Laparoscopic Vascular Surgery
Antoine Bouchard-Fortier, MD, BSc, Ricardo Ruz, MD, MSc, Yves-Marie Dion, MD, MSc, FACS, FRCSC
INTRODUCTION
Modern vascular surgery began in the early 1900s with Carell and Guthrie who studied techniques of vascular anastomosis in animals, which granted them a Nobel prize in 1912. It was not until November 14, 1950 that a French vascular surgeon, Jacques Oudot, performed the first aorto-bifemoral bypass for occlusive aorto-iliac disease.1 Three years later, another French surgeon, Charles Dubost, performed the first aortic aneurysm repair by using a retroperitoneal approach with a freeze-dried arterial homograft prepared by Oudot.2 Meanwhile, in New York City, Arthur Blakemore3 was the first to treat a patient presenting with a ruptured aortic aneurysm by using a bifurcated synthetic graft.
The use of endoprotheses was initiated in 1985, in Ukraine, where Volodos et al4 developed their implantation in dogs. They subsequently reported successful endoprosthesis implantation in humans.5-7 In 1988, his team inserted a thoracic endograft for a traumatic thoracic aneurysm.8 During the same period, an Argentinian surgeon, Juan Carlos Parodi, reported his clinical experience with 5 patients treated between 1990 and 1991 who received an endograft for an abdominal aortic aneurysm.9 His work became the first publication in the English literature of an endovascular treatment for aortic aneurysmal disease.
In the meantime, our research team began to study the use of laparoscopy for vascular disease as a minimally invasive therapeutic strategy. In 1993, we published the first clinical article referring to the laparoscopic-assisted technique.10 Soon after, in 1995, we published experimental results in animals with