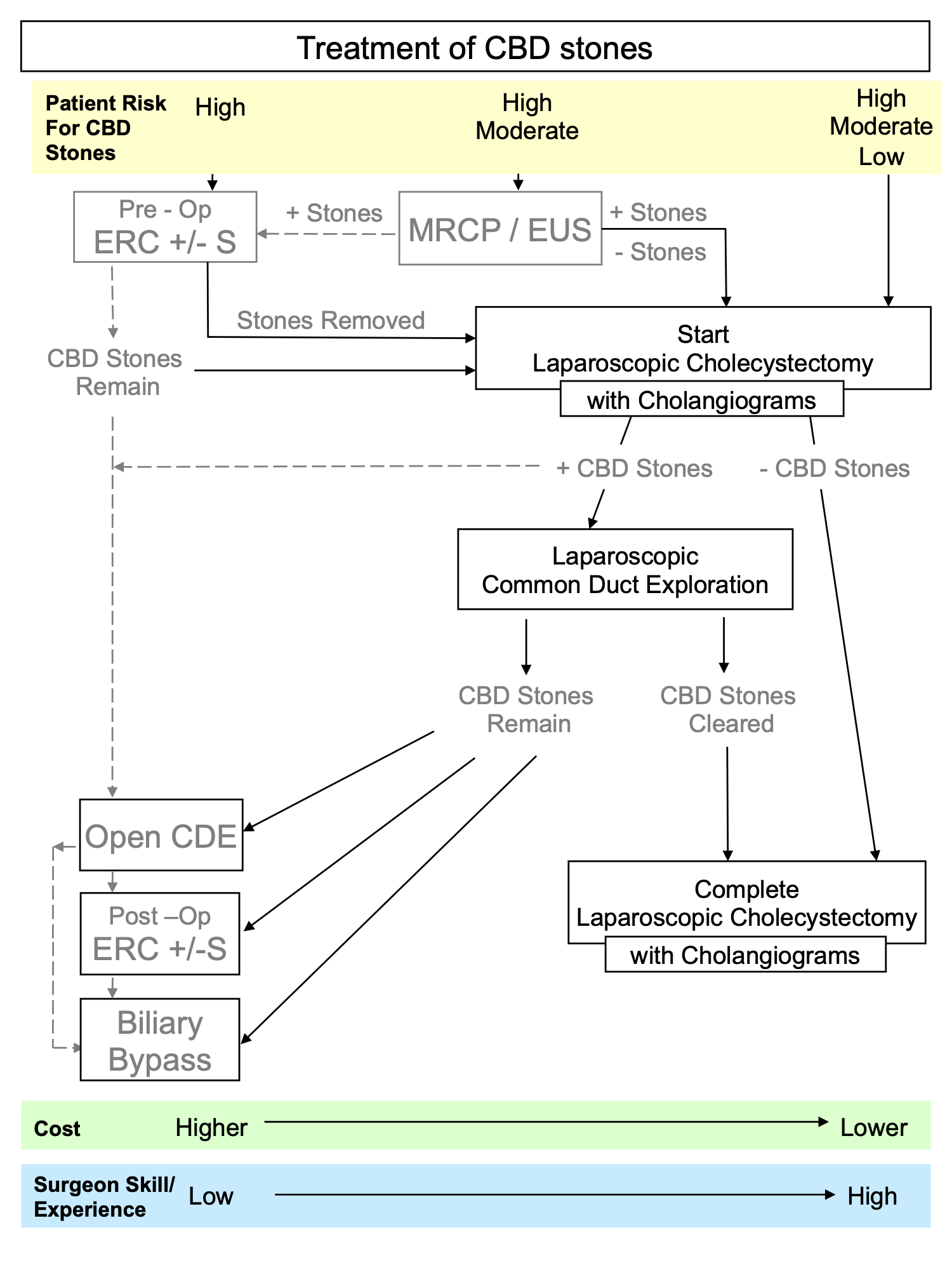 Figure 1. Surgical Decision-Making for the Management of Common Bile Duct Stones.
Figure 1. Surgical Decision-Making for the Management of Common Bile Duct Stones.
Indications
An abnormal intraoperative cholangiogram or sonogram is the most common indication for laparoscopic common bile duct exploration. Preoperative studies, including unexplained elevated liver function tests, a dilated ductal system, sonographic evidence of bile duct stones, scintigraphic, endoscopic, or radiographic evidence of common bile duct obstruction, or history of biliary pancreatitis may also warrant laparoscopic common bile duct exploration.
Contraindications
Inability of the surgeon to perform the maneuvers required for common bile duct exploration, an unstable patient, and local conditions in the porta hepatis that would make exploration hazardous are the primary contraindications to laparoscopic common bile duct exploration. However, the strongest contraindication to laparoscopic manipulation of the common bile duct is lack of training of the surgeon. There are also relative contraindications to specific approaches to ductal exploration, wherein either a transcystic approach or a direct choledochotomy approach may be preferred over the other. These specific situations will be discussed later.
Equipment
Items Needed
Laparoscopic common bile duct exploration usually requires more equipment than laparoscopic cholecystectomy. Some or all of the following items may be required for ductal exploration:
- 14 gauge IV catheter, 2 inches in length
- Fluoroscope (C-Arm type)
- Glucagon, 1 to 2 mg (given IV by the anesthetist)
- Balloon-tipped catheters (4 French preferred over 3 French and 5 French)
- Segura type baskets (4-wire, flat, straight, in-line configuration)
- 0.035 inch diameter long (>90 cm) guide wire
- Mechanical “over-the-wire” dilators (7 to 12 French) (found in most urology departments)
- High Pressure “over-the-wire” pneumatic dilator
- IV tubing (for saline instillation through the choledochoscope)
- Atraumatic grasping forceps (for choledochoscope manipulation)
- Flexible choledochoscope with light source (smaller < 3mm diameter, with >1mm working channel preferred)
- 2nd Camera
- 2nd Monitor (or 2nd viewing area on the primary laparoscopic monitor)
- Video Switcher (for simultaneous same monitor display of choledochoscopic, fluoroscopic, and/or laparoscopic images)
- WaterpikTM
- Electrohydraulic or pulsed dye lithotripter
- Absorbable Suture (polyglycolic acid suture, 4-0 or 5-0 size)
- T-Tube (transductal) or C-Tube (transcystic)
- Stent (straight, 7 French or 10 French)
- Sphincterotome (for antegrade sphincterotomy)
- Side-viewing endoscope (for antegrade sphincterotomy)
Table 1. Equipment Options for Laparoscopic Bile Duct Exploration
The standard 2 inch long 14 gauge IV catheter used to gain access to the peritoneal cavity for intraoperative cholangiography, represents an “added port” for introduction of balloon catheters and baskets (Figure 2).69
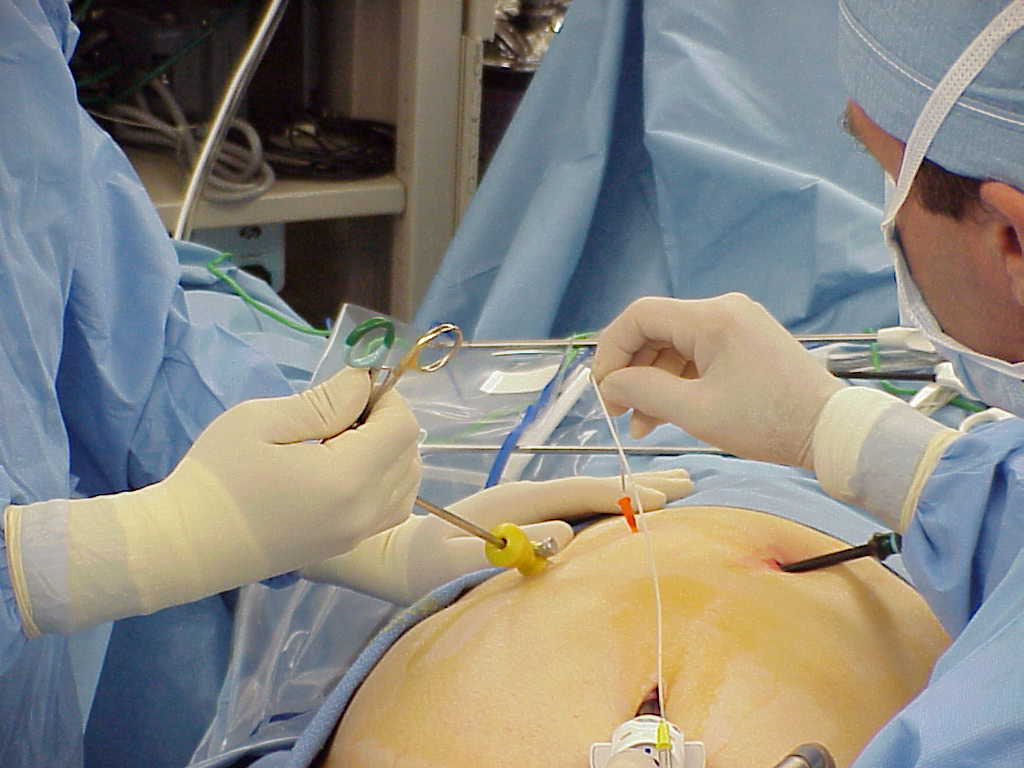 Figure 2. The 14 gauge sleeve is used to introduce the cholangiogram catheter.
Figure 2. The 14 gauge sleeve is used to introduce the cholangiogram catheter.
Stone retrieval baskets are usually essential. Straight and helical configurations with either flat or round wires are available. The straight flat wire basket, with its natural ability to expand the duct, presents large interstices for stones to enter the basket (Figure 3). The round wire helical basket is used by those who prefer a twisting maneuver to capture stones while trolling through the duct. It should be noted, however, that twisting maneuvers while the basket is in the channel of the scope are quite difficult to achieve.
This author prefers a 4-wire straight basket. Models with distal tip variations offer a wide variety of baskets from which to choose.
 Figure 3. The 4-Wire Segura-Type Basket.
Figure 3. The 4-Wire Segura-Type Basket.
Fogarty™ embolectomy catheters are often useful for LCDE. A standard vascular-type of catheter is preferred, because the “biliary” FogartyTM used in “open” CBD exploration is not long enough. Most commonly, a 4 French size proves adequate, but occasionally 3 French and 5 French models may be required.
A flexible choledochoscope is necessary to perform LCDE in approximately 60% of cases. The most versatile scopes feature a maximum outside diameter of 3 mm, tip deflection in at least one direction, a working channel of > 1 mm, excellent optics, and camera-ready capability. The scope requires a light source with automatic intensity control (Figure 4).
 Figure 4. The Olympus Flexible Choledochoscope.
Figure 4. The Olympus Flexible Choledochoscope.
Although not absolutely essential, a second camera directly connected to the choledochoscope significantly improves the performance of LCDE. This allows the surgeon the use of both of his hands during the manipulation; since he does not have to hold the scope to his eye during the exploration. Projection of the choledochoscopic image onto the video monitor allows other members of the operative team to assist more effectively. Obviously, this either requires an additional monitor or video switching equipment. A video switcher provides a picture-in-picture effect, where both the laparoscopic and choledochoscopic images may be viewed on the same monitor simultaneously. This reduces clutter in the OR since it eliminates the need for an additional trolley and monitor. It also improves efficiency of the operating surgeons since they can direct their gaze at one monitor, instead of alternating between two.42,70 Laparoscopic video systems today often incorporate an integrated video mixer for this purpose.
