Endoscopic Component Separation
Lawrence C. Biskin, MD
INTRODUCTION
The objective of abdominal wall reconstruction is to restore the structural and dynamic integrity of the abdominal wall that covers and protects the intra-abdominal viscera while minimizing complications and optimizing aesthetic body contour1.
Reconstruction of large and complicated abdominal wall hernias remains a challenging problem for surgeons. Primary suture repair of ventral hernias is associated with high recurrence rates2,3,4,5, but with the application of synthetic mesh the recurrence rates decreased significantly4,5,6,7,8,9,10. Mesh implantation may result in adhesions (despite the use of various mesh coatings), enterocutaneous fistulae, and infection. The incidence of mesh infection after open ventral hernia repair may be up to 15 percent11.
Dealing with infected mesh after hernia repair is a vexing problem and is one that surgeons would like to avoid. Autogenous tissue repair that reliably restores the structural integrity of the abdominal wall without the use of synthetic material is optimal.
The repair of infected ventral hernias has been successful with techniques using autogenous tissues such as the creation of myocutaneous and myofascial flaps, harvesting fascial grafts12, and component separation techniques. However, with extensive tissue dissection or the creation of donor sites, the associated wound complications and morbidity can be high13,14.
Endoscopically-assisted component separation minimizes the lengthy subcutaneous undermining with resulting minimal blood loss, elimination of tissue ischemia, and an improved recovery time by nearly 50 percent15,16,17,18.
This chapter will outline the technique for endoscopic component separation (ECS) and how to avoid the associated risks of the procedure.
HISTORY
In 1946, Wangensten reported the repair of large abdominal defects by pedicled to fascial flaps19. In 1983, Ger and Duboys13 described muscle transposition; however, denervation resulted in muscle atrophy and abdominal wallprotuberance.
In 1990, Ramirez and colleagues20 pioneered the open component separation (OCS) technique. They used bilateral abdominal musculofascial complexes transposed medially to reconstruct ventral abdominal wall defects up to 20 cm at the waistline without the use of prosthetic mesh.
This open technique requires the dissection of skin and subcutaneous fatty tissue off the anterior rectus sheath as well as the external oblique aponeurosis. This allows transaction of the external oblique aponeurosis longitudinally from the costal margin to the level of the inguinal ligament. The external oblique muscle is then separated from the underlying internal oblique muscle. The extensive tissue dissection and undermining not only leads to large seromas and hematomas, but by interrupting the blood supply to the abdominal wall there is an increased rate of tissue ischemia with resultant wound infection and wound dehiscence. Complication rates for open component separation up to 41 percent have been reported21, 22, 23, 24, 25, 26, 27, 28.
Saulis and Dumainian29 modified the open technique by preserving the peri-umbilical perforating vessels and noted a profound reduction in wound ischemia. However, the dissection still resulted in large hematomas and seromas.
In 1997, Lowe et al30 introduced the concept of using balloon dissectors and endoscopy to facilitate component separation. Around the same time modifications of the technique by Mass et al31 simplified the endoscopic approach.
In 2007, Rosen et al17 improved on the endoscopic technique by successfully avoiding the lengthy subcutaneous undermining with avascular separation of parts. Rosen and colleagues18 showed that there was no statistically significant difference in the amount of abdominal wall mobilization between ECS and OCS.
ABDOMINAL WALL ANATOMY
The external oblique muscle (EOM) originates from the outer surface of the lower eight ribs and is inserted into the anterior one-half of the iliac crest, the pubic tubercle and crest, the linea alba, and the xiphoid process. The lower border of the aponeurosis is folded backwards forming the inguinal ligament. The posterior border is free. Lateral dissection can stop at this border.
The internal oblique muscle (IOM) runs deep and at right angles to the external oblique muscle. It originates from the lateral inguinal ligament, anterior iliac crest, and lumbar fascia. It is inserted into the lower three ribs, xiphoid process, linea alba, and pubic symphysis.
The transversus muscle (TM) originates deep to the internal oblique muscle from the lower six costal cartilages, lumbar fascia, anterior iliac crest, and lateral inguinal ligament. It inserts into the pubic symphysis, linea alba, and the xiphoid process. The posterior borders of the internal oblique muscle and transversus muscle are attached to the lumbar vertebrae by the lumbar fascia.
The skin and subcutaneous tissues are supplied directly by the superficial circumflex iliac artery, the superficial inferior epigastric artery, and by the deep inferior and superior epigastric arteries. The perforators of the deep epigastric arcade supply the rectus muscle and the overlying skin in the midline.