a totally laparoscopic approach for aorto- iliac disease.11,12 The first patient to receive a totally laparoscopic aortic end-to-end anastomosis was operated on in Quebec in June of 1995.13 Our vascular service also published in 1997 the operative technique for totally laparoscopic treatment of occlusive and aneurysmal aorto-iliac disease.14 Then, in April of 1999, we successfully operated on the first patient for an aortic aneurysm by using the totally laparoscopic approach.15 Due to growing clinical experience, we published, in 2003, improvements to the totally laparoscopic technique we had previously described.16
At the beginning, laparoscopy for aorto-iliac disease relied on the early instrumentation designed for general surgery. Improvements in instrumentation during the mid 1990s made it possible to securely clamp laparoscopically a large vessel and perform a totally laparoscopic anastomosis. At the turn of the 21st century, several specifically designed instruments had been made available. The laparoscopic approach began to be performed in more centers throughout the world. Performance of the anastomosis, with or without endarterectomy, remains the most delicate part of the procedure. Today, the laparoscopic concept has translated into the recognition of a new minimally invasive technique.
Aorto-Iliac Laparoscopy is Now a Proven Technique
Laparoscopy in vascular surgery, just as in general surgery, gynecology, or urology, has shown significant clinical benefits compared with open surgery. It has been demonstrated that this minimally invasive approach has lower mortality rates; shorter hospital stay; less postoperative pain; faster return to a regular diet, ambulation, and work; lower incisional hernia rates; as well as better cosmetic results.17-21 It has the additional advantage of reproducing the quality and durability of the open technique.22
