Complications in Achalasia
Santiago Horgan, MD, Kari Thompson, MD
INTRODUCTION
Achalasia of the esophagus is a primary motor disorder of unknown etiology, characterized by inadequate lower esophageal sphincter (LES) relaxation, absence of peristalsis, and high basal pressure at the LES. It is associated with an inflammatory process with progressive neuronal degeneration in the myenteric plexus of Auerbach.
It has no predlication to gender or race and has an incidence of about 7 to 10 cases in 100,000 in North America.1 Achalasia was first described by AF Hurst who determined the etiology of the symptoms to be the inability of the lower esophageal sphincter to relax in 1927.
The diagnosis of achalasia is established by a history of dysphagia, first to solids, then progressing to liquids over time. Subsequent weight loss is common, as is retrosternal chest pain, reflux, and regurgitation of undigested food.1 Contrast esophagram, endoscopy, and esophageal manometry are the tests of choice for diagnosing achalasia.
Manometry is one of the most important diagnostic tools in achalasia. It provides information about the resting pressure of the LES, the absence of relaxation with swallowing, and the aperistalsis of the esophageal body, which is characteristic of achalasia.
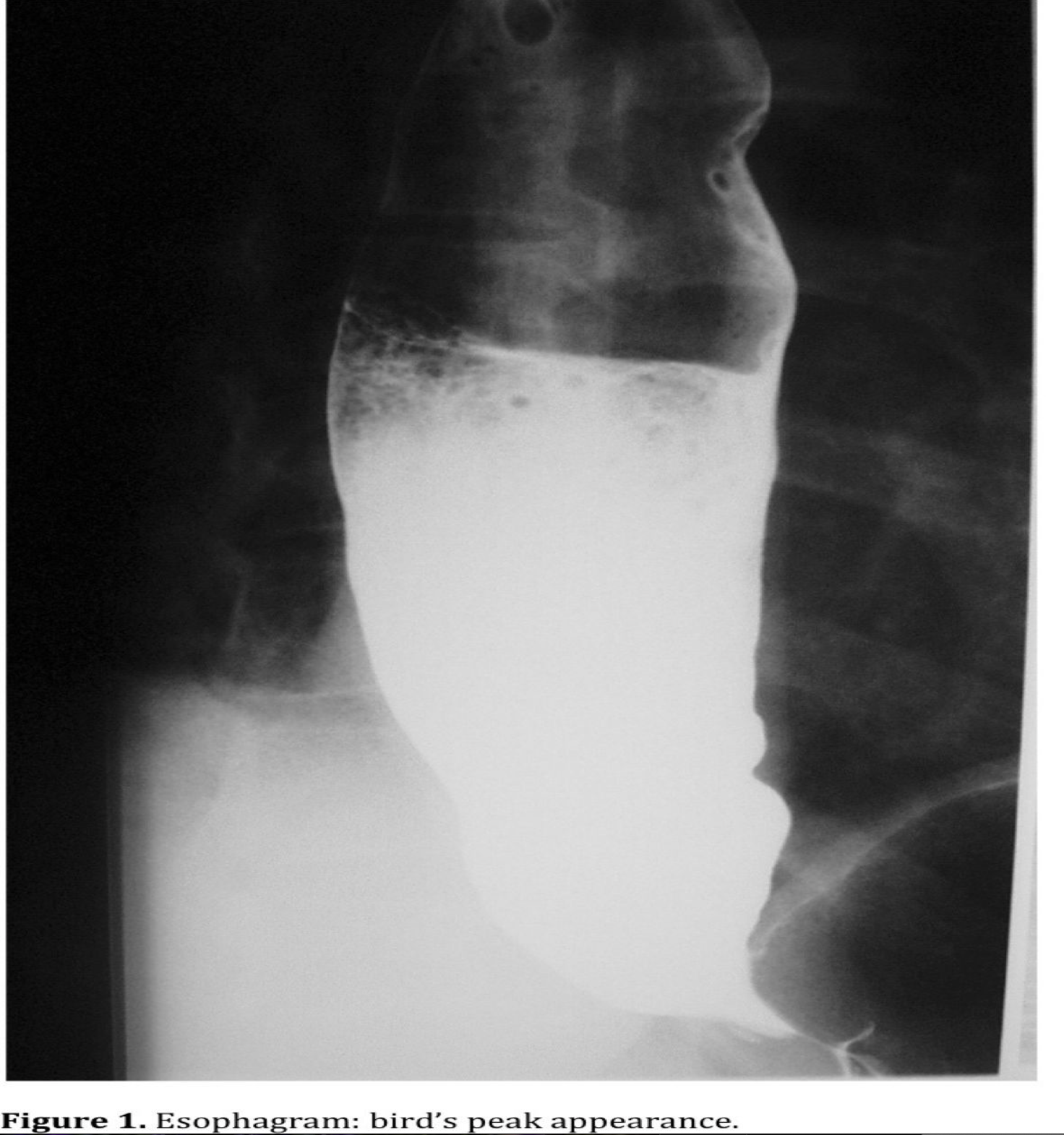
The contrast esophagram (Figure 1) is another important test to evaluate the anatomic characteristics of the esophagus: the typical distal narrowing of the LES (“bird’s beak”), the lack of peristalsis, and the dilatation of the esophagus caused by the hypertensive LES. In patients with end-stage disease, the continuous elongation of the esophagus creates a very dilated esophagus also known as “sigmoid esophagus.”
