The Pneumoperitoneum
Douglas E. Ott, MD, MBA
The peritoneal cavity is a privileged space. It is covered by a continuous intact sheet of mesothelial cells (peritoneum) having a surface area of 1.5 square meters (16 square feet).1 The peritoneum is covered by a thin film of peritoneal fluid.2 It is 37 degrees Centigrade (°C) and is inflammation and pain free. There are no foreign bodies, smoke or smoke by-products. There is a normal physiologic prevailing homeostatic steady state biochemical and physical condition of zero to three (3) millimeters (mm) mercury (Hg) of intra-abdominal pressure (IAP). Creating and maintaining a pneumoperitoneum alters these circumstances. The consequences of distending the abdomen with gas have physical, chemical and biologic effects. For safety, cost and convenience, carbon dioxide (CO2) is used almost exclusively to create and maintain the pneumoperitoneum for laparoscopy. Characteristics of the pneumoperitoneum are related to mechanical pressure effects regardless of the chemical formula of the gas used, the level of intra-abdominal pressure, length of time the pressure is exerted, the volume of gas consumed, the chemical formula of the gas and the gas quality (humidity and temperature). The gas is introduced at 21°C with almost zero percent humidity and 14°C lower than body temperature with the effects of the dry cool gas compared to body temperature condition being underestimated.3,4 Despite these changes, the effects can be mitigated to achieve safe improved laparoscopic clinical outcomes.
Introduction of gas that is volume and pressure controlled into the potential space of the abdominal cavity creates a purposeful pneumoperitoneum. This pressurized dome produces a dynamic environment encompassing the interaction of mechanical, chemical and biologic forces and processes. For purposes of this chapter the definition of a “pneumoperitoneum” is the placement of any gas into the peritoneal cavity causing increased IAP. Creating and maintaining a pneumoperitoneum for laparoscopy produces complex physiologic events and changes. It is a continuous process of dynamic events having cumulative effects extending beyond its creation and maintenance regardless of the surgical procedure for which it was created.
The effect of a pneumoperitoneum involves the interaction of IAP, mechanical stretching, the chemistry and chemical effects of the gas(es) used and the biologic tissue effects on the peritoneum and related tissues and structures. The method of gas delivery, dryness of the gas, length of gas exposure, patient position, the interaction of these components and pre-existing medical conditions of the patient determines and influences the effects of the pneumoperitoneum. The pneumoperitoneum and all the factors associated with this short- term compartment situation have immediate, transient, lasting, local and global consequences: some meaningful; others not. Recognizing these changes and conditions, once known, are manageable, benefit the patient and reduce undesirable side effects.
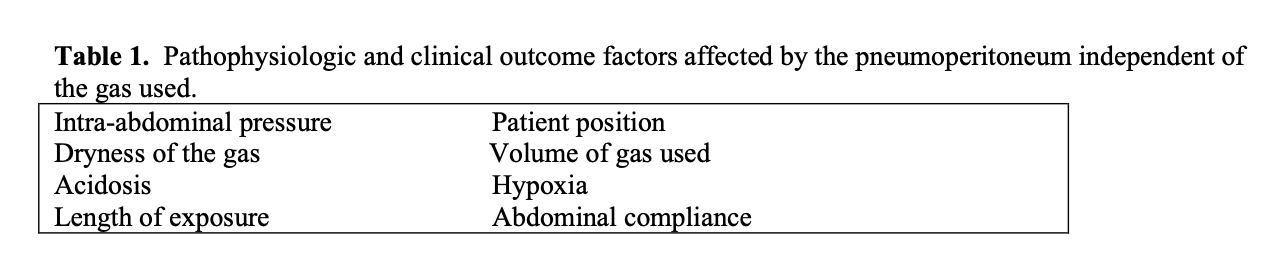
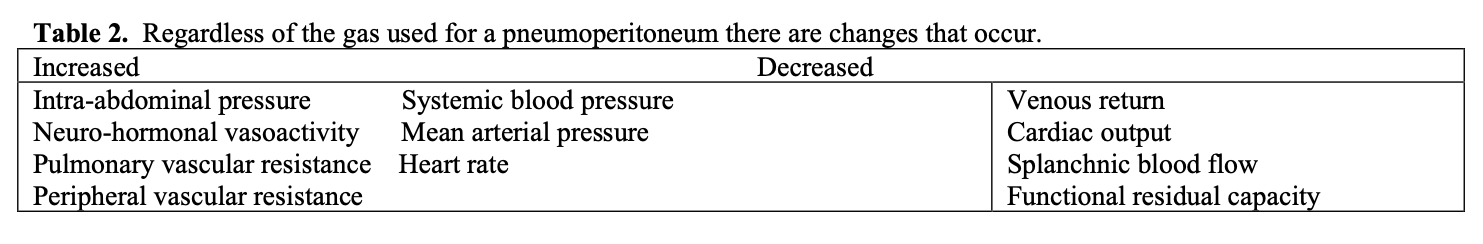
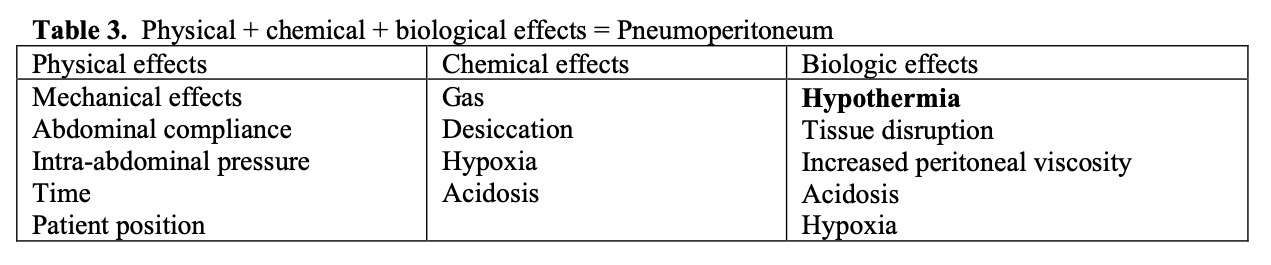
Factors affecting the pathophysiologic and clinical outcomes of the pneumoperitoneum are largely independent of the gas used. See Tables 1, 2, 3.