The most challenging approach is the totally laparoscopic technique. Efforts are currently being made to facilitate the technique by providing an easier and faster method of vascular anastomosis. Moreover, as with any other technique, the learning curve is estimated to be about 25 cases to 30 cases for the laparoscopic-assisted approach.23
We performed a systematic review of the literature to assess morbidity and mortality and to identify complications associated with laparoscopic surgery for aorto-iliac disease (occlusive and aneurysmal). Surgical techniques included in this review were totally laparoscopic, laparoscopic-assisted, and hand-assisted laparoscopic surgery.
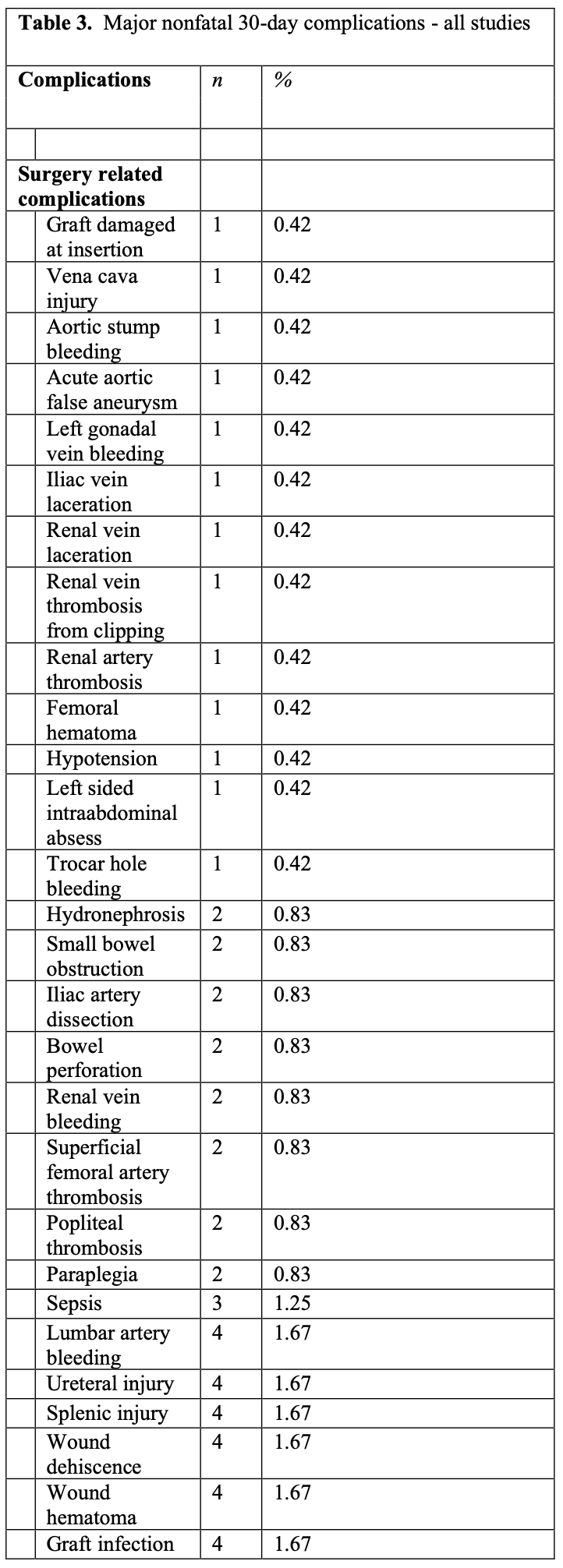
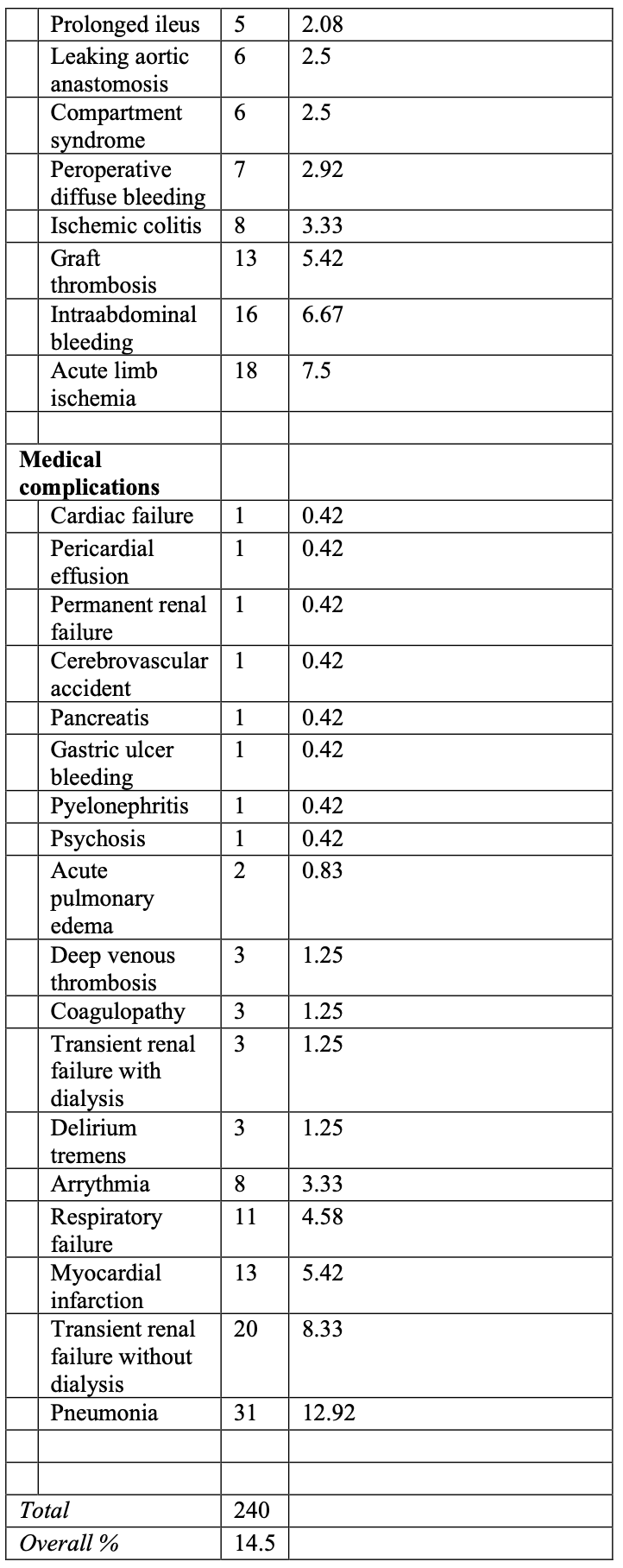
Table 1 summarizes relevant data from the retained studies on aortoiliac disease including 30-day mortality and major nonfatal complications at 30 days.
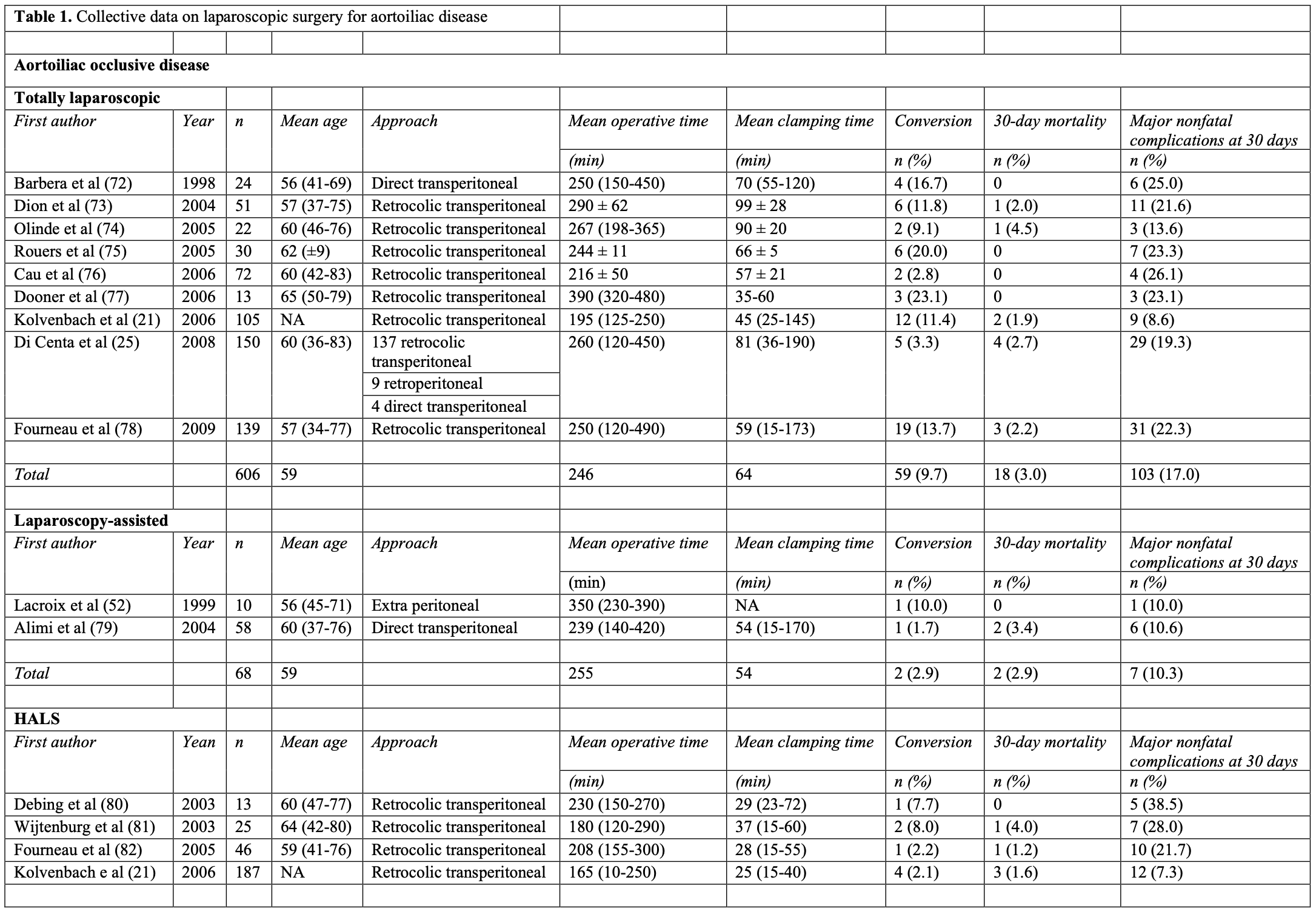
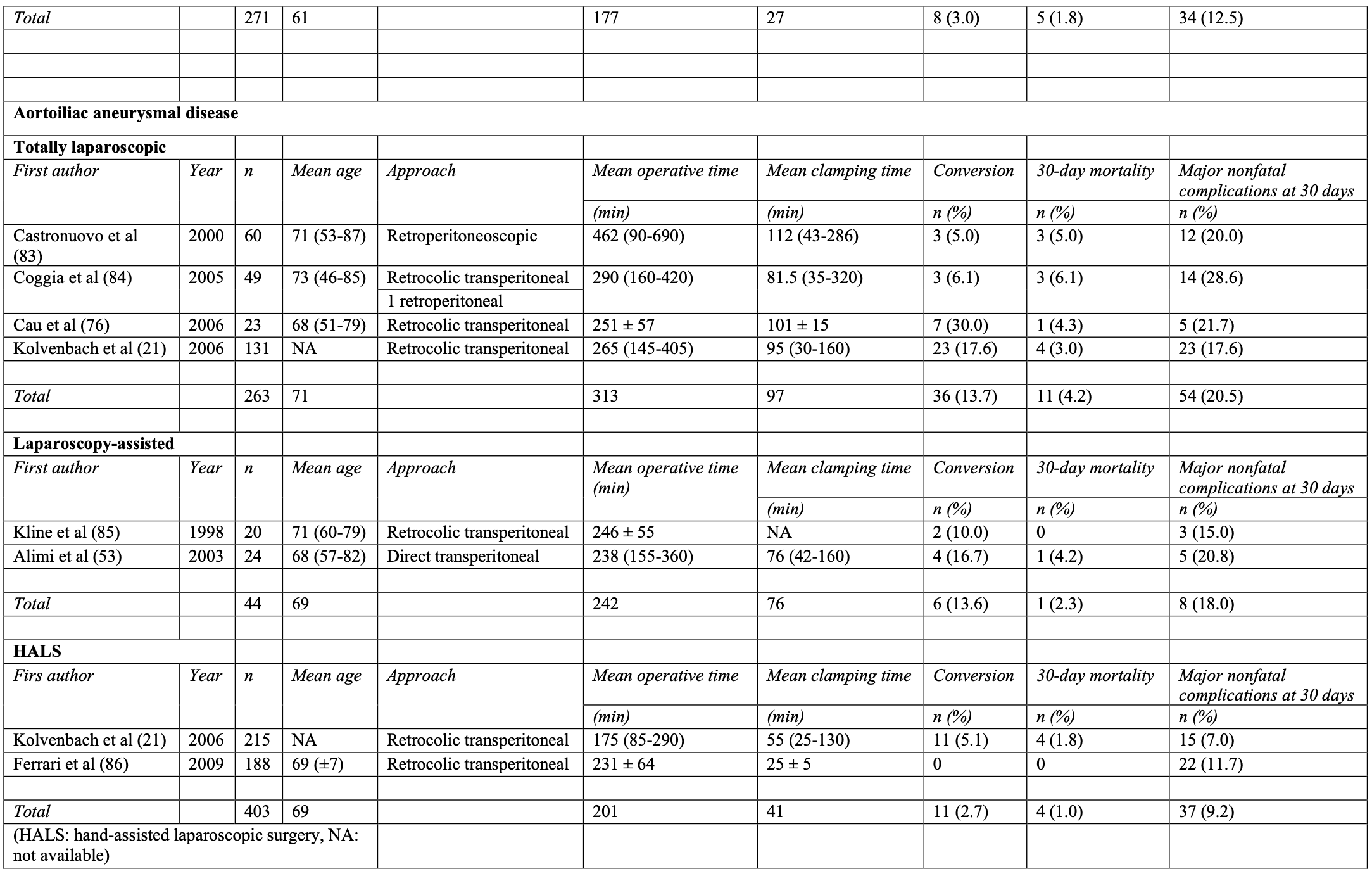
Assessment of the most common causes of mortality in laparoscopic aorto-iliac surgery is represented in Table 2. The overall 30-day mortality rate was 2.1% (range, 0 to 5.0). This represents 34 deaths from a total of 1655 patients. The most common primary causes of death were heart related (35.3% of all deaths) followed by bowel ischemia (20.6% of all deaths).
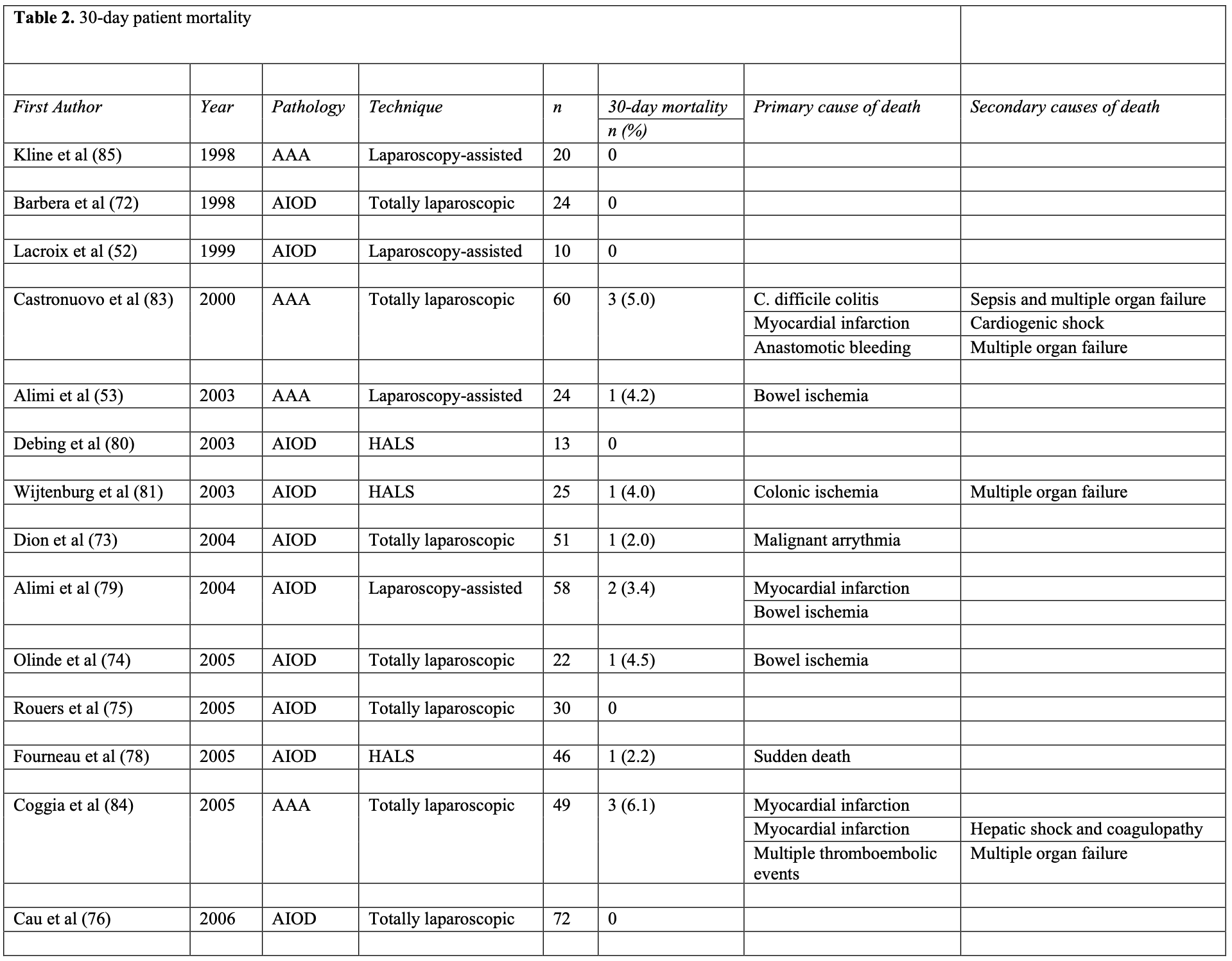
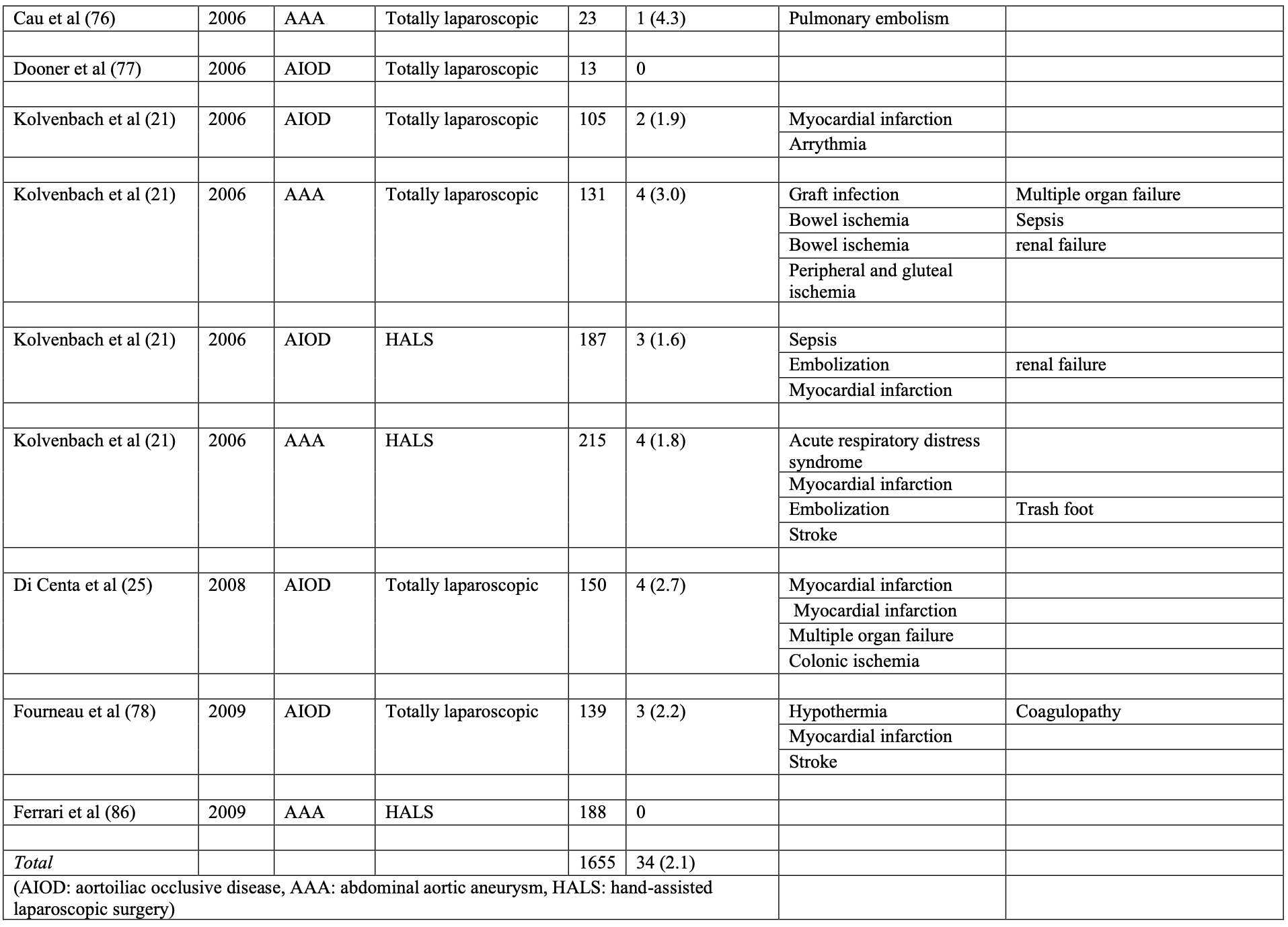