Large or impacted CBD stones may require intraoperative lithotripsy. Electrohydraulic and laser lithotripters are available, but the latter are prohibitively expensive. The energy from either of these sources is delivered to the stone via wires or optical fibers introduced through the working channel of the choledochoscope. The author finds both types to be of limited value in most cases because they multiply the debris present within the duct when fragmentation occurs. Nevertheless, in the infrequent case of impaction of a stone in the distal duct, they can be veryuseful.24,27,71
Dilators may be used to enlarge the cystic duct to a 12 French diameter, or greater, to allow easy introduction and manipulation of the choledochoscope. Graduated over-the-wire mechanical dilators have the advantage of being inexpensive and readily available in most urology departments. Pneumatic dilators, while more expensive, allow excellent control of the dilatation process with radially directed force rather than the shearing force of the former type.
In cases where choledochotomy is necessary, a laparoscopic scalpel or scissors is necessary to open the duct.17,70 Laparoscopic needle holders are used to close the choledochotomy. Standard T-tubes or pigtail or straight stents may be used for subsequent drainage of the duct. T-Tubes are most easily introduced through a 10 mm portal using an introduction sleeve. A variety of devices that reportedly facilitate T-Tube insertion have been developed.72,73
If a stent is used it is placed through the cystic duct or the choledochotomy, and advanced into the duodenum.74,75 Its location is documented either endoscopically or with intraoperative cholangiography. 10-French sized stents are preferred over 7-French stents in many cases because of better patency.76-78 Those that are placed percutaneously through the cystic duct allow access to the common bile duct for cholangiography and/or stone removal, just as a T-Tube would; and subsequent removal does not require upper endoscopy. The disadvantage of internal stents is that that they require subsequent endoscopy for their removal, although some have reportedly passed spontaneously.
Endoscopic sphincterotomes may be used for intraoperative antegrade or retrograde sphincterotomy. Intraoperative sphincterotomy, however, is usually a logistical tour de force, requiring an additional endoscopic team and their equipment for its performance. This technique has not found widespread acceptance.
Availability & Storage
The necessary tools for CBD exploration should be organized in a central location; a common bile duct exploration cart that may be moved from room to room is the best option. These carts promote efficient CBD exploration in the same way that “code carts” facilitate emergency cardio-respiratory resuscitation. However, even at the author’s institution the nursing management has seen fit to eliminate because of “space concerns” (Figure 5).
 Figure 5. The Common Bile Duct Exploration Cart.
Figure 5. The Common Bile Duct Exploration Cart.
Intraoperative Placement
Prior time spent in planning for equipment preparation and placement during LCDE scenarios is well worth the effort, and should be given the same importance as preparation of the surgeon’s skills to carry out the maneuvers once the equipment is available. This preparation decreases surgeon and staff tension, facilitates LCBDE, and reduces the likelihood of complications.
The choledochoscope and related equipment is placed on a separate Mayo stand, placed near the patient’s left shoulder, to the right of the surgeon in the sterile field (Figure 6a, 6b). The various cables, catheters, and tubes used in the duct exploration should be routed so as to minimize clutter in the operative field. Nursing staff or biomedical engineering personnel should be available and competent to connect the choledochoscope to its light source, the choledochoscopic camera to its processing unit, and the video cable to the appropriate location on the video mixer ormonitor.
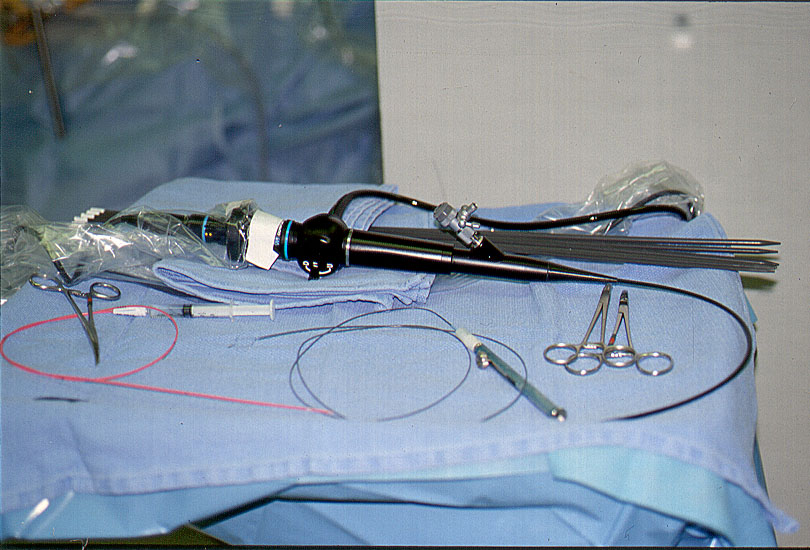 Figure 6a. Common bile duct exploration instruments are placed on a separate Mayo stand.
Figure 6a. Common bile duct exploration instruments are placed on a separate Mayo stand.
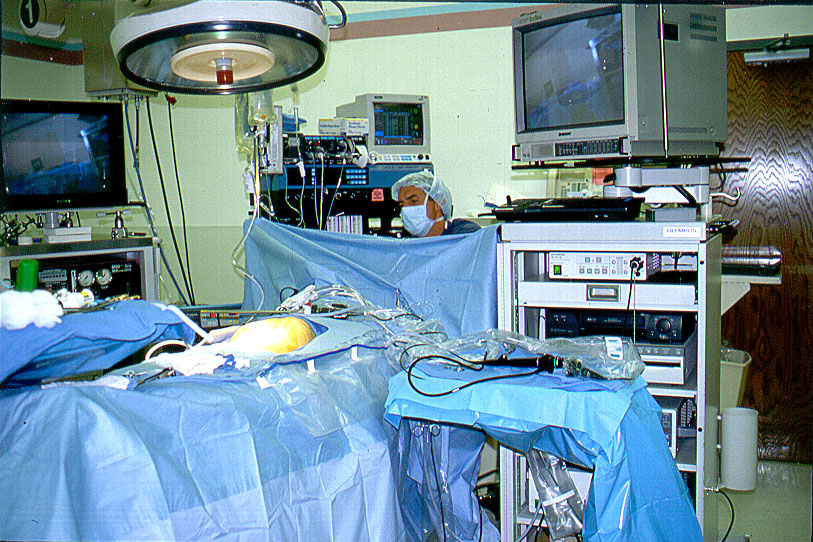 Figure 6b. Common Bile Duct Mayo stand at the surgeon’s side of the table.
Figure 6b. Common Bile Duct Mayo stand at the surgeon’s side of the table.
Approach
Laparoscopic common bile duct exploration may be accomplished through the cystic duct or through a choledochotomy. Stone size, configuration of the cystic duct-common duct junction, the course of the cystic duct, and the diameter of each of the ducts affect the decision as to which approach is best in a particular case. If a transcystic approach appears feasible, it is usually tried before choledochotomy, because it is less invasive and is associated with better patient satisfaction.
The author, and many other biliary tract surgeons, has found transcystic ductal exploration possible and has been highly successful in a large percentage of cases. In some cases, however, choledochotomy is necessary or even the preferred route. The following table summarizes characteristics, which are helpful in making the determination.
Operative Techniques
Ductal Imaging
Ductal imaging is an essential part of laparoscopic common bile duct exploration. While some surgeons prefer sonography, most surgeons favor fluoroscopic imaging. It is preferred for intraoperative radiological evaluation in contrast to static x-rays, because it is faster, more detailed, and allows surgeon interaction with the images in real time, i.e. the ductal system can be scanned by moving the C-arm while injecting contrast material (Figure 7).
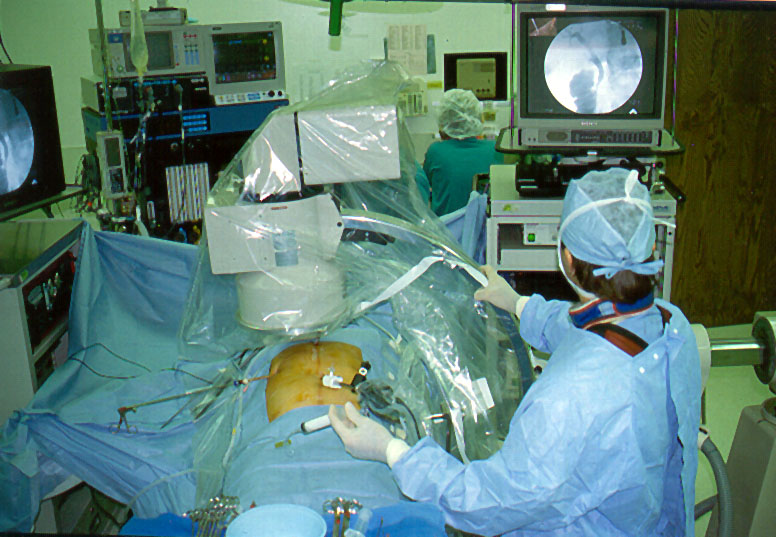 Figure 7. Percutaneous fluoroscopic cholangiography.
Figure 7. Percutaneous fluoroscopic cholangiography.
Cholangiography—Percutaneous Method
Percutaneous cholangiography is performed with a 14 gauge IV needle/catheter that is inserted through the abdominal wall approximately 3 cm medial to the mid-clavicular port. It is directed toward the Triangle of Calot. The needle is removed and the catheter is used as a sleeve for introduction of the actual cholangiogram catheter. This sleeve also acts as a “mini-port” which may be used for introduction of balloons and baskets during common bile duct exploration. The catheter is guided into the cystic duct with forceps introduced through the medial epigastric port. It is fixed into position with a clip applied transversely across the axis of the catheter at its insertion point into the cystic duct.
The author prefers this technique because it does not require removal of forceps controlling the gallbladder from another port (as is needed in the portal technique).
Cholangiography—Portal Method
With this method the catheter is introduced through the mid-clavicular port freely or with an instrument that directs it into the cystic duct. Some models also fix the catheter into the cystic duct.80 The major disadvantage of this technique is that it uses an existing port that would otherwise be occupied by an instrument providing exposure of the porta hepatis.
Intraoperative Sonography
Some authors advocate the use of intraoperative laparoscopic sonography to evaluate the ductal system, liver, and surrounding structures for abnormalities. Here the sonographic probe is inserted through a 10 mm port and is placed in direct contact with the tissues.
Proponents of this technology indicate that it is faster and more accurate than cholangiography in their hands. Critics have argued that fluoroscopic imaging is not only faster than sonographic imaging but doesn’t require additional equipment expense.
Additionally, cholangiogram films may be used as “maps” of the ductal anatomy in cases where intense inflammation obscures visual cues. In experienced hands intraoperative sonography has been demonstrated to be efficient, accurate, and effective. However, widespread use of this technology for ductal evaluation has not occurred.
Preparation of the Porta Hepatis
Dissection
Dissection of the porta hepatis in preparation for laparoscopic duct exploration is usually carried out more thoroughly than it is for routine laparoscopic cholecystectomy. This is required because access to the cystic duct-common duct junction or the anterior surface of the common duct itself is often necessary for ductal exploration. The dissection is started lateral to the Triangle of Calot in most cases and carried toward the cystic duct- common duct junction as the anatomy is further defined. Intraoperative cholangiography provides a “map” that proves useful in this sometimes tedious dissection.
