Laparoscopic Bariatric Surgery
Vincent W. Vanek, MD, FACS, CNSP
HISTORY
Obesity has been increasing at epidemic proportions and results in an increase in mortality and morbidity and a decrease in quality of life.1-8 Obesity is defined and classified based on an individual’s body mass index or BMI (Table 1). The incidence of obesity (BMI > 30 or about 30 pounds overweight) in adults in the United States (US) from 1976 to 1980 was estimated at 14.5%.2 The Center for Disease Control (CDC) has been tracking the incidence of obesity in the US since 1985.3 Between 2005-2006, 34% of adults in the US were obese accounting for over 72 million people.1 In addition, in 2003-2004 17.1% of children and adolescents were overweight.10
Bariatric surgery refers to surgical procedures designed to help people lose weight. Bariatric surgery has been around for over 50 years and includes a large number of different surgical, laparoscopic, and endoscopic procedures (Table 2). The first bariatric surgical procedure, the jejunoileal (JI) bypass, was performed in 1953 by Dr. Richard Varco at the University of Minnesota. This procedure did not limit the amount of Calories the patient could ingest but simply created significant malabsorption, bypassing over 90% of the small intestine, such that many of the Calories ingested were not absorbed. Multiple variations of this procedure were developed and much experience was gained with this procedure over the next 20 years. However, although the weight loss results were dramatic and sustained, the JI bypass was also associated with a high incidence of complications due to malnutrition and various deficiency states and was associated with a significant long-term mortality. So it was finally abandoned in the mid to late 1970’s.11

Because of the significant risks of complications and even death with the JI bypass, the focus of bariatric surgery turned to gastric procedures in an attempt to limit the amount of Calories that one could ingest. In 1966, Dr. Edward Mason at the University of Iowa developed the gastric bypass. This procedure entailed transection of the upper portion of the stomach forming an 100 ml proximal gastric pouch that was then attached to a loop of jejunum anywhere from 20 to 150 cm below the ligament of Treitz. The procedure was designed to limit the individual’s food intake by restricting the size of the stomach and the size of the opening between the stomach and the jejunum. While this procedure did create some element of dumping syndrome and malabsorption, this was not a major mechanism in the weight loss with this procedure. With the subsequent advent of gastrointestinal (GI) staplers, some surgeons would simply staple across the top of the stomach separating it into a proximal and distal gastric pouch without dividing the stomach.11
However, Dr. Mason and others were still concerned about the potential long term adverse effects of the physiological changes created by the gastric bypass so in 1973 he described the gastroplasty procedure. This surgery entailed partially dividing the stomach horizontally leaving a small proximal gastric pouch above the staple line and a narrow, 10 to 12 mm, opening on the greater curvature of the stomach resulting in a purely restrictive procedure with no risk of dumping syndrome or malabsorption. Various different techniques of horizontal gastroplasties were developed over the next several years. Although the short term weight loss was good, proximal gastric pouch dilation, stomal opening dilation, and/or staple line disruption resulted in frequent reoperation and poor long term weight loss. In 1981, Dr. Henry Laws reported better results using a vertical stapled gastroplasty along with wrapping a silastic ring around the outside of the stomal outlet to decrease the risk of stomal dilation. Then in 1982, Dr. Mason modified this technique to include using a circular stapler to make a hole through the stomach near the lesser curvature, which allowed the horizontal stapling to be done more easily, and to wrap a 1.5 cm wide piece of polypropylene mesh around the stoma to limit the opening to 12 mm in diameter, the vertical banded gastroplasty (VBG). The vertical gastric pouch did not dilate like the horizontal pouch and the mesh prevented stomal dilation so the VBG had better long-term weight loss then the previous gastroplasty techniques.11,21 The VBG became the most popular purely restrictive procedure over the next 10 to 15 years until less invasive and lower risk restrictive procedures were developed.
In 1977, Dr. Ward Griffen from the University of Kentucky published a randomized control trial (RCT) comparing JI bypass and gastric bypass. However, he modified Dr. Mason’s gastric bypass to a Roux-en-Y gastric bypass (RYGB) in which the jejunum was transected about 30 cm below the ligament of Treitz and a 75 cm Roux limb was connected to the proximal gastric pouch, creating a modest amount of malabsorption in addition to the gastric restriction.11 This procedure has gone through numerous modifications since then but is still the most common bariatric surgical procedure performed in the US.
In 1978, Wilkinson and Peloso first placed a nonadjustable band, 2 cm wide piece of polypropylene mesh, around the upper stomach for a purely restrictive bariatric surgical procedure. Then in 1983, Hallberg and Forsell, in Sweden, developed a silastic ring with an inflatable balloon on its inner surface that could be connected to a subcutaneous port and the balloon adjusted to provide the desired amount of restriction on the stomach. This device was known as the Swedish Adjustable Gastric Band (SAGB). Simultaneously, Kuzmak, a Ukrainian surgeon working in the US, developed a similar band, known initially as the “American Band” and later on as the “Lap Band.” In 1993, the first adjustable gastric bands were placed laparoscopically and following this both the SAGB and the Lap Band were all placed laparoscopically. In 2001, the Lap Band was approved for use in the US. Subsequently, Ethicon Endo-Surgery, Inc. bought the rights to the SAGB and developed it into the next generation, the REALIZE band. This band was approved for use in the US in 2007. Ethicon released the REALIZE-C band, its next generation band, in 2009. The generic term used to refer to both of these gastric bands is laparoscopic adjustable gastric band (LAGB). LAGB was much less invasive, easier, and adjustable so by the mid to late 1990s, it replaced the VBG as the preferred purely restrictive bariatric surgical procedure.13
Dr. Nicola Scopinaro, an Italian surgeon, first reported on a “second-generation” malabsorptive procedure known as the biliopancreatic diversion (BPD) in 1979. This procedure entailed resection of about half of the stomach, causing some restriction in intake of Calories, but was mainly dependent on malabsorption by bypassing 85% to 90% of the small intestine.11,21-23 This procedure was similar to the JI bypass but there was no stasis in the bypassed segment.21 All of the small intestinal mucosa was in continuity with either food, biliopancreatic secretions, or both. This procedure had excellent and sustained weight loss results but had a significant risk of malnutrition and other deficiency states requiring close and careful long-term follow-up and monitoring for early diagnosis and treatment of these complications.11,21
The American Society for Bariatric Surgery (ASBS) was founded in 1983. Its vision was to improve public health and well-being by lessening the burden of the disease of obesity and related diseases throughout the world. It has been active in advancing the science and practice of bariatric surgery through education, improvement in clinical care, promoting research, and advocating for health care policies that ensure patient access to high quality prevention and treatment of obesity. In 2007, the ASBS changed its name to the American Society for Metabolic and Bariatric Surgery (ASMBS) to emphasize that these surgical procedures are not only to help people lose weight but are mainly designed for treating the metabolic diseases that frequently accompany obesity (Personal Communications with ASMBS).24
In 1998, Dr. Douglas Hess in Bowling Green, OH and Dr Picard Marceau from Quebec, Canada, separately published the first description of the BDP with duodenal switch (BPD/DS), a modification of the classical BPD procedure.17,18 Dr. Hess reported on his first 440 DS procedures performed, with the first procedure being performed in 1988. Dr. Marceau reported on his 465 DS patients being performed since 1990. This procedure also resected more than 50% of the stomach, leaving a approximately 150 ml gastric pouch. But the resection was performed by a vertical sleeve gastrectomy, resecting the left lateral half of the stomach rather than the distal half of the stomach. This allowed anastomosis for the small intestinal bypass to be performed distal to the pylorus, preserving the pylorus and decreasing the risk of dumping syndrome.
One of the first endoscopic procedures for weight loss was the endoluminal gastric balloon known as the Garren-Edwards bubble. It was approved in the US by the Food and Drug Administration (FDA) in 1985 but was subsequently removed from the market in 1988 due to poor patient tolerance and lack of efficacy. An improved version, Orbera™ (Allergan, Irvine, CA), was released in the mid-1990s and has been in use in Europe but has not been approved yet in the US.16
The National Institutes of Health (NIH) convened a consensus panel to review the medical necessity and indications for bariatric surgery and published a consensus statement in 1991.25 This panel concluded that bariatric surgery is an acceptable form of treatment for obesity in patients who meet specific criteria. The panel considered gastric bypass and VBG as the only two acceptable procedures that had “advanced beyond the experimental stage.” The report mentioned the BPD but stated “Experience with this procedure in the United States is limited.” The report outlined the criteria for patients who are candidates for these procedures as follows:
- “Those patients judged by experienced clinicians to have a low probability of success with non-surgical measures, as demonstrated for example by failures in established weight control programs or reluctance by the patient to enter such a program, may be considered for surgery.”
- “A gastric restrictive or bypass procedure should be considered only for well-informed and motivated patients with acceptable operative risks. The patient should be able to participate in treatment and long-term follow-up.”
- “Patients whose BMI exceeds 40 are potential candidates for surgery if they strongly desire substantial weight loss, because obesity severely impairs the quality of their lives. They must clearly and realistically understand how their lives may change after operation. In certain instances less severely obese patients (with BMI between 35 and 40) also may be considered for surgery. Included in this category are patients with high-risk co-morbid conditions such as life-threatening cardiopulmonary problems (e.g., joint disease treatable but for the obesity, or body size problems precluding or severely interfering with employment, family function, and ambulation).”25
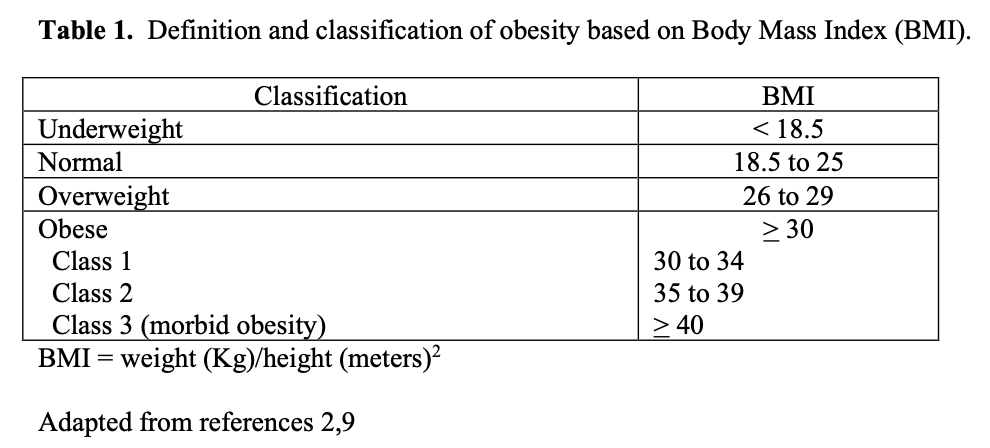
The NIH has not updated this consensus statement since then although there has been much more data collected and published on these and many other bariatric surgical procedures developed since that time. With the validation of bariatric surgery by the NIH consensus statement, the number of members in the ASMBS increased exponentially over the next 18 years from 177 in 1992 to 3,058 in 2008. Based on these membership numbers, the ASMBS estimates that the number of bariatric surgical procedures has also increased dramatically from about 16,200 in 1992 to about 220,000 in 2008 (Figure 1).
In 1992, Dr. Robert Brolin in New Brunswick, NJ published a RCT showing that a RYGB using a 150 cm Roux limb, thus creating more malabsorption, had better weight loss results then when the standard 75 cm limb was used.11 Subsequently, many surgeons have used the 150 cm limb routinely. Also, the size of the proximal gastric pouch has been steadily decreasing. Currently most surgeons aim for a gastric pouch of 10 to 20 ml in size instead of the original 30 to 50 ml pouches.
In 1994, Dr. Alan Wittgrove from San Diego, CA reported the first laparoscopic RYGB (lap RYGB).11,26 In 1995, Cigaina in Italy began implanting gastric pacing devices with the purpose of decreasing hunger and assisting patients in losing weight. Two electrodes were anchored in the wall of the stomach and connected to an electrical generator, similar to a cardiac pacemaker, that was placed in a subcutaneous pocket in the anterior abdominal wall. His initial results were subsequently published in 2002 and showed modest weight loss results.27 Clinical trials were subsequently performed in the US but the weight loss results were less than ideal and enthusiasm for this procedure has declined.
In 2008, the results of the first 31 patients utilizing a new implantable electrical device, VBLOC vagal blocking therapy, were published.14 Laparoscopically, the anterior and posterior vagus nerves are isolated and each is surrounded by an electrode and the electrodes are connected to an electrical generator placed in a subcutenous pocket in the anterior abdominal wall. The electrical stimulation blocks conduction of the vagus nerve. The electrical stimulation is alternated on for 5 minutes then off for 5 minutes and the stimulator is turned on for 12 hours during the day and off for 12 hours at night. Patients experienced decrease in hunger, decrease in Caloric intake, and modest weight loss.
Another trial involving 27 patients used a slightly different protocol, 3 minutes on and 5 minutes off for 12 hours a day, and showed significantly greater but still somewhat modest weight loss.28 This procedure remains under investigation.
In 1999, Gagner performed the first laparoscopic BPD/DS (lap BPD/DS).19,22 In 2003, in an attempt to reduce morbidity and mortality, in super (BMI 50-59) and super-super (BMI > 60) morbidly obese patients, the lap BPD/DS was performed as a two stage operation. The laparoscopic sleeve gastrectomy (LSG) was performed first with plans to perform the intestinal bypass part of the procedure laparoscopically in 6 to 12 months after the patient had lost some weight from the sleeve gastrectomy alone. However, many of these patients had such good weight loss results after the first stage of the procedure that either the patient or their insurance company would not approve of proceeding with the second stage.20,29-35 So some bariatric surgeons are utilizing LSG as a primary bariatric procedure. However, the long-term weight loss data is limited and some bariatric surgeons are concerned about weight regain with a purely restrictive procedure and a relatively large pouch that can dilate. For this reason, some advocate to use smaller sizing bouges and start closer to the pylorus in order to make a much smaller gastric pouch when using LSG as a primary operation.20,29,35
In 2002, Dr. Brolin reported on the distal RYGB for “super obese” patients, BMI > 50. The entero-enterostomy (EE) for the distal RYGB was performed 75 cm proximal to the ileocecal valve rather than 75 cm or 150 cm below the ligament of Treitz. In a RCT, he showed that the distal RYGB had significantly better peak weight loss and maintenance of weight loss compared to either the 75 cm or 150 cm limb RYGB. However, the distal RYGB has malabsorption similar in magnitude of that with the BPD or BPD/DS and therefore also had a higher incidence of malnutrition and other deficiency states compared to the standard RYGB.12
In 2003, the ASMBS started an independent not-for-profit company called Surgical Review Corporation (SRC) to designate hospitals, bariatric surgery programs, and bariatric surgeons as Bariatric Surgery Centers of Excellence (BSCOE) and Bariatric Surgeons of Excellence. This designation process began in 2004 (Communications with SRC). Simultaneously, the American College of Surgeons (ACS) developed a similar center of excellence program called the Bariatric Surgery Center Network (ACS BSCN) Accreditation Program.36 One of the requirements for designation as an ASMBS BSCOE is that the program must submit all of its bariatric surgery patient data to the Bariatric Outcomes Longitudinal Database (BOLD), which was established and is managed by the SRC. This requires Institutional Review Board (IRB) approval at each BSCOE participant program, and patients have to sign consent as to whether or not their data submitted to BOLD can be used for research purposes. Data began to be entered into BOLD in June 2007 and as of June 2009, over 450 BSCOE participant facilities had submitted data on over 129,000 patients into the database. Of these, about 64,000 patients were available for analysis of their data for research purposes (personal communication with SRC). The first analysis of the clinical data in BOLD was presented at the ASMBS 26th Annual Meeting on June 24, 2009, Grapevine Texas (Demerit EJ. Baseline Data from ASMBS-Designated Bariatric Surgery Centers of Excellence using the Bariatric Outcomes Longitudinal Database).
In 2007, Gersin reported on an investigational endoscopic bariatric procedure, EndoBarrier™ GI liner. A 60 cm long plastic sleeve was inserted endoscopically anchoring the proximal end to the duodenum just distal to the pylorus and extending the sleeve for 60 cm through the duodenum and jejunum. This created a physical barrier that kept the food from contacting the intestinal wall of the duodenum and upper jejunum and delayed food from mixing with the biliary and pancreatic secretions. Patients had a modest weight loss at 3 months but no long term data is available yet.15 Further investigation is ongoing.
Multiple different endoscopic suturing and stapling devices are currently under investigation as a means of restricting the volume of the stomach and assisting in weight loss. These include the RESTORe Suturing System™ (C.R. Bard, Inc., Murray Hill, New Jersey), Transoral Gastroplasty (TOGa™ ) System (Satiety Inc., Palo Alto, CA), SafeStitch device (SafeStitch Medical Inc., Miami, FL), as well as others that are under development. However, there are no long-term studies and none of these devices are yet approved for use in the US outside of an IRB approved study protocol.16
In summary, there is a continued growing need for bariatric surgery not only in the US, but around the world. In addition, there are so many different procedures to treat one disease process it means that there is no one procedure that is perfect. The rest of this chapter will focus on the prevention and treatment of complications for the 5 most commonly performed bariatric surgeries today, RYGB, LAGB, BPD, BPD with DS, and Sleeve Gastrectomy.
ANATOMY
Roux-en-Y Gastric Bypass (RYGB). Since RYGB was first described in 1977, the technique and the order in which steps of the procedure are performed have been modified and vary from surgeon to surgeon; however, the general principles have remained the same.11 As previously mentioned, it was originally performed using an open technique although now more and more bariatric surgeons are performing this surgery laparoscopically.
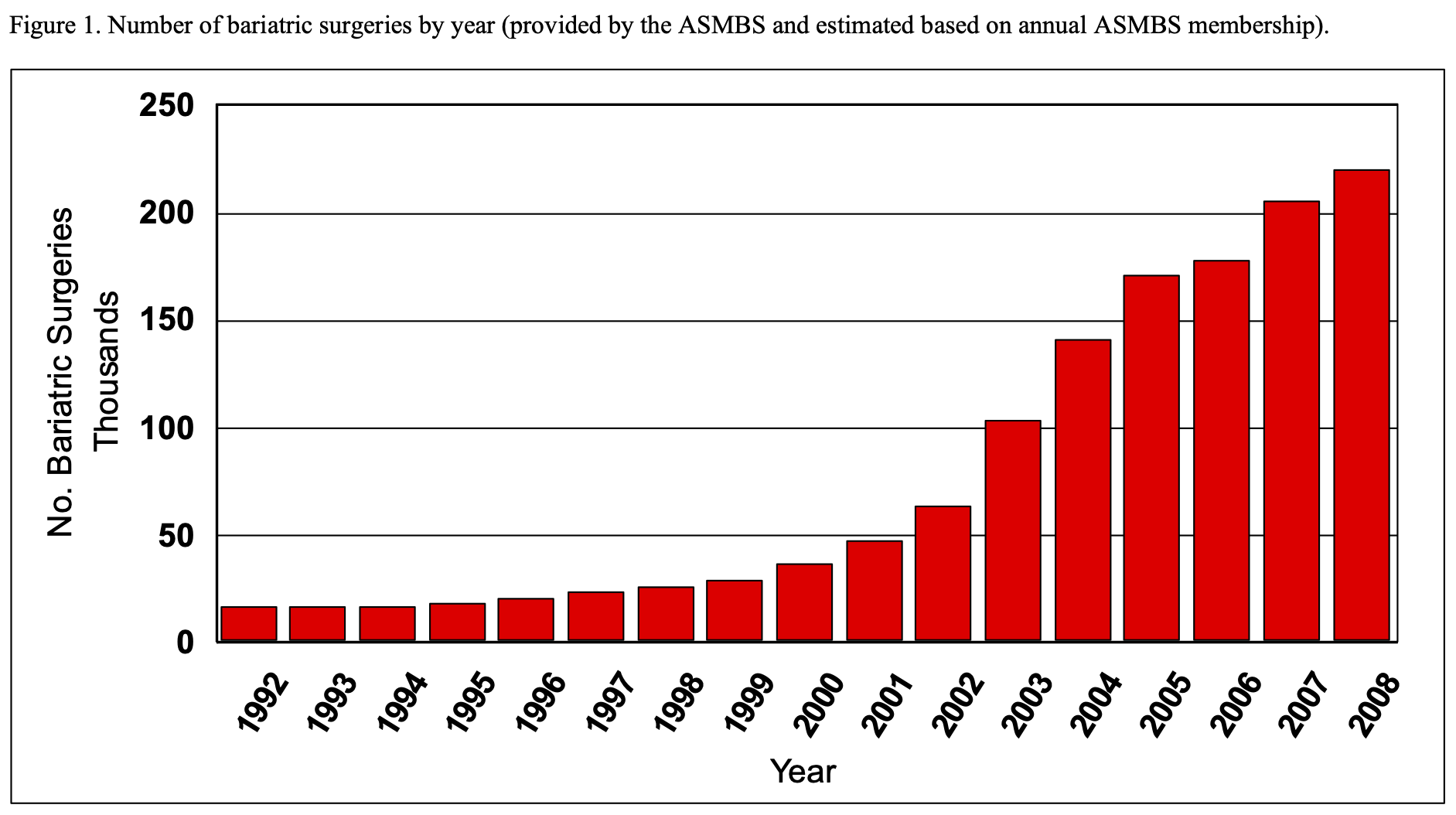
The size of the functional stomach is significantly reduced in order to restrict the amount of Calories that the patient can ingest at one time. This is accomplished by stapling across the top of the stomach separating it into the small upper gastric pouch and the much larger lower gastric remnant (Figure 2). In the earlier days of RYGB, many surgeons would staple across the stomach without dividing the stomach. However, the staple line would occasionally disrupt re-establishing a communication between the gastric pouch and the lower gastric remnant partially reversing the operation.37-40 Most surgeons now staple and divide the upper gastric pouch from the lower gastric remnant in an attempt to avoid forming a connection or a fistula between these two portions of the stomach, gastro-gastric (GG) fistula.21
As previously mentioned, the initial desired size of the gastric pouch was about 50 ml. But due to the concern over dilation of the pouch with time, surgeons decreased their pouch sizes to 30 ml and now most surgeons try to make the upper gastric pouch as small as possible, approximately 10 to 20 ml.21 Also, some bariatric surgeons have placed a silastic band around the pouch to decrease the risk of pouch dilation.21,41 However the addition of the band may be subject to long-term complications related to reintervention, reoperation, and decreased quality of life so it is unclear as to whether or not this is advantageous.41 In a survey study of bariatric surgeons, only 5% (10/215) were placing a band around the gastric pouch.42 Transaction of the stomach to form the gastric pouch is accomplished by opening the pars flacida, a clear area in the gastrohepatic ligament, and transversally dividing the lesser omentum starting approximately 2 to 3 cm below the gastroesophageal junction including dividing the lower branches of the anterior and posterior vagus nerves and the lower branches of the left gastric artery. The gastric stapling is started at this point on the lesser curvature of the stomach and continued to the angle of His, the junction of the fundus of the stomach and the esophagus.
Another important feature of the operation is creating malabsorption by bypassing a portion of the proximal small bowel. This is accomplished by forming a Roux-en-Y gastrojejunostomy (GJ) to the upper gastric pouch. The jejunum is usually transected approximately 30 to 75 cm below the ligament of Treitz. In a survey study of bariatric surgeons, this distance below the ligament of Treitz that the jejunum is transected varied from 10 cm to 250 cm with an average of 48 cm.42 The closer the jejunum is transected to the ligament of Treitz, the less mobile is the small bowel mesentery and the more difficult it may be to get the jejunum mobilized up to the gastrojejunostomy causing tension on the anastomosis. However, if a circular stapled GJ is being performed, care must be taken not to transect the jejunum too far distal as the diameter of the lumen of the small bowel decreases and it may be difficult to get the circular stapler inside the small intestine. The Roux-en-Y EE is then created 75 to 150 cm distal to the distal transected end of the jejunum. As previously mentioned, in the early days of RYGB, this length was standardized at 75 cm. But after a RCT showed better weight loss with a 150 cm versus a 75 cm Roux limb, many surgeons began using 75 cm for smaller patients and 150 cm for larger patients (BMI > 50).11 Subsequently many surgeons routinely make their gastric Roux limb 150 cm long. In the above-mentioned survey, the length of the gastric Roux limb ranged from 35 to 225 cm with an average of 114 cm.42
The length of the small bowel varies from patient to patient and on how much you stretch the small bowel out when measuring it but the average length is about 20 feet or 600 cm.43 So if the distance from the ligament of Treitz to the Roux-en-Y EE is 50 cm and the gastric limb is 150 cm, approximately one third of the proximal small bowel is bypassed before the food mixes with the digestive enzymes in the bile and pancreatic secretions creating significant malabsorption. Although the length of the common channel is the critical determinant on how much malabsorption will be created, the above survey study revealed that only 2% (4 out of 215 surgeons) actually measured the common channel.42 Consequently, the amount of malabsorption created can vary from patient to patient.
The last component of the RYGB is the GJ. When the gastric limb of jejunum is brought up to the upper gastric pouch for anastomosis, it can either be tunneled behind the transverse colon and lower gastric remnant, retrocolic and retrogastric, or be pulled up in front of these structures, antecolic and antegastric. The retrocolic technique was most often used with open RYGB while the antecolic technique is more commonly used with lap RYGB.42 There are advantages and disadvantages to each technique. The retrocolic, retrogastric technique requires the jejunum to traverse a shorter distance since it does not have to be draped over top of the omentum, transverse colon, and lower gastric remnant and therefore may result in less tension on the anastomosis. However, this technique requires more dissection, which may be more challenging especially laparoscopically, and creates a space, called Peterson’s space, for potential internal hernia, Peterson’s hernia, behind the jejunal limb just before it traverses the opening in the transverse mesocolon. This space must be sutured close to prevent this complication. This too may be challenging especially laparoscopically.
The advantage of the antecolic, antegastric technique is that it is much simpler and less time consuming. Also, if the patient does need revisional surgery, the gastric limb and the GJ is more readily accessible and identifiable. By dividing the omentum perpendicular to the transverse colon in line with the upper gastric pouch and by gently pulling the EE superiorly along with the gastric limb, the jejunum can usually be brought up to the upper gastric pouch without significant tension. The antecolic, antegastric technique also results in a Peterson’s space with the potential for a Peterson’s hernia and some bariatric surgeons again recommend closing this space with this technique as well, which again can be challenging especially laparoscopically. However, many bariatric surgeons do not routinely close this space and have not had a problem with Petersen’s hernias, especially if the proximal end gastric limb of jejunum is directed towards the patient’s left side rather than towards the right side.44 It appears that as the gastric limb of jejunum is pulled up to the upper gastric pouch, the jejunal mesentery is pulled against the transverse colon and mesocolon and these two structures adhere together and obliterate this space.
The GJ is purposely designed to be relatively narrow, about 1.5 cm in diameter, in order to prevent the food from exiting the pouch too quickly and therefore maintain satiety for a longer period of time after eating. Three different techniques have been used to perform this anastomosis, hand sewn, circular stapler, and linear stapler, and each has advantages and disadvantages. The circular staple technique is the most commonly used technique because it is fast, involves the least amount of suturing, and provides the most standardized diameter of anastomosis.42 However, it does require experience at getting the anvil for the stapler into position in the gastric pouch using either a transoral or transgastric technique. Also, if being performed laparoscopically, one of the cannula sites must be dilated large enough to allow passage of the circular stapler, which increases postoperative pain. The linear stapled technique is also commonly used.42 It avoids having to pass the anvil into the gastric pouch and the stapler can be inserted through one of the larger cannulas rather having to dilate a cannula site. But it is more difficult to create a standardized relatively small GJ and it requires significant amount of suturing, which may take longer and may be more challenging especially laparoscopically. Hand sewn anastomosis is the least commonly used technique, especially for lap RYGB, due to the increased amount of time required and, if performed laparoscopically, the need for significant experience at laparoscopic suturing.42
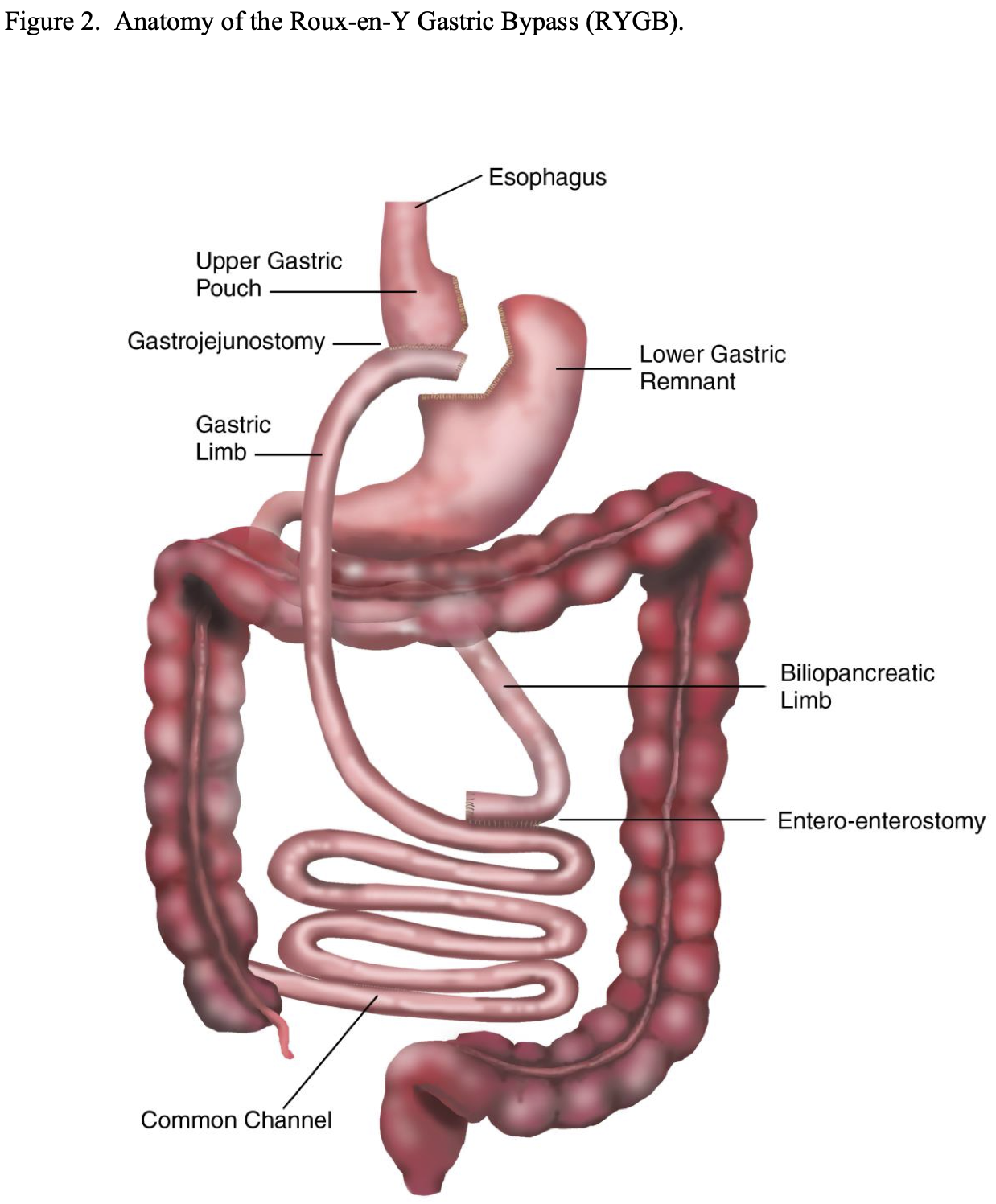
Laparoscopic Adjustable Gastric Band (LAGB). While the initial non-adjustable and adjustable gastric bands were performed open, all of these procedures are now done laparoscopically utilizing adjustable gastric bands. Initially, the LAGB bands were placed utilizing a perigastric technique. With this technique, the dissection was started right next to the lesser curvature side of the stomach a short ways below the gastroesophageal junction and a posterior tunnel was created behind the stomach through which the band was placed. Once the band was placed, there was approximately 50 ml gastric pouch above the band and the opening of the band could be adjusted by accessing the subcutaneous port and instilling or withdrawing fluid (Figure 3).
However, the perigastric technique had an unacceptably high rate of band slippage, causing malpositioning and obstruction, and erosion into the stomach.13 Due to this, the pars flacida technique was developed. This entailed opening the pars flacida, as previously described for the RYGB, and identifying the anterior border of the right crus of the diaphragm. Using electrocautery, a small opening is made in the retroperitoneum just anterior to the right crus and as inferior as possible. Then a blunt grasper is inserted into this opening and carefully passed blindly behind the upper stomach aiming for the angle of His. Once the tip of the grasper is seen through the loose connective tissue at the angle of His, the grasper can be advanced all of the way through the tissue. The gastric band is then inserted through a large cannula into the peritoneal cavity and passed to the grasper behind the stomach. The grasper and band is then pulled back through this posterior gastric tunnel. The band is buckled together anteriorly on the stomach. Since the early 2000s the pars flacida technique has been used almost exclusively of the LAGB procedures. Another technique that helps to decrease slippage of the band is suturing the stomach below the band to the gastric pouch above the band, creating a serosal tunnel around the anterior and left lateral aspects of the band. However, there is much debate as to if the band needs to be sutured into place and if so how many sutures and where these sutures should be positioned in order to most efficiently minimize slippage.45-50
Once the gastric band is in place, the tubing for the band is brought out through the abdominal wall and connected to a port that can be accessed with a Huber needle, a non- coring needle, to adjust the band. Finally, a subcutaneous pocket is created and the port is adequately secured to the fascia in order to prevent the port from migrating or flipping over. The technique and location of placement of the port is quite variable from surgeon to surgeon.
Biliopancreatic Diversion (BPD). This bariatric surgical procedure was designed to provide more malabsorption and less gastric restriction than the RYGB in an attempt to obtain and maintain better long-term weight loss. It is similar to a Billroth II Gastrectomy with a very long Roux-en-Y limb (Figure 4). A subtotal gastrectomy is performed leaving a 150 to 200 ml gastric pouch.23,51 Then a Roux-en-Y GJ is performed. However, the small bowel is transected about 250 cm proximal to the ileocecal valve instead of 30 to 75 cm below the ligament of Treitz as is done with the RYGB. The distal transected end of the small bowel is then used to perform the GJ. The proximal transected end of small bowel is used to perform the Roux-en-Y EE 50 cm from the ileocecal valve, leaving a 50 cm long common channel in which the food and digestive enzymes will come in contact.21,23,51 This results in bypassing about 85% of the small bowel compared to about 33% with the RYGB.
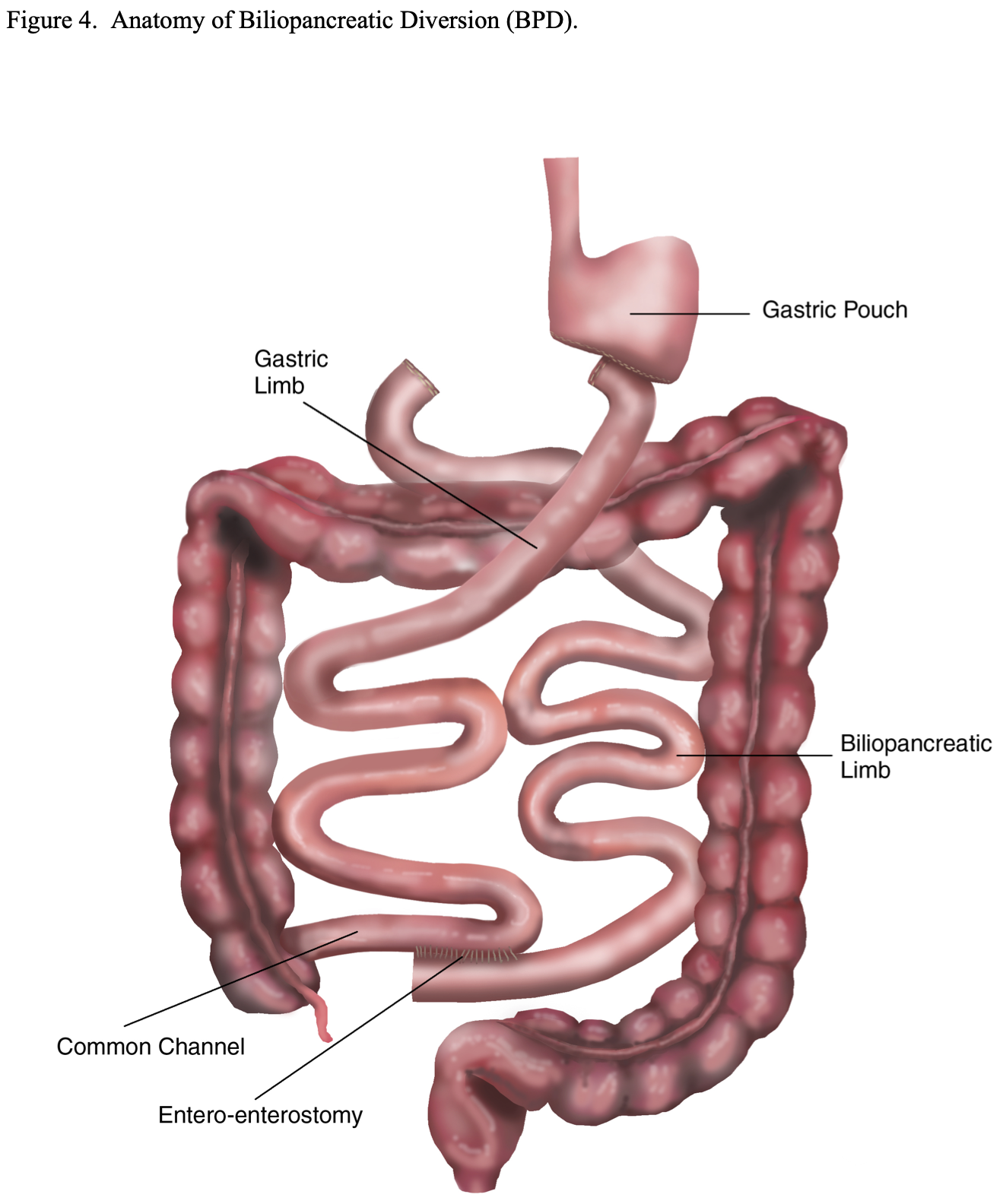
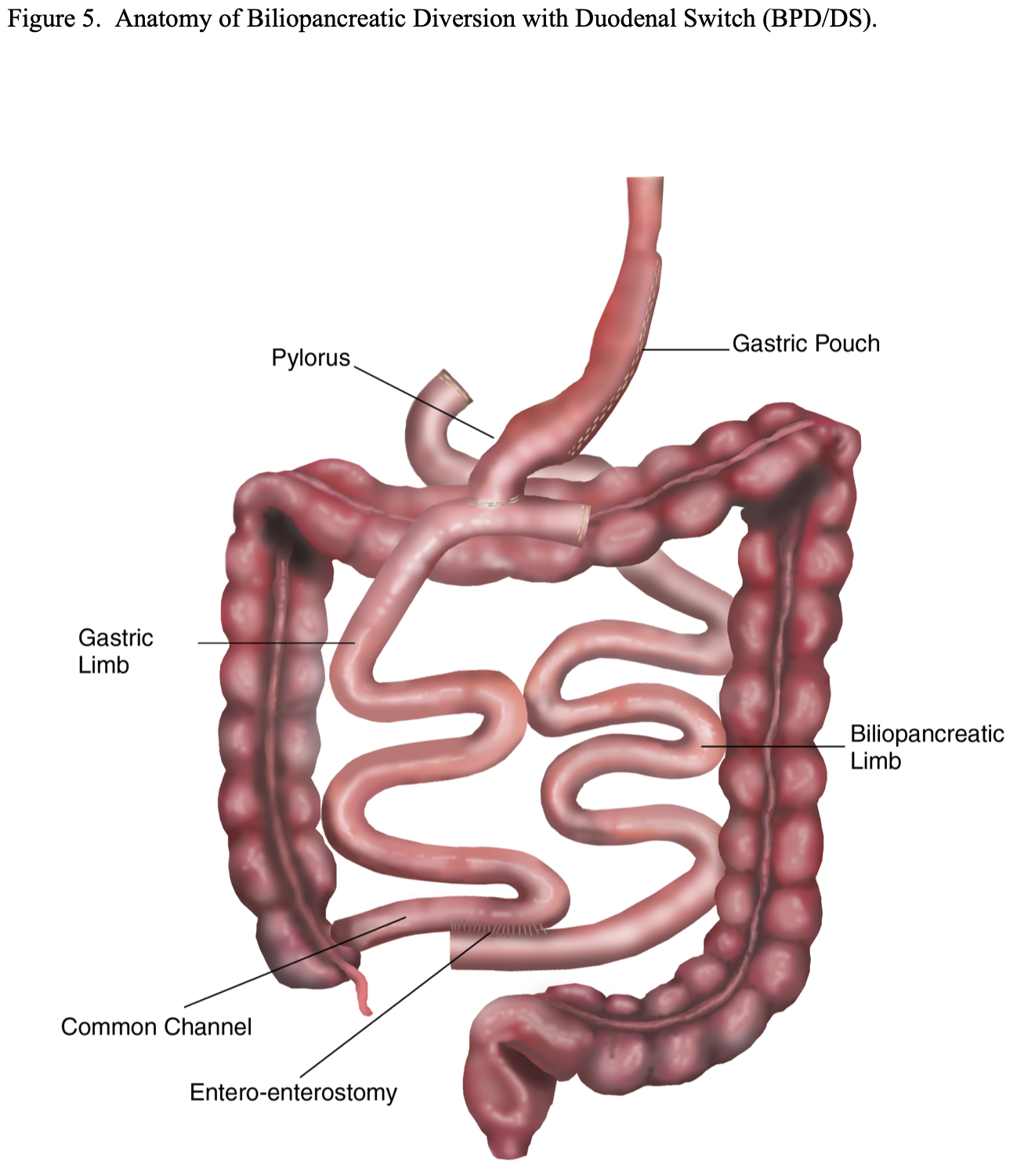
BPD with Duodenal Switch (BPD/DS). This modification of the BPD was developed in an attempt to decrease the incidence of dumping syndrome. A vertical sleeve gastrectomy, removing the lateral portion of the stomach, is performed instead of a distal gastrectomy (Figure 5). This is accomplished by transecting the greater omentum and the short gastric vessels off of the lateral aspect of the stomach from about 5 to 6 cm proximal to the pylorus all the way to junction of the funds to the esophagus, the angle of His. Then an esophageal dilator (size varies by surgeon from 40 to 52 French) is passed orally by anesthesia and the dilator is positioned along the lesser curvature of the stomach with the tip in or near the pylorus. The lateral portion of the stomach is then resected just laterally to the dilator leaving a 150 to 200 ml gastric pouch. The pylorus and duodenal bulb are mobilized and the duodenum is transected as far as possible from the pylorus, usually 4 to 5 cm. The small bowel is then transected 250 to 300 cm proximal to the ileocecal valve and the distal transected end of the small bowel is anastomosed to the proximal transected end of the duodenum. The proximal transected end of small intestine is used to perform the Roux-en-Y EE anywhere from 50 to 75 to 100 cm from the ileocecal valve depending on the preference of the surgeon. Some surgeons adjust the length of the common channel depending on the severity of the obesity.17,21,22,29 This procedure still bypasses about 80% to 85% of the small bowel similar to the original BPD procedure and results in significantly more malabsorption than the standard RYGB.
