Laparoscopic Operations on the Ovary
Arathi Veeraswamy, Tanjia Pejovic, Han Ying, Aixingzi Aili, Farr Nezhat, Camran Nezhat
Although most benign ovarian tumors can be treated laparoscopically, observation of the adnexa enables a gynecologist to decide whether laparotomy is indicated.
OVARIAN BIOPSIES
It is often difficult to immobilize the ovary because of its smooth surface and firm texture. The uterine-ovarian ligament can be grasped to lift and rotate it, or the ovary can be wedged against the pelvic side wall by using the flattened edges of open or closed forceps. Sometimes Morgagni paratubal cysts can be used as a handle or the uterus can be manipulated under the ovary to provide a shelf (Figure 1). Overly aggressive manipulation may cause lacerations in the capsule and result in bleeding from follicles or cysts.
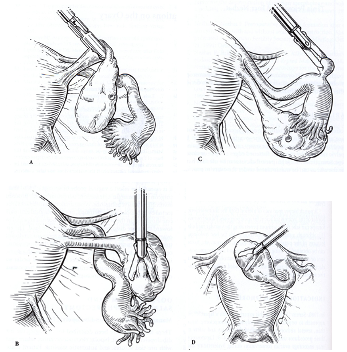 Figure 1. Different methods of immobilizing the ovary. (A) Grasping the utero-ovarian ligament.(B)Wedging the ovary against the pelvic side wall.(C) Grasping tubal or ovarian inclusion cysts.(D) Using the uterus as a platform.
Figure 1. Different methods of immobilizing the ovary. (A) Grasping the utero-ovarian ligament.(B)Wedging the ovary against the pelvic side wall.(C) Grasping tubal or ovarian inclusion cysts.(D) Using the uterus as a platform.
A punch biopsy specimen of a lesion from the antimesenteric border of the ovary is sufficient for most purposes. Palmer biopsy forceps can take tissue without penetrating the vascular medulla, although a small wedge resection yields the best histologic features of the ovarian stroma and cortex.1 Alternatively, tissue can be obtained by using toothed forceps and laparoscopic scissors or a laser (Figure 2). Bleeding is controlled with bipolar electrocoagulation; sutures should be avoided so that postoperative adhesions can be minimized.2
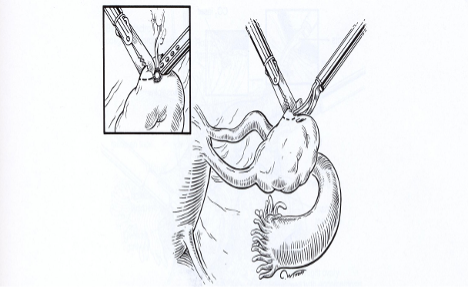 Figure 2. A small wedge biopsy specimen of the ovary is taken using a scissors or CO2 laser (insert).
Figure 2. A small wedge biopsy specimen of the ovary is taken using a scissors or CO2 laser (insert).
INDICATIONS FOR OOPHORECTOMY
In 1980, Semm reported his experience with a laparoscopic approach to oophorectomy and salpingo-oophorectomy.3 Since then, several authors have described the efficacy and safety of these procedures using different techniques.4-8 Laparoscopy may encourage ovarian conservation during hysterectomy and more conservative management of pain caused by adnexal disease. If necessary, oophorectomy maybe done laparoscopically at a later date, with a short hospital stay and recovery period. The indications for oophorectomy are as follows:
- Persistent localized pain despite previous lysis of adhesions or treatment of endometriosis
- Residual ovary syndrome
- Dysgenetic gonads
- Ovarian cysts 5cm or greater with ovarian damage, or when spillage of cystic contents increases the likelihood of complications (cystic teratomas, mucinous cystadenomas, malignancy)
- Unilateral tubo-ovarian abscess
- Prophylactic therapy for advanced breast cancer
- Early ovarian cancer in young women (StageІ)
A uterine manipulator is inserted for traction and counter traction to aid in the exposure and manipulation of the ovary. The pelvis and especially the adnexa are inspected to plan the surgical approach. Before starting the procedure, it is important to observe the course of the ureter as it crosses the external iliac artery near the bifurcation of the common iliac artery at the pelvic brim. The left ureter may be more difficult to find because the base of the sigmoid mesocolon often covers it. If the ureter cannot be seen through the intact peritoneum, it must be identified by retroperitoneal dissection. If the patient does not have a uterus, it is essential to insert a vaginal probe or sponge stick so that the surgeon can maintain orientation, particularly with procedures involving extensive adhesions.
When anatomic landmarks are distorted by adhesions, endometriosis, or prior surgical extirpation, one should begin the procedure at the most normal area and work toward the more distorted parts of the operative field. The entire ovary must be removed to prevent ovarian remnant syndrome or tumor development in a dysgenetic gonad. At the conclusion, the operative field is inspected and clots are removed with a suction irrigator or grasping forceps. Pedicles are inspected under water and with decreased pneumoperitoneum, and hemostasis is obtained with bipolar electrocoagulation.9
OOPHORECTOMY AND SALPINGO-OOPHORECTOMY
Management of the Infundibulopelvic Ligament
Three techniques have been described for managing the infundibulopelvic ligament: bipolar electrodessication, suture ligation with pre-tied sutures, and automatic stapling. Patient costs are approximately $600 for the linear stapler and $48 for each pre-tied ligature. There is no extra charge for bipolar electrocoagulation.
A bipolar forceps is preferable for hemostasis of the Infundibulopelvic ligament.4 Endoloop sutures (Ethicon) cannot be applied in the presence of adhesions that distort the anatomy, and it is difficult to place Endoloop sutures on large pedicles such as the mesovarium and infundibulopelvic ligament, even if the anatomy is normal. Once it is applied, the slipknot can loosen under the tension of the large pedicle, increasing the risk of intraoperative hemorrhage, or a piece of the ovary may be left in the pedicle, predisposing the patient to ovarian remnant syndrome.10
Aside from cost, there are several drawbacks to the stapling device. The instrument is bulky, and the operator must note its proximity to the ureter, bowel, and bladder.
Adequate desiccation of tissue with bipolar forceps by monitoring the flow of electrons on an ammeter has been suggested before transecting the pedicles. Excessive desiccation creates friable tissue and increases thermal damage, and the tissue may adhere to the forceps. A self-limiting bipolar electrocoagulator (Valleylab Force II series generator, Boulder, CO) provides controlled desiccation without charring the adjacent tissue. In this mode, the power peaks at 100 ohms instead of 300 to 500 ohms in typical generators. The power then “rolls off” to provide the desired surgical effect without excess drying, blanching, or destruction of tissue.
The mechanism of closure of large blood vessels with high-frequency electrocoagulation was described by Sigel and Dunn.11 Electrocoagulation begins with shrinkage of the vessel wall resulting from the denaturation of tissue proteins combined with the melting of the carbohydrate tissue components and the dehydration of tissue fluids. The resulting coagulum formed by this melting and fusion of the vessel wall obliterates the lumen. The most successful closures are characterized by low levels of heating that end before char is formed, which preserve the inherent fibrillar structure of the connective tissue. Too much heating destroys the inherent fibrillar structure, forming a more amorphous coagulum that is poorly penetrated by fibroblasts and capillaries and characterized by inflammatory-type reorganization and healing process that results in unsuccessful or weak closures. Further heating during electrocoagulation causes complete disintegration of the amorphous coagulum and carbonization.11,12 Ammeters or flow meters measure only the flow of current in relation to tissue resistance and have no value in assuring hemostasis. Pedicles are reinspected after the intraabdominal pressure has been lowered because they can bleed again at the termination of a procedure the haemostatic effects of the elevated abdominal pressure are lost.9
The technique for oophorectomy is similar to that for salpingo-oophorectomy except that the tube must be protected from thermal damage. The procedure begins at the utero- ovarian ligament (Figure 3A).The pedicles are desiccated and cut.4 The mesovarium is coagulated and cut into 2-cm bites, working from the uterine side to the fimbria until the ovary is removed (Figure 3B-D). The latter step may jeopardize the fallopian tube if the mesovarium is over desiccated. In some circumstances, it may be preferable to sharply incise the individual leaves of the mesovarium if the distance between the tube and the ovary is small. The underlying vascular tissue may be coagulated and divided to allow excision of the remaining ovary. Laparoscopic oophorectomy causes less morbidity than oophorectomy by laparotomy, and the patient’s recovery is shorter.4
An ovary and tube minimally involved with adhesions or endometriosis are approached from the infundibulopelvic ligament or the utero-ovarian ligament. Filmy adhesions that limit the mobility of the ovary are lysed. Ovarian cysts are aspirated and deflated, making removal of the ovary easier. The adnexa are removed by beginning with the infundibulopelvic ligament. This approach is preferable if the uterus is to be removed or significant disease is found in the uterine-ovarian ligament, in patients with a prior hysterectomy, or if hemostasis of the ovarian vessels is necessary. The procedure begins with ureteral identification through the peritoneum as it enters the pelvic brim and travels parallel to the infundibulopelvic ligament.