With any surgery patient co-morbidities must be determined, defined and assessed. Assessment and placement into the American Society of Anesthesiologists’ (ASA) classification system levels above III, chronic obstructive pulmonary disease and other co-morbidities may require invasive monitoring of blood pressure and circulating volume, urinary catheter, pharmacologic intervention, intermittent pneumatic compression or monitoring of end tidal CO2 concentration.
The pathophysiologic processes resulting from a pneumoperitoneum are directly related to and have a cause and effect relationship to clinical outcomes. Preventing or beneficially modifying these pathophysiologic processes directly affects clinical outcomes. The most common and significant co-morbidity factors for laparoscopy are cardiac, pulmonary, hepatic, renal and vascular.5 The goal of medicine and surgery is to comprehend the underlying causes of disease and correct problems that alter normal function or activity. The goal is to understand how surgery alters normal physiology and to re-establish or maintain as normal as possible the best physiologic conditions for the patient. Maintenance of as normal as possible physiologic state helps achieve the best clinical outcomes. A pneumoperitoneum that maintains body temperature, tissue hydration and integrity during laparoscopic surgery maximally improves clinical outcome.
The ideal gas for pneumoperitoneum insufflation should be non-toxic, colorless, readily soluble in blood, easily expelled from the body or expired through the lungs, non- flammable and inexpensive. CO2 best satisfies these characteristics. Oxygen and air are not readily absorbed through the peritoneum and can result in air embolism. Nitrous oxide (N2O) has unpredictable absorption. Helium is relatively insoluble in blood compared to CO2. Argon has a more significant depressant effect on hemodynamics than CO2. Oxygen and N2O, if mixed with methane, support combustion and are dangerous. The best compromise of factors of safety, utility and cost dictates using CO2.
Desiccation effects
One characteristic of any of the gases used for the pneumoperitoneum is they are all “bone dry” having a relative humidity (rh) of 0.0002 percent (%) (200 parts per million or less) of water vapor as mandated by regulatory agencies.6 The gas is passed through a throttling device (insufflator or down pressure regulator) and forced through trocars that usually contain an instrument or laparoscopic viewing device occupying the lumen further restricting the effective diameter of the orifice to 0.1 millimeter or less as an annular slot creating a jet stream of gas that touches and effects tissue surfaces in its path. The gas velocities can reach 20 meters per second.7 The effect of the jet stream of gas, being cooler than body temperature 20°C compared to 36°C and bone dry creates a constellation of cascading events resulting in peritoneal fluid changes and local, systemic and global hypothermic and acute inflammatory effects for the patient. As the dry cold gas enters the abdomen it touches tissue structures in its path having an immediate effect of increasing peritoneal fluid viscosity and causing peritoneal cell damage due to evaporation and desiccation. Tissue surfaces not in the direct line of gas flow are also affected; sustaining collateral damage albeit its slower but nonetheless continuous incessant and relentless stresses (See Figure 1). Desiccation is to be avoided as much as possible.8,9
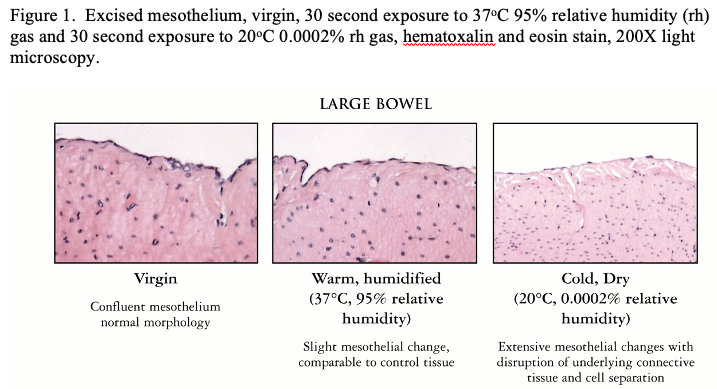
Peritoneal fluid covers peritoneal surfaces as a thin film (60 microns) with water content similar to serum. The fluid contains cellular components that are responsive and reactive to inflammation or cellular damage, proteins, coagulant precursors and surface-active lipoproteins in specific quantities to maintain normal peritoneal homeostasis. The flow of very dry gas, directly onto tissue surfaces or indirectly by deflection as the gas enters and circulates within the peritoneal cavity during gas flow, causes evaporation of water from the peritoneal fluid increasing its viscosity and altering its tribologic effects.10 The combination of rapid and dry gas flow causes a loss of 18°C in less than two (2) seconds in a tissue area of 2 square centimeters in the path of the gas stream.11 The rapid flow of dry gas causes peritoneal fluid viscosity to increase dramatically, losing its lubricating qualities, increasing its solute concentration and altering the normal inflammatory and fibrinolytic response. In addition, the surface evaporation and cooling effect decreases intestinal motility and increases the likelihood of ileus.