The anterior rami of the lower six thoracic and the first lumbar nerves pass forward between the internal oblique muscle and transversus muscle to supply the anterior abdominal wall muscles and parietal peritoneum. Sensory branches of the middle and lower abdomen, groin, and scrotum also lie between these muscles. It is therefore important not to separate the internal oblique muscle and transversus muscle to preserve abdominal wall sensation and function. Dissection in the avascular plane between the EOM and IOM preserves the innervation of the rectus muscle.
ENDOSCOPIC COMPONENT SEPARATION (ECS) TECHNIQUE
- The patient is positioned supine on the operating table with the arms tucked at their sides. This allows freedom of movement around the patient without the armboards interfering.
- In the non-infected field, the ventral hernia is first approached by resecting the cicatrix, excising the sac and reducing the hernia. The opening in the abdomen allows insertion of the surgeon’s hand into the abdominal cavity for abdominal wall manipulation during endoscopic component separation.
- With infected mesh it may be more prudent to perform the ECS first to avoid cross- contamination into the lateral compartments.
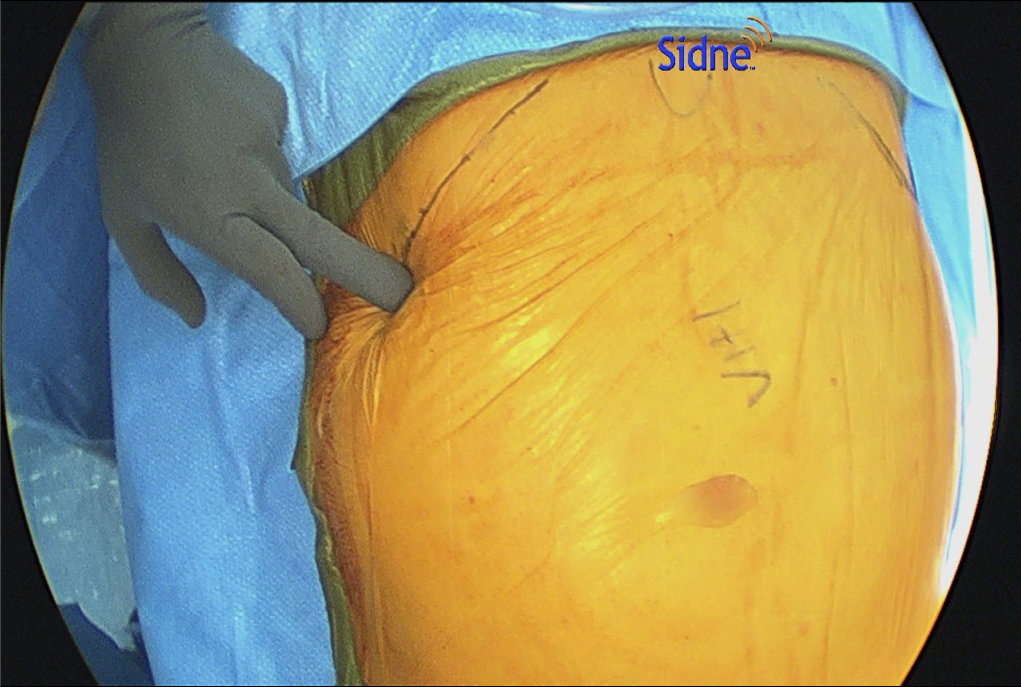
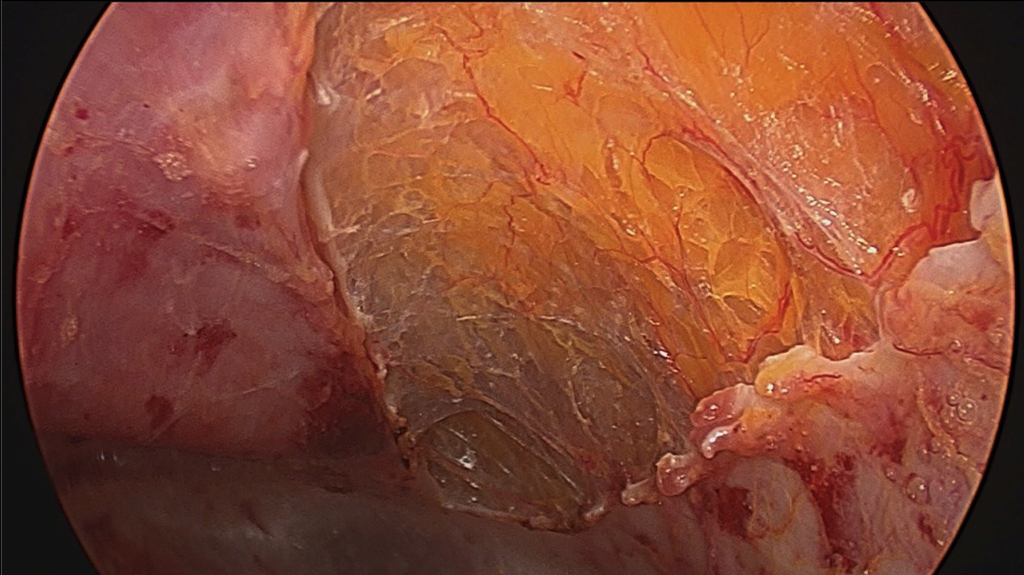