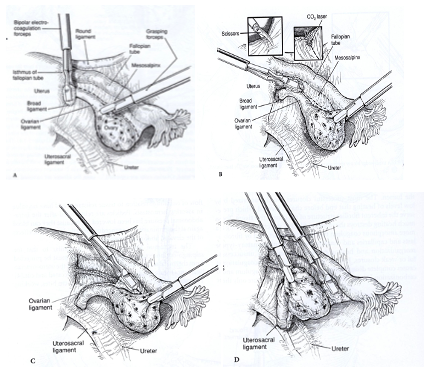 Figure 3. Oophorectomy is done by coagulating and cutting the mesovarium. (A). The procedure starts from the utero-ovarian ligament. (B-D) It continues toward the fimbria. Inserts show the use of scissors or a laser for completing the reparation.
Figure 3. Oophorectomy is done by coagulating and cutting the mesovarium. (A). The procedure starts from the utero-ovarian ligament. (B-D) It continues toward the fimbria. Inserts show the use of scissors or a laser for completing the reparation.
The isthmic portion of the tube and the ovarian ligament are desiccated and cut (Figure 4). The ovary is held with a grasping forceps, and the infundibulopelvic ligament is put under traction by elevating it and pulling it medially. The infundibulopilvic ligament is desiccated with bipolar forceps and cut with a laser or scissors in 1-to 2-cm increments, working from lateral to medial until the adnexa are removed (Figures 5, 6, 7, 8). To avoid damage to the lateral pelvic side wall, traction is used on the tube and ovary and excessive coagulation is avoided.
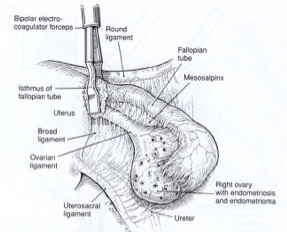 Figure 4. The procedure of salpingo-oophorectomy is begun by electrodesiccation and transection of the utero-ovarian ligament and the isthmic portion of the fallopian tube.
Figure 4. The procedure of salpingo-oophorectomy is begun by electrodesiccation and transection of the utero-ovarian ligament and the isthmic portion of the fallopian tube.
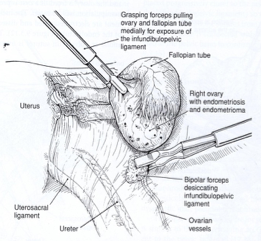 Figure 5. After identification of the ureter, the infundibulopelvic ligament is coagulated using a bipolar forceps while gentle traction is applied to the adnexa.
Figure 5. After identification of the ureter, the infundibulopelvic ligament is coagulated using a bipolar forceps while gentle traction is applied to the adnexa.
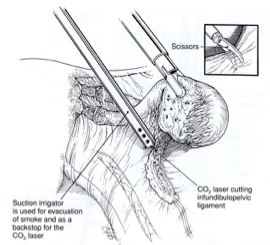 Figure 6. A laser or scissors (inset) are used to cut the coagulated infundibulopelvic ligament.
Figure 6. A laser or scissors (inset) are used to cut the coagulated infundibulopelvic ligament.
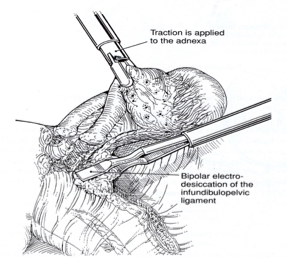 Figure 7. Coagulation and cutting of the infundibulopelvic ligament continue in 1- to 2-cm increments until it is removed completely.
Figure 7. Coagulation and cutting of the infundibulopelvic ligament continue in 1- to 2-cm increments until it is removed completely.
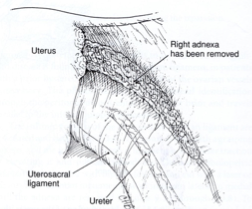 Figure 8. A view of the pelvic side wall after the adnexa is removed.
Figure 8. A view of the pelvic side wall after the adnexa is removed.
The Stapling Device
The laparoscopic linear stapling device used during gynecologic procedures is a modification of the stapling device used for bowel resection. The trocar site used to introduce the stapler is modified depending on the specific adnexal disease. The trocar is introduced between the symphysis pubic and the umbilicus lateral to the rectus muscle and inferior epigastric vessels, although injury to inferior epigastric vessels is possible. At the end of the procedure, the fascia is closed to prevent a hernia. After the stapler is introduced, the adnexa are grasped with laparoscopic forceps and retracted medially and caudally to stretch and outline the infundibulopelvic ligament. The ligament is grasped and secured with the stapler (Figure 9). The stapler is not fired until the contained tissue is identified and the ureter’s safety is assured. Once transected, the stapler line is examined for placement and hemostasis. Usually one or two stapler applications are required for each adnexa.
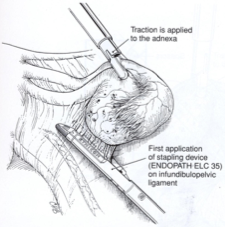 Figure 9. A right salpingo-oophorectomy is done using a stapling device. The tube and ovary are under traction as the Endopath ELC 35 is applied across the infundibulopelvic ligament.
Figure 9. A right salpingo-oophorectomy is done using a stapling device. The tube and ovary are under traction as the Endopath ELC 35 is applied across the infundibulopelvic ligament.
The Endoligature
Pre-tied Endoloop sutures may be used in an oophorectomy or a salpingo- oophorectomy.13 Peri-ovarian adhesions are lysed, and the ovary is freed. If a cyst is present, it is aspirated so that manipulation will be easier. The mesovarium and ovarian ligament are electrodesiccated and dissected to facilitate placement of the endoligature. The Endoloop (0 polydioxanone or polyglactin suture) is introduced into the abdominal cavity through the mid-suprapubic trocar sleeve. Using forceps, the ovary is pulled through the Endoloop. Atraumatic forceps are used to assist in placement. The suture is pushed onto the mesovarium while a knot pusher is used on the opposite side to place the slipknot at the most lateral position on the mesosalpinx and mesovarium. The suture is tightened as the ovary is pulled toward the midline, and the tube is retracted with the atraumatic foceps. A second and, if necessary, a third Endoloop are placed, each successively closer to the pelvic wall so that the mesovarian pedicle will be long. The mesovarium is transected with scissors. The pedicle is evaluated to confirm that the sutures have been placed beyond any ovarian tissue to avoid ovarian remnant syndrome.
For salpingo-oophorectomy, an Endoloop is placed over the adnexa after the ovarian ligament and tubal isthmus have been electrocoagulated and cut. The ovary and tube are grasped with forceps and pulled contra laterally. Simultaneously, the atraumatic forceps are used to push the Endoloop laterally, ensuring that the ligature is placed as far lateral as possible on the infundibulopelvic ligament. One or two additional sutures are placed progressively closer to the pelvic wall (at least 1 cm below the infundibulopelvic ligament) so that the pedicle will be long enough to prevent the sutures from slipping.
The adnexal pedicle is transected with scissors. The ureter is evaluated at the pelvic brim to confirm that it is not damaged.
A borderline tumor of the ovary is an epithelial tumor with a low rate of growth and a low potential to invade or metastasize. They account for 10%–15% of all ovarian tumors. In particular, this type of ovarian tumor is characterized by a degree of cellular proliferation (stratification of the epithelial lining of the papillae, and nuclear atypia and mitotic activity) in the absence of stromal invasion.88
Borderline epithelial ovarian tumors are generally discovered at an earlier stage than malignant tumors. They frequently affect young women in which conservative and minimally invasive surgery is required to preserve childbearing potential Laparoscopy can be used to perform all the surgical procedures needed in patients with early borderline ovarian tumors (BOT), including excision (adnexectomy and hysterectomy) and staging (peritoneal biopsies, omentectomy, appendectomy in patients with mucinous tumors, and removal of pelvic and paraaortic lymph nodes, although this last procedure has generated controversy in recent years). Laparoscopic management seems feasible in early BOT.77 Laparoscopic treatment of adnexal masses has proven to be a safe and effective diagnostic and therapeutic tool in the hands of experienced laparoscopists.88
All procedures are performed under general endotracheal anesthesia. Patients are usually placed in the dorsolithotomy position, with the legs in universal Allen stirrups. In addition, intraoperative lower extremity sequential compression devices for venous thrombosis prophylaxis are used.
The entire procedure is performed through laparoscopy and all patients are given antibiotic prophylaxis (cefoxitin 2 g IV). The vaginal cavity is cleansed with povidone- iodine solution and a Foley catheter is placed in the bladder.