The volume of gas exposure averages 50-60 liters per hour but it can easily exceed this (1) when tissue combustion plume is created by cautery or laser, (2) during suctioning with or without irrigation, (3) due to open trocar valves or (4) due to leaks around the trocars. The 50-liter per hour dry gas flow contributes an additional 0.3°C thermal loss to the patient over and above other surgical hypothermic stresses.12 The gas flow causes a tissue surface vapor pressure differential between the high water content inside the peritoneal cell and the low water content on the external surface of the peritoneal cell membrane. The result is peritoneal cell stress and the compromise, with desiccation and outflow of water from and rupture of the peritoneal cell with disruption of the underlying connective tissue.13 Cold dry or warm dry laparoscopic gas changes the normal peritoneal environment, causing it to lose its normal characteristics and to become like a desert.14 See Figure 2.
The stress on the peritoneal cell, either continuous or intermittent, is repetitive, having a cumulative effect resulting in peritoneal compromise with laparotomy or laparoscopy. Peritoneal stress and trauma releases acute phase proteins; C-reactive protein (CRP), interleukin-6 (IL-6), and other lymphokines and cytokines. These are mediators that initiate the inflammatory process, result in pain perception, and initiate the process of adhesion formation. There is a greater release of IL-6 and CRP by a factor of four when cold dry or warm dry gas is used compared to warm wet gas.15 Use of humidified warmed gas significantly decreases pain and narcotic use and shortens recovery time. The desiccating effects of dry cold gas explains the findings of intra-operative hypothermia, direct and peripheral peritoneal damage, peritoneal inflammation and shoulder pain.16-22 Since peritoneal trauma is a prerequisite for adhesion formation with mesothelial regeneration, a source of laparoscopic adhesion formation is the condition of the gas and changing it to being humidified reduced this possibility.4,23,24
The combination of gas insufflation flow rate, cool temperature, and severe dryness causes rapid desiccation and a host of subsequent cascading effects to the peritoneal fluid and peritoneal tissue resulting in localized and systemic effects to the patient. As the dry gas enters the abdomen it touches the peritoneal fluid and then the peritoneum. The gas stream directly affects the peritoneum in its path and, as it is deflected from an impact point, also causes collateral desiccation by evaporation from surrounding peritoneum as gas flows to maintenance the pneumoperitoneum throughout the procedure. The flow of very dry gas, whether by direct or indirect flow, causes evaporation of water from the thin 60 micron layer of peritoneal fluid and causes an immediate drop of tissue surface temperature. The result to the peritoneal fluid is a dramatic increase in viscosity, loss of its lubricating qualities, and an increase in solute concentration. These changes result in longer contact time between tissue surfaces and can alter tissue healing between damaged surfaces. Continuing dry gas exposure over a peritoneal cell results in a vapor pressure differential of the peritoneal cell with high water content internally and low water content on the external cell membrane resulting in cell bulging, outflow of water, and peritoneal cell rupture. Evaporation caused by the extremely low water content gas compared to the wet tissue surface of the peritoneum results in heat loss and hypothermia. “The decrease in intraoperative intra-abdominal gas temperature is remarkable and can potentially harm the patient. Substantial core body temperature drop should be prevented with appropriate heating and hydration devices.”25 “Insufflation with cold, dry CO2 lowers body temperature during laparoscopic surgery. Both humidifying and warming the insufflation gas can prevent hypothermia.”26 Hypothermia below 36°C is important because it (1) alters drug pharmacokinetics, (2) delays discharge to home or other hospital bed, (3) causes patient discomfort, and (4) increases costs associated with recovery room stay and correction of the problem.27 “Evaporative cooling accounts for significant hypothermia. The cooling is dependent on the lack of water vapor in the gases currently used during laparoscopy. Heating and hydrating the gas to a physiologic condition eliminates hypothermia and tissuedesiccation.”24
Desiccation injury in the presence of a small amount of blood causes loss of normal serosal fibrinolytic activity.28 This allows clotted blood to stimulate a response that can initiate adhesion formation. The effects from intermittent, continuous and repetitive gas insufflation are cumulative on peritoneum and organ structures predisposing them to inflammation. Direct surgical touching by instruments initiates an acute phase stress protein release. The same acute phase inflammatory response is caused by dry gas pneumoperitoneum desiccation and is directly related to the lack of water content. The most prevalent of these are CRP, IL-6, tumor necrosis factor and other lymphokines and cytokines that are mediators initiating the inflammatory process and result in pain perceived by the patient. It has been shown clinically that pre-conditioning the gas to 35°C and 95% rh reduces peritoneal cell damage, inflammation and inflammatory stress. “Post-laparoscopy pain is a serious problem. The following factors have been implicated in post-laparoscopy pain, (lack of) humidity of the insufflated gas. On the basis of the factors implicated in post-laparoscopy pain, the following recommendations can be made in an attempt to reduce such pain. Use humidified gas at body temperature.”16 Clinical studies comparing the effects of dry cold gas to wet warmed gas during awake laparoscopy using only local anesthesia for pain mapping show that with humidified warmed gas there was more operating space, less pain, and shorter postoperative recovery time.18,19 The thought that shoulder pain is related to distention was dispelled