Laparoscopic Appendectomy
Michael S. Kavic, MD, Stephen M. Kavic, MD, Suzanne M. Kavic, MD
INTRODUCTION
The appendix is a worm-like diverticulum of the cecum that arises at the confluence of the colonic longitudinal muscle fiber bands (taenia coli). More than 4000 years ago, the ancient Egyptians noted the presence of the appendix during their funereal preparations, and referred to it as the “worm” of the bowel.1 Its function and role in disease, however, remained obscure.
Most medical authorities thought that inflammation in the right lower quadrant originated in the cecum. They named this type of disease “typhlitis,” and infectious processes of the iliac fossa were named “peri-typhlitis.” Reginald H. Fitz was a pathologist at Harvard in the late nineteenth century and was the first to describe the signs and symptoms of acute appendicitis.2 He coined the term “appendicitis” in 1886 and advocated surgical intervention as a management option for this disease.
Charles McBurney was a general surgeon, and a contemporary of Fitz. He described the point of maximal tenderness in the right lower quadrant associated with acute appendicitis.3 This point that now bears his name is located one-third the distance between the right anterior superior iliac spine and the umbilicus, and corresponds to the anatomic base of the appendix. McBurney helped popularize the gridiron incision for appendectomy and, along with Fitz, was a strong advocate of early surgical intervention for acute appendicitis.4
Appendicitis is a common surgical emergency. In excess of 270 000 appendectomies are performed each year in the United States.5 Persons between 10 and 19 years of age have the highest incidence of appendicitis, with males having a higher rate than females for all age groups. The lifetime risk for appendicitis has been estimated at 8.6% for males and 6.7% for females.6 Overall, the incidence appears to be approximately 120 per 100 000 population.7
ANATOMY AND PHYSIOLOGY
The average appendix is approximately 9cm in length, although it can measure between 2cm and 22cm in the normal adult. The appendix is a retrocecal organ that typically lies within the peritoneal cavity. In a study of 10 000 cases, Wakeley8 identified 5 common positions of the appendix, listed in Table 1.
The appendix has long been thought to be a vestigial organ with little apparent clinical function. However, it may have an immunologic function, especially in children and adolescents. Lymphoid tissue first appears below the epithelium of the appendix during the 14th week of gestation.9,10 Lymphoid tissue gradually increases over time until adolescence, and then declines thereafter. Appendiceal lymphatic tissue, along with lymphatic tissue of the tonsils and Peyer’s patches, are different from typical lymph nodes in that no lymphatic vessels drain this tissue. Some have suggested that the unique tissue of the appendix has a role in immunologic surveillance of the gut.11
The blood supply to the appendix is via the appendiceal artery, an end branch of the posterior cecal branch of the ileocolic artery, itself derived from the superior mesenteric artery.12 Because the arterial supply is that of an end artery, perfusion cannot be increased in the setting of acute inflammation, and the potential for ischemia and subsequent perforation is then increased. This underappreciated structural characteristic of perfusion may be an important causal factor in the overall etiology of appendicitis.
APPENDICITIS
The underlying etiology of acute appendicitis is that of obstruction of the appendiceal lumen followed by inflammation and subsequent suppuration.13 Common causes of obstruction are fecalith, hypertrophy of submucosal lymphoid tissue, or kinking of the appendiceal wall. Less common causes of appendiceal obstruction include obstruction secondary to foreign bodies, intestinal worms, or vegetable seeds.
After obstruction of the lumen, intraluminal pressure continues to rise due to continued mucosal secretion. The normal appendix has a volume of 0.6mL, and mucus production of the appendix can approach 2mL to 3mL per day. Therefore, an obstructed appendix can distend within a matter of hours, which explains the sometimes rapid course of appendicitis.12 In effect, proximal occlusion of the appendix causes a closed loop obstruction. If unchecked, intraluminal pressures can exceed capillary venous and lymphatic pressures, leading to venous infarction.
Mucosal inflammation in the distal segment is followed by ulceration and ultimately inflammation of the entire appendiceal wall. Stagnation distal to the obstruction allows for overgrowth of both anaerobic and aerobic organisms. Anaerobes predominate in the appendix, and Escherichia coli, Bacteroides fragilis, and Pseudomonas species are commonly cultured. With ongoing bacterial proliferation and mucous production, arterial blood flow can be degraded, resulting in gangrenous appendicitis. Transmural necrosis leads to perforation, which may be contained as a localized abscess or progress to diffuse peritonitis.
Patients with acute appendicitis usually present with right lower quadrant pain that may have begun in the periumbilical region.14 Fever and emesis are common findings, often associated with anorexia. A leukocytosis of 11 000 to 18 000 WBCs per cubic milliliter is common in acute appendicitis with a “left shift” (increase in the percentage of neutrophils and immature band forms). Although plain abdominal radiographs may demonstrate a fecalith, for the most part these studies are nonspecific. Computerized tomographic (CT) studies are more accurate than plain films are in diagnosing and differentiating acute inflammatory processes in the right lower quadrant.15 Right lower quadrant ultrasound, which is very operator-dependent, can be useful and may demonstrate a “bulls-eye” in the right lower quadrant signifying a distended appendix.16
Two adverse outcomes must be considered in the management of the presumed acute appendicitis: first, perforation of the appendix with the possibility of contamination, peritonitis, abscess, and sepsis, and second, misdiagnosis that can result in the removal of a normal appendix and misidentification of the true pathology. To reduce the potential of a perforated appendix, surgeons have traditionally accepted that approximately 10% to 20% of explorations for possible acute appendicitis will reveal noninflamed, histologically grossly normal appendices.17,18 The advent of laparoscopy seems to have decreased this percentage radically.7
APPENDECTOMY
McBurney’s technique for operative removal of a diseased appendix, described in the late 1800s, remained relatively unchanged for almost a century. Through a right-sided gridiron incision, surgeons isolated and skeletonized the appendix, with control of its arterial supply. After amputation of the appendix itself, the stump was not buried in the cecum, which was returned to its normal anatomic position, and the incision closed. The open approach to appendectomy has remained the gold standard, because it is a safe and efficacious procedure that results in minimal morbidity and near-zero mortality.
Kurt Semm, a German gynecologist, was the first to radically change McBurney’s procedure when he performed the first laparoscopic appendectomy on May 30, 1980.19,20 Rather than traumatizing the abdominal wall for exposure, Semm’s technique used a laparoscope to visualize the appendix. Laparoscopic needle and suture (Endosutures) were used to secure the mesoappendix prior to division. Pretied Roeders loops were used to ligate the base of the skeletonized appendix. The appendix was amputated between the fixed loops. The technique was efficient, effective, and of course, minimally invasive.