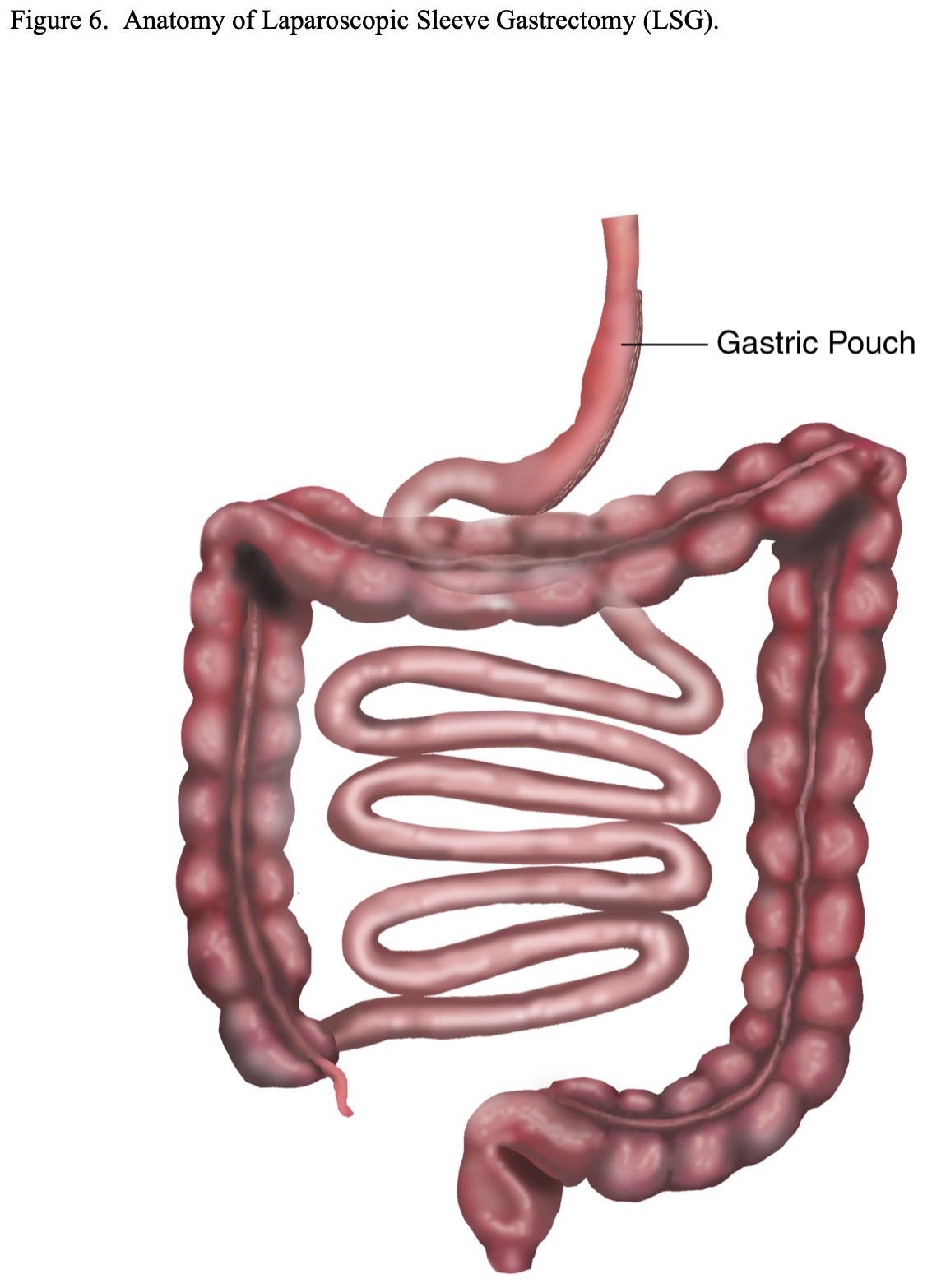
Laparoscopic Sleeve Gastrectomy (LSG). As previously mentioned, LSG alone has now become another option of a primary procedure for patients seeking bariatric surgery (Figure 6). The advantages are that there is no foreign bodies such as with LAGB; no mesenteric defects that could lead to internal hernias and obstruction or malabsorption that could lead to malnutrition or deficiency states such as with RYGB, BPD, or BPD/DS; and no risk of dumping syndrome such as with RYGB or BPD.32 However, there are no long-term follow-up studies available, raising the concern over possible gastric pouch dilation and weight regain. For this reason, some bariatric surgeons have suggested reducing the size of the gastric pouch by resecting more stomach. This can be accomplished by starting the resection closer to the pylorus and using a smaller dilator to size the pouch. However, this could increase the risk of stenosis of the gastric pouch and obstruction.32,35
PHYSIOLOGY/PATHOPHYSIOLOGY/DISEASE/DISORDERS
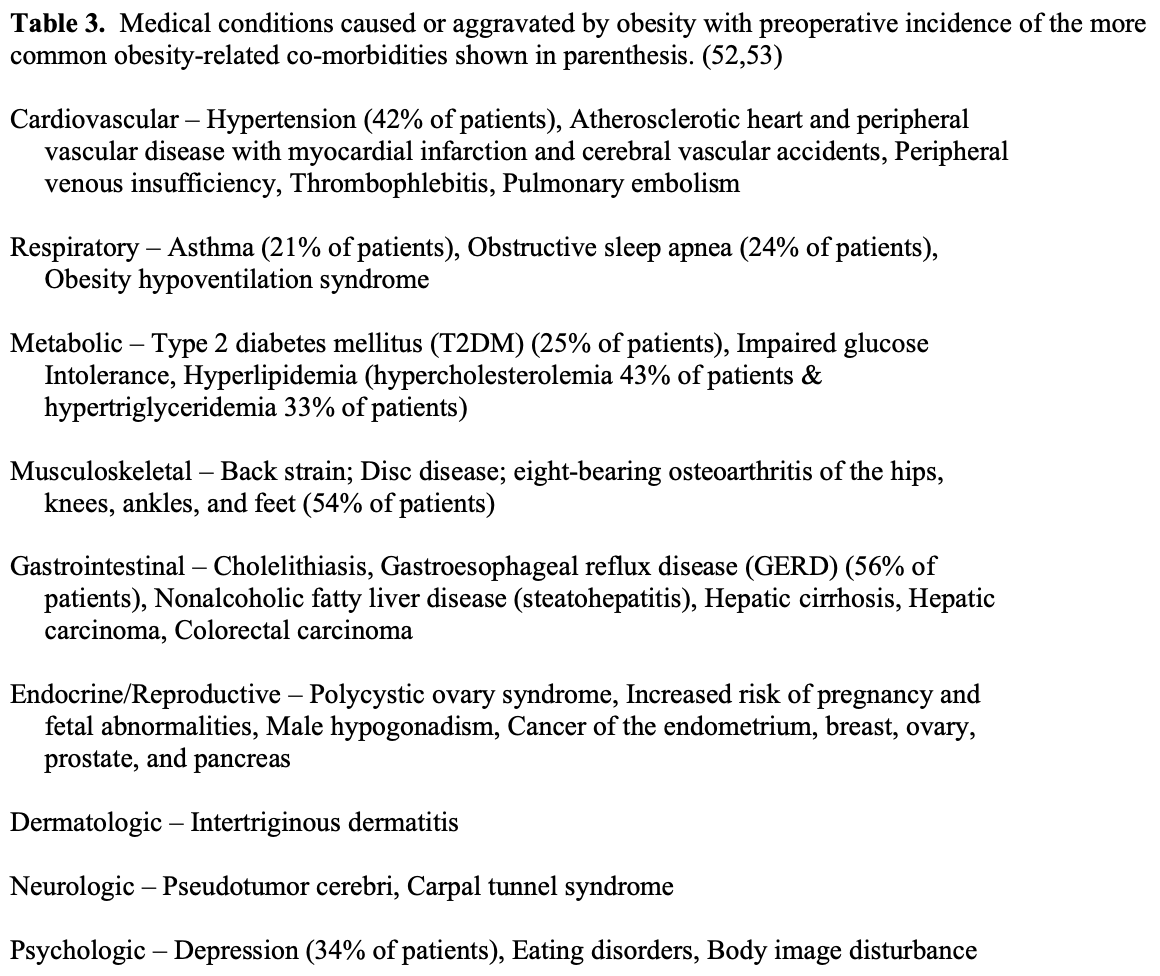
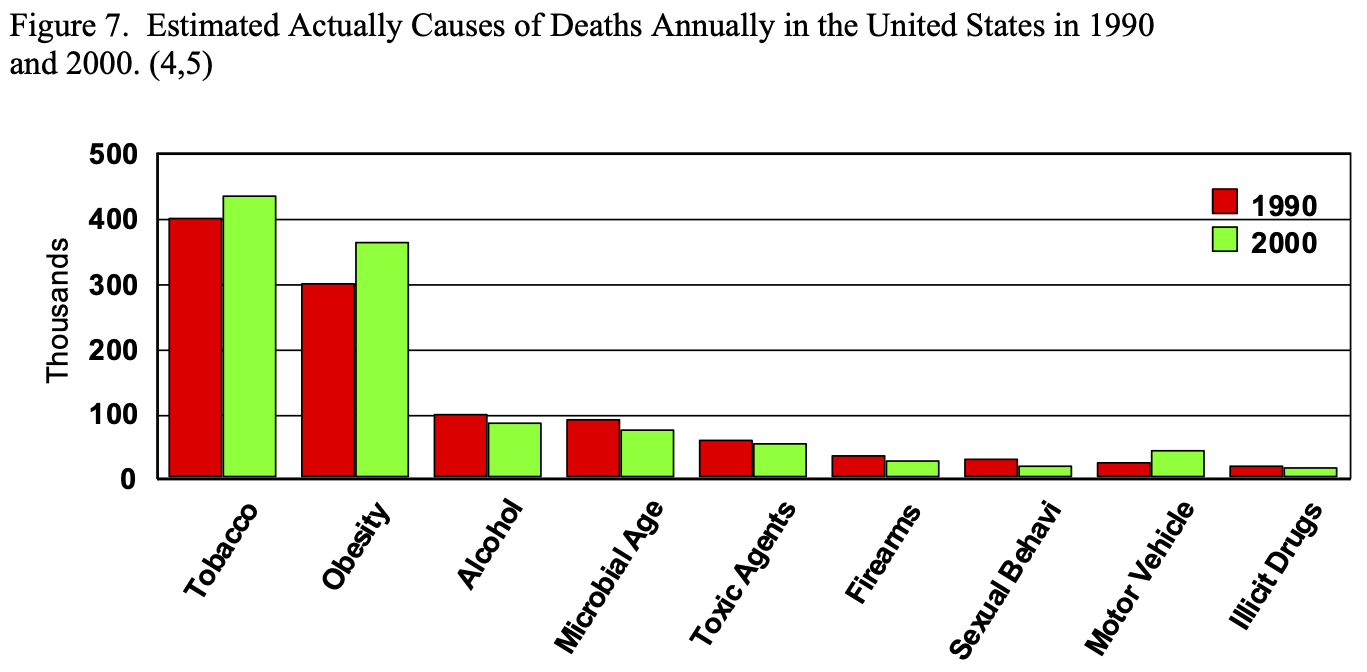
Obesity affects nearly every organ system and causes or aggravates a long list of medical problems (Table 3). The combination of central obesity, glucose intolerance, hypertension, and dyslipidemia is known as Metabolic Syndrome, which has an ICD-9 code (277.7) and is known to be a significant risk factor for T2DM and coronary heart disease.54,55 It was estimated that in 1990, approximately 300,000 people died of obesity related co-morbidities, second only to tobacco, and this number increased to 365,000 in 2000 (Figure 7).4,5 The type and number of obesity related co-morbidities also significantly affects the risk of postoperative complications following bariatric surgery.56
Metabolic factors, diet, and physical activity are major determinants in modulating body weight and each are influenced by genetic traits.57 However, the final determining factor whether someone will gain, lose, or maintain their weight is energy balance, how many Calories are taken in and how many Calories are burned off. The first line of therapy for obesity is modifying dietary habits and life style to decrease Caloric intake and increasing exercise and activity to increase Caloric expenditure. Numerous diets have been designed and utilized to lose weight and most of these diets are safe and effective. However, an individual has to choose a diet that they can follow life long or they will simply regain the weight once they go off the diet. Even morbidly obese patients can lose weight with diet and exercise short term but they cannot effectively maintain this weight loss long term.
The next option in the management of obesity is pharmacotherapy. Two medications are approved and available in the United States for the treatment of obesity. Sibutramin (brand name Meridia®) is a central acting appetite suppressant. It is a serotonin norepinephrine reuptake inhibitor (SNRI) causing increased satiation or a feeling of fullness. It also increases thermogenesis and therefore increases energy expenditure.58-60
The other available medication, orlistat (brand name Xenical®), blocks digestion and absorption of ingested dietary fat by reversibly inhibiting pancreatic and gastric lipases that are essential for the hydrolysis of dietary fat in the GI tract to allow for absorption.61-63
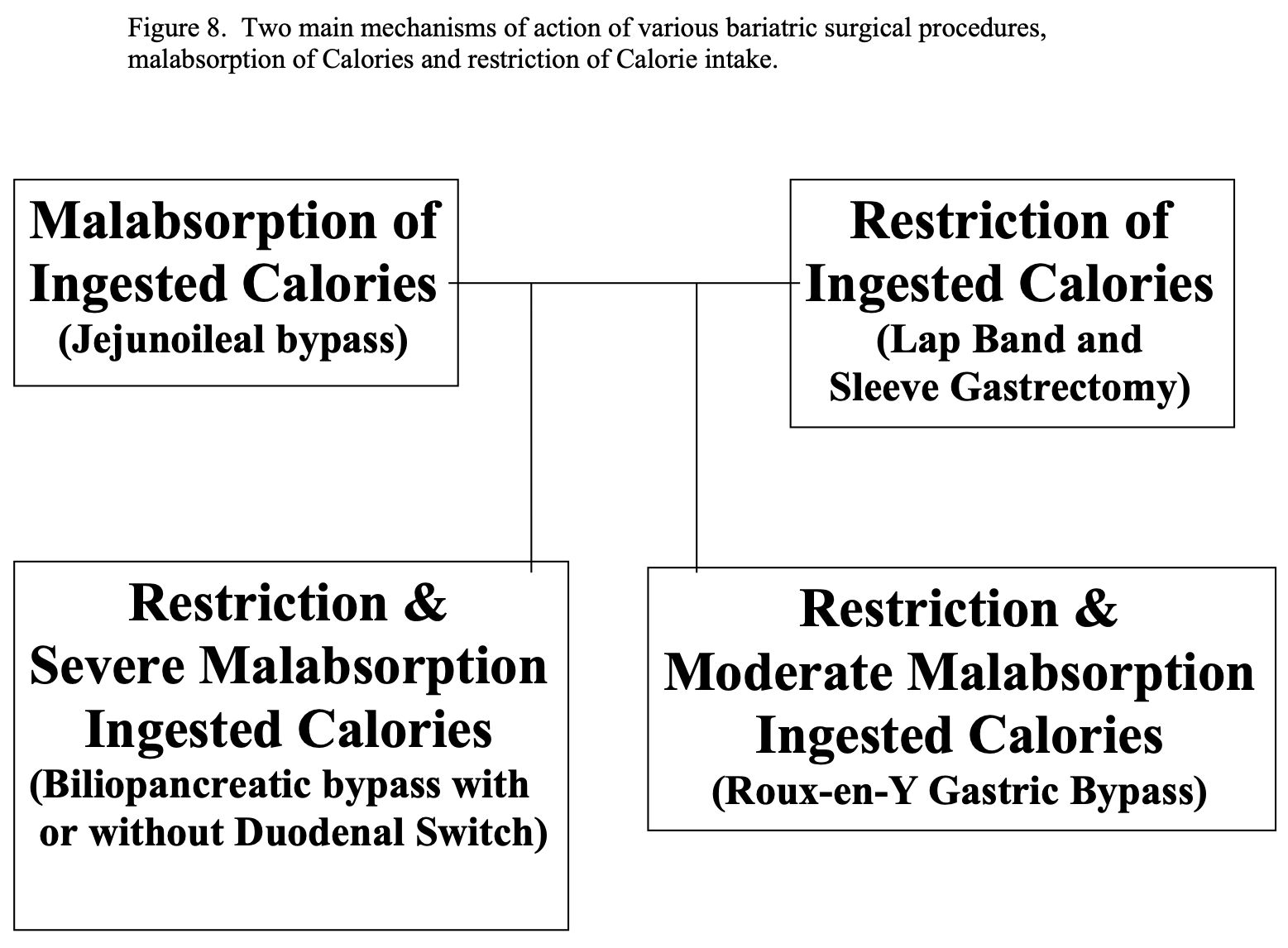
However, once an obese individual reaches a BMI of 35 or greater, the chances of obtaining and maintaining long term weight loss is low. This is where bariatric surgery may be helpful. There are 2 main ways that bariatric surgical procedures help people to lose weight, malabsorption of ingested Calories or restriction of ingested Calories, and some procedures use a combination of these 2 mechanisms (Figure 8). The first bariatric procedure performed, the JI bypass, relied on malabsorption alone without any restriction in the amount of Calories, or food, ingested. Of course, if JI bypass patients ingested large amounts of fatty food in their diet, they would experience steatorrhea with severe abdominal cramping and diarrhea, which would have a negative feedback mechanism to encourage the patient not to ingest these types of food. The next generation of bariatric surgical procedures, the gastric stapling and gastroplasty procedure were aimed at restricting the amount of Calories ingested by decreasing the functional capacity of the stomach. While these procedures had a high failure rate, two of the currently performed procedures, LAGB and LSG, rely solely on restriction of ingested Calories with no effect on digestion or absorption of ingested nutrients. RYGB, BPD, and BPD/DS result in weight loss from a combination of restricting the Calories ingested and causing malabsorption of the Calories that are ingested. RYGB relies on more restriction and less malabsorption with a smaller functional stomach and less length of small bowel bypassed where as BPD, with or without DS, relies more on malabsorption and less on restriction with longer length of small bowel bypassed and much larger functional stomach.
Bariatric surgery may also induce various hormonal changes that may contribute to postoperative weight loss. Ghrelin is a peptide that was first described in 1999. It is mainly produced in the fundus of the stomach but affects multiple organ systems besides the GI tract including the central nervous system, immune system, cardiovascular system, and adrenal glands. It is an orexigenic gut hormone that has a role in mealtime hunger and long–term regulation of body weight by stimulating appetite, increasing energy stores, and promoting the deposition of adipose tissue. Serum levels increase rapidly prior to meals and fall quickly following meals, which may be one of the body’s mechanisms to stimulate hunger.64,65
Obese individuals have lower serum ghrelin levels than normal weight individuals but their ghrelin levels increase with non-surgical weight loss. In one study, obese individuals who obtained 17% body weight loss with dieting alone had a 24% increase in their 24- hour serum ghrelin levels. The pre- and postprandial rise and fall in the ghrelin levels were maintained with higher baseline and peak levels suggesting that the body was trying to get the individual to eat more. In comparison, RYGB patients who obtained a 36% body weight loss had a 77% decrease in 24-hour ghrelin levels and the serum levels remained constant throughout the day despite ingestion of food, which could at least partially explain the decrease in appetite experienced by RYGB patients.65 However, the mechanism by which the ghrelin levels are decreased in gastric bypass patients is not known. Also, subsequently studies have been inconsistent with studies showing decreased levels, no change, or even an increase in serum ghrelin levels following RYGB.66-79 Some of these differences are attributed to differences in the method by which serum samples have been obtained and how the serum ghrelin was measured.
BPD/DS or SG alone has been shown to decrease ghrelin levels, which makes sense since these procedures involve resecting the portion of the stomach from which ghrelin is secreted.69,71,79-81 However, most studies have not shown significant changes in ghrelin levels following either standard BPD67,82 or LAGB77,81,83-85. Consequently, the true role of ghrelin in surgical weight loss remains unclear. Also, numerous other hormones, such as leptin, peptid YY (PYY), human apolipoprotein, obestatin, adiponectin, growth hormone, are being studied regarding how the various bariatric surgical procedures affect these hormones and what role these changes contribute to surgical weight loss.66-68,73,75,77,79,80,82
TREATMENT
Diet modification and exercise are the first line of therapy for obesity and, as previously mentioned, if these fail then there are two medications approved to assist with weight loss: sibutramin and orlistat. However, once patients meet the criteria for bariatric surgery, it is the only proven effective treatment for these clinically severely obese individuals. While bariatric surgery is expensive, studies have shown that it is a cost- effective treatment for clinically severe obesity and that after 2 to 4 years the decreased medical costs of obesity related medical problems offsets the cost of bariatric surgery.86,87
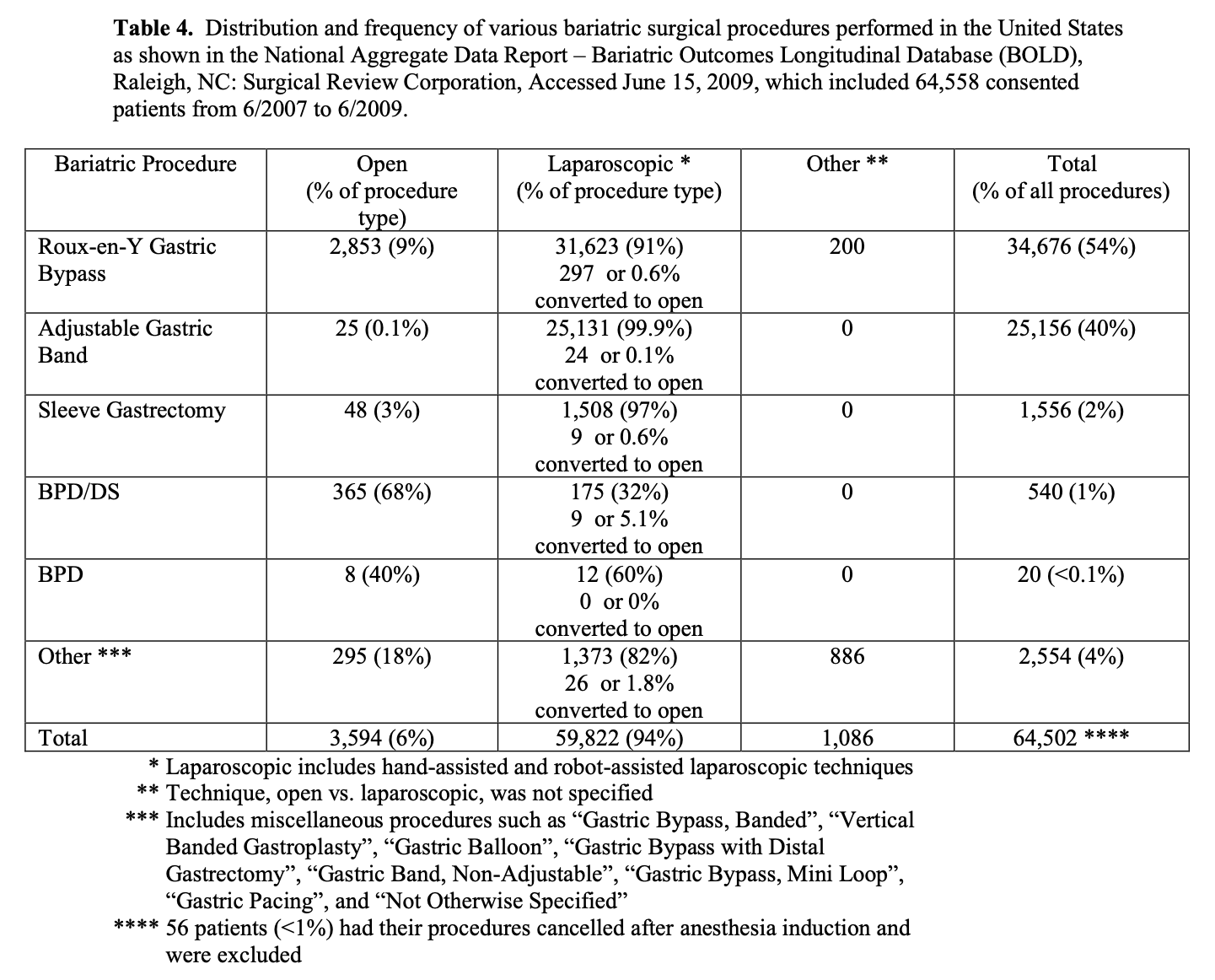
The bariatric procedure performed varies by surgeon and geography. Outside of the United States, the most common bariatric surgical procedure performed is the LAGB while in the United States; it is the RYGB, although the frequency of LAGB has been increasing. The distribution and frequency of the 5 most common bariatric surgical procedures and the technique used, open vs. laparoscopic, for the 64,558 patients in BOLD who have signed consent to have their data utilized for research purposes is shown in Table 4. By far, RYGB and LAGB are the most common bariatric surgical procedures and 94% of all of the bariatric surgical procedures were performed laparoscopically.
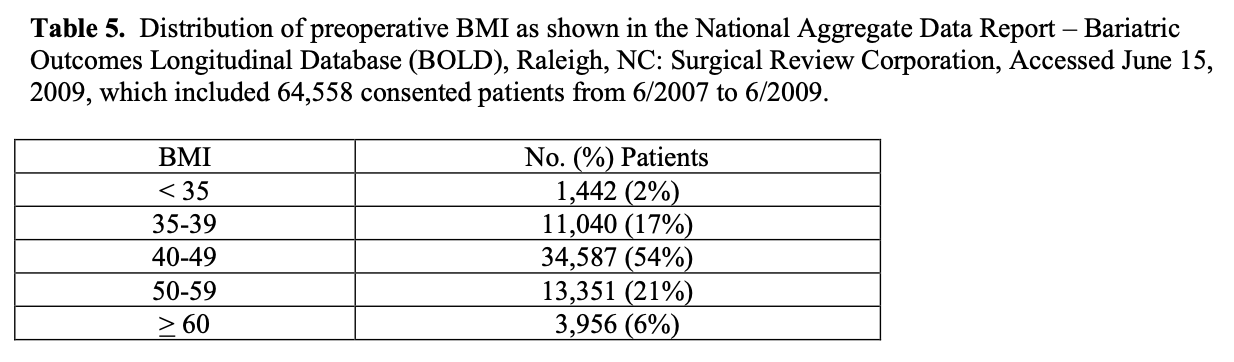
However, all of these patients were treated by an ASMBS BSCOE and accounts for about 15% of the estimated number of bariatric surgical procedures performed over this two- year period of time. It is possible that the distribution of open vs. laparoscopic cases and RYGB vs. LAGB may be significantly different for bariatric surgery programs that are not designated as a BSCOE. The average preoperative BMI and age of patients in BOLD was 46.5 + 8.3 and 45.8 + 11.8 years, respectively, with the distribution of BMI shown in Table 5 (National Aggregate Data Report – Bariatric Outcomes Longitudinal Database (BOLD), Raleigh, NC: Surgical Review Corporation, Accessed June 15, 2009). The majority of all bariatric surgery patients, about 80%, are female.88 However, the incidence of obesity in the US is the same for men and women indicating that women are much more likely to pursue bariatricsurgery.1
Each of the bariatric surgical procedures has their own advantages and disadvantages (Table 6). Sleeve gastrectomy alone is a relatively new procedure so was not included in the meta-analysis88,93 and collective reviews89-92 used to construct Table 6 but rather was obtained from a review of the results of 106 bariatric surgeons35, which included 14,776 LSG. In terms of weight loss and long-term maintenance of weight loss, BPD with or without DS is the best operation.21,41 RYGB has excellent early weight loss but most patients do have some weight regain with the long term weight loss less than that of BPD.41 The weight loss with LAGB is much slower, over a longer period of time and the long-term weight loss is more variable from patient to patient. The short-term weight loss for SG has been good 65%EBWL at 2 years but weight regain appears to be significant (49 %EBWL > 4 years after surgery). More long-term weight loss data with LSG procedures designed as primary treatment options are needed.35 Resolution and resolution/improvement in obesity related co-morbidities is best with BPD and BPD/DS, intermediate with RYGB, and lowest with LAGB (Tables 6 and 7). The most dramatic differences in co-morbidity resolution between malabsorptive/restrictive procedures and restrictive procedures alone is with diabetes mellitus and hypertension.90,94 In a series of 240 patients with Type 2 Diabetes Mellitus (T2DM) that underwent lap RYGB, Schauer found that patients with the shortest duration of T2DM (< 5 years), the mildest form of T2DM (diet controlled), and the greatest weight loss after surgery were most likely to achieve complete resolution of their diabetes, suggesting that earlier surgical intervention may increase the likelihood of preventing or resolving T2DM.95 Improvement in obesity- related co-morbidities after LSG has not been as well studied or compared to the other bariatric surgical procedures. But LSG has been reported to have an 80% to 85% resolution rate for T2DM, which is similar to that of RYGB.35
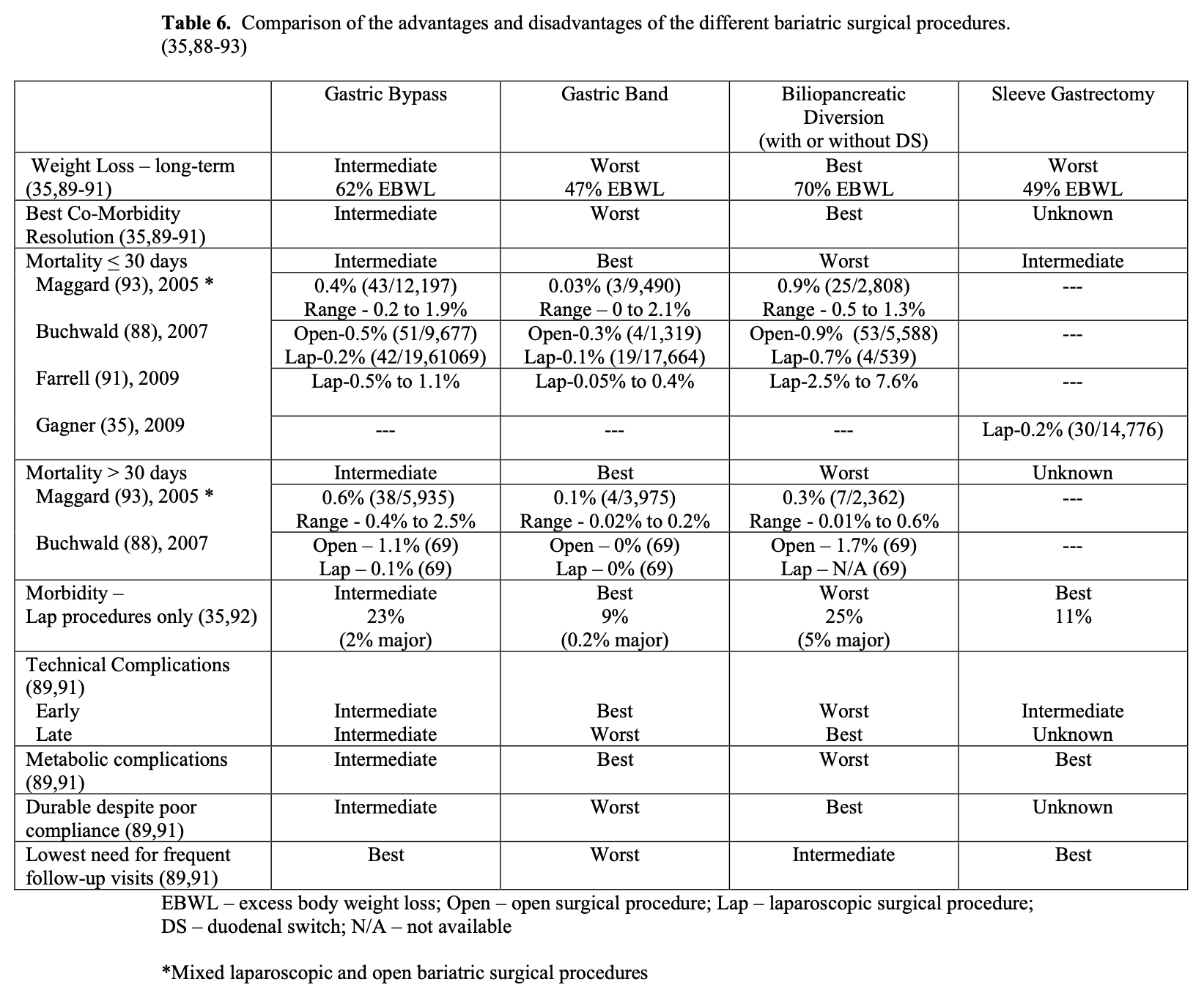
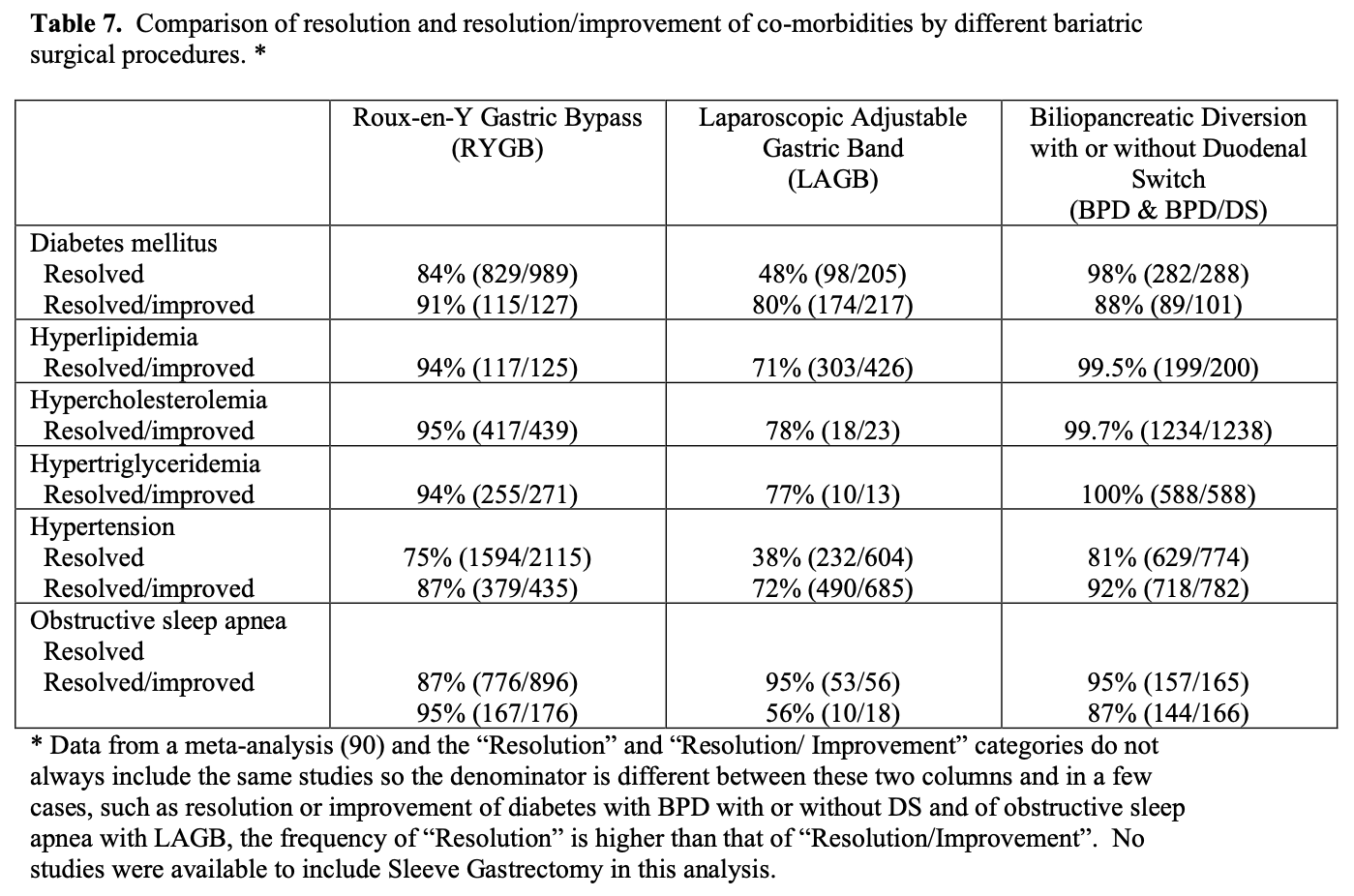
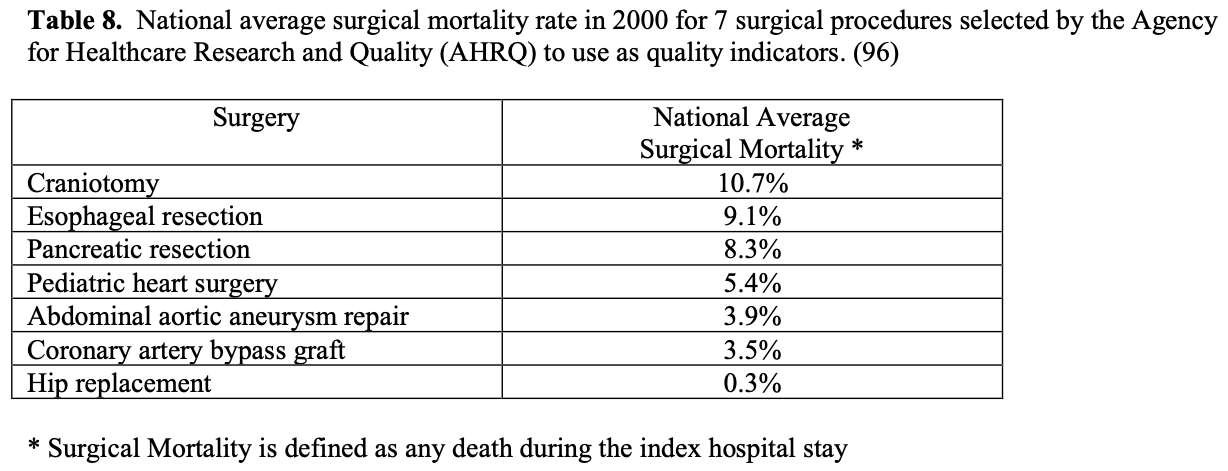
Some insurance companies and even some physicians and healthcare professionals claim that bariatric surgery is too high risk and therefore should not be done. However, a 2007 meta-analysis of mortality in bariatric surgery, which included laparoscopic and open gastric bands, gastroplasties, gastric bypasses, BPD, and revision bariatric surgeries, found an average perioperative mortality for the entire group of 0.4%. Considering this surgery is being performed in a high-risk patient population, this operative mortality is quite low especially when you compare it to the national average surgical mortality (any death during the index hospital stay) for the 7 surgical procedures that the Agency for Healthcare Research and Quality (AHRQ) selected to study quality between US hospitals (Table 8). Bariatric surgery has the operative mortality equivalent to an elective hip replacement, which has the lowest mortality by far of any of these 7 procedures.96
Obesity itself is associated with increased mortality. A collective review was performed of 57 prospective studies with 894,576 participants from western Europe and the US with a mean age of 46 years.6 The mortality in these patients from year 5 of follow-up to a mean of 13 years follow-up was correlated to their original BMI, adjusting for age, sex, and smoking status. Mortality was lowest in individuals with a BMI of 22.5 to 25. Each increasing increments of 5 in the BMI was associated with a 30% higher overall mortality. There was a 2 to 4 year reduction in median survival for individuals with a BMI of 30 to 35 and 8 to 10 year reduction in individuals with BMI 40 to 45.
However, bariatric surgery can reverse this trend. A Swedish multi-center prospective trial compared a cohort of 2010 bariatric surgery patients to a matched control group of morbidly obese patients treated with diet alone with a median follow-up of over 15 years.7 The control group remained within 2% of their original weight despite attempts at non-surgical weight loss while the surgery group loss a significant amount of weight at 15 years after surgery depending on the specific bariatric procedure performed (13% weight loss for gastric band, 18% for VBG, and 27% for gastric bypass). Even including the perioperative mortality, the bariatric surgery group had a significantly lower mortality compared to the control group, 5.0% vs. 6.3%, with a hazard ratio for cumulative mortality of 0.76 (95% CI, 0.59-0.99). Another prospective cohort study from Canada also showed a reduction in long-term mortality with bariatric surgery.97 A cohort of 1035 bariatric surgery patients were compared to an age and gender matched cohort of 5746 morbidly obese patients not treated with surgery and both groups were followed for 5 years. The bariatric surgery cohort had a mortality rate of 0.68% compared to 6.17% in the control group for a relative risk of mortality of 0.11 (0.04 to 0.27, 95% confidence interval) for an 89% reduction in the relative risk of death for morbidly obese patients who underwent bariatric surgery. Lastly, a retrospective cohort study of 7,925 patients who had gastric bypass performed by a single group of bariatric surgeons in Utah from 1984 to 2002 were matched to an equal number of severely obese individuals applying for driver’s license.98 The two groups were matched for sex, age, BMI, and year (year of surgery matched to year applied for driver’s license). With a mean follow-up of 7.1 years, the adjusted long-term mortality from all causes decreased by 40% in the bariatric surgery group (37.6 vs. 57.1 deaths/10,000 person-years, p<0.001). The bariatric surgery group also had significant reduction in cause-specific mortality, 56% for coronary artery disease, 92% for diabetes mellitus, and 60% for cancer. However, the bariatric surgery group did have an increase in deaths not caused by disease with hazard ratios of 1.22 for accident, 1.82 for poisoning, 2.03 for suicide, and 1.69 for other non-disease causes.
None of these hazard ratios were statistically significant but the combined hazard ratio for all non-disease deaths was significantly higher in the bariatric surgery group with a 58% increase (11.1 vs. 6.4 deaths/10,000 patient-yeas, p<0.05).
There are differences in morbidity and mortality between the different bariatric surgical procedures (Table 6). Since LAGB is a much simpler procedure with no transection or anastomosis of the GI tract it has the lowest mortality, perioperative morbidity, and early technical complications compared to RYGB, which has an intermediate incidence, and BPD with or without DS, which has the highest incidence. One study of 1,185 lap RYGB and 862 LAGB patients revealed a significantly higher 30-day readmission rate in lap RYGB patients (7.3% vs. 3.1%, p<0.001).99 However, LAGB has the highest risk of late technical complications, which include erosion or slippage of the band and breakage or leakage of the port, tubing, or balloon, with some studies showing up to 30% of LAGB patients require one or more major re-operative procedures to repair, replace, or remove the LAGB.100 Also, LAGB requires the most frequent follow-up visits with some LAGB programs seeing these patients every 4 to 6 weeks for the first year and every 2 to 3 months the second year. Since LAGB patients do not experience any malabsorption or dumping syndrome, it is much easier for them to “cheat” on their diet. So successful weight loss with LAGB requires the patient to have the greatest compliance with the postoperative diet recommendations. BPD with or without DS relies the least on dietary compliance since the majority of the weight loss with this procedure is based on malabsorption; however, these patients need to be followed more closely than the RYGB patients in order to diagnosis and treat the more frequent complications of malnutrition and nutritional deficiencies. LSG appears to have an operative mortality and morbidity between that of gastric bypass and gastric band and since it creates no malabsorption, it has few metabolic complications.