During this dissection, the location of the common duct may be temporarily demonstrated by depressing the first portion of the duodenum inferiorly with atraumatic forceps introduced through the mid-clavicular port. This straightens the common duct a bit and brings it anteriorly, which usually makes it easier to identify. If the cystic artery has not been divided yet, and if it obscures visualization of the common hepatic duct, then it may be divided at this time. Superior extension of the incision in the peritoneum lying medial to the neck of the gallbladder is also helpful in some cases. This often allows the extrahepatic ductal system to be delivered into a more anterior location as the gallbladder is displaced toward the right hemi-diaphragm. Dissection must be done carefully here in order to avoid vascular or ductal injury.
Although not always necessary, the anterior surface of the common duct may be cleared of its overlying fatty tissue near the cystic duct-common duct junction. In some instances, a posteriorly located junction requires the surgeon to dissect lateral to the common bile duct and inferior to the cystic duct in order to “unwrap” the cystic duct from the common bile duct. However, the author do not advocate “chasing” a long tortuous cystic duct down into the head of the pancreas. Correct interpretation of the cholangiogram is very helpful here. Occasionally, the lateral peritoneal attachments of the duodenum must also be severed, i.e. a Kocher maneuver, in order to achieve this effect. This facilitates introduction of instruments into the common duct via the cystic duct. The importance of these maneuvers cannot be overestimated.81
Dilatation of the cystic duct
In order to allow insertion of the choledochoscope through the cystic duct it will need to be > 3 mm in diameter. If the duct is not already large enough for scope insertion, it may be dilated either with mechanical over-the-wire graduated dilators or with pneumatic dilators. With either device, it is important to closely observe the physical changes in the duct while the dilatation proceeds, so that injury to the cystic duct-common duct junction may be avoided.17-19,24
A guide wire (0.028″ or 0.035″) is inserted through the mid-clavicular port, through the cystic duct, and into the common duct. If graduated dilators are used, a 9 French size is usually the first to be advanced over the wire into the duct. The authors have found that if a 9 French dilator will not enter the cystic duct relatively easily, then it is unlikely that dilatation to a large enough diameter (11 or 12 French) will occur. Each successively larger dilator is advanced over the wire until the duct is patulous enough to accept the scope.17,42,65
Pneumatic dilators may also be advanced over the wire into the cystic duct. The dilatation balloon is filled while observing both the video monitor and the pressure gauge on the screw-type syringe attached to the dilator.
The guide wire may be removed or left in place to facilitate guidance of the choledochoscope into the common bile duct. If left in place, the wire is back-loaded into the distal end of the working channel of the scope; the scope is then advanced into the ductal system over the wire.
Choledochotomy
This approach is used when the cystic duct cannot be dilated enough to accept passage of the scope or the largest common duct stone, or when intrahepatic pathology is suspected. Some authors prefer common duct access via a choledochotomy for all cases.43,82,83 A longitudinal incision approximately 1 cm in length, or as long as the diameter of the largest stone, is recommended by most authors. This limits the amount of time that will be spent later in closing the choledochotomy. Stay sutures, which are commonly used in open common duct exploration, are not necessary for laparoscopic common duct exploration.
One well-respected group in Ancona, Italy prefers a transverse choledochotomy, and has had excellent results with this approach, but this technique has the potential for compromise of the primary blood supply to the common duct (which parallels the duct on either side) with subsequent stricture.55 If it is to be used, great care should be exercised to avoid this problem.
Irrigation Techniques
Transcystic flushing of the duct with saline or contrast material is sometimes successful in forcing debris into the duodenum. The process is monitored fluoroscopically.23 This technique often works well for 1-2 mm stones, but surgeons should not expect this method to be successful in clearing stones 4 mm and larger from the duct. In order to relieve Sphincter of Oddi pressure and improve the results of this flushing maneuver, the anesthetist may administer glucagon, 1-2 mg, intravenously.
Balloon Techniques
A standard 4 French Fogarty balloon catheter may be inserted into the abdomen through the 14-gauge sleeve used to perform the percutaneous cholangiograms.17,69 The catheter is guided through the cystic duct into the common duct and into the duodenum if possible with forceps inserted through the medial epigastric port. The balloon is inflated in the duodenum and the catheter is withdrawn until resistance is met at the sphincter; the duodenum is observed to move with the catheter at this point. The balloon is deflated, the catheter is withdrawn 1 cm, and the balloon is re-inflated. This should position it in the most distal portion of the duct, just proximal to the sphincter. The catheter is then withdrawn through the cystic duct, using the forceps from the medial epigastric port. Stones retrieved through the cystic duct can then be removed with forceps introduced through the medial epigastric port. The surgeon should use great care and gentle manipulations with the catheter in order to avoid perforation of the ductal system during these maneuvers, especially when a stone is impacted in the distal duct. In the unusual event of displacement of the stone into the proximal hepatic duct, irrigation of the duct combined with operating table position changes will usually return the stone to the distal duct. The author has never “lost” a stone into the proximal system.
In most cases, the balloon catheter technique is employed without choledochoscopic or fluoroscopic monitoring, but either of these devices may aid in exact localization of the stone and the balloon. If a balloon catheter is used in conjunction with a choledochoscope, the balloon-tipped catheter must be inserted alongside the scope because the working channel of the scope is not large enough to accept the catheter. This obviously requires either a large diameter cystic duct or insertion through a choledochotomy.
The combined use of the choledochoscope and a balloon catheter is particularly useful for stones that defy capture with a basket, even under direct vision through the choledochoscope. The balloon is inserted alongside the scope (not in the scope channel). The balloon is advanced past the stone, inflated, and withdrawn toward the scope. The entire scope-stone-balloon ensemble is then withdrawn through the ductal orifice. This technique is especially useful when dealing with intrahepatic stones. Baskets are less useful in the intrahepatic ductal system because they cannot be fully deployed in the narrow diameter hepatic ducts.
Basket Techniques
Stone retrieval baskets may be used with or without the choledochoscope. When used alone the basket is inserted through the 14-gauge sleeve used for cholangiography. The basket is advanced into the common duct through the cystic duct, using forceps introduced through the medial epigastric port. If no fluoroscope is used during this maneuver, the surgeon should estimate the location of the basket tip in the common duct. This localization can be relatively accurate when the length of the duct has already been measured with the balloon-tipped catheter. The basket is opened immediately after it is advanced into the common bile duct. The deployed wires offer not only a “soft” distal end to the catheter, but also provide increased resistance when the catheter reaches the distal end of the bile duct. After the basket is positioned in the distal common duct it is moved back and forth in small increments while slowly withdrawing it as the wires of the basket are being closed. Capture of a stone is identified when the basket fails to close completely. The device is removed through the cystic duct, and the stones are delivered from the abdomen as described above. Great care must be exercised with this method so that accidental “capture” of the papilla of Vater does not occur.
The manipulations required for stone capture with the basket may be monitored in real time in the contrast-filled common duct using a fluoroscope.18 This technique, however, requires positioning of the fluoroscope in such a way as to avoid interference with movements of the forceps in the medial epigastric port. In some individuals, especially the obese, adequate fluoroscope position cannot be achieved. The surgeon must then either pursue basket extraction without it, or use a choledochoscope to monitor the procedure.
Although free basket techniques may seem rather simple, the author feels that they are one of the more difficult sets of maneuvers to master.
Choledochoscopic Techniques
Choledochoscopy provides the ultimate in visualization and control of CBD manipulations. Choledochoscopic techniques are used when the conservative measures described above fail to clear the common duct.
The choledochoscope is inserted in the most direct route to the porta hepatis, with or without wire guidance, into the cystic duct or the choledochotomy. The author finds that the mid-clavicular port is the best scope entry site for easy access to the ductal system. The surgeon initially uses an atraumatic forceps inserted through the medial epigastric port to help guide the scope into the common duct. Saline instillation through the working channel of the scope is employed at this time in order to distend the common duct and provide better visualization. Once a sufficient length of the choledochoscope is present within the common duct, the forceps is withdrawn. Further manipulations are most efficient if the surgeon controls the scope with both hands. One hand controls twisting maneuvers on the body of the scope at the cannula site, while the other holds the scope head and directs the tip of the scope with the deflection lever located there. The choledochoscopic and laparoscopic images must be kept in view, either on separate monitors or preferably on the same screen with a video switcher.
The distal portion of the common bile duct is usually the easiest to inspect and treat. When a cystic duct approach is employed, access to the proximal ductal system is usually not possible unless the cystic duct is very short or patulous and oriented at 90 degrees to the common duct. When a choledochotomy is used, the scope may be directed either into the proximal system or the distal bile duct.
If the scope will not traverse the cystic duct-common duct junction, further dissection along the lateral border of the cystic and common bile duct or a Kocher maneuver may allow the junction to “unwrap” thereby providing a less convoluted path into the common duct.
The surgeon manipulates the scope so that a stone is in direct view. Control of the body of the scope is then transferred to the assistant. It is essential that he or she maintain the same direction and the same amount of torque on the scope at the level of the mid- clavicular cannula as the surgeon had applied prior to the transfer; otherwise the location of the stone will be lost. If this happens after the basket is inserted into the working channel of the scope, it is very difficult to manipulate the scope to find it again without removing the basket from the channel. This difficulty occurs because the working channel of the scope is too small to allow installation of saline while a basket is in place.
Without saline installation, the common duct collapses and visualization is lost. This problem becomes compounded when multiple stones are present in the duct, since their manipulation and capture usually produces minute debris, which “clouds” the fluid in the duct. This debris, combined with loss of the exact location of the stone, makes it nearly impossible to capture the stone. In this case, the basket will need to be removed so that saline instillation through the basket channel may be used to clear the fluid in the duct.
Obviously, inefficient manipulations performed at this time create more debris and decrease the likelihood of a successful LCDE.
Stone capture is accomplished by advancing the basket past the stone, opening it, and then withdrawing it back to the stone. Often, the basket must be moved back and forth past the stone until it drops into the interstices. In some cases, this maneuver must be combined with twisting of the scope body or tip deflection in order to capture the stone (Figure 8). The basket is then slowly closed around the stone to secure it. As the basket is closed, it usually withdraws itself into the choledochoscope. Therefore, it is usually best to advance the basket out of the tip of the scope while the basket is being closed. (This maneuver seems to be one of the most difficult for the novice to master.) The entire scope-basket-stone ensemble is then removed from the duct and the stone temporarily deposited on the omentum. The stone may then be removed from the abdomen with forceps introduced through the medial epigastric port.
 Figure 8. Laparoscopic CBD stone capture with flexible choledochoscopy.
Figure 8. Laparoscopic CBD stone capture with flexible choledochoscopy.
Lithotripsy
The primary indications for intraoperative lithotripsy include an impacted stone that defies less aggressive removal techniques or a stone that is too large to be captured and removed through the cystic duct or the choledochotomy. Electro hydraulic lithotripters (EHL) are much less expensive than laser models, and consequently have been used somewhat more frequently. EHL devices must be used with great caution because they may cause unwanted ductal damage if the tip of the EHL probe is not accurately applied to the stone. However, with careful, direct visualization and application of EHL energy to the stone surface, stones may be safely fragmented without undue risk.24,27,71 Intraoperative electrohydraulic or laser lithotripsy techniques have seen limited use since the introduction of laparoscopic common bile duct exploration.
Application of a pulsatile saline jet, eg. Waterpik™, through the working channel of the scope may occasionally be useful in freeing stones or debris from the duct wall. Because there are no ready-made adapters for this application, the surgeon will have to configure his or her own adapter to connect the device to the scope.