After a carbon dioxide pneumoperitoneum by Veress needle is induced at the level of umbilicus, a 10-mm trocar that incorporates the zero-degree laparoscope is inserted through an umbilical vertical incision and the entrance into the abdominal cavity is made under direct visualization, the laparoscope is connected to a video monitor.
Once the trocar has been safely introduced into the abdominal cavity, the intra-abdominal pressure is maintained at 15 mmHg.
Three suprapubic ancillary trocars are used: one 5-mm trocar is inserted in the midline 3 cm under the umbilicus, and one in each iliac fossa (5 mm on the left side and 10 mm on the right size) laterally to the inferior epigastric vessels.
Before the operative procedure, all the pelvic structures are inspected and the abdomen explored through the laparoscope in a clockwise fashion.
Guidelines for surgical treatment of BOT in women who do not wish any other pregnancy include peritoneal washing, hysterectomy with bilateral salpingo- oophorectomy, omentectomy, and multiple peritoneal biopsies.79 As has already been discussed, patients with BOT tend to be younger than women with invasive ovarian cancer. For many of these patients, fertility is an important issue. Previous studies have suggested the safety of conservative surgery with unilateral salpingo-oophorectomy or cystectomy for patients with stage I BOT. They are characterized by an excellent long- term survival.80,81
The laparoscopic approach follows a standardized procedure. First, an observational time of adnexal masses in search of macroscopic findings suggestive of malignancy is performed. These features are: presence of extraovarian implants, presence of exophytic growth, anarchic vascularization on the surface of the ovary, and ascites.
The laparoscopic surgery includes a cytologic evaluation of pelvic and abdominal washings and treatment of adnexal mass by cystectomy or unilateral salpingo- oophorectomy in accordance with recommendations for laparoscopic treatment of benign adnexal mass. Tissue is removed from the abdominal cavity using a plastic bag to reduce the possibility of parietal implantation of neoplastic cells. An intraoperative histologic examination on a frozen section preparation is carried out in all patients.
Multiple pelvic and abdominal peritoneal and contralateral ovarian biopsies, cytologic evaluation of pelvic and abdominal washings, infracolonic omentectomy, and an accurate abdominal inspection to obtain the intraoperative staging of the disease are performed.
Systematic appendectomy is also a criterion for complete staging of mucinous borderline tumors. If there are no obvious abdominal or pelvic peritoneal lesions, random peritoneal biopsies are performed.
Laparoscopic salpingo-oophorectomy should be considered as the first choice of conservative treatment in most patients, because it seems to be associated with lower recurrence rates compared with laparoscopic cystectomy. The greatest risks of this procedure are cysts rupture, intra-abdominal spillage, and contamination of the abdominal wall. These risks can be reduced to an acceptable level by the use of a plastic bag, reducing the possibility of parietal implantation of neoplastic cells.78
The laparoscopic approach to borderline tumors, as any suspicious adnexal mass, should follow strict criteria to reduce the risk of spreading and relapse. Spillage should be minimized and in case of intraoperative rupture during laparoscopy or laparotomy, the treatment is the same: a copious washing of the pelvis and abdomen; manipulation of the tumor should be kept to the minimum; any biopsy specimen should be extracted with an endobag protecting the abdominal wall.
Laparoscopic cystectomy may have more chance of preserving a woman’s fertility compared with adnexectomy because of the removal of less ovarian tissue. Its greatest danger is the risk of inadvertently leaving behind some malignant cells. Therefore, this procedure should be reserved for patients with previous unilateral salpingo- oophorectomy or when bilateral lesions are present to preserve at least some ovarian tissue.78
If bilateral adnexal masses are present in a young patient wanting to preserve fertility, intraoperative decision-making is more difficult. Before any resection, the ovarian masses should be carefully inspected and assessed for adjacent normal ovarian tissue. In general, the more suspicious ovary should be removed using the most conservative means on the contralateral side (ovarian cystectomy, if possible) and the specimens sent for frozen section analysis. If a borderline tumor is diagnosed, then either ovarian cystectomy or oophorectomy should be performed on the contralateral side.82
The accuracy of frozen section diagnosis of ovarian tumors is important, especially in young women who may be managed conservatively with preservation of fertility. A recent series by Ilvan et al. confirmed that frozen section diagnosis is a reliable method for the surgical management of BOT with a sensitivity of 87 % and a specificity of 98 %.87 However, diagnostic problems can occur in mucinous borderline tumors during frozen section examination.87
Although several investigators would recommend routine biopsy of a normal-appearing contra lateral ovary, some investigators believe that careful macroscopic inspection of the normal ovary should be sufficient because unnecessary biopsy or wedge resection may result in peritoneal adhesions or ovarian failure.
In our opinion, conservative laparoscopic treatment of BOT is an appropriate and reasonable therapeutic option for young women with low-stage disease who wish to preserve their childbearing potential because the fertility results are encouraging.
Recurrence can be noted after this type of treatment, but the cases of recurrent disease can be detected with close follow-up and treated accordingly. For these reasons, careful selection of candidates for this kind of treatment is, of course, necessary and close follow-up is required.
Whenever possible, the treatment options for each possible operative finding should be discussed thoroughly with the patient and her family, including the advantages and disadvantages of each treatment approach.
The main objective of conservative treatment for women with BOT is to spare fertility without negatively affecting overall and disease-free survival. Our results are in keeping with previous studies in which recurrence rates were higher after cystectomy than after unilateral salpingo-oophorectomy.85,86 This suggests that laparoscopic cystectomy should be considered only for women with one ovary or with bilateral tumors who wish to preserve their childbearing potential. When BOTs are identified at surgery by intraoperative histology, the recommended conservative treatment should be laparoscopic salpingo-oophorectomy.
If these restrictions are rigorously applied, then fertility-sparing surgery may be considered a safe option for this pathology, but all laparoscopic procedures should be reserved for oncologic surgeons trained in extensive laparoscopic procedures.
Dysgenetic Gonads
Individuals with androgen insensitivity syndrome have a high risk (20-30%) of developing malignancy in their gonads.14 Phenotypic females with the XY karyotype require gonadectomy to protect them from developing gonadoblastoma. These gonads present as streaks, and the boundaries between the gonadal tissue and the peritoneum are not always clear. Because there is a chance that some of the dysgenetic gonadal tissue will be missed, the peritoneal borders must be kept wide. The laparoscopic approach for gonadectomy has been used in patients with male pseudohermaphroditism, including patients with pure gonadal dysgenesis, testicular feminization, and mixed gonadal dysgenesis and dysgenetic male pseudohermaphroditism.14-16
The laparoscopic procedure for removing a dysgenetic gonad is similar to that for removing an ovary that is densely adherent to the pelvic side wall.17,18 Bilateral laparoscopic gonadectomy was performed under general anesthesia in patients without complications, the procedure may be performed safely, even with gonads located in inguinal canals.99 Peritoneal insufflation with a Veress needle inserted.infraumbilically, insertion of a 5–10 mm umbilical trocar for laparoscopic evaluation, and two or three additional pelvic trocars for therapeutic procedures. The patient was then placed in a Trendelenburg position. The gonadal structures were evaluated initially, and when necessary, the bowel retracted. Both the utero-ovarian and infundibulopelvic ligaments are electrocoagulated and cut. The mesovarium above and below is incised with scissors or the CO2 laser with hydro dissection. The loose areolar tissue immediately below the gonad is dissected away from the gonad. In some cases when the gonads are not easily seen, the gonadal vessels may be identified and followed downwards. Most often, the gonads are identified near the inguinal region, eventually with normal testicular or ovarian appearance, but also with a dysplastic or tumoral aspect. In some cases the gonads are not clearly identified because of dysplasia, sometimes leading to confusion with ductal structures. Once identified, the gonads are resected, most often together with the ductal structures.
Adherent Adnexa
Adhesions between the ovary and pelvic side wall, broad ligament, and bowel are lysed with the CO2 laser or scissors until the ovary is freed. The ovary is grasped with a toothed forceps and elevated. It is put on stretch to create a plane between the ovary and the peritoneum. To avoid injury to ureters, blood vessels, and other underlying structures, the retroperitoneal area is entered and hydrodissection is carried out.19 Using the suction-irrigator probe as a backstop, the adhesions are lysed close to the ovary. Removal of ovarian tissue may require excision of the peritoneum attached to the ovary.
The ovary may be enlarged, may be adherent to the pelvic side wall and broad ligament, or may contain endometriomas, so the surgeon may need to enter the retroperitoneal space. In this case, the ovary is removed by retro peritoneal dissection. After hydrodissection is done, an incision is made between the round and Infundibulopelvic ligaments medial to the pelvic side wall. Blunt dissection, hydro dissection, and sharp dissection with the CO2 laser are used to lyse adhesions and separate the adnexa and peritoneum, ureter, and blood vessels. Hemostasis is achieved with bipolar forceps. After dissection of the pelvic side wall, the remaining infundibulopelvic ligament, the ovarian ligament, and the proximal portion of the tube are coagulated and cut. The ureter is dissected from the ovary, and the adnexa are removed.
