SURGICAL SIMULATION: SUPPORTING A CULTURE OF PATIENT SAFETY

Wm. LeRoy Heinrichs, MD, MS, PhD
“Videoendoscopy has changed everything!
Surgeons don’t operate on patients any more; they operate on (their) images.”
–Heinrichs 2004
INTRODUCTION
The apprenticeship model introduced by William Halsted for training surgical residents, learning by repeated practice on patients, served for over a century but is no longer tenable. Traditionally, trainee surgeons were exposed to innumerable operative cases over several years, with supervision tailored to their learning and skills. Experience was gained by learning to cope with a wide range of operative approaches and complications with the primary focus on trainee’s levels of capability, rather than on issues of patient inconvenience and safety. Over the past several decades, other modes of training (eg, the use of synthetic objects, animal models, and video training) have supplanted early phases of apprentice-style learning in the OR. Accordingly, clinical skills-labs, part-task trainers (defined below), and animal surgery have been introduced into training programs with increasing frequency for providing hands-on instruction of technical skills. On a parallel track following successful experience with objective, structured, clinical examinations (OSCEs) for assessing mastery of cognitive knowledge and decision-making, Martin et al1 and Wanzel et al2 developed a new assessment method that provides objective, structured assessments of technical skills (OSATS). First evaluated among general surgical trainees, this formative assessment method has also been used to guide learning and for assessing performance of trainees in obstetrics and gynecology.3
Nowadays, resident training is constrained by the 80-hour workweek, decreasing volumes of surgical cases, and greater complexity of patient’s surgical diseases in Level 3 training institutions. Also, financial constraints in hospitals have further limited opportunities for teaching in the operating room. Another important factor is the public’s heightened awareness of the occurrence of medical errors, placing resident training under intense scrutiny, emphasizing patient safety concerns. The same concern applies to the training of practicing surgeons in new procedures and new equipment, such as robotic surgical environments. The proposition of lengthening the period of training for gaining surgical proficiency is also untenable. Although the 80-hour workweek is the “reduced” learning time, it seems to have had a positive impact on lifestyle issues and the recruitment of medical students into surgical careers. However, the reduced training time has caused program directors to face substantial hurdles for training in the current healthcare environment.
NEW EMPHASIS ON ADULT EDUCATION
This emerging situation requires greater efficiency and reliance on the principles of adult education rather than on patient service to achieve the goal of graduating competent surgeons. A variety of newer methods to prepare trainees for the OR have been implemented in recent years, as surgical procedures are changing from open surgery to minimally invasive surgery (MIS), a practice requiring new technical skills. Thus, issues of patient safety4,5 and cost-effectiveness involved in MIS training are fostering alternative methods of instruction. A considerable burden is now placed on program directors to ensure that educational objectives are met, and that trainees gain adequate clinical experience, even as they regard patients’ interests as paramount. As a consequence, the manner in which training is conducted has changed dramatically; the technical skills’ learning curve has moved outside the operating room, into the simulation laboratory/center.
The process of surgical training has also changed with the introduction of a national system of the Accreditation Council on Graduate Medical Education (ACGME) mandated residency training programs. The ACGME Outlook Project seeks to broadly improve medical training of all physicians. The 6 competencies of physicians recently identified by ACGME6 are patient care, medical knowledge, practice-based learning and improvement, interpersonal and communication skills, professionalism, and systems-based practice. The acquisition and maintenance of surgical technical skills are included in patient care and practice-based learning and improvement requirements, but the inclusion is by interpretation.
WHAT IS SCORE?
A new (2006) organization, the Surgical Council on Resident Education (SCORE)7, comprising 7 surgical associations, has added technical ability as a seventh competency. SCORE emerged from a concerted desire to strengthen the graduate education of surgeons and to assure the competence of surgical trainees in the US:
A technical skills curriculum under development by the ACS (American College of Surgeons) and APDS (Association of Program Directors in Surgery) for each level of general surgery training will also eventually be integrated into the new curriculum. SCORE has also agreed to support an operative performance rating system currently in development at Southern Illinois University, which will be incorporated into the new curriculum as an assessment tool.8
In addition, SCORE is examining issues related to surgical training, such as the assessment of technical competency, the role of simulation in surgical education [emphasis by the author], the teaching and assessment of professional behaviors, the practicing surgeon’s view of the adequacy of residency training, and faculty development. Members of SCORE are also investigating best practices in surgical education in other countries.7-9
The American Board of Surgery has added a new requirement of FLS (Fundamentals of Laparoscopic Surgery)10 certification for all surgical residents seeking board certification in general surgery, effective July 1, 2009, and applicable to those completing a general surgery residency in the 2009 to 2010 academic year or thereafter.
WHERE ARE WE NOW?
At the beginning of the 21st century, progress in computer technology offers an adjunct remedy for surgical training. Basic and intermediate-level MIS maneuvers can be learned and practiced by residents and instructors using computer-based virtual environments, and performances can be assessed objectively before trainees proceed to patients in the OR. Studies reported during the recent past with simulation-based instruction using first-generation, part-task trainers have demonstrated enhanced performance of trainees in either animal or human OR settings. In early studies11-13 with the Minimally Invasive Surgical Trainer-Virtual Reality (MIST-VR) (Mentice, Sweden) in which safety was considered, the number of subsequent, intraoperative surgical errors and time utilized were reduced among the trainees who had simulation experience on a part-task trainer. Many have contributed to this early development phase.11-19
DEFINITIONS OF TRAINERS AND SIMULATORS
Part-task Trainers
The term “part-task” trainer, adopted from its original use in the psychology field where psychomotor procedures were deconstructed into their component parts and identified as tasks,20 applies to many surgical simulators. Part-task trainers include 2 types of simulators: those that afford practicing basic skills, the psychomotor actions, and those that focus on more complex tasks of basic surgical procedures, such as suturing. An example of a “basic-skills” trainer is one that teaches the eye-hand coordination required for using surgical instruments. Navigating cameras, grasping tissue, picking and placing, and handing of objects between instruments can be learned and must be practiced for proficient surgical performance. These skills are also called “surgical gestures,”21 much as a mime “goes through planned postures.” In practice, basic skills represent the enabling skills necessary to be learned before more comprehensive tasks are conducted. Not all basic-skills trainers afford examples of more complex tasks, such as dissection or suturing, that involve more than one basic skill necessary for performing a surgical task or manipulation. Over time, several basic-skills trainers have evolved, adding modules that support practicing manipulations of increasing complexity. For them, the exercise of deconstructing tasks into their individual components is beneficial, because practicing these technically demanding components is critical in the performance of instrument-tissue manipulations.