Sphincterotomy and Drainage Procedures
Intraoperative laparoscopic antegrade sphincterotomy was first described by DePaula in Brazil in 1993.84 Zucker also reported success with this procedure. Here, a sphincterotome is passed through the working channel of the choledochoscope and through the sphincter of Oddi. The cutting action of the device is monitored by simultaneous side-viewing endoscopy of the duodenum. This technique achieves excellent results as a drainage procedure, but it is logistically quite difficult to accomplish. It requires more equipment and an additional endoscopic team to be present in an already crowded operating theater. Endoscopists report that it is more difficult to perform this procedure with the patient supine rather than in the typical prone position. Surgeons indicate that excessive air insufflation by the endoscopist hampers laparoscopic visualization and manipulation. For these reasons, laparoscopic antegrade sphincterotomy has not gained widespread acceptance. Others have employed endoscopic retrograde sphincterotomy at the same time as the LCDE, but again this has not gained widespread acceptance.85
Surgical biliary bypass may be indicated for patients with a dramatically dilated common bile duct, multiple common duct stones, non-removable impacted distal common duct stones, retained common duct stones not amenable to ERC + sphincterotomy, and obstruction secondary to tumor. Three laparoscopic operations have proven feasible: cholecysto-enterostomy, choledocho-duodenostomy, and choledocho-enterostomy.
Cholecysto-enterostomy requires patency of the cystic duct.86 All of these procedures have been performed in the laboratory and in patients.87-89
These procedures require advanced technical skills including laparoscopic suturing and knotting. Therefore they should only be attempted by surgeons with proficiency in these techniques. In skilled hands, patency rates, morbidity, and mortality compare favorably with open techniques.
Completion Cholangiography
Cholangiograms are repeated after the ductal exploration is complete. If cholangiograms reveal residual stones, the surgeon must decide whether to proceed with LCDE again, convert to open CDE, perform a biliary bypass, or leave the stones in place for subsequent ERC +/- S. The decision is based on the expertise and equipment available.
After the duct is cleared
Ligation of the cystic duct
The cystic duct stump is secured either during the common duct exploration (in cases where a choledochotomy is made) or after the completion cholangiograms. If the cystic duct is dilated, >5 mm, or if subsequent ERC +/_ S in planned, then ligatures (instead of, or in addition to clips) should be used to secure the duct, in order to prevent subsequent leak.
T-Tube or C-Tube placement
T-Tubes are used for three reasons: 1) decompression of the duct, in the case of residual distal obstruction, 2) access for ductal imaging in the post-operative period, and 3) access for removal of residual common duct stones, should they be left after common bile duct exploration.90
If a T-Tube is used, a 14 French T-tube is prepared by removing the back wall of the T portion.17 The entire T-tube is placed into the abdomen through a 10 mm port; then the “T” is inserted into the common duct (Figure 9). This usually requires some effort, but it is not much more difficult than it is in open surgery. After the tube is in the duct, the outlying portion is temporarily occluded at its tip with a clip or ligature; this prevents bile drainage into the peritoneal cavity while the CBD is sutured. This clip is removed just prior to delivery of the tube through the abdominal wall. The common duct closure is completed with either 4-0 or 5-0 polyglycolic acid suture in either a continuous or interrupted fashion.91 The magnification afforded by the laparoscope and camera allows more precise placement of the sutures than in open surgery. The suture is secured with intracorporeal ligation techniques rather than extracorporeal techniques because of the fragility of the duct. The security of the closure is tested by temporarily advancing the tube out of one of the 5 mm right upper quadrant ports and injecting saline through the tube. If there is a leak, the common duct closure may be reinforced or re-sutured and then tested again.17 It is usually necessary to replace the tube into the peritoneal cavity until the cholecystectomy is completed, so that the port is available for retracting forceps, and so that the tube itself does not limit access to the porta hepatis. While the tube is completely inside the peritoneal cavity, its tip should remain occluded with a clip or ligature.
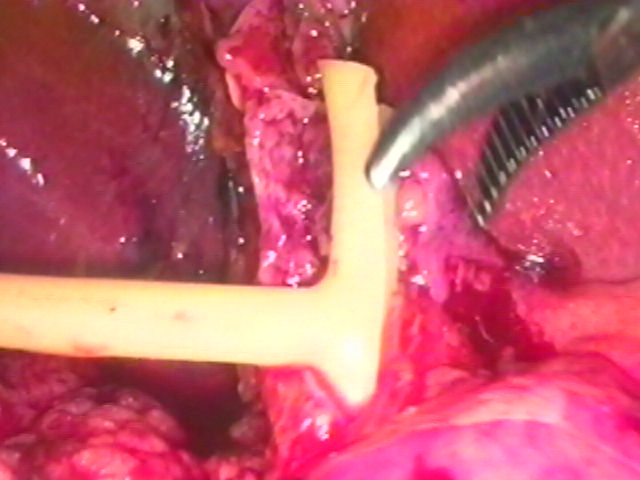 Figure 9. T-Tube insertion. The duct is closed with absorbable polyglycolic acid suture.
Figure 9. T-Tube insertion. The duct is closed with absorbable polyglycolic acid suture.
There are many potential disadvantages to the use of T-Tubes. T-Tube placement may be difficult at times. This difficulty may become even more pronounced in laparoscopic surgery.92,93 Surgeon patience and practice are the most important requirements for success with this maneuver.94-99 Most authors prefer a 14 French Latex T-Tube (or larger), and ductal closure with an absorbable fine suture such as 4-0 or 5-0 polyglycolic acid. Although silicone T-Tubes have been used by some authors, they are often not preferred because they do not excite the degree of tissue reaction necessary to produce a tract to the surface of the abdominal wall in the case of persistent bile leakage after removal. Silicone T-Tubes, however, have been associated with less bacterial contamination than Latex T-Tubes.100-106
In addition to bacteremia, dislodgment of the tube, obstruction by the tube, or fracture of the tube may occur.92,93,107 Some authors recommend broad-spectrum antibiotic coverage while the T-Tube is in situ.108 T-Tube cholangiography should be performed before removal of the tube. Removal of T-Tubes postoperatively has been suggested as early as 4 days, and as long as 6 weeks.109 The most appropriate management plan lies somewhere between these two extremes. Complications of T-Tube removal include bile leaks, peritonitis, and re-operation.104-106,108,110-112
Some authors have reported choledochotomy closure and placement of a transcystic tube, a C-tube, to decompress the common bile duct and provide access for subsequent cholangiography or ERC +/- S.113,114
Primary closure of the choledochotomy
Primary closure of the common bile duct without a T-Tube has been advocated by some authors in open biliary tract surgery because of the potential complications associated with T-Tube placement.90,100-106,115,116 Shorter operative times, and length of stay have been observed with primary closure. No increase in bile leak or peritonitis has been noted with primary closure in the “open” literature. Higher patient satisfaction has been associated with primary closure.
In the author’s series, primary closure of the choledochotomy laparoscopically was performed in over one third of cases where a choledochotomy was used, and did not result in any complications. There was no incidence of bile leak, peritonitis, or clinical evidence of retained bile duct stones. Patients reported a higher degree of comfort and satisfaction than those in whom T-Tubes had been placed.117 Other authors have had similar results.45,76,118,119
Cholecystectomy
Laparoscopic cholecystectomy is performed after completion of the common bile duct exploration and its associated tasks. If a C-Tube or a T-Tube is used, it may have to be temporarily replaced inside the abdomen and clipped, or deflected out of the way of the dissection. The gallbladder is removed through the umbilicus, and the umbilical wound closed at the fascial level. If a C-Tube or T-Tube has been placed, it is brought out of the abdomen through the most direct route through one of the existing port sites.
Drain Placement
Drains are not routinely used after transcystic laparoscopic common bile duct exploration. However, a drain may be indicated in cases where intense inflammation, infection, or contamination is present, where a choledochotomy has been performed, or where tissue integrity may be questionable. If used, a closed system suction drain is inserted through a 10 mm port into the porta hepatis and is usually brought out through the abdominal wall through one of the 5 mm port sites.
COMPLICATIONS: AVOIDANCE & MANAGEMENT
The most common complications that can occur with laparoscopic common bile duct exploration include failure to clear the common duct of stones, common duct injury, bile leak, abscess, and pancreatitis. It is obviously best to avoid these if possible, but if any of them occurs, there are methods to manage them.
Failure to clear the common duct
Failure to clear the common duct of its obstruction occurs for many reasons, including intense inflammation in the porta hepatis, obesity, intrahepatic stones, impacted stones, multiple stones, stones distal to a stricture, inadequate equipment, and surgeon inexperience.
Surgeon inexperience is the most important factor relating to inability to clear the duct of stones. For most surgeons who perform an average of 50 laparoscopic cholecystectomies per year, only 5 patients with common bile duct stones will be identified (10%). This suggests that a surgeon may have only an opportunity to hone his or her laparoscopic common bile duct exploration skills once every two months or so. While this suggests that it may be difficult to gain actual laparoscopic common bile duct exploration experience, surgeons who practice routine intraoperative cholangiography are using many of the same maneuvers that are used for transcystic ductal exploration, such as catheter and basket insertion into the ductal system. This should make them somewhat more adept at transcystic LCDE techniques than those who do not practice routine cholangiography. Participation in a laparoscopic common bile duct exploration course or laparoscopic fellowship is also extremely valuable in providing the training needed to perform successful LCDE.
After the duct has been explored, completion cholangiography is usually essential to document the status of the ductal system. Intraluminal opacities or failure of contrast to pass into the duodenum usually indicates retained intraductal material, mass, or stricture. If attempts to remove these are unsuccessful, then the surgeon must decide whether to convert to open common bile duct exploration or resort to post-operative endoscopic retrograde techniques for stone removal. It should be noted, however, as shown by Traverso, that either of the latter is significantly more expensive than LCDE.15 Therefore it behooves the laparoscopic biliary surgeon to become proficient in LCDE techniques.
Common duct injury
Improper identification of the anatomy during dissection may lead to injury to the duct. This may be more likely in cases where there is intense inflammation in the porta hepatis. The mechanism of injury usually involves one of two fairly reproducible scenarios. One episode begins with less than optimal camera work, which leads to poor visualization or distortion of the porta hepatis. A thorough knowledge of the anatomy and its multiple variants, as seen laparoscopically, is essential. In the second scenario, the case may be fairly well controlled until bleeding is encountered in the Triangle of Calot. This time however, it is controlled with electrocautery instead of clips. The patient initially does reasonably well post-operatively, but in a very short period of time develops jaundice secondary to a stricture of the common bile duct or the common hepatic duct.120,121,122
Intraoperative cholangiography via the cystic duct, or the gallbladder if necessary, may provide clues as to the location of the common bile duct and delineate the local anatomy. This should help the surgeon to avoid maneuvers that might injure the duct.123,124,125,126
During the ductal exploration itself, aggressive manipulation of instruments or the duct itself may lead to ductal injury. Introduction of instruments into the common bile duct must be done gently. This is especially true when using baskets in the ductal system because they may puncture the duct more easily than larger more blunt instruments.
Similarly, application of electrohydraulic lithotripsy to stones in the duct must be done accurately and under direct vision in order to avoid injury to the duct wall.