- When performing ECS, the most important part is entering the correct space. This is best done by making a 1.5-cm incision below the costal margin along the anterior axillary line. The surgeon must be sure to be lateral to the rectus muscle to avoid entering the wrong plane (Figure 1). Using S-retractors and a Kelly clamp, the subcutaneous tissues are bluntly dissected to expose the external oblique aponeurosis. The external oblique aponeurosis is incised and the internal oblique muscle is identified below. An excellent option, especially for obese patients, is the use of the optical trocar for muscle dissection. This trocar allows quick and easy tissue identification and navigates the abdominal wall layers for controlled entrance between the EOM and IOM (Figure 2).
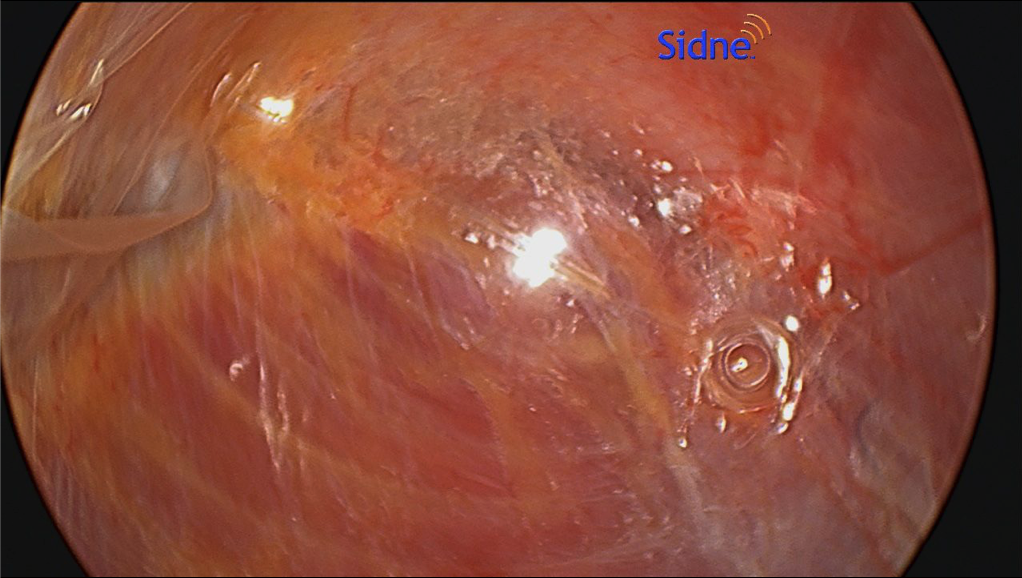
- Sterile lubricant is injected into the opening to facilitate balloon dissection of the space between the muscles. This is performed in a similar fashion as when creating the preperitoneal space in the inguinal TEP repair. Previous experience with the balloon dissector will make this part of the operation an easier transition. The unilateral balloon allows a more controlled dissection than the bilateral balloon; however, this is the surgeon’s preference.
- A blunt-tip or structural balloon port is then inserted and the space is insufflated at a pressure of 10 to 12 mmHg.
- One 5-mm port is inserted just cephalad to the inguinal ligament and another at approximately the level of the umbilicus at the mid- to post-axillary line (Figure 3). This will allow adequate longitudinal and transverse dissection. A 30-degree, 10-mm scope is utilized; however, a 5-mm angled scope is also required to alternate trocars.