Residual Ovary
In patients who have had a previous hysterectomy, many of the usual landmarks in the pelvic are absent and extensive adhesions.
May involve the left ovary and descending colon. Lysis of adhesions is carried out cautiously to avoid damaging the bowel. If the ureter cannot be identified, it is necessary to open the retroperitoneal space. A sponge stick placed in the vagina aids in orientation.
The ovary often is adherent to the vaginal cuff and is dissected from its attachment with scissors or the laser. The ureter is proximal to the lateral margins of the vaginal cuff, and its position can be altered from a previous operation. No ovarian fragments should remain on the pelvic side wall or vaginal cuff.
The ovary is immobilized with a grasping forceps and put on stretch, and the infundibulopelvic ligament is coagulated and transected in 1-to-2-cm increments until the ovary is removed. Depending on the pelvic anatomy, it is preferable to begin the oophorectomy from the infundibulopelvic ligament to improve the anatomic relationships.
The ovary is removed through an abdominal incision as was described previously. If the ovary is large, it is cut into pieces. Colpotomy is done if the ovary is 5 cm or greater.
However, colpotomy remote from a hysterectomy is technically difficult and is associated with significant risks if the bladder and rectosigmoid colon are adherent to the vaginal cuff.
Removal of Tissue
Removal of the ovary may be difficult if it is more than 5 cm in diameter. It can be removed through a 10-mm trocar sleeve placed in a suprapubic punctures, pulling the sleeve and forceps together and bringing the tissue to the incision. A Kelly or Kocher clamp is used to grasp the tissue to remove it from the abdomen. Alternatively, a long clamp is inserted through the accessory trocar incision and the tissue is grasped under direct observation and pulled from the abdomen through the trocar incision. The tube and ovary can be divided wit scissors, a morcellator, or a laser and removed through trocar sleeves if fragmentation is not contraindicated by the characteristics of the cyst. If an open laparoscopy is required, the ovary is removed through an enlarged umbilical incision. After the ovary is removed, the pelvic cavity is irrigated and the pelvis is examined to assure hemostasis. For endometriomas and there cysts 5 cm or larger, the tissue is removed by posterior colpotomy. The cul-de-sac is identified by placing a sponge stick in the vagina and applying pressure to the posterior fornix between the uterosacral ligaments. An incision is made between the uterosacral ligaments with a laser or unipolar knife electrode. Once the incision extends to the sponge stick, the ovary is brought to the incision and grasped vaginally with an allis clamp. If a cyst is present, it is deflated with a large-bore needle or trocar while traction is applied to the ovary. As the ovary collapses, it is pulled into the vagina intact, with minimal spillage of its contents. The colpotomy is closed vaginally or laparoscopically, using two to three sutures.
Removal the ovary and vaginal closure of the colpotomy are facilitated by placing the patient’s legs in the position for a vaginal hysterectomy.
If it is necessary to avoid spillage of the cyst contents, the ovary is removed in a specially designed laparoscopic bag (Endopouch Retriever™, Ethicon).20 The bag is removed through a posterior colpotomy incision or an extended suprapubic incision.21 When the ovary is large the cyst is drained, and deflated contained cyst is pulled from the abdominal cavity.
Ovarian Wedge Resection
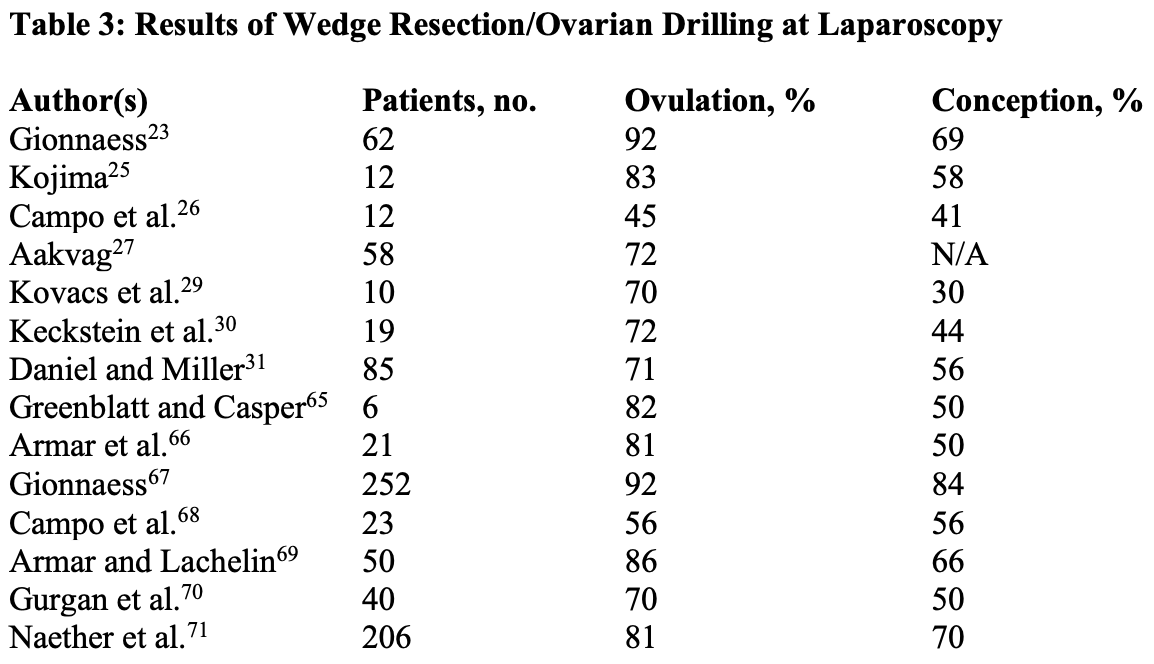
Stein and Leventhal described enlarged polycystic ovaries with the clinical features of menstrual aberrations, obesity, and hyperandrogenism.22 Although polycystic ovarian disease (PCOD) has variable manifestations, its hallmark is chronic anovulation. It was believed that the enlarged ovaries cause the condition, and so ovarian wedge resection was advocated. As ovulation induction agents were unavailable, ovarian wedge resection represented a major breakthrough, with ovulation and pregnancy rates of 80% and 50%, respectively. However, many patients who were initially ovulatory reverted to the previous anovulatory state after several months.23 Although most had apparently normal ovulatory cycles, only 50% conceived (Table 1) because postoperative adhesions developed in many of these women (Table 2).


The availability of ovulation-inducing medications in the 1960s and 1970s (clomiphene citrate [CC] and human menopausal gonadotropins [hMGs]) offered a nonsurgical approach to the treatment of anovulatory infertility that was safer than ovarian wedge resection. As a result, ovarian wedge resection was carried out rarely. CC therapy does not induce ovulation in all women. The alternative, hMG, is expensive, requires intensive monitoring, and may cause ovarian hyperstimulation. For clomiphene-resistant patients, laparoscopic techniques have many advantages over gonadotropin therapy, including serial repetitive ovulation events, no increased risk of ovarian hyperstimulation or multiple pregnancies, and a lower incidence of spontaneous abortion. These procedures are not the first-line treatment for anovulatory patients with polycystic ovarian syndrome (PCOS), for whom CC remains the primary therapy.24
Some endoscopists have accomplished ovarian wedge resection laparoscopically,25 whereas others have reduced ovarian volume by taking multiple biopsies,23,26 coagulating with monopolar current,27-29 and creating craters on the ovarian surface with lasers,30,31 or puncturing the small cysts on the ovarian surface.32 Broadly similar results have been obtained using biopsy, multielectrocoagulation, and laser surgery: 50% or greater ovulation rate and a mean pregnancy rate of 50%.24 Laparoscopic ovarian drilling appears to be associated with comparable rates of ovulation and conception (Table 3). Regardless of the method used to decrease ovarian mass, the hormonal changes observed with the laparoscopic procedure are similar to those observed after ovarian wedge resection by laparotomy.24,33Laparoscopic techniques offer cost savings and a lower risk of postoperative adhesions compared with wedge resection by laparotomy.24 A disappointing finding is that the risk of postoperative adhesions is high (average, 30%) in women undergoing ovarian drilling (Table 4). Although adhesions were less common after laparoscopic multiple biopsies, they were observed in about 90% of patients after resection by laparotomy, 30% after laparoscopic electrocoagulation, and 50% after laparoscopic vaporization.34 The possible effect of applying an oxidized regenerated cellulose (Interceed, Gynecare) barrier on postoperative surfaces after laparoscopic electrosurgical treatment for PCOS was studied in a prospective, randomized controlled study.35 After bilateral ovarian treatment, one ovary was chosen randomly to have Interceed applied to its surface, using a specially designed applicator, with the other ovary serving as a control. Periadnexal adhesions of significant extent and severity developed in 57% of the women and 38% of the adnexa. The incidence of adhesions on the Interceed-treated side was 43%, whereas on the control side, it was 33%. In addition, the extent and severity of the adhesions appeared to be similar on the Interceed-treated and control sides. Larger numbers are required to ascertain statistically the effects of Interceed on prevention of adhesions after laparoscopic electrosurgical treatment of PCOS.