Bariatric surgery preceded laparoscopic surgery so initially all these procedures were performed open. However, in the early 1990’s as more surgeons became experienced with advanced laparoscopic surgery, more and more bariatric surgical procedures were performed laparoscopically. Several studies of varying study design, methodology, and number of patients included (51 to 22, 422 patients) have been published comparing the outcomes of laparoscopic to open RYGB (Tables 9, 10, 11). The published conversion rate from lap RYGB to open RYGB ranged from 0% to 8% excluding Westling’s study, which had an unusually high conversion rate of 23%.106 The most recent conversion rate as reported in BOLD is 0.6% (Table 4) so the need for conversion from lap to open may be decreasing as surgeons become more experienced at laparoscopic bariatric surgery (National Aggregate Data Report – Bariatric Outcomes Longitudinal Database (BOLD), Raleigh, NC: Surgical Review Corporation, Accessed June 15, 2009). During the learning curve of laparoscopic RYGB, the operative times are usually much longer than for open RYGB; however, 2 studies showed with experienced laparoscopic surgeons a decreased operative time with laparoscopic compared to open RYGB.37,105 Blood loss is usually less with laparoscopic RYGB and the number of patients requiring ICU and the ICU length of stay (LOS) is shorter. Studies have demonstrated that patients have less postoperative incisional pain after lap RYGB compared to open and therefore have shorter hospital LOS and are able to return to activities of daily life and to work sooner than open RYGB patients.106-115

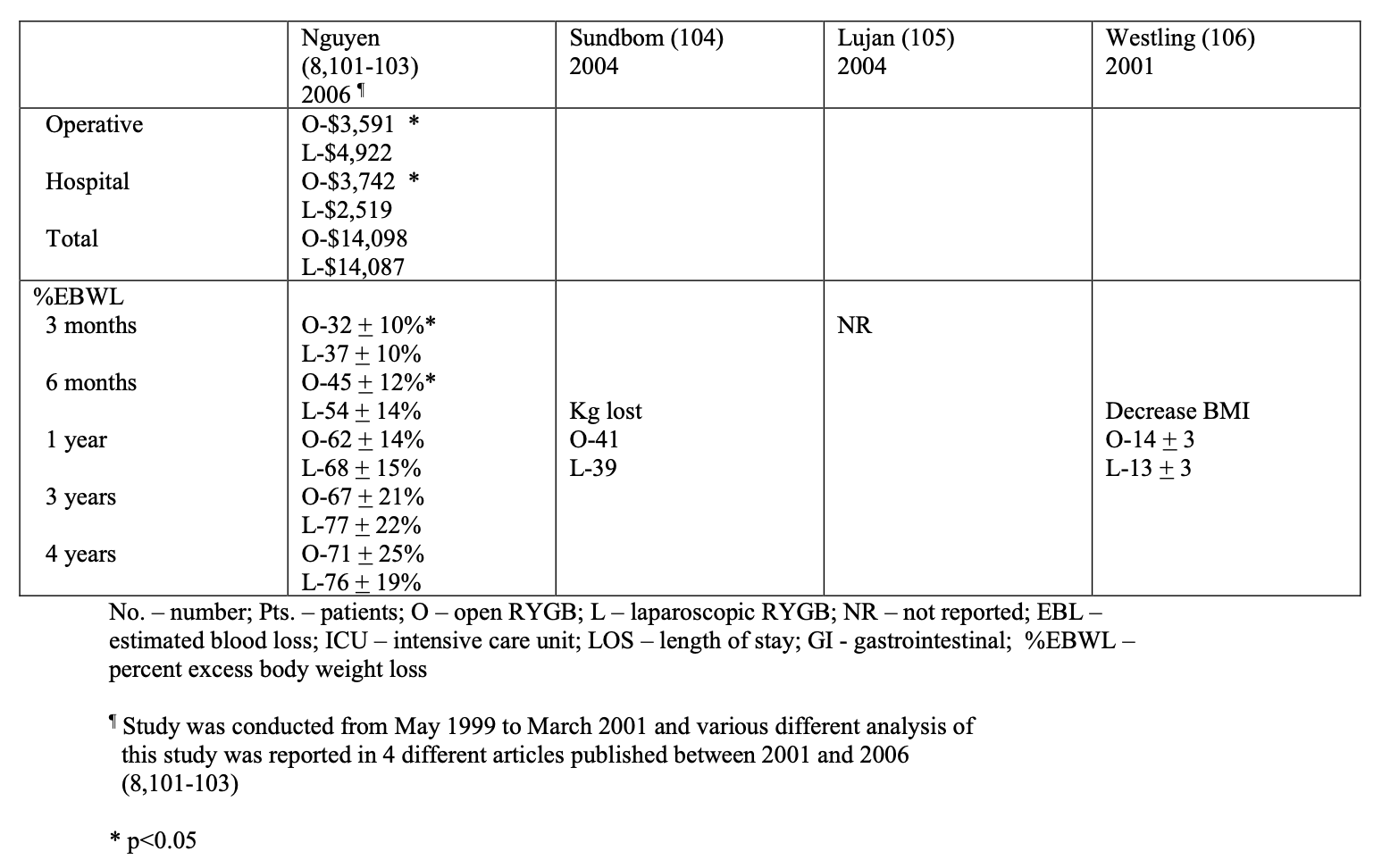
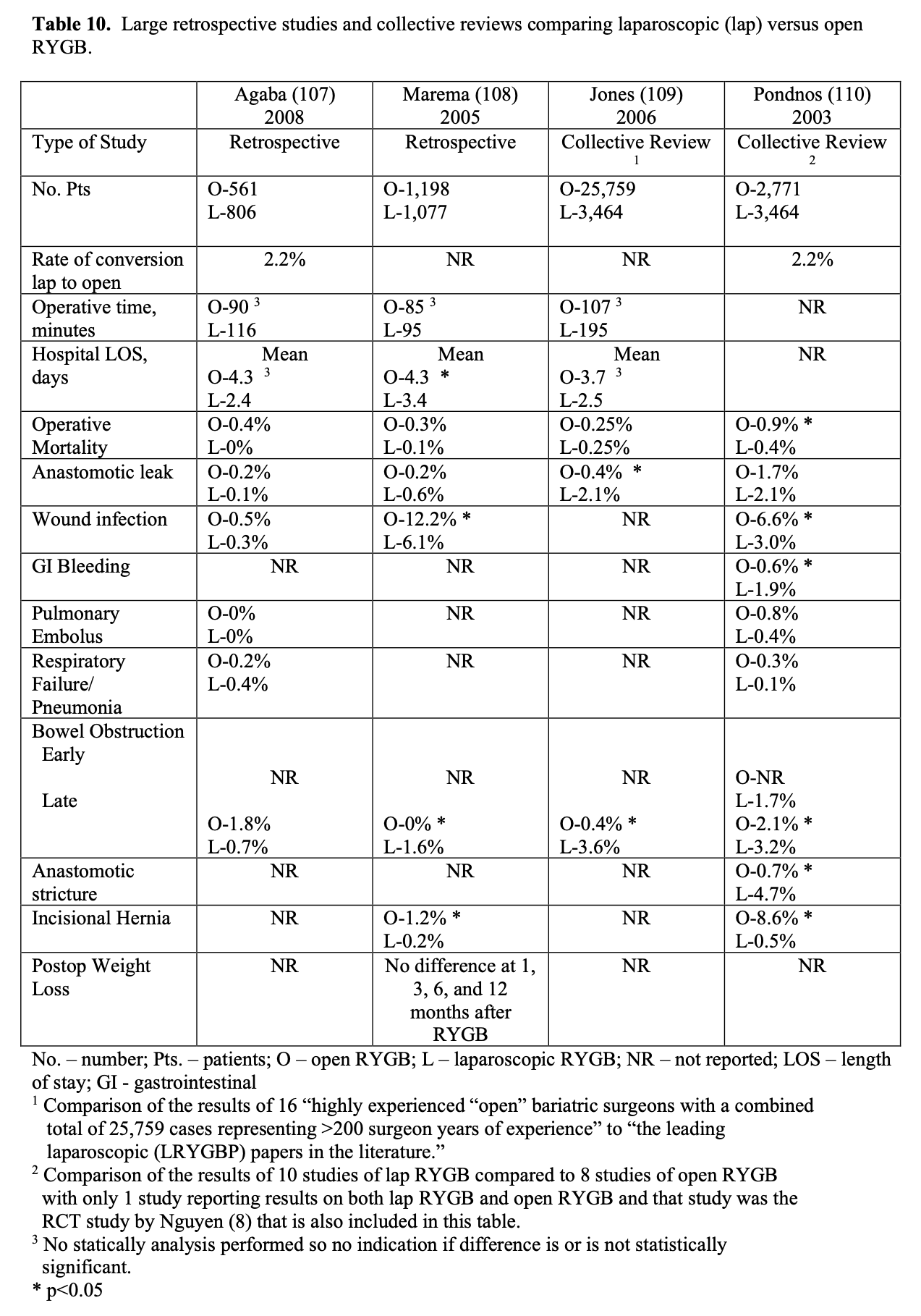
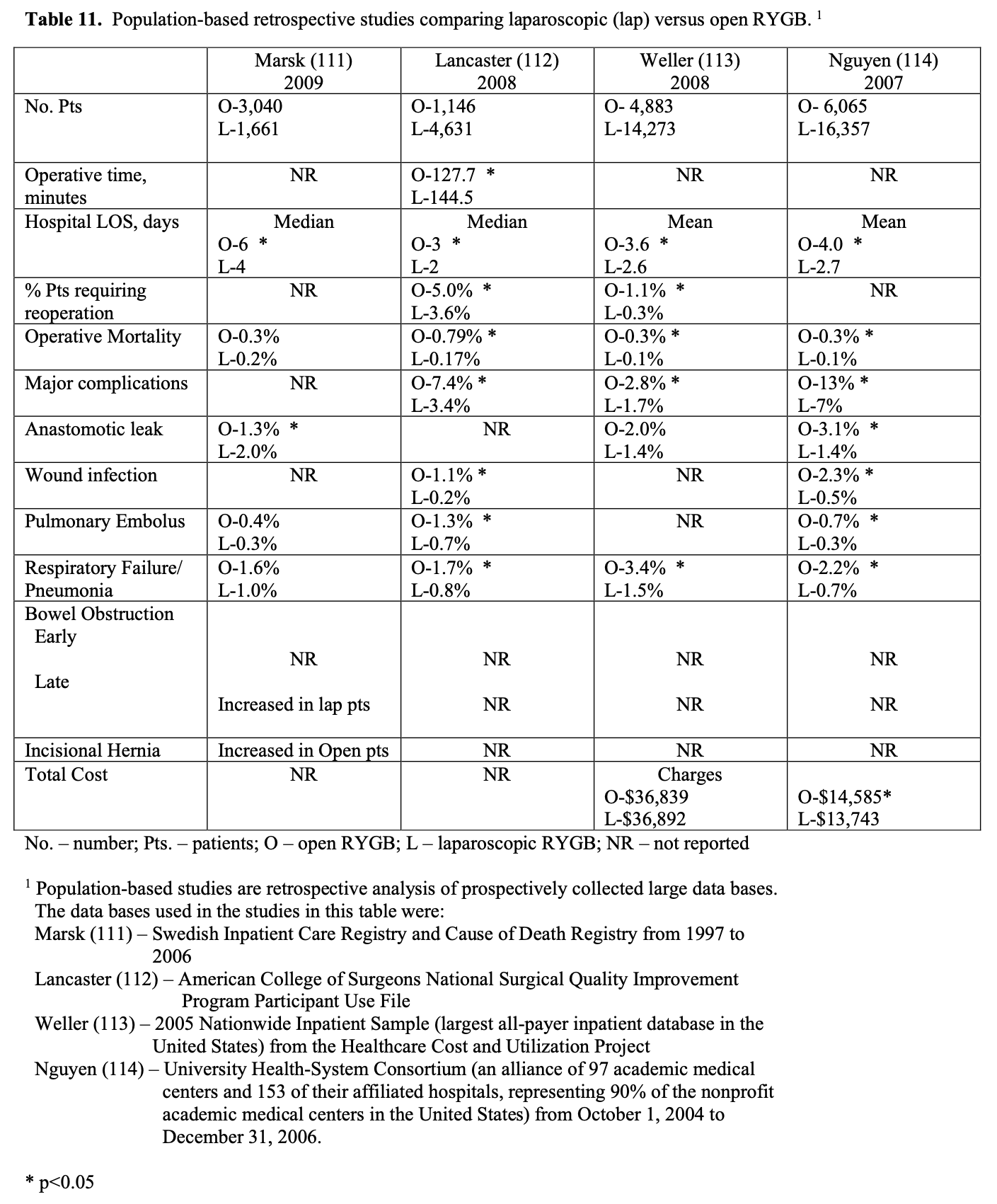
The operative mortality is low for both lap and open RYGB (Tables 9, 10, 11). In one collective review110 and two population-based studies113,114 operative mortality was significantly higher with open RYGB. However, it is possible that there was a procedure selection bias that could have affected these results. Three RCT,8,105,106 one prospective study with historical controls,37 one collective review,109 and one other population-based study111 showed no significant difference in mortality between lap and open RYGB. The early and late complication rate was similar for the two procedures. However, the open RYGB had significantly more major complications compared to lap RYGB, mainly due to an increased incidence of incisional hernia.
Initial reports of lap RYGB suggested a higher anastomotic leak rate than open RYGB; however, this was may have been due to the learning curve.115 Of the 5 large collective reviews or population based studies, two109,111 showed a significantly higher leak rate with lap RYGB, one114 showed a significantly higher leak rate with open RYGB, and two110,113 showed no significant difference (Tables 10, 11). So most likely the anastomotic leak rate is fairly equivalent for lap and open RYGB. Lap RYGB has a higher incidence of GJ stricture compared to open RYGB (Tables 9, 10). Lap RYGB may have a higher incidence of postoperative GI bleeding compared to open.110 Most studies showed that lap RYGB had a lower incidence of pneumonia and pulmonary complications (Tables 9, 10, 11). As previously mentioned, patients have less postoperative incisional pain after lap RYGB compared to open so there is probably less interference with coughing and deep breathing.106,115 The reported incidence of pulmonary embolus is slightly higher for open vs. lap RYGB (Tables 9, 10, 11) but venous thromboembolism prophylaxis needs to be applied equally for both of these procedures.115
One of the major advantages of the laparoscopic technique is that the smaller incisions result in a significantly lower incidence of wound-related complications such as wound infection and incisional hernia.115 All of the studies in Tables 9, 10, 11 showed a lower rate of major wound infections and this difference was statistically significant in two of the population-based studies. Also, there is a dramatic decrease in the rate of incisional hernias with lap RYGB. However, lap RYGB has been associated with a higher incidence of bowel obstructions. Early postoperative bowel obstruction is rarely seen with open RYGB but has been reported with lap RYGB,115 usually due to inadequate closure of trocar sites. Lap RYGB has also been found to have a higher rate of late bowel obstructions (Tables 9, 10, 11) due to technical narrowing of the EE, failure to close the mesenteric defects resulting in internal hernias, and failure to place the “anti-obstruction stitch” at the EE.115 But the incidence of bowel obstructions may be decreasing as more and more laparoscopic bariatric surgeons are closing the EE mesenteric defect. Lap RYGB does have a lower incidence of late bowel obstruction due to adhesions then open RYGB.21,44,116-118
The operative costs are much higher for lap RYGB because of the increased use of disposable equipment.8,101-103 However, the hospital costs for the postoperative care is higher for the open RYGB, mainly due to a longer hospital stay and an increased need for ICU care. So the overall hospital cost is about the same for the two procedures (Tables 9 and 11). There is no significant difference between lap and open RYGB regarding long term weight loss results (Tables 9 and 10).
The quality of life (QOL) as assessed by the Short Form 36 (SF-36) Health Survey is significantly lower in preoperative bariatric surgery patients compared to US norms.8 However, following RYGB the SF-36 scores improved rapidly. A RCT showed that compared to the open RYGB patients, the lap RYGB patients had significantly better SF- 36 scores in 4 of the eight domains (physical functioning, social functioning, general health, and bodily pain) at 1 month but was significantly better in only one domain (physical functioning) at 3 months. By 6 months, there was no significant difference in SF-36 scores between the lap and open RYGB groups and both groups’ scores were comparable to US norms.8 This study also showed that improvement or resolution of hypertension, depression, GERD, obstructive sleep apnea, diabetes mellitus, infertility, stress urinary incontinence, and lower extremity edema at 3-year follow-up was not different between the lap vs. open RYGB groups. However, the lap RYGB group had a significantly higher rate of improvement/resolution with osteoarthritis (80% vs. 61%, p<0.05) while the open RYGB group was significantly better at improvement/resolution of dyslipidemia (100% vs. 88%, p<0.01).103
This study also compared the postoperative systemic stress response between lap and open RYGB patients.102 Blood specimens were drawn after induction of anesthesia (baseline) and 1, 24, 48, and 72 hours postoperatively. Also nitrogen balance was performed 24 and 48 hours postoperatively. ostoperatively the plasma concentrations of insulin, glucose, epinephrine, dopamine, and cortisol increased; interleukin (IL)- 8 and tumor necrosis factor (TNF)-alpha remained unchanged; and the 24 and 48 hour nitrogen balances were negative. However, there was no significant difference in these variables between the lap and open RYGB groups. Concentrations of norepinephrine, adrenal corticotrophin hormone (ACTH), C-reactive protein, and IL-6 levels also increased, but these levels were significantly lower after lap RYBG compared to open RYGB (p < 0.05). Therefore, the systemic stress response is similar for both lap and open RYGB; however there may be a lower degree of operative injury with lap RYGB.
For these reasons, most RYGB and almost all adjustable gastric bands and sleeve gastrectomies are now being performed laparoscopically (Table 4). In comparison, there are much fewer BPD with or without DS being performed. According to BOLD only about one third of BPD or BPD/DS are currently being performed laparoscopically (National Aggregate Data Report – Bariatric Outcomes Longitudinal Database (BOLD), Raleigh, NC: Surgical Review Corporation, Accessed June 15, 2009). However, the proportion of these cases performed laparoscopically will most likely increase.
One study compared the results of 2,522 bariatric surgeries performed during a 17 month period of time in 2001-2002 (February 1, 2001 to July 1, 2002) and a 10 month period of time in 2005 to 2006 (February 1 to July 1 for 2005 and 2006) from a commercial insurance claims and encounter database that included 16 million enrollees under the age of 65 in employer-based benefit plans for 45 large employers in 49 states.119 The 180-day risk-adjusted complication rate declined by 21% (41.7% to 32.8%), the risk-adjusted readmission with complications declined by 31% (9.8% to 6.8%), the risk-adjusted hospital say declined from 6 to 3.7 days, and the risk-adjusted, inflation-adjusted hospital payments declined by 6% ($29,563 to $27,905 per patient). The authors concluded that these improvements in outcomes and cost were due to increases in within-hospital volume, laparoscopic versus open procedures, and gastric band versus gastric bypass.
Another study based on the American College of Surgeons Quality Improvement Program Participant Use File showed that LAGB had a similar operative mortality compared to lap RYGB (0.09% vs. 0.14%,p=1.0) but that LAGB had a significantly lower major complication rate (1.0% vs. 3.3%, p<0.0001), lower total complication rate (2.6% vs. 6.7%, p<0.0001), lower short-term return visits to surgery (0.94% vs. 3.6%, p<0.0001), and shorter hospital LOS (median 1 vs. 2 days, p<0.001).112 A systematic review comparing the results of the SAGB and the Lap Band showed that at 1, 2 and 3 years after surgery, there was no significant difference between the two gastric bands in regards to weight loss, complications and resolution of diabetes and hypertension.120
While short-term complications are lower and the hospital LOS is shorter with the gastric band compared to the gastric bypass, there is concern over the long-term complications, the incidence of weight loss failure, and the need for re-operation and/or band removal with the gastric band.121-123 There has only been one small (51 patients) RCT comparing gastric band to gastric bypass and with 5 years follow-up, the gastric bypass had better weight loss and less weight loss failures (defined as BMI>35 at 5 years) compared to the gastric band.123 In 2008, a systematic review of 14 comparative studies, all retrospective except for the above RCT that was included in this review, concluded that while gastric band has a lower operative time, short-term morbidity, and hospital LOS, gastric bypass had better weight loss results and lower re-operation rates with a trend towards higher patient satisfaction.122 A study of 380 LAGB patients in Switzerland assessed predictors of successful outcomes with LAGB.124 With a median follow-up of 5 years, 128 (34%) of the patients required band removal and 97.5% of these patients were converted to another procedure, BPD/DS (58% of the patients), lap RYGB (23% of the patients), or lap SG (19% of the patients). LAGB was found to be less successful in older patients and in patients with binge eating disorder or sweet-eating behavior so the authors recommended that these types of patients might be better candidates for other types of bariatric surgeries.
Consequently, controversy remains as to which if any of these bariatric surgical procedures is “the best” and it is the role of the bariatric surgeon to discuss with the patient the advantages and disadvantages of these various procedures in order to allow the patient to make an informed decision as to which procedure is “best” for them.
COMPLICATIONS AND RESCUE STRATEGIES
The complications and how to prevent and treat them, i.e. rescue strategies, will be discussed separately for each of the most commonly performed bariatric procedures.
Roux-en-Y Gastric Bypass (RYGB)
Bleeding. This is a risk with any surgical procedure. Most bariatric surgery patients receive perioperative anticoagulants for venous thromboembolism prophylaxis, which will be discussed later, placing them at higher risk for bleeding. Patients may experience intraabdominal bleeding from omental or mesenteric vessels encountered during the dissection or iatrogenic injuries. Also, injuries to the spleen or liver can cause bleeding. A liver retractor is used to retract the left lobe of the liver and expose the upper stomach.
Care must be taken when inserting and positioning the liver retract to avoid puncture or laceration of the liver. This can also occur with the needle when suturing close to the liver. Preoperative weight loss of 5% to 10% of initial body weight is recommended41 and has been shown to reduce the size of the liver125-128 and visceral adipose, including the mesentery and omentum,125 making the surgery technically easier. One study showed a statistically significant decrease in blood loss with preoperative weight loss (72 vs. 102 ml, p<0.05).127 When dissecting, stapling, and dividing the gastric pouch from the lower gastric remnant, care must be taken not to injury the spleen or short gastric vessels which are in close proximity. Also, bleeding can occur from trocar sites so all but the last cannula can be removed while observing the trocar site for bleeding and control the bleeding if it occurs.
Patients can also experience postoperative GI bleeding, which is usually related to one of the staple lines either in the gastric pouch or the EE. All staple lines should be examined for bleeding at the time of surgery and hemostasis controlled if necessary using cautery, clips, or suture ligation. Many surgeons at the end of the procedure perform intraoperative upper GI endoscopy to examine for staple line bleeding within the gastric pouch. If bleeding is seen, it can be controlled with endoscopic cauterization or, once localized, by suture ligation of the staple line from the intraperitoneal side. The internal EE staple lines can be examined prior to closure of the enterostomy used to create the anastomosis. If the patient develops intraabdominal or GI bleeding postoperatively, all anticoagulants are immediately discontinued and the patient is closely observed for hemodynamic instability and falling hemoglobins. Patients are transfused if necessary. If the bleeding persists or the patient develops hemodynamic instability then the patient needs to be taken back to surgery for laparoscopy or laparotomy and control of bleeding. If the patient is having signs of GI bleeding, hemetemesis or bloody bowel movements, an upper GI endoscopy can be performed to diagnose and treat a bleeding source in the gastric pouch. If the patient is hemodynamically stable, a nuclear GI bleeding scan can also be helpful to decide if the patient is still actively bleeding in the GI tract and, if so, where.
Oral or Nasal Gastric Tube (OGT/NGT) Complications. Anesthesia usually inserts an OGT to decompress the patient’s stomach following induction of anesthesia. The surgeon must remember to have anesthesia remove the gastric tube prior to any stapling or suturing of the stomach to prevent transecting the tube or suturing or stapling the tube into the gastric pouch and/or gastric remnant. While this has happened to almost every bariatric surgeon, it is rarely reported. But in one retrospective review of 727 lap RYGB patients looking specifically for this complication, the incidence of OGT complications was 1.2%, in 7 patients the OGT was stapled and transected and in 2 patients it was incorporated into the gastrojejunal anastomosis sutures.129 This significantly increases the operative time, may require conversion to an open procedure, and may be associated with significant morbidity. All of these cases occurred early in a surgeon’s lap RYGB experience or early in a surgeon’s experience in a new hospital setting. Due to this, the surgeon and anesthesia staff must maintain good communication and have established procedures in an attempt to avoid OGT complications. Also, it is not recommended to blindly insert a NGT in a patient who has had gastric bypass for concern of possible perforation of the gastric pouch, GJ, or the upper gastric Roux limb; although, I am not aware of any reported cases of this complication.
Anastomotic/staple line leaks, perforations, and intra-abdominal sepsis. A collective review of the literature including 32 studies revealed a GJ leak rate of 2.8% with a range of 0.3% to 8.3%.130 Some of the studies were mixed lap and open RYGB. The incidence of GJ leak was the same for lap and open RYGB, 2.1%, when the purely lap and purely open RYGB studies were compared with a range of 0.5% to 6.1% for open RYGB and 0.3% to 5.8% for lap RYGB. However, combining the results of the studies comparing lap and open RYGB in Tables 9, 10, and 11, lap RYGB has a small but statistically significant increase in anastomotic leak rate (1.5% (583/39,668) vs. 1.0% (461/44,176), p<0.001) with a range of 0% to 4.8% for open RYGB and 0% to 3.8% for lap RYGB).
Leaks most commonly occur at the GJ although they can occur at the EE, other staple lines, or due to GI perforations.21,131,132 Lee retrospectively analyzed their database of primary open RYGB (2,337 patients) and lap RYGB (1,080 patients) and found a significantly higher GJ leak rate with lap vs. open RYGB (4.1% vs. 1.7%, p<0.001).131 This study also found a 0.5% EE leak rate with the leak rate exactly the same for both lap and open RYGB. Seventeen (0.5%) patients had other types of leaks including other staple line leaks and/or perforations of the esophagus, gastric remnant, or jejunum and again there was no significant difference in incidence between lap and open RYGB (0.6% vs. 0.4%, p=0.39).
GJ leaks can occur early, within 48 hours, or late, 5 to 10 days.21 In Lee’s study, GJ leaks were detected an average of 4.3 + 5.6 days after RYGB with a median of 2 days with a longer detection time for open RYGB GJ leaks compared to lap RYGB (mean 4.9 + 6.7 days vs. 2.3 + 2.6 days, p<0.05; median 3 vs. 1 days, p<0.001).131 EE leaks are more difficult to diagnose than GJ leaks resulting in a longer detection time for the EE leaks (mean 5.9 + 5.6 days vs. 4.3 + 5.6 days, p=0.29; median 4 vs. 2 days, p<0.05). The detection time for EE leaks was longer in lap RYGB patients compared to open RYGB patients but due to the low number of EE leaks, the differences were not statistically significant (10.0 + 9.8 days vs. 4.6 + 1.9 days, p=0.35; median 8 vs. 4 days, p=0.92).
Possible etiologies for leaks include local factors (ischemia, tension, and infection), patient factors (advanced age, male gender, BMI>50, impaired wound healing, diabetes mellitus, cardiac failure, renal failure, peripheral arterial occlusive disease) and surgical factors (type of operation, operative technique, surgeon’s learning curve).133,134 In a multivariant analysis of 3000 open and laparoscopic RYGB, increasing age, male gender, preoperative obstructive sleep apnea, and type of procedure (open RYGB – 2.3%, lap RYGB – 4.2%, revisional RYGB – 6.9%) were independent risk factors of a leak.
Preoperative weight, BMI, and presence of hypertension or obstructive sleep apnea were not significant independent risk factors.135 Lee also found that leaks were more common in older patients and in males but found no relationship to preoperative weight or BMI.131
Surgeons have tried multiple different techniques in an attempt to decrease the incidence of anastomotic leak. Care must be taken to preserve the blood supply to the gastric pouch by preserving branches of the left gastric artery and to the jejunum by being careful not to compromise its blood supply in the process of transecting the mesentery to mobilize the jejunum.134 Also, if the end of the jejunum is resected after the GJ is constructed, which is typically done with the circular stapled GJ, the jejunum and its mesentery should not be transected too close to the GJ. Also, tension on the GJ needs to be minimized. This can be accomplished by ensuring that the small bowel is transected far enough away from the ligament of Treitz that the mesentery is sufficiently mobile.
Another technical factor that can affect tension on the GJ is whether the gastric Roux limb is positioned retrocolic or antecolic. The retrocolic approached was most commonly used for open RYGB but with the increasing use of lap RYGB, the antecolic approach is more commonly used. The sited reasons for using the antecolic approach include reduced operative time, less internal hernias at Peterson’s space, easier technique to teach, and easier to revise GJ if needed.133 However, the antecolic technique may place more tension on the GJ since the small bowel must extend over the top of the omentum, transverse colon, and gastric remnant to reach the gastric pouch. This may be at least partially relieved by splitting the omentum perpendicular to the transverse colon, in line with the GE junction, passing the Roux limb thru this split so it does not have to stretch over the thick fatty omentum.134 One retrospective study showed a trend for a lower GJ leak rate in lap RYGB patients who had the Roux limb placed retrocolic compared to antecolic.133 The retrocolic group had a lower intraoperatively-tested leak rate (0.5% (1/218) vs. 8.1% (11/135), p<0.001) and postoperative clinical leak rate (0.5% vs. 3.0%, p=0.07) but the difference in clinical leak rate did not quite meet statistical significance. However, the GJ in these patients were performed with either a circular or linear stapled anastomosis at the discretion of the surgeon. All of the clinical leaks occurred in linear stapled GJ and it was not stated how many GJ in the two groups, retrocolic and antecolic, had linear versus circular stapled GJ. So this may have significantly affected the results. Another retrospective study had the opposite finding with a significantly lower leak rate with the antecolic technique (0.1% (1/1000) vs. 1.9% (2/108), p<0.05).136 It is unclear as to the whether or not there is a difference in leak rate between the retrocolic and antecolic techniques.
The technique used in constructing the GJ could affect the leak rate. However, the leak rate appears to be similar for hand sewn, circular stapled, and linear stapled GJ (Table 12). Also, the size of circular stapler used does not seem to affect the leak rate (Table 12). However, when using linear GI staplers, the surgeon must choose the correct height of staples for the tissues being stapled to ensure secure staple lines. When using multiple stapler loads to transect the gastric pouch, the surgeon needs to make sure that there are no loose staples on the stapler arms are on the tissue, especially at the crotch of the previous staple line, which can cause a stapler misfire.130 Other techniques that have been used to decrease GJ leak rates include over sewing the staple lines, using staple line reinforcements, wrapping the anastomosis with omentum, and/or applying fibrin glue and other sealants to the anastomosis.147-154 However, more studies are needed to further delineate the effectiveness of these maneuvers in decreasing the leak rate.132
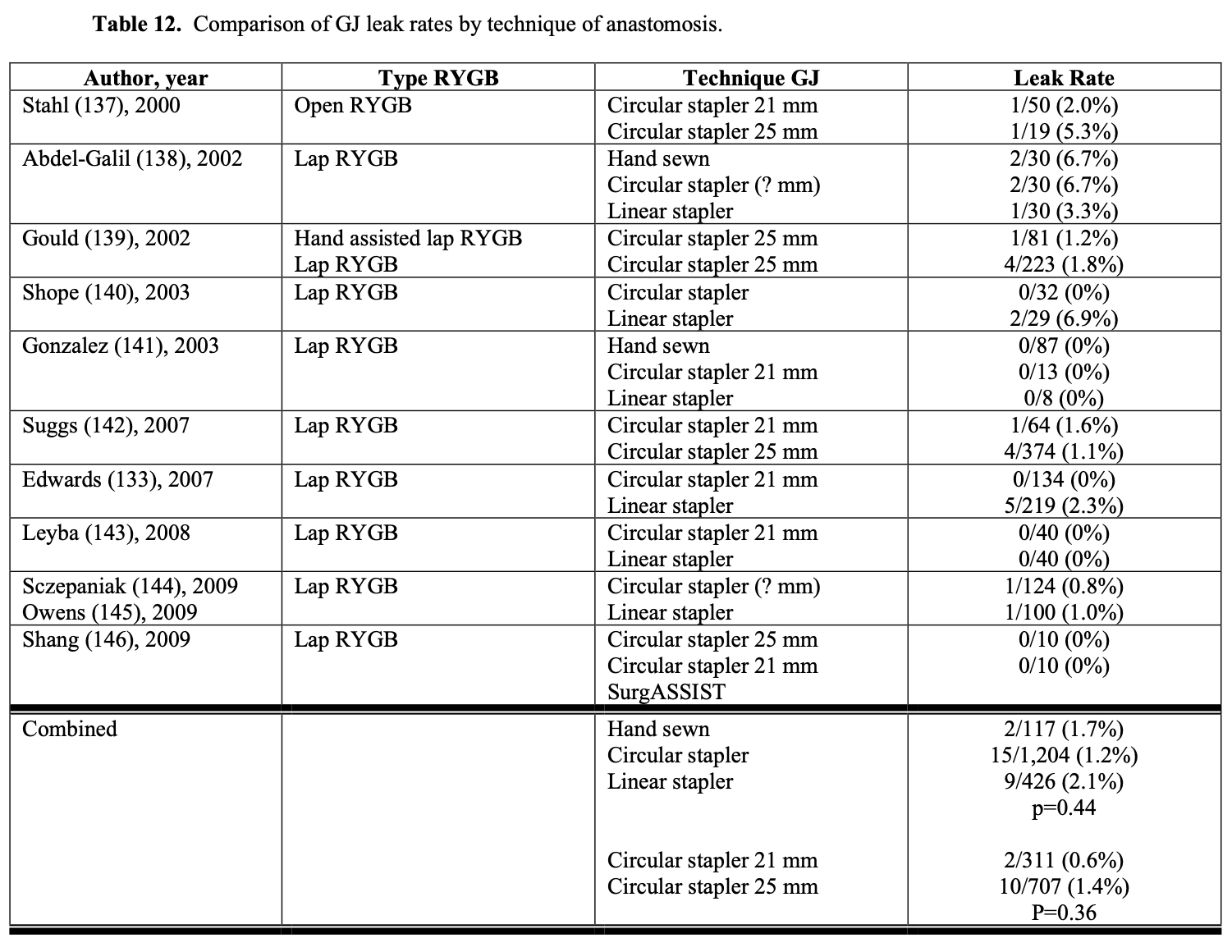
In a survey study of bariatric surgeons performing lap (98%) and open (2%) RYGB, 93% (186/201) of the surgeons’ routinely tested for a gastrojejunal anastomotic leak at the time of the RYGB procedure and some had used multiple different techniques (so the total percentage of techniques was > 100%).42 The most common method was by filling the gastric pouch with air, either by inserting an endoscope (38% of surgeons) or an nasogastric (NG) tube (45% of surgeons) into the pouch, while occluding the jejunum just below the anastomosis and filling the upper abdomen with saline solution.42,132 If no bubbling is seen thru the saline then the anastomosis and gastric pouch staple lines are airtight. If bubbling is seen then the staple line can be reinforced with sutures in the area of bubbling and the leak test repeated. Another technique of checking for a leak entails filling the gastric pouch with methylene blue dye through a NG tube (26% surgeons) and observing for any extravasation of dye intraperitoneally. Again, if there is dye extravasation then the area of leak can be closed with sutures and the leak test repeated. However, even if the intraoperative leak test is negative, patients can still develop anastomotic leaks postoperatively.132,133,155
In a study of 340 consecutive lap RYGB patients, intraoperative leak was detected via endoscopic insufflation of the gastric pouch in 16.4% of the patients.156 Patients older than 40 years of age had a significantly higher leak rate then younger patients (21% vs. 11%, p<0.05) and linear stapled GJ had a higher leak rate than circular stapled GJ although the difference was not statistically significant (18% vs. 12%, p=0.18). None of the patients developed postoperative leaks. The postoperative anastomotic stricture rate was the same for those patients who had an intraoperative leak detected and repaired and those patients who did not have a leak (5.4% vs. 5.6%, p-0.93). Champion found a postoperative GJ leak rate of 2.9% (1/34) in patients who had repair of an intraoperative leak found by endoscopic insufflation compared to a leak rate of 0.25% (2/791) in patients without an intraoperative leak (p=0.12).155 Another retrospective study showed a significantly higher postoperative leak rate in patients who had repair of an intra- operative leak found on routine testing (20% (3/12) vs. 0.6% (2/341), p<0.001).133
Some bariatric surgeons routinely place a closed suction drain posterior to the gastrojejunal anastomosis extending up into the left subdiaphragmatic space to better diagnosis and/or control an anastomotic leak if one occurs. However, other surgeons feel that having a drain in proximity of an anastomosis may increase the risk of leak or is simply not necessary.21,132,134,157 Kligman proposed a protocol for selective use of drains based on the findings of the intraoperative leak test via endoscopic insufflation of the gastric pouch: 1) if no air leak occurs, then no drain is needed; 2) if non-reproducible air leak (initially see air bubbles but stops and can not be reproduced), then place a closed suction drain at the surgeon’s discretion; 3) if persistent air leak, repair the leak and place a closed suction drain; and 4) postoperative contrast x-ray study should be considered before removal of the drain.158
Postoperatively, some bariatric surgeons routinely perform a modified upper GI (UGI) x- ray anywhere from postoperative day 1 to 3 to check for obstruction or leakage of the GJ.21,134,159,160 However, even if this x-ray is normal, a delayed leak can still occur several days later. Also, this x-ray procedure usually will not show the EE so is not good for detecting this type of leak.21,132,134,160 Carter reported the results of routine UGI water- soluble contrast x-ray studies on 654 consecutive RYGB patients.161 The test was done on postoperative day one for lap RYGB and day one or two for open RYGB. The UGI x-ray detected 3 out of 7 leaks (43% sensitivity) with the four non-detected leaks presenting at 4, 5, 34, and 56 days postoperatively. The UGI x-ray was read as showing a leak in 2 patients that were later reinterpreted as artifact or misreading after a repeat UGI x-ray was performed. Overall, 99% of the UGI x-rays were normal. He concluded that UGI x- rays should be reserved for patients with clinical evidence of possible leak. Katasani utilized a selective protocol and found that 78% of 553 lap RYGB patients did not need UGI x-ray and none developed a clinical leak.162 Of the 122 patients who met at least one criteria for possible leak, only four (3.2%) were confirmed to have a leak. None of the patients with a negative UGI x-ray subsequently developed a clinical leak. Several other authors have also advocated for selective use of postoperative UGI x-rays.163-165
Tachycardia, tachypnea, fever, hypoxia, and extreme anxiety are the earliest signs of anastomotic leak. Abdominal pain rarely occurs prior to the patient developing diffuse peritonitis and sepsis.21,134 Lee found that tachycardia, heart rate over 120 beats per minute, was present in 65% of patients with a GJ leak and 83% of patients with a EE leak. If a patient is suspected of having an anastomotic leak postoperatively, a modified contrast UGI x-ray can be performed using a water soluble contrast.131 Lee found that the UGI x-ray was diagnostic much more often for GJ leaks than for EE leaks (80.9% vs. 10.0%, p<0.001).131 The other option is to perform a computerized tomographic (CT) scan of the abdomen and pelvis with intravenous (IV) and water soluble oral contrast, which is probably more sensitive for a leak especially an EE leak.21,132
If a leak is detected by x-ray, management depends on the patient’s clinical condition. If the patient is showing signs of diffuse peritonitis, systemic sepsis, and/or hemodynamic instability then the patient needs urgent surgery.131,134However, if the leak is small and contained, a drain is already in place, and the patient has no signs of generalized peritonitis or systemic sepsis, the patient can be treated with broad spectrum IV antibiotics, nothing by mouth (NPO), nutrition support (either parenteral nutrition or enteral nutrition through a radiologically placed nasojejunal tube), and close observation.131,160,166 If a drain was not inserted at the time of the primary surgery, a radiologically guided percutaneous drain can be placed at the time of diagnosis of the leak.131 Most of the time these leaks will heal within a few weeks; however, there is a risk of developing a gastro-gastric fistula.21,131,160,167 If the x-ray examinations are negative and the patient continues to have signs and symptoms of an anastomotic leak or the x- rays show a leak and there is any question as to whether or not the leak is contained, then exploratory laparoscopy or laparotomy is indicated.21,132,134 Endoscopic stents have also been utilized in the non-operative management of GJ leaks.168,169
In Lee’s study, 69% of the GJ leaks only were treated with re-operation and this was similar for both lap and open RYGB patients (67% vs. 71%, p=0.72).131 None of the patients treated non-operatively subsequently required re-operation. The rate of operative treatment of the GJ leak was not significantly different between those patients who had a drain placed at the time of the initial operation or those that did not (67% vs. 78%). All patients that did not have a drain placed at the time of RYGB and were treated non- operatively had successful percutaneous radiologically guided drains inserted and responded to non-operative treatment.
At the time of reoperation, after thorough irrigation and identification of the leak, closure of the leak can be attempted. However, sometimes this is not feasible or it has a high risk of recurrent leak due to the dense inflammatory changes in the tissues around the leak.134 A patch of omentum or adjacent gastric remnant can be used to reinforce the close. The area needs to be well drained in attempt to establish a controlled fistula if re-leak does occur. Also, the lower gastric remnant should be decompressed with insertion of a gastrostomy tube. The gastrostomy tube can also latter be used for enteral nutrition support if necessary.134
Anastomotic leaks are associated with a significant risk of mortality and are one of the top 3 causes of operative mortality following RYGB, with the other two being pulmonary embolism (PE) and myocardial infarction (MI).21,110Lee found a leak rate of 3.4% (117/3,417) with a mortality of 17% (20/117).131 These leaks included GJ only (69%),EE only (10%), and other (21%), which included combined GJ and EE leaks, other staple line leaks, and/or perforations of the esophagus, gastric remnant, or jejunum. Even though the lap RYGB had a significantly higher GJ leak only rate compared to open RYGB (1.6% vs. 4.0%, p<0.001), the lap RYGB GJ leak only patients had a significantly lower mortality compared to the open RYGB (2.3% vs. 18.4%, p<0.05). The mortality of patients with GJ leaks treated operatively was higher than those treated non-operatively (16% vs. 0%), which is intuitive since the more severely ill, septic patients were more likely to receive surgical treatment and the stable controlled leak patients were more likely to be treated non-operatively. The mortality of the EE leaks only was significantly higher than the GJ leaks only (50% vs. 9.9%, p<0.01). This was probably due to the increase in difficulty diagnosing the EE leaks and therefore longer delay in diagnosis and treatment of these leaks. The incidence of EE leaks only and the mortality for these leaks was the same or similar comparing lap RYGB to open RYGB, 0.4% vs. 0.3% (p=1.00) and 50% vs. 50% (p=1.00), respectively.131
Venous Thromboembolism (VTE). Deep vein thrombosis (DVT) occurs in 1% to 3% of the patients and PE occurs in 0.3% to 2% and is fatal about 30% of the time, being one of the top three causes of operative mortality.21,110,170 In June 2007, the ASMBS released a position statement on “Prophylactic Measures to reduce the Risk of Venous Thromboembolism in Bariatric Surgery Patients” that stated “Early post-operative ambulation and peri-operative use of lower extremity sequential compression devices are safe and suggested for all bariatric patients when feasible. Unless contraindicated, chemoprophylaxis using various anticoagulant regimens is an important adjunct to these methods which should be routinely administered to bariatric surgery patients.” However, the position statement acknowledged that the choice of anticoagulant, anticoagulant dosage regimen, duration of prophylaxis (including post-hospital prophylaxis), and use of inferior vena cava filter remains “controversial.”171
A survey of ASMBS members regarding VTE prophylaxis was conducted in 1998172 and repeated in 2007170. The most recent survey was completed by 35% of the membership. Routine mechanical prophylaxis for VTE was used by 98% of the respondents with sequential calf compression devices being used by 61%. Chemical prophylaxis for VTE was used by 94% of the respondents with 58% using enoxaparin, a low molecular weight heparin, and 31% using unfractionated heparin. The dose of enoxaparin ranged from 40 mg daily (21%) to 30 mg twice a day (26%) to 40 mg twice a day (45%). The dose of unfractionated heparin ranged from 5,000 unit every 12 hours (30%) to 5,000 units every 8 hours (62%). In bariatric surgery patients that were at a “high risk” of VTE, as defined by personal history of previous DVT/PE, prolonged immobilization, or leg swelling, 60% of respondents would continue chemical prophylaxis post-hospital discharge for any where from 2 to 4 weeks with most (86%) using low molecular weight heparin and some using warfarin (14%) or unfractionated heparin (1.5%). In these “high risk” patients, 55% of the respondents had used a prophylactic inferior vena cava (IVC) filter in at least one patient but it was not clear exactly how often this form of prophylaxis was actually used. With the newer temporary removable IVC filters, this option is more attractive then previously.
Mechanical prophylaxis was used by most bariatric surgeons in both surveys; however, the use of chemoprophylaxis increased dramatically with heparin use increasing 94% and the use of low molecular weight heparin increasing from 13% of respondents to 58% of respondents. The incidence of DVT and PE decreased between these two surveys from 2.6% and 0.95% to 0.93% and 0.7%, respectively; however, it is unclear how accurate these figures are since they were self-reported. Despite these encouraging results, other bariatric surgeons are concerned about the potential increased risk of bleeding with chemoprophylaxis.
Clements reported their results in 957 laparoscopic RYGB patients treated with mechanical prophylaxis alone.173 Patients at “high risk” of VTE, history of previous VTE, hypercoagulable state, or strong family history of VTE, received either anticoagulants or IVC filters and were excluded from the study. Their patients developed clinical DVT in 3 patients (0.31%) and one patient had a non-fatal PE (0.1%). Six of their patients (0.6%) developed bleeding complications requiring either reoperation (0.2%) or blood transfusions alone (0.4%). Clements compared their results to 5 other recent published bariatric surgery series, which showed an incidence of DVT between 0% and 1.0% and PE between 0% and 1.2% with an incidence of major postoperative bleeding between 0.9% and 5.9%. Clements concluded that in selected patients mechanical prophylaxis alone could obtain similarly low incidence of DVT and PE with a much lower incidence of complications of postoperative bleeding.
Cardiopulmonary complications. Perioperative MI occurs in 0.5% to 1% of bariatric surgery patients and is one of the top three causes of operative mortality.21 Obesity alone is an independent risk factor for coronary artery disease and bariatric surgery patients frequently have a family history of cardiac disease. Some bariatric surgery patients have severe cardiomyopathy that can increase their risk of complications such as pulmonary edema, hypotension, and renal insufficiency. However, bariatric surgery can be safely performed in most of these individuals resulting in a significant improvement in their cardiac function with surgical weight loss.174 Preoperative cardiac risk assessment is important including the use of consultation with a cardiologist, cardiac stress test, echocardiogram, and even cardiac catheterization in selected patients based on their individual risk factors. Also, appropriate management or treatment of cardiac risk factors pre- and intraoperatively is essential in minimizing perioperative cardiac complications.175 Symptoms may be atypical, especially in diabetic patients, so you must maintain a high index of suspicion.21
Obese patients are at increased risk of pulmonary complications intra- and postoperatively so preoperative assessment and treatment of risk factors is important. Patients should be encouraged, and some bariatric surgeons even require, to cease smoking at least 6 weeks or longer before surgery to decrease the risk of pulmonary complications; although, the evidence that this is beneficial is limited.21,176 Patient’s should also continue cessation of smoking indefinitely following surgery in order to decrease the risk of pulmonary disease, lung cancer, and anastomotic ulcer formation.21,41,177,178
Obstructive sleep apnea (OSA) is common in obese individuals and increases the risk of perioperative pulmonary complications.179-182 Most bariatric surgeons screen patients for OSA preoperatively. The Epiworth Sleepiness Scale is one screening tool and involves patients completing a survey of how often they fall asleep in 6 different daily situations resulting in a score of 0 to 24 with a score of 10 or greater being at risk for OSA.175,183 In addition, patients can be asked if their family or friends have noted excessive snoring or periods of apnea while they sleep. Patients at risk of sleep apnea should undergo polysomnography (sleep study) to confirm the diagnosis and document the severity and need for treatment of OSA with either continuous positive airway pressure (CPAP) or bi- level positive airway pressure (BIPAP).41,175
If patients are CPAP or BIPAP dependent, most surgeons advocate continuing these treatments in the postoperative care recovery unit while the patients are emerging from anesthesia and subsequently whenever the patients are sleeping. However, other surgeons are concerned that this positive pressure may be transmitted via the esophagus to the gastric pouch and increase the risk of perforation, although this has never been shown.