Major nonfatal complications at 30 days are summarized in Table 3. Complications noted were categorized as either surgery related or medical. An overall complication rate of 14.5% was observed (240/1655). The most frequent surgery related complications were acute limb ischemia (7.5%) followed by graft thrombosis (5.4%) and ischemic colitis (2.9%). The most predominant medical complications were pneumonia (12.9%) followed by renal failure (8.3%) and myocardial infarction (5.4%). These results compare to those published in 2007 by Nio et al,24 which revealed a similar overall mortality rate of 2.0% (26/1044). In addition, recent data25 show 5-year primary and secondary permeability rates of 93% and 95.6%, respectively. These results could be interpreted as equivalent to those proposed for endovascular aortic repair (EVAR). The Lifeline registry26 clearly reveals that effect.
Over the years, indications for laparoscopic surgery have broadened and contraindications decreased. Even type IV thoraco-abdominal aneurysms and juxtarenal aneurysms have been treated.27,28 We expect that in the future, with improving knowledge of vascular laparoscopy and better instrumentation, morbidity and mortality rates will decrease.
LAPAROSCOPY AND ENDOVASCULAR THERAPY
One may wonder whether 2 minimally invasive vascular techniques have a role without florid competition. The answer to that question is yes. However, as these 2 techniques evolve, indications, patency rates, patient survival, and cost-effectiveness will be among the important variables to follow.
Occlusive Aorto-Iliac Disease
Therapeutic indications for occlusive disease can be found in the TASC II (Trans- Atlantic inter-Society Consensus) guidelines.29 Although indications for treatment are the result of expert opinions instead of randomized controlled studies, it remains nevertheless the best tool we have at this moment to treat patients according to evidence-based principles.