An example of a part-task trainer supporting complex manipulations is one that focuses on one critical component of a procedure, such as a laparoscopic cholecystectomy. A critical component of that procedure is dissection of the triangle of Calot, the anatomical region where the cystic duct and cystic artery emerge from the gallbladder adjacent to each other in unpredictable anatomical configurations, and are at risk for inadvertent injury during dissection. Several manipulations are necessary for surgically exposing these structures; grasping and retracting, incising sharply with a scissors or with a selected energy modality (electrosurgery, ultrasound, or laser), pushing, pulling, or both pushing and pulling, to extend an initial incision and separating the fibrous tissues that encase them, elevation of the identified tubular structures to assure their separation/isolation, clipping or suturing to interrupt the lumina, and cutting to divide the structures. Inspection for hemostasis and irrigation of blood and possibly bile from the dissection site are additional necessary manipulations. The several actions required to accomplish these manipulations exceed those offered by basic-skills trainers.
The Fundamentals of Surgical Manipulations
The 8 fundamental manipulations of tissues by surgical instruments that surgeons must learn are those that have been taught since 500 BC, first described by Sushruta, the Father of Surgery.22,23 These are exploration [both visual and haptic (touch)], aspiration/injection, incision, excision, extraction, evacuation, scarification (purposeful injury), and closure (including suturing, clips, etc).24,25 We add implantation/transplantation, new manipulations developed during the 20th century, as the ninth. Vascular cannulation, another modern procedure, is considered an extension of the aspiration/injection manipulation.
These familiar manipulations, known as the Hidden Technical Curriculum of Surgery,26 must be mastered by junior trainees, yet they are rarely taught explicitly– rather trainees learn by observation or in simulators, but few have had methods for practicing them. Such manipulations when choreographed into a series of appropriate surgical actions required to accomplish the objective of a surgical prescription are integrated into a surgical procedure or operation. The vocabulary that is used to describe these common manipulations is very familiar to surgeons, but not to computer scientists and cognitive scientists who prize their elaboration as a guide during simulator development. Often, more than one method using different steps or approaches may be used to accomplish a similar surgical objective. Heinrichs26 has pointed out the similarity of surgery to figure skating. The professional skater studies, learns, and practices to perform the required steps and jumps, inserts them into a program, choreographs them to appropriate music, and practices again and again preparing for the actual, live performance, then dons appropriate garments, and steps onto the ice for the performance–the equivalent of a surgical operation. Practicing this routine repetitively is the essential ingredient for success in figure skating and offers a model for surgical professionals. Another similar, commonly rehearsed skill is playing musical instruments by professional musicians. Each discipline has its own vocabulary. The macro to micro progression of graphical components begins with surgical procedures done by different methods and moves deeper to the tissue/instrument manipulations, and finally collisions of instruments with polygons of the virtual organs/tissues.27,28 (Table 2)
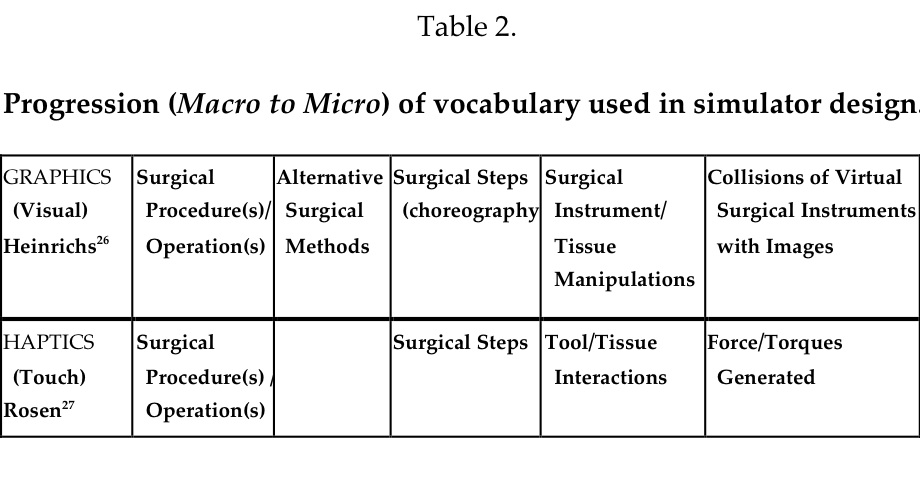
Procedure Trainers
Beyond part-task trainers, procedure simulators enable users/learners to practice the more comprehensive and higher level target skills that enable tissue-instrument interactions, such as multiple dissections, development of vascular pedicles, or electrosurgical, ultrasonic, or laser coagulation of pathological surfaces. A number of second-generation systems that may be considered procedure-simulators are now available as training systems. Most offer multiple elementary surgical tasks that comprise the many manipulations that comprise a procedure, and also include VR anatomic images on which to integrate the tasks. Two systems, the LapSim trainer (Surgical-Science AB, Goteburg, Sweden) and the LapMentor (Simbionix, Ltd, Lod, Israel) have labeled the above exercise as Dissection. The requisite set of surgical manipulations requires using target skills (see below), but this dissection component of the surgical procedure is but one part of a laparoscopic cholecystectomy. Exploration of the common bile duct may be needed as another component, and dissection of the gallbladder from the liver and removal of the excised structure from the abdomen are additional manipulations. Many simulators designed to support dissection of Calot’s triangle have been offered as LapChole simulators, but most are part-task trainers that are not comprehensive for all of the necessary actions/manipulations of a LapChole. For Ob/Gyn surgeons, the LapSim Trainer and the LapVR Trainer (Immersion Medical, Inc. San Jose, CA) both offer salpingectomy as an equivalent procedure. Other procedures of the female reproductive tract, including excision of an ectopic pregnancy, adhesiolysis, and salpingo-oophorectomy are available. Remarkably, efforts to simulate the introduction of trocars into surgical spaces, the chest and abdomen, have not been commercialized. This complex, coordinated action is another example of a laparoscopy partial task (Table 1).
