Ovarian Remnant
Ceana Nezhat, MD, Michael Lewis, MD
Ovarian remnants are the result of ovarian tissue that is inadvertently left in the pelvic cavity after oophorectomy. Responding to hormonal stimulation, the remaining piece of functional ovarian tissue can have growth, cystic degeneration, or hemorrhage and produce pain.1-3 In the rat model, Minke and colleagues showed that devascularized ovarian tissue can reimplant on intact or denuded peritoneal surfaces and the revascularized tissue can become functional as evidenced by follicle formation and vaginal cornification.4 Predisposing risk factors include increased vascularity causing hemostasis, endometriosis, pelvic inflammatory disease/ tubo-ovarian abscess, pelvic adhesions, and altered anatomy.5-6
As many patients have had prior surgeries or suffered from preexisting conditions, it is not surprising to discover the ovarian remnants encased in adhesions (Figure 1). For example, a patient with a previous tubo-ovarian abscess is at high risk of having an ovarian remnant after an attempted oophorectomy. Moreover, it is not unusual for these patients to have had previous attempts to excise the tissue.
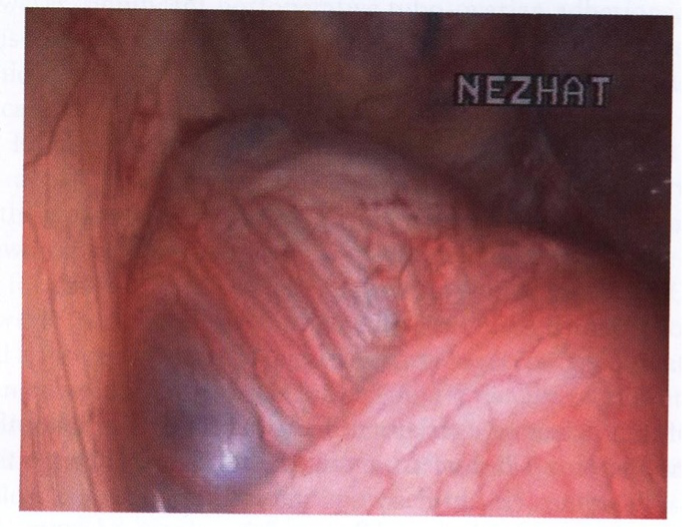 Figure 1. Ovarian remanent encased with adhesions.
Figure 1. Ovarian remanent encased with adhesions.
DIAGNOSIS
Diagnosis is most often made from the history and localization of pelvic pain. Symptoms of ovarian remnant usually occur within 5 years of oophorectomy.5,7 The most frequent symptom is chronic pelvic pain, being described as both cyclic and acyclical. In addition, patients characterize the pain as dull and aching to sharp and stabbing.5 They may also experience low back pain, variable bowel symptoms, dyspareunia, pelvic mass, or ureteral compression.7 Some patients have cystic adnexal structures or ill-defined fixed masses, whereas others have normal pelvic findings. Imaging studies, including vaginal ultrasound, CT, and MRI, are useful but not always indicative of ovarian remnant.8 Preoperative follicle-stimulating hormone (FSH) levels can contribute to the diagnosis when found in the premenopausal range (<40) in patients who have undergone bilateral oophorectomy. However, the remaining ovarian tissue can produce estradiol levels incapable of suppressing gonadotropin; therefore, an FSH level of 40 mIU/ml or greater does not exclude the diagnosis.1,9 In cases of unilateral oophorectomy, FSH levels are of no value. Hormonal suppression with oral contraceptives or GnRH agonists provides no relief in most patients.2,10 Ovarian stimulation may be used to increase ovarian remnant size to confirm the diagnosis preoperatively or to aid in locating the tissue intraoperatively.11 Laparoscopic ultrasonography may be helpful in detecting ovarian remnants in patients in whom the pelvic anatomy is distorted by multiple adhesions.12
TREATMENT
As definitive treatment, surgical excision of the ovarian remnant is the best choice in the majority of cases. Older literature considered laparoscopy ineffective in the management of ovarian remnant because of the presence of dense adhesions.1,5,13,14 Since the publication of those reviews, the advancement in the field of operative laparoscopy, including improved instrumentation, robotics and training has overcome previous obstacles. Laparoscopy offers additional benefits over laparotomy for treating ovarian remnants. The magnification provided by the laparoscope facilitates identification of the remnant tissue. Increased intra-abdominal pressure helps reduce blood loss, is less traumatic, and the distention causes the retroperitoneal space to unfold, thus allowing for better visualization.10,15 Also in the literature, reports have shown that the number of recurrences of ovarian remnant and the number of conversion to laparotomy, along with postoperative complications, decreased as the surgeon’s experience and technical advancements increased.8
Because most of these patients are at higher risk for both intra and postoperative complications, all patients undergo a thorough 1- or 2-day bowel prep (both mechanical and antibiotic). Before signing the consent form, the patient should be thoroughly counseled regarding the possibility of proctosigmoidoscopy, cystoscopy, or resection of portions of the bowel, bladder, or ureters, as well as the possibility of conversion to laparotomy, if indicated. With ovarian remnant, the patient must understand that the goal is complete resection of the ovarian tissue to prevent recurrence.