Larson et al21 elegantly iterated several reasons why a laparoscopic approach seems preferable to open appendectomy: superior visualization, identification of lesions in structures other than the appendix, reduced tissue trauma, the potential for more rapid return to normal activity, good exposure in obese patients, and decreased wound surface area to serve as a focus for infection. Of particular importance is the capability of establishing a diagnosis in female patients of childbearing years, in whom the diagnosis may be less certain.22
Technique of Laparoscopic Appendectomy
Since Kurt Semm’s initial description of laparoscopic appendectomy in 1980, numerous revisions and adaptations of his technique have been described.23 Instruments and energy sources have been developed and refined into more sophisticated devices. Additionally, some have modified access, performing the procedure through fewer ports, smaller ports, or with NOTES.24,25 What follows is one common approach to laparoscopic appendectomy.
Standard preoperative preparation is used, including anesthetic clearance. A preoperative antibiotic, usually a broad-spectrum cephalosporin, is given intravenously. After induction of general anesthesia, the patient remains in the supine position on the operating table. The abdomen is prepped with antiseptic (povidone-iodine), and the umbilicus is carefully cleansed. Pneumoperitoneum is then established, either through the “closed” technique (use of the Veress needle) or the “open” technique (Hasson blunt port insertion).
In the “closed” technique, a Veress needle is passed transumbilically or through the left upper quadrant. A 10-mm to 12-mm trocar and cannula may then be introduced through the umbilicus. Alternately, an “open” laparoscopy technique may also be used to initiate laparoscopic access. A 2-cm incision is made in the skin about the umbilicus. Dissection is carried down through all layers of the abdomen to the peritoneum, which is opened under direct vision. A Hasson-type cannula is inserted and secured to the abdominal wall or the underlying fascia.26
The camera is then introduced via the umbilical cannula. Laparoscopic examination of the entire abdomen is first performed to rule out other pathology, such as a Meckel’s diverticulum, Crohn’s disease, mesenteric adenitis, or pelvic genital tract disease in the female patient. This important step helps confirm the region of pathology and serves as a guide for placement of additional working ports.
Additional 5-mm trocars and cannulae are brought in under direct vision. Operating ports are sited so that the surgeon can appropriately triangulate the diseased organ. Generally, cannulae should be placed at least 4 fingerbreadths distant from each other to prevent the instruments from interfering with one another (“sword fighting”). A 3-port technique may be used with ports in the mid left lower quadrant, and suprapubic or right lower quadrant position.
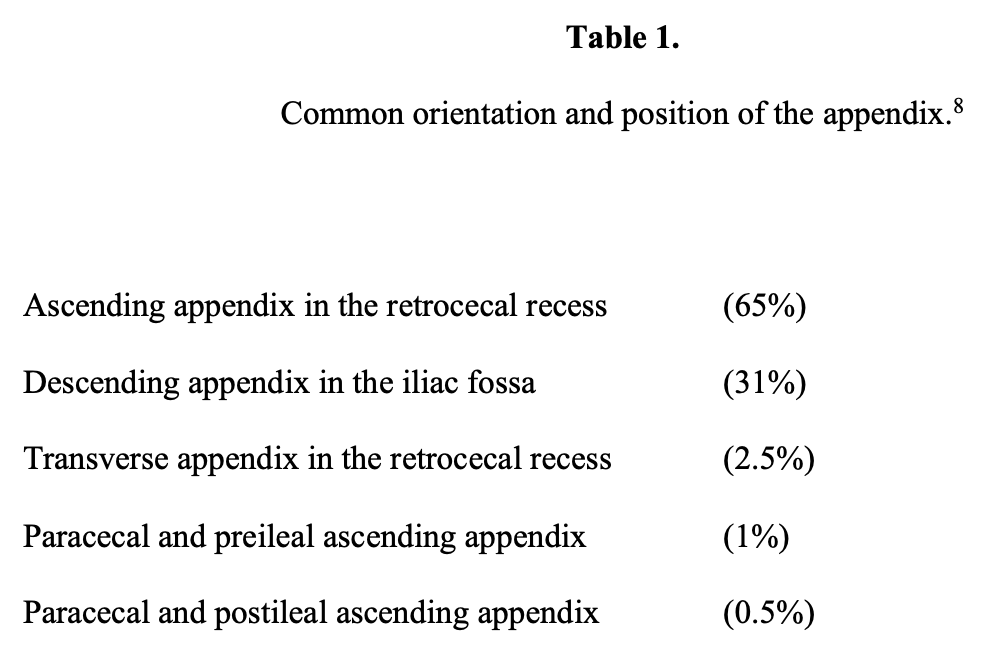
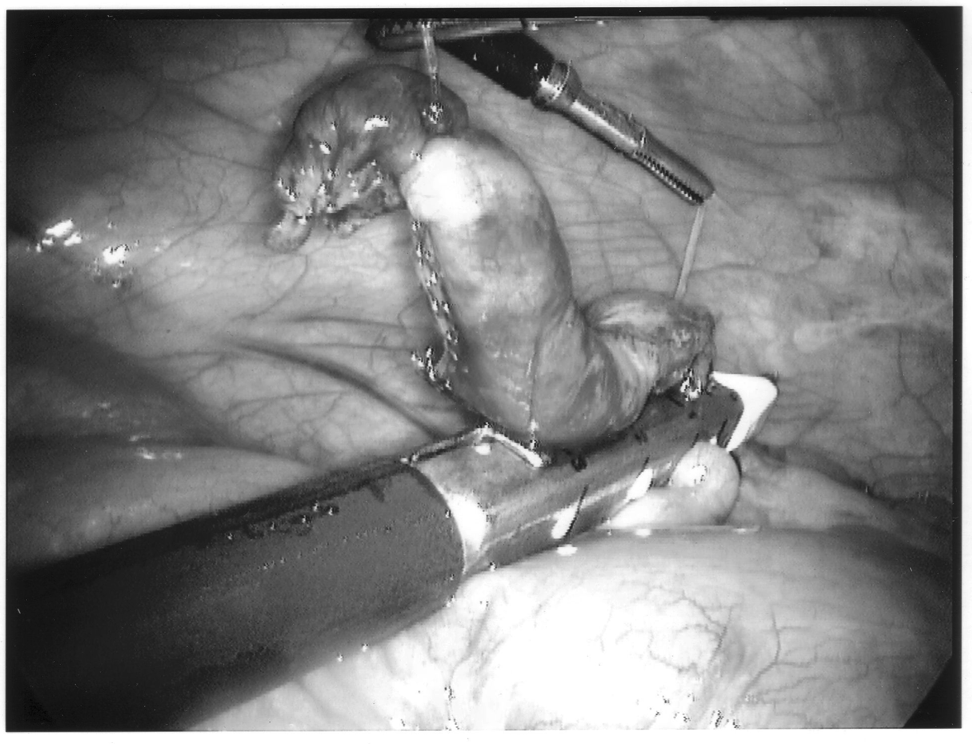
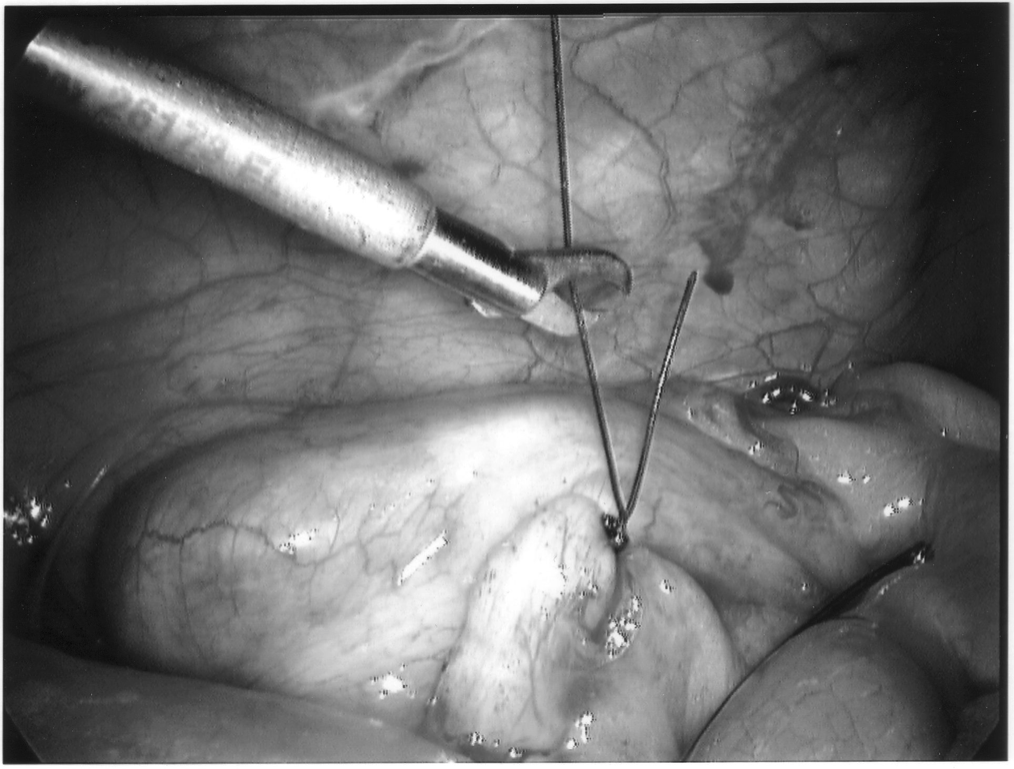
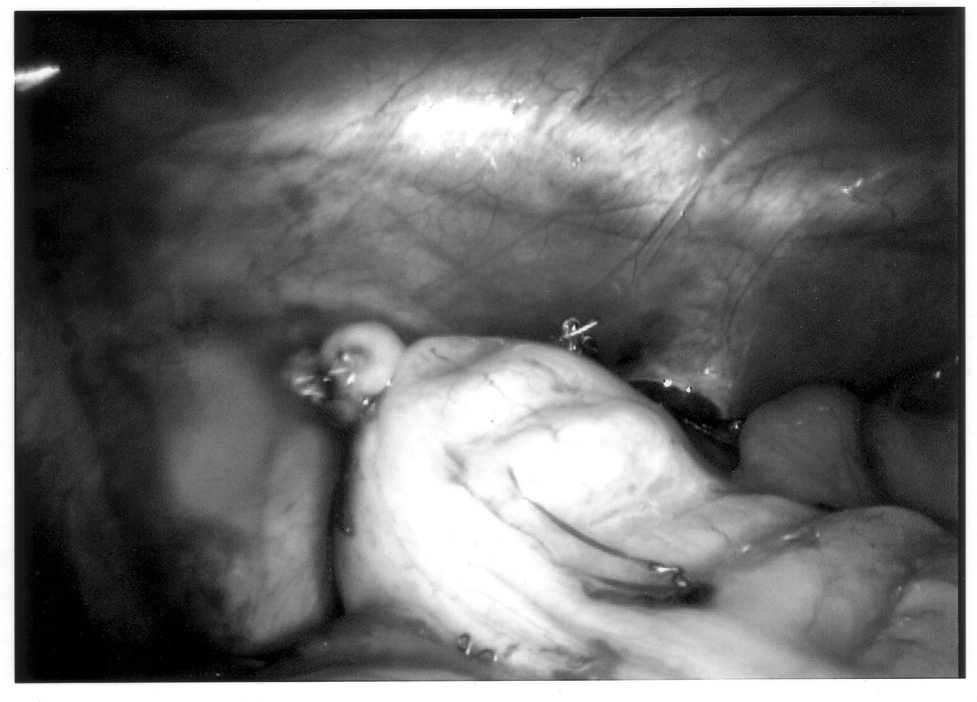
The appendix is then mobilized. It may be necessary to divide the lateral peritoneal attachments of the cecum and right colon to expose the appendix completely (Figure 1). The tip of the appendix is usually friable in acute appendicitis and easily torn with laparoscopic graspers. To minimize this possibility, a pretied Roeders endoloop may be applied to the tip of the appendix. The loop is secured, and the ends of the suture cut long to provide a handle for manipulation of the appendix (Figure 2).