The upper endoscopy is valuable in ruling out pseudo-achalasia and other diseases known to cause dysphagia. The occurrence of pseudoachalasia in patients with dysphagia mimicking achalasia has been reported to be 2.4-4%.2 These patients present with dysphagia and diagnostic studies consistent with achalasia, but the symptoms are related to an occult malignancy (most frequently adenocarcinoma of the gastroesophageal junction). Usually patients tend to present with shorter durations of symptoms, greater weight loss, and a more advanced age at presentation than do patients with primary achalasia. Contrast radiography and endoscopy may fail to differentiate these 2 diseases, and patients with presumed achalasia meeting the above criteria who are referred for surgery should undergo additional imaging to rule out an occult malignancy.
Achalasia is an incurable disease and the treatment focuses on symptom relief. The treatment modalities that are available consist of reducing the LES pressure in order to eliminate or decrease the esophageal outflow resistance responsible for most of the symptoms. Treatment options include medication (calcium channel blockers, nitrates and injected Botox), pneumatic dilation of the LES, stenting of the LES, and surgery to divide the muscle fibers of the LES and the proximal stomach. In this chapter, we will discuss the complications associated with each treatment and the possible solutions.
BOTULIN TOXIN INJECTION (BOTOX)
The Botulin toxin inhibits the release of acetylcholine from the presynaptic nerve endings, restoring the balance between excitatory and inhibitory neurotransmitters in the LES. The injection of Botox in the lower esophageal sphincter produces a substantial and sustained reduction in basal pressure and improves the emptying of the esophagus.3
The use of Botox rapidly became popular because of the simplicity of the technique and the low risk of complications. After many years of experience with Botox, it was evident that to achieve good results the toxin had to be injected in several areas of the gastroesophageal junction, required several applications, lost efficacy over time, worked for an average of 6 to 9 months in most patients, and was only effective in 60% to 65% of the patients. The symptoms recurred and the applications had to be repeated several times within the first 2 years.
Due to the initial widespread use of Botox, many patients underwent injection as the first approach in the treatment of achalasia, but some of these patients were later referred for surgery because of the recurrence of symptoms. At the University of Washington (Seattle, WA) it was shown that a previous application of Botox made the myotomy much more difficult, increasing the operative time, and increased the risk of mucosal perforation. In their initial experience with 57 patients undergoing surgery, 15 had previous Botox injections; 2 (13.3%) of these patients experienced a mucosal perforation during the myotomy due to the impossibility of operating on the mucosa from the muscularis.3 None of the patients without Botox had a mucosal perforation. The perforation rate in patients with Botox injection is significantly higher than in patients without Botox (13.3% vs. 0%).
Patti et al. also showed similar results, wherein the injection of Botox created inflammation and fibrosis, obliteration of the submucosal plane, and loss of definition of the anatomical planes, all of which increased the risk of mucosal perforation.4
Other complications related to the injection of Botox are acid reflux, esophagitis, pneumothorax, heart block, and myasthenic crisis.5-7
We feel that the application of Botox may not be appropriate as a first line of treatment, due to the short interval of symptom relief, the high relapse rates, the requirement of several applications, and the potential associated risks, especially in those patients who will subsequently undergo surgery.
The application of Botox should be reserved for elderly patients or patients with multiple medical problems who are poor candidates for more invasive procedures and for those patients unwilling to undergo either surgery or pneumatic dilatation.