One group of bariatric surgeons who did not routinely screen for OSA and did not routinely use CPAP or BIPAP in the postoperative period after laparoscopic RYGB showed no difference in pulmonary complications between the patients who had CPAP or BIPAP dependent OSA, non-dependent OSA, or no history of OSA (1/144 (0.7%) vs. 3/140 (2.1%) vs. 6/811 (0.7%), p=0.26 comparing all 3 groups and p=0.37 comparing the two OSA groups).184 However, these bariatric surgeons emphasized that all OSA patients were observed in a monitored unit maintaining their oxygen saturation above 92%. Also, all patients were trained in incentive spirometry and ambulated within a few hours of surgery.
Airway management can be very difficult in obese patients. Fortunately, there are multiple techniques and newer equipment available to deal with the difficult airway.41 The anesthesiologist must assess the patient preoperatively for this situation and have all of the necessary equipment and staff available and ready at the time of induction. Also, the patients must be closely monitored following extubation to ensure maintenance of a patent airway.181,185,186 Postoperatively, hypoventilation, atelectasis, pulmonary edema, and/or pneumonia can result in acute respiratory insufficiency and hypoxemia. One study showed that transient oxygen desaturation occurs frequently in patients with and without OSA and with or without routine oxygen supplementation following laparoscopic bariatric surgery.179 Also placing the patient in at least 25 degree head- position can improve lung function, atelectasis, and shunting.180 So all patients following bariatric surgery should be given supplement oxygen, placed in head-up position, and monitored for hypoxemia and treated appropriately. Routine postoperative incentive spirometry and early ambulation are also important in decreasing the risk of pulmonary complications.
Wound infection. This is one of the most common complications after gastric bypass ranging in incidence from 5% to 20% and varying in severity. Since the incisions are much smaller, lap RYGB has a lower incidence and severity of wound infections compared to open procedures. Showering with antibacterial soap the night before and the day of surgery may decrease the skin flora somewhat and decrease the risk of postoperative wound infection. Since this is a clean-contaminated procedure, prophylactic antibiotics given at the time of induction of anesthesia and for 24 hours following surgery can decrease the risk of wound infection. In the case of open RYGB, a closed suction drain in the large subcutaneous space may also decrease the incidence of wound infection. Treatment depends on the severity of the infection. If there is only mild erythema without fluctuance or drainage then local wound care and oral antibiotics may be adequate. However, if there is any drainage, fluctuance, or question of a wound abscess then thorough incision, drainage, and irrigation of the wound needs to be performed. Following this the wound should be packed open with saline wet-to-wet dressing changed 3 to 4 times a day and the wound allowed to heal by secondary intention. The patient should also be treated with antibiotics and, if the wound infection is severe, the patient may need to be hospitalization for IV antibiotics for a few days followed by oral antibiotics for 7 to 10 days total.21
Nausea and Vomiting. Most patients will experience nausea and many will have vomiting at some time after bariatric surgery. All currently performed bariatric procedures have some degree of gastric restriction as a part of the procedure so patients have to be thoroughly instructed prior to surgery and continually reminded after surgery that they have to take smaller bites, chew their food more thoroughly (25 to 30 chews per bite), eat slower, eat less amounts at one time, and avoid dry foods or foods that are prone to get stuck in the pouch such as red meat, bread, pasta, and solid vegetables.21 If the nausea and vomiting is mild and intermittent, the bariatric dietitian can work with the patient regarding their dietary habits. However, if the nausea and vomiting is severe or persistent then the patient needs further evaluation to rule out obstruction. Also, bariatric surgery patients can become dehydrated and develop acute renal failure quickly. If adequate oral intake can not be maintained, the patient should be admitted for IV fluid hydration and correction of any electrolyte abnormality.
Dehydration and electrolyte disorders. Dehydration is a common problem in RYGB patients especially in the immediate postoperative period but can also occur later.21 Patients are recommended to drink 48 to 64 ounces of fluid daily. With the small gastric pouch and the usual postoperative edema that occurs at the GJ, patients must drink small amounts frequently. Once the patients have advanced to solid food, they are instructed to not to drink fluids for 1 hour after eating solid food in order to avoid rapid emptying of the gastric pouch and maintain satiety. Patients who are experiencing nausea or vomiting, are already dehydrated, or are exposed to hot environments may not be able to meet their fluid needs orally and may need IV hydration. Patients that are on diuretics preoperatively should be instructed to discontinue use or use judiciously postoperatively to avoid dehydration and even possible acute renal failure. Patients can also develop problems with electrolyte deficiency especially potassium, phosphorous, andmagnesium.
Anastomotic (Marginal) Ulcers. Anastomotic or marginal ulcers occur in 1% to 16% of patients, with most studies reporting an incidence of approximately 2%.21,177,178 Combining the results of the studies in Table 9, the incidence of anastomotic ulcers was 3.1% with a range of 0% to 10% and no significant difference in incidence between open and lap RYGB (4.0% (5/122) vs. 2.2% (3/134), p=0.48) The ulcers can present any time from weeks to years after surgery.21,177 In a study of 3285 lap RYGB patients with a linear stapled GJ, Sacks found an 1.7% incidence of anastomotic ulcers with symptoms appearing a mean of 11.2 months after surgery with a range of 0.5 to 36 months.178 The majority of patients (63.2%) presented within the first 12 months after surgery.
The ulcers are usually located on the jejunal side of the GJ. The etiology of anastomotic ulcers is often multifactorial and is still not completely understood. Potential etiologies include use of non-steroidal anti-inflammatory drugs (NSAID), large proximal gastric pouch, development of gastro-gastric fistulas, and smoking.21,177,178 In a study involving 57 anastomotic ulcers following lap RYGB, 40% smoked and 16% were taking NSAID preoperatively, no mention was made if the patients were still smoking or using NSAID at the time of presentation of the ulcer.178 In addition to exposure of gastric irritants such as NSAIDs, the etiology of these ulcers is thought to be due to increased acid exposure and poor acid protective mechanisms of the jejunum.21,177 Suboptimal perfusion to the GJ may also be a contributing facture, especially if there is some tension on the GJ and/or the end of the jejunum is transected closely to the GJ.177,178
Helicobacter pylori (H pylori) infection may also be a causative agent.21,177,178 Combining the results of 7 studies, H pylori was present in 22% (192/882) of the patients with a range of 10% to 67%.187-192 Four studies have shown or suggested a relationship between the presence of H pylori preoperatively and the risk of postoperative GI complications. One study found an increased incidence of marginal ulcers in a group of patients not tested for H pylori compared to a group of patients who tested positive for H pylori and were treated prior to RYGB (6.8% (24/354) vs. 2.4% (5/206), p<0.05).190 Another study showed that patients who were H pylori negative had a lower incidence of postoperative marginal ulcers compared to a group of untreated H pylori positive patients (19% (10/53) vs. 48% (10/21), p<0.05).193 Rasmussen found a significantly higher incidence of preoperative H pylori seropositivity in patients who developed anastomotic ulcers after surgery compared to those patients who did not (32% (6/19) vs. 12% (29/241), p<0.05).188 The fourth study found a higher incidence of postoperative GI bleeding or ulcers in patients who were H pylori positive and treated preoperatively compared to those that were H pylori negative but this difference was not statistically significant (18.2% (2/11) vs. 5.8% (5/85), p=0.18).187
The technique of constructing the GJ may affect the incidence of anastomotic ulcers. In a study of open RYGB patients, Capella found a higher incidence of anastomotic ulcers if the gastric pouch was stapled in continuity rather than stapled and divided but the difference was not statistically significant (8.5% (16/189) vs. 5.8% (18/311) , p=0.25).40 The GJ was constructed with a circular stapler in both groups. The incidence of anastomotic ulcers was significantly lower when a two layered hand sewn anastomosis was compared to the circular stapler GJ with divided gastric pouch (5.8% (18/311) vs. 1.2%% (5/403), p<0.001). Table 13 shows the results of 5 studies that examined the relationship between the technique used to construct the GJ and the incidence of anastomotic ulcer. While the numbers of patients in these studies are low, the linear stapled GJ seems to have the lowest incidence of anastomotic ulcer while the circular stapled GJ the highest. In circular stapled GJ, there is no difference in the incidence of anastomotic ulcer between the 21 mm circular stapler and the 25 mm circular stapler.
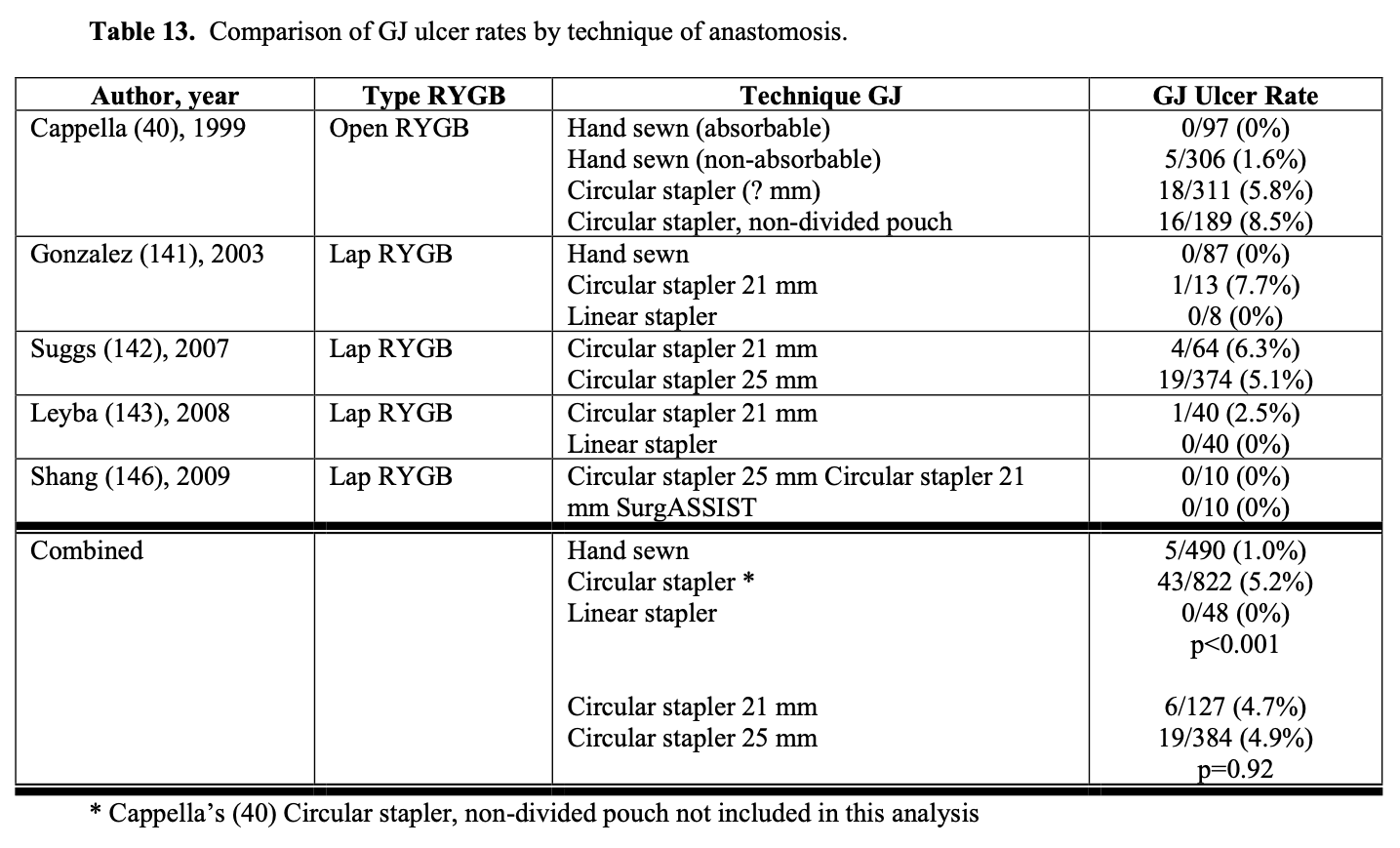
The presence of foreign bodies at the GJ (staples or suture material) and the use of non- absorbable sutures on the GJ have also been associated with anastomotic ulcers.21,177,178 In Sacks study, the enterotomy from the linear stapled GJ was closed by nonabsorbable suture in an initial series of 1,095 patients and then by absorbable suture in the next 2,190 patients.178 The anastomotic ulcer rate was significantly lower in the absorble suture group (1.3% vs. 2.6%, p<0.05). Also, the incidence of endoscopically finding suture material at the site of the marginal ulcer was significantly lower in the absorbable suture group (3.4% vs. 64.3%, p<0.001). In another retrospective study, the GJ was created with a 25 mm circular staple and then reinforced with an outer layer of sutures, using nonabsorbable sutures in the first 231 patients and absorbable sutures in the next 84 patients. Again, the absorbable suture group had a significantly lower incidence of anastomotic ulcer (2.3% vs. 13.4%, p<0.05).177 In Cappella’s study, there was a trend toward a lower incidence of ulcers in the hand sewn GJ group when absorbable sutures were used for both layers of the anastomosis compared to non-absorbable sutures used for the outer layer (0% (0/97) vs. 1.2%% (5/306) , p=0.34).40 However, Rasmussen in a study of 260 lap RYGB patients found no significant difference in the incidence of anastomotic ulcer when absorbable sutures were used to close the gastroenterostomy of a linear stapled GJ compared to non-absorbable (8.1% (10/124) vs. 6.6% (9/136), p=0.65).188
Symptoms of anastomotic ulcer include epigastric abdominal pain, dysphagia, nausea & vomiting, heartburn, and upper GI bleeding.21,177,178 Upper GI endoscopy is the best modality for diagnosing anastomotic ulcers and, frequently, associated anastomotic stricture may also be seen.21,177,178 Treatment includes avoidance of contributing factors (NSAIDs, smoking, other gastric irritants), acid suppression with proton pump inhibitors, and barrier protection of the ulcer with sucralfate (recommend using liquid slurry rather than tablets in order to better coat the ulcer).21,178 Endoscopic removal of any foreign bodies, staples or suture material, associated with the ulcer can be beneficial for healing of the ulcer.178,194 Anastomotic ulcers usually respond to medical therapy; however, if refractory, surgery may be required to resect the ulcer and reconstruct the GJ.21,194
Anastomotic strictures. The incidence of GJ stricture varies widely from 0% to 35.8% (Table 14). Combining the results of 6 studies, the mean time from surgery to dilation of stricture was 53.4 days with a range of 17 to 197 days.225,227,229-231,239 The proposed mechanisms for stricture formation are ischemia, excessive scar formation, technique used for construction of the GJ, and ulceration from gastric acid hypersecretion, non- steroidal anti-inflammatory medications (NSAID), alcohol, or other irritants.225,239,242
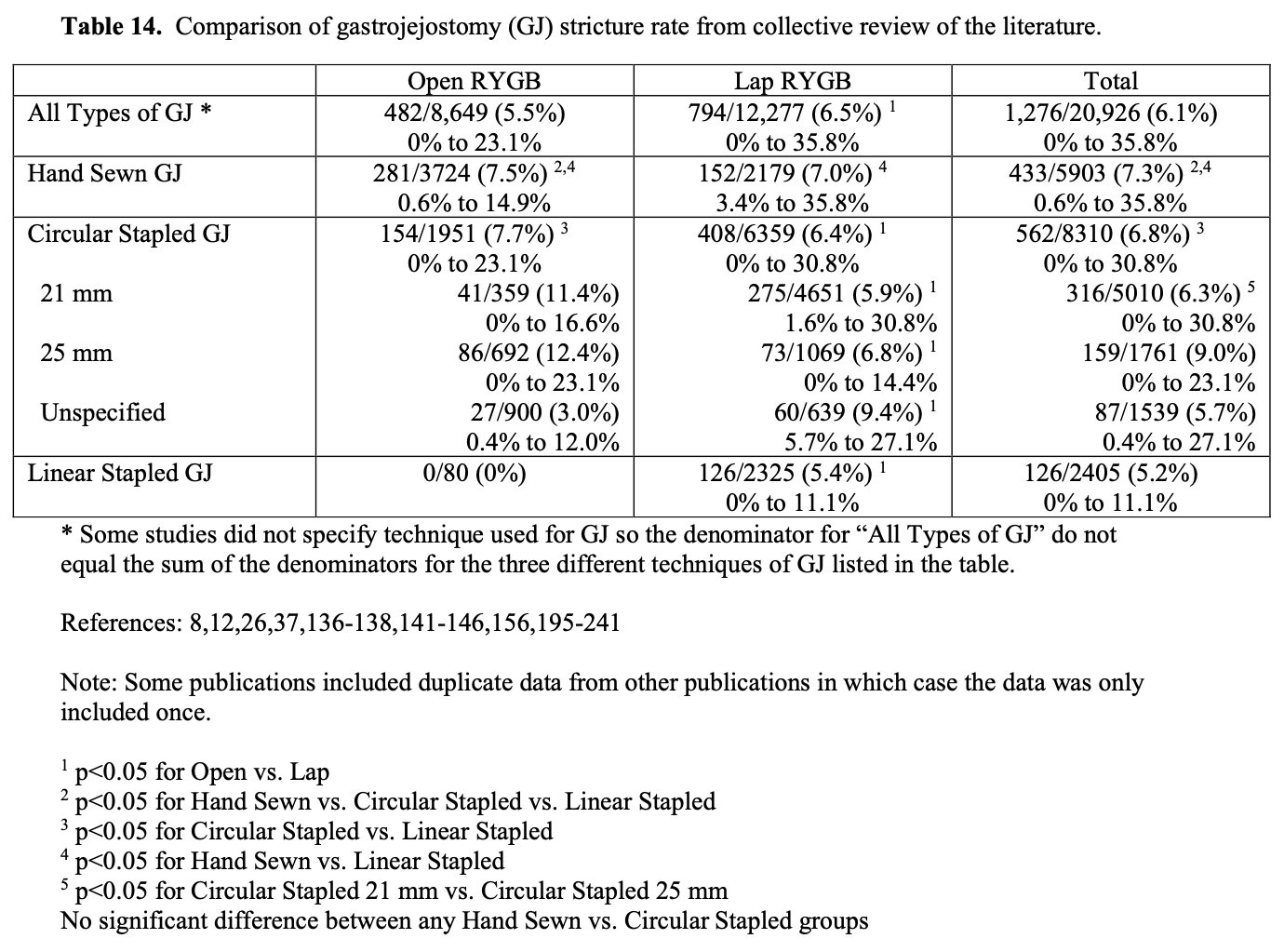
Blackstone conducted a multivariant analysis of the predictors of GJ stricture on 1,351 lap RYGB patients.228 Younger age and the preoperative history of GERD significantly increased the risk of stricture while gender and preoperative BMI were not significant predictors. Patients who were 35 years or younger had a two time greater risk of developing stricture compared to patients 36 to 45 years of age and a 2.32 times greater risk of stricture compared to patients 46 to 55 years of age, the risk of stricture leveled off after age 55. There is no readily apparent explanation for this finding and it is counterintuitive as younger individuals should have better circulation and better perfusion to a fresh anastomosis and therefore, if anything, should have a lower rate of ischemia and stricture. On the other hand, GERD is known to cause inflammation in the lower esophagus and fundus of the stomach and therefore, may predispose to more vigorous scarring at the GJ. However, Blackstone instituted a protocol of routine administration of H2 antagonist for 30 days following RYGB and this did not seem to decrease the risk of GJ stricture.
Based on a collective review of the literature (Table 14), lap RYGB had a significantly higher stricture rate compared to open RYGB (6.5% vs. 5.5%, p<0.05) with a range of 0% to 35.8% for lap RYGB and 0% to 23.1% for open. However, only 8 studies directly compared lap to open RYGB (Table 15) and only 1 of these studies was a randomize controlled trial.8 Also, all but two of these studies used different techniques in constructing the GJ.213,229 Despite these shortcomings, 6 of the studies showed a higher stricture rate with lap RYGB with statistically significant differences in 3 of these studies. One study showed no difference in stricture rate between lap and open RYGB while one study showed a significantly lower stricture rate with lap RYGB but again the GJ was constructed differently in these two groups of patients. Combining the results of the lap versus open RYGB studies in Tables 9 and 10, the incidence of stricture was significantly increased in the lap RYGB group (4.8% (176/3651) vs. 0.9% (23/2670), p<0.001). One study of 379 lap and open RYGB showed no significant difference between these two approaches but did show a higher incidence of stricture when the Roux limb was positioned retrocolic, retrogastric versus antecolic, antegastric but this difference did not quite meet statistical significance (7.3% vs. 3.0%, p=0.08).242 This study also showed a higher stricture rate in the first 50 cases of a surgeon’s experience but again the difference was not statistically significant (6.0% vs. 2.6%, p=0.08).
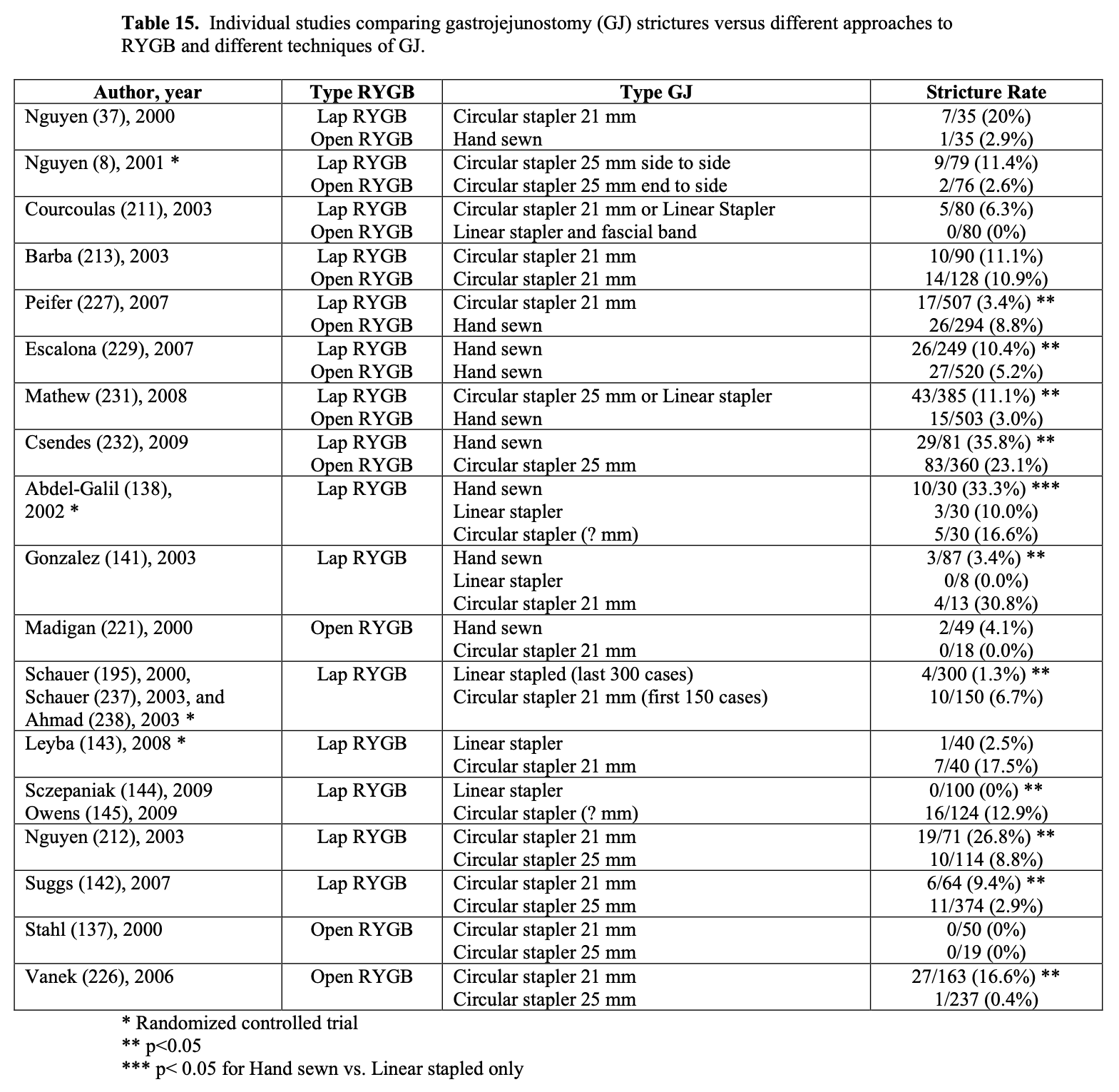
Several different techniques have been used to create the GJ. In a survey of 202 bariatric surgeons doing lap (98%) and open (2%) RYGB, 43% had used a circular stapler, 41% had used a linear stapler, and 21% had used totally hand sewn (some surgeon’s marked more than one technique so the total percentage is >100%).42 In the surgeons that have used a circular stapler, 61% had used a 25 mm circular stapler and 43% had used a 21 mm circular stapler.42 The incidence of strictures also varies depending on the technique used to construct the GJ (Table 14). Linear stapled anastomosis has the lowest risk of stricture (5.2%) with hand sewn and circular stapled anastomosis having similar stricture rates (7.3% vs. 6.8%). Again, only 6 studies have directly compared either two or all three of these techniques of GJ (Table 15) and only two of these studies were RCT.138,143 In the five studies that included a linear stapled group, this group had the lowest stricture rate and the difference was statistically significant in four of the five studies. Three studies included hand sewn and circular stapler groups. One of these studies showed a significantly lower stricture rate for the hand sewn group while the circular stapler group had a lower but not statistically significant stricture rate. The lap RYGB with hand sewn GJ has the widest variation in reported incidence of anastomotic strictures. This method of constructing the GJ during a lap RYGB is the most technically challenging and so may have the one of the largest variations from surgeon to surgeon. Over half of the patients in this category in Table 14, 69% (1500/2179), were performed by one surgeon, Higa, who is very skilled at laparoscopic suturing.199,234,235 His GJ stricture rate was 4.9% compared to 11.6% for the rest of the hand sewn laparoscopic GJ.
It is difficult to say which method and technique for constructing the GJ is the best in regards to minimizing the risk of postoperative stricture formation. In addition to the two methods, lap vs. open RYGB, and the three techniques of constructing the anastomosis, hand sewn, circular stapler, or linear stapler, there are many other variations including whether the hand sewn anastomosis were one layered or two layered, whether the stapled anastomosis were reinforced with sutures and how many and with what kind of suture, whether staple line reinforcement strips were used, whether any fibrin sealant was used, etc. Also as mentioned above, the study designs vary dramatically with few RCT. Even in the comparative studies (Table 15), there seem to be multiple differences between the two studygroups.
For example, in our study, we experienced a high stricture rate (16%) in our first 163 open RYGB performed using a retrocolic, retrogastric Roux limb and a 21 mm circular stapled GJ with over sowing of the staple line with interrupted silk sutures.226 So we changed to a 25 mm circular stapled GJ and our stricture rate decreased to 0.4%. But we also made several other changes in our technique. We stopped using staple line reinforcements for division of the stomach in the formation of the gastric pouch in order to perform an end-to-side GJ rather than a side-to-side GJ. Instead of transecting the small bowel 30 cm below the ligament of Treitz, we divided it 100 cm distal where the small bowel mesentery is more mobile. Also we divided more of the small bowel mesentery perpendicular to the small bowel at the site of transection. All of these maneuvers would theoretically decrease the tension on the GJ and therefore could have also contributed to the decrease in GJ stricture rate. Interestingly, when we started performing lap RYGB, we continued using the 25 mm circular stapler for the GJ but transected the small bowel 30 cm from the ligament of Treitz and placed the Roux limb antecolic and antegastric. Our GJ stricture rate increased to 14.3% in our first 21 cases. When we started transecting the small bowel 100 cm distal to the ligament of Treitz again, our stricture rate decreased to 5.5% in our next 163 lap RYGB (unpublished data). In conclusion, there are probably multiple factors that can affect the perfusion of and tension on the GJ and subsequently influence the rate of GJ stricture. Probably the most important thing is to monitor your GJ stricture rate and if it is too high, to consider modifying your technique in order to optimize your outcomes.
Patients with GJ strictures usually present with regurgitation of saliva, dysphagia, nausea and vomiting, and/or upper abdominal pain.21,225,230 UGI x-ray is not usually very helpful as the obstruction is usually partial.21,231,243 In one study of 87 post RYGB patients that were assessed for GJ stricture, the UGI had a positive predictive value of 66%, negative predictive value of 83%, and an accuracy of only 72%.231 Another study of 20 GJ strictures out of 119 lap RYGB showed that UGI had a sensitivity of 55%, specificity of 100%, and a negative predictive value of 53% in diagnosing GJ stricture.243 Upper GI endoscopy cannot only diagnose this complication but utilizing endoscopic balloon dilation can relieve the obstruction and symptoms rapidly.225,227,229-231,233,239
The severity of the stricture varies with the stoma frequently narrowing down to a size of 2 to 8 mm diameter.21 Several grading systems have been proposed. Goitein proposed the following system: Grade I – mild stenosis allowing passage of a 10.5 mm diameter endoscope; Grade II – moderate stenosis only accommodating an 8.5 mm diameter pediatric endoscope; Grade III – severe stenosis only allowing a guidewire to pass through the stricture; and Grade IV – complete or near complete obstruction in which nothing can be passed thru the stricture.239 Swartz graded GJ stenosis as: Low – endoscope able to traverse stricture; Medium – endoscope cannot traverse the stricture but can visualize at least 2 adjacent mucosal folds thru the stricture; or High – endoscope cannot traverse the stricture and little if any mucosa visible thru the stricture.233
A single endoscopic balloon dilation will frequently cure the stricture permanently.21 The endoscopic appearance of a GJ stricture and results of endoscopic balloon dilation are shown in Figure 9A, 9B, 9C. Combining the results of five studies224,227,229,230,239 involving endoscopic balloon dilation of GJ strictures of varying severity, 64% (97/152) of the patients required only one dilation and in four of these studies,227,229,230,239 88% (123/139) required only two dilations or less. Depending on the original diameter of the anastomosis, it should be dilated up to 15 to 20 mm diameter. Dilation to diameters less than 15 mm may be associated with a high recurrence rate.21 Swartz endoscopically dilated 110 post-RYGB strictures with a 12 mm diameter balloon irrespective of the grade of the stricture, low (39 patients), medium (32 patients), or high (39 patients).233 The recurrence rate was 2.6% for low grade strictures, 34.4% for medium grade, and 35.9% for high grade. He then initiated a protocol in which a 12 mm balloon was used for low-grade strictures, 13.5 mm for medium grade, and 15 mm for high grade. Although the recurrence rate remained high following initiation of this protocol, 9.7% for 31 low grade, 26.3% for 19 medium grade, and 43.6% for high grade, the number of dilations per patient decreased from 1.0 per patient for low, 1.5 per patient for medium, and 2.1 per patient for high to 1.0, 1.0, and 1.2 per patient, respectively. Peifer also found that 80% (4/5) patients dilated with a 12 mm or less balloon required repeat dilation compared to only 14% (5/36) of patients initially dilated with a 15 mm or greater balloon (p<0.01).227 Out of the 152 patients with GJ stricture in the above combined five studies, only 1 patient (0.7%) failed endoscopic dilation and required surgical treatment of the stricture. 224,227,229,230,239 The perforation rate of endoscopic balloon dilation ranges from 0% to 3.2% with a combined frequency of 1.8%.224,225,227,229-231,239 Endoscopic stents have also been used in the treatment of GJ stricture but further investigation regarding the indications and role of this therapy for anastomotic strictures is stillneeded.168
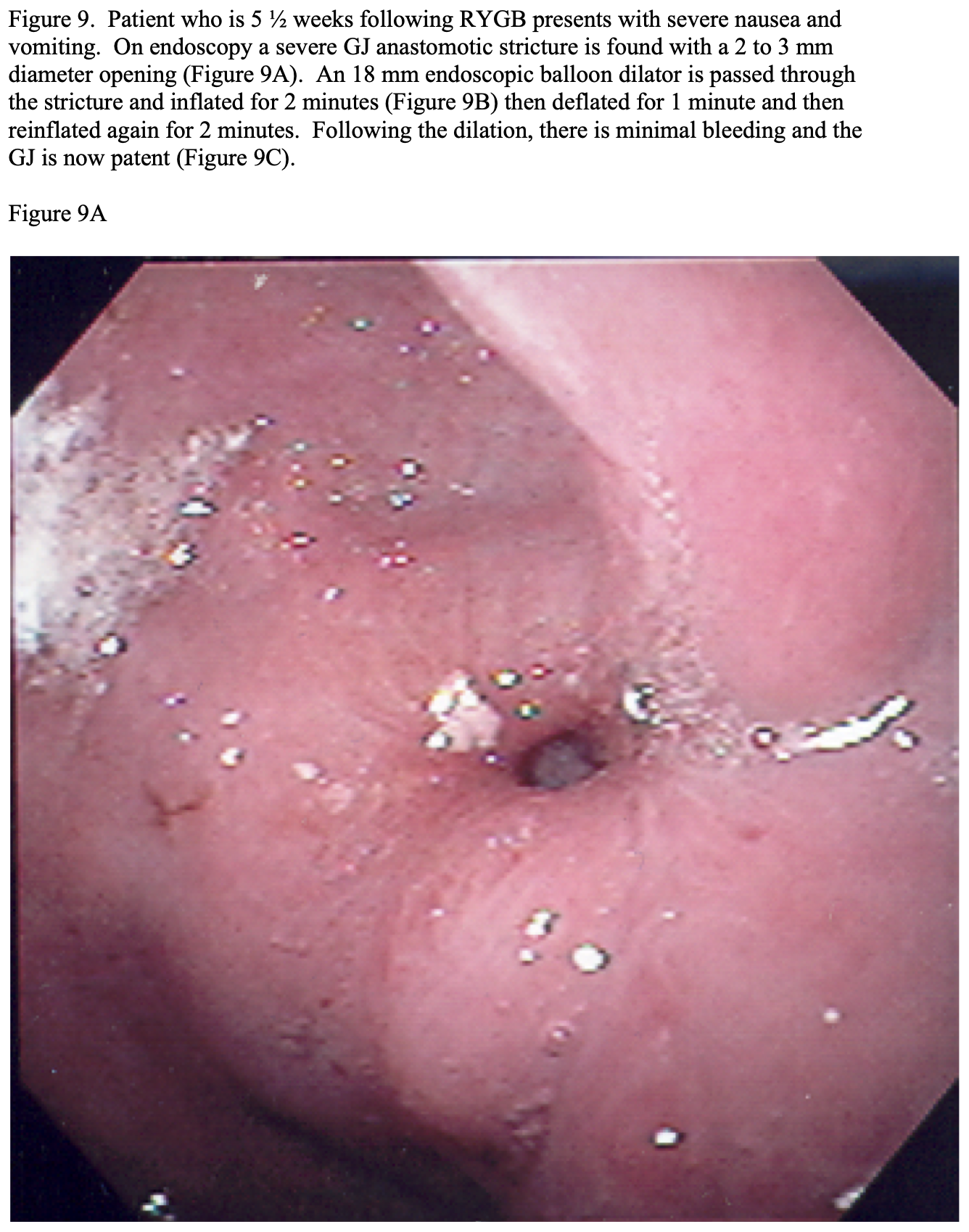
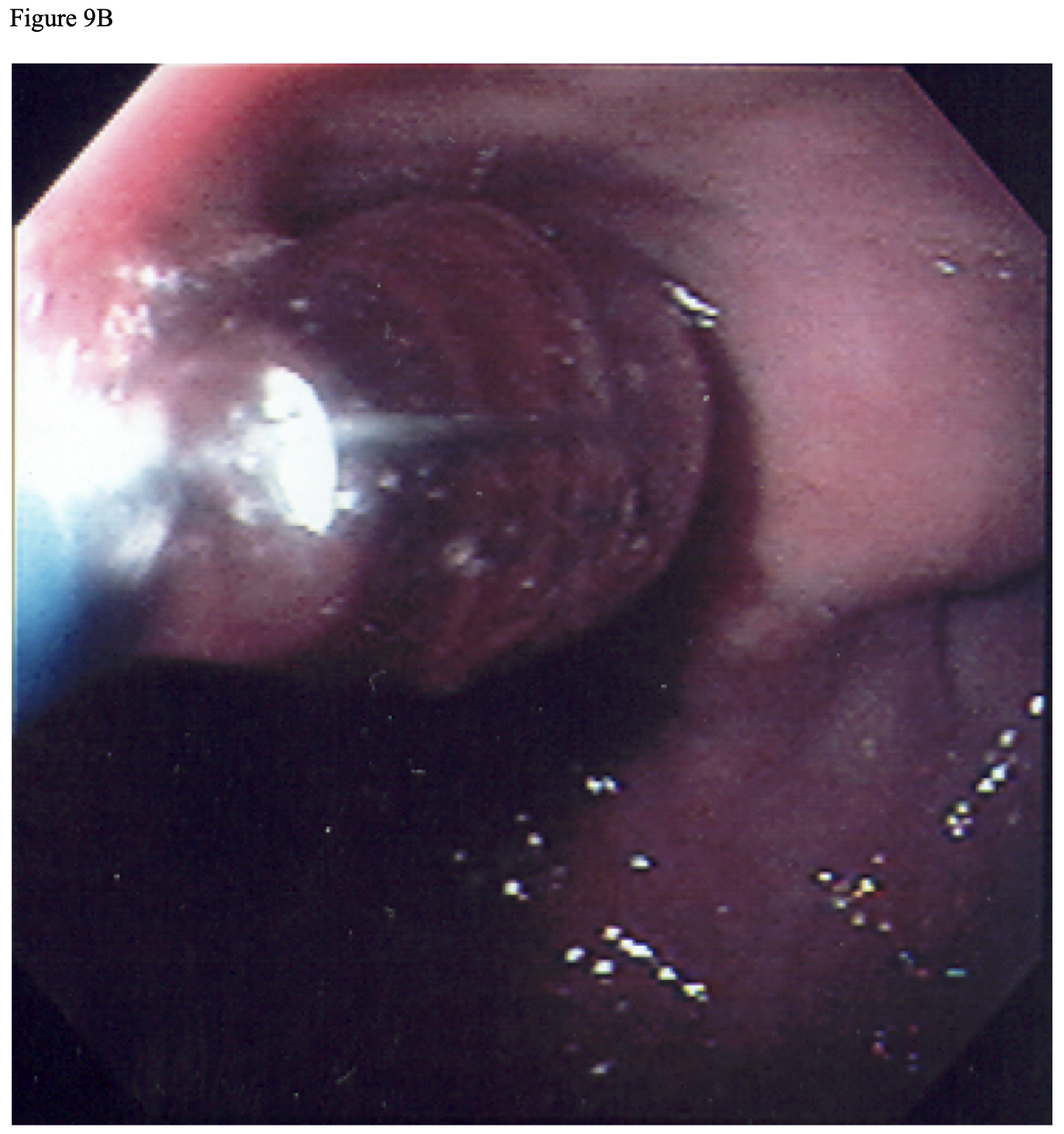
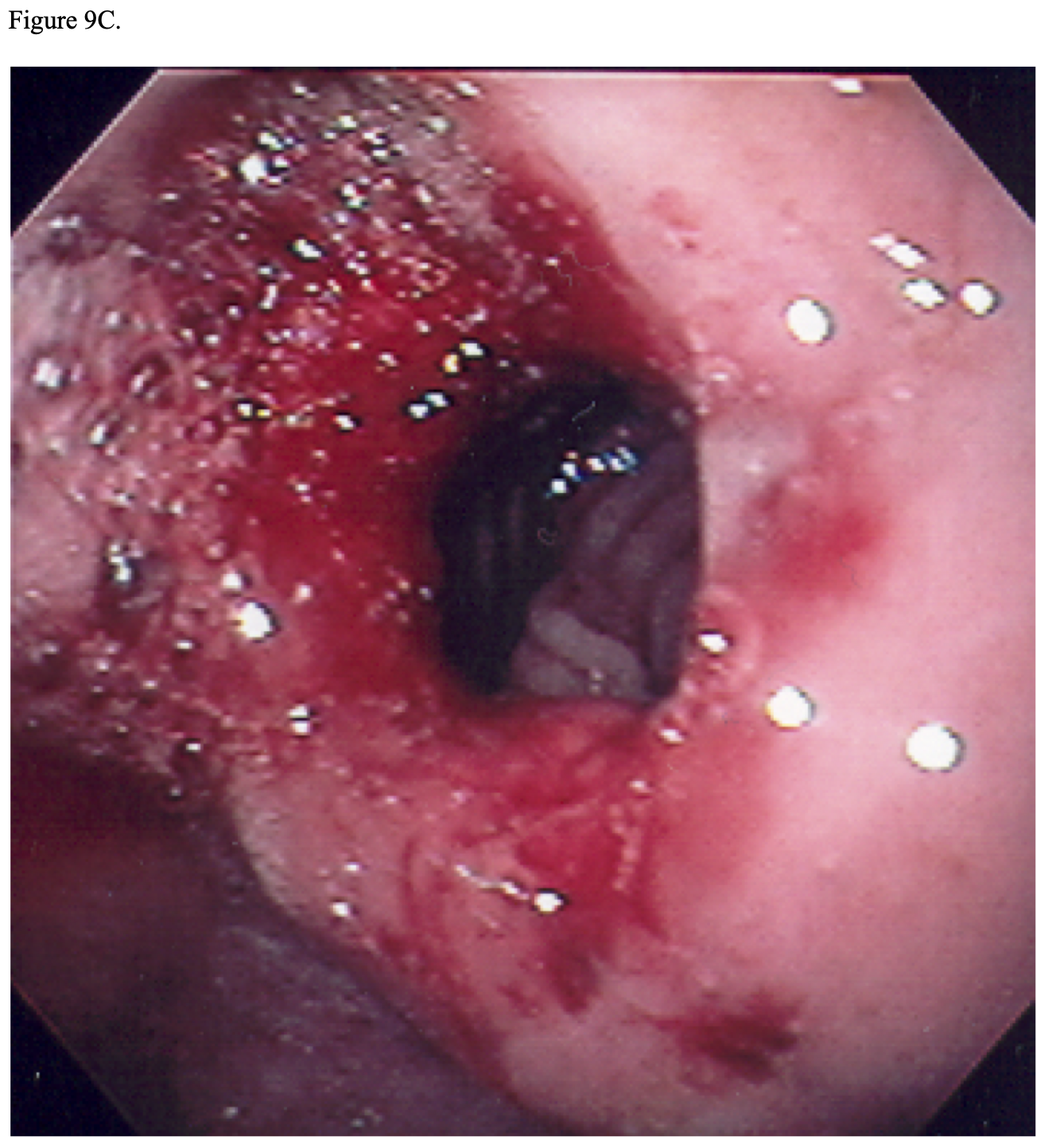
Some patients fear that if the GJ is dilated too much it will adversely affect their weight loss. However, in our bariatric surgery patients, patients with GJ strictures and treated with endoscopic balloon dilation had significantly greater weight loss at 6 weeks, 3 months, and 6 months after RYGB compared to patients who did not develop a stricture but there was no significant difference in weight loss between these two groups of patients after 1 year following surgery.226 Several other studies have also had the same or similar findings.225,227,228
Much less commonly, obstruction can occur at the EE.42,116,244,245 Several different techniques have been used to form this anastomosis. One survey of 215 bariatric surgeons showed that 53% performed a linear stapled anastomosis with hand suturing of the enterostomy site, 36% used a “double staple” technique (the anastomosis was formed with a linear stapler then the enterotomy site was closed with another linear stapler), 13% used a “triple staple” technique (enterotomy made some distance from the end of the biliopancreatic limb then the linear stapler was fired twice, once proximally through the enterotomy and once distally, then the enterotomy was closed with a third firing of the linear stapler transversely across the jejunum), and 1% performed a completely hand sewn anastomosis.42 When using one of the first two techniques, especially the double staple technique, care must be taken not to compromise the lumen of the jejunum at the enterostomy closure site causing a stricture. This is avoided altogether with the “triple staple technique.”246 Also, the gastric Roux limb can get kinked where it meets the EE. Dr. Brolin described a technique in which a stitch, now termed the Brolin antiobstruction stitch, is placed between this Roux limb of jejunum and the end of the biliopancreatic limb just proximal to the EE preventing this kinking and obstruction.245,247
Gastro-gastric (GG) fistula. This is a communication between the gastric pouch and the lower gastric remnant. The exact incidence is unknown, as most bariatric surgeons do not routinely check for this complication unless the patient is symptomatic. However, the reported incidence was as high as 49% when the gastric pouch and gastric remnant were stapled but not divided and decreased to 1.5% to 6% with a divided pouch technique.38,39,167
Capella found that the incidence of GG fistula decreased from 49% for gastric bypasses stapled in continuity (189 patients) to 2.6% when stapled and divided (222 patients) and 0% when stapled, divided, and the Roux limb of jejunum interposed between the gastric pouch and gastric remnant (492 patients), p<0.001 between all three groups and between the latter 2 groups.40 Also an inadvertent incomplete division of the gastric pouch and remnant results in a GG fistula. This can be avoided by performing an adequate dissection of the angle of His and ensuring good visualization of the end of the gastric staple line ensuring complete separation between the gastric pouch and distal gastric remnant staple lines.