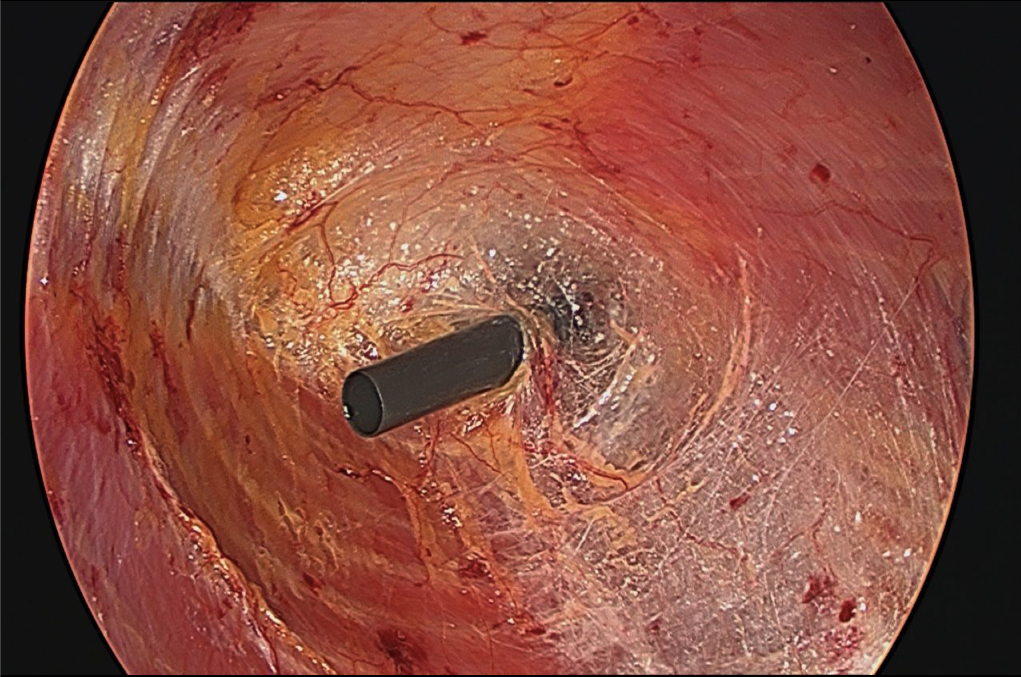
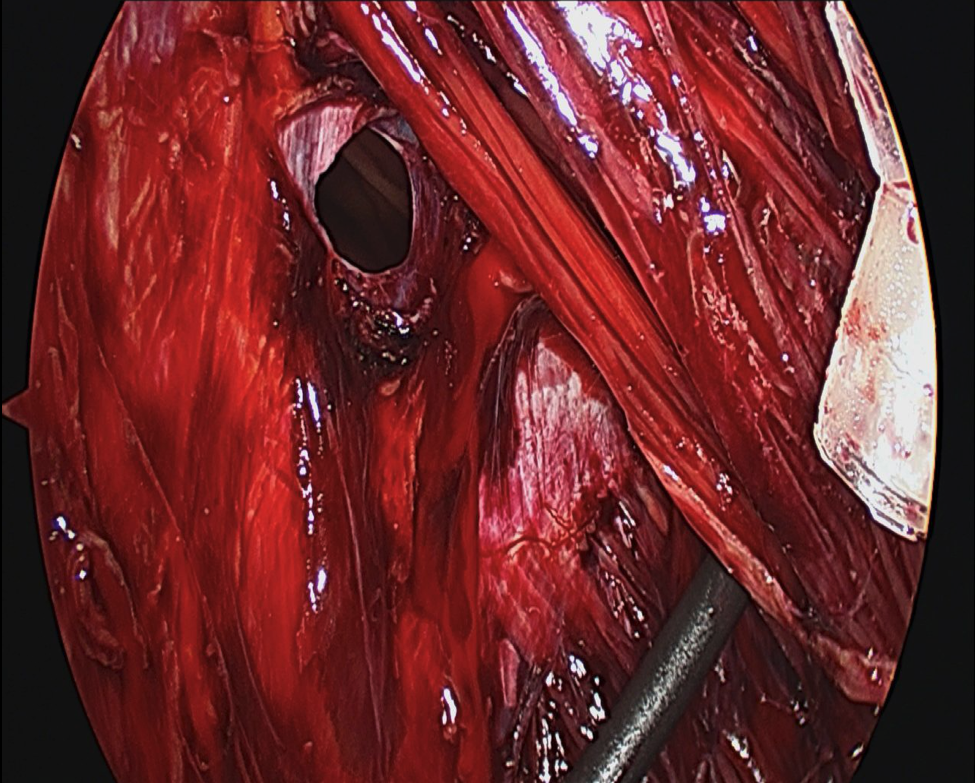
- Approximately 2 cm lateral to the linea semilunaris, using cauterizing sharp dissection, the external oblique aponeurosis is released longitudinally from the costal margin to the inguinal ligament (Figure 4).