Anterior abdominal wall adhesions are common with prior surgeries, and an open laparoscopy, left upper quadrant entry, or mapping technique is advisable.16 After all the instruments are inserted, intra-abdominal adhesions are lysed and normal anatomy is restored as much as possible. The remnant tissue is identified and dissected. Extensive and careful retroperitoneal dissection is required to facilitate identification and removal of the ovarian remnant tissue.6,15 Chao recently described a case report of ovarian remnant at a previous port site.17 Careful attention needs to be given when removing specimens through an endoscopic bag or trocar sleeve. Just as it is possible to leave an endometrial implant in an incision, it is possible to remove a part but not all of it though a port. As described in later chapters, there are many means to carefully extract specimens intact and complete from the abdominal cavity.
The anatomy of the retroperitoneal space is identified when the ovarian remnant is adherent to the lateral pelvic wall (Figure 2). The technique of hydrodissection is applied to open the retroperitoneal space and identify both the infundibulopelvic ligament and its remnant. Adjustable pressure pumps for hydrodissection can be used to establish various pressures to assist in injecting physiologic solutions, such as lactated Ringer’s, retroperitoneally to create a plane of dissection. Even in cases with retroperitoneal fibrosis, the combination of hydrodissection and sharp dissection with CO2 laser is beneficial.8 Adhesions are lysed until the course of the major pelvic blood vessels and the ureter can be traced and, if necessary, dissected. The ovarian blood supply is cauterized, and the ovarian tissue is excised and submitted for histologic examination. In cases where the ovarian remnant is adhered to the vaginal cuff and bladder area (Figure 3 A and B), extensive dissection and ureterolysis may not be necessary; however, identification of the anatomy and a plane of dissection is essential.
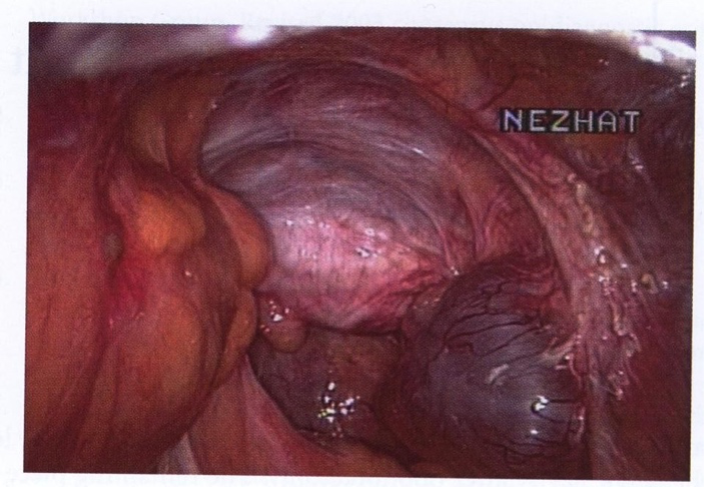 Figure 2. Ovarian remanent on the lateral pelvic side wall.
Figure 2. Ovarian remanent on the lateral pelvic side wall.