GJ leak and/or ulcer also predisposes to GG fistula. In one study, 27% of patients who had a GG fistula had previously had a GJ leak.167 Perforation of the gastric pouch or remnant and gastric wall tissue migration also predisposes to GG fistula.38,39,167 Silastic bands or rings placed on the gastric pouch or GJ to prevent dilation have been found to cause GG fistula.38,39,167
GG fistulas may be asymptomatic and require no treatment. However, the most common symptom is epigastric abdominal pain secondary to GJ ulcer and other symptoms may include nausea, vomiting, hematemesis, or hematochezia.21,39 Patients with GG fistula may also have heartburn or other symptoms of GERD. The ulcers and GERD are due to increased amounts of acid in the gastric pouch and esophagus that can reflux from the lower gastric remnant.21 Another common presentation of patients with GG fistula is suboptimal weight loss or weight regain due to an increased capacity to ingest food and the ingested food that passes through the lower gastric remnant is more completely digested and absorbed.21,38,39
UGI x-rays, upper GI endoscopy, or CT scans of the abdomen can detect GG fistulas. UGI x-ray will show flow of contrast into the gastric remnant and can demonstrate how much of ingested contents are going through the GG fistula versus the GJ.21,39 The GG fistula can most often be directly visualized by upper GI endoscopy and the scope can usually be passed through the fistula with visualization of the gastric remnant and upper duodenum.39 CT scans of the abdomen are not the best test for demonstrating a GG fistula but may show a significant amount of air or oral contrast in the gastric remnant indicating that a GG fistula is likely.21,39
Symptomatic GG fistulas should be first treated medically with PPI and, if an ulcer is present, sucralfate.39,167 About 25% to 33% of GG fistulas can be managed with symptomatic control with medical therapy. However, if the patient fails medical therapy or is experiencing weight regain then the surgical options include resection of the fistula alone or resection of the fistula and gastric remnant.21,38,39 Either of these surgeries may or may not require resection and reconstruction of the GJ depending on the site of the fistula and the size of the gastric pouch.39 Also, there are endoscopic equipment and procedures that are under development that have been used in an attempt to occlude the fistula.16,21,168
Bowel obstruction. This complication has been reported in 2% to 9% of patients.21,44,116,244,248 Combining the studies in Tables 9 and 10 comparing the results of lap versus open RYGB, lap RYGB has a significantly higher incidence of early (4.5% (6/132) vs. 0% (0/127), p<0.05) and late (3.1% (332/10,849) vs. 1.1% (72/6296), p<0.001) bowel obstruction.
Obstruction can be due to a variety of etiologies including intrabdominal adhesions, internal hernias, incisional hernias (which will be discussed in the next section), anastomotic strictures (either at the GJ or EE as discussed in the previous section), intussusception, food or foreign bodies, or miscellaneous causes.116 Obstruction due to adhesions is most common after open procedures where as obstruction due to an internal hernia is most common after laparoscopic procedures.21,44,116-118 Some surgeons propose that the higher internal hernia rate in lap RYGB compared to open RYGB is the decrease in adhesion formation allowing increased mobility of the small bowel leading to an increased risk of entrapment in an internal defect.117,245,249,250 Also, especially earlier on, surgeons performing lap RYGB did not routinely close all internal defects.249 In one review of the etiology of postoperative bowel obstructions in 5 lap RYGB series, the obstruction was caused by internal hernia in 75% of the cases, adhesions 13%, incisional hernia 3%, and other causes 9%.251
Internal hernias occur in 0.2% to 9.1% of laparoscopic RYGB.118,248,252-254 Potential internal hernia locations include: 1) opening in the transverse mesocolon for the retrocolic gastric Roux limb; 2) EE mesenteric defect; and 3) the space between the posterior aspect of the mesentery of the gastric limb and the transverse mesocolon (Peterson’s space).117,118,249-252 The distribution of the site of internal hernias varies from study to study but in one review of retrocoloic RYGB patients, the hernia was at the transverse mesocolon defect in 63% of the patients, the EE mesenteric defect in 28% of the patients, and at Peterson’s space in 9% of the patients.251 Also, internal hernias usually do not present for months or years after surgery, so it has been postulated that postoperative weight loss results in reduced intraperitoneal fat and may cause these potential defects to enlarge, even if the defects were originally sutured closed.117,255,256
The incidence of internal hernias significantly correlates with the position of the gastric limb (retrocolic vs. antecolic) and to whether or not the EE mesenteric defect is sutured closed (Table 16). Many surgeons prefer a retrocolic, retrogastric RYGB because it is the shortest route to the gastric pouch and may decrease tension on the GJ.248,251 However, the antecolic RYGB eliminates the transverse mesenteric defect and has a significantly lower incidence of internal hernia then retrocolic RYGB (Table 16, 2.0% vs. 3.6%, p<0.001).245 Likewise, many surgeons when initially performing lap RYGB chose not to close any of the internal defects or spaces created during this procedure that could result in an internal hernia because it was technically difficult to do so and significantly increased the operative time. Also, closure of mesenteric defects can cause tension on the GJ or EE and injure mesenteric blood vessels that can cause bleeding or mesenteric hematomas, all of which can result in bowel ischemia, leak, and/or stricture.259 Also non- absorbable sutures can result in more intraabdominal adhesions and even cause an adhesive bowel obstruction.259 However, closing the EE mesenteric defect has a significantly lower incidence of internal hernia compared to no closure in antecolic procedures (1.6% vs. 2.2%, p<0.05) and a trend in that direction in retrocolic procedures (3.6% vs. 4.8%, p=0.19) (Table 16).
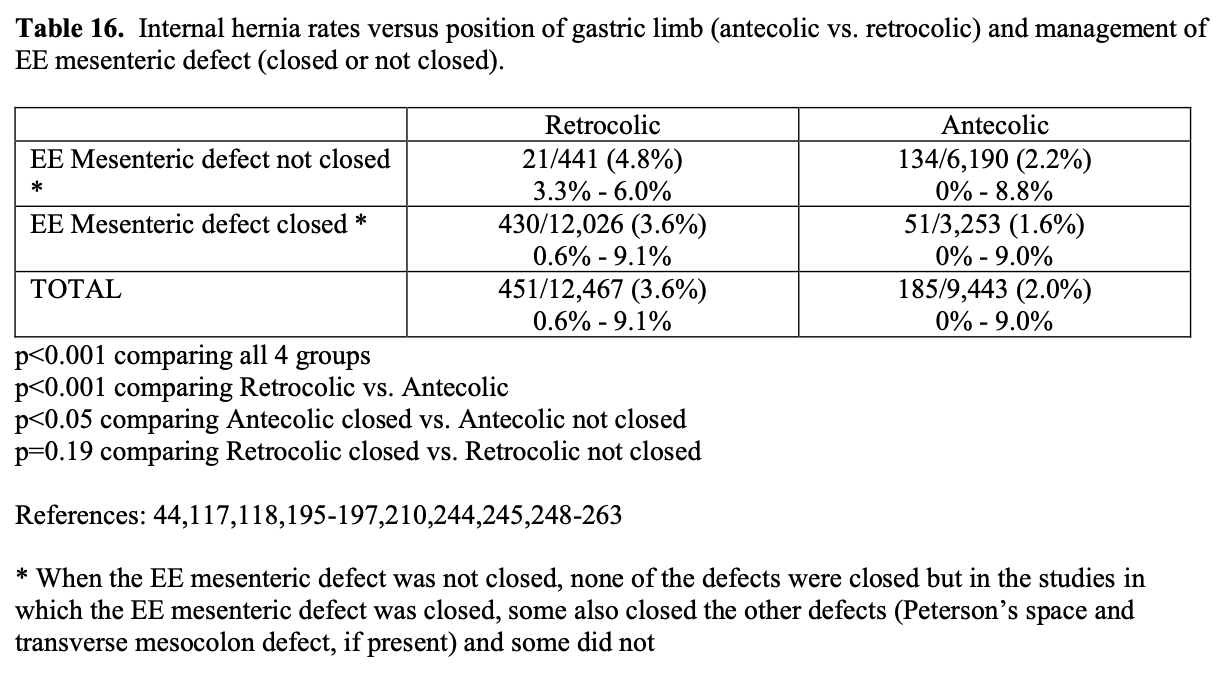
The type of suture and technique of suture closure is also significant. One retrospective study showed that a running, non-absorbable suture closure of the EE mesenteric defect had a significantly lower internal hernia rate compared to either absorbable or non- absorbable interrupted sutures (22/604 (3.6%) vs. 19/230 (8.2%) vs. 16/181 (8.8%), p<0.01); however, an absorbable running suture was not tried.253 Also when closing the EE mesenteric defect, care must be taken to continue the closure all the way to the EE as Paroz reported 3 cases of internal hernia between the mesenteric closure and the end of the EE.253
Antecolic RYGB with closure of the EE mesenteric defect has the lowest incisional hernia rate compared to the other 3 combinations of technique (1.4% vs. 2.0% vs. 3.2% vs. 4.4%, p<0.01, Table 16). In a survey study of 200 bariatric surgeons, 64% use the antecolic, antegastric technique, 12% used the retrocolic, antegastric technique, and 24% used the retrocolic, retrogastric technique.42 While some surgeons recommend closing all potential internal defects to prevent hernia formation, in practice this is variable.255 In surgeons using an antecolic technique, 9% did not close any defects, 88% closed the EE mesenteric defect, and only 9% closed Peterson’s space but 24% sutured the gastric limb to the transverse colon. In surgeons using the retrocolic approach, only 1% (1 surgeon) did not close any defects, 96% closed the EE mesenteric defect, 96% closed the defect in the transverse mesocolon, 93% closed Peterson’s space, and 8% sutured the gastric limb to the transverse colon.42 In summary, while most surgeons now close the EE mesenteric defect, many still do not close Peterson’s space, since this is technically challenging and time consuming.
The orientation of the gastric limb also significantly affects the internal hernia rate from a Peterson’s hernia. A left-oriented gastric limb is when the stapled end of the jejunum is pointing towards the lesser curvature of the stomach and the antimesenteric border is towards the patient’s left side and a right-oriented gastric limb is when the stapled end of the jejunum is pointing towards the greater curvature and the anti-mesenteric border is towards the patient’s right side.44 The incidence of internal hernia from a Peterson’s hernia without suture closure of this defect is significantly lower with a right-oriented gastric limb compared to a left-oriented gastric limb (0.5% vs. 9%, p<0.01).44
Intussusception is the most common cause of intestinal obstruction in early childhood and is usually due to an ileocecal intussusception with no identifiable pathologic lead point.264 However, intussusception is rare in adults, accounting for 0.003% to 0.02% of hospital admissions and 1% to 5% of bowel obstructions and is usually associated with a pathologic lead point such as a polyp or tumor.265,266 There have been numerous case reports and small case series of intussusception as a late complication of laparoscopic RYGB.116,265,267-281 The largest of these was published by a bariatric surgery program that had 15,553 gastric bypass patients in their database from 1979 to 2008. The incidence of bowel obstruction from all causes was 2% (330 patients) and from intussception was 0.1% (20 patients). Three additional cases of intussusception were included in this report that had their gastric bypass performed elsewhere. The intussusception occurred an average 77 months after RYGB with a range of 6 to 288 months.271 In our bariatric surgery program, two out of 737 RYGB patients (0.3% incidence) presented with a bowel obstruction secondary to intussusception years following the surgery (unpublished data).
RYGB-associated intussusception cases are unique in that usually there is no identifiable lead point and it occurs retrograde, i.e. the distal small bowel (the common channel) most often intussuscepts into the proximal small bowel at the EE site, rather than the usual antegrade intussusception (Figure 10).267,271 However, some antegrade entero-enteric intussusceptions have been reported in gastric bypass patients.273-275 Also, there has been one report of two cases of retrograde jejunogastric intussusception.282 The etiology of intussusception in gastric bypass patients is unknown. One theory is that the EE suture line acts as a lead point; however, this would result in an antegrade intussusception rather than retrograde.265 Studies in animals and humans after RYGB found multiple motor abnormalities in the Roux limb including ectopic pacemakers resulting in retrograde peristalsis, which is a normal occurrence but may be accentuated after RYGB.265 Also, there are areas of dysmotility in the small intestine following RYGB with normal peristalsis in proximal segments and relaxation with no motility in distal segments, which could predispose to a retrograde intussusception.265,278
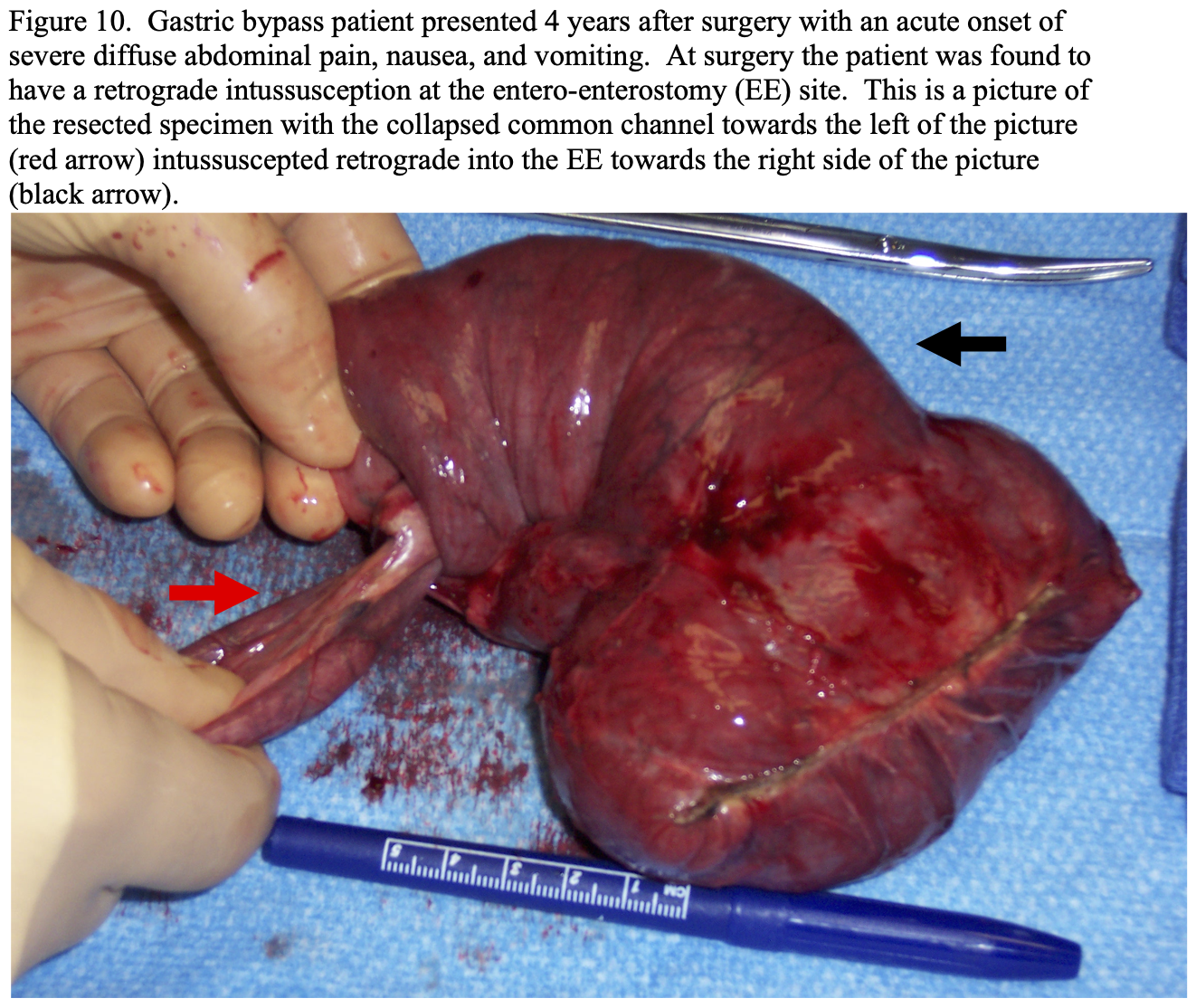
Patients with intermittent intussusception may present with chronic and intermittent abdominal pain while patients with an acute incarcerated, strangulated intussusception will present with acute, severe, unrelenting abdominal pain.270 CT scan is the best diagnostic test and, if performed during an acute intussception, will frequently reveal a “target sign” (Figure 11); however, a normal CT scan does not exclude an intussusception.265,267,270-274,276 The treatment depends on the presentation. If it is chronic, then the surgeon has to have a high index of suspicion and the patient can be treated with elective or semi-elective exploratory laparoscopy or laparotomy, depending on the preference and experience of the surgeon, and the intussusception can be reduced and the bowel plicated in an attempt to prevent recurrent intussusceptions.278 If it is acute, which is the most common situation, then treatment is urgent exploratory laparoscopy or laparotomy. Manual reduction of the intussusception at the time of surgery can be carefully attempted but perforation of the bowel needs to be avoided.265,272,274 If the intussuception is successfully reduced, the bowel can be plicated in an attempt to prevent recurrent intussusception.271,274,278 But most often the intussusception cannot be reduced or if reduced the bowel has irreversible ischemia so resection of the intussusception and reconstruction of the Roux-en-Y anastomosis is required.265,271-273,275 Recurrence of the intussusception is common ranging from 13% in patients treated initially with resection to 40% with reduction and plication to 100% in patients with simple reduction.271
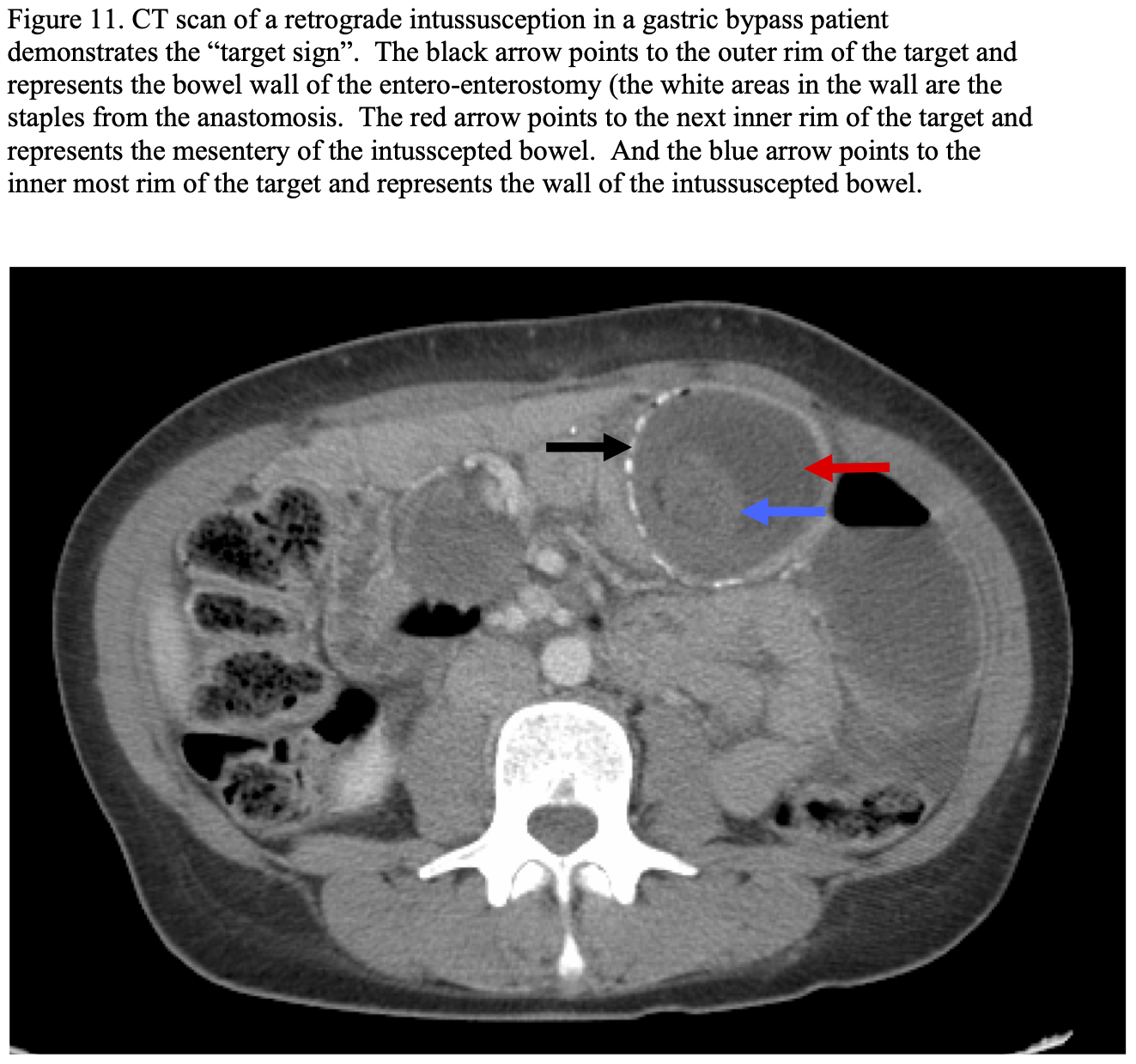
Obstructions can also occur due to food or foreign bodies becoming lodged in the gastric pouch or bezors in the small intestine.116 In the case of food, it will usually be digested with time and eventually pass on through. One of our lap RYGB patients was 1 year following gastric bypass when she presented with obstruction of her GJ due to a Bay leaf that she swallowed while eating vegetable soup (Figure 12A, 12B, 12C). It was removed endoscopically and the GJ was normal with no stricture. Undigested food can also form a bezoar in the gastric Roux limb causing obstruction.116
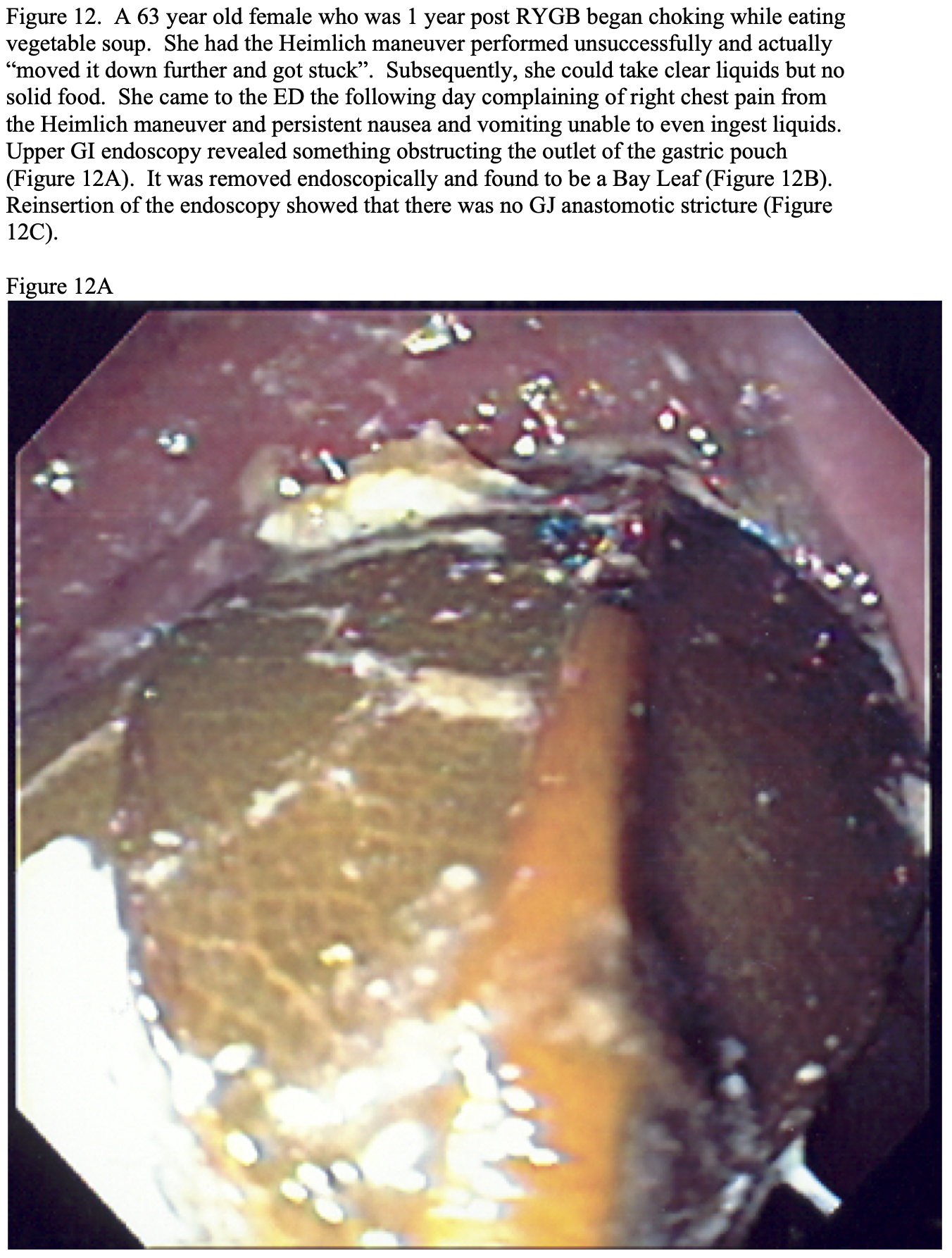
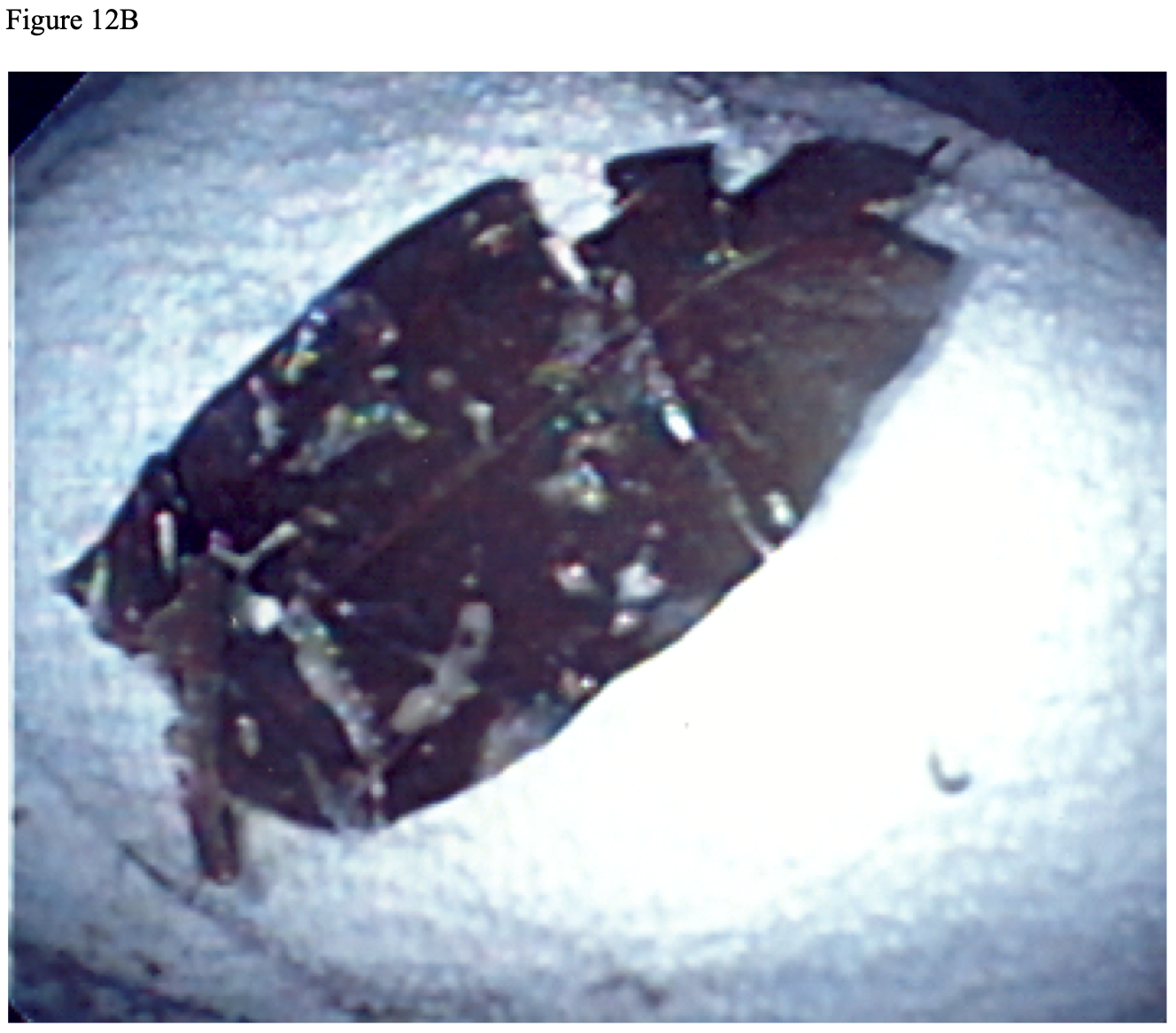
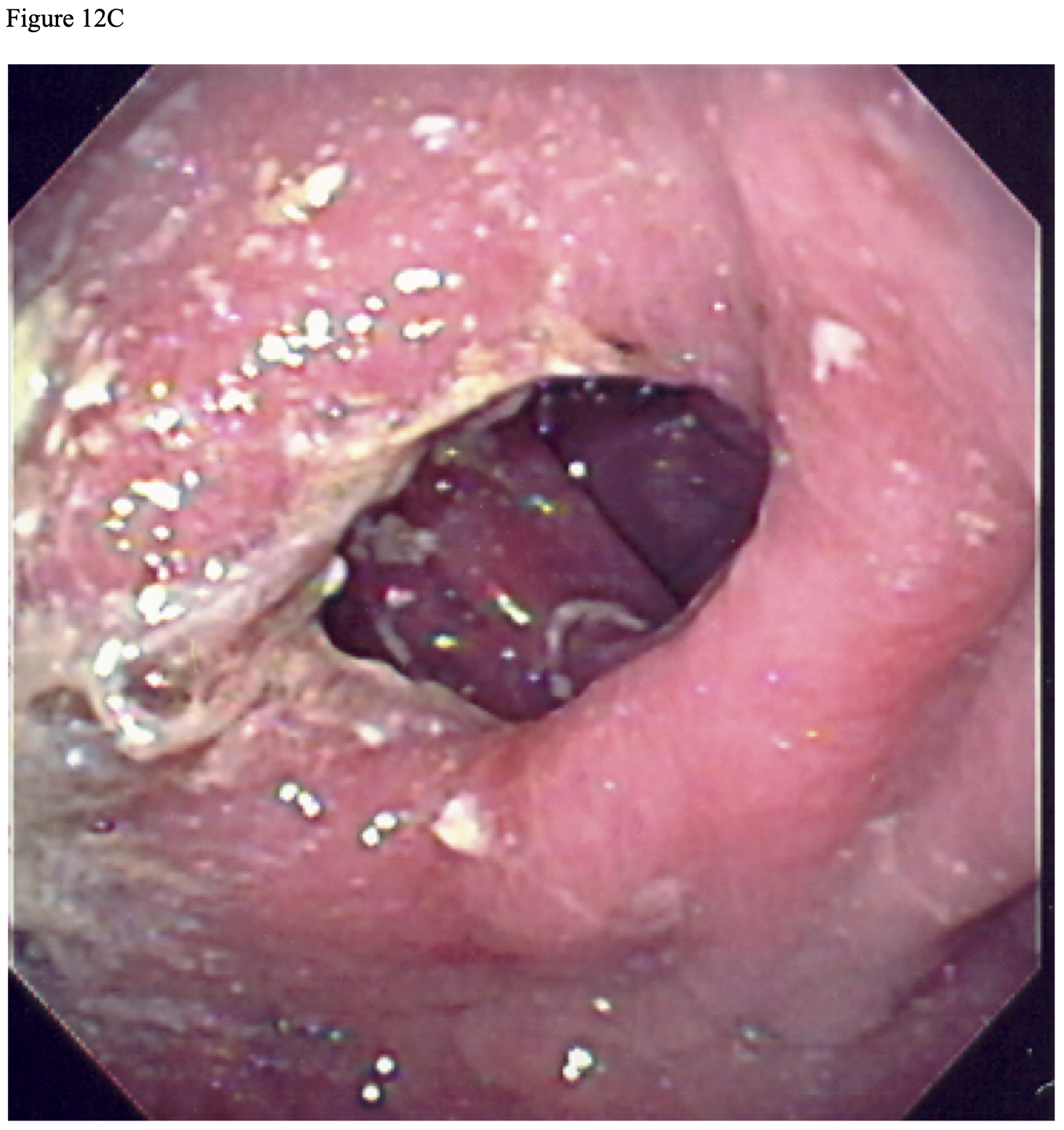
The recommendations for minimizing postoperative bowel obstructions include using the antecolic, antegastric, right-oriented gastric Roux limb; secure closure of internal defects, especially the EE mesenteric defect, with a running non-absorbable suture; being careful not to narrow the lumen when performing the EE; and use of the antiobstruction stitch at the EE.41,44,117,244,245,250,252 Other techniques that have been suggested to decrease internal hernia rate are to divide the omentum only when too thick,250 minimal or no division of the small bowel mesentery,118 and positioning the EE above the transverse colon.249,259
If a patient presents with obstructive symptoms, such as nausea, vomiting, and abdominal pain, either an UGI and small bowel x-ray series or a CT scan of the abdomen and pelvis with IV and oral contrast can be helpful and complimentary for diagnosis.251 However, the CT scan is most often a better choice as it will detect dilation of the lower gastric remnant, bowel dilated with air or fluid, transition points in the bowel dilation, “target sign”, etc.116,244 However, the radiologic findings of bowel obstruction can be subtle and easily missed so if patient has severe unrelenting pain, surgical exploration with laparoscopy or laparotomy may be the diagnostic procedure of choice. If the obstruction is thought to be in the gastric pouch then upper GI endoscopy would be the diagnostic procedure of choice and may also be therapeutic.
In general, patients who present with a bowel obstruction can usually be given a trial of NG decompression and IV hydration. However, unlike patients who present with bowel obstruction without prior surgery or who have had other types of abdominal surgery, RYGB patients who present with signs or symptoms of bowel obstruction need rapid diagnosis and urgent surgical treatment with either exploratory laparoscopy or laparotomy, depending on the skill and experience of the surgeon.251 Since the lower gastric remnant is no longer in continuity with the esophagus, any obstruction in the biliopancreatic limb or distal to the EE results in a closed loop obstruction of the biliopancreatic limb and distal gastric remnant and can result in rapid development of ischemia, infarction, and perforation.117,251,283
Incisional Hernia. A collective review of the incidence of incisional hernias for lap and open RYGB is shown in Table 17. The incidence is much higher with open RYGB compared to lap RYGB (9.1% vs. 1.1%, p<0.001) with a range of 1.0% to 23.9% for open RYGB, 0% to 13.6% for hand-assisted lap RYGB, and 0% to 3.9% for totally laparoscopic RYGB. One possible reason for the wide range of incidence of incisional hernias is the variation in the length of follow-up and the intensity with which patients are examined for incisional hernias, both of which can significantly affect the reported incidence.
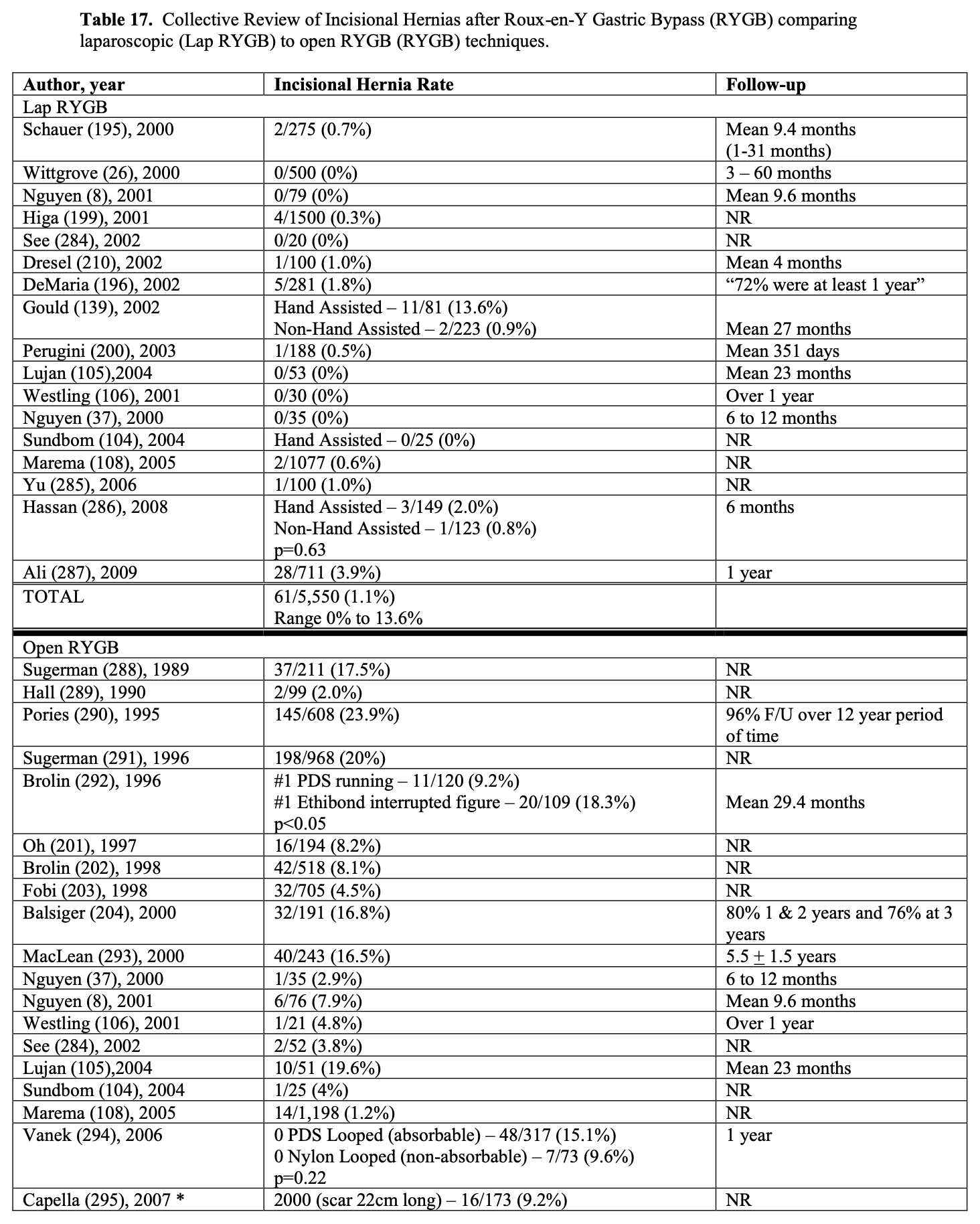
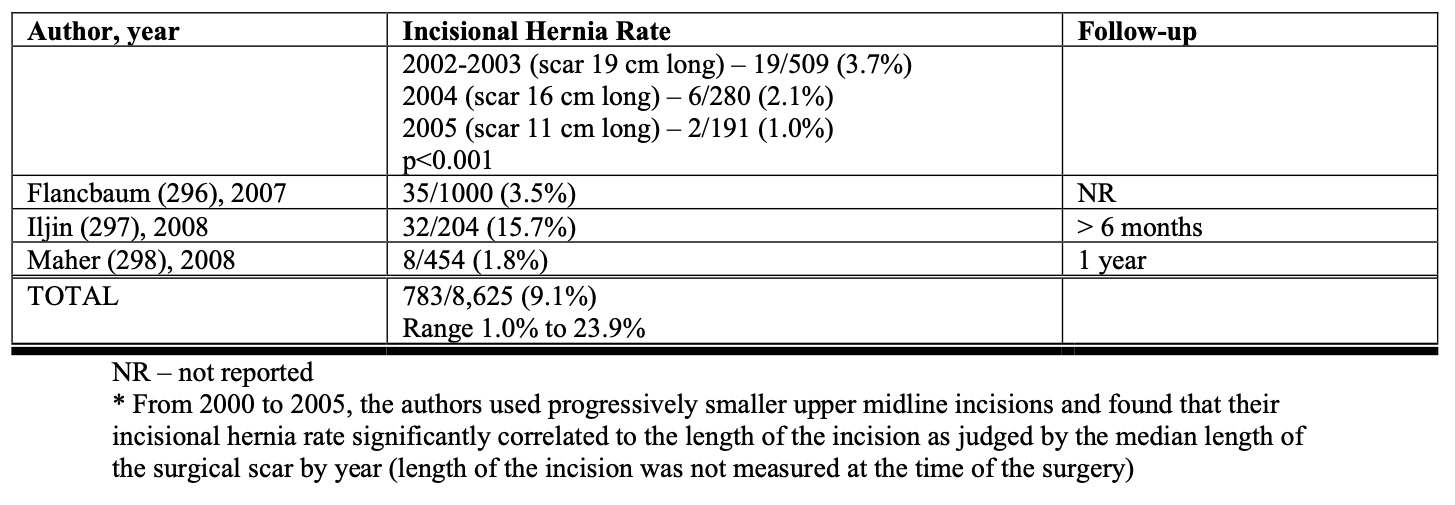
At least in open RYGB, postoperative wound infections increases the risk of subsequently developing an incisional hernia, 27% vs. 13% (p<0.05) in one study294 and 38% vs. 14% (p<0.001) in another study.299 Also, the type of suture and suture technique used for fascial closure may affect the incidence of incisional hernia. Brolin showed that a running suture has a lower incisional hernia rate compared to an interrupted figure of 8 suture (9.2% vs. 18.3%, p<0.05).292 In our program, a running non-absorbable suture showed a trend towards lower incisional hernia rated compared to running long-acting absorbable suture (9.6% vs. 15.1% at 1 year, p=0.22).294
About 5% of patients being evaluated for RYGB will have a ventral, incisional, or umbilical hernia.198,294,300 While the ideal repair for a significant abdominal wall hernia involves prosthetic mesh, there may be an increased risk of mesh infection and subsequent complications if mesh repair is performed at the same time as the RYGB. In our bariatric surgery program, patients who had primary repair of an abdominal wall hernia at the time of RYGB had a significantly higher incidence of subsequent incisional hernia compared to patients who did not have a hernia at the time of RYGB (33% vs. 14%, p<0.05).294 Herbert prophylactically placed a prosthetic mesh in a subfascial location in 16 open RYGB patients.301 However, with a mean follow-up of 6 months, four patients (25%) required excision of the mesh, three for infection and one for persistent symptomatic seroma. Another patient had the mesh removed when the patient was re-explored for persistent nausea and vomiting and another patient developed an incisional hernia despite the prophylactic mesh placement. Schuster reported concomitant repair of abdominal wall hernia and RYGB in 12 patients.300 Nine of the hernias were incisional hernias and the other three were umbilical. Ten of the RYGB were laparoscopic while the other two were open. Primary repair was performed in one patient but prosthetic mesh repair was used in the other 11 patients. There were no mesh infections and only 2 recurrent hernias (17%). But, one of the recurrences was in the patient who had primary repair without mesh and the other recurrence was in a patient who had two previous failed incisional hernia repairs. Consequently, there is no standard approach as to what to do with a patient who has an abdominal wall hernia and is being scheduled for RYGB.
Incisional hernias with lap RYGB are associated with the trocar sites and can occur early, within hours, or late, months to years, after surgery. An entire loop of bowel can protrude through the trocar fascial defect or just a portion of the wall, a Richter’s hernia. The reported trocar site hernia rate for laparoscopic surgery in general ranges from 0.2% to 3%.302 Generally, it is recommended to close all trocar sites that are 10 mm diameter or larger to decrease the risk of trocar site hernia.302 Matthews reported an unusual trocar hernia after a lap RYGB in which the fascia was closed on a 10 mm trocar site but not the peritoneal opening and the patient subsequently developed a symptomatic Richter’s hernia through the peritoneal opening into the preperitoneal space.303 So he recommended closing all trocar sites 10 mm or larger incorporating both the fascia and the peritoneum in the closure. Eid reported a 0.3% trocar hernia rate using the standard Carter-Thomason CloseSure System to close 12 mm trocar sites but the trocar hernia rate decreased to 0% in a series of 250 lap RYGB patients using the newer Carter-Thomason CloseSure System XL™.304 He attributed the lower rate to a more consistent and wider purchase on the fascia and peritoneum duringclosure.