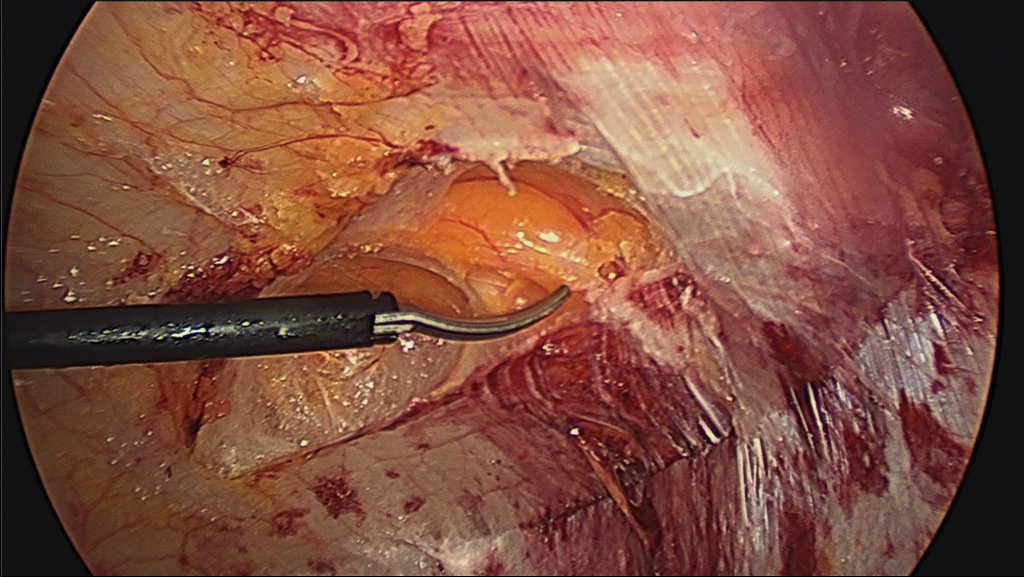
- The same technique is performed on the contralateral side.
- Closed suction drains are not necessary since the space is essentially avascular32.
- The muscular complex is then transposed to the midline. On each side approximately 4 cm can be gained in the upper third abdomen, 8 to 10 cm in the middle, and 3 cm in the lower third18 (Figure 5). If additional fascial translation is required, the posterior rectus sheath can be separated from the rectus muscle for 2 cm further advancement. It is possible to close very large abdominal wall defects with minimal morbidity11.
- Reinforcement with mesh (biologic or synthetic) is at the discretion of the Whether the mesh is placed in an overlay, underlay, open, or laparoscopic fashion is determined by the surgeon at the time of repair.
ABDOMINAL WALL DYNAMICS
Increased compliance of the abdominal wall is more important than the rate of recovery of wound tensile strength as a mechanical property to predict low hernia recurrence rates33. As the size of implanted mesh increases, the abdominal wall becomes less pliable resulting in a rigid, fixed abdominal wall.
Shestak et al26 found that after component separation for abdominal wall reconstruction several patients showed a 40 percent improvement in their ability to generate truncal flexion force. Patients with large defects have poor dynamics of the abdominal wall impairing some of their activities of daily living; however, with myofascial medial transposition the mechanics of the abdominal wall improve greatly.
Component separation provides a more anatomically correct and innervated abdominal wall reconstruction over the adynamic mesh repair by mobilizing the EOM. Accidental dissection between the internal oblique and transversus muscles may potentially injure the neurovascular bundles of the rectus muscle and interfere with muscle dynamics.
INDICATIONS FOR COMPONENT SEPARATION
The technique is not indicated for small or average size, uncomplicated hernias since these can be repaired with the standard open or laparoscopic techniques.
Component separation should be considered in the following situations:
- Infected abdominal wall with or without mesh.
- Patients with hernias who are also having a colostomy reversed.
- Very large ventral hernias.
- Multiple defects.
- Multiple failed attempts at previous repairs.
- Treatment or prevention of the abdominal compartment syndrome (ACS). May avoid open wound management which usually results in a large ventral hernia and potential skin deficit.