The best time to recognize this injury is at the time of surgery, when it can be either repaired primarily or bypassed if necessary. Unfortunately, most injuries are not recognized at this time and present themselves later with fever, tachycardia, abdominal pain, ileus, and jaundice. At that point, after stabilization of the patient, referral to a center specializing in biliary tract surgery is the best option. Roux en Y hepatico- jejunostomy is usually the procedure of choice for most severe common duct injuries.127,128,129
Bile leak
Bile may leak from the gallbladder bed, the cystic duct orifice, the cystic duct-common duct junction, or the common duct itself. This may be the result of dissection and manipulation of these structures during LCDE.130
Good visualization and gentle tissue handling techniques may help reduce the incidence of this problem. Additionally, the cystic duct should be secured adequately. This may require suture ligation in cases where the cystic duct is large, thickened, or short, significant distal common duct manipulation was used, or where recent pancreatitis may cause temporary distal ductal hypertension. Suture ligation or “loop” ligation of the cystic duct stump should also be considered in cases where the surgeon suspects that post- operative ERCP may be required.
The diagnosis of a bile leak may be suggested by postoperative fever, excessive bilious drain output, ileus, generalized abdominal pain and elevated liver function studies. A radionuclide scan may confirm the presence of a leak, and the possibility of a distal bile duct obstruction. Sonography or CT scanning of the abdomen may help localize a bile collection if there is one.
If a drain is already in place, and if no evidence of distal obstruction of the common bile duct exists, observation, intravenous fluids, and antibiotic coverage may be all that is necessary. If no drain is in place, and if a bile collection is localized, then radiographically directed placement of a drain may be adequate to allow a period of observation. Postoperative ERCP with stent placement across the sphincter of Oddi may be necessary to relieve a relative distal obstruction caused by spasm, edema, or debris.131 Surgical intervention may be warranted if the leak will not seal or if generalized peritonitis is present.
Abscess
Patients requiring LCDE are often older with more intense gallbladder inflammation than those requiring a simple laparoscopic cholecystectomy. Hence, they may be more prone to the development of postoperative infectious problems at the surgical site. While prophylactic antibiotics are usually used here, the finding of acute or gangrenous cholecystitis or cholangitis indicates the need for therapeutic antibiotic coverage, which should be continued into the postoperative period.
The perihepatic space should also be thoroughly freed of debris and stones prior to completion of the case. In cases where there is severe inflammation or where there has been spillage of bile or stones, placement of a closed system suction drain may be prudent.
Postoperative fever, tachycardia, ileus, and abdominal pain may signal the presence of a problem at the surgical site. Sonography or CT scanning may confirm the presence of an abscess. Intravenous antibiotics are usually necessary here. Radiographically directed percutaneous drain placement may be the first interventional step in management. If this is not successful in reducing or eliminating the above symptoms, surgical intervention may be warranted.
Pancreatitis
Common duct stones themselves may cause pancreatitis preoperatively. Intraoperatively, it may be caused by manipulations in the distal duct. Baskets, balloons, and the choledochoscope must be gently manipulated to avoid such injury.
High-pressure balloon dilatation of the sphincter of Oddi has been used by some authors, but may cause hyperamylasemia or frank pancreatitis.54 While this technique may occasionally be successful, it is not widely recommended.
Passage of the choledochoscope into the duodenum is similarly a potentially hazardous practice and should only be used when necessary to gently push debris into the duodenum, or when the orifice into the duodenum is widely patent, such as after preoperative sphincterotomy or intraoperative intravenous glucagon administration.
Pancreatitis may present postoperatively with excessive abdominal or back pain, fever, ileus, anorexia, or failure to thrive. The diagnosis may be confirmed with amylase or lipase measurements. CT scanning of the abdomen may be necessary if the patient does not improve with intravenous fluids, NPO status, and NG suction. Antibiotics may be required if pancreatic abscess is suspected or confirmed with imaging studies.
RESULTS
General
Thousands of successful laparoscopic common bile duct explorations have been reported since the introduction of laparoscopic cholecystectomy in the late 1980s. During this time techniques have evolved that enhance the likelihood of success of the procedure. In experienced hands successful ductal clearance rates exceed 90%.3,17,18,24,33,42,79,82-84,132-139 Morbidity rates have been low in these series. Mortality has occurred in less than 1% of patients.
Access Route
Most laparoscopists have generally preferred the transcystic route for ductal exploration when it is feasible. In most series it is successful in 80 to 90% of cases.42,135,136 In some authors’ experience, Franklin for example, the type and size of the ductal stones dictates the need for a transductal approach in approximately 90 % of cases.139 As discussed above, there are well-defined criteria that should lead a surgeon to one or the other approach.
Operative Times
Laparoscopic choledocholithotomy takes longer than straightforward laparoscopic cholecystectomy. Assuming that mean operative time for laparoscopic cholecystectomy in less than one hour, it appears that LCDE adds approximately one hour or more to the procedure time. Interestingly, this added time is not solely due to technical manipulations, but includes equipment set up time, and often the need to perform additional surgery. Additionally, these patients are often older, with more chronic changes in the tissues in the porta hepatis, making dissection more difficult.3,42,45,46,50,52,135,136,139
Length of Stay
Whereas the length of stay (LOS) for laparoscopic cholecystectomy is generally less than 24 hours, the LOS for patients undergoing LCDE ranges from 1.3 to 7 days, depending on the severity of the disease, co-morbid factors, access route, whether or not a T-tube was placed, and whether or not a biliary enteric anastomosis was created. For transcystic LCDE, the mean length of stay is 1.5 days in many large series. Length of stay for LCDE via choledochotomy is generally longer than that for the transcystic approach.
Complications
Morbidity associated with LCDE occurs in approximately 8 to 10% of patients, and includes those problems typically associated with general surgery and laparoscopy: nausea, diarrhea, ileus, ecchymosis, atelectasis, fever, phlebitis, urinary retention, urinary tract infection, wound infection/inflammation, biliary leak, dislodged T-tube, sub-hepatic fluid collection, pulmonary embolus, and myocardial infarction. It is generally believed that the incidence of complications is less with a laparoscopic approach than an open approach to common bile duct stones.
Mortality associated with LCDE is zero to 1% in the hands of experienced laparoscopic biliary tract surgeons. This incidence is similar to that found in open surgery and relates more to the general health status of these patients than to laparoscopic common bile duct exploration.
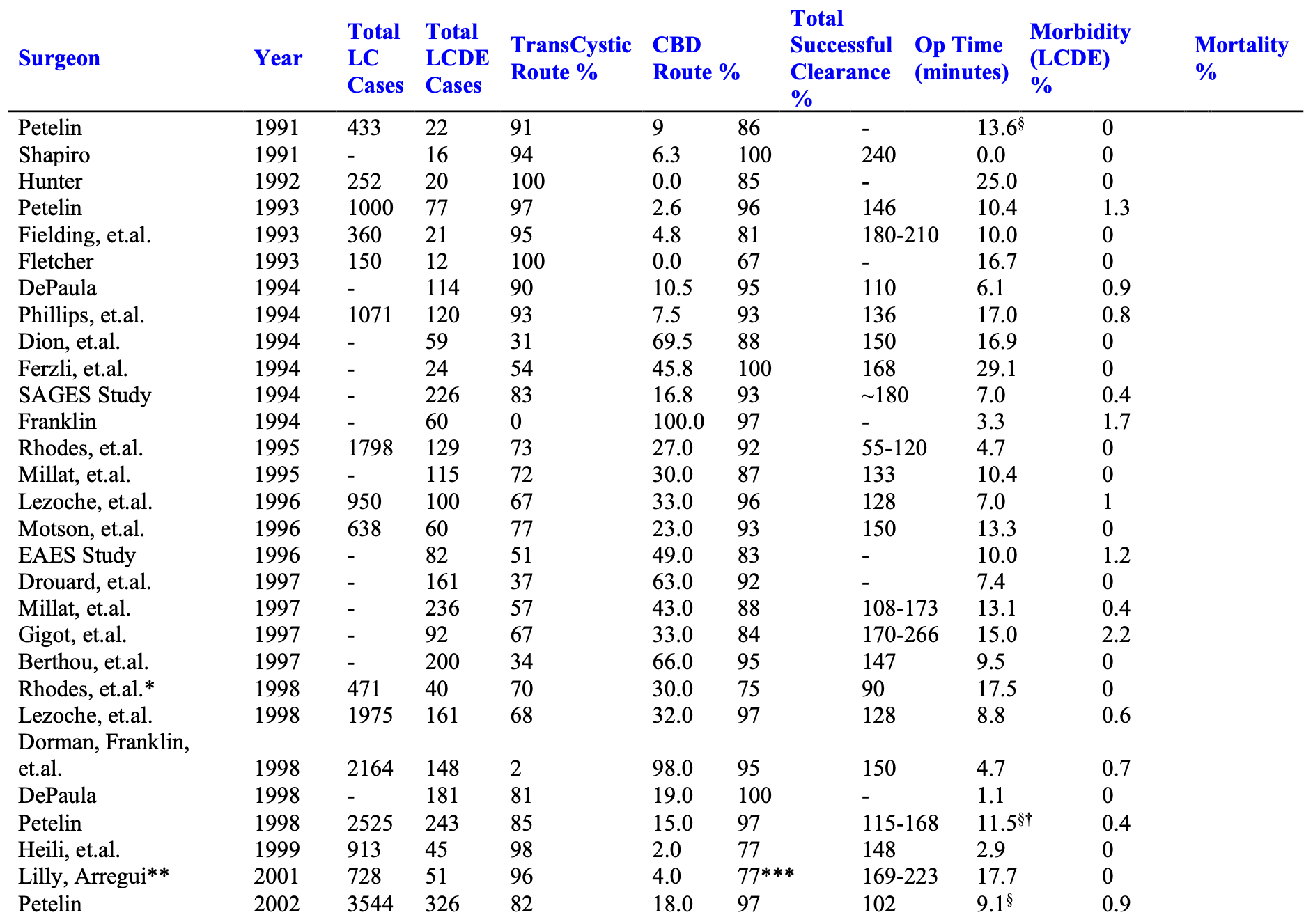
The Author’s Experience
The experience of many advanced biliary tract surgeons is shown in Table 3.