Another technique to decrease trocar hernia rates and avoid taking the additional time for trocar closure is to use non-bladed or dilating trocars, which when removed leave a much smaller defect in the abdominal wall especially if used off the midline and inserted at a slight angle to offset the openings in the different fascial and muscles layers of the abdominal wall.245,302,305,306 In one study of 747 lap RYGB cases, an open technique with the Hassan cannula was used to initially enter the peritoneal cavity followed by insertion of dilating trocars (three 5 mm and two 12 mm trocars).305 The Hassan cannula site was closed with a figure of 8 absorbable suture while the other trocar sites were not closed. At a mean follow-up of 20 months, there were no trocar hernias at any of the 5 mm or 12 mm dilating trocar sites but there were 9 (1.2%) hernias at the Hasson cannula site.
Combining the results of this study with three other studies involving lap RYGB cases, the incidence of trocar hernias with 12 mm dilating or bladeless trocars was 0.1% of the patients (4/3,318 patients with a range of 0% to 0.2%) or 0.03% of trocar sites (4/13,500 trocar sites with a range of 0% to 0.1%).245,302,306
Chiu reported on another novel approach to decreasing trocar hernias for both bladed and nonbladed trocars.307 Prior to removal of the trocar, a piece of Surgical® was folded into a plug and inserted into the peritoneal cavity through the cannula with a grasper. The cannula was then pulled back into the muscular layer of the abdominal wall followed by pulling the Surgical® plug up into the muscular layer as well. The cannula was then completely removed. Using this technique, the trocar hernia rate was 0.33% of patients (2/610); however, he did not specify how many of these trocars were bladed or non- bladed and which trocars were used in the 2 cases of trocar hernias. So, non-bladed or dilating trocar sites that are off of the midline and the trocar is 12 mm or less in diameter do not need to be closed. However, if these trocars are inserted in the midline where there are no muscle layers or the trocar is larger than 12 mm then the trocar site should be sutured closed.306 Also, all bladed trocar sites made with trocars that are 10 mm diameter or larger should be sutured closed.
Acute symptomatic incisional hernias, usually trocar hernias, need to be taken to the operating urgently and repaired. However, many late incisional hernias are relatively asymptomatic. Patients may develop abdominal pain (especially with vigorous activity), nausea or vomiting, or change in bowel habits and incarceration with or without strangulation can result in a bowel obstruction, ischemia, and/or perforation. Incisional hernias should be repaired with open or laparoscopic incisional hernioplasty with mesh. However, if the hernia is asymptomatic or minimally symptomatic, it is best to wait until 12 to 18 months after RYGB when the patient has achieved maximal weight loss.21 A panniculectomy can also be done at the same time as incisional hernioplasty to address the loose, excess skin.21
Dumping syndrome. This can occur whenever the pyloric sphincter is ablated or bypassed as occurs with RYGB and the standard BPD. Most RYGB patients will develop dumping syndrome if they do not follow their bariatric diet, which is a beneficial effect resulting in negative feedback and behavior modification. While most of the time the dumping syndrome is self-limited and responds to dietary modification, in a small number of patients, it can be severe and some would consider it a complication.21 There are two forms of dumping syndrome, early (30 to 40 minutes after a meal) and late (2 to 4 hours after a meal). The early dumping syndrome is due to hypertonic food and liquids rapidly entering the jejunum causing an acute shift of intravascular fluid into the lumen of the bowel due to the osmotic gradient. This results in symptoms of acute intravascular volume depletion, such as lightheadedness, dizziness, and sweating, and acute increase in volume in the lumen of the bowel, such as abdominal pain, bloating, and diarrhea. The late dumping syndrome is due to reactive hypoglycemia due to an over secretion of insulin from the pancreas in response to a rapid rise in the blood glucose as a result of simple carbohydrates rapidly passing from the gastric pouch into the jejunum and being rapidly broken down and absorbed. These symptoms include lightheadedness, dizziness, tachycardia, palpitations, and syncopal or near syncopal episodes.21 Most of the time these symptoms can be avoided or treated with dietary counseling.
Protein-Calorie Malnutrition and other nutritional deficiencies. This can occur with any bariatric surgery procedure, especially if the patient is experiencing nausea or vomiting, but is more common with the malabsorptive procedures and correlates directly with the amount of malabsorption created. Protein-Calorie malnutrition and hypoproteinemia is uncommon with RYGB as long as the patient follows the bariatric diet including protein supplements. However, a small percentage of RYGB patients may lose too much weight, dropping below their ideal body weight and becoming cachexic. This is usually associated with persistent nausea, vomiting, and/or anorexia in which case the patient should be assessed, and if needed, treated for chronic partial small bowel obstruction or GJ ulcers or strictures. If no anatomical cause is found then the patient needs to be evaluated, and if needed, treated for depression and other psychiatric illnesses as some of these patients may develop anorexia nervosa. Usually these patients with excessive weight loss can be treated with dietary counseling by the bariatric dietitian with 6 small meals a day including protein supplements. Oral pancreatic enzymes can also be helpful to improve the digestion and absorption of oral nutrients and partially reverse the malabsorption created by the RYGB. In rare situations a 3-month course of parenteral nutrition may be indicated to improve the patient’s nutrition status. If all else fails, the patient may need to be considered for reversal of the RYGB; however, this is a major procedure with significant morbidity.21
Micronutrient deficiencies are common following RYGB.21 Iron deficiency and anemia is one of the most common symptomatic micronutrient deficiencies occurring in 13% to 47% of RYGB patients. Iron is mostly present in meats, which many RYGB patients have difficulty ingesting, so their iron intake is generally less than before surgery.308Also, normally the gastric acid reduces dietary ferric iron (Fe3+) to the more absorbable ferrous iron (Fe2+).21,308,309 Following RYGB there is much less acid in the gastric pouch so less of the iron in the diet is converted to ferrous iron.309 Another contributing factor to iron deficiency is that the chief sites of iron absorption in the GI tract, the duodenum and proximal jejunum, are bypassed in RYGB patients.309 RYGB patients should routinely take iron supplements following surgery and their complete blood count (CBC) and serum ferritin levels should be routinely monitored. If despite this, patients develop iron deficiency, additional iron supplements should be ordered. Taking vitamin C (ascorbic acid) along with the oral iron supplements can increase iron absorption.(21,309) IV iron supplementation may occasionally be required.21
Calcium and vitamin D deficiencies are also common before and after RYGB and can lead to osteopenia, osteoporosis, and increased risk of fractures and problems with the patient’s teeth.21,309 Between 25% and 50% of preoperative RYGB patients have calcium and/or vitamin D deficiency and, despite recommending supplementation, up to 50% of postoperative RYGB patients are found to be vitamin D deficient.309 Like iron, the principle site of calcium absorption is the duodenum and proximal jejunum, which are bypassed by RYGB.309 Vitamin D is a fat soluble vitamin absorbed in the jejunum and ileum309 but absorption is decreased following RYGB due malabsorption of fats.308 RYGB patients should take calcium and vitamin D supplementation following surgery to provide 1500 mg of calcium per day, preferably as calcium citrate since it is better absorbed than calcium carbonate.21,308,309 Intact parathyroid hormone (PTH) and 25- hydroxy vitamin D levels should be periodically monitored and if abnormal, additional oral vitamin D 50,000 units weekly should be ordered for 8 weeks then the levels repeated.
Vitamin B12 is primarily absorbed in the terminal ileum but does require intrinsic factor, which is secreted from the antrum of the stomach.309 Since the terminal ileum and gastric antrum are intact following RYGB, deficiency of this vitamin should be uncommon. However, there is a complex chain of events that normally occurs in the stomach and duodenum to release vitamin B12 from proteins in the food to which it is bound.308 So bypassing these areas can result in a vitamin B12 deficiency in 5% to 36% of RYGB patients 1 to 9 years after surgery but is symptomatic, peripheral neuropathy or megaloblastic anemia, in only about 5% of patients.21,309 Usually oral vitamin B12 supplementation can prevent or treat this deficiency with only about 10% of patients requiring parenteral vitamin B12.309 Folate deficiency is rare after RYGB as long as patients take their supplemental vitamins.309
Vitamin B1 (Thiamine) is absorbed throughout the duodenum especially in the more acidic environment of the proximal duodenum. Deficiency is unusual after RYGB as long as the patients take their vitamin supplements unless they experience vomiting and have decreased food intake. While serum levels of thiamine may be low, the clinical complications of deficiency, Wernicke’s-Korsikoff’s encephalopathy or Beri-Beri, are rare.309
Zinc absorption is dependant on fat absorption so deficiency can be associated with malabsorptive procedures and cause alopecia but this is unusual as long as patients properly take their vitamin, mineral, and trace element supplements. While fat malabsorption may result in low serum levels of the other fat-soluble vitamins, vitamins A, E, and K, clinically significant deficiency states are rare. Deficiencies of the other water soluble vitamins and trace elements are likewise rare.309
Cholelithiasis and Cholecystitis. Rapid weight loss and a low fat diet result in formation of cholelithiasis in up to 50% of RYGB patients with 10% or more of RYGB patients developing symptomatic cholelithiasis.21 Since obesity is a predisposing factor for cholelithiasis, 14% to 30% of RYGB patients have already had their gallbladder removed prior to their bariatric surgery.187 Some bariatric surgeons recommend routine cholecystectomy either before or during the bariatric procedure to avoid this complication.21,187 Other surgeons screen for cholelithiasis and if present (17% to 26% of preoperative RYGB patients will have cholelithiasis) then perform cholecystectomy either before or during the bariatric procedure while still other surgeons do not evaluate the gallbladder at all before surgery unless the patient has symptoms of biliary disease.187
Ursodiol (ursodeoxycholic acid) 300 mg twice a day for 6 months after RYGB has been shown in a multi-center randomized controlled trials to significantly decrease the incidence of cholelithiasis formation at 6 months postoperative from 32% with placebo to 2% with the Ursodiol 600 mg/day.310 However, compliance with the 6-month course of Ursodiol is poor.187 In patients who develop symptomatic cholelithiasis after RYGB, laparoscopic cholecystectomy can be performed as usual. But the management of choledocholiathiasis is complicated by the fact that endoscopic retrograde cholangiopancreatography (ERCP) is usually technically not possible due to the gastric bypass. However, some centers have performed laparoscopic-assisted, percutaneous, trans-gastric remnant ERCP to diagnose and treat choledocholithiasis.21
Nephrolithiasis. Morbidly obese patients have a higher risk of nephrolithiasis compared to normal weight individuals. One study performed a urine StoneRisk® Diagnostic Profile on 45 preoperative bariatric surgery patients who had a mean BMI of 49.5 and a mean age of 47 years.311 At least one risk factor for nephrolithiasis was present in 98% of the patients and 3 or more was present in 80% of the patients. In the 1970’s, JI bypass was found to result in hyperoxaluria and an increased risk of nephrolithiasis with up to 39% of the patients having nephrolithiasis at 15 years after surgery.312,313 Although RYGB results in significantly less malabsorption than the JI bypass, it has also been associated with nephrolithiasis occurring months to years following surgery and some of these patients will develop oxalate nephropathy and acute renal failure.312-314 A population based study of 4,639 RYGB patients revealed an increased risk of symptomatic nephrolithiasis compared to a matched control group (7.7% vs. 4.6%, p<0.0001).314 In another study of 972 lap RYGB patients, 8.8% were found to have kidney stones prior to RYGB and 3.2% of all the patients develop new stones postoperatively. Of the 85 patients who had stones preoperatively, 31.4% developed new stones after RYGB.315
The etiology of this increased risk of nephrolithiasis is not completely understood. However, one of the main factors is hyperoxaluria. This is caused by increased binding of calcium to malabsorbed free fatty acids in the lumen of the intestine. This results in decreased availability of calcium to bind with oxalate in the diet forming an insoluble product that is excreted in the feces. Oxalate absorption and urinary excretion is increased resulting in formation of calcium oxalate stones.312-314 Increased delivery of bile salts and fatty acids to the colon also increases oxalate absorption.313
The combination of calcium binding to malabsorbed free fatty acids in the intestine, reduced calcium absorption in bypassed duodenum, and reduced absorption of vitamin D causes decreased urinary excretion of calcium resulting in supersaturation of calcium oxalate in the urine.312,313 In one study, hyperoxaluria increased from 8% of patients before RYGB to 29% three months after surgery.313 Oxalate not only binds to calcium but also binds to magnesium, which inhibits stone formation.312 Increased amounts of oxalate in the intestine can bind magnesium decreasing magnesium absorption, lowering urinary magnesium levels, and increasing the risk of stone formation.312 Also, the urinary oxalate/creatinine ratio is increased in RYGB patients, most likely due to decreased protein consumption, decreased muscle mass, and mild malnutrition; this increases the risk of stone formation.313 RYGB can also alter the gut microbiota including inhibition of a specific endogenous bacteria Oxalobacter formigenes that is important in regulating oxalate metabolism.313
Recommendations to prevent or treat nephrolithiasis following RYGB include limiting dietary oxalate and fats, administering calcium supplements, increasing oral fluid intake, and alkalinizing the urine.313,314 Dietary oxalate consumption accounts for 10% to 20% of daily-excreted oxalate with the remainder coming from endogenous sources. Major dietary sources of oxalate include spinach, rhubarb, tea, nuts, and cocoa.313 Intuitively, if a patient is developing calcium oxalate stones, one would be tempted to decrease oral calcium intake. However, in this situation, the opposite is true. It is even more important that the patient take their oral calcium supplements in order to meet or surpass the recommended daily allowance of 1200 mg of calcium a day in order to have more calcium to bind to the oxalate in the lumen of the intestine and decrease oxalate absorption.313 Also, the calcium should be in the form of calcium citrate, which is better absorbed through the intestine and the citrate will also help to alkalinize the urine.312,313 Patients need to increase their oral fluid intake to achieve a target of two liters of urine output a day.313 Treatment for symptomatic nephrolithiasis is extracorporal shock wave lithotripsy; however, increasing BMI decreases the effectiveness of this therapy.313
Inaccessibility to lower gastric remnant. Following RYGB, the lower gastric remnant is relatively inaccessible to routine diagnostic tests to detect pathology in this part of the stomach. For this reason, some surgeons recommend routine preoperative esophagogastroduduodenoscopy to assess for any pathology.187 The incidence of abnormal EGD ranges from 56% to 91% depending on the criteria used to define an abnormal EGD.187 In a study of 94 consecutive preoperative EGD, 164 abnormalities were found in 79 (84%) of the patients and included gastritis (70% of patients), duodenitis (43% of patients), hiatal hernia (23% of the patients), esophagitis (59% of the patients), gastric polyps (16% of the patients), gastric ulcer (1% of patients), duodenal ulcer (1% of patients), and masses (2% of patients). All of the gastric polyps were inflammatory or fundic glandular polyps with no malignant potential and the two masses were both benign (one was small inflammatory tissue and the other was a pancreatic rest on the pylorus that was excised at the time of open RYGB).187 However, several other studies have reported isolated cases of gastric cancer found on EGD during the preoperative evaluation.316-318 Sohn performed 427 consecutive open RYGB in which they routinely resected the lower gastric remnant and the pathology found in this portion of the stomach included gastritis (16% of patients), fundic polyp (2% of patients), intestinal metaplasia associated with chronic gastritis (0.7% of patients), gastric ulcer (0.5% of patients), lymphoid aggregate (0.5% of patients), diverticulum (0.2% of patients), developmental cyst (0.2% of patients), and leiomyoma (0.2% of patients).319 No malignancies were found. If a malignancy or pre-malignancy is found then the lower gastric remnant can be resected at the time of RYGB.316,317,320
Even if the lower gastric remnant is normal prior to RYGB, pathology can subsequently develop in the future. Even though gastric bypass for treatment of morbid obesity has been performed since 1977, the first reported case of cancer in the gastric remnant was in 1991321 and there have only been a few cases reported since that time.322,323This would suggest that the gastric remnant is not at any increased risk of malignancy. In 2007, Inoue et al randomized 55 rats to either RYGB, duodenojejunal bypass, or a sham operation then over a 16 week period of time the rats were subjected to a protocol of cancer induction with N-methyl-N-nitrosurea added to their water supply and once a week injected into the esophagus.324 Seventeen weeks after surgery the rats were sacrificed and the entire stomach examined pathologically. Gastric cancer developed in 85% of the rats in the sham group and 75% in the duodenojejunal bypass group but in only 23% of the RYGB group. The authors concluded that RYGB may reduce the risk of gastric cancer by lack of direct contact with carcinogens, lower incidence of bile reflux, and lower bacteria concentration in the gastric remnant.
If a patient does develop problems requiring assessment of the lower gastric remnant or duodenum, a CT scan of the abdomen may be helpful but will be limited by the inability to fill the stomach and duodenum with contrast. Another option is accessing the gastric remnant percutaneously under fluoroscopic guidance and then injecting contrast for an UGI x-ray. However, the resolution may be limited on these examinations. More recently, utilizing virtual three-dimensional CT, virtual colonoscopy and now virtual gastroscopy can be performed.325 However, this is diagnostic only as no biopsies or therapeutic interventions can be performed. In these situations, a double-balloon enteroscopy passed orally through the gastric Roux limb, through the EE, and up into the biliopancreatic limb was able to access the gastric remnant in 87.5% (35/40) of the patients.326 However, this is very technically demanding and it remains to be seen if these results can be duplicated. Another option is laparoscopic-assisted, percutaneous, trans- gastric remnant endoscopy or even endoscopic retrograde cholangiopancreatography for treatment biliary or pancreaticpathology.327,328
Suboptimal Weight Loss Results. Weight loss after bariatric surgery has been reported and measured in many different ways such as number of pounds or kilograms weight lost, percent body weight loss, percent decrease in BMI, etc. However, the most common and the current standard for measuring weight loss after bariatric surgery is percent excess body weight loss (% EBWL). The weight loss after RYGB is extremely variable from patient to patient and depends on how closely they follow their bariatric diet, how much they increase their exercise and Caloric expenditure, and what their individual genetically pre-determined metabolism is like. But on average, patients following RYGB will lose about 33% EBWL in the first 3 months, 67% EBWL in the first year, and about 75% EBWL in the first 18 months. Frequently there will be some weight regain and the long- term average weight loss is about 60% to 65% EBWL. Somewhat arbitrarily, 50% EBWL or greater has been established as “success” for bariatric surgery. However, the key is how much weight an individual needs to lose in order to improve their obesity related co-morbidities and their quality and quantity of life and this is unknown.
Despite the significant gastric restriction and malabsorption created by RYGB, some patients will not obtain the above weight loss goals or will regain a significant about of their weight loss. If this occurs, the patient should be examined for an anatomical reason for this such as a GG fistula, dilated gastric pouch, or dilated GJ. If any of these are present, revisional bariatric surgery may be helpful to correct these problems and help the patient to start losing weight again. If none of these situations are found then from a surgical standpoint, the only other option is to revise the EE, moving it more distal in the small intestine and creating more malabsorption. However, revisional bariatric surgery has a longer hospital length of stay and a higher rate of complications and mortality.41,111 It may also have a lower success rate then the primary bariatric surgical procedure.
Laparoscopic Adjustable Gastric Band (LAGB)
Bleeding. Since LAGB is a simpler procedure requiring much less dissection, the incidence of bleeding complications is less. However, the surgeon still must be careful to avoid injury to the nearby spleen and liver that can result in bleeding. The incidence of postoperative GI bleeding is very low since there is no stapling or transection of the stomach or small intestine. Bleeding from trocar sites and/or from the subcutaneous pouch formed for the injection port can occur.
Infection. Intraabdominal infections, abscesses or peritonitis, is rare due to the fact that the procedure does not involve transection of any portion of the GI tract. However, care must be taken not to perforate the stomach in the dissection of the angle of His and the rather blind dissection when creating the tunnel for the passage of the band behind the stomach. This can result in infection of the band requiring subsequent removal of the band or, less commonly, intraabdominal abscess or peritonitis. Wound infections can occur and can have more serious sequela then after RYGB especially if it results in an infection of the subcutaneous injection port. If the port does get infected, it may be possible to remove the port but leave the band and intraperitoneal tubing in place. Once the infection is resolved, laparoscopically the intraperitoneal tubing can be tunneled through the abdominal wall in a new site and new port place. However, if the band or intraperitoneal tubing is infected then the entire system has to be removed.
VTE and Cardiopulmonary complications. The risk of VTE and cardiopulmonary complications is less for LAGB since it is a short and less involved procedure. However, all bariatric surgery patients are at increased risk of VTE and require prophylaxis and require that appropriate measures, as previously discussed, be taken to prevent,diagnosis, and treat cardiopulmonary complications.
Nausea and Vomiting. Most LAGB patients will experience nausea and many will have vomiting some time after surgery and usually it is due to eating too much, too fast, or wrong foods that get stuck in the gastric pouch.21 If the nausea and vomiting is mild and intermittent, the bariatric dietitian can work with the patient regarding their dietaryhabits. However, if the nausea and vomiting is severe or persistent then the patient needs further evaluation to ensure that they have not developed an obstruction. LAGB patients can become dehydrated and develop acute renal failure quickly so if adequate oral intake cannot be maintained, the patient should be admitted for IV fluid hydration and correction of any electrolyte abnormality.
Acute Band Obstruction. This can occur immediately after surgery due to either too small of a band being placed, band not being emptied at time of surgery, or edema or hematoma of the wall of the stomach around the band. This can usually be managed by keeping the patient NPO and administering IV fluids. IV non-steroidal anti-inflammatory medications may be helpful to more quickly resolve the edema and inflammation around the band. Some surgeons also use a short course of IV steroids in this situation. If the obstruction persists then the patient will need repeat surgery with excision of some of the perigastric fat under the band or replacement of the band with a larger band. This can also occur following a band adjustment if the band is tightened too much, in which case the port can be re-accessed and the volume of the band decreased.21
Band “Slip” or pouch dilation. Band “slip,” or more appropriately called gastric prolapse, is when the stomach below the band prolapses or herniates superiorly up through the band causing the band to tilt down to the left or “slip” resulting in a partial or complete obstruction at the level of the band (Figure 13). Patients with a “slipped band” will commonly present with nocturnal reflux/regurgitation, night cough, dysphagia, or persistent vomiting, even to the point where the patient cannot even drink water. Pouch dilation is a concentric enlargement of the gastric pouch with the band still in good position, titled up towards the left shoulder. Patients with pouch dilation can complain of lack of satiety, heartburn, reflux, regurgitation, or intermittent chest pain.329
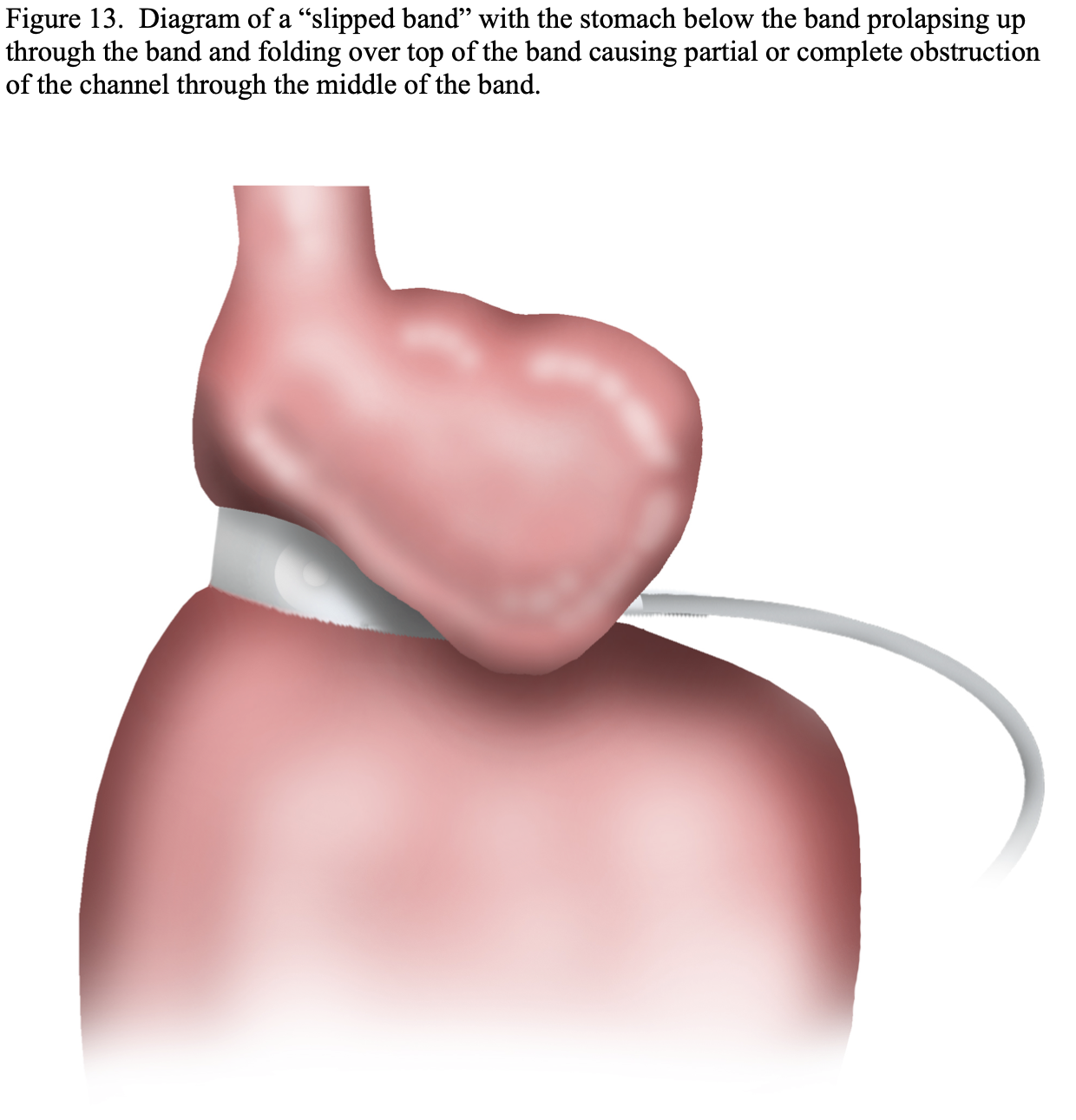
A band “slip” occurs in 2% to 25% of band patients with most series reporting a 2% to 4% “slip” rate.21,330,331 It is usually associated with vomiting from some other cause, such as eating too much or too fast, getting food stuck in the gastric pouch, viral gastroenteritis, pregnancy, post-anesthesia vomiting, etc., and the retrograde peristalsis causes the distal stomach to prolapse or “slip” up through the band.21 This results in a change in the orientation of the band from pointing up towards the left shoulder to a more horizontal orientation or even pointing down towards the left hip and the prolapsed portion of the stomach causes a partial or complete obstruction (Figure 14A, 14B, 14C, 14D).
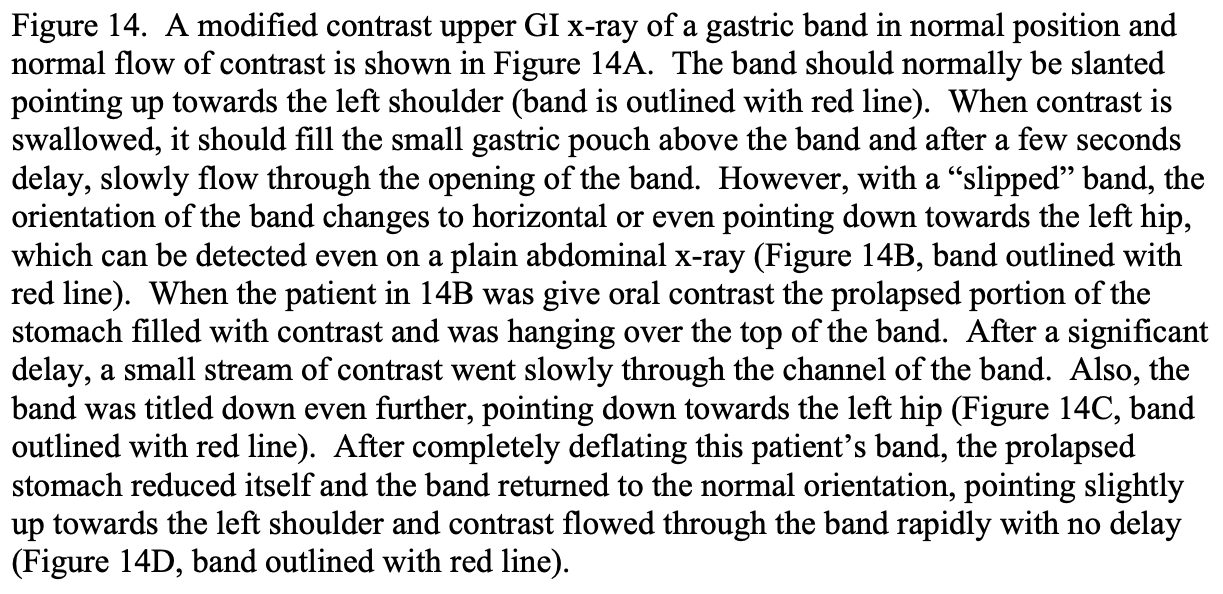
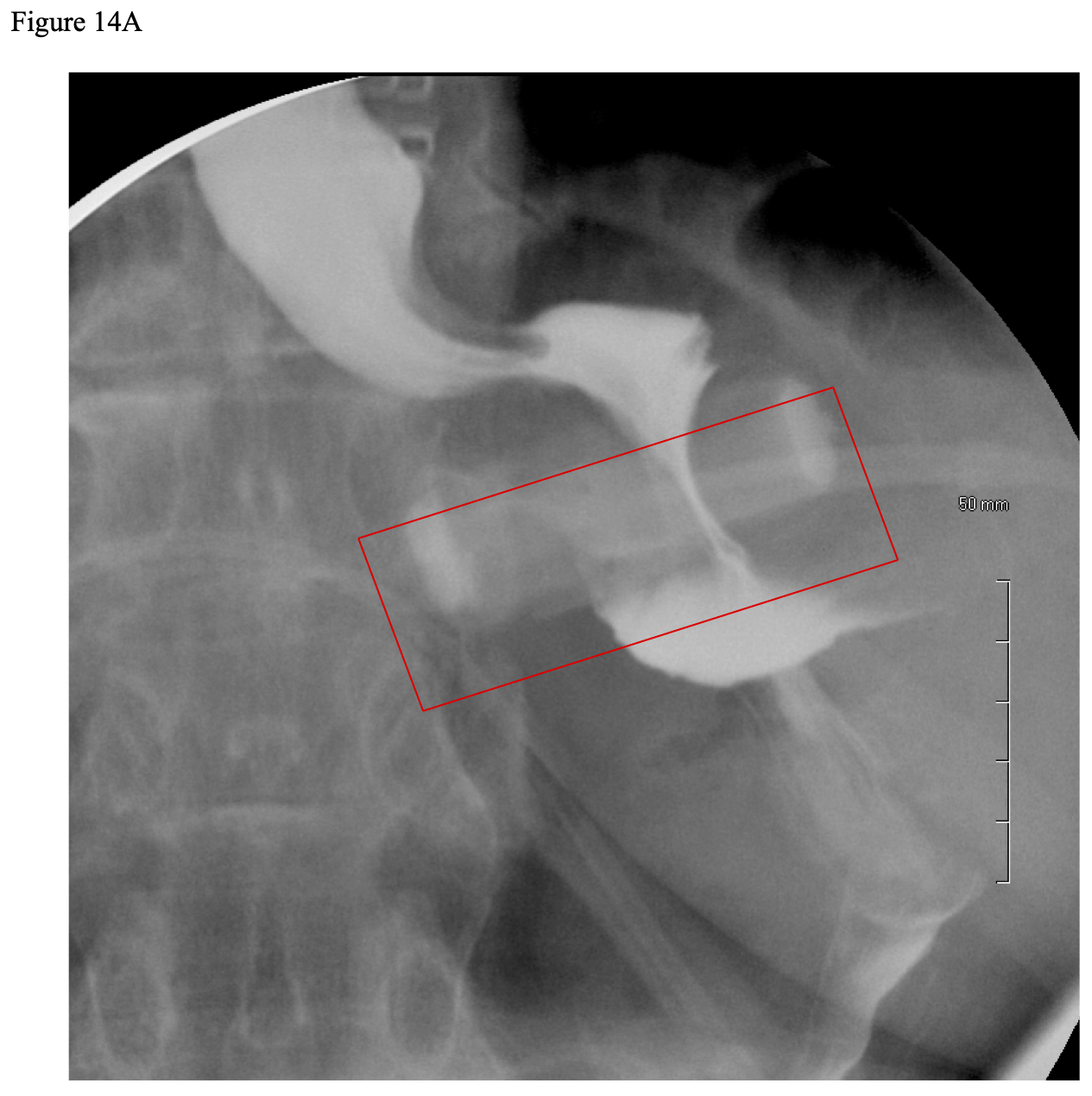
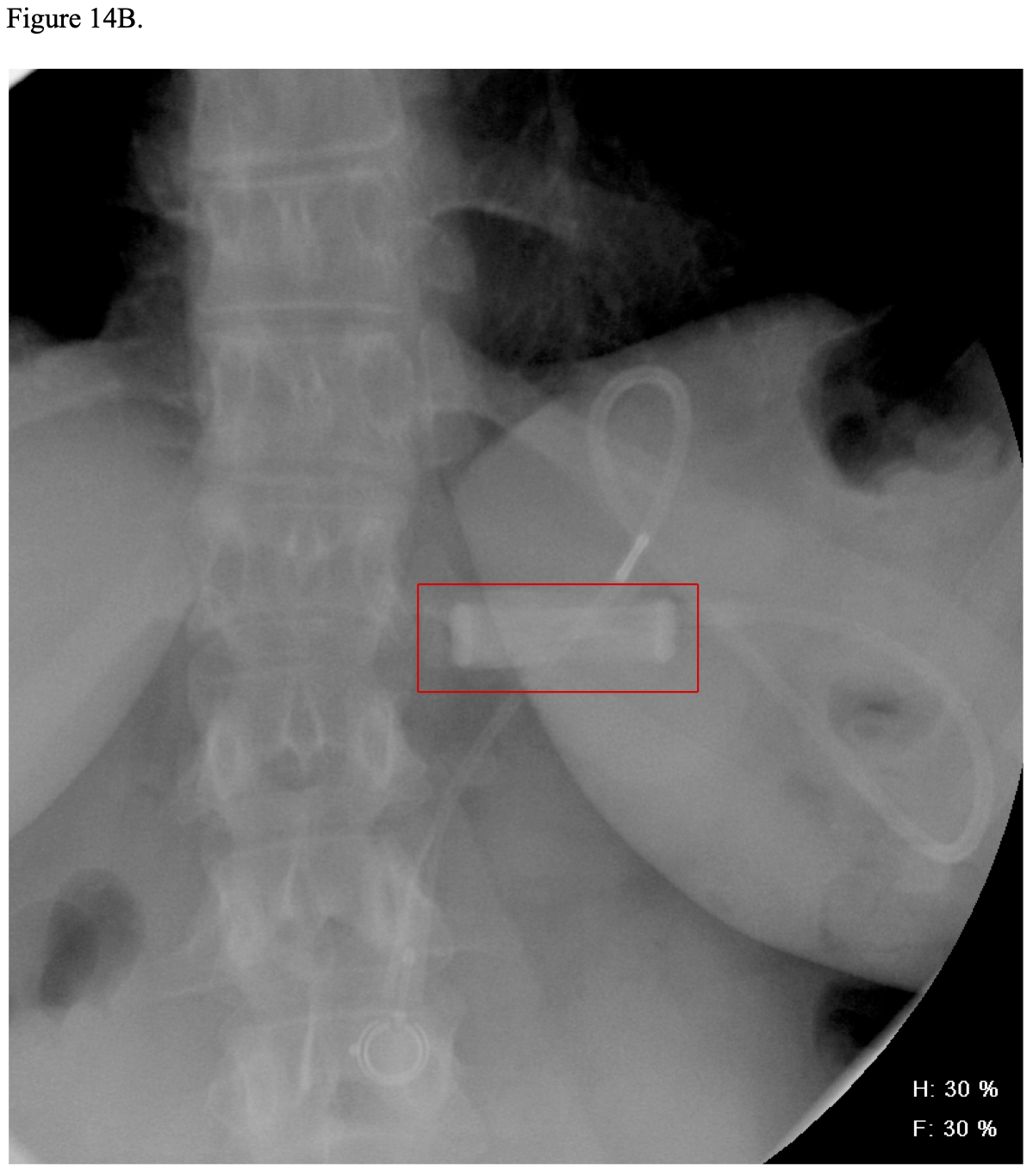
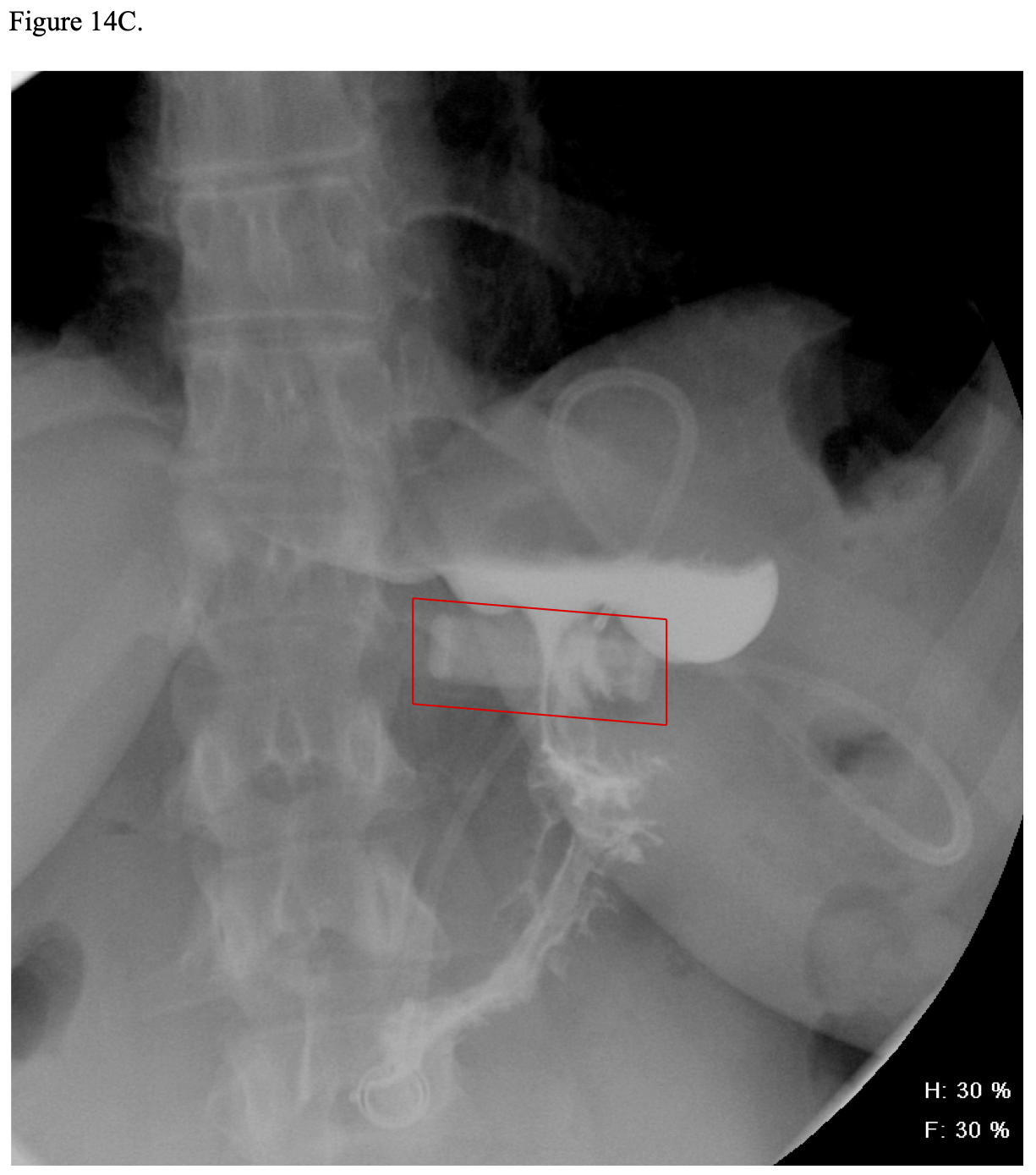
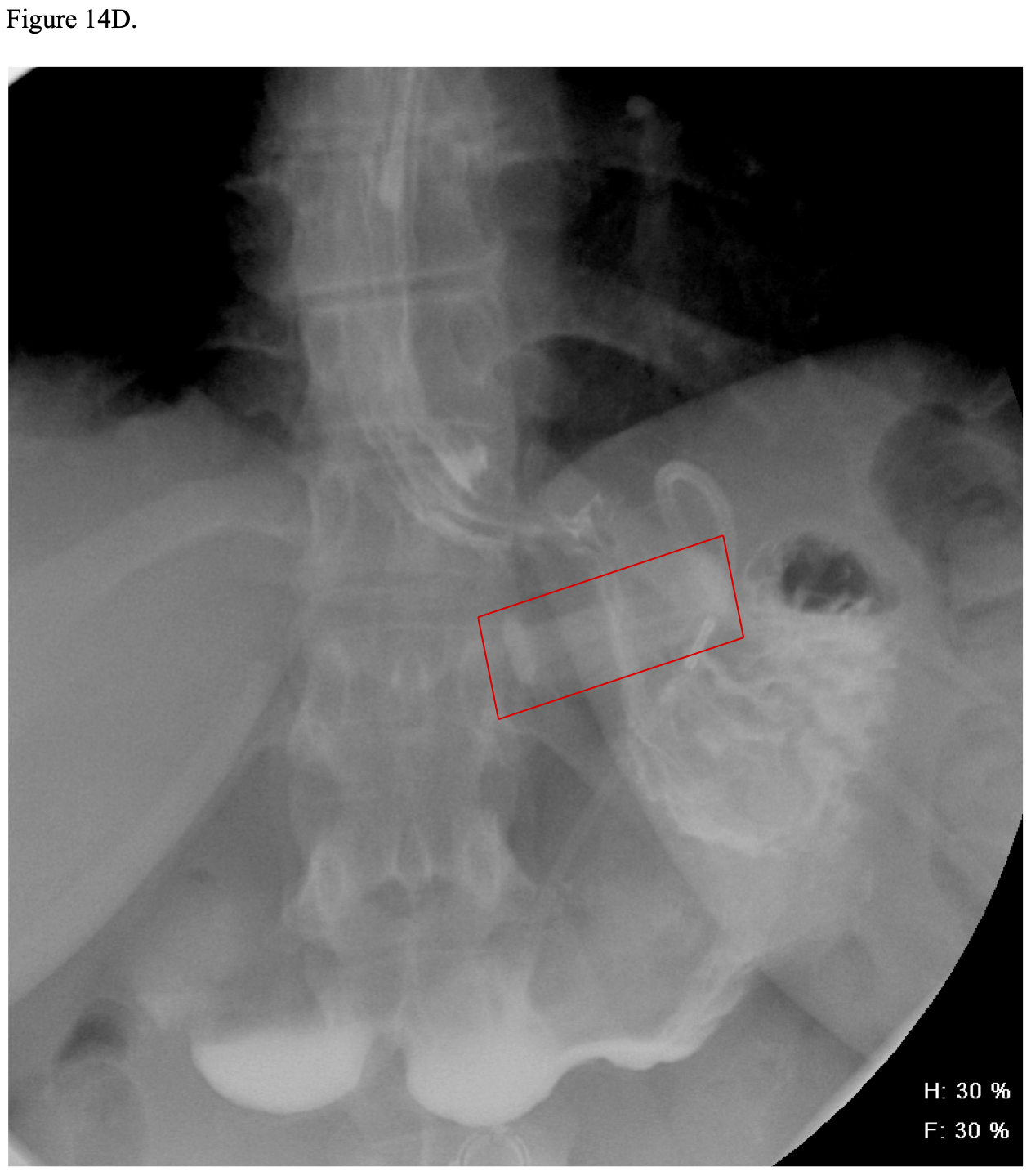
With the previous perigastric insertion technique, the slip most often occurred in posterior aspect of the band. However, with the current pars flacida technique the slip rate is less since there is minimal dissection and better fixation posteriorly and if a “slip” occurs, it usually is at the anterior portion of the band.21 In order to decrease anterior band “slips” most surgeons utilize seromuscular sutures to approximate the stomach below the band to the gastric pouch above the band, gastro-gastric sutures. However, there is marked variation in the number (1, 2, 3, 4, etc.) and type (interrupted or running) of gastro-gastric sutures.49,50 Four different methods of gastro-gastric suture techniques were compared in an in vivo canine model.46 The first group had no gastro-gastric sutures placed (no wrap group), the second group had three sutures placed on the left lateral aspect of the stomach (fundic wrap group), the third group had 2 sutures placed anteriorly and towards the lesser curvature of the stomach (anterior wrap), and the last group had 4 sutures placed from the greater curvature to the lesser curvature (complete wrap group).