Recommendations are graded according to levels of evidence. It is well established that published literature and select statements are rated according to guidance issued by the former US Agency for Health Care Policy and Research, renamed the Agency for Healthcare Research and Quality.30
While the specific lesions stratified in the TASC II classification schemes have been modified from the original TASC guidelines to reflect technological advances, the principles behind the classification remain unchanged. Thus, type A lesions should be treated by endovascular means. Type B lesions offer sufficiently good results with endovascular methods that this approach is preferred, unless an open revascularization is required for other associated lesions in the same anatomic area. Type C lesions produce good enough long-term results with open revascularization that endovascular methods should be used only in patients at high risk for open repair. Finally, type D lesions do not yield good enough results with endovascular methods to justify them as primary treatment (Figure 1). What precedes is considered a Grade C recommendation.
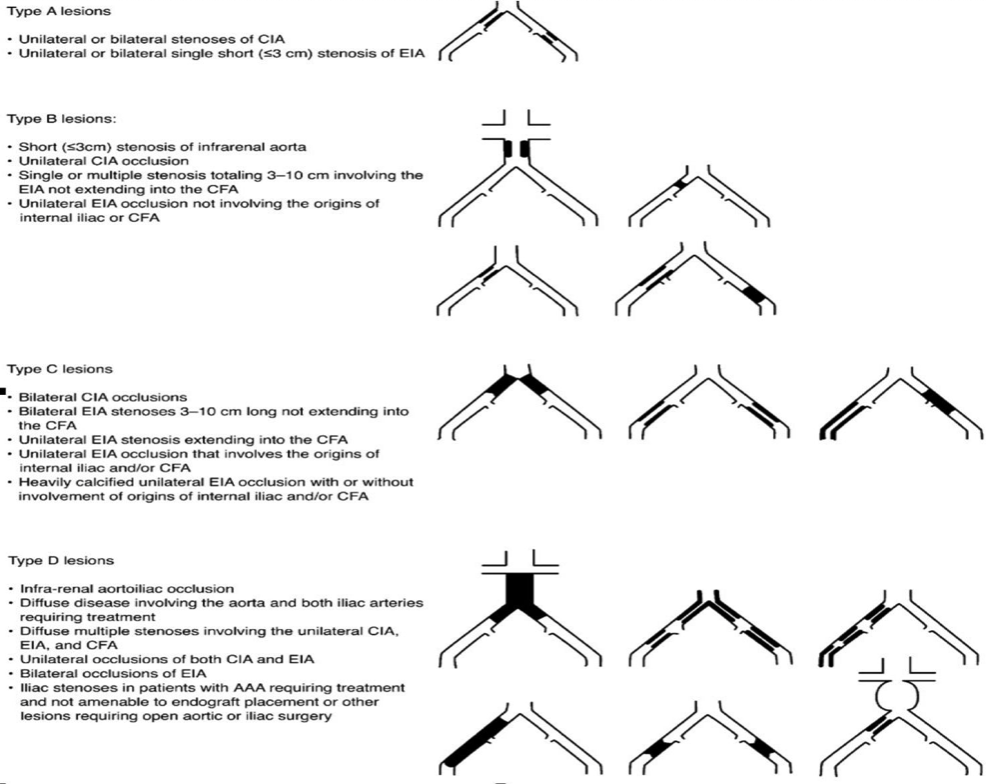 Figure 1. TASC II classification of aorto-iliac occlusive disease.
Figure 1. TASC II classification of aorto-iliac occlusive disease.
Finally, it must be understood that peripheral arterial disease requiring intervention is mostly characterized by more than one lesion, at more than one level, so these schemes are limited by the necessity to focus on individual lesions. With broadening indications for laparoscopic treatment, indications found in the TASC II document for open surgery could be extrapolated for laparoscopic surgery, in view of the good results obtained with the latter technique. In the future, even some TASC B lesions could be advantageously treated laparoscopically.
Aneurysmal Aorto-Iliac Disease
The gold standard presently recognized for treatment of aorto-iliac aneurysmal disease is the open repair. The Food and Drug Administration and other regulatory bodies are known to require open surgical controls as part of well-structured randomized controlled studies. However, as new EVAR devices are developed, the clinical disparity, recruiting difficulty, and significant expense associated with the open surgical controls might be avoided by using the pooled data set provided by the Lifeline registry, which was supported exclusively by funds from the Society for Vascular Surgery (SVS).
According to the Lifeline registry, operative mortality at 30 days for open surgery was 2.8%. Operative mortality for patients with large abdominal aortic aneurysms (AAA) (>5.5cm, 3.6%) was not different than for patients with small AAA (<5.5cm, 2.4%, P=0.54).26 In addition, operative mortality for men and women undergoing elective open surgical repair has been reported in population-based reports to be 3.5% to 4.6%.31-35 It can now be stated that open surgical repair of AAA is safe and effective in preventing aneurysm rupture and avoiding death.
In a recent analysis of open AAA repair compared with EVAR (677 patients),36 it was concluded that operative mortality was similar between the 2 treatment groups (3.5% for open surgery and 2.7% for EVAR, P=0.627). Other authors37-39 have shown a significant short-term survival benefit for EVAR, reducing operative mortality by up to 66%.
Interestingly, when EVAR demonstrated benefit compared with open repair, the mortality in patients undergoing open repair approached 5%. If an operative mortality of <4% can be demonstrated, it is likely that procedure-related mortality will not be significantly different between the 2 techniques.
Reduced length-of-stay is a consistent observation in EVAR compared with open repair.37,39,40 However, Carpenter et al40 reported that the length-of-stay advantage of EVAR was lost during the first year of follow-up owing to readmissions required for subsequent procedures.
Many believe EVAR to be the preferred method of treatment for octogenarians requiring AAA repair. However, it is well recognized that studies show conflicting results about this patient population. Henebiens et al41 performed a systematic review of AAA repair in octogenarians and found a pooled 30-day mortality rate after open repair of 7.5% compared with a pooled mortality rate after EVAR of 4.6%. On the other hand, Paolini et al42 showed no differences in operative and long-term survival between open aneurysm repair and EVAR in octogenarians (P=0.13). Although age itself is an independent risk factor for death after AAA repair, age is not a factor that would favor EVAR compared with open repair. It seems apparent from many studies including the EVAR II trial that neither of the 2 techniques is better than conservative treatment in high-risk patients.43-46
Over the past 20 years, endovascular grafting seems to have brought better results, mainly due to improvements in new generations of endografts. There still remains a significant portion of patients with infrarenal aneurysms that cannot be treated endovascularly, mainly for anatomical reasons. The role of laparoscopy then becomes clearer, and it appears that the 2 techniques become complementary. Laparoscopic treatment of aneurysms with short proximal necks using existing technology has already been reported.47
The cost of laparoscopic treatment has not been fully evaluated. However, it is expected that the procedure will be found to be cost effective when appropriate trials will be designed. In fact, hand instrumentation needed for laparoscopy is reusable and inexpensive. In addition, length-of-stay seems to be shorter than for open surgery. The Dutch DREAM trial48 addressed this issue and concluded that EVAR does not result in quality-adjusted life-year gain at 1 year, does provide only a marginal overall survival benefit, and is associated with a substantial, if not prohibitive, increase in costs. Hayter et al49 also concluded that EVAR results in significantly greater hospital costs compared with open repair, despite reduced length-of-stay. As Noll et al50 showed that efforts at minimizing cost should emphasize technical and device modifications aimed at reducing endoleaks and the need for secondary procedures.