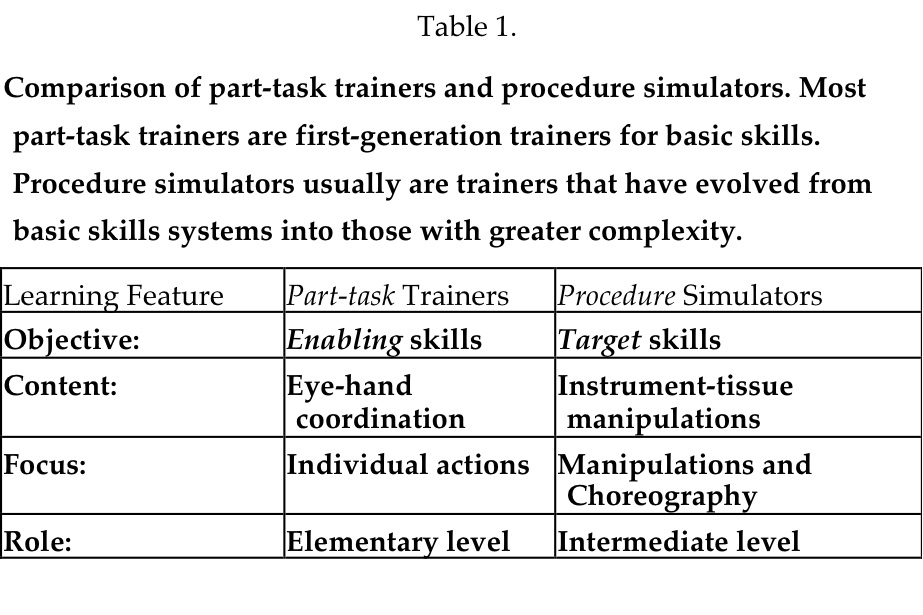
CLASSIFICATION AND DESCRIPTION OF SIMULATORS
An important feature that distinguishes some trainers and simulators from others is the type of construction with physical components such as manikins into which hardware is installed and surgical instruments are inserted. These are labeled physical reality systems in contrast to virtual reality (VR) systems that are software-based. Physical reality trainers (sometimes called mixed reality trainers) may be designed with video cameras or camcorders that provide a graphical image on a display, utilize electronic sensors that are activated upon touching with an instrument or a physical object, and are able to provide data and reports about performance. The earliest box trainers were simple Lucite or plastic shells into which laparoscopic instruments were inserted for manipulating physical objects, animal tissues, or even placenta. Some commercial physical reality trainers incorporate a computer that compiles performance data that can be displayed on a monitor screen.
A well-validated physical system, the Fundamentals of Laparoscopic Surgery (FLS) trainer developed by Gerald Fried, MD, and colleagues at the University of McGill11 has become a widely adopted system because of its low cost, reliability, and simplicity. The Society of Gastro-intestinal Endoscopic Surgeons (SAGES, Los Angeles, CA) has promoted its use,15 establishing dozens of test centers in the US and Canada, and the American College of Surgeons (ACS) has adopted it as an obligatory trainer for surgical residents. An outstanding, multi-authored, comprehensive set of presentations, including illustrations, of its application organized by Nathaniel Soper, MD, is available.29,30 Another widely used box trainer, the SurgicalSim Laparoscopy Training Simulator31,32 (LTS– METI, Inc., Sarasota, FL) developed by Harry Hasson, MD, offers the advantage of incorporating the McGill scoring system, and an electronic interface for record keeping. Still another device, the ProMIS Trainer33 (Haptica, Inc., Dublin, IR), described as a hybrid trainer, offers either physical or virtual objects to be manipulated, and a scoring system. It alone has a module for colorectal surgical simulation. The SkillSetPro Trainer34 (Verefi Technologies, Inc., Elizabethtown, PA) offers virtual nonanatomic objects in a physical trainer with an electronic display.
Simulator systems are not static; many continue to be developed as commercial products with new features and additional modules. Also, many systems have been developed as research projects in academic institutions and have not emerged as products. The process of design to commercialization is difficult to track, particularly for those systems being developed outside the US. Table 3 lists most of the trainer or simulator systems commercially available for each category of procedures, but the list is not exhaustive. It is restricted to vascular access and catheterization and endoscopic and laparoscopic surgery excluding other simulators for thoracic surgery, ophthalmology, otolaryngology, orthopedics, and neurosurgery. A valuable annotated comprehensive listing of available medical/surgical simulation devices is maintained by Penn State’s Simulation Center.35
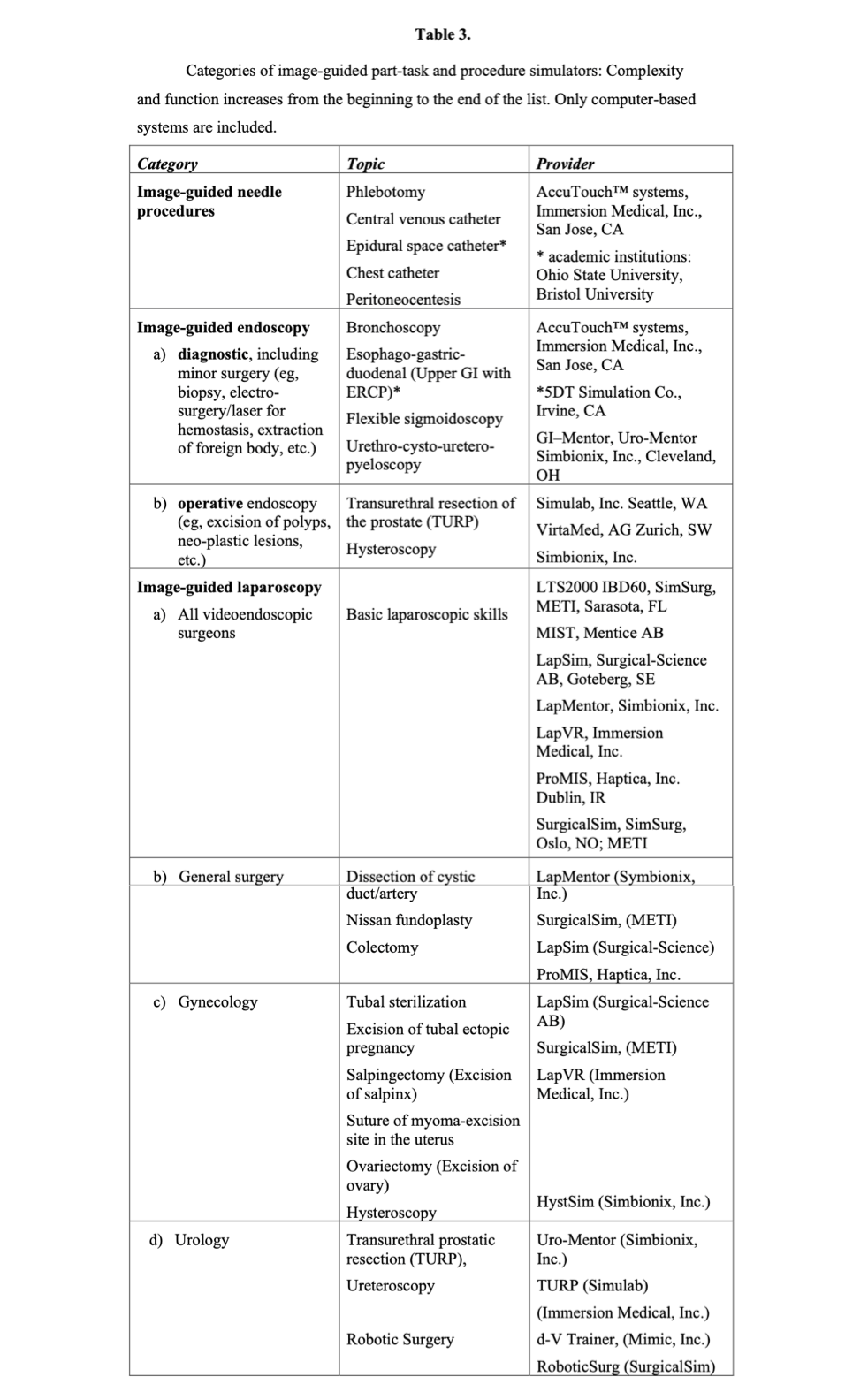
VALIDATION OF SIMULATOR SYSTEMS
Validation of surgical simulators refers to the process of evaluating these systems to determine their quality and value as training and/or assessment tools.30 Much has been accomplished during the past 5 years on validating various systems. Initial evaluation begins when the system is under development and includes beta-testing to check that the system performs according to the design specifications and usability testing to ensure the trainees find it easy to use. In addition, content experts, in this case experienced surgeons, must review the system to check the accuracy and realism of the simulated tasks and procedures, as well as the usefulness of the internal metrics or scoring mechanisms, if these features are included.