since there was 3 times greater distention in the humidified group. The myth that shoulder pain is caused by carbonic acid is false since CO2 and water (H2O) are in constant equilibrium CO2 + H2O ↔ H2CO3 ↔ H2O + CO2 and would occur earlier with water vapor being added to the gas stream on entering the abdomen which doesn’t happen. Rapid gas insufflation does cause a peritoneal stretch reflex and should be avoided. The cause of shoulder pain is mostly related to the drying effect caused by dry gas desiccation with release of chemically active noxious inflammatory chemical production and is probably mediated via nerves to the right shoulder. Patients having less disruption to the peritoneum when humidified gas is used have less pain and require less postoperative narcotic and consequently fewer anti-emetics. This coupled with maintenance of body temperature in the normal range accounts for shortened recovery.17,20,27 Similar findings have been found in patients having cholycystectomy, Rouen-Y bypass, gastric banding and donor nephrectomy.21,22,29-31
The gas (CO2)
A CO2 pneumoperitoneum causes transient hypercapnia and respiratory acidosis made worse with increased IAP above 12 mmHg and head-down position but is compensated for in healthy adults and can be avoided by controlled hyperventilation and increased respiratory oxygen concentration. The use of other gases or gasless laparoscopy in randomized trials has not shown to be of any benefit for this occurrence. When IAP is above 12 mmHg and the Trendelenburg position is employed, there is a reduction in pulmonary compliance of at least 30% with associated pulmonary-perfusion impairment suggesting that a pressure below 12 mmHg for the pneumoperitoneum is the safest.
Cardiovascular effects induced by a pneumoperitoneum are most frequently associated with induction and are related to IAP mostly due to hypercarbia leading to acidosis and IAP.32,33 The length of time the patient is exposed to a pneumoperitoneum and the level of IAP influences the extent of hypercarbia and acidosis. IAP increases the absorption and decreases respiratory release of CO2.34
In ASA I and II patients 12-14 mmHg has no significant clinical effects. In ASA III and IV patients’ adequate pre-operative volume loading, beta-blockers and lower limb intermittent sequential pneumatic compression can be helpful and should be considered. Initial insufflation rate should be low flow (one liter per minute or less) to allow equilibration of splanchnic vascular capacity and be maintained at the lowest pressure to achieve an adequate operating space. Other parameters not related to the chemical composition of the gas but having an effect the patient are length of operation, patient positioning, tissue desiccation and peritoneal stretching.
Increased IAP effects
Regardless of the chemical composition of the gas used for the pneumoperitoneum the result is IAP. Regardless of the chemical formula (CO2, N2O, helium (He) argon (Ar) or air) of the gas used it causes an increase in IAP, heart rate, mean arterial pressure, systemic vascular and pulmonary vascular resistance and decreased venous return and cardiac output.35 Increased IAP within the closed peritoneal cavity transmits the mechanical pressure equally against all tissues and structures within the cavity. The pressure is transmitted into the tissue with its effect based on its composition and compliance. This results in reduction of tissue perfusion in conjunction with the blood supply and distance from the compressive force. Exchange of gases, nutrients and metabolites between blood and tissues occurs almost exclusively in the microcirculation with inadequate perfusion resulting in improper organ function. Peritoneal cavity gas clearance in humans estimates peritoneal blood flow to be 2-7% of cardiac output or approximately 100 milliliters per minute.36,37
The most common change due to reduced renal perfusion resulting from increased IAP to the kidneys is oliguria caused by activation of renin-angiotensin-aldosterone system and increasing renal cortical vasoconstriction.38,39 At 15 mmHg the cortical renal flow decreases by 28%, medullar renal flow decreases by 31% and glomerular filtration rate decreases to 18–31% of normal values.40,41
The easiest method for maintaining renal perfusion is sufficient intravascular volume before and during pneumoperitoneum. Urine output and reduction of local hypothermia are significantly improved with insufflation gas pre-conditioned to body moisture and temperature compared with room temperature dry gas insufflation because of reduced vasoconstriction caused by evaporative cooling and perfusion changes.11,42,43
Oxidative stress is induced by sudden changes in pressure during laparoscopy.44 This occurs with a sudden drop in IAP from normal operating pressures of 10-12 mmHg within a few seconds of rapid desufflation followed by a rapid return to 10–12 mmHg insufflation.45 Sudden changes in IAP trigger ischemia/reperfusion and oxidative damage to portions of the splanchnic system demonstrated by histologic changes in the terminal ileum even when safe IAP ranges are used. Animal studies suggest that there is a protective effect of oxidative stress by pre-conditioning or lowering IAP after five (5) minutes of a normal IAP pneumoperitoneum and then resuming normal pneumoperitoneum pressures.46
Tissue oxygenation level is an important determinant of adhesion formation, wound healing and tumor growth.47 An increase in adhesion formation follows prolonged pneumoperitoneum due to increased IAP pneumoperitoneum. This is due to peritoneal hypoxemia and the combined series of local events of peritoneal damage, mesothelial cell hypoxia, reactive oxygen species production and desiccation trauma lead to adhesion formation.48 It is therefore incumbent on the surgeon to do laparoscopy using the lowest possible IAP to accomplish the surgical task. Increasing the duration of laparoscopic procedures and or increasing IAP adds additional stress to tissues, organ systems, increases acute inflammatory cellular response, and effects healing and adhesion formation.