- In those patients with Loss of Domain, component separation allows placement of a smaller piece of mesh, thereby minimizing eventration.
Caution should be exercised in those patients who have scarring from lateral incisions as well as those with stomas since dissection will be restricted and tedious. Also, lateral hernias prevent the use of bilateral fascial transposition and surrounding tissue mobility will be limited by the ribs and iliac crest.
Delaying the operation until any risk factors (obesity, smoking, COPD, malnutrition, steroids) can be eliminated will greatly improve the outcome of the operation.
RECURRENCE
Re-creation of the linea alba via tensionless coaptation is important to achieve wound healing34 and provides an anchor for the lateral abdominal myofascial tissue which minimizes the chance for recurrence.
Adequate transection of the external oblique muscle on the thoracic wall over a 5-cm distance cephalad of the costal margin is of utmost importance to prevent undue tension on the fascia in the upper abdomen35. Placing the cephalad trocar too high will impede proper dissection and restrict transection of the muscle.
To achieve a tension-free closure, dissection to the posterior axillary line may be necessary for adequate midline mobilization. Further mobilization is achieved with separation of the posterior rectus sheath from the rectus muscle with attention to avoid injury to the neurovascular supply that enters the rectus muscle posteriorly.
If dissection extends too deep and into the internal oblique muscle (Figure 6), the innervation to the rectus muscle may be injured, or the spigelian fascia may be damaged which can predispose the patient to a spigelian hernia.
The average recurrence rate for open component separation is around 6 percent36. For endoscopic component separation it is as low; however, follow-up time isn’t as long17. Consistent patient and disease factors must be taken into account to achieve optimal comparison.
TISSUE NECROSIS
Tissue ischemia and necrosis is a complication rarely seen with endoscopic component separation. ECS preserves the blood supply and transposes innervated and vascularized tissue.
In a patient with prior lateral scarring the blood supply from the intercostal arteries may be compromised and if the musculocutaneous perforators of the epigastric arteries are transected, as in OCS, ischemia will result.
INFECTION
The component separation technique offers several advantages over other hernia repairs, most notably avoidance of mesh in a potentially contaminated field. Advantages of ECS versus OCS are mostly related to wound problems.
Gonzalez et al37 determined that the infection rate was significantly higher in OCS (33 percent) versus ECS (2 percent).
In the case of abdominal wall infection, the mesh is usually removed and primary closure at the midline is the optimal treatment. ECS done in a non-contaminated space prior to manipulation of the infected field provides a major benefit over OCS and should theoretically eliminate cross-contamination into the dissected space.
If an infection, hematoma, or a seroma develops postoperatively after ECS, subcutaneous progression will be minimized and a complex wound infection would be significantly reduced and more easily managed.
STOMAS
The OCS is not conducive in patients with stomas since it destabilizes the outer layer of the abdominal wall allowing shifting of the skin in relation to the underlying myoaponeurotic tissue31. With the ECS, the dissection plane is lateral to the rectus muscle which allows a stoma to be left in position or a new stoma can be fashioned since there is no shifting of skin in relation to the rectus muscle32.
OPERATING TIME AND COST
Any technical difficulty of endoscopic component separation is overcome with experience. The optical trocar permits rapid entrance between the muscle layers and the use of balloon dissection as in the preperitoneal inguinal hernia repair allows the surgeon to quickly and effectively develop the fascial plane providing an overall reduction in operative time over the open technique. The decrease in operative time overcomes the increased cost of the laparoscopic equipment.
CONCLUSIONS
The search continues for reliable techniques that will provide long-term success in the repair of ventral hernias. Although there is no universal technique or algorithm for repair of complex abdominal wall hernias, the objective remains the same – minimizing recurrence and morbidity. Proper patient selection is the key to the appropriate technique (Figure 7). The OCS is an excellent technique that allows tension-free closure of the abdominal wall while maintaining a dynamic and stable repair without the use of mesh.