Surgical Technique
Four laparoscopic techniques have been described to date. As previously mentioned, the totally laparoscopic procedure is the most demanding but remains our preferred method (Figures 2 and 3). Most authors now use the transabdominal retrocolic approach to the aorto-iliac vessels similar to ours.16,51 Other procedures have been introduced to reduce the technical difficulties associated with the former technique. The goal is to allow the surgeon to gain greater ease with laparoscopy to progress from a laparoscopic-assisted approach toward a totally laparoscopic approach. The 3 techniques now recognized are the totally laparoscopic approach, the laparoscopic-assisted approach,52,53 and the hand- assisted laparoscopic approach.54,55 We will not discuss the use of robotic devices in vascular laparoscopy.56
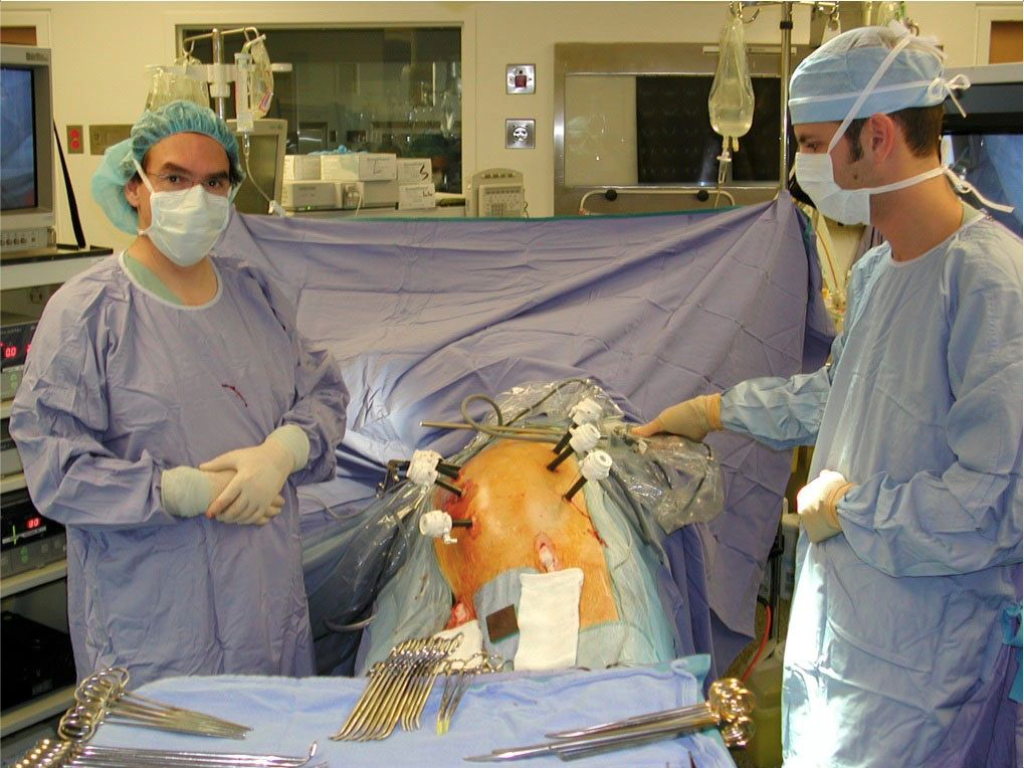 Figure 2. Patient position and trocar sites for a totally laparoscopic technique. The patient’s left side is elevated using cushions. Note that the table can be rotated toward the left to allow femoral dissection when necessary. It is usually rotated toward the right to ensure that intraperitoneal organs will not interfere with adequate visualization of the operative field. The location of the seven 10-mm trocars is shown: 4 are located in the midline (1 infraumbilical, 1 umbilical, and 2 supraumbilical) and 3 laterally on a line slightly medial to the anterosuperior iliac spine.
Figure 2. Patient position and trocar sites for a totally laparoscopic technique. The patient’s left side is elevated using cushions. Note that the table can be rotated toward the left to allow femoral dissection when necessary. It is usually rotated toward the right to ensure that intraperitoneal organs will not interfere with adequate visualization of the operative field. The location of the seven 10-mm trocars is shown: 4 are located in the midline (1 infraumbilical, 1 umbilical, and 2 supraumbilical) and 3 laterally on a line slightly medial to the anterosuperior iliac spine.
 Figure 3. Aorto-aortic bypass shown in a totally laparoscopic repair of an abdominal aortic aneurysm.
Figure 3. Aorto-aortic bypass shown in a totally laparoscopic repair of an abdominal aortic aneurysm.
Surgical Challenges During Laparoscopic Vascular Surgery
Potential complications of laparoscopic surgery for aorto-iliac disease can be divided in 2 main categories. Complications related to access of the aorto-iliac vessels and complications related to surgery on the infrarenal aorta and iliac arteries. The reader should know that most complications occur early in one’s experience, and the following information comes from our personal experience and should help avoid some difficulties that can occur during vascular laparoscopy.
Complications Related to Access of the Aortoiliac Segment
Prior to surgery, careful evaluation of previous abdominal operations is done and scars looked at. As a rule, previous midline infraumbilical incisions are not contraindications to Veress needle use to create the pneumoperitoneum. For other more extensive or left-sided incisions, the aponeurosis should be opened and access to the abdominal cavity guaranteed before trocars are inserted. Prior left hemicolectomy will render retrocolic access more difficult. Therefore, beginners should select their first patients.
Once trocars have been inserted, care should be taken not to injure the small bowel when pushing it away from the operative field. Inadequate handling may cause enterotomies. If this happens, we suggest proceeding to a small laparotomy for repair of the laceration. If there is no gross bile spillage, the aortic bypass can be performed.
After dissection of the peritoneum at Toldt’s line, the Gerota’s fascia covering the kidney, and more distally, the gonadal vessels and ureter become visible. Every effort should be made to keep the left kidney in situ by not dissecting behind and lateral to it. The plane of dissection should be kept anterior where the left renal vein will bedissected. If the kidney is mobilized, it will have a tendency to fall medially over the aorta because of the patient’s position. The other reason to keep the kidney away from the aorta is to keep the ureter at a distance. In an early description of our technique, the kidney was mobilized cephalad and adopted a more medial location, bringing the ureter closer to the aorta.14 This can lead to 2 complications: direct trauma to the ureter due to its proximity to the aorta and possible ischemia due to traction and skeletonization. We have never injured the ureter, although others have reported this complication for both conventional surgery and laparoscopic surgery.57 Hence, meticulous dissection is recommended. We have encountered 2 cases of early transient hydronephrosis of the left ureter that could be attributed to the second mechanism mentioned.58 Although these patients can be watched for resolution of the hydronephrosis, we have modified this part of the technique by first identifying the gonadal vein and minimizing manipulation of the ureter that can be seen under and lateral to these vessels.