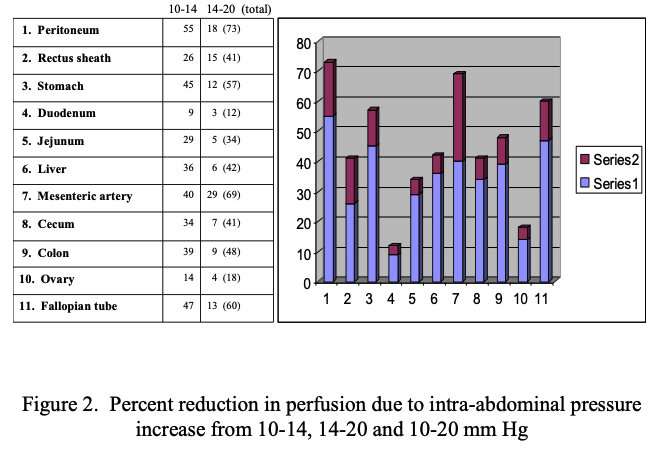
Maintaining IAP under 12 mmHg reduces the reduction in perfusion and resulting compensatory changes. A report on splanchnic circulatory changes during CO2 pneumoperitoneum showed that an increase of 5 mmHg from 10 to 15 mmHg of IAP causes a decrease in blood flow of 40–54% to the stomach, 32% to the jejunum, 44% to the colon, 39% to the liver and 60% to the peritoneum.49 Simultaneous measurement of organ perfusion changes between 10-14 mmHg pressure as a % change and from 14-20 mmHg change and total cumulative change from baseline to 20 mmHg is shown in Table 4 and Figure 1. The largest % changes and the most significant decrease from baseline perfusion to 14 mm Hg and 14 to 20 mmHg was for parietal peritoneum, mesenteric artery, fallopian tube and stomach. The least percentage loss of perfusion from opening pressure to 14 mmHg and from 14 to 20 mmHg was the duodenum and ovary. See Table 4.50 A study measuring direct intestinal mucosal blood flow at 20 mmHg showed a decrease in perfusion of 61% from baseline. Experiments in pigs demonstrate that increasing supplemental oxygen increases intestinal intramural oxygenation.51 As IAP increases and perfusion decreases there is progressive intramucosal acidosis with pH falling from normal of 7.27 to 7.15 (p<0.05).52
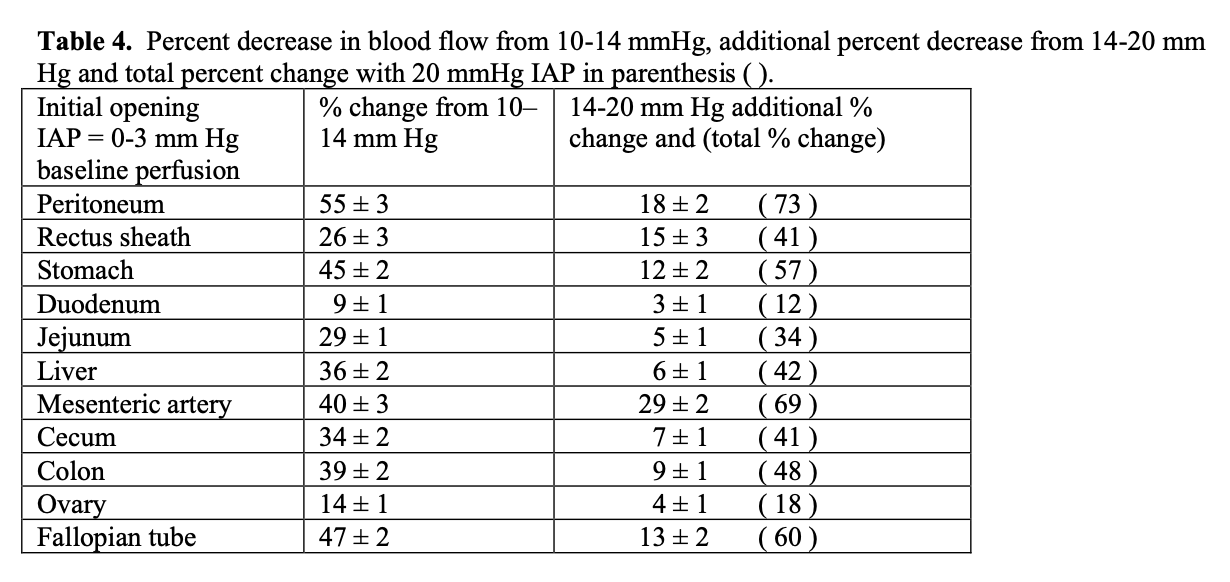
Complications secondary to transient acute ischemia and perfusion/reperfusion conditions related to IAP due to splanchnic hypo-perfusion lead to metabolic, immune and oxidative stress response disturbances.44,53-55 IAP increases can spread tumor cells, intestinal bacterial translocation causing septicemia secondary and increase the postoperative incidence of adhesions.56-58 These changes are reported as minimal when laparoscopic surgery is performed at 8–12 mmHg of IAP.59,60
The pneumoperitoneum effects post-operative adhesion formation. It is known that the mesothelium suffers desiccation damage and exposure of the underlying connective tissue due to drying during laparotomy with air exposure (40-50% rh and 20°C) and during laparoscopy (at the same temperature but 0.0002% rh CO2) in 30 seconds with direct gas flow and in 8-10 minutes with indirect gas flow.5,13,17,23,26,61 These facts dictate a laparoscopic technique (1) using the lowest intra-abdominal pressure, lowest flow rate, shortest duration, least amount of direct tactile peritoneal touching, fewest foreign bodies, least amount of blood loss and least amount of peritoneal cell damage, (2) avoiding tissue desiccation by using hydrated gas, (3) preventing hypothermia and (4) maintaining normal peritoneal fluid viscosity to achieve maximal benefits.
Conclusion