PNEUMATIC DILATION
The dilation of the LES is considered the most effective nonsurgical treatment of achalasia. It creates a controlled disruption of the LES muscle, resulting in relief of the distal esophageal obstruction with secondary symptomatic improvement. It has been commonly used since the late 1970s as the initial treatment of achalasia.
The success rate for pneumatic dilation is between 55% and 70% with symptom improvement in 50% to 89% of patients. Some patients will require a second or third dilatation to achieve up to 90% relief of symptoms.4,8
Esophageal perforation is the most important and potentially life-threatening complication following pneumatic dilation, occurring predominantly above the cardia on the left side of the esophagus. The reported perforation rate after dilatation is between 2% and 12% of patients with a mortality rate of 1% to 2%.9 Several factors may influence the risk of perforation. Those factors include the technique and the type of dilator used for the procedure. Barotto in France reported a perforation rate of 2.4% with the Rigiflex dilator and 5.2% with the Witzel dilator. It has also been found that a small esophageal diameter and high amplitude of esophageal contractions increase the risk for an esophageal perforation during pneumatic dilation.10
Csendes et al. from Chile published in 1989 a prospective randomized trial comparing pneumatic dilation and surgery.11 They included 81 patients divided into 2 groups, 39 patients treated by dilation using a Mosher bag and 41 patients treated with surgical myotomy via an abdominal approach. After a mean follow-up of 62 months, they obtained good results after one or more dilations in 65% of the patients in the first group compared with 95% of patients after a surgical myotomy. In the dilation group, a perforation rate of 5.6% occurred. The study showed that surgical treatment offered a better final clinical result than did pneumatic dilatation.
Metman et al. from France showed the results of balloon dilation in 237 patients with achalasia between 1980 and 1994, using a progressive dilation technique.10 They experienced 15 complications (6% of patients): 7 perforations (3%), 3 asymptomatic esophageal mucosal tears, and 4 esophageal hematomas.
An esophagram with water-soluble contrast material should be performed after each pneumatic dilation in order to rule out mucosal perforation. In the presence of a perforation, surgery is required. Therapy consists of oversewing the perforation, completion of the myotomy, and creation of a partial wrap to prevent gastroesophageal reflux. In select patients and in the presence of nontransmural tears, conservative treatment is an alternative to urgent surgery.
Scatton et al. from France published the results of their experience with 524 dilations in 412 patients, with 13 esophageal perforations (3%).12 All the perforations were diagnosed by a postprocedure esophagogram. They medically treated 6 patients with nasogastric suction, antibiotics, and pleural drainage. Surgical decision was based on clinical and radiological parameters. Surgical treatment included left thoracophrenotomy, perforation closure, contralateral myotomy, and anterior fundoplication. They also noted that the presence of a pneumomediastinum at the initial presentation seemed to predict failure of conservative medical management, requiring immediate surgical intervention.
Other complications following pneumatic dilation include prolonged chest pain, gastroesophageal reflux, gastrointestinal hemorrhage, esophageal mucosal tear, and intramural esophageal hematoma. Almost 30% of patients undergoing pneumatic dilation for achalasia develop either prolonged pain or morphologic lesions.
Regarding the interference of pneumatic dilation with further surgical treatment, Patti et al. showed that a previous pneumatic dilation did not affect the results of myotomy and that a surgical myotomy can give excellent results after failure of pneumatic dilatation.4
Pneumatic dilation is an effective therapeutic alternative for the treatment of achalasia, with symptomatic improvement close to 85%. Although dilation does initially reduce LES pressure in the long-term, it has not been as successful as surgical myotomy. A recent study by Parkman et al. reported less than 50% success following initial dilation and less than 70% success after 2 or more dilations.13 Pneumatic dilation should be attempted in patients with extreme dysphagia while awaiting surgery, for patients who are not good candidates for surgery, and for patients that underwent surgical myotomy and are experiencing recurrent dysphagia.
MINIMALLY INVASIVE HELLER MYOTOMY
Thoracoscopic Heller Myotomy
The technique popularized by Ellis consists of a 7-cm myotomy in the thoracic esophagus extending into the gastric wall for 5 mm. The operation is performed through the left thorax. Because the operation is done via the chest, an antireflux procedure cannot be performed. Single lung ventilation and a chest tube are required. An endoscope is also required to facilitate the exposure and to evaluate the extent of the myotomy into the stomach. At the same time, the endoscope can identify a mucosal perforation.