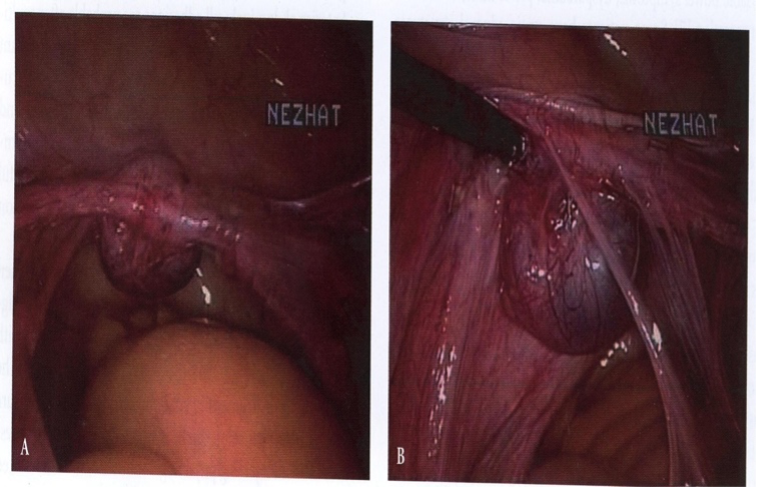 Figure 3 A,B. Ovarian remnant adherent to the vaginal cuff(A) and bladder(B).
Figure 3 A,B. Ovarian remnant adherent to the vaginal cuff(A) and bladder(B).
Unfortunately, the remnant may also be adherent to the bowel (Figures 4 and 5), in which case adhesions are lysed using hydrodissection or sharp dissection. Ovarian tissue embedded in the superficial muscularis of the bowel is removed using a shaving technique. When deeply embedded in the bowel or bladder or when the ureter is involved or possibly obstructed, partial resection of the invaded structure and repair are necessary. The serosa and muscularis layers are reinforced with one to three interrupted 4-0 polydioxanone or 0 polyglactin 910 sutures in one layer. To confirm that the repair is airtight, sigmoidoscopy and examination under water are performed. Bipolar forceps or new sealing devices are used to desiccate the ovarian blood supply and the ovarian remnant, and the contiguous peritoneum and surrounding tissue are meticulously excised and submitted for histologic examination. Although the likelihood is low, the possibility for a malignancy exists. A variety of findings, including follicular cysts, endometriosis, corpus luteum, and ovarian cancer, have been revealed during histologic examination of the ovarian remnant tissue. In fact, there are several documented cases of ovarian cancer developing in an ovarian remnant after total abdominal hysterectomy and bilateral salpingoophorectomy.18
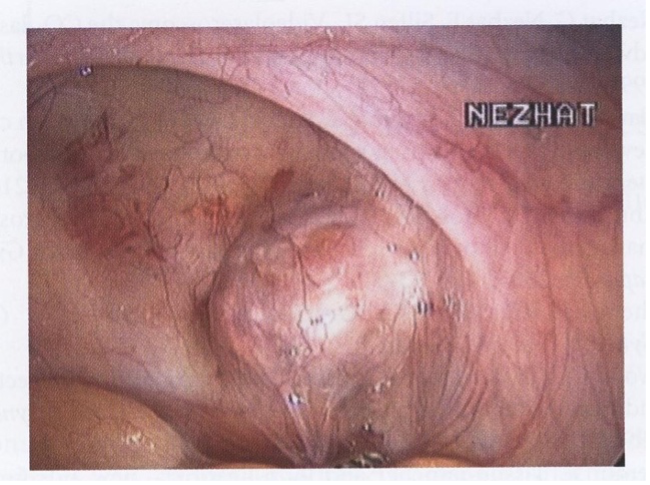 Figure 4. Retroperitoneal mass on the rectum.
Figure 4. Retroperitoneal mass on the rectum.
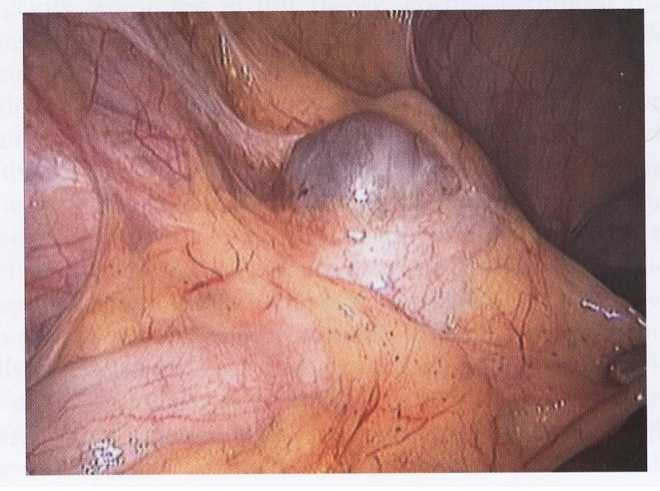 Figure 5. Ovarian remnant embedded in the sigmoid wall.