Theoretically, wedge resection and ovarian drilling work by reducing androgen production in the ovarian stroma. Appropriate patients are women who fail to ovulate after 3 to 4 months on clomiphene and do not respond to HMG. The procedure is achieved by using a 10-mm video laparoscope coupled to a CO2 laser. A 5-mm second puncture is placed suprapubically in the midline and is used for a suction-irrigator or grasping instrument. Associated pelvic abnormalities are corrected before ovarian coagulation. Each ovary is fixed in the anterior cul-de-sac or held by the utero-ovarian ligament during treatment. The ultra pulse (40 to 80 W, 25 to 200 millijoules [mJ]) or superpulse (25 to 40W) CO2 laser is used. All visible sub capsular follicles are vaporized and drained, and randomly placed 2- to 4-mm- diameter craters are made in the ovarian stroma (Figure 10). Each ovary is treated symmetrically, and cysts are vaporized. The ovaries are irrigated, and hemostasis is obtained with bipolar forceps.
 Figure 10. Ovarian drilling is used for PCOD. While the ovarian ligament is grasped and the ovary is held, multiple surface craters are created by the CO2 laser, fiber laser, or needle electrode (inset).
Figure 10. Ovarian drilling is used for PCOD. While the ovarian ligament is grasped and the ovary is held, multiple surface craters are created by the CO2 laser, fiber laser, or needle electrode (inset).
A potassium titanyl phosphate, neodymium: yttriumaluminum-garnet (Nd: YAG), or argon laser can be used also.30,31 The fiber is threaded through the central channel of a special 5-mm dual-channel suction-irrigation probe. When the dual-channel probe is used, it is possible to suction the smoke from vaporization at the site of occurrence.Holes are drilled in the ovary in a manner similar to that described for the CO2 laser.
Ovarian coagulation has been done using unipolar punch biopsy forceps23 or a needle electrode.26-28 The power setting for the monopolar current is 20 to 30 W in a cutting mode to minimize thermal damage, and the power is activated just before the ovary is touched. The ovary is penetrated in approximately 10 to15 sites at a depth of 3 to 5 mm.
Ovarian Torsion
Adnexal torsion is a surgical emergency. When this is diagnosed early, the adnexa can be unwound.36,37 However, the diagnosis often is delayed because of the inconsistent presenting symptoms and signs and intermittent pain. When the diagnosis is delayed, the adnexa become congested, ischemic, hemorrhagic, and necrotic.38 Gynecologists have been taught to remove tissue that has undergone torsion and ischemia because of the risk of thrombotic embolism arising from the ovarian vein. Way reported successful conservative management of adnexa torsion.39 The affected structure was straightened to assess the viability, and even ovaries that appeared infracted at laparoscopy regained normal color after untwisting. No complications related to the procedure were reported. Because adnexa torsion produces no path gnomonic clinical findings, laparoscopy is used for diagnosis and treatment. Prompt laparoscopic examination is essential because delay is associated with gangrene.
A prospective, controlled follow-up study to designed to examine the effects of adnexal torsion on long-term ovarian histology and free radical scavenger (FRS) activity and subsequent viability after the detorsion of twisted ischemic adnexa. Adnexal torsion was created by twisting the adnexa three times and fixing onto the side wall or by applying vascular clips in cycling female rats at 70 days of age. After an ischemic period of 4 to 36 hours, the twisted adnexa were removed and fixed. In the second group of rats, after the above ischemic periods, the torsions were relieved by untwisting or removing the vascular clips. Then the animals were perfused for a week, and adnexa were extirpated.
After both ischemia and reperfusion, the removed adnexa were examined histologically and tissue concentrations of glutathione peroxidase, superoxide dismutase, catalase and glutathione were ascertained. Regardless of the ischemic time, all the twisted adnexa were black-bluish. Despite the gross ischemic time, all the twisted adnexa were black- bluish. Despite the gross ischemic-hemorrhagic features, histologic sections revealed negligible changes, with intact ovarian structure similar to that of controls in 4- to 24- hour groups. Although decreased compared with controls, the change in tissue concentrations of FRS was not significant in the 4-to-24-hour groups. Only the 36–hour group showed prominent congestion on all sections and a significant decrease in all FRS concentrations studied. Although no long-term reperfusion injury was observed histologically in the 4-to 24-hour groups, the 36-hour group ended up with adnexal necrosis. These findings support the importance of early diagnosis and conservative surgical management (detorsion) in adnexal torsion. Lack of histologic changes and unimpaired FRS metabolism are consistent with recent data showing that vascular compromise is caused by venous and lymphatic stasis in early torsion; adnexal integrity is not correlated with gross ischemic appearance, thus providing evidengce of adnexal resistance against ischemia.40
The cause of ovarian or adnexal torsion are Para ovarian cysts, functional and pathologic ovarian cysts, ovarian hyper stimulation, tubal pregnancy, adhesions, and congenital malformation.41,42 The ischemic structures are straightened gently with atraumatic forceps to avoid additional adnexal damage. In women with ovarian hyper stimulation, the functional cysts are drained before untwisting.43 The abnormalities contributing to torsion should be treated. One should shorten the utero-ovarian ligament if its length may have contributed to ovarian torsion. A running suture of monofilament material is placed along the length of the utero-ovarian ligament (Figure 11) and tied to shorten it, limiting ovarian mobility.
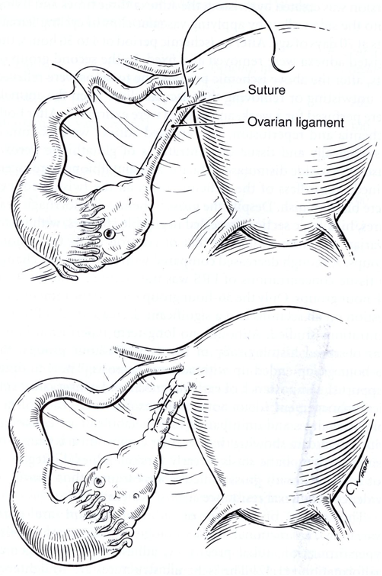 Figure 11. Ovarian suspension is achieved with a monofilament suture placed along the length of the utero-ovarian ligament and tied.
Figure 11. Ovarian suspension is achieved with a monofilament suture placed along the length of the utero-ovarian ligament and tied.
Mage and associates, in a report of 35 patients, noted that 21 women showed no gross evidence of ischemia of mild changes, with immediate and complete recovery within 10 minutes of untwisting.41 In eight, the tube or ovary was dark red or black, but partial recovery was apparent after the pedicle was untwisted. Six had gangrenous adnexa that required salpingectomy or oophorectomy. The first two groups were managed conservatively; the third group underwent excision of the involved organ(s). The postoperative course in all patients was uneventful. Six of the eight women in the intermediate group underwent a second-look laparoscopy that showed complete recovery.
Ovarian Remnant Syndrome
In premenopausal women who have had a bilateral oophorectomy, a small piece of functional ovarian tissue may respond to hormonal stimulation with growth, cystic degeneration, or hemorrhage and produce pain.44-46 In a rat model, Minke and colleagues showed that devascularized ovarian tissue can reimplant on intact or denuded peritoneal surfaces and that the revascularized tissue can become functional as evidenced by follicle formation and vaginal cornification.47
Ovarian remnants remain because of dense adhesions, and distorted anatomic relationships invariably worsen with subsequent operations. It is not unusually for these patients to have had previous attempts to excise an ovarian remnant. Removal of the ovarian remnant is preferred, although the reported incidence of complications with laparotomy ranges from 16% to 30%.48 The challenge and complications are related to the presence of extensive pelvic and abdominal adhesions multiple previous operations, endometriosis, pelvic inflammatory disease, or ovarian cysts.