Residency training program directors and others who are interested in adopting these technologies will want to know how the simulator has performed in formal validation studies with trainees, such as studies of construct validity, teaching effectiveness, skills transfer, and curriculum integration, or field studies. In Table 4, these types of evaluation studies are defined by the evaluation question they seek to answer. The last column in the table identifies the person(s) whose simulator performance and/or opinion is sought.
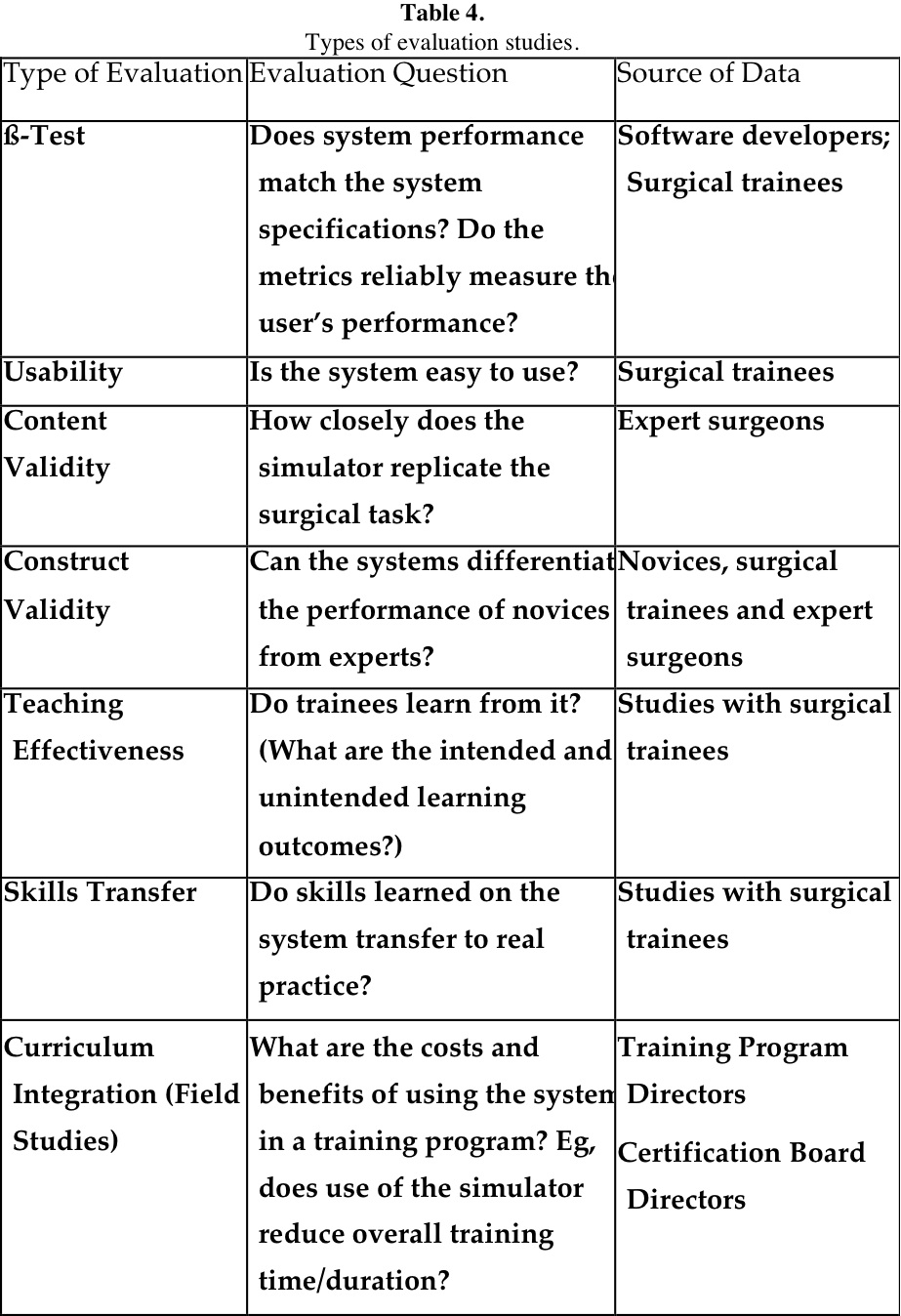
The first 2 types of evaluation presented in Table 4 are usually conducted when systems are under development; content validity and curriculum integration are based on the judgment of experienced professionals, and those remaining are based on educational research using a true experimental design. Meaningful studies of this type usually incorporate a control group that receives either no training, or preferably standard training either in the OR, or on a comparable simulator system. Another investigative approach is using an interrupted time series design in which the subjects serve as their own control. It is also important to control for the amount of training received by each group and to ensure that observers/raters are trained for consistent scoring and blinded for which group received the training.36 Some reports37-39 emphasize that variability in performance may stem from different visual perception capabilities.
Conducting validation studies of these new technologies is imperative, particularly in medicine and surgery where established methods developed over decades effectively protect the interest of patients. Introduction of simulation technologies must enhance, not jeopardize, this commitment to patient care and safety.
HOW MUCH TRAINING MAKES A DIFFERENCE?