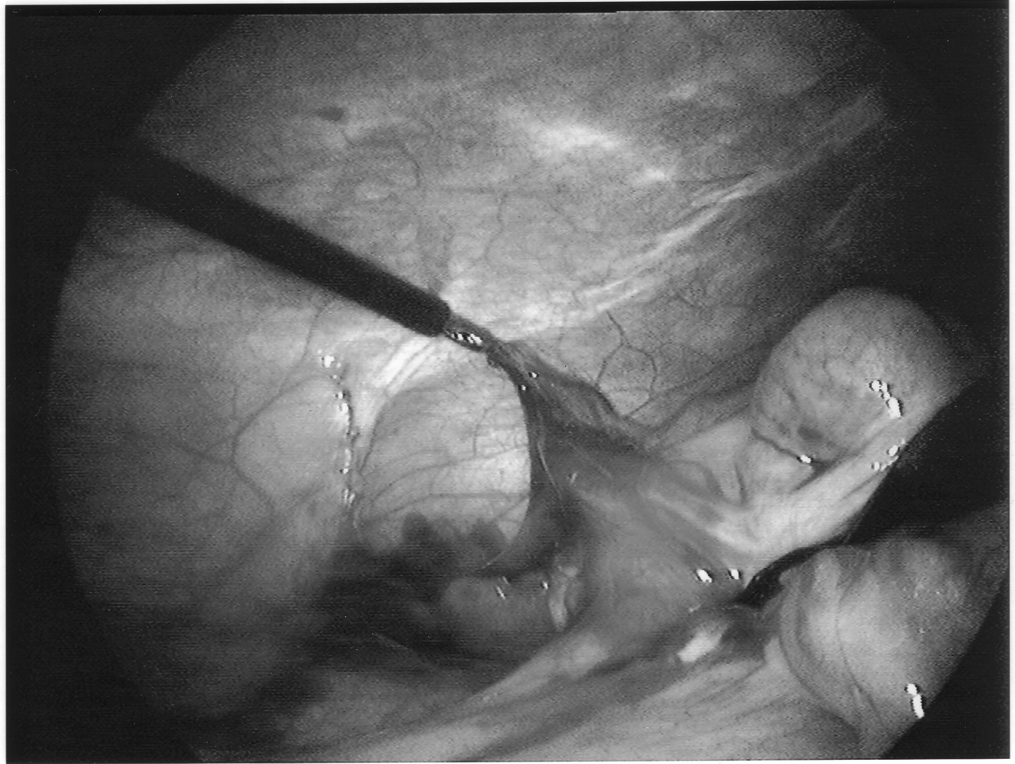
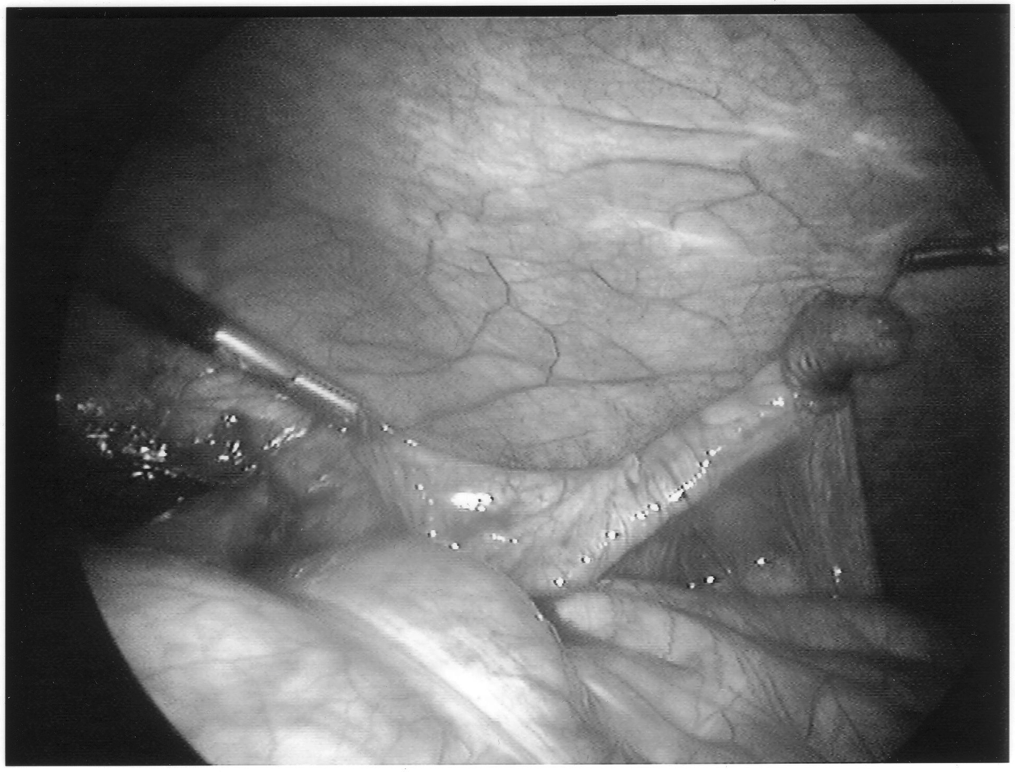
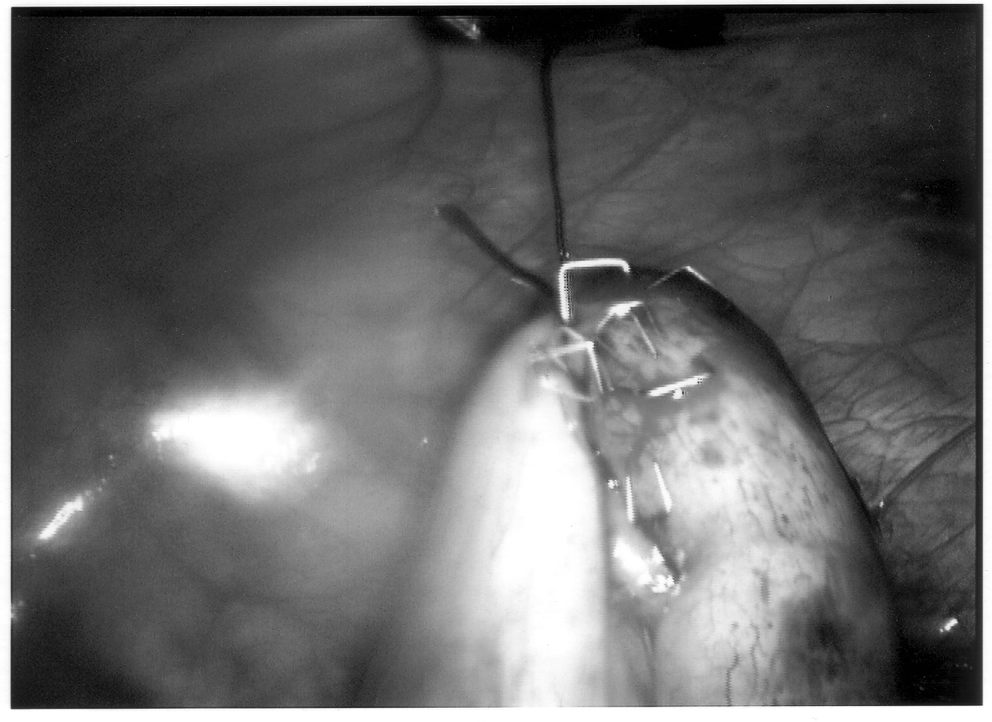
Once the appendix is stretched, an opening is made into the mesoappendix at the base of the appendix, and the mesoappendix is divided with an EndoGIA stapling unit (US Surgical Corporation, Norwalk, CT), Harmonic scalpel, ultrasonic dissector, or serial clamping, divided, and tied with pretied suture loops (Figures 3 and 4). If the appendix is extensively bound, it may be necessary to remove it in a retrograde fashion. In this circumstance, the base of the appendix is first divided between endoloops or with an EndoGIA stapler (Figures 5 and 6). With 2 points of access to the mesoappendix, it is then divided as described above.