Diagnosis is based on history and localization of pelvic pain. Whereas some patients have cystic adnexal structures or ill-defined fixed masses, others have normal pelvic findings. Vaginal ultrasound can help locate the ovarian remnant.49 Low or borderline levels of follicle-stimulating hormone in patients with documented bilateral oophorectomy are consistent with the presence of active ovarian tissue.44 Hormonal suppression with oral contraceptives or a gonadotropin-releasing hormone agonist provides no relief in most patients.10,50 CC or hMG is used to increase ovarian remnant size to confirm the diagnosis preoperatively or to aid in locating the tissue intraoperatively.51 Laparoscopic ultrasonography is used to detect ovarian remnants in patients in whom the pelvic anatomy is distorted by multiple adhesions.52
Past reviews have considered laparoscopy ineffective in the management of ovarian remnant syndrome because of the presence of dense pelvic adhesions.48 However, the absence of complications in series of 22 patients attests to the feasibility of the laparoscopic approach.10
Attention should be focused on prevention. Factors associated with ovarian remnant syndrome are the use of Endoloops for laparoscopic oophorectomy, multiple operative procedures with incomplete removal of pelvic organs, densely adherent ovaries, and multiple ovarian cystectomies for functional cysts.10 When pre-tied sutures are used for the infundibulopelvic ligament, they should be placed below the ovarian tissue.
Electrocoagulation and transaction of the infundibulopelvic ligament or the application of clips is preferred.
We believe that ORS is often the result of blunt dissection of ovarian adhesions. Failure to sharply dissect ovarian adhesions risks tearing the ovarian cortex and leaving a fragment of the ovary attached to either the surrounding adherent peritoneum or the viscera. We therefore avoid the blunt dissection of these adhesions. In addition, failure to open the retro peritoneum at adnexectomy most likely increases the risk of ORS. During any adnexectomy, whether by laparotomy or by laparoscopy, we incise the peritoneum lateral to the ovarian vessels and widely open the retroperitoneum. This approach allows easy and accurate identification of vital structures, including the ureters and the pelvic vessels.98 The surgeon can then safely ligate the ovarian blood supply well cephalad to the ovary and remove the adnexa and surrounding peritoneum en masse. We do not advocate the common technique of retracting the ovary medially with the transection of the vascular pedicle, using endoscopic staplers or surgical loops. Doing so risks leaving behind a fragment of the ovary behind. When the ovary is adherent to the pelvic side wall, retroperitoneal hydro dissection, meticulous adhesiolysis, and removal of the peritoneum underlying the ovary are essential in achieving a laparoscopic oophorectomy. The need for restraint in managing functional cysts is underscored by the fact that some patients in the author’s series had only a corpus luteum resected at first laparotomy.10
A preoperative bowel preparation of GoLYTELY (Braintree Laboratories), enemas, and oral metronidazole is indicated. Anterior abdominal wall adhesions are probable after multiple laparotomies, and an open laparoscopy or mapping technique is advisable.53
After all instruments are inserted, intra-abdominal adhesions are lysed and the ovarian remnants are dissected. Extensive and careful retroperitoneal dissection is required to facilitate identification and removal of the ovarian remnant tissue.54,55 The pelvic peritoneum lateral and parallel to the ovarian vessels is incised, and the retroperitoneum is opened widely. The paramedical and Para rectal spaces are dissected, and the anterior division of the internal iliac artery is divided. After the ureters are identified, the ovarian vessels are isolated and relegated with excision cephalad to the pelvic brim, above the level of the aortic bifurcation. High religation of the ovarian vessels ensures that no remnant ovarian tissue is missed along the vascular pedicle. This vascular segment is sent for routine pathologic assessment, because it may contain residual ovarian cortical tissue. The ureters are then mobilized laterally, off the pelvic sidewall peritoneum, and traced down to their entrance into the bladder. The dissection and ligation of the anterior division of the internal iliac artery facilitates the dissection of the ureters to their point of entry into the bladder. Dissection at this point allows complete resection of the pelvic sidewall peritoneum. In most patients, the remnant ovary lies on the pelvic sidewall peritoneum, near the angle of the vaginal vault, and it is encased in dense, fibrotic scar tissue. The residual ovary, however, may be located anywhere along the pelvic sidewall; thus, we recommend the stripping and excision of the entire pelvic sidewall peritoneum. The bladder is then dissected off the vaginal vault, and wide excision of the remnant is performed en bloc with the peritoneum. To ensure complete resection of all residual ovarian tissue, the surgeon should perform segmental resection of a portion of the vaginal vault, bladder, ureters, or adjacent bowel when the ovarian remnant is densely adherent to any of these surrounding structures. Any compromise of, or deviation from, these surgical principles risks potential persistence of the patient’s symptoms.
When the remnant is adherent to the bowel, adhesions are lysed using hydrodissection and the CO2 laser. Ovarian tissue embedded in the muscularis of the bowel is removed superficially, skinning the mucosa beneath it. The serosa and muscularis layers are imbricated with one to three interrupted 4-0 polydioxanone sutures in one layer. All remnant ovarian tissue should be removed. When the lesion is embedded in the bowel or bladder muscularis or when the ureter is involved or possibly obstructed, partial removal of the organ and repair are necessary.
Oophoropexy
In an attempt to protect ovarian function in young females given radiation therapy for HL, oophoropexy was first performed at the time of surgical staging in 1968 at Stanford University Medical Center, where the efficacy of this procedure in retaining ovarian function and enabling pregnancy was demonstrated in 60% of patients.92 Recent studies showed that ovarian function was preserved in 88.6% of patients under 40 years old.93,97
The debate concerning long-term cancer survivors and their future state of health and fertility is gaining more attention.
The use of oophoropexy is not restricted to HL but could be considered in all cases for which pelvic irradiation is planned. The technique is feasible with use of a laparoscopic approach; it is technically straightforward, and a portion of ovary can be collected for cryopreservation. Indications for laparoscopic ovarian transposition cover a fairly wide spectrum of neoplastic diseases in the literature: cervical cancer94,95 Hodgkin’s disease95 cauda equina ependymoma,95 and anal canal cancer.95
Successful preservation of ovarian function is dependent on two factors: the dose of radiation delivered to the ovaries and the age of the patient.96 Because the rate of ovarian preservation is low after the age of 40 years, ovarian transposition should be performed only in patients who are <40 years of age.
A pneumoperitoneum was achieved and a 10-mm trocar was inserted just below the umbilicus. The uterus was not systematically probed. Three suprapubic 5-mm trocars were placed (one medially and two laterally). When laparoscopic lymphadenectomy was performed, a 10-mm trocar was introduced medially to extract the lymph nodes. The operative procedure began with complete surgical staging and careful inspection of the ovaries, peritoneum, and entire abdominopelvic cavity.
Peritoneal fluid sampling and peritoneal washing were performed. The ovaries were mobilized and grasped. The ureters were identified through the peritoneum. The uteroovarian ligament was cauterized and severed using bipolar forceps for coagulation and scissors alternatively. The fallopian tubes were separated from the ovaries through the mesovarium. The peritoneum then was incised along the infundibulopelvic ligament to mobilize the ovaries completely.
Dissection of the ovarian vessels was performed up to the level of the aortic bifurcation. The ovaries were transposed laterally to the paracolic gutters and fixed securely with the use of two transaponeurotic sutures. The ovarian vessels were not tunneled. The left ovary was placed at the level of the aortic bifurcation and the right ovary was placed above the pelvic brim, between the level of the aortic bifurcation and the lower pole of the right kidney.
Two metal clips were applied to each transposed ovary to guide subsequent roentgenographic localization. When the ovaries were macroscopically normal, they were both transposed. Only the remaining ovary was transposed after contralateral adnexectomy in the patient who had an ovarian dysgerminoma.
Evaluation of ovarian function
Ovarian function was assessed by a routine postoperative ultrasonographic (US) scan and by measurement of gonadotropin and E2 levels 6 months after the transposition. This assessment was repeated when menstrual disorders and/or hot flashes were observed.
Ovarian function was considered normal when the FSH level was <10 mIU/mL, when the E2 level was >50 pg/mL, and when follicles were present on the US scan.97
References
- Yuezpe AA, Rioux JE. The value of laparoscopic ovarian biosy. J Reprod Med. 1975;15:57.
- Nezhat C, Nezhat F. Postoperative adhesion formation after ovarian cystectomy with and without ovarian reconstruction. Abstract presented at: American Fertility Society Annual Meeting; October 21, 1991; Orlando,FL.
- Semm K. Course of endoscopic abdominal surgery. In:Friedrich E, ed. Operative Manual for Endoscopic Abdominal Surgery. Chicago:Year Book;1984.
- Nezhat F, Nezhat C, Silfen SL. Videolaseroscopy for oophorectomy. Am J Obstet Gynecol. 1991; 77:482.
- Silva PD, Juffel ME, Beguin EA. Open Laparoscopy simplifies instrumentation required for laparoscopic oophorectomy and salpingo-oophorectomy. Obstet Gynecol. 1971;77:482.
- Ressell JB. Laparoscopic oophorectomy. CurrOpin Obstet Gynecol. 1995;7:295.