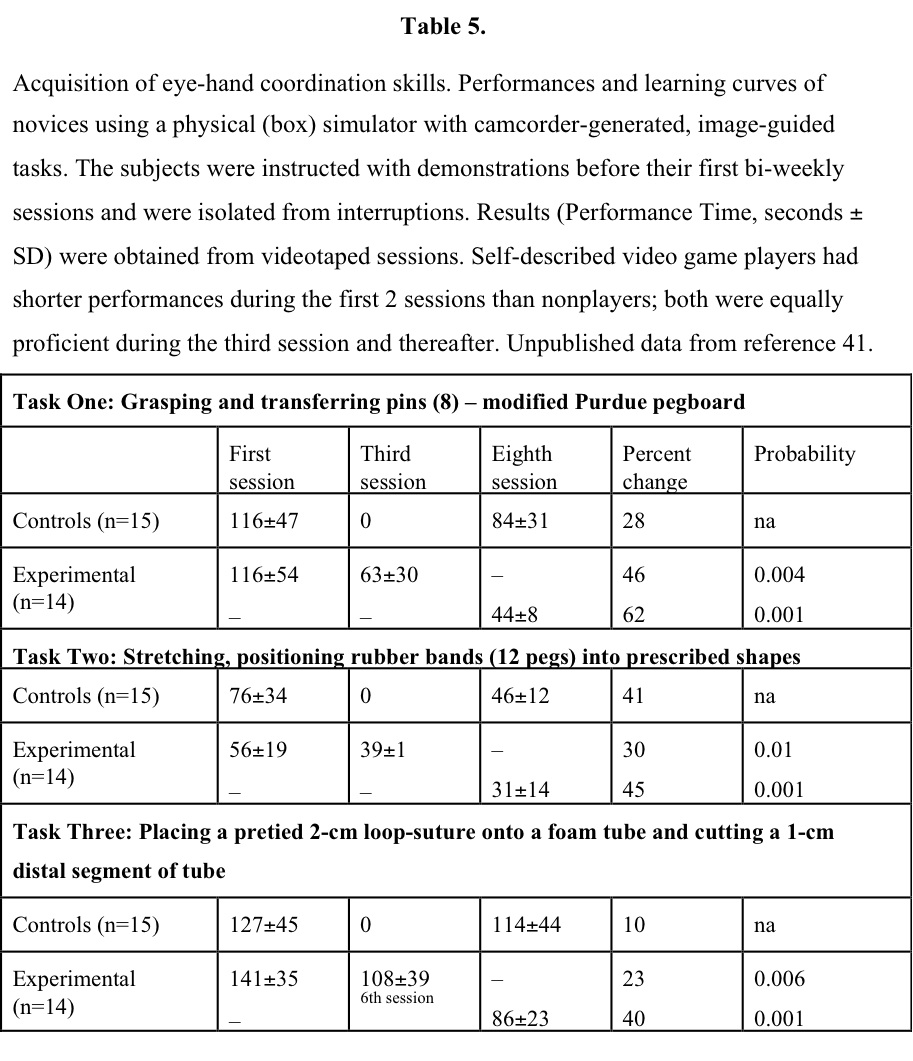
Several studies using physical-reality systems or simple tasks, such as grasping and placing a ball into a cylinder, using 3 or fewer repetitive practice experiences one day or a few days apart, were ineffective in demonstrating an effect of training. Therefore, 3 or fewer experiences can be thought of as familiarization with the system, rather than producing a change in skill level, but this hypothesis needs further study. Early (1994) data41 in a trial comparing no training with twice-weekly practice sessions for 2 weeks with either a latex box trainer or a VR trainer demonstrated significant differences produced in VR trainees (Table 5). Data obtained with a latex physical simulator42 indicate that an average of at least 9 practice sessions over a 2-week period are necessary before performing laparoscopic cholecystectomies (latex models) when proficiency is reached. A benchmark study43 among practicing laparoscopic surgeons clearly indicates that repetitive practice on simulators for at least 3, and preferably 4, sessions closely linked in time was necessary before their performance skills were evident. These data obtained for 6 widely distributed simulation systems having the electronic capability of collecting performance results are interpreted to mean that studies without a rigorous familiarization phase for subjects are unlikely to yield reliable results about the performance of surgeon subjects. This experience could be considered an OR-to-VR demonstration, indicating the training required by skilled laparoscopic surgeons for efficient use of surgical simulators.
Transfer of skills to real-life situations has been analyzed in several studies in which training was assessed by performances in the operating room, either with patients or with animals. The obvious difficulty with scheduling patients with comparable pathologies, accessibility, and other variables, for conducting VR-to-OR transferability and having resident physicians with equivalent, prior surgical experiences has prolonged such studies with patients. Animal surgery has the limitations of species-specific anatomy, usually no pathology, and lack of the urgency and responsibility of human ORs. However, with forethought, relevant performance objectives can be constructed in animals, such as “pick-and-placing” beans as gallstones, “grasping-and transferring” colored stitches placed on the antimesenteric portion of bowel to mimic “running-the-bowel,” and exposing mesenteric vessels for “grasping-clipping-cutting” exercises.44 In vivo simulations of exercises are often instantiated into box trainers with inanimate objects, such as string, beans, cloth, and latex tubing, but real equivalency is very difficult to achieve between these static environments. For example, a string target placed on the floor of a box trainer remains stationary until it is moved, but the bowel’s peristaltic action presents a more challenging moving target. Thus, mobility of targets may be a major advantage of VR systems. Validation studies, like clinical trials of any type, benefit from being conducted in several centers simultaneously. Caution is warranted for qualifying novice trainees who are recruited by their prior experience with video games,43-45 visual perception ability,46-48 and prior surgical “exposure.” In summary, one can say from these data that 4 practice sessions of 45 minutes to 60 minutes duration at 3-day to 4-day intervals make a significant difference in performance of simple part-tasks, and that after 8 to 10 sessions of the same duration and interval, the learning curve becomes nearly flat, indicating that the simple task has been mastered.
In 2007, Heinrichs et al43 conducted a study among experienced laparoscopic surgeons (general, gynecological, and urological) who practiced on 6 widely available, commercial part-task trainers long enough to establish performance levels that can serve as “training criteria” to guide simulator trainees and their mentors. These data from expert surgeons that define “criterion-based training” agree with the prior observation that repetitive practice on simulators is needed for obtaining “best-practice” use. We look forward to performance data from experienced surgeons with learning curves established on part-task trainers, as they practice on procedure simulators. Such observations are needed to guide program directors in criteria- or proficiency-based training.
VR-TO-OR STUDIES
VR-to-OR studies are important for assessing the value of simulators in surgical education. One step short of obtaining the most desirable confirmation of patient outcomes data, VR-to-OR studies demonstrate whether the immediate, intraoperative performance by simulation-trained surgeons is facilitated during OR practices. These have been conducted in both human and animal ORs.