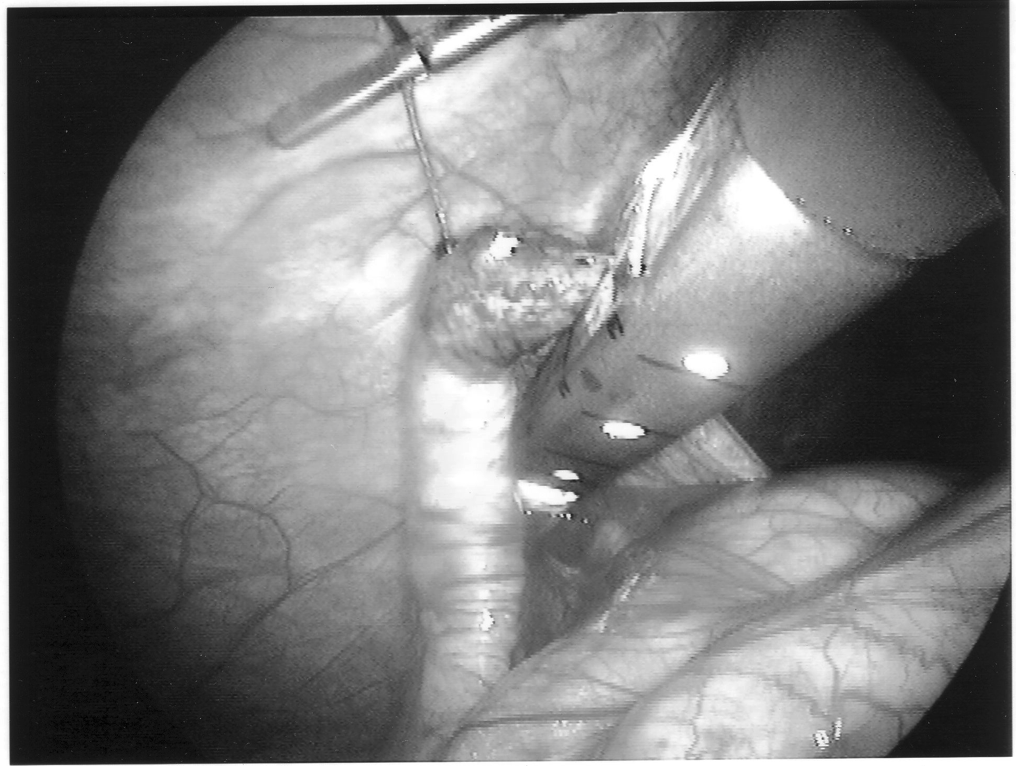
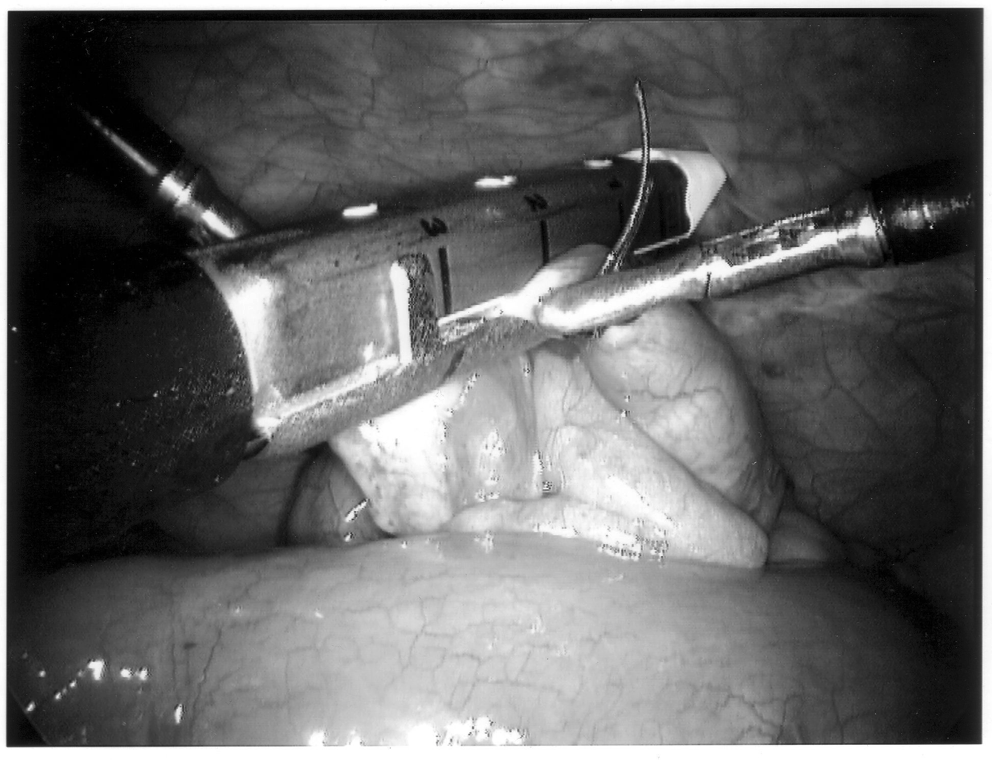
Staple leg length should be considered when using the EndoGIA device. Standard intestinal staple cartridges have a leg length of 3.5mm and are suitable for congested or edematous tissues. Vascular staples with a shorter leg length of 2.5mm should be used on isolated vascular structures or tissue without significant bulk, edema, or swelling.
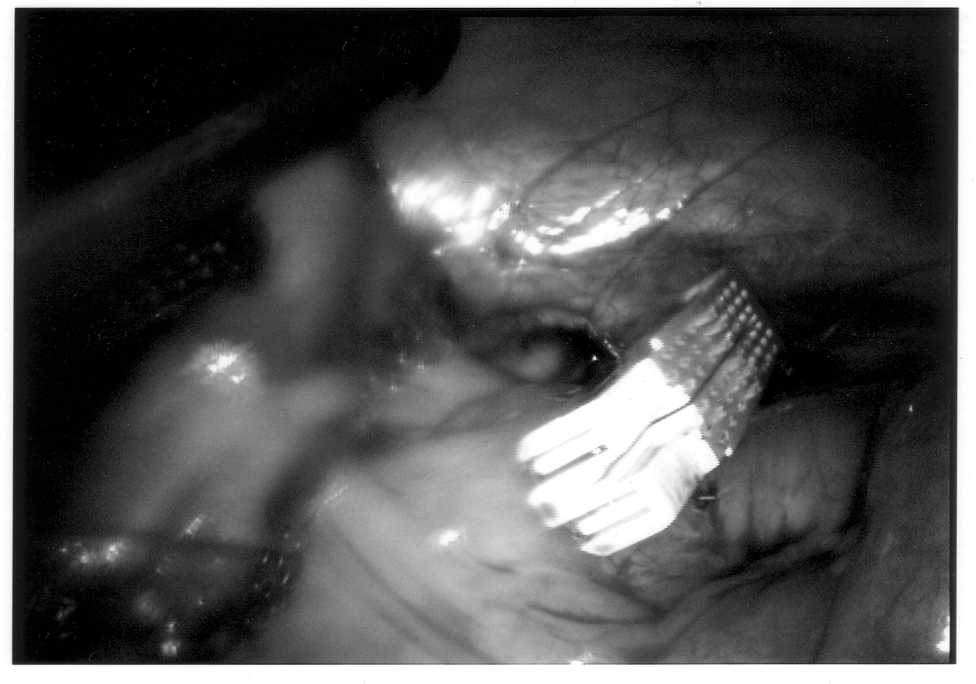
Any mechanical device has a failure rate, and endomechanical staplers are no exception. Improper loading of misapplication of the stapling unit can cause failure. In the instance depicted in Figure 7, the stapling cartridge broke free of the stapler housing, and all staples were discharged into the abdominal cavity. All foreign bodies should be removed, especially undeployed staples that may cause perforation (Figures 8 and 9).