Retroperitoneal bleeding is usually venous and related to trauma to the gonadal vein or a branch of this vein. These episodes of bleeding can rapidly be stopped using hemoclips. Once one learns to dissect and follow the gonadal vein from its distal location upward to the left renal vein, these minor complications should not occur.
Another less frequent source of bleeding may be the presence of a retroaortic left renal vein. Today, this complication is usually avoided with its identification on preoperative CT-scans. If such a vein is traumatized, laparoscopic control is obtained by first applying pressure with the instruments already present in the abdomen and carefully using the suction device. At this point, one has the choice of proceeding to a laparotomy to repair the laceration or to do it laparoscopically by applying hemoclips on the renal vein. In fact, kidney edema will develop in 2% to 3% of patients who undergo renal vein ligation done during aneurysm repair.
One potentially lethal complication is trocar-site bleeding. Although a rare occurrence, this complication can easily be avoided by looking at the sites at the end of the procedure. Heparinization during the procedure can be incriminated. The use of an endostitch will quickly resolve the matter. It must be emphasized that perfect hemostasis should always be obtained at the end of the procedure.
Complications Related to Surgery of the Infrarenal Aorta and Iliac Arteries
When dissecting the aorta, one must be careful not to traumatize the lumbar veins, which could lead to major bleeding. When this occurs, tamponade should first be performed followed by clipping. Adequate visualization of the juxtarenal region will be obtained by clipping and incising the gonadal and lumbar veins at the level of the left renal vein.
During the arteriotomy, oozing may appear from the proximal stump. In that case, a second aortic clamp should be inserted at the level of the arteriotomy. One can reposition the first aortic clamp to take care of the bleeding lumbar artery at which point the second aortic clamp could be removed.
During the performance of a laparoscopic anastomosis, the quality of the aortic stump must be inspected and endarterectomy performed when necessary. The running suture should always be followed up by the assistant holding and tightening it with a laparoscopic nerve hook. Leaks can be corrected easily by using a variety of techniques in both end-to-end and end-to-side anastomoses. From our experience, the most difficult anastomoses are those made in calcified aortas. In such cases, one should consider a limited laparotomy, suprarenal clamping, or both of these. In some cases, the laparoscopic gastrointestinal anastomotic (GIA) device cannot penetrate the calcified distal aorta to allow its occlusion, at which point intracorporeal suturing should be attempted laparoscopically or conversion to laparotomy should be considered.
WHAT DOES THE FUTURE HOLD FOR VASCULAR LAPAROSCOPY?
Over the past few years, numerous difficulties have been resolved. The totally retroperitoneal approach has virtually been abandoned, because of specific problems related to working in a small cavity. For the totally laparoscopic technique, most authors work through a transabdominal retrocolic approach. A direct transabdominal approach has been described for the robotic-assisted technique. Surgeons practicing hand-assisted laparoscopy will select 1 of the 2 mentioned approaches for a given patient.14,16,17,51,55,56
To make the less traumatic totally laparoscopic technique easier and more attractive to vascular surgeons, work is underway to evaluate the possibility of simplifying the anastomosis using automated devices. This is the focus of the following section.
“Handmade” Laparoscopic Anastomosis
Development of dedicated instrumentation has made possible the safe performance of laparoscopy for aorto-iliac disease.16,59 We recognized during our early animal work that the original technique of utilizing one continuous suture had to be modified for laparoscopic use.11 We realized that utilizing one long suture in a relatively confined space inevitably led to suture entanglement. To prevent this problem, we used two 24-cm sutures which, for end-to-end anastomoses, would begin posteriorly and run anteriorlyon either side of the anastomosis.13,14 For end-to-side anastomoses, the 2 hemi-running sutures begin at the heel of the anastomosis. Blocking the end of the running suture with a pledget leads to improvement in suturing time because only one knot is needed.51 Others have proposed an anastomosis using 9 U-shaped sutures reinforced with pledgets.60 This technique proved rather cumbersome and is not in use today. To perform a faster anastomosis, many authors will transect the proximal aorta so that the proximal aortic stump can be mobilized, thus facilitating the procedure. Also, beginning the end- to-end anastomosis at 9 o’clock facilitates the technique.
Alternatives to the “Handmade” Laparoscopic Anastomosis
In the 1950s, Soviet surgeons already contemplated the possibility of using an automated suturing device.61 In the early 1960s, Japanese surgeons proposed utilization of 2 rings to perform an end-to-end anastomosis.62 In 2003, Zeebregts63 presented a review of all methods of sutureless anastomoses. He divided these various devices into 5 distinct groups: devices using rings, staples, adhesive glues, stents, and laser welding. All of these techniques offer the advantage of a significant reduction in anastomotic time and appear to offer the added benefit of less trauma to the arterial wall compared with the handmade suture. However, each of these devices has some drawbacks. Because of their rigidity, the rings could pose a difficulty when the diameters of tissues to be anastomosed are not equal. Staples involve vessel wall eversion that in theory avoids creation of neointimal hyperplasia. In the early days, their use was hampered by the presence of atheromatous plaque in the human aorta, allowing for uneven coaptation of the staples around the anastomosis. In the case of glues, it appears they could be used on small vessels but would most likely lead to false aneurysms and anastomotic disruption in larger vessels.
As for stents, permeability rates have been shown to be quite low. Laser welding has not been studied rigorously for large-caliber anastomoses. It seems that these techniques would be difficult to introduce in the setting of large aortic anastomoses, considering that most of them have been designed for small-caliber anastomoses.
Over the past 5 years, with increasing developments in vascular laparoscopy, a number of devices have been tested. Below, the most promising devices available are presented.
- The staple-free device, also known as the SuDyn device, has the advantage of using interrupted polypropylene sutures that are automatically locked in place by using a specific instrument.64
- Nakano et al65 and Scarcello et al66 propose to use 2 stents (one intraaortic and one extraaortic) to maintain a graft in close approximation to the aortic wall. In the case of Nakano’s device, a reabsorbable polyglactic internal stent is deployed, while Scarcello uses a self-expanding intraaortic stent.
- Yoffe et al67 designed a device that can be affixed to the end of any prosthesis, tubular or bifurcated. The device holds the graft in place by a series of hooks penetrating the aortic wall (Figure 4).
 Figure 4. Hermetic Docking Head® device.
Figure 4. Hermetic Docking Head® device.
The previous 3 devices are not in contact with blood flow, thus limiting neointimal hyperplasia.
- Stapling devices
- In 2004, Elkouri et al68 demonstrated in an animal study using a stapler available in general surgery, the potential of aortic stapling for end-to-side anastomosis. He conducted his study in sheep.