The pneumoperitoneum creates a complex dynamic set of changing conditions having pathophysiologic consequences during laparoscopy. The effects of unseen tissue touching due to gas flow, cellular stress and inflammation caused by lack of water in the gas combined with increased intra-abdominal pressure can be minimized and corrected. Maintaining intra-abdominal pressure below 12 mmHg, using humidified warmed gas, and adhering to microsurgical principles of tissue handling can be controlled and result in improved clinical outcomes.
References
- Albanese A, Albanese E. Mino J, Gomez E, Gomez M, Zandomeni M, Merlo A. Peritoneal surface area: measurements of 40 structures covered by peritoneum: correlation between total peritoneal surface area and the surface calculated by Surg Radiol Anat. 2009;31:369-77.
- Ott DE. Desertification of the peritoneum by thin-film evaporation during laparoscopy. JSLS.2003;7:189-195.
- Sammour T, Kahokehr A, Hill A. Meta-analysis of the effect of warm humidified insufflation on pain after laparoscopy. Br J Surg. 2008;95:950-956.
- Binda M, Molinas C, Hansen P, Koninckx P. Effect of desiccation and temperature during laparoscopy on adhesion formation in mice. Fertil Stertil. 2006;86:166-75.
- Volz J, Koster S, Spacek Z, and Paweletz. Characteristic alterations of the peritoneum after carbon dioxide pneumoperitoneum. Surg Endosc. 1999;13:611-614.
- United States Pharmacopoeia and National Formulary and supplements, XXI-NF, 1984.
- Lackey LW, Ott DE. Terminal gas velocity during laparoscopy. J Am Assoc Gynecol Laparosc. 2002;9:297-305.
- Holmdahl L, Risberg B, Beck DE, Burns JW, Chegini N, diZerega GS. Adhesions: Pathogenesis and Prevention – Panel Discussion. Eur J Surg. 1997; Suppl 577:56-62.
- Gomel V. Peritoneal surgery. Eds Gere S. diZerega. Springer Verlag. New York, NY, 2000.
- Ott DE. Laparoscopy and tribology: The effect of laparoscopic gas on peritoneal fluid. J Am Assoc Gynecolo Laparosc. 2001;8:117-123.
- Gray R, Ott D, Henderson A, Cochran S, and Roth C. Severe local hypothermia from laparoscopic gas evaporative jet cooling: A mechanism to explain clinical observations. JSLS. 1999;3:171-177.
- Ott DE. Laparoscopic hypothermia. J Laparosco Surg. 1991;3:127-131.
- Wiseman D, Richardson J. Humidity and temperature of insufflation gas on intact peritoneum. JAAGL. 2002;9(3) S61supplement, and 2003 PAX – VIth Symposum on Peritoneum, Amsterdam.
- Ott DE. Desertification of the peritoneum by thin-film evaporation during laparoscopy. JSLS.2003;7:189-195.
- Ott D. Reduced Peritoneal Inflammation Using Wet Gas Compared to Cold Dry Gas as Measured by C-Reactive Protein and Interleukin-6. JSLS. 2003;7:S1.2.
- Mouton W, Bessell J, Otten K, and Madern G. Pain after laparoscopy. Surg Endosc 1999;13:445-8.
- Mouton W, Bessell J, Millard S, Baxter P, Maddern G. A randomized controlled trial assessing the benefit of humidified insufflation gas during laparoscopic surgery. Surg Endosc. 1999;13:106-8.