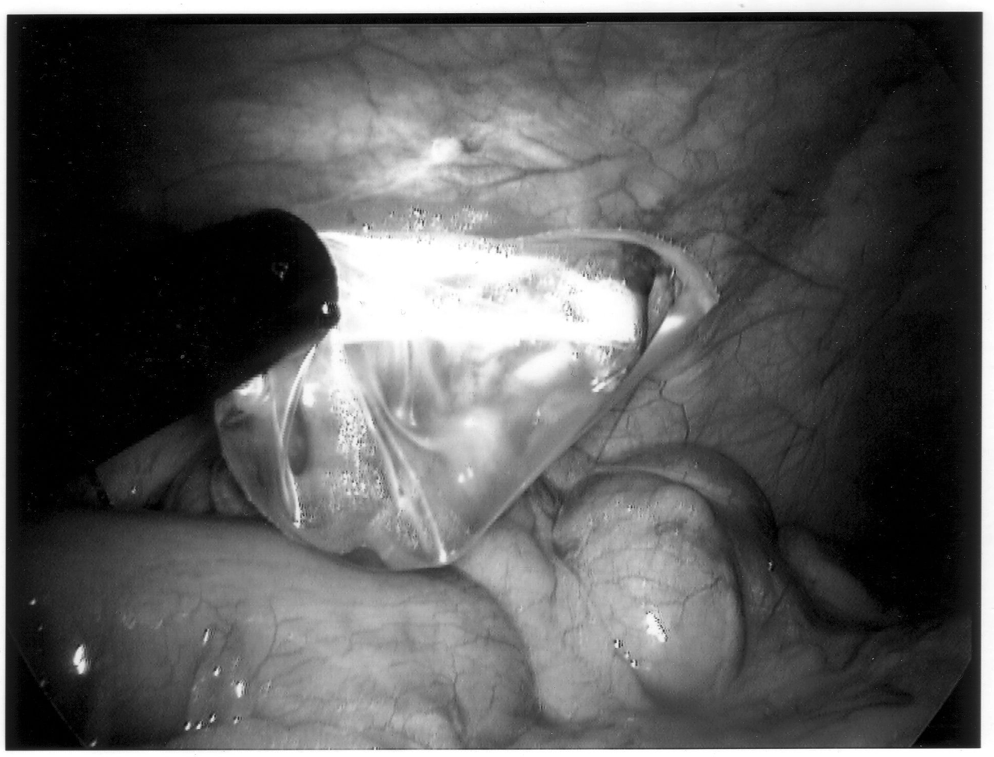
To limit contamination and protect a friable appendix, the specimen should be placed in an endoscopic retrieval bag and removed through the 10-mm cannula site (Figure 10). If there has been any significant spillage, the operative site must be irrigated with a copious amount of saline, typically several liters in volume. Antibiotics should also be continued throughout the postoperative period. Evidence exists that the addition of a local anesthetic to this irrigation may even decrease postoperative shoulder pain, providing an added benefit.27
After removal, the operative site is inspected, and all port sites ≥10mm in diameter have had fascial sutures placed, the cannulae are withdrawn. CO2 is allowed to evacuate, and the sutures are tied with the knots sited in a subcutaneous position. It is important to maintain upward traction on the fascial sutures while CO2 is being evacuated to prevent omentum or intestine becoming entangled in the cannula site. Subcutaneous and subcuticular suture complete the wound closure. Steri-strips help maintain the skin edges in apposition.
Many variations of this basic technique have been developed. Some surgeons have aimed to determine the optimal number of ports required for appendectomy, including in the extreme only a single-port site.28 Others have performed the procedure during transvaginal hysterectomy.29 Laparoscopic appendectomy has also been performed using 2-mm mini-laparoscopy instruments.30 The appendix itself has been removed by using a wire snare31 or ultrasonically activated scalpel.32 An additional maneuver has been the introduction of a finger through a port site in cases of complicated appendicitis.33
Complications of Laparoscopic Appendectomy
It is important to bear in mind that a laparoscopic approach to abdominal disease frequently results in reduced morbidity: decreased postoperative pain, decreased recovery time, and earlier return to normal activities. However, regardless of the approach, the primary disease process and its effect on the patient must be constantly born in mind.
Infection is the main problem associated with appendicitis, and the overall level of patient comfort is not important if the infectious process is not controlled. If the disease is confined to the organ itself, surgeons need only remove the appendix and anticipate complete recovery. However, if the appendix has perforated, management is more complex. Minimal access techniques do not change disease pathophysiology.
It cannot be overemphasized that the principles of good surgical practice exist independent of the approach involved. Specifically, careful handling of tissues, precise dissection, adequate hemostasis, secure closure of the appendiceal stump, and anatomic wound approximation must all occur to minimize the overall risk of complications.
In addition, a laparoscopic approach incurs potential complications inherent in performing an intracavitary procedure remote from without that body cavity. These complications have been reviewed elsewhere.34 Complications associated with laparoscopic access to the abdominal cavity may be broadly classified into 3 groups: abdominal wall injuries, vascular injuries, and visceral injuries,35 and are outlined in Table 2. A more complete listing of complications specifically reported after laparoscopic appendectomy is presented as Table 3.


Access Complications
Subcutaneous emphysema may arise from initial superficial placement of the insufflation needle or dislodgement of the cannula used for intraoperative insufflation into the subcutaneous tissues. Carbon dioxide (CO2) is injected under pressure into subcutaneous spaces, which may ascend into the neck and face. The body usually rapidly reabsorbs CO2, and tissue emphysema is usually well tolerated. Hypercarbia, however, can occur with a rise in end-tidal CO2 and a fall in pH. These changes can often be easily managed with controlled ventilation. It may be necessary to discontinue CO2 insufflation during this time and evacuate the pneumoperitoneum while the anesthesiologist manages the patient’s ventilation needs.
Gas embolism can result from insufflation of CO2 or other gases into a major vessel or venous channel. Devices such as the argon beam coagulator utilize a low-flow stream of inert gas to maintain the beam of electrical energy in position. This gas, if directed at a venous channel, may gain entrance and lead to a gas embolism. Obstruction of right heart outflow can occur with gas embolism and subsequent cardiovascular collapse.