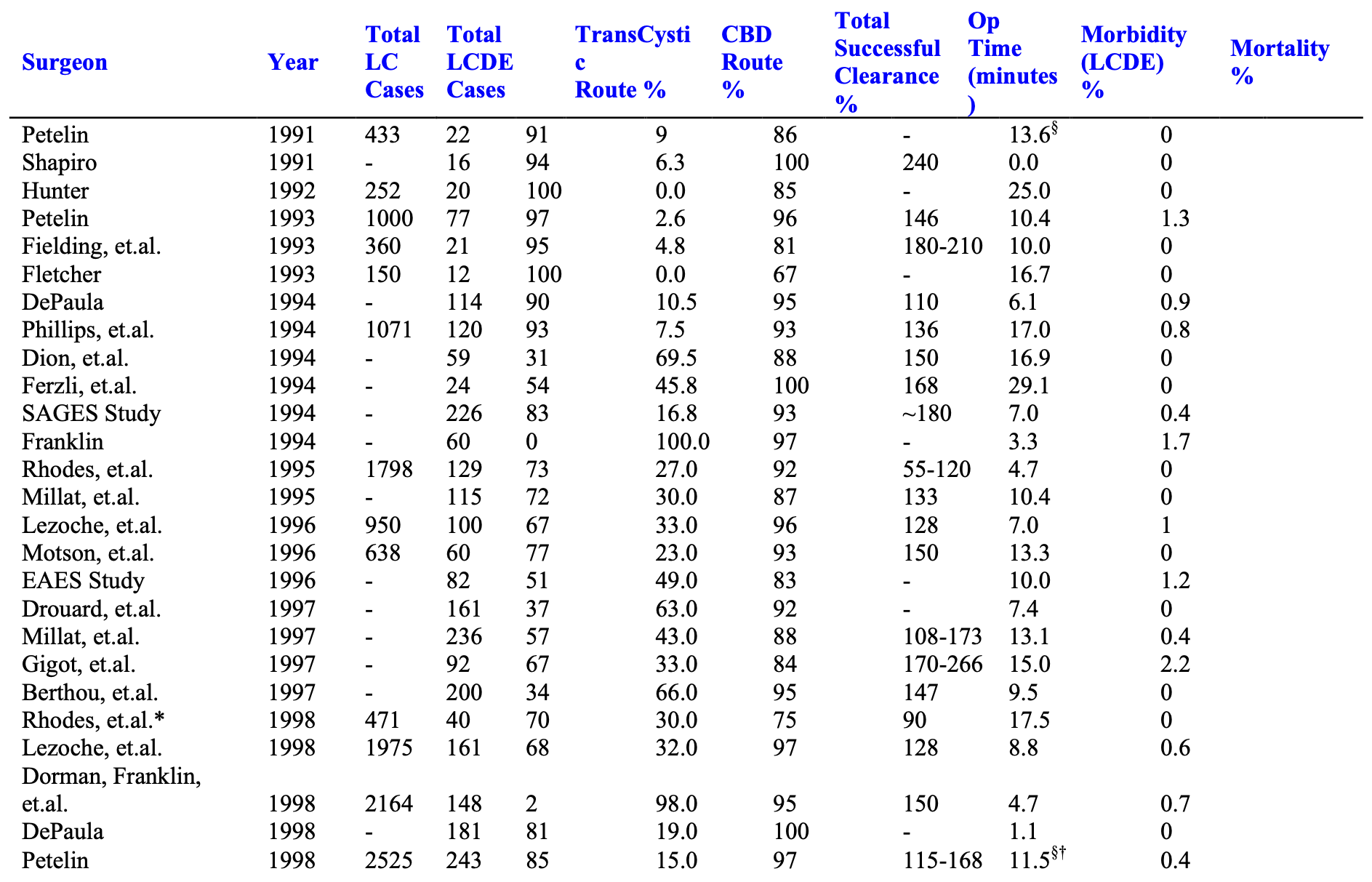
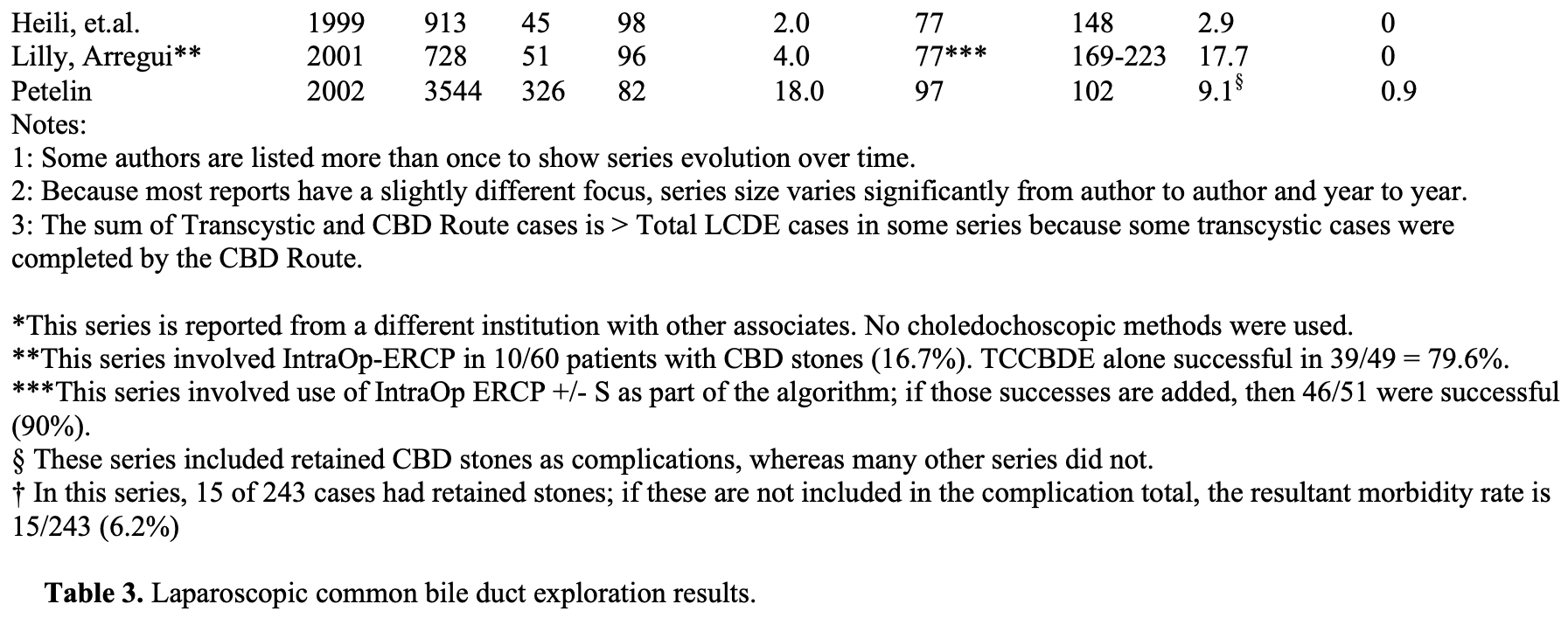
From September 21, 1989 through the present, over 5,000 patients presented to the senior author with symptomatic biliary tract disease. Laparoscopic cholecystectomy (LC) was attempted in over 99%, and completed in over 99.5%. Laparoscopic cholangiograms (IOC) were performed in 98%. Over 9.5% were abnormal. Pre-operative ERCP was performed in approximately 1.4% of patients and 0.9% underwent postoperative ERCP. Laparoscopic common bile duct exploration (LCDE) was attempted and completed in 98.5%. It was successful in clearing the duct in 97% of patients with abnormal cholangiograms.
Mean operating times for all patients undergoing laparoscopic cholecystectomy with or without cholangiograms or common bile duct exploration (LCDE) or other additional surgery was 55 minutes. Mean length of stay was 22 hours. Mean operating times for “LC only” patients, not undergoing LCDE or any other additional procedure, was 45 minutes, and mean post operative length of stay was 17 hours.
Ductal exploration was performed via the cystic duct in 82% of cases, and through a choledochotomy in 18% of the cases. T-Tubes were used in patients in whom there was concern for possible retained debris or stones, distal spasm, pancreatitis, or general poor tissue quality secondary to malnutrition or infection.
In cases where choledochotomy was used, a T-Tube was placed in 67%, and primary closure without a T-Tube occurred in 33%. There were no duct-related complications in either group.3
SUMMARY
Since 1990, surgeons throughout the world have developed a comprehensive laparoscopic solution to the problem of common bile duct stones. The success rate among accomplished laparoscopic surgeons approaches 90% or better. This compares favorably with treatment expectations in the pre-laparoscopic era, and addresses Perissat’s challenge, “We must move towards a management policy …, which prevents patients from needing a dangerous and debilitating second operation…”16
Biliary tract surgeons practicing in this era should have the ability to treat all benign biliary tract pathology laparoscopically in one setting, not requiring a series of patient manipulations.
References
- Nahrwold D. The biliary system. In: Sabiston X, ed. Textbook of surgery. Philadelphia: WE Saunders, 1986.
- Way LW, Admirand WJ, Dunphy JE: Management of choledocholithiasis. Ann Surg. 1972; 176: 347-359.
- Petelin JB. Laparoscopic common bile duct exploration: Lessons learned from > 12 years experience. Surg Endosc. 2003, Nov: 17(11): 1705-15.
- Beal J. Historical perspective of gallstone disease. Surg Gynecol Obstet. 1984; 158: 181-9.
- Escat J, Fourtanier G, Maigne C, Vaislic C, Fournier D, Prevost F. Choledochoscopy in common bile duct surgery for choledocholithiasis: a must. Am Surg. 1985: 51 (3): 166-7.
- Fink AS. Current dilemmas in management of common duct stones, Surg Endosc. 1993, 7: 285-91
- Pappas TN, Slimane TB, Brooks DC. 100 consecutive common duct explorations without mortality. Ann Surg. 1990, 211: 260-62.
- Fink AS. To ERCP or not to ERCP? That is the question. Surg Endosc. 1993, 7: 375- 376.
- Newman KD, Powell DM, Holcomb III GW. The Management of Choledocholithiasis in Children in the Era of Laparoscopic Cholecystectomy. J Ped Surg. 1997: 32 ( 7): 1116-1119.
- Cox MR, Wilson TG, Toouli J. Peroperative endoscopic sphincterotomy during laparoscopic cholecystectomy for choledocholithiasis. Br J Surg. 1995: 82: 257-259.
- Petelin J. Laparoscopic common bile duct exploration: evolution of a protocol which minimizes the need for ERCP. Abstract & paper presented at SAGES ‘94-Nashville, April 18, 1994. Submitted for publication to SurgEndosc.
- Traverso LW, Roush TS, Koo K. CBD stones—outcomes and costs: Laparoscopic transcystic techniques other than choledochoscopy. Surg Endosc. 1995: 1242-1244.
- Liberman MA, Phillips EH, Carroll BJ, Fallas MJ, Rosenthal R, Hiatt J. Cost- Effective Management of Complicated Choledocholithiasis: Laparoscopic Transcystic Duct Exploration or Endoscopic Sphincterotomy. J Am Coll Surg. 1996: 182: 488-494.
- Phillips EH. Laparoscopic transcystic duct common bile duct exploration—outcome and costs. Surg Endosc. 1995: 1240-1242.
- Traverso LW. A cost-effective approach to the treatment of common bile duct stones with surgical versus endoscopic techniques. 1996. In Berci G, Cuschieri A (ed): Bile Ducts and Bile Duct Stones. Philadelphia, PA, WB Saunders, pp 154-160.
- Perissat J, Huibregtse K, Keane FV, Russell CG, Neoptolemos JP. Management of bile duct stones in the era of laparoscopic cholecystectomy. BJS. 1994: 799-810.
- Petelin J: Laparoscopic approach to common duct pathology. Surg Lap & Endosc. 1991, 1:33-41.
- Hunter J: Laparoscopic transcystic common bile duct exploration. Am J Surg. 1992, 163:53-58.
- Carroll BJ, Phillips EH, Daykhovsky L, Grundfest WS, Gersham A, Fallas M, Chandra M: Laparoscopic choledochoscopy: an effective approach to the common duct. Journal of Laparoendoscopic Surgery. 1992, 2:15-21.
- Sackier JM, Berci G, Pas-Partlow M. Laparoscopic transcystic choledochotomy as an adjunct to laparoscopic cholecystectomy. The American Surgeon. 1991, 57: 323-326.
- Jacobs M, VCerdeja JC, Goldstein HS. Laparoscopic choledocholithotomy. Jnl Laparoendoscopic Surgery. 1991, 1:2: 79-82.
- Quattlebaum JK, Flanders HD. Laparoscopic treatment of common bile duct stones. Surg Lap & Endosc. 1991, 1:1:26-32.
- Appel S, Krebs H, Fern D. Techniques for laparoscopic cholangiography and removal of common duct stones. Surg Endosc. 1992, 6: 134-137.
- Birkett D. Technique of cholangiography and cystic-duct choledochoscopy at the time of laparoscopic cholecystectomy for laser lithotripsy. Surg Endosc. 1992, 6: 252- 254.