As early as 2001, surgical simulation-based training on the MIST was shown by Grantcharov12 to significantly improve performances in the porcine model. In 2002, two such studies13,14 were reported based on either the MIST or the LapSim simulators; one by Seymour et al among 12 surgery residents conducting cholecystectomies in humans, and the other by Hyltander et al, among 24 medical students performing surgical tasks in a porcine model. In both instances, completion of objectives judged by experienced observers indicated improved performance, and reduced time consumption. In a comparison study (2005) by Youngblood et al44, 48 medical students trained on either a Tower Trainer or the LapSim simulator demonstrated that naive medical student subjects trained on the VR part-task trainer performed better on live surgical tasks in a porcine model compared with those trained with a traditional box trainer. This study highlights the complementary use of different simulators, one a low-tech, low-cost device, and the other a high-tech, more expensive device, within a structured curriculum.
In a 2007, both Cosman46 et al and Ahlberg47 et al demonstrated that the beneficial effect–fewer errors and reduced time–of simulator training extended into the OR, the latter group evaluating a trainee’s first 10 cholecystectomies. In 2009, the Grantcharov team published 2 studies about VR-to-OR transfer, the first with cholecystectomies,48 and the second with salpingectomies49; both used the LapSim (Surgical Science, Goteburg, Sweden). The latter study included a trained group of Ob/Gyn resident physicians (n=11) with 7 hours of simulator practice, who when conducting a laparoscopic salpingectomy reached a median total score of 33 points (interquartile range, 32 to 36), equivalent to the experience gained after 20 to 50 laparoscopic procedures. A control group with standard training (n=10) reached a median total score of 23 points (range, 22 to 27), equivalent to the experience gained from fewer than 5 procedures (P<0.001). The median total operation time in the simulator-trained group was 12 minutes (interquartile range, 10 to 14) and in the control group was 24 minutes (range, 20 to 29) (P<0.001). Observers’ inter-rater agreement was 0.79. The investigators concluded that skills in laparoscopic surgery could be increased in a clinically relevant manner by using proficiency based virtual reality simulator training. The performance level of novices was increased to that of intermediately experienced laparoscopists, and operation time was halved.
This important although small study, similar in design to the 2002 study of Seymour et al13 among general surgeons conducting a laparoscopic cholecystectomy after training on a box simulator (MIST), achieved similar skills transfer to the operating room. These VR-to-OR studies support the claims that as in the aviation industry, practice on simulators is effective in reducing errors related to passenger or patient risk and saving time, both factors in supporting a culture of patient safety. Seymour has recently reviewed VR to OR studies,50 and Mettler and Dewan51 have reviewed the case for VR simulators in gynecology. A review of the trainers available for transurethral resection of the prostate has been published by Sweet.52
PRESENT STATUS OF VR SIMULATION
The present status of VR simulation has recently been subjected to a Cochrane Database Systematic Review.53 The findings are described below:
To determine whether virtual reality training can supplement or replace conventional laparoscopic surgical training (apprenticeship) in surgical trainees with limited or no prior laparoscopic experience, the team in the Department of Surgery at the Royal Free Hospital in the UK examined 23 trials with 612 participants reported in the literature. Four trials compared virtual reality versus video trainer training. Twelve trials compared virtual reality versus no training or standard laparoscopic training. Four trials compared virtual reality, video trainer training, and no training, or standard laparoscopic training. Three trials compared different methods of virtual reality training. Most of the trials were of high risk of bias. In trainees without prior surgical experience, virtual reality training decreased the time taken to complete a task, increased accuracy, and decreased errors compared with no training; the virtual reality group was more accurate than the video trainer training group was. In the participants with limited laparoscopic experience, virtual reality training reduces operating time and error better than standard training in the laparoscopic training group; composite operative performance score was better in the virtual reality group than in the video trainer group. The authors conclude that, “virtual reality training can supplement standard laparoscopic surgical training of apprenticeship and is at least as effective as video trainer training in supplementing standard laparoscopic training. Further research of better methodological quality and more patient-relevant outcomes are needed.”53
In efforts to establish criterion-based skills training for surgeons,43 we assessed the performance of 17 experienced laparoscopic surgeons on basic technical surgical skills recorded electronically in 26 modules selected in 6 commercially available, computer-based simulators. Performance data were derived from selected surgeons randomly assigned to simulator stations, and practicing repetitively during one and one-half day sessions. We measured surgeon proficiency defined as efficient, error-free performance and developed proficiency score formulas for each module. Demographic and opinion data were also collected. Surgeons’ performance demonstrated a sharp learning curve with the most performance improvement seen in early practice attempts. Median scores and performance levels at the 10th, 25th, 75th, and 90th percentiles are provided for each module. Construct validity was examined for 2 modules by comparing experienced surgeons’ performance with that of a convenience sample of less-experienced surgeons. A simple mathematical method for scoring performance is applicable to these simulators. Proficiency levels for training courses can now be specified objectively by residency directors and by professional organizations for different levels of training or posttraining assessment of technical performance. But data users should be cautious due to the small sample size in this study and the need for further study into the reliability and validity of the use of surgical simulators as assessment tools.
Inspection of the data from these experienced surgeons indicates that the process of “familiarization” with the simulators requires at least 4 practice sessions before surgeons’ proficiency becomes evident. Studies that omit this familiarization step are likely to present flawed data and conclusions. Proficiency based training effectively differentiates experienced and novice surgical trainees.43
SIMULATION SYSTEMS OF THE FUTURE
Surgical simulators have a checkered past with many efforts attempted, but only a few survive. One cannot predict which systems will survive the slowly emerging demand, and prevail into the future. The following systems are innovative and offer new features that may make them competitive enough to survive in the marketplace.
Newly Available Simulators
The LapVR system (Immersion Medical, Inc., San Jose, CA), available during the past 2 years, is an essential skills trainer, plus offering tools and anatomy for cholecystectomies, and the following gynecologic procedures: excision of an ectopic pregnancy, tubal occlusion, and salpingo-oophorectomy.54 Validation data have not been identified with PubMed or Google searches.
A VR robotic simulator, dV-Trainer, for the da Vinci Surgical System in development by Mimic Technologies, Inc. (Seattle, WA) during this decade is undergoing beta validation studies with plans for release in 2010.55 This device has been evaluated by several groups.56-59 SurgicalSim (Oslo, Norway) also offers a robotic surgery simulator recently studied by Lin et al.60
A new hysteroscopic surgical simulator developed and released by VirtaMed AG (Zurich, Switzerland) has been licensed for use by Conceptus, Inc. (Mountain View, CA) to demonstrate insertion of the company’s spring device for transcervical sterilization. It is distributed by Simbionix, Ltd (Lod, Israel), and has been evaluated by Bajka et al.61
Summary Opinion
In a discussion that took place during an October 2009 Mini-Seminar “Standing on the Shoulders of Dummies: Simulation Education 2009,” 5 US speakers in a San Diego, California workshop examined simulation from many different angles:
“The Big Picture” was presented by Prof. Lord Ara Darzi, HonFREng, FMedSc, the Hamlyn Chair of Surgery at Imperial College, London, and Past (2009) Parliamentary Under-Secretary of State (Lords) at the Department of Health (NHS). Prof. Darzi reviewed the recent history of surgical simulation:
“The debate started in the early 1990s to teach old dogs new tricks (about laparoscopic surgery),” he said, adding that in some circles simulation was not viewed seriously until a framework for the assessment of technical skills was developed. For example, a mathematical formula, the “Transfer-effectiveness Ratio for Virtual Reality Simulation,” which was developed by the airline industry to measure the effect of its simulation training, was applied to simulation for teaching laparoscopic surgery.