Emergency treatment of gas embolism is outlined in Table 4.
Hemorrhage from the abdominal wall secondary to port placement is usually due to transection of the inferior epigastric vessels or their branches in the lower abdomen. Inferior epigastric vessels (the inferior epigastric artery is accompanied by 2 epigastric veins) may be avoided by inserting trocars lateral to the rectus sheath under direct laparoscopic vision. Epigastric vessels as well as unnamed vascular channels of the abdominal wall may also be transilluminated with the laparoscope and thereby avoided during trocar insertion.
The risk of injury to the aorta or external iliac vessels can be lessened by strict and constant attention to technique. No trocar and cannula should be inserted without countertraction on the abdominal wall. A tense pneumoperitoneum may provide sufficient countertraction; however, it may be safer to have the surgeon apply countertraction with the aid of a towel clip using the nondominant hand to support the abdominal wall. Additionally, if the surgeon extends the third digit of the thrusting hand, this finger will help limit incursion of the trocar and provide an additional measure of safety.
Minor bleeding from abdominal wall trocar sites can be controlled with bipolar or monopolar desiccation. Larger transected vessels should be secured with suture ligature. There are special needles for passage of suture via port sites including the Semm emergency needle, Gore needle, and Carter-Thomason closure system. In general, cannula at the bleeding port site should be left in place. Using any of the above needles, suture is passed through the fascia into the abdomen where it is caught and held with a laparoscopic grasping instrument. The needle is repositioned and passed through the fascia 180 degrees opposite to the initial suture placement. Under direct laparoscopic visualization, the intraabdominal suture is passed to the needle now in an intraabdominal position, secured to the needle, and withdrawn. Several sutures can be passed in this fashion allowing the bleeding vessel to be secured and fascia of the port site closed. The long ends of the suture are held with hemostats and the needle transferred to other cannula sites for fascial closure.
Visceral trauma can occur at any time during a laparoscopic procedure from the initial introduction of trocar and cannulae to the completion of the procedure. Visceral injuries may be life threatening but indolent, as they may occur away from the region of interest. Distended hollow organs, such as the stomach and urinary bladder, can be protected from injury by preoperative decompression, ie, orogastric tube and Foley catheter. Patients who have undergone previous open operative procedures are at increased risk for visceral injury because of the presence of adhesions. Careful dissection of the bowel and closure of all iatrogenic serosal tears are necessary when dense adhesions are encountered.
Intestinal or visceral trauma secondary to puncture with the Veress needle can usually be managed expectantly. If there is little lateral damage from passage of the Veress needle, the intestine will usually seal spontaneously without untoward sequelae. Of course, larger tears must be repaired primarily. The repair can be performed laparoscopically or via open laparotomy. Lacerations to solid organs like the liver and spleen must be managed according to the degree of injury. As important as managing an injury is, it is perhaps more important to recognize that an injury has occurred. For this reason, a diagnostic abdominal survey visualizing all of the abdomen and its content before and after the operative procedure should be routine during any laparoscopic operation.
Persons of small body habitus should be carefully assessed before a laparoscopic procedure. A small frame and thin abdominal wall mandate that appropriately sized laparoscopic instrumentation be used. There is a decreased intraabdominal working space in patients of small build and incursions of trocars and laparoscopic instruments must be carefully managed lest intraabdominal vascular and visceral injury occur. The principles of countertraction and extension of the third digit must be followed.
Port-site hernias have been an ongoing problem since the advent of laparoscopic surgery. Any port site ≥10mm in diameter should be closed with fascial suture reliably placed under laparoscopic visualization. Port sites <10mm in diameter if vigorously manipulated and stretched, should also be closed with fascial suture.
Procedural Complications
A systematic review of studies comparing laparoscopic and open appendectomies was performed by Sauerland et al36 and published in 2002. The metaanalysis of 39 separate investigations concluded that wound infections were significantly reduced in laparoscopic appendectomy (odds ratio 0.5), but abscess formation was significantly increased (odds ratio 2.8).
Intraabdominal abscess remains one of the more feared complications of appendectomy, be it performed by laparoscopic or open techniques. The responsible organisms include those typically associated with the gastrointestinal tract. In one of the largest published series,37 the overall rate of abscess formation was 0.4% in laparoscopic procedures, including perforated and gangrenous appendicitis. The rate of abscess formation in this and in many centers is comparable to abscess formation following open procedures.38,39 More recently, laparoscopy has been advocated in a Cochrane Database Systematic Review.40
Nordentoft et al41 conducted a randomized study in 23 adult patients of bacteremia at the time of appendectomy. In this study, half of the patients who underwent laparoscopic appendectomy had culture-documented bacteremia, whereas none of the patients who underwent open appendectomy had bacteremia. The significance of this finding may be debated, as there was no increase in rates of abscess formation in the laparoscopic group, as well as the obvious size limitations of the study.
However, in a separate report, one patient developed a left-sided scrotal abscess following laparoscopic appendectomy.42 This does implicate pneumoperitoneum as a possible causal factor in facilitating transit through a processus vaginalis and subsequent abscess development.
Wound infection, which can be as high as 30% following open appendectomy, is reported to be about 0.1% following laparoscopic procedures.43 Strict attention to cleansing the umbilicus of all debris and proper sterilization of laparoscopic instruments may lessen this incidence even further. The majority of studies suggest that wound infections occur infrequently, at rates similar to those of open surgery, with most randomized studies demonstrating fewer infections in laparoscopic appendectomy than in the open cohort.44,45
CONCLUSION
In experienced hands, laparoscopic appendectomy is a safe, efficient alternative to open appendectomy for the treatment of acute appendicitis. Regardless of the approach, the potential of infection must always be borne in mind by the operating surgeon. The fact that minimally invasive surgery is accompanied by lessened immediate postoperative pain does not diminish the importance of treating the patient for infection and its sequelae. Intravenous antibiotic, adequate hydration, and support of nutrition are all- important. By treating the whole patient and anticipating the possibility of infectious sequelae, the likelihood of complications will be minimized.
Address correspondence to: Michael S. Kavic, MD, Director of Education, General Surgery, Saint Elizabeth Health Center, 1044 Belmont Ave., PO Box 1790, Youngstown, OH 44501-1790, USA.
References
- Herrington JL The vermiform appendix: its surgical history. Contemp Surg. 1991;39:36-43.
- Fitz RH. Perforating inflammation of the vermiform appendix with special reference to its early diagnosis and treatment. Trans Assoc Am Phys. 1886;1:107-144.
- McBurney C. The incision made in the abdominal wall in cases of appendicitis with a description of a new method of operating. Ann Surg. 1894;20:38.
- McBurney C. Experience with early operative interference in cases of disease of the vermiform appendix. NY State Med J. 1889;50:676-687.