- Fletcher D, Jones RM, O’Riordan B, Hardy KJ. Laparoscopic cholecystectomy for complicated gallstone disease. Surg Endosc. 1992, 6: 179-182.
- Dion YM, Morin J, Dionne G, Dejoie C. Laparoscopic cholecystectomy and choledocholithiasis. CJS. 1992, 35: 67-74.
- Arregui M, Davis CJ, Arkush AM, Nagan RF. Laparoscopic cholecystectomy combined with endoscopic sphincterotomy and stone extraction or laparoscopic choledochoscopy and electrohydraulic lithotripsy for management of cholelithiasis with choledocholithiasis. Surg Endosc. 1992, 6: 10-15.
- Cotton PB. Endoscopic management of bile duct stones (apples and oranges). Gut. 1984, 25: 587-597.
- Carr-Locke DL. Acute gallstone pancreatitis and endoscopic therapy. Endoscopy. 1990, 22: 180-183.
- Vitale GC, Larson GM, Wieman TJ, Cheadle WG, Miller FB. The use of ERCP in the management of common bile duct stones in patients undergoing laparoscopic cholecystectomy. Surg Endosc. 1993, 7: 9-11.
- Fanning NF, Horgan PG, Keane FBV. Evolving management of common bile duct stones in the laparoscopic era. J R Coll Surg Endinb. 1997: 389-394.
- Heili MJ, Wintz NK, Fowler DL. Choledocholithiasis: Endoscopic versus Laparoscopic Management. Am Surg. 1999: 65: 135-138.
- Newman KD, Powell DM, Holcomb III GW. The Management of Choledocholithiasis in Children in the Era of Laparoscopic Cholecystectomy. J Ped Surg. 1997: 32 (7): 1116-1119.
- Rhodes M, Sussman L, Cohen L, Lewis MP. Randomised trial of laparoscopic exploration of common bile duct versus postoperative endoscopic retrograde cholangiography for common bile duct stones. Lancet. 1998: 351: 159-161.
- Arregui ME, Navarrete JL, Davis CJ, Hammond JC, Barteau J. The Evolving Role of ERCP and Laparoscopic Common Bile Duct Exploration in the Era of Laparoscopic Cholecystectomy. Int Surg. 1994: 79: 188-194.
- Croce E, Faggioni a, Jakimowicz J, lacy A, Lezoche E, Morino M, Ribeiro VM, Toouli J, Visa J, Wayand W. EAES ductal stone study: Preliminary findings of multi- center prospective randomized trial comparing two-stage vs. single-stage management. Surg Endosc. 1996: 10: 1130-1135.
- Bonatsos G, Leandros E, Polydorou A, Romanos A, Dourakis N, Birbas C, Golematis B. ERCP in association with laparoscopic cholecystectomy. Surg Endosc. 1996: 10: 37-40.
- Phillips EH, Liberman M, Carroll BJ, Fallas MJ, Rosenthal RJ, Hiatt JR. Bile Duct Stones in the Laparoscopic Era: Is Preoperative Sphincterotomy Necessary? Arch Surg. 1995: 130: 880-886.
- Frazee RC, Roberts J, Symmonds R, Hendricks JC, Snyder S, Smith R, Custer MD, Stoltenberg P, Avots A. Combined Laparoscopic and Endoscopic Management of Cholelithiasis and Choledocholithiasis. Am J Surg. 1993: 166: 702-706.
- Rieger R, Sulzbacher H, Woisetschlager R, Schrenk P, Wayand W. Selective Use of ERCP in Patients Undergoing Laparoscopic Cholecystectomy. World J Surg. 1994: 18: 900-905.
- Francheschi D, Brandt C, Margolin D, Szopa B, Ponsky J, Priebe P, Stellato T, Eckhauser ML. The Management of Common Bile Duct Stones in Patients Undergoing Laparoscopic Cholecystectomy. Am Surg. 1993: 59: 525-532.
- Petelin J. Laparoscopic approach to common duct pathology. Am J Surg. 1993, 165: 487-491.
- Franklin ME, Pharand D, Laparoscopic common bile duct exploration. Surg Lap & Endosc. 1994, 4(2): 119-124.
- Phillips E, Carroll BJ, Pearlstein R, Daykhovsky L, Fallas MJ, Laparoscopic choledochoscopy and extraction of common bile duct stones. World J Surg. 1993, 17: 22- 28.
- Drouard F.,Passone-Szerzyna N., Berthou JC. Laparoscopic Treatment of Common Bile Duct Stones, Hepato-Gastroenterology. 1997: 44: 16-21.
- Millat B, Atger J, Deleuze A, Briandet H, Fingerhut A, Guillon F, Marrel E, DeSeguin C, Soulier P. Laparoscopic Treatment for Choledocholithiasis: a Prospective Evaluation in 247 Consecutive Unselected Patients, Hepato-Gastroenterology. 1997: 44: 28-34.
- Helms B, Czarnetzki HD. Strategy and Technique of Laparoscopic Common Bile Duct Exploration. End Surg. 1993: 1: 117-124.
- Arvidsson D, Berggren U, Haglund U. Laparoscopic Common Bile Duct Exploration. Eur J Surg. 1998: 164: 369-375.
- Paganini AM, Lezoche E. Follow-up of 161 unselected consecutive patients treated laparoscopically for common bile duct stones. Surg Endosc. 1998: 12: 23-29.
- Martin IJ, Bailey IS, Rhodes M, O’Rourke N, Nathanson L, Fielding G. Towards T- Tube Free Laparoscopic Bile Duct Exploration: A Methodologic Evolution During 300 Consecutive Procedures. Ann Surg. 1998: 228 (1):29-34.
- Dorman JP, Franklin ME, Glass JF. Laparoscopic common bile duct exploration by choledochotomy: An effective and efficient method treatment of choledocholithiasis. Surg Endosc. 1998: 12: 926-928.
- Rhodes M, Nathanson L, O’Rourke N, Fielding G. Laparoscopic exploration of the common bile duct: lessons learned from 129 consecutive cases. Br J Surg. 1995: 82: 666-668.
- Millat B, Fingerhut A, Deleuze A, Briandet H, Marrel E, DeSeguin C, Soulier P. Prospective evaluation in 121 consecutive unselected patients undergoing laparoscopic treatment of choledocholithiasis. Br J Surg. 1995: 82: 1266-1269.
- Carroll BJ, Phillips EH, Rosenthal R, Liberman M, Fallas M. Update on Transcystic Exploration of the Bile Duct. Surg Lap & Endosc. 1996: 6 (6): 453-458.
- Lezoche E, Paganini AM, Carlei F, Feliciotte F, Lomanto D, Guerrieri M. Laparoscopic Treatment of Gallbladder and Common Bile Duct Stones: A Prospective Study. World J Surg. 1996: 20: 535-542.
- Swanstrom LL, Marcus DR, Kenyon MT. Laparoscopic treatment of known choledocholithiasis. Surg Endosc. 1996: 10: 526-528.
- Ferzli GS, Massaad A, Kiel T, Worth MH. The utility of laparoscopic common bile duct exploration in the treatment of choledocholithiasis. Surg Endosc. 1994: 8: 296-298.
- Berci G, Morgenstern Laparoscopic management of common bile duct stones: a multi-institutional study. Surg Endosc. 1994: 8: 1168-1175.
- DePaula AL, Hashiba K, Bafutto M. Laparoscopic management of choledocholithiasis. Surg Endosc, 1994: 8: 1399-1403.
- Gigot JF, Navez B, Etienne J, Cambier E, Jadoul P, Guiot P, Kestens PJ. A stratified intraoperative surgical strategy is mandatory during laparoscopic common bile duct exploration for common bile duct stones. Surg Endosc. 1997: 11: 722-728.
- Stoker M. Common Bile Duct Exploration in the Era of Laparoscopic Surgery. Arch Surg. 1995: 130: 265-269.
- Khoo DE, Walsh CJ, Cox MR, Murphy CA, Moston RW. Laparoscopic common bile duct exploration: evolution of a new technique. Br J Surg. 1996: 83: 341-346.
- Kelley WE, Sheridan VC. Laparoscopic choledochoscopy with a small-caliber endoscope. Surg Endosc. 1995: 9: 293-296.
- Dion YM, Ratelle R, Morin J, Gravel D. Common Bile Duct Exploration: The Place of Laparoscopic Choledochotomy. Surg Lap & Endosc. 1994: 4 (6): 419-424.
- O’Doherty D, Neoptolemos J, Carr-Locke D. Endoscopic sphincterotomy for retained common bile duct stones in patients with T-tube in situ in the early postoperative period. Br J Surg. 1986; 73: 454-6.
- Broughman T, Sivak M, Herman R. The management of retained and recurrent bile duct stones. Surgery. 1985: 98(4): 746-51.
- Petelin J. Clinical results of common bile duct exploration. Endoscopic Surgery and Allied Technologies. Gerog Thieme Verlag Publishers, Stuttgart-New York. 1993, 1:3: 125-129.
- Martin IG, Curley P, McMahon MJ. Minimally invasive treatment for common bile duct stones. Br J Surg. 1993: 80: 103-106.
- Petelin J. The argument for contact laser laparoscopic cholecystectomy. Clin Laser Monthly. 1990, 71-74.
- Petelin J. Laparoscopic common duct exploration. American College of Surgeons Motion Picture Library 1992, 16 minute comprehensive video presented at ACS Meeting October, 1992 and selected for addition to the library of the college.
- Swanstrom Laparoscopic approaches to the common bile duct stone: transcystic bile duct exploration, choledochotomy and stone fragmentation. Bailliere’s Clinical Gastroenterology. 1993: 7(4): 897-919.
- Kitano S, Iso Y, Moriyama M, Sugimachi K. A rapid and simple technique for insertion of a T-tube into the minimally incised common bile duct at laparoscopic surgery. Surg Endosc. 1993, 7: 104-105.
- Mooney MJ, Deyo G, O’Reilly MJ. T-tube placement during laparoscopic cholecystectomy. Surg Endosc. 1992, 6: 32.
- Gersin KS, Fanelli RD. Laparoscopic endobiliary stenting as an adjunct to common bile duct exploration. Surg Endosc. 1998: 12: 301-304.
- Rhodes M, Nathanson L, O’Rourke N, Fielding G. Laparoscopic Antegrade Biliary Stenting. Endoscopy. 1995: 27: 676-678.
- DePaula AL, Hashiba K, Bafutto M, Machado C, Ferrari A, Machado MM. Results of the routine use of a modified endoprosthesis to drain the common bile duct after laparoscopic choledochotomy. Surg Endosc. 1998: 12:933-935.
- Hensman C, Crosthwaite G, Cuschieri A. Transcystic biliary decompression after direct laparoscopic exploration of the common bile duct. Surg Endosc. 1997: 11: 1106- 1110.
- Fitzgibbons RJ, Ryberg AA, Ulualp KM, Nguyen NX, Litke BS, Camps J, McGinn TR, Jenkins JX, Filipi CJ. An Alternative Technique for Treatment of Choledocholithiasis Found at Laparoscopic Cholecystectomy. Arch Surg. 1995: 130: 638-642.
- Petelin JB. Laparoscopic Ductal Stone Clearance: Transcystic Approach, in Bile Ducts and Bile Duct Stones, Berci G & Cuschieri A, eds. 97-108, WB Saunders, Philadelphia, 1996.