“The data showed evidence that the simulation tools were no longer pretty, expensive toys,” Prof. Darzi said. “That is why the American College of Surgeons and other institutions take this seriously. Eventually, simulation advanced into patient-specific simulation, using scans of a patient to ‘practice on the patient before doing the procedure,’” he said. Other advances include eye tracking so teachers can see what a trainee looks at during a simulation, versus where an expert surgeon looks. This allows teachers to learn how an individual acquires skills.
Surgical simulation is an established technique for learning and assessment of the technical skills of surgery. The field is in its infancy and is being embraced by a few of the key players responsible for the quality of surgical training. But other professional societies and certifying boards have yet to implement programs of modern training that benefit from simulations’ affordances. Because the abundant studies of simulation-augmented training in videoendocsopic surgery to date are so repetitive and compelling, the next few years will surely see expansion into most, if not all, surgical specialties. This technique is an important tool for reducing healthcare costs and promoting a culture of patient safety across the globe.
References
- Martin JA, Regehr G, Reznick RK, et al. Objective structured assessment of technical skill (OSATS) for surgical residents. Br J Surg. 1997;84(2):273-278.
- Wanzel KR, Ward M, Reznick RK. Teaching the surgical craft: from selection to certification. Curr Probl Surg. 2002;39:573-659.
- Goff BA, Lentz GM, Lee D, Fenner D, Morris J, Mandel LS. Development of bench stations for objective, structured assessment of technical skills. Obstet Gynecol. 2001;98(3) 412-416.
- Bridges M, Diamond DL. The financial impact of teaching surgical residents in the operating room. Am J Surg. 1999; 177(1):28-32.
- Sachdeva AK. Acquisition and maintenance of surgical competence. Semin Vasc Surg. 2002;15:182-190.
- ACGME Outcome General Competencies Standards are available at http://www.acgme.org/outcome/comp/GeneralCompetenciesStandards21307.pdf. Accessed Nov 2009.
- Bell RH. Surgical Council on Resident Education: a new organization devoted to graduate surgical education. J Am Coll Surg. 2007 Mar;204(3):341-6 http://www.surgicalcore.org/about.html Accessed Nov 2009.
- Scott DJ, Dunnington G. The new ACS/APDS skills curriculum: moving the learning curve out of the operating room. J Gastrointest Surg. 2008;12:213-221.
- Britt LD. Acute care surgery: the curriculum should address the needs. J Surg Educ. 2007;64(5):300-301.
- Sachdeva AK, Philibert I, Leach DC, et al. Patient safety curriculum for surgical residency programs: results of a national consensus conference. Surgery. 2007 Apr;141(4):427-441.
- Fried GM, DeRossis AM, Bothwell J, Sigman HH. Comparison of laparoscopic performance in vivo with performance in a laparoscopic simulator. Surg Endosc. 1999;13:1077-1081.
- Grantcharov TP, Rosenberg J, Pahle P, Funch-Jensen P. Virtual reality computer simulation. Surg Endosc. 2001;15(3):242-244.
- Seymour NE, Gallagher AG, Roman SA, et al. Virtual reality performance improves operating room performance: Results of a randomized, double-blinded trial. Ann Surg. 2002;236:458-464.
- Scott DJ, Bergen PC, Rege RV, et al. Laparoscopic training on bench models: better and more cost effective than operating room experience? J Am Coll Surg. 2000;191(3):272-283.
- Hamilton EC, Scott DJ, Fleming JB, et al. Comparison of video trainer and virtual reality training systems on acquisition of laparoscopic skills. Surg Endosc. 2002;16(3):406-411.
- Hyltander A, Liljegren E, Rhodin PH, Lonroth H. The transfer of basic skills learned in a laparoscopic simulator to the operating room. Surg Endosc. 2002;16:1324-1329.
- Hasson HM, Kumari NA, Eekhout J. Training simulator for developing laparoscopic skills. JSLS. 2001;5:255-265.
- Datta VK, Mandalia M, Mackay SD, Darzi AW. The PreOp flexible sigmoidoscopy trainer. Validation and early evaluation of a virtual reality based system. Surg Endosc. 2002;16(10):1459-1463.
- Montgomery K, Heinrichs WL, Bruyns C, Wildermuth S, Hasser C, Bailey OS. Surgical simulator for diagnostic and operative hysteroscopy. In: Lemke H, ed. Proceedings of the 15th Annual International Computer-assisted Radiology & Surgery (CARS) Conference. Amsterdam, The Netherlands: Elsevier Science Press; 2001:79-84.
- Fitts PM, Posner MI. Human Performance. Monterey, CA: Brooks/Cole Publ;1967.
- Dubois P, Thommen Q, Jambon AC. In vivo measurement of surgical gestures. IEEE Trans Biomed Eng. 2002 Jan;49(1):49-54.
- Garrison FE. An Introduction to the History of Medicine. 4th ed. Philadelphia, PA: WB Saunders Co.; 1929:70-73.
- Zimmerman LO, Veith I. Great Ideas in the History of Surgery. Baltimore, MD: Williams & Wilkins Co.; 1961:56-67.
- Heinrichs WL, Srivastava S, Pugh C, et al. Visual language to interface users of educational surgical simulators for fundamental surgical manipulations. In: Proceedings of 14th Annual International Meeting of Computer-assisted Radiology & Surgery (CARS). Lemke H-J, ed. San Francisco, Amsterdam: Elsevier Publishers; 2000;29-34.
- Satava RM, Cuschieri A, Hamdorf J. Metrics for objective assessment of surgical skills workshop. Surg Endosc. 2003;17(2):220-226.
- Heinrichs WL, Srivastava S, Montgomery K, Dev P. The fundamental manipulations of surgery: A structured vocabulary for designing surgical curricula and simulators. Special Article; J Am Assoc Gynecol Laparosc. 2004;11(4):450-456.
- Rosen J, MacFarlane M, Richards C, Hannaford B, Sinanan M. Surgeon-tool force/torque signatures-Evaluation of surgical skills in minimally invasive surgery. In: Westwood JD, et al, eds. Medicine Meets Virtual Reality. The Netherlands: IOS Press 1999;9:290-206.