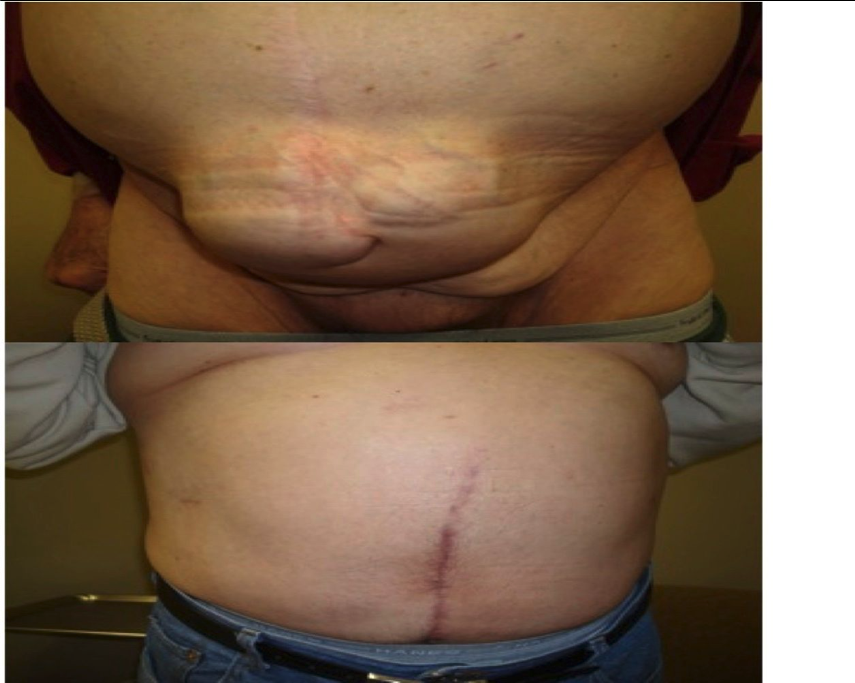
The drawbacks of OCS led to the evolution of ECS, which supports the concept of decreasing wound complications by preserving tissue vascularity. The technique of ECS is relatively easy to learn for laparoscopic surgeons familiar with the use of balloon dissectors and the development of the intermuscular abdominal wall spaces and is a welcome addition to the armamentarium of hernia surgeons.
References
- Rohrich RJ, Lowe JB, Hackney FL, et al. An algorithm for abdominal wall reconstruction. Plast Reconstr Surg 2000; 105:202-16 (quiz 17).
- Burger JW, Luijendijk RW, Hop WC, et al. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg 2004;240:578- 85.
- Millikan KW. Incisional hernia repair. Surg Clin North Am 2003;83:1223-34.
- Sauerland S, Schmedt CG, Lein S, et al. Primary incisional hernia repair with or without polypropylene mesh: a report on 384 patients with 5-year follow-up. Langenbecks Arch Surg 2005;390:408-12.
- Luijendijk RW, Hop WC, van den Tol MP, et al. A comparison of suture repair with mesh repair for incisional hernia. N. Engl J Med 2000; 343:392-8.
- Lomanto D, Iyer SG, Shabbir A, et al. Laparoscopic versus open ventral hernia mesh repair; a prospective study. Surg Endosc 2006;20:1030-5.
- Novitsky YW, Porter JR, Rucho ZC, et al. Open preperitoneal retrofascial mesh repair for multiply recurrent ventral incisional hernias. J Am Coll Surg 2006;203:283-9.
- Usher FC, Ochsner J, Tuttle LL Jr. Use of Marlex mesh in the repair of incisional hernias. Am Surg. 1958;24:969-974.
- Usher FC. Further observations on the use of Marlex mesh: a new technique for the repair of inguinal hernias. Am Surg. 1959;25:792-795.
- Usher FC. New technique for repairing incisional hernias with Marlex mesh. Am J Surg. 1979;138:740-741.
- Cobb WAS, Harris JB, Lokey JS, McGill ES, Klove KL (2003) Incisional herniorrhaphy with intraperitoneal composite mesh: a report of 95 cases. Am Surg 69:784-787.
- Baghai, M, Ramshaw, BJ, Smith, CD, et al. 2009. Technique of laparoscopic ventral hernia repair can be modified to successfully repair large defects in patients with loss of domain. Surgical Innovation. Vol 16-1;38-45.
- Ger R, Duboys E. The prevention and repair of large abdominal-wall defects by muscle transposition: a preliminary communication. Plast Reconstr Surg. 1983;72:170- 178.
- Williams JK, Carlson GW, deChalain T, et al. Role of tensor fasciae latae in abdominal wall reconstruction. Plast Reconstr Surg. 1998;101:713-718.
- Lowe JB, Garza JR, Bowman JL, Rohrich RJ, Strodel WE (2000) Endoscopically assisted “components separation” for closure of abdominal wall defects. Plast Reconstr Surg 105(2):720-729.