In the early 90s, Oelschlager et al. also described the thoracoscopic approach for the treatment of achalasia.14 They performed 15 thoracoscopic myotomies, achieving excellent results in 70% of the patients. Ramacciato from Italy compared the outcomes of the thoracoscopic and laparoscopic approach finding that the laparoscopic myotomy with an antireflux procedure had a shorter hospital stay, shorter operative time, and was superior to the thoracoscopic approach in relieving dysphagia (5.7% vs 31.2% failure).16 Vaezi et al. reported that when using the thoracoscopic approach the incidence of persistent postoperative dysphagia was 18% with more than 50% of patients experiencing gastroesphageal reflux.8 This can be explained in part because an antireflux operation is complicated to perform through the chest, and the myotomy cannot be extended more than 1 cm distally into the stomach. Additionally, an endoscopy is needed to evaluate the position of the LES and to confirm the extent of the myotomy due to poor exposure of the gastroesophageal junction during the thoracoscopic approach. Other features of the operation such as single lung ventilation and the need of a chest tube together with the high incidence of reflux and higher rates of dysphagia convinced many surgeons to move toward the laparoscopic approach.
Laparoscopic Heller Myotomy
In 1991, Cuschieri first described the laparoscopic Heller myotomy.17 The operation is performed through 5 trocars placed in the upper quadrants of the abdomen. The myotomy extends 5 cm into the thoracic esophagus and 2 cm to 3 cm into the stomach. An antireflux procedure is then completed. This procedure became more attractive to patients due to its low morbidity, fast recovery, and superior results.
Patti et al. reported the results of 168 patients over an 8-year period; 21% were operated on with thoracoscopy and 79% with laparoscopy.15 Good to excellent relief of the dysphagia was achieved in 85% of patients in the thoracoscopic group and in 93% in the laparoscopic group. Gastroesophageal reflux was experienced in 60% of the patients that received a thoracoscopic myotomy, and in only 17% in the laparoscopic myotomy plus fundoplication group.15 It is evident that the laparoscopic Heller myotomy plus the addition of an antireflux operation is the best treatment for achalasia, due to the good outcomes and low complication rates.
In recent years, robotic technology has been applied to the minimally invasive field. In October 2000, the first robotic Heller myotomy was performed at the University of Illinois, Chicago.18 Laparoscopic techniques, the myotomy, and Dor fundoplication were performed as the same techniques used in the conventional laparoscopic approach were mimicked in the robotic approach. The initial steps were performed with the standard robotic system (Figure 2). The articulating wrists of the robotic system enable the surgeon to operate in the narrow field around the thoracic esophagus. Because robotic instruments have wrists, the operative field is not obstructed by the instrument, as happens with laparoscopic surgery, allowing for better visualization. This not only allows for proper extension of the myotomy but also lengthens the intraabdominal portion of the esophagus, which, with the addition of a fundoplication, significantly decreases the risk of postoperative gastroesophageal reflux. Additionally, the 10X, 3-D vision allows each individual muscle fiber to be visualized and divided ensuring a proper myotomy. This view, along with electronic filtering that removes tremor, allows precise dissection reducing the risk of perforation of the esophageal mucosa.

A multicenter study conducted by the University of Illinois at Chicago, Ohio State University, and Johns Hopkins School of Medicine, showed that when using robotic technology for the treatment of achalasia, the perforation rate decreased to 0%. This is in contrast with the reported perforation rates for the laparoscopic techniques that vary from 5% to 10%, independent of surgeon experience.
Complications of Esophageal Myotomy
Postoperative Dysphagia
When dysphagia persists or recurs after the operation, an esophagogram, manometry, and eventually an endoscopy should be performed to diagnose the cause of the symptoms.
The reasons for immediate postoperative dysphagia may be technical, including the length of the myotomy and the construction of the antireflux procedure. Recurrent dysphagia is believed to be secondary to healing or peptic stricture secondary to reflux. One of the causes of persistent dysphagia is an incomplete myotomy. It is accepted that the myotomy should be performed 6 cm to 7 cm above the LES and 2 cm into the stomach to completely disrupt the muscle of the LES.
Pellegrini et al. showed that an extended myotomy 3cm into the stomach improved the results of the treatment of achalasia for dysphagia without increasing the rate of abnormal gastroesophageal reflux.19 Patti also found that a short myotomy was responsible for persistent dysphagia after the operation.20 This could be avoided by the routine use of intraoperative endoscopy to evaluate the completion of the myotomy.