- Kowalewski TM, Rosen J, Chang L, Sininan M, Hannaford B. Optimization of a vector quantization codebook for objective evaluation of surgical skill. In: JD Westwood, et al, eds. Medicine Meets Virtual Reality. The Netherlands: IOS Press. 2004;12:174-179.
- Soper N, et al. Fundamentals of Laparoscopic Surgery Hands-On Course www.sages.org/meetings/annual_meeting/2008/syllabi/2008_FLS_Course.pdf (http://www.flsprogram.org/ ). Accessed Nov 2009.
- Soper N, Fried GM. The fundamentals of laparoscopic surgery: its time has come. Bulletin of the American College of Surgeons. 2008;93(9):30-32.
- Hasson HM. Simulation training in laparoscopy using a computerized physical reality simulator. JSLS. 2008 Oct-Dec;12(4):363-367.
- http://www.meti.com/products_ss_lts.htm Accessed Nov 2009.
- http://www.haptica.com/index.htm Accessed Nov 2009.
- http://www.verefi.com/ssp.php Accessed Nov 2009.
- http://www.pennstatehershey.org/web/simlab/home/available Accessed Nov 2009.
- Gagne RM, Briggs LJ. Principles of Instructional Design. 2nd ed. NY, NY: Holt, Rinehart, Winston, Publishers;1979:90,97.
- Popham WJ. Data-gathering designs. In: Educational Evaluation. 2nd ed. NJ: Prentice Hall;1988:174.
- Eyal R, Tendick F. Spatial ability and learning the use of an angled laparoscope in a virtual environment. Stud Health Technol Inform. 2001;81:146-152.
- Waller D. Individual differences in spatial learning from computer-simulated environments. J Exp Psychol Appl. 2000;6(4):307-321.
- Keehner M, Cohen C, Hegerty M, Montello DR. Cognitive factors and interactivity: Implications for the design and implementation of 3D computer visualizations for medical education. Presented at: 12th Annual Meeting of Medicine Meets Virtual Reality; January 15-18, 2004;17 [abstract]; Newport Beach, CA.
- Tsai C, Heinrichs WL Acquisition of eye-hand coordination skills for videoendoscopic surgery. J Am Assoc Gynecol Laparosc. 1994;1(4 Part2): S37.
- Pohl D, Eubanks TR, Kao CC, et al. Synthetic material simulation improves performance of laparoscopic cholecystectomy in an animate model. Available at: http://www.simulab.com/clinical-studies. Accessed Nov 2009.
- Heinrichs WL, Lukoff B, Youngblood P, Dev P, Shavelson R. Criterion-based training with surgical simulators: proficiency of experienced surgeons. JSLS. 2007;11:273-302.
- Youngblood PL, Srivastava S, Curet M, Heinrichs WL, Dev P, Wren SM. Comparison of training on two laparoscopic simulators and assessment of skills transfer to surgical performance. J Am Coll Surg. 2005 Apr;200(4): 546-551.
- Rosser JC, Lynch PJ, Cuddihy L, Gentile DA, Klonsky J, Merrell R. The impact of video games on training surgeons in the 21st century. Arch Surg. 2007;142:181-186.
- Cosman PH, Hugh TJ, Shearer CJ, Merrett ND, Biankin AV, Cartmill JA. Skills acquired on virtual reality laparoscopic simulators transfer into the operating room in a blinded, randomised, controlled trial. Stud Health Technol Inform. 2007;125:76-81.
- Ahlberg G, Enochsson L, Gallagher AG, et al. Proficiency-based virtual reality training significantly reduces the error rate for residents during their first 10 laparoscopic cholecystectomies. Am J Surg. 2007 Jun;193(6):797-804.
- Kundhal PS, Grantcharov TP. Psychomotor performance measured in a virtual environment correlates with technical skills in the operating room. Surg Endosc. 2009 Mar;23(3):645-9. Epub 2008 Jul 12.
- Larsen CR, Soerensen JL, Grantcharov TP, et al. Effect of virtual reality training on laparoscopic surgery: randomised controlled trial. BMJ. 2009 May;14:338.
- Seymour NE. VR to OR: a review of the evidence that virtual reality simulation improves operating room performance. World J Surg. 2008 Feb;32(2):182-188.
- Mettler LL, Dewan P. Virtual reality simulators in gynecological endoscopy: a surging new wave. JSLS. 2009;13:279-286.
- Sweet RM. Review of trainers for transurethral resection of the prostate skills. J Endourol. 2007 Mar;21(3):280-284.
- Gurusamy KS, Aggarwal R, Palanivelu L, Davidson BR. Virtual reality training for surgical trainees in laparoscopic surgery. Br J Surg. 2008 Sep; 95(9):1088-97; Cochrane Database Syst Rev. 2009 Jan 21;(1):CD006575.
- http://www.immersion.com/markets/medical/products/laparoscopy/index.html Accessed Nov 2009
- http://www.mimic.ws Accessed Nov 2009
- Wignall G, Denstedt J, Preminger G, et al. Surgical simulation: a urological perspective. J Urol. 2009;179(5):1690-1699.
- Lendvay TS, Casale P, Sweet R, Peters C. VR robotic surgery: randomized blinded study of the dV-Trainer robotic simulator. Stud Health Technol Inform. 2008;132:242-244.
- Kenney P, Wszolek M, Gould J, Libertino J, Moinzadeh A. Face, content, and construct validity of dV-Trainer, a novel virtual reality simulator for robotic surgery. Urology. 2009;73(6):1288-1292.
- Sethi A, Peine W, Mohammadi Y, Sundaram C. Validation of a novel virtual reality robotic simulator. J Endourol. 2009;23(3):503-508.
- Lin DW, Romanelli JR, Kuhn JN, Thompson RE, Bush RW, Seymour NE. Computer-based laparoscopic and robotic surgical simulators: performance characteristics and perceptions of new users. Surg Endosc. 2009;3(1):209-214.
- Bajka M, Tuchschmid S, Streich M, Fink D, Székely G, Harders M. Evaluation of a new virtual-reality training simulator for hysteroscopy. Surgical Endoscopy. 2009 Sep;23(9):2026-33
- http://news.bbc.co.uk/2/hi/health/8050633.stm Accessed Nov 2009.
Address correspondence to: Wm. LeRoy Heinrichs, MD, PhD, Innovation in Learning, Inc., CA 94022, USA. Telephone: (650) 854-7410, E-mail: wlh@stanford.edu
Acknowledgments: Patricia Youngblood, PhD, kindly assisted with the section on evaluation and assessment. Other colleagues at SUMMIT, Sakti Srivastava, MBBS, and Parvati Dev, PhD, have assisted with our simulation-based training of students, residents, and fellows. The collaboration of Camran Nezhat, MD, and fellows in Ob/Gyn has sustained our effort.
Sources of Support: Sponsored by Innovation in Learning, Inc.