- Almeida O. Awake microlaparoscopy with the Insuflow® JSLS. 2002;6:199- 201.
- Demco L. Effect of heating and humidifying gas on patients undergoing awake laparoscopy. J Am Assoc Gynecolo Laparosc. 2001;8:247-251.
- Ott D, Reich H Love B, et al. Reduction of laparoscopic-induced hypothermia, postoperative pain and recovery-room length of stay by pre-conditioning gas with the Insuflow® device: A prospective randomized controlled multi-center study. JSLS. 1998;2:321-9.
- Beste T, Daucher J, Holbert D. Humidified Compared With Dry, Heated Carbon Dioxide at Laparoscopy to Reduce Pain. Obstet Gynecol. 2006;107:263-8.
- Benavides R,Wong A, Nguyen H. Improved outcomes for Lap-banding using the Insuflow device compared with heated-only gas. JSLS. 2009;13:302-5.
- Peng Y, Zheng M, Ye Q, Chen X, Yu B, Liu B. Heated and humidified CO2 preventshypothermia, peritoneal injury and intra-abdominal adhesions during prolonged laparoscopic insufflations. J Surg Res. 2009;151:40-7.
- Ott D. Lapaorsocpy and adhesion formation, adhesions and laparoscopy. Semin Reprod Med. 2008;26:322-30.
- Jacobs V, Morrison J, Mettler L, Mundhenke C, and Jonat W. Measurement of CO2 hypothermia during laparoscopy and pelviscopy: How cold it gets and how to prevent it. JAAGL. 1999;6:289-295.
- Hazebroek EJ, Schreve MA, Visser P, De Bruin RW, Marquet RL, Bonjer HJ. Impact of temperature and humidity of carbon dioxide pneumoperitoneum on body temperature and peritoneal morphology. J Laparoendosc Adv Surg Tech A. 2002;12:355- 64.
- Lenhardt R, Marker E, Goll V, Tschernich H, Kurz A, Sessler D, Narzt E. Lackner Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology. 1997;87:1318-23.
- Ryan G, Grobety J, Majno G. Mesothelial Injury and Recovery. Am J Pathol.1973;71:93-112.
- Hamza M, Recart A, White P, Ogunnaike T, Schneider B. Role of Heated and Humidified Intraperitoneal Gases Using an Insuflow® Device During Laparoscopic Roux- En-Y Surgery – Effect on Temperature, Post-operative Pain and Recovery. International Anesthesia Research Society. 2003; S 121.
- Farley D, Greenlee S; Larson D; Harrington J. Doubled Blind, Prospective, Randomized Study of Warmed, Humidified CO2 Insufflation versus Standard CO2 forPatients Undergoing Laparoscopic Cholecystectomy, Western Surgical Association, 2003.
- Kandaswamy R. Laparoscopic vs open nephrectomy in 210 consecutive patients. Surg Endosc. 2004;18:1684 and Decreased Incidence of Hypothermia and Peritoneal Irritation in Laparoscopic Donor Nephrectomy Using a Filter-Hydrator-Heating Device (Insuflow®) SAGES, 2003, PO69.
- Laureano B, Andrus C, Kaminski D. Cardiovascular changes during laparoscopy. The Pathophysiology of Pneumo-peritoneum. Rosenthal R, Friedman R, Philips E. eds. Springer-Verlag 1998.
- Gutt C, Oniu T, Mehrabi A, Schemmer P, Kashfi A, Kraus T, Buchler M. Circulatory and Respiratory Complications of Carbon Dioxide Insufflation. Dig Surg. 2004;21:95– 105.
- Neudecker J, Sauerland S, Neugebauer E, Bergamaschi R, Bonjer H, Cuschieri A, Fuchs K, Jacobi C, Jansen F, Koivusalo A, Lacy A, McMahon M, Millat B, Schwenk The EAES clinical practice guidelines on pneumoperitoneum for laparoscopic surgery. Surg Endosc. 2002;16:1121–1143.
- Lacy A, Sala Blanch X, and Visa J. Alternative Gases In Laparoscopic Surgery. The Pathophysiology of Pneumo-peritoneum. Rosenthal R, Friedman R, Philips E. eds. Springer-Verlag 1998.
- Grzegorzewska A, Antoniewicz K. Effective peritoneal blood flow and patient characteristics. In: Khanna R, ed. Advances in Peritoneal Dialysis. Toronto: Peritoneal Dialysis Publications, 1994;10:27-29.
- Kim M, Lofthouse J, Flessner M. A method to test blood flow limitation of peritoneal-blood solute transport. J Am Soc Nephrol. 1997;8:471-4.
- Nguyen NT, Perez RV, Fleming N, Rivers R, Wolfe BM: Effect of prolonged pneumoperitoneum on intraoperative urine output during laparoscopic gastric bypass. J Am Coll Surg. 2002;195:476–483.
- Chiu AW, Azadzoi KM, Hatzichristou DG, Siroky MB, Krane RJ, Babayan RK: Effects of intra-abdominal pressure on renal tissue perfusion during laparoscopy. J Endourol. 1994;8:99–103.