The other technical problem may be related to the construction of the antireflux procedure. Usually this dysphagia is related to the position of the sutures through the edges of the myotomy and the crura, the tension on the fundus exerted by the short gastric vessels, or the orientation of the fundoplication.
The division of the short gastric vessel should be done when tension is suspected, but if a floppy fundus is present, with no tension from the short gastric vessels, this step could be avoided.
Pneumatic dilatation can be used as a first approach for patients with persistent or recurrent dysphagia. In most cases, the dysphagia will resolve with one or multiple dilatations. If the balloon dilatations are not successful, then a second operation may be required, either to extend the myotomy, to rearrange the antireflux operation, or to redo the myotomy. In patients with persistent symptoms, a total esophagectomy may be reserved for severe uncorrectable dysphagia following a myotomy.19
Esophageal Perforations
The mucosal perforation is the most common complication of the laparoscopic myotomy, ranging from 1% to 15% of cases or even higher (9.5% to 25%).9,21 We found that the preoperative use of Botox is a predisposing factor for intraoperative mucosal perforation.3 Usually the perforations occur at the GE junction. They are small and can be closed laparoscopically during the same procedure with no change in the outcome. The creation of an anterior fundoplication provides additional support. Also, mucosal burn injuries can be caused by accidental contact with the hook cautery. These are not complete perforations and can be handled by oversewing the burn site.17
With the addition of robotic technology to the treatment of the achalasia, we documented that the perforation rate decreased, probably due to the enhanced 3-D vision and the elimination of tremor.
The concomitant use of intraoperative endoscopy, at the beginning of a surgeon’s experience, is helpful in identifying small perforations or a short or incomplete myotomy. To confirm the integrity of the mucosa, every patient who suffers a perforation requiring an intraoperative repair should undergo a Gastrografin swallow the following day before resuming oral intake.
Gastroesophageal Reflux
One of the adverse effects of the myotomy is postoperative gastroesophageal reflux (GERD) produced by the disruption of the LES muscles. Due to the incompetent LES and the aperistalsis of the body, the esophagus is unable to clear the refluxed gastric contents. Serious complications may develop due to the chronic acid exposure, including esophagitis, peptic strictures, Barrett’s esophagus, and carcinoma of the esophagus.
During the early experience with esophageal myotomy, when the procedure was performed through the chest without any antireflux operation, the postoperative incidence of GERD was 35-40%.9 Abnormal pH studies were found in patients that underwent a myotomy without funduplication, even in patients who were completely asymptomatic.20
In patients with a Heller myotomy with an antireflux procedure, gastroesophageal reflux is reported to be 6% to 10%.4,21 De Meester demonstrated that adding an antireflux procedure to the myotomy decreased the reflux rate to 4%.9 The reduction in the incidence of reflux is the reason why an antireflux procedure should be added.
Many reports have shown that a Toupet fundoplication might have better results relative to reflux and dysphagia.8 But the anterior fundoplication (Dor) does not require a posterior dissection of the esophagus, preserves all the posterior attachments that contribute to the antireflux mechanism, and does not angulate the distal esophagus. The Dor fundoplication is a simple and efficient procedure that prevents gastroesophageal reflux in the majority of patients.9,17,20
Zaninotto reported his experience with 142 patients who underwent Heller myotomy with a Dor fundoplication.22 A 24-hr pH test was performed 6 months after the procedure in 73% of the patients to evaluate abnormal reflux. He found only 7 patients (6.7%) with abnormal reflux, all of which were treated with medical therapy (H2 blockers or proton pump inhibitors).
Donahue showed that dysphagia was effectively relieved in all patients after myotomy followed by floppy Nissen fundoplication, and neither postoperative reflux nor esophageal obstruction was observed.23 He concluded that myotomy with a floppy Nissen fundoplication was an effective treatment for achalasia, without clinical evidence of obstruction of the esophagus.
It is important to evaluate patients carefully to treat reflux effectively and avoid the development of Barrett’s esophagus and adenocarcinoma. To assess the severity and the causes of postoperative reflux, the pH study is the gold standard. In most patients, postoperative reflux can be treated with a proton pump inhibitor with excellent results.21,24
The great challenge in the surgical treatment of achalasia is to perform an operation that will not create any dysphagia and at the same time will not increase acid reflux.