- Maas SM, de Vries Reilingh TS, van Goor H, de Jong D, Bleichrodt RP (2002) Endoscopically assisted “components separation technique” for the repair of complicated ventral hernias. J Am Coll Surg 194(3):388-390.
- Rosen MJ, Jin J, McGee MF, et al. Laparoscopic component separation in the single- stage treatment of infected abdominal wall prosthetic removal. Hernia (2007)11:435-440.
- Rosen MJ, Williams C, Jin J, et al. Laparoscopic versus open-component separation: a comparative analysis in a porcine model. Am J Surg 194(2007) 385-389.
- Wangensteen, O.H. Repair of large abdominal defects by pedicled to fascial Surg. Gynecol. Obstet. 82:144. 1946.
- Ramirez OM, Rusa E, Dellon AL. “Components separation” method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg. 1990;86:519-526.
- Dibello JN, Moore JH (1996) Sliding myofascial flap of the rectus abdominis muscle for the closure of recurrent ventral hernias. Plast Reconstr Surg 98(3):464-469.
- Girotto JA, Ko MJ, Redett R, Muehlberger T, Talamini M, Chang B (1999) Closure of chronic abdominal wall defects: a long term evaluation of the components separation method. Ann Plast Surg 42(4):385-395.
- DiBello JN Jr, Moore JH Jr (1996) Sliding myofascial flap of the rectus abdominus muscles for the closure of recurrent ventral hernias. Plast Reconstr Surg 98:464-469.
- Lowe JB, Garza JR, Bowman JL, Rohrich RJ, Strodel WE (2000) Endoscopically assisted “components separation” for closure of abdominal wall defects. Plast Reconstr Surg 105:720-729;quiz 730.
- de Vries Reilingh TS, van Goor H, Rosman C, Bemelmans MH, de Jong D, van Nieuwenhoven EJ, van Engeland MI, Bleichrodt RP (2003) “Components separation technique” for the repair of large abdominal wall hernias. J Am Coll Surg 196:32-37.
- Shestak KC, Edlingotn HJ, Johnson RR The separation of anatomic components technique for the reconstruction of massive midline abdominal wall defects: anatomy, surgical technique, applications, and limitations revisited (2000). Plast Reconstr Surg 105:731-738.
- Girotto JA, Ko MJ, Redett R, Muehlberger T, Talamini M, Chang B (1999) Closure of chronic abdominal wall defects: a long-term evaluation of the components separation method. Ann Plast Surg 42:385-394; discussion 394-395.
- Cohen M, Morales R Jr, Fildes J. Barrett J (2001) Staged reconstruction after gunshot wounds to the abdomen. Plast Reconstr Surg 108:83-92.
- Saulis AS, Dumanian GA (2002) Periumbilical rectus abdominis perforator preservation significantly reduces superficial wound complications in “separation of parts” hernia repairs. Plast Reconstr Surg 109:2275-2280; discussion 2281-2282.
- Lowe JB, Garza JR, Bowman JL, et al. Endoscopically assisted “components separation” for closure of abdominal wall defects. Plast Reconstr Surg 2000;105:720-30.
- Maas SM, van Engerland M, Bleichrodt RP. A modification of the “components separation” technique for closure of abdominal wall defects in the presence of an enterostomy. J Am Coll Surg 1999;189:138-40.
- Maas SM, de Vries Reilingh TS, van Goor H, et al. Endoscopically assisted “components separation technique” for the repair of complicated ventral hernias. J Am Coll Surg 2002;194:388-390.
- DuBay DA, Wang X, Adamson B, Kuzon WM Jr., Dennis RG, Franz MG. Mesh incisional herniorrhaphy increases abdominal wall elastic properties: a mechanism for decreased hernia recurrences in comparison with suture repair. Surgery 2006;140:14.
- Abrahamson J, Eldar S. Shoelace repair of large postoperative ventral abdominal hernias: a simple extraperitoneal technique. Contemp Surg. 32:24, 1988.
- de Vries Reilingh TS, van Goor H, Rosman C, et al. “Components separation technique” for the repair of large abdominal wall hernias. Am Coll Surg 2003; 196;1:32.
- Ewart CJ, Lankford AB, Gamboa MG. Successful closure of abdominal wall hernias using the components separation technique. Med Coll of Georgia 2002;50;3:269-274.
- Gonzalez R, Rehnke RD, Ramaswamy A, et al. Components separation technique and laparoscopic approach: a review of two evolving strategies for ventral hernia Am Surg 2005;71:598-605.