Figure 5. Ovarian remnant embedded in the sigmoid wall.
The incidence of injury to the bladder, ureter, and bowel at laparotomy for ovarian remnant is estimated to be 3% to 33%, with injuries to the ureter significantly greater by laparotomy than by laparoscopy.15 A recent study of ovarian remnant managed by laparoscopy reported the rate of intraoperative complications at 5.8%, with four intraoperative complications in 69 laparoscopies. However, there were no ureteral injuries.8 This series and others have demonstrated that the rate of complications with laparoscopic treatment of ovarian remnant is comparable or lower when compared with those reported in laparotomy.15,19 And now with the advancement of robotic assisted surgery, it is likely that these numbers shall drop further.
The following are key steps to safe, complete resection of ovarian remnant. First, in those patients who have undergone prior bilateral salpingoophorectomy, the anatomy should be restored as completely as possible with bilateral side wall and cul-de-sac dissection. Any other pelvic pathology, such as endometriosis, should be treated.
Second, the ureter should be identified and dissected completely, to the pelvic brim, if necessary. The utility of ureteral stents is controversial given reports in the literature of ureteral injury due to its rigidity.1
Third, complete excision of ovarian remnant, including a wide margin of healthy tissue, must be executed. Resection of the adherent structures, such as portions of bowel or bladder, may be necessary to completely resect the tissue; however, this is necessary to prevent subsequent surgery due to recurrence. Fourth, the ovary should be removed in one piece, placed in a surgical specimen bag, and extracted from the abdominal cavity through an enlarged trocar site, posterior colpotomy, or large canula. However, in patients with severe endometriosis and para-ovarian adhesions, the ovary may be fragmented and removed in pieces, and great care must be taken to assure complete removal. The ability of devascularized ovarian tissue to reimplant on peritoneal surfaces has been shown in animal studies as well as the gynecologic population.4,20 Wood et al. described one case that required reoperation after laparoscopic adnexectomy to remove a small portion of ovary that had been left in the abdomen and apparently implanted on the bladder.21 Fragments of ovarian tissue left in the abdomen may implant and become hormonally active. Lastly, a complete and thorough examination of the abdominopelvic cavity should be performed before completing the procedure to identify any other possible sites of ovarian remnant.
PREVENTION
Attention should be focused on prevention. Aside from poor operative technique, factors associated with ovarian remnant are:
- the use of Endoloops (Ethicon) for laparoscopic oophorectomy
- multiple operative procedures with incomplete removal of pelvic organs
- densely adherent ovaries
- multiple ovarian cystectomies for functional cysts.14
When pre-tied sutures or stapling devices are used for the infundibulopelvic ligament, they should be placed well below the ovarian tissue. As described by Semm, the infundibulopelvic ligament must be freely mobile if an Endoloop is used to ligate the ovarian blood vessels.22 Otherwise, the most proximal suture ligature may trap ovarian tissue on excision from this pedicle. Electrocoagulation and transection of the infundibulopelvic ligament or the application of clips is preferred. When the ovary is adherent to the pelvic side wall, retroperitoneal hydrodissection, adhesiolysis, and removal of the contiguous peritoneum underlying the ovary are essential for the prevention of this complication. The need for restraint in managing functional cysts is underscored by the fact that some patients in one series had only a corpus luteum resected at first laparotomy.14
CONCLUSION
Despite new innovations and the introduction of robotic assisted surgery for complex procedures, laparoscopic removal of the ovary does not necessarily ensure its complete removal. In certain cases, ovarian remnant may not be preventable. In cases where the ovary is densely adherent to the pelvic sidewall or other visceral surfaces, the necessity to use adjunctive medical therapy, such as clomid, may be necessary to identify the suspected ovarian remnant.