Unfortunately, this balance is not always achieved. The creation of a Dor, Toupee, or even a Nissen fundoplication will prevent acid reflux to different degrees but may also create some degree of dysphagia in the patient. As we stated above, some controversy still exists about which of the fundoplications is best. We believe that the Dor fundoplication is the procedure of choice due to its effectiveness in preventing acid reflux and the low incidence of postoperative dysphagia.16
TRANSESOPHAGEAL ENDOSCOPIC ESOPHAGEAL MYOTOMY
The least invasive and most current treatment of achalasia is the transesophageal endoscopic esophageal myotomy (TEEM). This approach is a totally endoscopic treatment for achalasia without any surgical incisions. New technology and studies are emerging allowing a surgeon to perform an endoscopic myotomy and intralumenal fundoplication. Finding an endoscopic treatment to rival surgery would be beneficial to the patient to reduce their overall morbidity. Woodard et al. recently described their method for transesophageal surgery where they were able to, endoscopically, create an esophageal myotomy (mimicking the Heller myotomy) in a porcine model. Here a submucosal tunnel is formed using standard endoscopic instrumentation.25 Other groups have performed similar survival animal studies with encouraging outcomes.26-28
Under IRB protocol at University of California San Diego we have performed a TEEM on the first two human patients in the United States. The procedure included making a submucosal tunnel in the esophagus, identifying the circular muscles of the esophagus and making a myotomy through only those fibers extending 3 centimeters onto the stomach. Both patients were admitted to the hospital for observation overnight and were discharged on postoperative day one without complication. Subjectively, the patients both claimed their dysphagia was 100% improved. Barrium swallow studies were performed postoperatively without evidence of leak. At two week follow up, both patients are without complication and doing well. Once the patient has completely healed from their myotomy, we plan to perform an endoscopic intralumenal fundoplication for antireflux purposes.
Although we are just beginning to explore using a natural orifice translumenal endoscopic surgery (NOTES) approach to achalasia, our preliminary results are very promising. The complications we anticipate would be similar to a surgical myotomy including: bleeding, perforation, dysphagia, and gastroesophageal reflux. As aforementioned, if complications were to occur, they would be treated in a conservative manner.
CONCLUSION
Surgical myotomy should be the first therapeutic approach for patients who are good surgical candidates. It has been shown that surgery has a high long-term success with a low complication rate and almost no mortality. Balloon dilation, although a good temporary treatment for achalasia, is not superior to surgery. Esophageal dilatations must be performed in a center with access to surgeons specially trained in esophageal surgery. If a perforation occurs without access to adequate treatment, the results can be catastrophic. Botulinum toxin should be reserved for elderly or high-risk patients who are poor candidates for dilatation or surgery. The application of Botox increases the risk of a perforation with low effectiveness in the medium- and long-term. Robotic Heller myotomy is a newer approach to treat achalasia, and has been shown to have a perforation rate of 0% in multiple centers across the country. As NOTES becomes a reality in multiple academic centers worldwide, TEEM may become a procedure with low morbidity for the patient in the treatment of achalasia. Increasing the clinical experience with long term follow up is needed at this time.
A very well-trained team should perform the surgical treatment of achalasia, with extensive experience in the treatment of the disease and the related complications.
References
- Barreca M, Oelschlager BK, Pellegrini C. Laparoscopic Heller myotomy: the treatment of choice of Achalasia. Surgical Rounds. 2001;24(8):395-402.
- Moonka R, Patti M, Horgan S, et al. Clinical presentation and evaluation of malignant pseudoachalasia. J Gastrointest Surg. 1999;3:456-461.
- Horgan S, Hudda K Pellegrini C, et al. Does botulinum toxin injection make esophagomyotomy a more difficult operation. Surg Endosc. 1999;13:576-579.
- Patti M, Feo CV, Arcerito M, et al. Effects of previous treatment on results of laparoscopic Heller myotomy for achalasia. Dig Dis Sci. 1999;44(11):2270-2276.
- Weusten B, Samson M, Smout A. Pneumothorax complicating botulinum toxin injection in the body of a dilated oesophagus in achalasia. Eur J Gastroenterol Hepatol. 2003;15(5):561-564.
- Borodic G. Myasthenic crisis after botulin toxin. Lancet.1998;352:1832.
- Malnick SD, Metchnick Fatal heart block following treatment with botulinum toxin for achalasia. Am J Gastroenterol. 2000:95:3333-3334.