The band was inflated to its maximal volume and a meat bolus was placed in the esophagus. Then an insufflation catheter and pressure transducer was placed in the esophagus and the esophagus inflated until prolapse of the stomach (a “slip”) occurred and the maximum pressure prior to the prolapse was recorded. “Slip” occurred at a pressure of 50 to 70 mm Hg in the no wrap group, 70 to 90 mm Hg in the fundic wrap group, and 90 to 110 mm Hg in the anterior wrap group. Prolapse did not occur with the complete wrap group. The authors recommended performing as complete an anterior wrap as possible to decrease the risk of prolapse or “slip.” However, another study from France found that the slip rate was higher in patients with gastro-gastric suturescompared to patients with no sutures (3% (64/2001) vs. 1% (18/1501), p<0.001).45 Some surgeons have also proposed suturing the gastric fundus to the left hemidiaphragm, a gastropexy, in order to decrease the incidence of “slippage.”47,48 There remains much controversy and no standardization in regards to how to fixate the band or whether to fixate it at all.
In one large bariatric surgery program, intractable GERD was the most common indication for reoperation or removal of LAGB.329 At reoperation, 27% of patients with prolapse and 53% of patients with pouch dilation were associated with a hiatal hernia.332 In general, several different surgical procedures have been described for repair of a hiatal hernia; however, the most common repair is the Nissen fundoplication, which usually incorporates a posterior crural repair to narrow the esophageal hiatal opening.333 However, Fielding and Ren began incorporating an anterior crural repair of any hiatal hernias found at the time of initial LAGB placement.329 A retrospective comparison of their 1,298 LAGB without hiatal hernia repair (HHR) and 520 LAGB with HHR performed between 2001 and 2006 revealed a significantly lower rate of reoperation in the LAGB/HHR group (3.5% vs. 7.9%, p<0.001) although the incidence of band “slip” was similar in both groups (1.7% vs. 2.3%, p=0.44).329 Performing the HHR at the time of LAGB added only 11 minutes to the mean operative time for this group.329 Initially, they only repaired the hiatal hernia if it was obviously apparent. But because of the findings of their study, they now routinely dissect off the epiphrenic fat pad and perform an anterior repair if a hiatal hernia is present unless there is a large posterior defect, in which case a posterior crural repair is performed.329 Many bariatric surgeons have incorporated this anterior crural repair with LAGB insertion; however, there is marked variability in how aggressive the hiatal hernias are searched for and repaired. Although most bariatric surgeons ignored small to moderate sized hiatal hernias at the time of RYGB, many are now incorporating this anterior crural repair with RYGB when a hiatal hernia is present in an attempt to decrease the incidence of postoperative GERD in RYGB patients as well.
Nausea and vomiting need to be avoided in LAGB to prevent “slippage” of the band. In an attempt to maximize weight loss, the LAGB can be over-filled, tightening the band too much resulting in nausea and vomiting and precipitating a “slip.” One study involving patients with a SAGB showed that patients that developed a “slip” had a significantly higher mean fill volume compared to patients that did not develop a “slip” (12.6 ml vs. 8.7 ml).330 So if the patient is not following their bariatric diet, simply continuing to tighten the band only increases their risk of “slip” and other complications related to the LAGB.
The management of an acute “slipped” band is complete deflation, which usually relieves the obstruction (Figure 14B, 14C, and 14D). If the slip is mild then the gastric prolapse may even reduce spontaneously and after a few weeks, the band can be readjusted being careful not to get the band too tight and causing further vomiting and recurrent band “slippage.” However, if the “slip” does not reduce or the patient has recurring problems with “slippage” then the patient will require laparoscopy and repositioning of the band, replacement of the band, or removal of the band depending on the patient’s preference and the technical feasibility of repositioning or replacement of the band.21
Esophageal dilation/megaesophagus. LAGB impairs esophageal peristalsis and can also cause a lack of relaxation of the lower esophageal sphincter, possibly leading to esophageal dilation.334 Severe esophageal dilation due to aperistalsis and associated secondary and tertiary contractions is called megaesophagus.334 About 25% of LAGB patients can develop this complication but most patients have mild or no symptoms.21 In a study of 257 LAGB patients, all patients had esophageal manometry performed as a part of their routine preoperative evaluation.334 Postoperatively, 1.9% (3 men and 2 woman) developed megaesophagus. Four of these five patients had normal preoperative esophageal manometry while 1 patient had a nonspecific motility disorder showing diffuse esophageal spasms. The authors concluded that routine preoperative esophageal manometery was not cost effective and was not predictive as to which patients may develop megaesophagus. Another study performed esophageal manometry preoperatively and at 6 and 12 months postoperatively in 22 LAGB patients and found that abnormal manometric findings are frequently encountered long-term after LAGB including increased LES residual pressure and peristaltic wave duration but there was no increase in total esophageal acidification time.335
The etiology of esophageal dilation and megaesophagus remains unclear. In general, obesity has been associated with esophageal disorders including esophageal motility disorders, hiatal hernia, and GERD.334 Esophageal dilation or megaesophagus could be caused by a LAGB that is too tight or malpositioned causing chronic obstruction resulting in food backing up into the esophagus and esophageal dilation. However, Wiesner found a 7.5% incidence of esophageal dilation in a series of 120 LAGB patients.336 All 9 patients had a well-positioned, well-adjusted band with appropriate stoma width, had preexisting insufficiency of the lower esophageal sphincter (LES) on preoperative endoscopy, and were non-compliant with their bariatric diet. Seven of the 9 patients with esophageal dilation presented with insufficient weight loss while only one of three other patients with preoperative LES insufficiency and who were compliant with their diet showed insufficient weight loss. In summary, one of the major etiologies of esophageal dilation and megaesophagus is poor dietary compliance with over-eating causing chronic overfilling of the gastric pouch with food backing up into the esophagus using it as additional space for food.334,336 This may explain why patients with esophageal dilation and megaesophagus frequently have poor weight loss results.334,336 One study showed a significantly lower weight loss at 2 years for patients with megaesophagus compared to those who did not develop this complication (25% vs. 58% EBWL).334
Patients with esophageal dilation can present with achalasia-like symptoms including dysphagia, vomiting, and/or GERD.21,334 If a LAGB patient develops any of these symptoms then a barium UGI x-ray should be obtained to diagnose or rule out this condition.21 The initial treatment of esophageal dilation or megaesophagus is partial or complete deflation of the band and dietary counseling.21,334 However, the treatment of LAGB patients with this condition and associated poor weight loss is difficult. If the band is tightened further in attempt to improve weight loss, the esophageal dilation or megaesophagus and associated symptoms will worsen but if the LAGB is even partially deflated, the patient is going to regain more weight.334 So if the patient does not respond to intensified dietary counseling then the LAGB will need to be removed and the patient will need to decide whether they want to be converted to another bariatric surgical procedure.334 In the above study, the LAGB was removed in all 5 patients with megaesophagus with 2 patients electing for conversion to LSG with good early weight loss results while the other 3 patients chose not to pursue any further bariatric surgery.334
Band Erosion. Anytime there is foreign material against the stomach or bowel, it can erode into and through the wall. This occurs in 1% to 11% of LAGB patients.21,330,331,337,338 It usually presents with either epigastric abdominal pain or persistent or recurring subcutaneous port infections.21 The diagnosis is made by seeing a black foreign body inside the stomach at the level of the band on upper GI endoscopy.21 The treatment is removal of the band. This is most often performed with laparoscopy although there are case reports of eroded bands being removed endoscopically in a piece meal fashion.338 Usually these erosions are chronic so the perforation may not require suture closure. The decision to insert another LAGB either at the time of removal of the eroded band or at a later time depends on patient preference and technical factors such as the degree of inflammation, infection, scarring, and need for suture closure of the perforation.
Tear, break, or leak of band, tubing or port and port displacement. Over the years, both of the commercially available LAGB have undergone design improvements and the current LAGB are fairly durable. However, any mechanical device with time is prone to failure.339-341 In a study of 286 consecutive LAGB patients, at a mean follow-up of 3.3 years, 104 mechanical complications occurred in 70 patients at a mean of 2.2 years, including port displacement (10% of patients), port disconnection (13% of patients), and band rupture (1% of patients).340 In another study of 489 LAGB patients with a mean follow-up of 41 months, 71 patients (14.5%) presented with port or connecting tubing complications that required 82 revisional surgeries. These complications included system leaks (54 patients), mechanical problems (14 patients), and infected port or tubing (3 patients).339 These complications frequently result in weight regain and/or corrective surgery and sometimes require removal of the band.339 Careful attention to technique can minimize these complications. During band insertion, care must be taken not to damage the balloon portion of the band during passage of the band through the trocar, with passage of the band behind the stomach, and when placing the seromuscular sutures between the stomach below the band and the gastric pouch above the band. Also these seromuscular sutures must be securely tied with good approximation of the two serosal surfaces so the suture does not come in contact with the balloon of the band. The surgeon must ensure that the tubing is properly secured to the port to avoid dislodgement of the tubing from the port. Also the port needs to be securely attached to the abdominal fascia to prevent the port from flipping over, which would require surgical correction. Lastly, during LAGB adjustments, a Huber needle should always be used to decrease the risk of damaging the port membrane causing a leak. In addition, it is critical that the person performing the adjustment knows the position and orientation of the port in order to avoid puncturing the tubing near the port, which will cause a leak and require re-operation to replace the port and proximal tubing.
Protein-Calorie Malnutrition and other nutritional deficiencies. This is rarely seen with LAGB as no malabsorption is created with this procedure. However, it can be associated with maladaptive eating, inadequate oral vitamin and mineral supplements, or if the patient develops a complication such as band “slip,” esophageal or pouch dilation, band erosion, etc. resulting in nausea, vomiting, and inability to eat.
Cholelithiasis and Cholecystitis. Postoperative formation of cholelithiasis can also occur with LAGB. However, one retrospective study examined the incidence of symptomatic cholelithiasis after 496 RYGB, 36 LAGB, and 54 LSG with mean follow-up of 25.9 months.342 The combined group of LAGB and LSG patients had a lower incidence of forming symptomatic cholelithiasis compared to the RYGB patients but it did not quite meet statistical significance (3.3% vs. 8.7%, p=0.09). However, the only factor in univariant and multivariant analysis that correlated to symptomatic gallstone formation was a postoperative weight loss of greater than 25% of original weight. So it make sense that since the restrictive procedures, LAGB & SG, generally lose less weight and the weight loss is slower postoperatively then the combined restrictive and malabsorptive procedures, RYGB and BPD with or without DS, that they would have a lower incidence of postoperative symptomatic cholelithiasis.
Suboptimal Weight Loss Results. The weight loss with the LAGB is much slower, over a longer period of time, and the ultimate weight loss on average is less than compared to RYGB.21,90 Also, since LAGB results in restriction alone without any malabsorption, it is easier for patients to “cheat” on their diet because they will not experience the symptoms of dumping syndrome.21 So LAGB patients must be even more motivated to follow their bariatric diet then the RYGB patients. Some LAGB patients try to rely solely on the band to loose weight rather than changing their diet. In this situation, when the patient does not lose weight, they keep requesting that the band be tightened more and more until they get to the point where they cannot tolerate solid food. Then they begin consuming only liquids, frequently high caloric liquids, and snacks. When they still do not lose weight, they request the band to be tightened further until they cannot consume anything at all and present with nausea, vomiting, GERD, esophageal dilation, etc., increasing the risk for “slippage” of the band.330 So the failure rate in obtaining and maintaining weight loss is higher with LAGB than RYGB. The LAGB patients need to be seen much more frequently than the RYGB patients in the first 1 to 2 years after surgery to ensure that the band is properly adjusted and to re-enforce the appropriate changes in their diet and exercise. LAGB patients that are not progressing towards their weight loss goals despite appropriate adjustments of their band need to keep accurate food records and work with the bariatric dietitian to change their diet. If a patient is unable to meet their weight loss goals despite all of these measures then conversion from LAGB to RYGB can be considered.
Biliopancreatic Diversion (BPD) with or without Duodenal Switch (DS)
The prevention, diagnosis, and treatment of complications for BPD with or without DS are very similar to RYGB. The main difference is the significant increased risk of protein-calorie malnutrition, nutritional deficiencies, and fluid and electrolyte abnormality related to greater magnitude of malabsorption created by this procedure. So it is even more important that BPD patients take their protein, vitamin, and mineral supplements and that they are compliant with their routine clinical and laboratory surveillance for these complications. The BPD/DS preserves the pylorus so it has a significantly lower incidence of dumping syndrome compared to RYGB or BPD without DS. The main advantage of BPD with or without DS compared to RYGB, LAGB, or LSG is the significantly lower risk of weight regain. 21-23
Laparoscopic Sleeve Gastrectomy (LSG)
Bleeding. Bleeding from the long gastric staple line is one of the most common complications. However, using reinforcement pads on the stapler can decrease bleeding and blood loss.343 Bleeding can also occur during the dissection of the stomach and from injuries to the liver or spleen.35
OGT Complications. Most surgeons have anesthesia staff insert an oral gastric sizing bougie during the procedure to assist in the sizing of the gastric pouch. If this is not inserted properly and the position properly maintained, damage can occur to the esophagus or stomach. Also, the tube can be stapled and transected or incorporated into suture reinforcement of the gastric staple line. Again, the surgeon and anesthesia staff must maintain good communication and have established procedures in an attempt to avoid these complications.
Anastomotic leaks and intraabdominal sepsis. The long gastric staple line is also prone to leak, which has been reported in 0.7% to 5.2% of the cases.35 Staple line reinforcements and/or oversowing of the gastric staple line have been tried in order to reduce the incidence of leaks. If the leak is small and contained, the leak can be treated with drainage, NPO, IV antibiotics, parenteral nutrition, and close observation. A stent can be placed via fluoroscopy or endoscopy to contain the leak and endoscopic fibrin sealants have also been tried.344 However, if the patient does not respond to non-surgical treatment or has signs of peritonitis exploratory laparoscopy or laparotomy is indicated for identification and closure of the leak as well as irrigation and drainage.
VTE and Cardiopulmonary complications. The risk of VTE and cardiopulmonary complications for SG is probably intermediated between RYGB or BPD and LAGB. So again, the patient should receive appropriate VTE prophylaxis and appropriate measures need to be taken to prevent, diagnose, and treat cardiopulmonary complications.
Wound infection. This is a clean contaminated procedure so patients are at risk for wound infections and need to receive prophylactic antibiotics and observation and prompt treatment if a wound infection does occur.
Nausea, vomiting, dehydration and electrolyte disorders. These complications can occur and should be managed as previously discussed above for RYGB.
Strictures of the Gastric Pouch. Incidence is about 1% and it usually occurs at the incisura of the stomach. As the use of LSG as a primary bariatric surgical procedure, surgeons have been using smaller sizing bougies in order to obtain smaller gastric pouches and more gastric restriction, which increases the risk of stricture.35 Strictures can usually be treated with endoscopic balloon dilation. However, if this fails, the stricture may need to be resected and the patient converted to a RYGB.
Bowel obstruction. The incidence of bowel obstruction is low and is usually secondary to adhesions. Since there are no defects created during this procedure, internal hernias are avoided.
Incisional/Trocar Hernia. As previously discussed for lap RYGB and LAGB.
Protein-Calorie Malnutrition and other nutritional deficiencies. This is rarely seen with LAGB as no malabsorption is created with this procedure. However, it can be associated with maladaptive eating, inadequate oral vitamin and mineral supplements, or the patient can develop a complication such as band “slip,” esophageal or pouch dilation, band erosion, etc. resulting in nausea, vomiting, and inability to eat.
Cholelithiasis and Cholecystitis. Again, this complication can occur with rapid weight loss and a low fat diet result but the incidence is not well documented. Prophylaxis and treatment is similar to that of RYGB and LAGB.
Suboptimal Weight Loss Results. As previously stated, the long-term weight loss of LSG is yet to be determined. Some long-term weight loss results are becoming available now but this is from the early days of the intended two stage BPD/DS procedures when the gastric pouches were large. Long-term weight loss results for the patients treated with a primary LSG procedure with smaller pouches is still needed.
References
- Ogden CL, Carroll MD, McDowell MA, Flegal KM. Obesity among adults in the United States – no change since 2003-2004. NCHS data brief no. 1. Hyattsville, MD: National Center for Health Statistics. 2007.
- Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960-1994. Int J Obes Relat Metab Disord. 1998;22(1):39-47.
- Centers for Disease Control and Prevention. US Obesity Trends. Overweight and Obesity. Available at: http://www.cdc.gov/obesity/data/trends.html
- McGinnis JM, Foege WH. Actual causes of death in the United States. JAMA. 1993;270:2207-12.
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238-45.
- Prospective Studies Collaboration, Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083-96.
- Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Agren G, Carlsson LM; Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741-52.
- Nguyen NT, Goldman C, Rosenquist CJ, Arango A, Cole CJ, Lee SJ, Wolfe BM. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann Surg. 2001;234(3):279-91.
- Body mass index. Wikipedia: The Free Encyclopedia. Available at: http://en.wikipedia.org/wiki/Body_mass_index.
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549-1555.
- Martin LF. The evolution of surgery for morbid obesity. In: Martin LF., ed. Obesity Surgery. New York: Mc-Graw Hill; 2004:15-48.
- Brolin RE, LaMarca LB, Kenler HA, Cody RP. Malabsorptive gastric bypass in patients with superobesity. J Gastrointest Surg. 2002;6(2):195-205.
- Steffen R. The history and role of gastric banding. Surg Obes Relat Dis. 2008;4(3 Suppl):S7-13.
- Camilleri M, Toouli J, Herrera MF, Kulseng B, Kow L, Pantoja JP, Marvik R,Johnsen G, Billington CJ, Moody FG, Knudson MB, Tweden KS, Vollmer M, Wilson RR, Anvari M. Intra-abdominal vagal blocking (VBLOC therapy): clinical results with a new implantable medical device. Surgery. 2008;143(6):723-31.
- Gersin KS, Keller JE, Stefanidis D, Simms CS, Abraham DD, Deal SE, Kuwada TS, Heniford BT. Duodenal-jejunal bypass sleeve: a totally endoscopic device for the treatment of morbid obesity. Surg Innov. 2007;14(4):275-8.
- Cha A, Brethaueer SA, Pryor A, Chand B. Emerging technologies: update on endoluminal treatment options for morbid obesity. Bariatric Times. 2009;6(6):1,8-13.
- Hess DS, Hess DW. Biliopancreatic diversion with a duodenal switch. Obes Surg. 1998;8(3):267-82.
- Marceau P, Hould FS, Simard S, Lebel S, Bourque RA, Potvin M, Biron S. Biliopancreatic diversion with duodenal switch. World J Surg. 1998;22(9):947-54.
- Ren CJ, Patterson E, Gagner M. Early results of laparoscopic biliopancreatic diversion with duodenal switch: a case series of 40 consecutive patients. Obes Surg. 2000;10(6):514-24.
- Brethauer SA, Hammel JP, Schauer PR. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis. 2009;5(4):469-75.
- Tessier DJ, Eagon JC. Surgical management of morbid obesity. Curr Probl Surg. 2008;45(2):68-137.
- Gagner M, Matteotti R. Laparoscopic biliopancreatic diversion with duodenal switch. Surg Clin North Am. 2005;85(1):141-49.
- Van Hee RH. Biliopancreatic diversion in the surgical treatment of morbid obesity. World J Surg. 2004;28(5):435-44.
- American Society for Metabolic and Bariatric Surgery. Mission and Purpose. Available at: http://www.asmbs.org/Newsite07/aboutasbs/asbs_mission.htm
- NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115(12):956-61.
- Wittgrove AC, Clark GW. Laparoscopic gastric bypass, roux-en-y- 500 patients: technique and results, with 3-60 month follow-up. Obes Surg. 2000;10:233-39.
- Cigaina V. Gastric pacing as therapy for morbid obesity: preliminary results. Obes Surg. 2002;12(Suppl 1):12S-16S.
- Camilleri M, Toouli J, Herrera MF, Kow L, Pantoja JP, Billington CJ, Tweden KS, Wilson RR, Moody FG. Selection of electrical algorithms to treat obesity with intermittent vagal block using an implantable medical device. Surg Obes Relat Dis. 2009;5(2):224-30.
- Tucker ON, Szomstein S, Rosenthal RJ. Indications for sleeve gastrectomy as a primary procedure for weight loss in the morbidly obese. J Gastrointest Surg. 2008;12(4):662-7.
- Givon-Madhala O, Spector R, Wasserberg N, Beglaibter N, Lustigman H, Stein M, Arar N, Rubin M. Technical aspects of laparoscopic sleeve gastrectomy in 25 morbidly obese patients. Obes Surg. 2007;17(6):722-7.
- Frezza EE, Reddy S, Gee LL, Wachtel MS. Complications after sleeve gastrectomy for morbid obesity. Obes Surg. 2009;19(6):684-7.
- Fuks D, Verhaeghe P, Brehant O, Sabbagh C, Dumont F, Riboulot M, Delcenserie R, Regimbeau JM. Results of laparoscopic sleeve gastrectomy: a prospective study in 135 patients with morbid obesity. Surgery. 2009 Jan;145(1):106-13.
- Arias E, Martínez PR, Ka Ming Li V, Szomstein S, Rosenthal RJ. Mid-term follow- up after sleeve gastrectomy as a final approach for morbid obesity. Obes Surg. 2009;19(5):544-48.
- Rubin M, Yehoshua RT, Stein M, Lederfein D, Fichman S, Bernstine H, Eidelman LA. Laparoscopic sleeve gastrectomy with minimal morbidity. Early results in 120 morbidly obese patients. Obes Surg. 2008;18(12):1567-70.
- Gagner M, Deitel M, Kalberer TL, Erickson AL, Crosby RD. The Second International Consensus Summit for Sleeve Gastrectomy, March 19-21, 2009. Surg Obes Relat Dis. 2009;5(4):476-85.
- American College of Surgeons Bariatric Surgery Center Network. About ACS BSCN. http://www.acsbscn.org/Public/AboutBSCN.aspx
- Nguyen NT, Ho HS, Palmer LS, Wolfe BM. A comparison study of laparoscopic versus open gastric bypass for morbid obesity. J Am Coll Surg. 2000;191(2):149-57.
- Tucker ON, Szomstein S, Rosenthal RJ. Surgical management of gastro-gastric fistula after divided laparoscopic Roux-en-Y gastric bypass for morbid obesity. J Gastrointest Surg. 2007;11(12):1673-79.
- Salimath J, Rosenthal RJ, Szomstein S. Laparoscopic remnant gastrectomy as a novel approach for treatment of gastrogastric fistula. Surg Endosc. 2009 May 22. [Epub ahead of print]
- Capella JF, Capella RF. Gastro-gastric fistulas and marginal ulcers in gastric bypass procedures for weight reduction. Obes Surg. 1999;9(1):22-28.
- Blackburn GL, Hutter MM, Harvey AM, Apovian CM, Boulton HR, Cummings S, Fallon JA, Greenberg I, Jiser ME, Jones DB, Jones SB, Kaplan LM, Kelly JJ, Kruger RS Jr, Lautz DB, Lenders CM, Lonigro R, Luce H, McNamara A, Mulligan AT, Paasche- Orlow MK, Perna FM, Pratt JS, Riley SM Jr, Robinson MK, Romanelli JR, Saltzman E, Schumann R, Shikora SA, Snow RL, Sogg S, Sullivan MA, Tarnoff M, Thompson CC, Wee CC, Ridley N, Auerbach J, Hu FB, Kirle L, Buckley RB, Annas CL. Expert panel on weight loss surgery: executive report update. Obesity (Silver Spring). 2009;17(5):842- 62.
- Madan AK, Harper JL, Tichansky DS. Techniques of laparoscopic gastric bypass: on-line survey of American Society for Bariatric Surgery practicing surgeons. Surg Obes Relat Dis. 2008;4(2):166-73.
- Small Intestine. Wikipedia: The Free Encycolopedia. Available at: http://en.wikpedia.org/wiki/Small_intestine
- Quebbemann BB, Dallal RM. The orientation of the antecolic Roux limb markedly affects the incidence of internal hernias after laparoscopic gastric bypass. Obes Surg. 2005;15(6):766-70.
- Frering V, Fontaumard E. Does stitching band increase slipping? Surg Obes Relat Dis. 2008;4(3)294.
- Sherwinter DA, Gupta A, Cummings LS, Brejt SZ, Brejt SZ, Macura JM, Adler H. Experimental in vivo canine model for gastric prolapse of laparoscopic adjustable gastric band system. Surg Obes Relat Dis. 2009 Sep 10. [Epub ahead of print]
- Singhal R, Kitchen M, Ndirika S, Hunt K, Bridgwater S, Super P. The “Birmingham stitch”–avoiding slippage in laparoscopic gastric banding. Obes Surg. 2008;18(4):359- 63.
- Boschi S, Fogli L, Berta RD, Patrizi P, Di Domenico M, Vetere F, Capizzi D, Capizzi FD. Avoiding complications after laparoscopic esophago-gastric banding: experience with 400 consecutive patients. Obes Surg. 2006;16(9):1166-70.
- Srikanth MS, Oh KH, Keskey T, Rumbaut R, Fox SR, Fox ER, Fox KM. Critical extreme anterior slippage (paragastric Richter’s hernia) of the stomach after laparoscopic adjustable gastric banding: early recognition and prevention of gastric strangulation. Obes Surg. 2005;15(2):207-15.
- Favretti F, Cadiere GB, Segato G, Himpens J, Busetto L, De Marchi F, Vertruyen M, Enzi G, De Luca M, Lise M. Laparoscopic adjustable silicone gastric banding (Lap- Band): how to avoid complications. Obes Surg. 1997;7(4):352-58.
- Holian DK. Biliopancreatic bypass. In: Deitel M., ed. Surgery for the Morbidly Obese Patient. Toronto, Canada: FD-Communications Inc., 1998:105-111.
- Buchwald H; Consensus Conference Panel. Consensus conference statement bariatric surgery for morbid obesity: health implications for patients, health professionals, and third-party payers. Surg Obes Relat Dis. 2005;1:371-81.
- Peluso L, Vanek VW. Efficacy of gastric bypass in the treatment of obesity-related comorbidities. Nutr Clin Pract. 2007;22(1):22-8.
- Pi-Sunyer X. The metabolic syndrome: how to approach differing definitions. Med Clin North Am. 2007;91(6):1025-40.
- Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231-7.
- Cawley J, Sweeney MJ, Kurian M, Beane S; New York State Bariatric Surgery Workgroup. Predicting complications after bariatric surgery using obesity-related co- morbidities. Obes Surg. 2007;17(11):1451-56.
- Weinsier RL, Hunter GR, Heini AF, Goran MI, Sell SM. The etiology of obesity: relative contribution of metabolic factors, diet, and physical activity. Am J Med. 1998;105(2):145-50.
- Finer N. Sibutramine: its mode of action and efficacy. Int J Obes Relat Metab Disord. 2002;26 Suppl 4:S29-33.
- James WP, Astrup A, Finer N, Hilsted J, Kopelman P, Rössner S, Saris WH, Van Gaal LF. Effect of sibutramine on weight maintenance after weight loss: a randomized trial. STORM Study Group. Sibutramine Trial of Obesity Reduction and Maintenance. Lancet. 2000;356(9248):2119-25.
- Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA. 2001;286(11):1331-9.
- Lucas KH, Kaplan-Machlis B. Orlistat–a novel weight loss therapy. Ann Pharmacother. 2001;35(3):314-28.
- Cavaliere H, Floriano I, Medeiros-Neto G. Gastrointestinal side effects of orlistat may be prevented by concomitant prescription of natural fibers (psyllium mucilloid). Int J Obes Relat Metab Disord. 2001;25(7):1095-9.
- Heymsfield SB, Segal KR, Hauptman J, Lucas CP, Boldrin MN, Rissanen A, Wilding JP, Sjöström Effects of weight loss with orlistat on glucose tolerance and progression to type 2 diabetes in obese adults. Arch Intern Med. 2000;160(9):1321-6.
- Camilleri M, Papathanasopoulos A, Odunsi ST. Actions and therapeutic pathways of ghrelin for gastrointestinal disorders. Nat Rev Gastroenterol Hepatol. 2009;6(6):343-52.
- Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. NEJM. 2002;346(21):1623-30.
- Oliván B, Teixeira J, Bose M, Bawa B, Chang T, Summe H, Lee H, Laferrère B. Effect of weight loss by diet or gastric bypass surgery on peptide YY3-36 levels. Ann Surg. 2009;249(6):948-53.
- Garcia-Fuentes E, Garrido-Sanchez L, Garcia-Almeida JM, Garcia-Arnes J, Gallego- Perales JL, Rivas-Marin J, Morcillo S, Cardona I, Soriguer F. Different effect of laparoscopic Roux-en-Y gastric bypass and open biliopancreatic diversion of Scopinaro on serum PYY and ghrelin levels. Obes Surg. 2008;18(11):1424-9.
- Roth CL, Reinehr T, Schernthaner GH, Kopp HP, Kriwanek S, Schernthaner G. Ghrelin and obestatin levels in severely obese women before and after weight loss after Roux-en-Y gastric bypass surgery. Obes Surg. 2009;19(1):29-35.
- Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247(3):401-7.
- Foschi D, Corsi F, Colombo F, Vago T, Bevilaqua M, Rizzi A, Trabucchi E. Different effects of vertical banded gastroplasty and Roux-en-Y gastric bypass on meal inhibition of ghrelin secretion in morbidly obese patients. J Invest Surg. 2008;21(2):77- 81.
- Peterli R, Wölnerhanssen B, Peters T, Devaux N, Kern B, Christoffel-Courtin C, Drewe J, von Flüe M, Beglinger C. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250(2):234-41.
- Ybarra J, Bobbioni-Harsch E, Chassot G, Huber O, Morel P, Assimacopoulos- Jeannet F, Golay A. Persistent correlation of ghrelin plasma levels with body mass index both in stable weight conditions and during gastric-bypass-induced weight loss. Obes Surg. 2009;19(3):327-31.
- Pardina E, López-Tejero MD, Llamas R, Catalán R, Galard R, Allende H, Vargas V, Lecube A, Fort JM, Baena-Fustegueras JA, Peinado-Onsurbe J. Ghrelin and Apolipoprotein AIV Levels Show Opposite Trends to Leptin Levels During Weight Loss in Morbidly Obese Patients. Obes Surg. 2009;19(10):1414-23.
- Liou JM, Lin JT, Lee WJ, Wang HP, Lee YC, Chiu HM, Wu MS. The serial changes of ghrelin and leptin levels and their relations to weight loss after laparoscopic minigastric bypass surgery. Obes Surg. 2008;18(1):84-9.
- Whitson BA, Leslie DB, Kellogg TA, Maddaus MA, Buchwald H, Billington CJ, Ikramuddin S. Entero-endocrine changes after gastric bypass in diabetic and nondiabetic patients: a preliminary study. J Surg Res. 2007l;141(1):31-39.
- Sundbom M, Holdstock C, Engström BE, Karlsson FA. Early changes in ghrelin following Roux-en-Y gastric bypass: influence of vagal nerve functionality? Obes Surg. 2007;17(3):304-10.
- Korner J, Inabnet W, Conwell IM, Taveras C, Daud A, Olivero-Rivera L, Restuccia NL, Bessler M. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring). 2006;14(9):1553-61.
- Engström BE, Ohrvall M, Sundbom M, Lind L, Karlsson FA. Meal suppression of circulating ghrelin is normalized in obese individuals following gastric bypass surgery. Int J Obes (Lond). 2007;31(3):476-80.
- Kotidis EV, Koliakos G, Papavramidis TS, Papavramidis ST. The effect of biliopancreatic diversion with pylorus-preserving sleeve gastrectomy and duodenal switch on fasting serum ghrelin, leptin and adiponectin levels: is there a hormonal contribution to the weight-reducing effect of this procedure? Obes Surg. 2006;16(5):554- 59.
- Kotidis EV, Koliakos GG, Baltzopoulos VG, Ioannidis KN, Yovos JG, Papavramidis ST. Serum ghrelin, leptin and adiponectin levels before and after weight loss: comparison of three methods of treatment–a prospective study. Obes Surg. 2006;16(11):1425-32.
- Langer FB, Reza Hoda MA, Bohdjalian A, Felberbauer FX, Zacherl J, Wenzl E, Schindler K, Luger A, Ludvik B, Prager G. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg. 2005;15(7):1024-29.
- Valera Mora ME, Manco M, Capristo E, Guidone C, Iaconelli A, Gniuli D, Rosa G, Calvani M, Mingrone G. Growth hormone and ghrelin secretion in severely obese women before and after bariatric surgery. Obesity (Silver Spring). 2007;15(8):2012-18.
- Shak JR, Roper J, Perez-Perez GI, Tseng CH, Francois F, Gamagaris Z, Patterson C, Weinshel E, Fielding GA, Ren C, Blaser MJ. The effect of laparoscopic gastric banding surgery on plasma levels of appetite-control, insulinotropic, and digestive hormones. Obes Surg. 2008;18(9):1089-96. Erratum in:Obes Surg. 2008;18(9):1097-98.
- Uzzan B, Catheline JM, Lagorce C, Airinei G, Bon C, Cohen R, Perret GY, Aparicio T, Benamouzig R. Expression of ghrelin in fundus is increased after gastric banding in morbidly obese patients. Obes Surg. 2007;17(9):1159-64.
- Ram E, Vishne T, Diker D, Gal-Ad I, Maayan R, Lerner I, Dreznik Z, Seror D, Vardi P, Weizman A. Impact of gastric banding on plasma ghrelin, growth hormone, cortisol, DHEA and DHEA-S levels. Obes Surg. 2005;15(8):1118-23.
- Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, Clegg AJ. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess. 2009;13(41):1-190, 215-357, iii-iv.
- Cremieux PY, Buchwald H, Shikora SA, Ghosh A, Yang HE, Buessing M. A study on the economic impact of bariatric surgery. Am J Manag Care. 2008;14(9):589-96.
- Buchwald H, Estok R, Fahrbach K, Banel D, Sledge I. Trends in mortality in bariatric surgery: a systematic review and meta-analysis. Surgery. 2007;142(4):621-35.
- Kohn GP, Haggerty SP, Overby DW, Fanelli RD, Farrell TM. Implementing an evidence-based approach to selection of type of laparoscopic bariatric surgery. Bariatric Times. 2009;6(7):1,18-20.
- Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724- 37. Erratum in: JAMA. 2005;293(14):1728.
- Farrell TM, Haggerty SP, Overby DW, Kohn GP, Richardson WS, Fanelli RD. Clinical application of laparoscopic bariatric surgery: an evidence-based review. Surg Endosc. 2009;23(5):930-49.
- Parikh MS, Laker S, Weiner M, Hajiseyedjavadi O, Ren CJ. Objective comparison of complications resulting from laparoscopic bariatric procedures. J Am Coll Surg. 2006;202(2):252-61.
- Maggard MA, Shugarman LR, Suttorp M, Maglione M, Sugerman HJ, Livingston EH, Nguyen NT, Li Z, Mojica WA, Hilton L, Rhodes S, Morton SC, Shekelle PG. Meta- analysis: surgical treatment of obesity. Ann Intern Med. 2005;142(7):547-59.
- Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta- analysis. Am J Med. 2009;122(3):248-256.
- Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, Eid GM, Mattar S, Ramanathan R, Barinas-Mitchel E, Rao RH, Kuller L, Kelley D. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238(4):467-85.
- Dimick JB, Welch HG, Birkmeyer JD. Surgical mortality as an indicator of hospital quality: the problem with small sample size. JAMA. 2004;292(7):847-51.
- Christou NV, Sampalis JS, Liberman M, Look D, Auger S, McLean AP, MacLean LD. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004;240(3):416-24.
- Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM, Hunt SC. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753-61.
- Saunders JK, Ballantyne GH, Belsley S, Stephens D, Trivedi A, Ewing DR, Iannace V, Capella RF, Wasielewski A, Moran S, Schmidt HJ. 30-day readmission rates at a high volume bariatric surgery center: laparoscopic adjustable gastric banding, laparoscopic gastric bypass, and vertical banded gastroplasty-Roux-en-Y gastric bypass. Obes Surg. 2007;17(9):1171-7.
- Balsiger BM, Ernst D, Giachino D, Bachmann R, Glaettli A. Prospective evaluation and 7-year follow-up of Swedish adjustable gastric banding in adults with extreme obesity. J Gastrointest Surg. 2007;11(11):1470-6; discussion 1446-7.
- Nguyen NT, Lee SL, Goldman C, Fleming N, Arango A, McFall R, Wolfe BM. Comparison of pulmonary function and postoperative pain after laparoscopic versus open gastric bypass: a randomized trial. J Am Coll Surg. 2001;192(4):469-77.
- Nguyen NT, Goldman CD, Ho HS, Gosselin RC, Singh A, Wolfe BM. Systemic stress response after laparoscopic and open gastric bypass. J Am Coll Surg. 2002;194(5):557-67.
- Puzziferri N, Austrheim-Smith IT, Wolfe BM, Wilson SE, Nguyen NT. Three-year follow-up of a prospective randomized trial comparing laparoscopic versus open gastric bypass. Ann Surg. 2006;243(2):181-8.
- Sundbom M, Gustavsson S. Randomized clinical trial of hand-assisted laparoscopic versus open Roux-en-Y gastric bypass for the treatment of morbid obesity. Br J Surg. 2004;91(4):418-23.
- Luján JA, Frutos MD, Hernández Q, Liron R, Cuenca JR, Valero G, Parrilla Laparoscopic versus open gastric bypass in the treatment of morbid obesity: a randomized prospective study. Ann Surg. 2004;239(4):433-7.
- Westling A, Gustavsson S. Laparoscopic vs open Roux-en-Y gastric bypass: a prospective, randomized trial. Obes Surg. 2001;11(3):284-92.
- Agaba EA, Shamseddeen H, Gentles CV, Sasthakonar V, Gellman L, Gadaleta D. Laparoscopic vs open gastric bypass in the management of morbid obesity: a 7-year retrospective study of 1,364 patients from a single center. Obes Surg. 2008;18(11):1359- 63.
- Marema RT, Perez M, Buffington CK. Comparison of the benefits and complications between laparoscopic and open Roux-en-Y gastric bypass surgeries. Surg Endosc. 2005;19(4):525-30.
- Jones KB Jr, Afram JD, Benotti PN, Capella RF, Cooper CG, Flanagan L, Hendrick S, Howell LM, Jaroch MT, Kole K, Lirio OC, Sapala JA, Schuhknecht MP, Shapiro RP, Sweet WA, Wood MH. Open versus laparoscopic Roux-en-Y gastric bypass: a comparative study of over 25,000 open cases and the major laparoscopic bariatric reported series. Obes Surg. 2006;16(6):721-7.
- Podnos YD, Jimenez JC, Wilson SE, Stevens CM, Nguyen NT. Complications after laparoscopic gastric bypass: a review of 3464 cases. Arch Surg. 2003;138(9):957-61.
- Marsk R, Tynelius P, Rasmussen F, Freedman J. Short-Term Morbidity and Mortality After Open Versus Laparoscopic Gastric Bypass Surgery. A Population-Based Study from Sweden. Obes Surg. 2009 Aug 29. [Epub ahead of print]
- Lancaster RT, Hutter MM. Bands and bypasses: 30-day morbidity and mortality of bariatric surgical procedures as assessed by prospective, multi-center, risk-adjusted ACS- NSQIP data. Surg Endosc. 2008;22(12):2554-63.
- Weller WE, Rosati C. Comparing outcomes of laparoscopic versus open bariatric surgery. Ann Surg. 2008;248(1):10-5.
- Nguyen NT, Hinojosa M, Fayad C, Varela E, Wilson SE. Use and outcomes of laparoscopic versus open gastric bypass at academic medical centers. J Am Coll Surg. 2007;205(2):248-55.
- Nguyen NT, Wolfe BM. Laparoscopic versus open gastric bypass. Semin Laparosc Surg. 2002;9(2):86-93.
- Gunabushanam G, Shankar S, Czerniach DR, Kelly EE, Perugini RA. Small-bowel obstruction after laparoscopic Roux-en-Y gastric bypass surgery. J Comput Assist Tomogr. 2009;33(3):369-75.
- Ahmed AR, Rickards G, Husain S, Johnson J, Boss T, O’Malley W. Trends in internal hernia incidence after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2007;17(12):1563-6.
- Steele KE, Prokopowicz GP, Magnuson T, Lidor A, Schweitzer M. Laparoscopic antecolic Roux-en-Y gastric bypass with closure of internal defects leads to fewer internal hernias than the retrocolic approach. Surg Endosc. 2008;22(9):2056-61.
- Encinosa WE, Bernard DM, Du D, Steiner CA. Recent improvements in bariatric surgery outcomes. Med Care. 2009;47(5):531-55.
- Cunneen SA, Phillips E, Fielding G, Banel D, Estok R, Fahrbach K, Sledge I. Studies of Swedish adjustable gastric band and Lap-Band: systematic review and meta- analysis. Surg Obes Relat Dis. 2008 Mar-Apr;4(2):174-85.
- Guller U, Klein LV, Hagen JA. Safety and effectiveness of bariatric surgery: Roux- en-Y gastric bypass is superior to gastric banding in the management of morbidly obese patients. Patient Saf Surg. 2009;3(1):10.
- Tice JA, Karliner L, Walsh J, Petersen AJ, Feldman MD. Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures. Am J Med. 2008;121(10):885-93.
- Angrisani L, Lorenzo M, Borrelli V. Laparoscopic adjustable gastric banding versus Roux-en-Y gastric bypass: 5-year results of a prospective randomized trial. Surg Obes Relat Dis. 2007;3(2):127-33.
- Wölnerhanssen BK, Peters T, Kern B, Schötzau A, Ackermann C, von Flüe M, Peterli R. Predictors of outcome in treatment of morbid obesity by laparoscopic adjustable gastric banding: results of a prospective study of 380 patients. Surg Obes Relat Dis. 2008;4(4):500-6.
- Colles SL, Dixon JB, Marks P, Strauss BJ, O’Brien PE. Preoperative weight loss with a very-low-energy diet: quantitation of changes in liver and abdominal fat by serial imaging. Am J Clin Nutr. 2006;84(2):304-11.
- Lewis MC, Phillips ML, Slavotinek JP, Kow L, Thompson CH, Toouli J. Change in liver size and fat content after treatment with Optifast very low calorie diet. Obes Surg. 2006;16(6):697-701.
- Liu RC, Sabnis AA, Forsyth C, Chand B. The effects of acute preoperative weight loss on laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2005;15(10):1396-402.
- Fris RJ. Preoperative low energy diet diminishes liver size. Obes Surg. 2004;14(9):1165-70.
- Sanchez BS, Safadi BY, Kieran JA, Hsu GP, Brodsky JB, Curet MJ, Morton Orogastric tube complications in laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2006;16(4):443-7.
- Baker RS, Foote J, Kemmeter P, Brady R, Vroegop T, Serveld M. The science of stapling and leaks. Obes Surg. 2004;14(10):1290-8.
- Lee S, Carmody B, Wolfe L, Demaria E, Kellum JM, Sugerman H, Maher JW. Effect of location and speed of diagnosis on anastomotic leak outcomes in 3828 gastric bypass cases. J Gastrointest Surg. 2007;11(6):708-13.
- The American Society for Metabolic and Bariatric Surgery Clinical Issues Committee. ASMBS Position Statement on Prevention and Detection of Gastrointestinal Leak After Gastric Bypass Including the Role of Imaging and Surgical Exploration. Approved by the ASMBS Executive Council, January 2009. Available at: http://www.asmbs.org/Newsite07/resources/leak_management_position.pdf.
- Edwards MA, Jones DB, Ellsmere J, Grinbaum R, Schneider BE. Anastomotic leak following antecolic versus retrocolic laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2007;17(3):292-7.
- Gonzalez R, Nelson LG, Gallagher SF, Murr MM. Anastomotic leaks after laparoscopic gastric bypass. Obes Surg. 2004;14(10):1299-307.
- Fernandez AZ Jr, DeMaria EJ, Tichansky DS, Kellum JM, Wolfe LG, Meador J, Sugerman HJ. Experience with over 3,000 open and laparoscopic bariatric procedures: multivariate analysis of factors related to leak and resultant mortality. Surg Endosc. 2004;18(2):193-97.
- Carrasquilla C, English WJ, Esposito P, Gianos J. Total stapled, total intra- abdominal (TSTI) laparoscopic roux-en-y gastric bypass: one leak in 1000 cases. Obes Surg. 2004;14:613-17.
- Stahl RD, Sherer RA, Seevers CE, Johnston D. Comparison of 21 vs. 25 mm gastrojejunostomy in gastric bypass procedure – early results. Obes Surg. 2000;10:540- 42.
- Abdel-Galil E, Sabry AA. Laparoscopic roux-en-y gastric bypass – evaluation of three different techniques. Obes Surg. 2002;12:639-42.