References
- Petit PD, Lee RA. Ovarian remnant syndrome: Diagnostic dilemma and surgical challenge. Obstet Gynecol. 1988;71 (4): 580-583.
- Siddall-Allum J, Rae T, Rogers V, et al. Chronic pelvic pain caused by residual ovaries and ovarian remnants. Br J Obstet Gynaecol. 1994;101:979.
- Orford V, Kuhn R. Management of ovarian remnant syndrome. Aust N Z J Obstet Gynaecol. 1996;36(4):468-471.
- Minke T, DePond W, Winkelmann T, Blythe J. Ovarian remnant syndrome: study in laboratory rats. Am J Obstet Gynecol. 1194;171:1440
- Symmonds RE, Petit P. Ovarian remnant syndrome. Obstet Gynecol. 1979;54(2):174- 177.
- Lafferty HW, Angioli R, Rudolph J, Penalver MA. Ovarian remnant syndrome: experience at Jackson Memorial Hospital, University of Miami, 1985 through 1993. Am J Obstet Gynecol. 1996;174(2):6471-645.
- Johns DA, Diamond MP. Adequacy of laparoscopic oophorectomy. J Am Assoc Gynecol Laparosc. 1993;1(1):20-23.
- Nezhat CH, Kearney S, Malik S, Nezhat C, Nezhat F. Laparoscopic management of ovarian remnant. Fertil Steril. 2005;83(4):973-978.
- Scott RT, Beatse SN, Illions EH, Snyder RR. Use of GnRH agonist stimulation test in the diagnosis of ovarian remnant syndrome: a report of three cases. J Reprod Med. 1995;40(2):143-146.
- Nezhat F, Nezhat C. Operative laparoscopy for the treatment of ovarian remnant syndrome. Fertil Steril. 1992;57(5):1003-1007.
- Kaminski PF, Sorosky JI, Mandell MJ, Broadstreet RP, Zaino RJ. Clomiphene citrate stimulation as an adjunct in locating ovarian tissue in ovarian remnant syndrome. Obstet Gynecol. 1990;76:924-926.
- Nezhat F, Nezhat C, Nezhat CH, et al. Use laparoscopic ultrasonography to detect ovarian remnants. Am J Obstet Gynecol. 1996;174:641.
- Price FV, Edwards R, Buchsbaum HJ. Ovarian remnant syndrome: difficulties in diagnosis and management. Obstet Gynecol Surv.1990;45:151.
- Webb MJ. Ovarian remnant syndrome. Aust N Z J Obstet Gynaecol. 1989;29(4):433- 435.
- Kamprath S, Possover M, Schneider A. Description of a laparoscopic technique for treating patients with ovarian remnant syndrome. Fertil Steril. 1997;68(4):663-667.
- Nezhat C, Nezhat F, Silfen SL. Videolaseroscopy: the CO2 laser for advancedoperative laparoscopy. Obstet Gynecol Clin North Am. 1991;18:3:585.
- Chao HA. Ovarian remnant at the port site. J Minim Invasive Gynecol. 2008 Jul- Aug;15(4):505-7.
- Narayansingh G, Cumming G, Parkin D, Miller I. Ovarian cancer developing in the ovarian remnant syndrome. A case report and literature review. Aust N Z J Obstet Gynecol. 2000;40(2):221-223.
- Abu-Rafeh B, Vilos GA, Misra M. Frequency and laparoscopic management of ovarian remnant syndrome. J Am Assoc Gynecol Laparosc. 2003;10(1):33-37.
- Shemwell RE, Weed JC. Ovarian remnant syndrome. Obstet Gynecol. 1970;36:299- 303.
- Wood C, Hill D, Maher P, Lolatgis N. Laparoscopic adnexectomy: indications, techniques and results. Aust N Z J Obstet Gynaecol. 1992;2:363-366.
- Semm K. Tissue-puncher and loop-ligation – new aids for surgical therapeutic pelviscopy (laparoscopy) – endoscopic intra-abdominal surgery. Endoscopy. 1978;10:119-124.
- Mahdavi A, Berker B, Nezhat C, Nezhat F, Nezhat C. Laparoscopic management of ovarian remnant. Obstet Gynecol Clin North Am. 2004 Sept;31(3):593-7.