- Vaezi MF, Richter JE. Current therapies for achalasia: comparison and efficacy. J Clin Gastroenterol. 1998;27(1):21-35.
- Sharp K, Khaitan L, Richards W, et al. 100 consecutive minimally invasive Heller myotomies: lessons learned. Ann Surg. 2002;235(5):631-639.
- Metman EH, Lagasse JP, d’Alteroche L, et al. Risk factors for immediate complications after progressive pneumatic dilation for achalasia. Am J Gastroenterol. 1999;94(5):1179-1185.
- Csendes A, Braghetto I, Henriquez A, Cortes C. Late results of a prospective randomised study comparing forceful dilatation and oesophagomyotomy in patients with achalasia. Gut. 1989;30(3):299-304.
- Scatton O, Gaudric M, Massault PP, Chaussade S, Houssin D, Dousset B. Conservative management of esophageal perforation after pneumatic dilatation for achalasia. Gastroenterol Clin Biol. 2002;26(10):883-887.
- Parkman HP, Ogorek CP, Harris AD, et al. Pneumatic dilation or esophagomyotomy treatment for idiopathic achalasia: clinical outcomes and cost analysis. Dig Dis Sci. 1993;38:75-85.
- Pellegrini C, Wetter LA, Patti M, Way L. Thoracoscopic esophagomyotomy. Initial experience with a new approach for the treatment of achalasia. Ann Surg. 1992;216(3):291-296.
- Patti M, Pellegrini C, Horgan S, Way L. Minimally invasive surgery for achalasia: an 8-year experience with 168 patients. Ann Surg. 1999;230(4):587-593.
- Ramacciato G, Mercantini P, Amodio PM, et al. The laparoscopic approach with antireflux surgery is superior to the thoracoscopic approach for the treatment of esophageal achalasia. Experience of a single surgical unit. Surg Endosc. 2002;16(10):1431-1437.
- Hunter J, Trus T, Branum G, Waring J. Laparoscopic Heller myotomy and fundoplication for achalasia. Ann Surg. 1997;225(6):655-665.
- Horgan S, Vanuno D. Robots in laparoscopic surgery. J Laparoendosc Adv Surg Tech A. 2001;11(6):415-419.
- Oelschlager B, Chang L, Pellegrini C. Improved outcome after extended gastric myotomy for achalasia. Arch Surg. 2003;138:490-497.
- Patti M, Molena, D, Fisichella M. Laparoscopic Heller myotomy and Dor fundoplication for achalasia: analysis of successes and failures. Arch Surg. 2001;136(8):870-877.
- Wiechmann R, Ferguson M, Naunheim K, et al. Video-assisted surgical management of achalasia of the esophagus. J Thorac Cardiovasc Surg. 1999;119(5):916-923.
- Zaninotto G, Costantini M, Molena D, et al. Minimally invasive surgery for esophageal achalasia. J Laparoendosc Adv Surg Tech A. 2001;11(6):351-359.
- Donahue PE, Schlesinger PK, Nyhus LM. Esophagocardio-myotomy-floppy Nissen fundoplication effectively treats achalasia without causing esophageal obstruction. Surgery. 1994;116(4):719-724.
- Zaninotto G, Costantini, M, Portale G. Etiology, diagnosis, and treatment of failures after laparoscopic Heller myotomy for achalasia. Ann Surg. 2002;235(2):186-192.
- Woodward T, McCluskey D, Wallace MB, et al. Pilot Study of Transesophageal Endoscopic Surgery: NOTES Esophagomyotomy, Vagotomy, Lymphadenectomy. JLAST. 2008;18:743-745.
- Sumiyama K, Gostout CJ, Rajan E, et al. Submucosal endoscopy with mucosal flap safety valve. Gastrointest Endosc. 2007 Apr;65:688-94.
- Sumiyama K, Gostout CJ, Rajan E, et al. Pilot study of transesophageal endoscopic epicardial coagulation by submucosal endoscopy with the mucosal flap safety valve technique (with videos). Gastrointest Endosc. 2008 Mar;67(3):497-501.
- Sumiyama K, Tajiri H, Goustout CJ. Submucosal endoscopy with mucosal flap safety valve (SEMF) technique: a safe access method into the peritoneal cavity and mediastinum. Minim Invasive Ther Allied Technol. 2008;17(6):365-9.