- Gould JC, Needleman BJ, Ellison EC, Muscarella P, Schneider C, Melvin WS. Evolution of minimally invasive bariatric surgery. Surgery. 2002;132:565-72.
- Shope TR, Cooney RN, McLeod J, Miller CA, Haluck RS. Early results after laparoscopic gastric bypass: EEA vs GIA stapled gastrojejunal anastomosis. Obes Surg. 2003;13(3):355-59.
- Gonzalez R, Lin E, Venkatesh KR, Bowers SP, Smith CD. Gastrojejunostomy during laparoscopic gastric bypass: analysis of 3 techniques. Arch Surg. 2003;138:181- 84.
- Suggs WJ, Kouli W, Lupovici M, Chau WY, Brolin RE. Complications at gastrojejunostomy after laparoscopic Roux-en-Y gastric bypass: comparison between 21- and 25-mm circular staplers. Surg Obes Relat Dis. 2007;3(5):508-14.
- Leyba JL, Llopis SN, Isaac J, Aulestia SN, Bravo C, Obregon F. Laparoscopic gastric bypass for morbid obesity-a randomized controlled trial comparing two gastrojejunal anastomosis techniques. JSLS. 2008;12(4):385-88.
- Sczepaniak JP, Owens ML. Results of gastrojejunal anastomotic technique designed to reduce stricture. Surg Obes Relat Dis. 2009;5(1):77-80.
- Owens ML, Sczepaniak JP. Size really does matter-role of gastrojejunostomy in postoperative weight loss. Surg Obes Relat Dis. 2009;5(3):357-61.
- Shang E, Hasenberg T, Magdeburg R, Keese M, Post S, Weiner R. First experiences with A circular stapled gastro-jejunostomy by a new transorally introducible stapler system in laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2009;19(2):230-36.
- Ibele A, Garren M, Gould J. Effect of circular staple line buttressing material on gastrojejunostomy failure in laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2009 Jun 23. [Epub ahead of print]
- Basu NN, Leschinskey D, Heath DI. The use of Seamguard to buttress the suture repair of a staple line leak following laparoscopic gastric bypass for obesity. Obes Surg. 2008;18(7):896-7.
- Silecchia G, Boru CE, Mouiel J, Rossi M, Anselmino M, Morino M, Toppino M, Gaspari A, Gentileschi P, Tacchino R, Basso N. The use of fibrin sealant to prevent major complications following laparoscopic gastric bypass: results of a multicenter, randomized trial. Surg Endosc. 2008;22(11):2492-7.
- Saber AA, Scharf KR, Turk AZ, Elgamal MH, Martinez RL. Early experience with intraluminal reinforcement of stapled gastrojejunostomy during laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2008;18(5):525-29.
- Miller KA, Pump A. Use of bioabsorbable staple reinforcement material in gastric bypass: a prospective randomized clinical trial. Surg Obes Relat Dis. 2007;3(4):417-22.
- Saber AA, Jackson O. Omental wrap: a simple technique for reinforcement of the gastrojejunostomy during Roux-en-Y gastric bypass. Obes Surg. 2007;17(1):15-8.
- Silecchia G, Boru CE, Mouiel J, Rossi M, Anselmino M, Tacchino RM, Foco M, Gaspari AL, Gentileschi P, Morino M, Toppino M, Basso N. Clinical evaluation of fibrin glue in the prevention of anastomotic leak and internal hernia after laparoscopic gastric bypass: preliminary results of a prospective, randomized multicenter trial. Obes Surg. 2006;16(2):125-31.
- Sapala JA, Wood MH, Schuhknecht MP. Anastomotic leak prophylaxis using a vapor-heated fibrin sealant: report on 738 gastric bypass patients. Obes Surg. 2004;14(1):35-42.
- Champion JK, Hunt T, DeLisle N. Role of routine intraoperative endoscopy in laparoscopic bariatric surgery. Surg Endosc. 2002 Dec;16(12):1663-65.
- Sekhar N, Torquati A, Lutfi R, Richards WO. Endoscopic evaluation of the gastrojejunostomy in laparoscopic gastric bypass. A series of 340 patients without postoperative leak. Surg Endosc. 2006;20(2):199-201.
- Dallal RM, Bailey L, Nahmias N. Back to basics–clinical diagnosis in bariatric surgery. Routine drains and upper GI series are unnecessary. Surg Endosc. 2007;21(12):2268-71.
- Kligman MD. Intraoperative endoscopic pneumatic testing for gastrojejunal anastomotic integrity during laparoscopic Roux-en-Y gastric bypass. Surg Endosc. 2007;21(8):1403-5.
- Raman R, Raman B, Raman P, Rossiter S, Curet MJ, Mindelzun R, Morton JM. Abnormal findings on routine upper GI series following laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2007;17(3):311-6.
- Csendes A, Burdiles P, Burgos AM, Maluenda F, Diaz JC. Conservative management of anastomotic leaks after 557 open gastric bypasses. Obes Surg. 2005;15(9):1252-56.
- Carter JT, Tafreshian S, Campos GM, Tiwari U, Herbella F, Cello JP, Patti MG, Rogers SJ, Posselt AM. Routine upper GI series after gastric bypass does not reliably identify anastomotic leaks or predict stricture formation. Surg Endosc. 2007;21(12):2172- 77.
- Katasani VG, Leeth RR, Tishler DS, Leath TD, Roy BP, Canon CL, Vickers SM, Clements RH. Water-soluble upper GI based on clinical findings is reliable to detect anastomotic leaks after laparoscopic gastric bypass. Am Surg. 2005;71(11):916-19.
- Kolakowski S Jr, Kirkland ML, Schuricht AL. Routine postoperative upper gastrointestinal series after Roux-en-Y gastric bypass: determination of whether it is necessary. Arch Surg. 2007 Oct;142(10):930-34.
- Lee SD, Khouzam MN, Kellum JM, DeMaria EJ, Meador JG, Wolfe LG, Maher JW. Selective, versus routine, upper gastrointestinal series leads to equal morbidity and reduced hospital stay in laparoscopic gastric bypass patients. Surg Obes Relat Dis. 2007;3(4):413-6.
- Doraiswamy A, Rasmussen JJ, Pierce J, Fuller W, Ali MR. The utility of routine postoperative upper GI series following laparoscopic gastric bypass. Surg Endosc. 2007;21(12):2159-62.
- Ballesta C, Berindoague R, Cabrera M, Palau M, Gonzales M. Management of anastomotic leaks after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2008;18(6):623-30.
- Carrodeguas L, Szomstein S, Soto F, Whipple O, Simpfendorfer C, Gonzalvo JP, Villares A, Zundel N, Rosenthal R. Management of gastrogastric fistulas after divided Roux-en-Y gastric bypass surgery for morbid obesity: analysis of 1,292 consecutive patients and review of literature. Surg Obes Relat Dis. 2005;1(5):467-74.
- Eubanks S, Edwards CA, Fearing NM, Ramaswamy A, de la Torre RA, Thaler KJ, Miedema BW, Scott JS. Use of endoscopic stents to treat anastomotic complications after bariatric surgery. J Am Coll Surg. 2008;206(5):935-39.
- Salinas A, Baptista A, Santiago E, Antor M, Salinas H. Self-expandable metal stents to treat gastric leaks. Surg Obes Relat Dis. 2006;2(5):570-72.
- Barba CA, Harrington C, Loewen M. Status of venous thromboembolism prophylaxis among bariatric surgeons: have we changed our practice during the past decade? Surg Obes Relat Dis. 2009;5(3):352-6.
- The American Society for Metabolic and Bariatric Surgery Clinical Issues Committee. ASMBS Position Statement on Prophylactic Measures to Reduce the Risk of Venous Thromboembolism in Bariatric Surgery Patients. Approved by the ASMBS Executive Council June 2007. http://www.asbs.org/Newsite07/resources/vte_statement.pdf.
- Wu EC, Barba CA. Current practices in the prophylaxis of venous thromboembolism in bariatric surgery. Obes Surg. 2000;10(1):7-14.
- Clements RH, Yellumahanthi K, Ballem N, Wesley M, Bland KI. Pharmacologic prophylaxis against venous thromboembolic complications is not mandatory for all laparoscopic Roux-en-Y gastric bypass procedures. J Am Coll Surg. 2009;208(5):917-23.
- McCloskey CA, Ramani GV, Mathier MA, Schauer PR, Eid GM, Mattar SG, Courcoulas AP, Ramanathan R. Bariatric surgery improves cardiac function in morbidly obese patients with severe cardiomyopathy. Surg Obes Relat Dis. 2007;3(5):503-7.
- Catheline JM, Bihan H, Le Quang T, Sadoun D, Charniot JC, Onnen I, Fournier JL, Bénichou J, Cohen R. Preoperative cardiac and pulmonary assessment in bariatric surgery. Obes Surg. 2008;18(3):271-7.
- Lawrence VA, Cornell JE, Smetana GW; American College of Physicians. Strategies to reduce postoperative pulmonary complications after noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144(8):596-608.
- Vasquez JC, Wayne Overby D, Farrell TM. Fewer gastrojejunostomy strictures and marginal ulcers with absorbable suture. Surg Endosc. 2009;23(9):2011-15.
- Sacks BC, Mattar SG, Qureshi FG, Eid GM, Collins JL, Barinas-Mitchell EJ, Schauer PR, Ramanathan RC. Incidence of marginal ulcers and the use of absorbable anastomotic sutures in laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2006;2(1):11-16.
- Ahmad S, Nagle A, McCarthy RJ, Fitzgerald PC, Sullivan JT, Prystowsky J. Postoperative hypoxemia in morbidly obese patients with and without obstructive sleep apnea undergoing laparoscopic bariatric surgery. Anesth Analg. 2008;107(1):138-43.
- Kaw R, Aboussouan L, Auckley D, Bae C, Gugliotti D, Grant P, Jaber W, Schauer P, Sessler D. Challenges in pulmonary risk assessment and perioperative management in bariatric surgery patients. Obes Surg. 2008;18(1):134-8.
- Passannante AN, Rock P. Anesthetic management of patients with obesity and sleep apnea. Anesthesiol Clin North America. 2005;23(3):479-91.
- Neligan PJ, Malhotra G, Fraser M, Williams N, Greenblatt EP, Cereda M, Ochroch EA. Continuous positive airway pressure via the Boussignac system immediately after extubation improves lung function in morbidly obese patients with obstructive sleep apnea undergoing laparoscopic bariatric surgery. Anesthesiology. 2009;110(4):878-84.
- Serafini FM, MacDowell Anderson W, Rosemurgy AS, Strait T, Murr MM. Clinical predictors of sleep apnea in patients undergoing bariatric surgery. Obes Surg. 2001;11(1):28-31.
- Jensen C, Tejirian T, Lewis C, Yadegar J, Dutson E, Mehran A. Postoperative CPAP and BiPAP use can be safely omitted after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2008;4(4):512-4.
- McGlinch BP, Que FG, Nelson JL, Wrobleski DM, Grant JE, Collazo-Clavell Perioperative care of patients undergoing bariatric surgery. Mayo Clin Proc. 2006;81(10 Suppl):S25-33.
- Dhonneur G, Ndoko SK, Yavchitz A, Foucrier A, Fessenmeyer C, Pollian C, Combes X, Tual Tracheal intubation of morbidly obese patients: LMA CTrach vs direct laryngoscopy. Br J Anaesth. 2006;97(5):742-5.
- Vanek VW, Catania M, Triveri K, Woodruff RW Jr. Retrospective review of the preoperative biliary and gastrointestinal evaluation for gastric bypass surgery. Surg Obes Relat Dis. 2006;2(1):17-23.
- Rasmussen JJ, Fuller W, Ali MR. Marginal ulceration after laparoscopic gastric bypass: an analysis of predisposing factors in 260 patients. Surg Endosc. 2007;21(7):1090-94.
- Frigg A, Peterli R, Zynamon A, Lang C, Tondelli P. Radiologic and endoscopic evaluation for laparoscopic adjustable gastric banding: preoperative and follow-up. Obes Surg. 2001;11(5):594-99.
- Schirmer B, Erenoglu C, Miller A. Flexible endoscopy in the management of patients undergoing Roux-en-Y gastric bypass. Obes Surg. 2002;12(5):634-38.
- Papavramidis ST, Theocharidis AJ, Zaraboukas TG, Christoforidou BP, Kessissoglou II, Aidonopoulos AP. Upper gastrointestinal endoscopic and histologic findings before and after vertical banded gastroplasty. Surg Endosc. 1996;10(8):825-30.
- Madan AK, Speck KE, Hiler ML. Routine preoperative upper endoscopy for laparoscopic gastric bypass: is it necessary? Am Surg. 2004;70(8):684-6.
- Ramaswamy A, Lin E, Ramshaw BJ, Smith CD. Early effects of Helicobacter pylori infection in patients undergoing bariatric surgery. Arch Surg. 2004;139(10):1094- 96.
- Frezza EE, Herbert H, Ford R, Wachtel MS. Endoscopic suture removal at gastrojejunal anastomosis after Roux-en-Y gastric bypass to prevent marginal ulceration. Surg Obes Relat Dis. 2007;3(6):619-22.
- Schauer PR, Ikramuddin S, Gourash W, Ramanathan R, Luketich J. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 2000;232(4):515- 29.
- DeMaria EJ, Sugerman HJ, Kellum JM, Meador JG, Wolfe LG. Results of 281 consecutive total laparoscopic Roux-en-Y gastric bypasses to treat morbid obesity. Ann Surg. 2002;235(5):640-47.
- Suter M, Giusti V, Héraief E, Zysset F, Calmes JM. Laparoscopic Roux-en-Y gastric bypass: initial 2-year experience. Surg Endosc. 2003;17(4):603-9.
- Taylor JD, Leitman IM, Hon P, Horowitz M, Panagopoulos G. Outcome and complications of gastric bypass in super-super obesity versus morbid obesity. Obes Surg. 2006;16(1):16-8.
- Higa KD, Ho T, Boone KB. Laparoscopic roux-en-y gastric bypass: technique and 3-year follow-up. J Laparoendosc Adv Surg Tech A. 2001;11:377-82.
- Perugini RA, Mason R, Czerniach DR, Novitsky YW, Baker S, Litwin DE, Kelly JJ. Predictors of complication and suboptimal weight loss after laparoscopic roux-en-y gastric bypass: a series of 188 patients. Arch Surg. 2003;138:541-46.
- Oh, CH, Kim HJ, Oh S. Weight loss following transected gastric bypass with proximal roux-en-y. Obes Surg. 1997;7:142-48.
- Brolin RE. Gastrointestinal surgery for obesity. Semin Gastrointest Dis. 1998;9:163-75.
- Fobi MA, Lee H, Holness R, Cabinda D. Gastric bypass operation for obesity. World J Surg. 1998;22:925-35.
- Balsiger BM, Kennedy FP, Abu-Lebdeh HS, Collazo-Clavell M, Jensen MD, O’Brien T, Hensrud DD, Dinneen SF, Thompson GB, Que FG, Williams DE, Clark MM, Grant JE, Frick MS, Mueller RA, Mai JL, Sarr MG. Prospective evaluation of roux-en-y gastric bypass as primary operation for medically complicated obesity. May Clin Proc. 2000;75:673-680.
- Ruiz-de-Adana JC, López-Herrero J, Hernández-Matías A, Colao-Garcia L, Muros- Bayo JM, Bertomeu-Garcia A, Limones-Esteban M. Laparoscopic hand-sewn gastrojejunal anastomoses. Obes Surg. 2008;18(9):1074-6.
- Kligman MD, Thomas C, Saxe J. Effect of the learning curve on the early outcomes of laparoscopic Roux-en-Y gastric bypass. Am Surg. 2003;69(4):304-10.
- Sundbow M, Gustavsson S. Hand-assisted laparoscopic roux-en-y gastric bypass: aspects of surgical technique and early results. Obes Surg. 2000;10:420-27.
- Oliak D, Ballantyne GH, Davies RJ, Wasielewski A, Schmidt HJ. Short-term results of laparoscopic gastric bypass in patients with BMI > or = 60. Obes Surg. 2002;12:643-47.
- Papasavas PK, Hayetian FD, Caushaj PF, Landreneau RJ, Maurer J, Keenan RJ, Quinlin RF, Gagné DJ. Outcome analysis of laparoscopic roux-en-y gastric bypass for morbid obesity. The first 116 cases. Surg Endosc. 2002;16:1653-57.
- Dresel A, Kuhn JA, Westmoreland MV, Talaasen LJ, McCarty TM. Establishing a laparoscopic gastric bypass program. Am J Surg. 2002;184:617-20.
- Courcoulas A, Perry Y, Buenaventura P, Luketich J. Comparing the outcomes after laparoscopic versus open gastric bypass: a matched paired analysis. Obes Surg. 2003;13:341-46.
- Nguyen NT, Stevens CM, Wolfe BM. Incidence and outcome of anastomotic stricture after laparoscopic gastric bypass. J Gastrointest Surg. 2003;7:997-1003.
- Barba CA, Butensky MS, Lorenzo M, Newman R. Endoscopic dilation of gastroesophageal anastomosis stricture after gastric bypass. Surg Endosc. 2003;416-20.
- Bell RL, Reinhardt KE, Flowers JL. Surgeon-performed endoscopic dilation of symptomatic gastrojejunal anastomotic strictures following laparoscopic roux-en-y gastric bypass. Obes Surg. 2003;13:728-33.
- Papasavas PK, Caushaj PF, McCormick JT, Quinlin RF, Hayetian FD, Maurer J, Kelly JJ, Gagné DJ. Laparoscopic management of complications following laparoscopic roux-en-y gastric bypass for morbid obesity. Surg Endosc. 2003;17:610-14.
- Gould JC, Garren MJ, Starling JR. Lessons learned from the first 100 cases in a new minimally invasive bariatric surgery program. Obes Surg. 2004;14:618-25.
- Atuso D, Wayne M, Kaul A, Bairamian M, Teixeira J, Cerabona T. Extremely high body mass index is not a contraindication to laparoscopic gastric bypass. Obes Surg. 2004;14:750-54.
- Rossi TR, Dunda DI, Estes NC, Marshall JS. Stricture dilation after laparoscopic roux-en-y gastric bypass. Am J Surg. 2005;189:357-60.
- Sanyal AJ, Sugerman HJ, Kellum JM, Engle KM, Wolfe Stomal complications of gastric bypass: incidence and outcome of therapy. Am J Gastroenterol. 1992;87:1165- 69.
- Curry TK, Carter PL, Porter CA, Watts DM. Resectional gastric bypass is a new alternative in morbid obesity. Am J Surg. 1998;175:367-70.
- Madigan JD, Morales DL, Bessler M. A technique of stapled gastrojejunostomy for open gastric bypass results in increased wound complication rate. Obes Surg. 2000;10:230-32.
- Livingston EH, Huerta S, Arthur D, Lee S, De Shields S, Heber D. Male gender is a predictor of morbidity and age a predictor of mortality for patients undergoing gastric bypass surgery. Ann Surg. 2002;236:576-82.
- Salinas A, Santiago E, Yeguez J, Antor M, Salinas H. Silastic ring vertical gastric bypass: evolution of an open surgical technique, and review of 1588 cases. Obes Surg, 2005, 1403-07.
- Matthews BD, Sing RF, DeLegge MH, Ponsky JL, Heniford BT. Initial results with a stapled gastrojejunostomy for the laparoscopic isolated roux-en-Y gastric bypass. Am J Surg. 2000;179(6):476-81.
- Carrodeguas L, Szomstein S, Zundel N, Lo Menzo E, Rosenthal R. Gastrojejunal anastomotic strictures following laparoscopic Roux-en-Y gastric bypass surgery: analysis of 1291 patients. Surg Obes Relat Dis. 2006;2(2):92-7.
- Vanek WV. Decreasing the incidence of gastrojejunal anastomotic stricture after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2006;2(3):332.
- Peifer KJ, Shiels AJ, Azar R, Rivera RE, Eagon JC, Jonnalagadda S. Successful endoscopic management of gastrojejunal anastomotic strictures after Roux-en-Y gastric bypass. Gastrointest Endosc. 2007;66(2):248-52.
- Blackstone RP, Rivera LA. Predicting stricture in morbidly obese patients undergoing laparoscopic Roux-en-Y gastric bypass: a logistic regression analysis. J Gastrointest Surg. 2007;11(4):403-9.
- Escalona A, Devaud N, Boza C, Pérez G, Fernández J, Ibáñez L, Guzmán S. Gastrojejunal anastomotic stricture after Roux-en-Y gastric bypass: ambulatory management with the Savary-Gilliard dilator. Surg Endosc. 2007;21(5):765-68.
- Fernández-Esparrach G, Bordas JM, Llach J, Lacy A, Delgado S, Vidal J, Cárdenas A, Pellisé M, Ginès A, Sendino O, Zabalza M, Castells A. Endoscopic dilation with Savary-Gilliard bougies of stomal strictures after laparosocopic gastric bypass in morbidly obese patients. Obes Surg. 2008;18(2):155-61.
- Mathew A, Veliuona MA, DePalma FJ, Cooney RN. Gastrojejunal stricture after gastric bypass and efficacy of endoscopic intervention. Dig Dis Sci. 2009;54(9):1971-78.
- Csendes A, Burgos AM, Burdiles P. Incidence of anastomotic strictures after gastric bypass: a prospective consecutive routine endoscopic study 1 month and 17 months after surgery in 441 patients with morbid obesity. Obes Surg. 2009;19(3):269-73.
- Swartz DE, Gonzalez V, Felix EL. Anastomotic stenosis after Roux-en-Y gastric bypass: A rational approach to treatment. Surg Obes Relat Dis. 2006;2(6):632-37.
- Higa KD, Boone KB, Ho T, Davies OG. Laparoscopic roux-en-y gastric bypass for morbid obesity: technique and preliminary results of our first 400 patients. Arch Surg. 2000;135:1029-34.
- Higa KD, Boone KB, Ho T. Complications of the laparoscopic roux-en-y gastric bypass: 1040 patients – what have we learned? Obes Surg. 2000;10:509-13.
- Oliak D, Ballantyne GH, Weber P, Wasielewski A, Davies RJ, Schmidt HJ. Laparoscopic roux-en-y gastric bypass: defining the learning curve. Surg Endosc. 2003;17:405-08.
- Schauer PR, Ikramuddin S, Hamad G, Gourash W. The learning curve for laparoscopic gastric bypass is 100 cases. Surg Endosc. 2003;17:212-15.
- Ahmad J, Martin J, Ikramuddin S, Schauer PR, Slivka A. Endoscopic balloon dilation of gastroenteric anastomotic strictures after laparoscopic gastric bypass. Endoscopy. 2003;35:725-28.
- Goitein D, Papasavas PK, Gagné D, Ahmad S, Caushaj PF. Gastrojejunal strictures following laparoscopic Roux-en-Y gastric bypass for morbid obesity. Surg Endosc. 2005;19(5):628-32.
- Szomstein S, Whipple OC, Zundel N, Cal P, Rosenthal R. Laparoscopic Roux-en-Y gastric bypass with linear cutter technique: comparison of four-row versus six-row cartridge in creation of anastomosis. Surg Obes Relat Dis. 2006;2(4):431-4.
- Ukleja A, Afonso BB, Pimentel R, Szomstein S, Rosenthal R. Outcome of endoscopic balloon dilation of strictures after laparoscopic gastric bypass. Surg Endosc. 2008;22(8):1746-50.
- Takata MC, Ciovica R, Cello JP, Posselt AM, Rogers SJ, Campos GM. Predictors, treatment, and outcomes of gastrojejunostomy stricture after gastric bypass for morbid obesity. Obes Surg. 2007;17(7):878-84.
- Daylami R, Rogers AM, King TS, Haluck RS, Shope TR. Accuracy of upper gastrointestinal swallow study in identifying strictures after laparoscopic gastric bypass surgery. Surg Obes Relat Dis. 2008;4(2):96-99.
- Cho M, Carrodeguas L, Pinto D, Lascano C, Soto F, Whipple O, Gordon R, Simpfendorfer C, Gonzalvo JP, Szomstein S, Rosenthal RJ. Diagnosis and management of partial small bowel obstruction after laparoscopic antecolic antegastric Roux-en-Y gastric bypass for morbid obesity. J Am Coll Surg. 2006;202(2):262-8.
- Hwang RF, Swartz DE, Felix EL. Causes of small bowel obstruction after laparoscopic gastric bypass. Surg Endosc. 2004;18(11):1631-5.
- Madan AK, Frantzides CT. Triple-stapling technique for jejunojejunostomy in laparoscopic gastric bypass. Arch Surg. 2003;138(9):1029-32.
- Brolin RE. The antiobstruction stitch in stapled Roux-en-Y enteroenterostomy. Am J Surg. 1995;169(3):355-7.
- Cho M, Pinto D, Carrodeguas L, Lascano C, Soto F, Whipple O, Simpfendorfer C, Gonzalvo JP, Zundel N, Szomstein S, Rosenthal RJ. Frequency and management of internal hernias after laparoscopic antecolic antegastric Roux-en-Y gastric bypass without division of the small bowel mesentery or closure of mesenteric defects: review of 1400 consecutive cases. Surg Obes Relat Dis. 2006;2(2):87-91.
- Finnell CW, Madan AK, Tichansky DS, Ternovits C, Taddeucci R. Non-closure of defects during laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2007;17(2):145-8.
- Iannelli A, Buratti MS, Novellas S, Dahman M, Amor IB, Sejor E, Facchiano E, Addeo P, Gugenheim J. Internal hernia as a complication of laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2007;17(10):1283-86.
- Eckhauser A, Torquati A, Youssef Y, Kaiser JL, Richards WO. Internal hernia: postoperative complication of roux-en-Y gastric bypass surgery. Am Surg. 2006;72(7):581-85.
- Carmody B, DeMaria EJ, Jamal M, Johnson J, Carbonell A, Kellum J, Maher Internal hernia after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005;1(6):543-8.
- Paroz A, Calmes JM, Romy S, Giusti V, Suter M. A new type of internal hernia after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2009;19(4):527-30.
- Müller MK, Guber J, Wildi S, Guber I, Clavien PA, Weber M. Three-year follow- up study of retrocolic versus antecolic laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2007;17(7):889-93.
- Comeau E, Gagner M, Inabnet WB, Herron DM, Quinn TM, Pomp A. Symptomatic internal hernias after laparoscopic bariatric surgery. Surg Endosc. 2005;19(1):34-9.
- Bauman RW, Pirrello JR. Internal hernia at Petersen’s space after laparoscopic Roux-en-Y gastric bypass: 6.2% incidence without closure–a single surgeon series of 1047 cases. Surg Obes Relat Dis. 2009;5(5):565-70.
- Champion JK, Williams M. Small bowel obstruction and internal hernias after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2003 Aug;13(4):596-600.
- Husain S, Ahmed AR, Johnson J, Boss T, O’Malley W. Small-bowel obstruction after laparoscopic Roux-en-Y gastric bypass: etiology, diagnosis, and management. Arch Surg. 2007;142(10):988-93.
- Madan AK, Lo Menzo E, Dhawan N, Tichansky DS. Internal Hernias and Nonclosure of Mesenteric Defects During Laparoscopic Roux-en-Y Gastric Bypass. Obes Surg. 2009;19(5):549-52.
- Escalona A, Devaud N, Pérez G, Crovari F, Boza C, Viviani P, Ibáñez L, Guzmán S. Antecolic versus retrocolic alimentary limb in laparoscopic Roux-en-Y gastric bypass: a comparative study. Surg Obes Relat Dis. 2007;3(4):423-27.
- Nguyen NT, Huerta S, Gelfand D, Stevens CM, Jim J. Bowel obstruction after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2004;14(2):190-6.
- Higa KD, Ho T, Boone KB. Internal hernias after laparoscopic Roux-en-Y gastric bypass: incidence, treatment and prevention. Obes Surg. 2003;13(3):350-4.
- Felsher J, Brodsky J, Brody F. Small bowel obstruction after laparoscopic Roux-en- Y gastric bypass. Surgery. 2003;134(3):501-5.
- Warner BW. Pediatric Surgery. In: Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL., ed. Sabiston Textbook of Surgery, 18th Edition. Philadelphia: Saunders Elsevier; 2008:2047-2089.
- Edwards MA, Grinbaum R, Ellsmere J, Jones DB, Schneider BE. Intussusception after Roux-en-Y gastric bypass for morbid obesity: case report and literature review of rare complication. Surg Obes Relat Dis. 2006;2(4):483-9.
- Evers BM. Small Intestine. In: Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL., ed. Sabiston Textbook of Surgery, 18th Edition. Philadelphia: Saunders Elsevier; 2008:1278-1332.
- Kasotakis G, Sudan R. Retrograde intussusception after Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2009;19(3):381-4.
- Efthimiou E, Court O, Christou N. Small bowel obstruction due to retrograde intussusception after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2009;19(3):378-80.
- Pauli EM, Haluck RS. Antiperistaltic (retrograde) intussusception after laparoscopic Roux-en-Y gastric bypass procedure. Surg Obes Relat Dis. 2008;4(4):567-8.
- Lessmann J, Soto E, Merola S. Intussusception after Roux-en-Y gastric bypass for morbid obesity. Surg Obes Relat Dis. 2008;4(5):664-7.
- Simper SC, Erzinger JM, McKinlay RD, Smith SC. Retrograde (reverse) jejunal intussusception might not be such a rare problem: a single group’s experience of 23 cases. Surg Obes Relat Dis. 2008;4(2):77-83.
- Al-Sabah S, Christou N. Intussusception after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2008;4(2):205-9.
- Zainabadi K, Ramanathan R. Intussusception after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2007;17(12):1619-23.
- Coster DD, Sundberg SM, Kermode DS, Beitzel DT, Noun SH, Severidt M. Small bowel obstruction due to antegrade and retrograde intussusception after gastric bypass: three case reports in two patients, literature review, and recommendations for diagnosis and treatment. Surg Obes Relat Dis. 2008;4(1):69-72.
- Bocker J, Vasile J, Zager J, Goodman E. Intussusception: an uncommon cause of postoperative small bowel obstruction after gastric bypass. Obes Surg. 2004;14(1):116- 19.
- Srikanth MS, Keskey T, Fox SR, Oh KH, Fox ER, Fox KM. Computed tomography patterns in small bowel obstruction after open distal gastric bypass. Obes Surg. 2004;14(6):811-22.
- Majeski J, Fried D. Retrograde intussusception after Roux-en-Y gastric bypass surgery. J Am Coll Surg. 2004;199(6):988-89.
- Hocking MP, McCoy DM, Vogel SB, Kaude JV, Sninsky CA. Antiperistaltic and isoperistaltic intussusception associated with abnormal motility after Roux-en-Y gastric bypass: a case report. Surgery. 1991;110(1):109-12.
- Duane TM, Wohlgemuth S, Ruffin K. Intussusception after Roux-en-Y gastric bypass. Am Surg. 2000as;66(1):82-4.
- Ver Steeg K. Retrograde intussusception following Roux-en-Y gastric bypass. Obes Surg. 2006;16(8):1101-3.
- Papadia F. Intussusception after gastric bypass. Obes Surg. 2004;14(5):705.
- Goverman J, Greenwald M, Gellman L, Gadaleta D. Antiperistaltic (retrograde) intussusception after Roux-en-Y gastric bypass. Am Surg. 2004;70(1):67-70.
- Keyser EJ, Ahmed NA, Mott BD, Tchervenkov J. Double closed loop obstruction and perforation in a previous Roux-en-Y gastric bypass. Obes Surg. 1998;8(4):475-9.
- See C, Carter PL, Elliott D, Mullenix P, Eggebroten W, Porter C, Watts D. An institutional experience with laparoscopic gastric bypass complications seen in the first year compared with open gastric bypass complication during the same period. Am J Surg. 2002;183:533-38.
- Yu SC, Clapp BL, Lee MJ, Albrecht WC, Scarborough TK, Wilson EB. Robotic assistance provides excellent outcomes during the learning curve for laparoscopic Roux- en-Y gastric bypass: results from 100 robotic-assisted gastric bypasses. Am J Surg. 2006 Dec;192(6):746-9.
- Hassan M, Kerlakian G, Curry T, Engel A, Bollmer C. Comparing outcomes of hand-assisted versus total laparoscopic gastric bypass. Surg Obes Relat Dis. 2008;4(2):91-5.
- Ali MR, Tichansky DS, Kothari SN, McBride CL, Fernandez AZ Jr, Sugerman HJ, Kellum JM, Wolfe LG, Demaria EJ. Validation that a 1-year fellowship in minimally invasive and bariatric surgery can eliminate the learning curve for laparoscopic gastric bypass. Surg Endosc. 2009 Jun 11. [Epub ahead of print]
- Sugerman HJ, Londrey GL, Kellum JM, Wolf L, Liszka T, Engle KM, Birkenhauer R, Starkey JV. Weight loss with vertical banded gastroplasty and Roux-Y gastric bypass for morbid obesity with selective versus random assignment. Am J Surg. 1989;157:93- 102.
- Hall JC, Watts JM, O’Brien PE, Dunstan RE, Walsh JF, Slavotinek AH, Elmslie RG. Gastric surgery for morbid obesity. The Adelaide Study. Ann Surg. 1990;211:419- 27.
- Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339-52.
- Sugerman HJ, Kellum JM Jr., Reines HD, DeMaria EJ, Newsome HH, Lowry JW. Greater risk of incisional hernia with morbidly obese than steroid-dependent patients and low recurrence with prefascial polypropylene mesh. Am J Surg. 1996;171:80-84.
- Brolin RE, Prospective, randomized evaluation of midline fascial closure in gastric bariatric operations. Am J Surg. 1996;172:328-31.
- MacLean LD, Rhode BM, Nohr CW. Late outcome of isolated gastric bypass. Ann Surg. 2000;231:524-28.
- Vanek VW. Risk factors for development of incisional hernia following open Roux- en-Y gastric bypass. Surg Obes Relat Dis. 2006;2(3):332-333.
- Capella RF, Iannace VA, Capella JF. Reducing the incidence of incisional hernias following open gastric bypass surgery. Obes Surg. 2007;17(4):438-44.
- Flancbaum L, Belsley S. Factors affecting morbidity and mortality of Roux-en-Y gastric bypass for clinically severe obesity: an analysis of 1,000 consecutive open cases by a single surgeon. J Gastrointest Surg. 2007;11(4):500-7.
- Iljin A, Szymanski D, Kruk-Jeromin J, Strzelczyk J. The repair of incisional hernia following Roux-en-Y gastric bypass-with or without concomitant abdominoplasty? Obes Surg. 2008;18(11):1387-91.
- Maher JW, Martin Hawver L, Pucci A, Wolfe LG, Meador JG, Kellum JM. Four hundred fifty consecutive laparoscopic Roux-en-Y gastric bypasses with no mortality and declining leak rates and lengths of stay in a bariatric training program. J Am Coll Surg. 2008;206(5):940-45.
- Christou NV, Jarand J, Sylvestre JL, McLean AP. Analysis of the incidence and risk factors for wound infections in open bariatric surgery. Obes Surg. 2004;14(1):16-22.
- Schuster R, Curet MJ, Alami RS, Morton JM, Wren SM, Safadi BY. Concurrent gastric bypass and repair of anterior abdominal wall hernias. Obes Surg. 2006;16(9):1205-8.
- Herbert GS, Tausch TJ, Carter PL. Prophylactic mesh to prevent incisional hernia: a note of caution. Am J Surg. 2009;197(5):595-98.
- Rosenthal RJ, Szomstein S, Kennedy CI, Zundel N. Direct visual insertion of primary trocar and avoidance of fascial closure with laparoscopic Roux-en-Y gastric bypass. Surg Endosc. 2007;21(1):124-8.
- Matthews BD, Heniford BT, Sing RF. Preperitoneal Richter hernia after a laparoscopic gastric bypass. Surg Laparosc Endosc Percutan Tech. 2001;11(1):47-9.
- Eid GM, Collins J. Application of a trocar wound closure system designed for laparoscopic procedures in morbidly obese patients. Obes Surg. 2005;15(6):871-3.
- Johnson WH, Fecher AM, McMahon RL, Grant JP, Pryor AD. VersaStep trocar hernia rate in unclosed fascial defects in bariatric patients. Surg Endosc. 2006;20(10):1584-6.
- Liu CD, McFadden DW. Laparoscopic port sites do not require fascial closure when nonbladed trocars are used. Am Surg. 2000;66(9):853-4.
- Chiu CC, Lee WJ, Wang W, Wei PL, Huang MT. Prevention of trocar-wound hernia in laparoscopic bariatric operations. Obes Surg. 2006;16(7):913-8.
- Tucker ON, Szomstein S, Rosenthal RJ. Nutritional consequences of weight-loss surgery. Med Clin North Am. 2007;91(3):499-514.
- Davies DJ, Baxter JM, Baxter JN. Nutritional deficiencies after bariatricsurgery. Obes Surg. 2007;17(9):1150-8.
- Sugerman HJ, Brewer WH, Shiffman ML, Brolin RE, Fobi MA, Linner JH, MacDonald KG, MacGregor AM, Martin LF, Oram-Smith JC, et al. A multicenter, placebo-controlled, randomized, double-blind, prospective trial of prophylactic ursodiol for the prevention of gallstone formation following gastric-bypass-induced rapid weight loss. Am J Surg. 1995;169(1):91-7.
- Duffey BG, Pedro RN, Kriedberg C, Weiland D, Melquist J, Ikramuddin S, Kellogg T, Makhlouf AA, Monga M. Lithogenic risk factors in the morbidly obese population. J Urol. 2008;179(4):1401-6.
- Whitson JM, Stackhouse GB, Stoller ML. Hyperoxaluria after modern bariatric surgery: case series and literature review. Int Urol Nephrol. 2009 Jul 2. [Epub ahead of print]
- Duffey BG, Pedro RN, Makhlouf A, Kriedberg C, Stessman M, Hinck B, Ikramuddin S, Kellogg T, Slusarek B, Monga M. Roux-en-Y gastric bypass is associated with early increased risk factors for development of calcium oxalate nephrolithiasis. J Am Coll Surg. 2008;206(6):1145-53.
- Matlaga BR, Shore AD, Magnuson T, Clark JM, Johns R, Makary MA. Effect of gastric bypass surgery on kidney stone disease. J Urol. 2009;181(6):2573-77.
- Durrani O, Morrisroe S, Jackman S, Averch T. Analysis of stone disease in morbidly obese patients undergoing gastric bypass surgery. J Endourol. 2006;20(10):749-52.
- Muñoz R, Ibáñez L, Salinas J, Escalona A, Pérez G, Pimentel F, Guzmán S, Boza C. Importance of routine preoperative upper GI endoscopy: why all patients should be evaluated? Obes Surg. 2009;19(4):427-31.
- Sevá-Pereira G, Trombeta VL. Early gastric cancer found at preoperative assessment for bariatric surgery. Obes Surg. 2006;16(8):1109-11.
- Boru C, Silecchia G, Pecchia A, Iacobellis G, Greco F, Rizzello M, Basso N. Prevalence of cancer in Italian obese patients referred for bariatric surgery. Obes Surg. 2005;15(8):1171-76.
- Sohn VY, Arthurs ZM, Martin MJ, Sebesta JA, Branch JB, Champeaux AL. Incidental pathologic findings in open resectional gastric bypass specimens with routine cholecystectomy and appendectomy. Surg Obes Relat Dis. 2008;4(5):608-11.
- Voellinger DC, Inabnet WB. Laparoscopic Roux-en-Y gastric bypass with remnant gastrectomy for focal intestinal metaplasia of the gastric antrum. Obes Surg. 2002;12(5):695-98.
- Raijman I, Strother SV, Donegan WL. Gastric cancer after gastric bypass for obesity. Case report. J Clin Gastroenterol. 1991;13(2):191-94.
- Khitin L, Roses RE, Birkett DH. Cancer in the gastric remnant after gastric bypass: a case report. Curr Surg. 2003;60(5):521-3.
- Lord RV, Edwards PD, Coleman MJ. Gastric cancer in the bypassed segment after operation for morbid obesity. Aust N Z J Surg. 1997;67(8):580-82.
- Inoue H, Rubino F, Shimada Y, Lindner V, Inoue M, Riegel P, Marescaux J. Risk of gastric cancer after Roux-en-Y gastric bypass. Arch Surg. 2007;142(10):947-53.
- Alva S, Eisenberg D, Duffy A, Roberts K, Israel G, Bell R. A new modality to evaluate the gastric remnant after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2008;4(1):46-49.
- Kuga R, Safatle-Ribeiro AV, Faintuch J, Ishida RK, Furuya CK Jr, Garrido AB Jr, Cecconello I, Ishioka S, Sakai P. Endoscopic findings in the excluded stomach after Roux-en-Y gastric bypass surgery. Arch Surg. 2007;142(10):942-46.
- Patel JA, Patel NA, Shinde T, Uchal M, Dhawan MK, Kulkarni A, Colella JJ. Endoscopic retrograde cholangiopancreatography after laparoscopic Roux-en-Y gastric bypass: a case series and review of the literature. Am Surg. 2008;74(8):689-94.
- Ceppa FA, Gagné DJ, Papasavas PK, Caushaj PF. Laparoscopic transgastric endoscopy after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2007;3(1):21-24.
- Gulkarov I, Wetterau M, Ren CJ, Fielding GA. Hiatal hernia repair at the initial laparoscopic adjustable gastric band operation reduces the need for reoperation. Surg Endosc. 2008;22(4):1035-41.
- Forsell P, Hallerbäck B, Glise H, Hellers G. Complications following Swedish adjustable gastric banding: a long-term follow-up. Obes Surg. 1999;9(1):11-16.
- O’Brien PE, Dixon JB. Weight loss and early and late complications–the international experience. Am J Surg. 2002;184(6B):42S-45S.
- Parikh MS, Fielding GA, Ren CJ. US experience with 749 laparoscopic adjustable gastric bands: intermediate outcomes. Surg Endosc. 2005;19(12):1631-35.
- Oelschlager BK, Eubanks TR, Pellegrini CA. Hiatal Hernia and Gastroesophageal Reflux Disease. In: Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL., ed. Sabiston Textbook of Surgery, 18th Edition. Philadelphia: Saunders Elsevier; 2008:1108- 1125.
- Arias IE, Radulescu M, Stiegeler R, Singh JP, Martinez P, Ramirez A, Szomstein S, Rosenthal RJ. Diagnosis and treatment of megaesophagus after adjustable gastric banding for morbid obesity. Surg Obes Relat Dis. 2009;5(2):156-59.
- Gamagaris Z, Patterson C, Schaye V, Francois F, Traube M, Fielding CJ, Fielding GA, Youn AH, Weinshel EH. Lap-band impact on the function of the esophagus. Obes Surg. 2008;18(10):1268-72.
- Wiesner W, Hauser M, Schöb O, Weber M, Hauser RS. Pseudo-achalasia following laparoscopically placed adjustable gastric banding. Obes Surg. 2001;11(4):513-8.
- Niville E, Dams A, Vlasselaers J. Lap-Band erosion: incidence and treatment. Obes Surg. 2001;11(6):744-47.
- Silecchia G, Restuccia A, Elmore U, Polito D, Perrotta N, Genco A, Bacci V, Basso N. Laparoscopic adjustable silicone gastric banding: prospective evaluation of intragastric migration of the lap-band. Surg Laparosc Endosc Percutan Tech. 2001;11(4):229-34.
- Lattuada E, Zappa MA, Mozzi E, Antonini I, Boati P, Roviaro GC. Injection Port and Connecting Tube Complications after Laparoscopic Adjustable Gastric Banding. Obes Surg. 2008 Jun 10. [Epub ahead of print]
- Launay-Savary MV, Slim K, Brugère C, Buc E, Nini E, Forestier D, Chipponi Band and port-related morbidity after bariatric surgery: an underestimated problem. Obes Surg. 2008;18(11):1406-10.
- Reijnen MM, Naus JH, Janssen IM. Mechanical evaluation of a ruptured Swedish adjustable gastric band. Obes Surg. 2004;14(2):253-55.
- Li VK, Pulido N, Fajnwaks P, Szomstein S, Rosenthal R. Predictors of gallstone formation after bariatric surgery: a multivariate analysis of risk factors comparing gastric bypass, gastric banding, and sleeve gastrectomy. Surg Endosc. 2009;23(7):1640-4.
- Consten EC, Gagner M, Pomp A, Inabnet WB. Decreased bleeding after laparoscopic sleeve gastrectomy with or without duodenal switch for morbid obesity using a stapled buttressed absorbable polymer membrane. Obes Surg. 2004;14(10):1360- 66.
- Serra C, Baltasar A, Andreo L, Pérez N, Bou R, Bengochea M, Chisbert JJ. Treatment of gastric leaks with coated self-expanding stents after sleeve gastrectomy. Obes Surg. 2007;17(7):866-72.
Address all correspondence to:
Vincent W. Vanek, MD, FACS, CNSP Department of Surgical Education
St. Elizabeth Health Center
1044 Belmont Avenue, P.O. Box 1790
Youngstown, Ohio 44501
Phone: 330-480-2371
Fax: 330-480-3640
E-mail: vince_vanek@hmis.org