- McDougall E, Bennett H, Monk T, Siegel C, Li D, McFarland E, Clayman R, Sharp T, Rayala H, Miller S, Haacke E. Functional MR imaging of the porcine kidney: Physiologic changes of prolonged pneumoperitoneum. JSLS. 1997;1:29–35.
- Cisek L, Gobet R, Peters C. Pneumoperitoneum produces reversible renal dysfunction in animals with normal and chronically reduced renal function. J Endourol. 1998;12:95–100.
- Backlund M, Kellokumpu I, Scheinin T, von Smitten K, Tikkanen I, Lindgren L. Effect of temperature of insufflated CO2 during and after prolonged laparoscopic surgery. Surg Endosc. 1998;12:1126–1130.
- Bessell JR and Maddern GJ (1996) The influence of insufflated gas on temperature balance during laparoscopy. In Rosenthal RJ, Friedman RL and Philips EH (eds) The Pathophysiology of Pneumoperitoneum. Springer Verlag, Los Angeles, pp. 18–27.
- Cay A, Imamoglu M, Unsal M, Aydin S, Alver A, Akyol A, Sarihan H. Does anti- oxidant prophylaxis with melatonin prevent adverse outcomes related to increased oxidative stress caused by laparoscopy in experimentalrat model? J Surg Res. 2006;135:2–8.
- Unsal M, Guven S, Imamoglu M, Aydin S, Alver A. The effect of CO2 insufflation- desufflation attacks on tissue oxidative stress markers during laparoscopy: a rat model. Fertil Steril. 2009;92:363-8.
- Arioz D, Polat C, Tokyol C, Kakraman A, Yilmaz S, Demirel R, Saylan A, Yilmazer M, Tekin A. What should be the ideal time for ischemic preconditioning in a laparoscopic rat model? J Laparoendosc Adv Surg Tech A.2009;19:141-7.
- Crowther M, Brown N, Bishop E, Lewis C. Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumours. J Leukoc Biol. 2001;70:478–490.
- Binda M, Koninckx P. Prevention of adhesion formation in a laparoscopic mouse model should combine local treatment with peritoneal cavity conditioning. Hum Reprod. 2009;24:1473-9.
- Schilling M, Redaelli C, Krahenbuhl L, Signer C, Buchler M. Splanchnic microcirculatory changes during CO2 laparoscopy. J Am Coll Surg. 1997;184:378–382.
- Ott D. Changes in organ perfusion during laparoscopy. JSLS. 2009;13:S76-S98.
- Ratnaraj J, Kabon B, Talcott M, Sessler D, Kurz A. Supplemental oxygen and carbon dioxide each increase subcutaneous and intestinal intramural oxygenation. Anesth Analg. 2004;99:207–211.
- Diebel L, Dulchavsky S, Wilson R. Effects of increased intra-abdominal pressure on mesenteric arterial and intestinal mucosal blood flow. Trauma. 1992;33:45–49, 1992.
- Eleftheriadis E, Kotzampassi K, Papanotas K, Heliadis N, Sarris K. Gut ischemia, oxidative stress, and bacterial translocation in elevated abdominal pressure in rats. World J Surg. 1996;20:11–6.
- Glantzounis G, Tselepis A,Tambaki A,TrikalinosT, Manataki A, Galaris D, Tsimoyiannis E, Kappas A. Laparoscopic surgery–induced changes in oxidative stress markers in human plasma. Surg Endosc. 2001;15:1315–9.
- Kirsch A, Hensle T, Chang D, Kayton M, Olsson C, Sawczuk Renal effects of CO2 insufflation: oliguria and acute renal dysfunction in a rat pneumoperitoneum model. Urology. 1994;43:453–9.
- Schachtrupp A, Toens C, Hoer J, Klosterhalfen B, Lawong A, Schumpelick V. A 24- h pneumoperitoneum leads to multiple organ impairment in a porcine model. J Surg Res. 2002;106:37–45.
- Unsal M, Imamoglu M, Cay A, Kadioglu M, Aydin S, Ulku C, Kesim M, Alver A, Bokaya H. Acute alterations in biochemistry, morphology and contractility of rat isolated urinary bladder via increased intra-abdominal pressure. Gynecol Obstet Invest. 2006;61:179–87.
- Unsal M, Imamoglu M, Kadioglu M, Aydin S, Ulku C, Kesim M, Alver A, Kalyoncu N, Bozkaya H. The acute alterations in biochemistry, morphology, and contractility of rat-isolated terminal ileum via increased intra-abdominal pressure. Pharmacol Res. 2006;53:135–41.
- Cevrioglu A, Yilmaz S, Koken T, Tokyol C, Yilmazer M, Fenkci I. Comparison of the effects of low intra-abdominal pressure and ischaemic preconditioning on the generation of oxidative stress markers and inflammatory cytokines during laparoscopy in rats. Hum Reprod. 2004;19:2144–51.
- Ozmen M, Kessaf Aslar A, Besler H, Cinel I. Does splanchnic ischemia occur during laparoscopic cholecystectomy? Surg Endosc. 2002;16:468–71.
- Volz J, Koster S, Weis M, Schmidt R, et al. Pathophysiologic features of a pneumoperitoneum at lapaparoscopy: A swine model. Am J Obstet Gynecol. 1996;174:132-140.