- Nezhat C, Nezhat F, Silfen SL. Laparoscopic hysterectomy and bilateral salpingo- 00phorectomy using multifire GIA surgical stapler. J Gynecol Surg. 1990; 6:287.
- Daneill JF, Jurtz BR, Lee JY. Laparoscopic oophorectomy:comparative stady of ligatures, bipolar coagulation and automatic stapling devices. OBstect Gynecol. 1992;80:325.
- Nezhat C, Nezhat F, Winer W. Salpingectomy via laparoscopy: a new surgical approach. J Laparosc Surg. 1991;1:91.
- Nezhat C, Nezhat F. Operative laparoscopy for the management fo ovarian remnant syndrome. Fertil Steril. 1992;57:1003.
- Sigel B, Dum MR. The mechanism of blood vessel closure by high frequency electrocoagulation. Surg Gynecol Obstet. 1965;121(4):823.
- Nezhat C, Nezhat F. Laparoscopic electrosurgical oophorectomy; risk of using “blanching” as the end point [letter to the editor]. Am J Obstet Gynecol. 1992;167:1151.
- Semm K. Operative Manual for Endoscopic Abodominal Surgery. Chicago:Year Book;1984.
- Campo S, Garcia N. Laparoscopic gonadectomy in two patients with gonadal dysgenesis. J Am Assoc Gynecol Laparoc. 1998;5:305.
- Kriplani A, Abbi M, Ammini AC, et al. Laparoscopic gonadectomy in male pseudohermaphrodites. Eur J Obstet Gynecol Reprod Biol. 1998; 81:37.
- Ulrich U, Keckstein J, Buck G. Removal of gonads in Y-chromosome-bearing gonadal dysgenesis and in androgen insensitivity syndrome by laparoscopic surgery. Surg Endosc. 1996; 6:566.
- Droesch K, Dorexch J, Chuma J, et al. Laparoscopic gonadectomy for gonadal dysgenesis. Fertil Steril. 1990; 53:360.
- Seifer DB. Laparoscopic adnexectomy in a prepubertal Turner mosaic female with isodicentric Y. Hum Reprod. 1991;6:566.
- Nezhat C, Nezhat F. Safe laser excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149.
- Nezhat C, Nezhat F,Welander CE, et al. Four Ovarian cancers diagnosed during laparoscopic management of 1011 adnexal massed. Am J Obstet Gynecol. 1992;167:790.
- Nezhat F, Brill AI, Nezhat CH, et al. Adhesion formation after endoscopic posterior colpotomy. J Reprod Med. 1993; 38:534.
- Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181.
- Gjonnaess H. Polycystic ovarian syndrome treated by ovarian electrocautery through the laparoscope. Fertil Steril. 1984;41:20.
- Judd HL, Rigg LA, Anderson DC, et al. The effects of ovarian wedge resection on circulating gonadotropin and ovarian steroid levels in patients with polycystic ovarian syndrome. J Clin Endocrinol Metab. 1976; 43:347.
- Kojima E. Ovarian wedge resection with contact Nd:YAG laser irradiation used laparoscopically. Reprod Med. 1989;34:444.
- Campo S, Garcia N, Caruso A, et al. Effect of celioscopy ovarian resection in patients with polycystic ovaries. Gynecol Obstet Invest. 1983;15:213.
- Aakvaag A. Hormanal response to electrocautery of the ovary in patients with polycystic ovarian disease. Br J Obstet Gynaecol. 1985;92:1258.
- Casper RF, Greenblatt EM. Laparoscopic ovarian cautery for induction of ovulation in women with polycystic ovary disease. Semin Reprod Endocrinol. 1990;8:209.
- Kovacs G, Buckler H, Bangah M, et al. Treatment of anovulation due to polycystic ovarian syndrome by laparoscopic ovarian electrocautery. J Obstet Gynecol. 1991;98:30.
- Keckstein G, Rossmainith W, Spatzier K, et al. The effect of laparoscopic treatment of polycystic ovarian disease by CO2 laser or ND-YAG laser. Surg Endosc. 1990;4:10-3.
- Daniell JF, Miller W. Polycystic ovaries treated by laparoscopic laser vaporization. Fertil Steril. 1989;51(2):232.
- Sumioki H, Utsunomyiya T, Matsuoka K, et al. The effect of laparoscopic multiple punch resection of the ovary on the hypothalamo-pituitary axis in polycystic ovary syndrome. Fertil Steril. 1988;50:567.
- Adashi EY, Bock JA, Guzick D, et al. Fertility following bilateral ovarian wedge resection:a critical analysis of 90 consecutive cases of the polycystic ovary syndrome. Fertil Steril. 1981;36:320.
- Campo S. Ovulatory cycles, pregnancy outcome and complications after surgical treatment of polycystic ovarian syndrome. Obstet Gynecol Surv. 1998; 53:297.
- Saravelos H, Li TC. Post-operative adhesions after laparoscopic electrosurgical treatment for polycystic ovarian syndrome syndrome with the application of Interceed to one ovary: a prospective randomized controlled study. Hum Reprod. 1996;11:1992.
- Oelsner G, Bider D, Goldenberg M, et al. Long-term follow-up of the twisted ischemic adnexa manged by detorsion. Fertil Steril. 1993;60:976.
- Shalev E, Bustan M, Yarom I, Peleg D. Recovery of ovarian function after laparoscopic detorsion. Hum Reprod. 1995;10:2965.
- Steyaert H, Meynol F, Valla JS. Torsion of the adnexa in children: the value of laparoscopy. Pediatr Surg Int. 1998;13:384.
- Way S. Ovarian cystectomy of twist cysts. Lancet. 1996; 2:47.
- Taskin O, Bririnciogulu M, Aydin A, et al. The effects of twisted ischaemic adnexa managed by detorsion on the ovarian viability and histology: an ischaemia-reperfusion rodent model. Hum Reprod. 1998;13:2823.
- Mage G, Ganis M, Manhes H, et al. Laparoscopic management of adnexal torsion. J Reprod Med. 1989;34:520.
- Wagaman R, Williams RS. Conservative therapy for adnexal torsion. J Reprod Med. 1990;35:833.
- Ben-Rafael Z, Bider D, Mashiach S. Laparoscopic unwinding of twisted ischemic hemorrhagic adnexum after in vitro fertilization. Fertil Steril. 1990;53:569.
- Petit PD, Lee RA. Ovarian remnant syndrome: diagnostic dilemma and surgical challenge. Obstet Gynecol. 1988;71:580.
- Siddall-Allum J, Rae T, Rogers V, et al. Chronic pelvic pain caused by residual ovaries and ovarian remnants. J Obstet Gynecol. 1994;101:979.
- Orford VP, Kuhn RJ. Management of ovarian remnant syndrome. Aust N Z J Obstet Gynecol. 1996;36:468.
- Minke T, DePond W, Winkelmann T, Blythe J. Ovarian remnant sysndrome:study in laparatory rats. Am J Obstet Gynecol. 1994;171:1440.
- Price FV, Edwards R, Buschsbaum HJ. Ovarian remnant syndrome:difficulties I diagnosis and management. Obstet Gynecol Surv. 1990;45:151.
- Fleischer AC, Tait D, Mayo J, et al. Sonographic features of ovarian remnants. J Ultrosound Med. 1998;17:551.
- Siddall-Allum J, Rae T, Rogers V, et al. Chronic pelvic pain caused by redidual ovaries and ovarian remnants. Br J Obstet Gynaecol. 1994;101:979.
- Kaminski PF, Sorosky JI, Mandell MJ, et al. Clomiphene citrate stimulation as an adjunct in locating ovarian tissue in ovarian remnat syndrome. Obstet Gynecol.1990;76:924.
- Nezhat F, Nezhat C, Nezhat CH, et al. Use of laparoscopic ultrasonography to detect ovarian remnants. Am J Obstet Gynecol. 1996;174:641.
- Nezhat C, Nezhat F, Silfen SL. Videolaparoscopy: the CO2 laser for advancer operative laparodcopy. Obstet Gynecol Clin North Am. 1991;18:3:585.
- Lafferty HW, Angioli R, Rudolph J, Penalver MA. Ovarian remnant syndrome: experience at Jackson Memorial Hospital, University of Miami, 1985 through 1993. Am J Obstet Gynecol. 1996;174:641.
- Kamprath S, Possover M, Schneider A. Description of a laparoscopic technique for treating patients with ovarian remnant syndrome. Fertil Steril. 1997;68:663.
- Goldzieher JW, Green JA. The polycystic ovary: clinical and histogical features. J Clin Endocrinol Metab. 1962;22:325.
- Buttram VC, Vaquero C. Post-ovrian wedge resection adhesive disease. Fertil Steril. 1975;26:874.
- Lunde O. Polycystic ovarian syndrome: a retrospective study of the therapeutic effect of ovrian wedge resection after unsuccessful treatment with clomiphere citrate. Ann Chir Gynaecol. 1982;71:330.