- Hall MJ, Owings MF. 2000 National Hospital Discharge Survey. Advance data from vital and health statistics; no 329. Hyattsville, Maryland: National Center for Health Statistics. 2002.
- Addis DG, Shaffer N, Fowler B, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990;132:910-925.
- Andreu-Ballester JC, Gonzalez-Sanchez A, Ballester F, et al. Epidemiology of appendectomy and appendicitis in the Valencian community (Spain), 1998-2007. Dig Surg. 2009;26:406-412.
- Wakeley CPG. Position of the vermiform appendix as ascertained by the analysis of 10,000 cases. J Anatomy. 1933;67:277.
- Kyriazis AA, Esterly JR. Development of lymphoid tissues in the human embryo and early fetus. Arch Pathol. 1970;90:348.
- Archer OK, Sutherland DE, Good RA. Appendix of the rabbit: a homologue of the bursa in the chicken? Nature. 1963;200:337-339.
- Fisher AC. Acute appendicitis. In Cameron, JL, ed. Current Surgical Therapy. St. Louis, MO: Mosby; 2001;267-272.
- Schumpelick V, Dreuw B, Ophoff K, Prescher A. Appendix and cecum: embryology, anatomy, and surgical applications. Surg Clin N Am. 2000;80:295-318.
- Lally KP, Cox CS, Andrassy RJ. Appendix. In: Townsend CM, ed. Sabiston Textbook of Surgery. 16th ed. Philadelphia: WB Saunders Co.; 2001:917-928.
- Silen W. Cope’s Early Diagnosis of the Acute Abdomen. 18th ed. New York: Oxford University Press; 1991:67-84.
- Birnbaum BA, Balthazar EJ. Computed tomography of appendicitis and diverticulitis. Radiol Clin North Am. 1994;32:885-898.
- Rossi P, Covarelii P, Mosci F, Bisacci R, Sensi B, Moggi L. Ultrasonography in management of acute appendicitis. Surg Endosc. 1996;149:619-621.
- Detmer DE, Nevers LE, Sikes ED Jr. Regional results of acute appendicitis care. JAMA. 1981;246:1318-1320.
- Flum DR, Morris A, Koepsell T, Dellinger EP. Has misdiagnosis of appendicitis decreased over time? JAMA. 2001;286:1784-1753.
- Semm K. Endoscopic appendectomy. Endoscopy. 1983;15:59-64.
- Litynski GS. Highlights in the History of Laparoscopy. Frankfurt: Barbara Bernert Verlag; 1996:136.
- Larson GM, Cheadle WG, Polk HC Jr. Appendectomy for acute appendicitis. In Ballantyne GH, Leahy PF and Modlin IM, eds. Laparoscopic Surgery. Philadelphia: WB Saunders and Company; 1994:220.
- Kavic MS. Laparoscopic appendectomy. In Grochmal S, ed. Minimal Access Gynecology. Oxford: Radcliffe Medical Press; 1995:149-162.
- Kavic MS, Semm K. Laparoscopic appendectomy. In Kavic MS, Levinson CJ, Wetter PA, eds. Prevention and Management of Laparoendoscopic Complications. Miami: Society of Laparoendoscopic Surgeons; 1999:35.
- Roberts KE. True single port appendectomy: first experience with the “puppeteer technique.” Surg Endosc. 2008;23:1825-1830.
- Horgan S, Cullen JP, Talamini MA, et al. Natural orifice surgery: initial clinical experience. Surg Endosc. 2009;23:1512-1518.
- Hasson HM. Modified instrument and method for laparoscopy. Am J Obstet Gynecol. 1971;110:886-887.
- Cunniffe MG, McAnena OJ, Dar MA, Calleary J, Flynn N. A prospective randomized trial of intraoperative bupivacaine irrigation for management of shoulder-tip pain following laparoscopy. Am J Surg. 1998;176:258-261.
- Rispoli G, Armellino MF, Esposito C. One-trocar appendectomy. Surg Endosc. 2002;16:833-835.
- Pelosi MA 3d, Pelosi MA. Vaginal appendectomy at laparoscopic-assisted vaginal hysterectomy: a surgical option. J Laparoendosc Surg. 1996;6:399-403.
- Schier F. Laparoscopic appendectomy with 1.7mm instruments. Pediatr Surg Intl. 1998;14:142-143.
- Chikamori F, Kuniyoshi N, Shibuya S, Takase Y. Laparoscopic appendectomy with the help of a wire snare. Surg Today. 2001;31:560-563.
- Martin del Olmo JC, Blanco Alvarez JI, Carbajo Caballero MA, de la Cuesta de la Llave C, Vaquero Peurta C, Arenal Laparoscopic appendectomy by ultrasonically activated scalpel in acute appendicitis: preliminary report. J Laparoendosc Adv Surg Tech A. 2002;12:111-113.
- Katkhouda N, Mason RJ, Mavor E, et al. Laparoscopic finger-assisted techniques (fingeroscopy) for treatment of complicated appendicitis. J Am Coll Surg. 1999;189:131- 133.
- Schafer M, Lauper M, Krahenbuhl L. Trocar and Veress needle injury during laparoscopy. Surg Endosc. 2001;15:275-280.
- Miranda CS, Carvajal AR, Escobar P. Complications of operative Gynaecologic Endosc. 2000;9:161-165.
- Sauerland S, Lefering R, Neugebauer EA. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev. 2002;1:CD001546.
- Katkhouda N, Friedlander MH, Grant SW, et al. Intraabdominal abscess rate after laparoscopic appendectomy. Am J Surg. 2000;180:456-461.
- Golub R, Siddiqui F, Pohl D. Laparoscopic versus open appendectomy: a metaanalysis. J Am Coll Surg. 1998;186:545-553.
- Chung RS, Rowland DY, Li P, Diaz J. A meta-analysis of randomized controlled trials of laparoscopic versus conventional appendectomy. Am J Surg. 1999;177:250-256.
- Sauerland S, Lefering R, Neugebauer EA. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev. 2004;18:CD001546.
- Nordentoft T, Bringstrup FA, Bremmelgaard A, Stage JG. Effect of laparoscopy on bacteremia in acute appendicitis: a randomized controlled study. Surg Laparosc Endosc Percut Tech. 2000;10:302-304.
- Kollis J, Gallery RM. Left scrotal abscess complicating laparoscopic appendicectomy. Aust N Z J Surg. 1996;66:568-569.
- Phillips JM. Complications in laparoscopy. Int J Gynaecol Obstet. 1977;15:157-162.
- Merhoff AM, Merhoff GC, Franklin Jr ME. Laparoscopic versus open appendectomy. Am J Surg. 2000;179:375-378.
- Ozmen MM, Zulfikaroglu B, Tanik A, Kale IT. Laparoscopic versus open appendectomy: prospective, randomized trial. Surg Laparosc Endosc. 1999;9:187-189.