- Reddick EJ, Olsen DO, DAniell JF, Saye WB, McKernan B, Miller W, Hoback Laparoscopic laser cholecystectomy. Laser Medicine & Surgery News and Advances. 1989, 38-40.
- Klaiber C, Metzger A, Petelin J. Laparoscopic cholecystectomy. Manual of Laparoscopic Surgery, Verlag Hans Huber Publishers 1993, 97-148.
- Ferzli GS, Massaad A, Ozuner G, Worth MH. Laparoscopic exploration of the common bile duct. S G & O. 1992, 174: 419-421.
- Stoker ME, Leveillee RJ, McCann JC, Maini BS. Laparoscopic common bile duct exploration. Journal of Laparoendoscopic Surgery. 1991, 1:5: 287-293.
- DePaula AL, Hashiba K, Bafutto M, Zago R, Grecco E. Laparoscopic treatment of choledocholithiasis. Surg Lap & Endosc. 1993, 3: 157-60.
- Gagner M, Deslandres E, Pomp A, Rheault M, Potvin C, Leduc R. Double endoscopy: the role of intraoperative ERCP during laparoscopic cholecystectomy for choledocholithiasis. Proceedings–III International Congress of Laparoscopic Surgery- Brazil 1993, 126-127.
- Tarnasky PR, England RE, Lail LM, et.al. Cystic duct patency in malignant obstructive jaundice. An ERCP-based study relevant to the role of laparoscopic cholecystojejunostomy. Ann Surg. 1995 Mar:221(3):265-71.
- Schob OM, Schmid RA, Morimoto AK, et.al. Laparoscopic Roux-en-Y choledochojejunostomy. Am J Surg. 1997 Apr: 173(4):312-9.
- Farello GA, Cerofolini A, Bergamaschi G, et.al. Choledochoduodenal anastomosis by laparoscopy. J Chir Paris. 1993 May: 130(5): 226-30.
- Rhodes M, Nathanson Laparoscopic choledochoduodenostomy. Surg Laparosc Endosc. 1996 Aug:6(4):318-21.
- Williams JAR, Treacy PJ, Sidey CS, et.al. Primary duct closure versus T-tube drainage following exploration of the common bile duct. Aust NZ J Surg. 1994. 64:823- 826.
- Lezoche E, Paganini A, Feliciotte F, Chan R. Laparoscopic Suture Technique After Common Bile Duct Exploration. Surg Lap & Endosc. 1993: 3 (3): 209-212.
- Bernstein DE, Goldberg RI, Unger SW. Common bile duct obstruction following T- tube placement at laparoscopic cholecystectomy. GastroIntest Endosc. 1994. 40(3): 362- 365.
- Elewaut A, de Vos, M, Huble F, de Cock J. Unusual migration of a straight Amsterdam-type endoprosthesis for bile duct stones. Am J Gastroenterol. 1989. 84(6): 674-676.
- Kram HB, Garces MA, Klein SR, Shoemaker WC. Common bile duct anastomosis using fibrin glue. Arch Surg. 1985. 120: 1250-1256.
- Lange V, Rau HG, Schardey HM, Meyer G. Laparoscopic stenting for protection of common bile duct sutures. Surg Lap & Endosc. 1993. 3(6): 466-469.
- Kelly TR, Fink JA. A new inflatable T-tube for completion cholangiography. SG & O. 1983. 157(4): 374-376.
- Jacob ET, Bronsther B. A double ballooned inflatable and collapsible T-tube for selective proximal or distal cholangiography. SG & O. 1988. 166: 85-86.
- Fine AP. Laparoscopic, wire-guided insertion of biliary T-tubes. Surg Lap & Endosc, 1993. 3(2): 147-148.
- Lezoche E, Paganini AM, Guerrieri M. A new T-tube applier in laparoscopic surgery. Surg Endosc. 1996. 10: 445-448.
- Lygidakis NJ. Choledochotomy for biliary lithiasis: T-tube drainage or primary closure-Effects on postoperative bacteremia and T-tube bile infection. Am J Surg. 1983. 146:254-256.
- Lygidakis NJ. Operative risk factors of cholecystectomy-choledochotomy in the elderly. SG&O. 1983. 157:15-19.Payne RA, Woods WGA.
- Lygidakis NJ. Incidence of bile infection in biliary lithiasis. Effects on postoperative bacteremia of choledochoduodenostomy, T-tube drainage, and primary closure of the common bile duct after choledochotomy–a prospective trial. Am Surg. 1984. 50: 236-240.
- Koivusalo A, Makisalo H, Talja A, et.al. Bacterial adherence and biofilm formation on latex and silicone T-tubes in relation to bacterial contamination of bile. Scand J Gastroenterol. 398-403.
- Horgan PG, Campbell AC, Gray GR, Gillespie G. Biliary leakage and peritonitis following removal of T tubes after bile duct exploration. Br J Surg. 1989. 76: 1296- 1297.
- Galan CP, Alonso AC. Bile leakage after removal of T tubes from the common bile duct. Br J Surg. 77(9): 1075.
- Ryttov N, Rasmussen L, Pedersen SA, Oster-Jorgensen E. 99m Tc-labelled HIDA scintigraphy in assessment of bile leakage after removal of T tube from the common bile duct. Br J Surg. 1989. 76: 1319.
- Thors H, Gudjonsson H, Oddsson E, Cariglia N. Endoscopic retrieval of a biliary T- tube remnant. GastroIntest Endosc. 1994. 40(2)(1): 241-242.
- Gillatt DA, May RE, Kenedy R, Longstaff AJ. Complications of T-tube drainage of the common bile duct. Ann Royal Coll Surg England. 1985. 67: 369-371.
- Norrby S, Heuman R, Anderberg B, Sjodahl R. Duration of T-tube drainage after exploration of the common bile duct. Acta Chir Scand. 1988. 154: 113-115.
- Cohen Z, Rosenman H, et. al. Transient elevation of serum alkaline phosphatase after choledochotomy and T-tube placement. J Clin Gastroenterol. 1986. 8(4): 495 496.
- Lygidakis NJ. Hazards following T-tube removal after choledochotomy. SG & O. 1993. 163: 153-155.
- Thors H, Gudjonsson H, Oddsson E, Cariglia N. Endoscopic retrieval of a biliary T- tube remnant. GastroIntest Endosc. 1994. 40(2)(1): 241-242.
- Shimi S, Banting Fracs S, Cuschieri A. Transcystic drainage after laparoscopic exploration of common bile duct. Minimally Invasive Therapy. 1992, 1: 273-276.
- Hensman C, Crosthwaite G, Cuschieri A. Transcystic biliary decompression after direct laparoscopic exploration of the common bile duct. Surg Endosc. 1997 Nov: 11(11): 1106-10.
- Payne RA, Woods WGA. Primary Suture or T-tube drainage after choledochotomy. Ann Royal Coll Surg Engl. 1986. 68:196-198.
- Shyr-Ming sheen-Cheng, Fong-Fu Chou. Choledochotomy for biliary lithiasis: Is routine T-Tube drainage necessary? A prospective controlled trial. Acta Chir Scand. 1990. 156:387-390.
- Petelin JB, Lechleitner RA. Laparoscopic choledochotomy without T-Tube drainage is effective and safe. Abstract and Poster. SAGES Annual Scientific Session, 1997.
- Martin IJ, Bailey IS, Rhodes M, O’Rourke N, Nathanson L, Fielding G. Towards T- tube free laparoscopic bile duct exploration: a methodologic evolution during 300 consecutive procedures. Ann Surg. 1998 Jul: 228(1): 29-34.
- Croce E,Golia M, Azzola M, et.al. Laparoscopic choledochotomy with primary closure. Follow-up (5-44 months) of 31 patients. Surg Endosc. 1996 Nov: 10(11): 1064- 8.
- Olsen DO. Bile duct injuries during laparoscopic cholecystectomy: a decade of experience. J Hepatobiliary Pancreat Surg. 2000: 7(1): 35-9.
- Olsen DO. Bile duct injuries during laparoscopic cholecystectomy. Surg Encosc. 1997 Feb: 11(2): 133-8.
- Carroll BJ, Birth M, Phillips EH. Common bile duct injuries that result in litigation. Surg Endosc. 1998 Apr: 12 (4): 310-3; discussion 314.
- Hookman P, Unger SW, Barkin JS. Laparoscopic cholecystectomy should be routinely performed with intraoperative cholangiography. Am J Gastroenterol. 2000 Nov: 95(11): 3299-302.
- Carroll BJ, Friedman RL, Liberman MA, Phillips EH. Routine cholangiography reduces sequelae of common bile duct injuries. Surg Endosc. 1996 Dec: 10(12): 1194-7.
- Kuster GG, Gilroy SB. Intraoperative trans-gallbladder cholangiography intended to delineate bile duct anatomy. J Laparoendosc Surg. 1995 Dec: 5(6): 377-84.
- Podnos YD Gelfand DV, Dulkanchainum TS, et.al. Is intraoperative cholangiography during laparoscopic cholecystectomy cost effective? Am J Surg. 2001 Dec: 182(6): 663-9.
- Macfadyen BV, Vecchio R, Ricardo AE, Mathis CR. Bile duct injury after laparoscopic cholecystectomy. The United States experience. Surg Endosc. 1998 Apr: 12(4): 315-21.
- Moore DE, Feurer ID, Holzman MD, et.al. Long-term Detrimental effect of Bile Duct Injury on Health-Related Quality of Life. Arch Surg. 2004: 139: 476-482.
- Sarmiento JM, Farnell MB, Nagorney DM, et.al. Quality of Life Assessment of Surgical Reconstruction After laparoscopic Cholecystectomy-Induced Bile Duct Injuries. What Happen at 5 Years and Beyond? Arch Surg. 2004: 139: 483-489.
- Peters JH, Ollila D, Nichols KE, et.al. Diagnosis and management of bile leaks following laparoscopic cholecystectomy. Surg Laparosc & Endosc. 1994 Jun: 4(3): 163- 170.
- Ponsky JL. Endoscopic approaches to common bile duct injuries. Surg Clin North Am. 1996 Jun: 76(3): 505-13.
- Shapiro SJ, Gordon LA, Daykhovsky L, et. al.. Laparoscopic Exploration of the Common Bile duct: experience in 16 selected patients. J Laparoendosc surg. 1991, (1)6: 333-41.
- Fielding GA, O’Rourke NA. Laparoscopic common bile duct exploration. Aust NZ J Surg. 1993, 63:113-115.
- Fletcher DR. Common bile duct calculi at laparoscopic cholecystectomy: a technique for management. Aust NZ J Surg. 1993, 63: 710-14.
- DePaula AL, Hashiba K, Bafutto M. Laparoscopic management of choledocholithiasis. Surg Endosc. 1994, 8: 1399-1403.
- Phillips EH, Rosenthal RJ, Carroll BJ, et.al.. Laparoscopic trans-cystic duct common bile duct exploration. Surg Endosc. 1994 8: 1389-1394.
- Dion YM, Ratelle R, Morin J, et.al.. Common bile duct exploration: the place of laparosocpic choledochotomy. Surg Lap & Endosc. 1994, (4)6: 419-424.
- Ferzli GS, Massaad A, Kiel T, et.al.. The utility of laparoscopic common bile duct exploration in the treatment of choledocholithiasis. Surg Endosc. 1994, 8: 296-298.
- Dorman JP, Franklin ME, Glass JL. Laparoscopic common bile duct exploration by choledochotomy: an effective and efficient method of treatment of choledocholithiasis. Surg Endosc. 1998: (12):926-928.