- Hjortrup A, Kehlet H, Lockwood K et al. Long term clinical effects of ovarian wedge resection in polycystic ovarian syndrome. Acta Obstet Gynecol Scand. 1983;62:55.
- Ronnberg L, Ylostalo P, Ruokonen A. Hormonal parameters and conception rate during 5 different types of treatment of polycystic ovarian ayndrome. Int J Gynaecol Obstet. 1985;23:177.
- Stein Wedge resection of the ovaries: the Stein Leventhal syndrome. In: Greenblatt RB, ed. Ovulation. Philadelphia: Lippincott;1966.
- Weinstein D, Polishuk WZ. The role of wedge resection of the ovary as a cause for mechanical sterility. Surg Gynecol Obstet. 1975;141:417.
- Toaff R, Toaff ME, Peyser MR. Infertility following wedge resection of the ovaries. Am J Obstet Gynecol. 1976;124:92.
- Portuondo JA, Melchor JC, Neyro JL, et al. Periovarian adhesions following ovarian wedge resection or laparoscopic biopsy. Endoscopy. 1984;16:143.
- Greenblatt R, Casper RF. Endocrine changes after laparoscopic ovarian cautery in polycystic ovary syndrome. Am J Obstet Gynecol. 1987;156:279.
- Armar NA, McGarrigle HHG, Honour J, et al. Laparoscopic ovarian diathermy in the management of ovulatory infertility in women with polycystic ovaries: endocrine changes and clinical outcome. Fertil Steril. 1990;53:45.
- Gjonnaess H. Ovarian electrocautery in the treatment of women with polycystic ovary syndrome(PCOS): factors affecting the results. Acta Obstet Gynecol Scand. 1994;73:407.
- Campo S, Felli A, Lamanna MA, et al. Endocrine changes and clinical outcome after laparoscopic ovarian resection in women with polycystic ovaries. Hum Reprod. 1993;8:359.
- Armar NA, Lachelin GC. Laparoscopic ovarian diathermy: an effective treatment for anti-oestrogen resistant anovulatory infertility in women with polycystic ovaries. Br J Obster Gynaecol. 1993;100:161.
- Gurgan T, Urman B, Aksu T, et al. The effect of short-interval laparoscopic lysis of adhesions on pregnancy rates following Nd-YAG laser photocoagulation of polycystic ovaries. Obstet Gynecol. 1992;80:45.
- Naether OG, Baukloh V, Fischer R, Kowalczyk T. Long-term Follow-up in 206 infertility patients with polycystic ovarian syndrome after laparoscopic electrocautery of the ovarian surface. Hum Reprod. 1994;9:2342.
- Gurgan T, Kisnici H, Yarali H, et al. Evaluation of adhesion for mation after laparoscopic treatment of polycystic ovarian disease. Fertil Steril. 1991;56:1176.
- Dabirashrafi H, Mohamad K, Behjanatia Y, et al. Adhesion formation after ovarian electrocauterization on patients with polycystic ovarian syndrome. Fertil Steril. 1991;55:1200.
- Naether OG, Fischer R. Adhesion formation after laparoscopic electrocoagulation fo the ovarian surface in polycystic ovary patients. Fertil Steril. 1993;60:95.
- Naether OG, Fischer R, Weise HC, et al. Laparoscopic electrocoagulation of the ovarian surface in infertile patients with polycystic ovarian disease. Fertil Steril. 1993;60.
- Keckstein G, Rossmanith W, Spatzier K, et al. The effect of laparoscopic treatment of polycystic ovarian disease by CO2-leser or Nd:YAG laser. Surg Endosc. 1990;4:103.
- M. Gershenson. Contemporary management of borderline ovarian tumors, Cancer Invest. 1999;17:206-210.
- Seracchioli, S. Venturoli, F.M. Colombo, F. Govoni, S. Missiroli and A. Bagnoli. Fertility and tumor recurrence rate after conservative laparoscopic management of young women with early-stage borderline ovarian tumors, Fertil Steril. 2001;76:999–1004.
- T. Creasman, R. Park and H. Norris. Stage I borderline ovarian tumors. Obstet Gynecol. 1982;59:93–96.
- D. Tazeelar, D.G. Bostwick and S.C. Ballon. Conservative treatment of borderline ovarian tumors. Obstet Gynecol. 1985;66:417–422.
- R. Barnhill, R.J. Kurman and M.F. Brady. Preliminary analysis of the behaviour of stage I ovarian serous tumors of low malignant potential: a Gynecologic Oncology Group study. J Clin Oncol. 1995;13:2752–2756.
- Wakahara, F. Kikkawa, A. Nawa, K. Tamakoshi, K. Ino and O. Maeda et al. Diagnostic efficacy of tumor markers, sonography, and intra-operative frozen section for ovarian tumors. Gynecol Obstet Invest; 2001;52:147–152.
- Maneo, M. Vignali, S. Chiari, A. Colombo, C. Mangioni and F. Landoni. Are borderline tumors of the ovary safely treated by laparoscopy? Gynecol Oncol. 2004;24:387–392.
- Nezhat, F. Nezhat and M. Burrel. Laparoscopically assisted hysterectomy for the management of a borderline ovarian tumor: a case report. J Laparoendosc Surg. 1992;2:167–169.
- Camatte, P. Morice, D. Atallah, A. Thoury, P. Pautier and C. Lhommé et al.. Clinical outcome after laparoscopic pure management of borderline ovarian tumors: results of a series of 34 patients. Ann Oncol. 2004;15:605–609.
- Fauvet, J. Boccara, C. Dufournet, C. Poncelet and E. Darai. Laparoscopic management of borderline ovarian tumors: results of a French multicenter study. Ann Oncol. 2005;16:403–410.
- Ilvan, R. Ramazanoglu, E. Ulker Akyildiz, Z. Calay, T. Bese and N. Oruc. The accuracy of frozen section (intraoperative consultation) in the diagnosis of ovarian masses, Gynecol Oncol. 2005;97:395–399.
- Tinelli R. Malzoni M. Cosentino F. Perone C. Tinelli A. Malvasi A. Cicinelli E. Feasibility, safety, and efficacy of conservative laparoscopic treatment of borderline ovarian tumors. Fertility & Sterility. 2009 Aug;92(2):736-41.
- Bisharah and T. Tulandi. Laparoscopic preservation of ovarian function: an underused procedure. Am J Obstet Gynecol. 2003;188:367–370.
- Le Floch, S.S. Donaldson and H.S. Kaplan. Pregnancy following oophoropexy and total nodal irradiation in women with Hodgkin’s disease. Cancer. 1976;38:2263– 2268.
- Morice, D. Castaigne, C. Haie-Meder, P. Pautier, J. El Hassan and P. Duvillard et al., Laparoscopic ovarian transposition for pelvic malignancies: indications and functional outcomes, Fertil Steril. 1998;70:956–960.
- Le Floch, S.S. Donaldson and H.S. Kaplan. Pregnancy following oophoropexy and total nodal irradiation in women with Hodgkin’s disease. Cancer. 1976;38:2263– 2268.
- Husseinzadeh, W.A. Nahhas, D.E. Velkley, C.W. Whitney and R. Mortel. The preservation of ovarian function in young women undergoing pelvic irradiation therapy. Gynecol Oncol. 1984;18:373–379.
- A. Prouvost, M. Canis, G. Le Bouedec, J.L. Achard, G. Mage and J. Dauplat. Laparoscopic ovariopexy before vaginal brachytherapy in stage IA and IB cervical cancer. J Gynecol Obstet Biol Reprod. 1991;20:361–365.
- B. Clough, F. Goffinet, A. Labib, C. Renolleau, F. Campana and A. De la Rochefordière et al., Laparoscopic unilateral ovarian transposition prior to irradiation. Prospective study of 20 cases, Cancer. 1996;77:2638–2645.
- Haie-Meder, N. Mlika-Cabanne, G. Michel, E. Briot, A. Gerbaulet and C. Lhommé et al. Radiotherapy after ovarian transposition: ovarian function and fertility preservation. Int J Radiat Oncol Biol Phys. 1993;25:419–424.
- U. Bieler, T. Schnabel and J. Knobel. Persisting cyclic ovarian activity in cervical cancer after surgical transposition of the ovaries and pelvic irradiation. Br J Radiol. 1976;49:875–879.
- Magtibay PM, Nyholm JL, Hernandez JL, et al. Ovarian remnant syndrome. Am J Obstet Gynecol. 2005;193:2062–2066.
- Denes FT. Cocuzza MA. Schneider-Monteiro ED. Silva FA. Costa EM. Mendonca BB. Arap S. The laparoscopic management of intersex patients: the preferred approach. BJU International. 2005 Apr;95(6):863-7.