- Schifrin,69 using the principle of circular stapling, performed 12 end-to-end anastomoses on a porcine model. Following these experiments, Kolvenbach70 presented a human case series utilizing this stapler. The results seem satisfying although some issues have to be resolved with this type of instrumentation before it becomes available for laparoscopic use.
- The VCS clipping device has been used extensively to secure infrainguinal grafts. However, it seems unlikely that it will be used in aorto-iliac surgery, because of concerns linked to its configuration made to avoid contact with blood flow by clips that do not penetrate the arterial wall. Such a configuration may contribute to weaken the anastomosis.71
One may speculate that one or more devices mentioned previously will eventually permit automated, easier, and faster laparoscopic anastomoses.
CONCLUSION
Two minimally invasive vascular techniques have been under scrutiny for almost 20 years. Both have their own advantages and potential disadvantages as discussed in this chapter. Much more has been studied on endovascular therapy and endografting for aorto-iliac disease compared with laparoscopy. Immeasurable investments have been made to develop endovascular therapy. However, laparoscopy reproduces the advantages recognized for open surgery without its disadvantages. As discussed earlier, less postoperative pain, decreased mortality, decreased hospital stay and return to work, better cosmesis and decreased incisional hernia rates have been reported compared with open surgery.
As previously stated, studies have demonstrated a significantly decreased 30-day mortality rate for EVAR compared with open surgery. One must be acquainted with the fact that statistical significance is only reached in studies demonstrating mortality rates >4% for open surgery.
Therefore, the 2 minimally invasive techniques discussed in this chapter must be thought of as complementary to each other in both occlusive and aneurysmal aorto-iliac disease. In addition, the fenestrated technology designed to include patients with aneurysms presenting with an infrarenal neck <10mm in length is expensive and requires expertise. This problem will be supplanted by the development of well-designed laparoscopic anastomotic devices.
Minimally invasive techniques will play an increasing role in the treatment of patients with aorto-iliac disease. Time and rigorous work will place each of these techniques in the vascular therapist’s armamentarium.
Address for correspondence: Yves-Marie Dion, MD, Centre Hospitalier Universitaire de Québec, Pavillon St-François d`Assise, 10, de l`Espinay, Québec, Qc, Canada, G1L 3L5. Fax: (418) 871-9678, E-mail: dion.yves@videotron.ca
References
- Oudot J. [Graft of the aortic bifurcation from the renal arteries to external iliac arteries for thromboarteritis.]. Mem Acad Chir (Paris). 1951 Jun 6;77(20-21):642-644.
- Dubost C, Allary M, Oeconomos N. [Treatment of aortic aneurysms; removal of the aneurysm; re-establishment of continuity by grafts of preserved human aorta.]. Mem Acad Chir (Paris). 1951 Apr 11;77(12-13):381-383.
- Voorhees AB Jr, Jaretzki A, III, Blakemore AH. The use of tubes constructed from vinyon “N” cloth in bridging arterial defects. Ann Surg. 1952 Mar;135(3):332-336.
- Volodos NL, Shekhanin V, Karpovich I. Self-fixing prosthesis for remote endoprosthetics of aorta and large artery. In: Moscow, ed. Actual Questions of Organizing, Prevention and Surgical Treatment of Vascular Disease. 1985:217-218.
- Volodos NL, Shekhanin VE. Device for insertion of a prosthesis in a vessel. USSR Patent. 1984.
- Volodos NL, Karpovich IP, Troyan VI, et al. Clinical experience of the use of self- fixing synthetic prostheses for remote endoprosthetics of the thoracic and the abdominal aorta and iliac arteries through the femoral artery and as intraoperative endoprosthesis for aorta reconstruction. Vasa Suppl. 1991;33:93-95.
- Volodos NL, Karpovich L, Troyan V, et al. Endovascular stented grafts for thoracic, abdominal aortic, and iliac arterial disease: clinical experience in the Ukraine from Seminars in Interventional Radiology. 1985.
- Volodos NL, Karpovich IP, Shekhanin VE, Troyan VI. A case of remote transfemoral endoprosthetics of the thoracic aorta with a synthetic self-fixing prosthesis for traumatic aneurysm. Grudnaya Khirurgia (Russia). 1988;6:84-86.
- Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991 Nov;5(6):491-499.
- Dion YM, Katkhouda N, Rouleau C, Aucoin A. Laparoscopy-assisted aortobifemoral bypass. Surg Laparosc Endosc. 1993 Oct;3(5):425-429.
- Dion YM, Chin AK, Thompson TA. Experimental laparoscopic aortobifemoral bypass. Surg Endosc. 1995 Aug;9(8):894-897.
- Dion YM, Gracia C. Experimental laparoscopic aortic aneurysm resection and aortobifemoral bypass. Surg Laparosc Endosc. 1996 Jun;6(3):184-190.
- Dion YM, Gaillard F, Demalsy JC, Gracia CR. Experimental laparoscopic aortobifemoral bypass for occlusive aortoiliac disease. Can J Surg. 1996 Dec;39(6):451- 455.
- Dion YM, Gracia CR. A new technique for laparoscopic aortobifemoral grafting in occlusive aortoiliac disease. J Vasc Surg. 1997 Oct;26(4):685-692.
- Dion YM, Gracia CR, Ben El Kadi HH. Totally laparoscopic abdominal aortic aneurysm repair. J Vasc Surg. 2001 Jan;33(1):181-185.
- Dion YM, Thaveau F, Fearn SJ. Current modifications to totally laparoscopic “apron technique”. J Vasc Surg. 2003 Aug;38(2):403-406.
- Alimi YS, Di ML, Hartung O, et al. Laparoscopy-assisted abdominal aortic aneurysm endoaneurysmorraphy: early and mid-term results. J Vasc Surg. 2003 Apr;37(4):744-749.
- Ferrari M, Adami D, Del CA, et al. Laparoscopy-assisted abdominal aortic aneurysm repair: early and middle-term results of a consecutive series of 122 cases. J Vasc Surg. 2006 Apr;43(4):695-700.
- Fourneau I, Sabbe T, Daenens K, Nevelsteen A. Hand-assisted laparoscopy versus conventional median laparotomy for aortobifemoral bypass for severe aorto-iliac occlusive disease: a prospective randomised study. Eur J Vasc Endovasc Surg. 2006 Dec;32(6):645-650.
- Kolvenbach R, Schwierz E, Wasilljew S, Miloud A, Puerschel A, Pinter Total laparoscopically and robotically assisted aortic aneurysm surgery: a critical evaluation. J Vasc Surg. 2004 Apr;39(4):771-776.
- Kolvenbach R, Puerschel A, Fajer S, et al. Total laparoscopic aortic surgery versus minimal access techniques: review of more than 600 patients. Vascular. 2006 Jul;14(4):186-192.
- Dion YM, Griselli F, Douville Y, Langis P. Early and mid-term results of totally laparoscopic surgery for aortoiliac disease: lessons learned. Surg Laparosc Endosc Percutan Tech. 2004 Dec;14(6):328-334.
- Fourneau I, Lerut P, Sabbe T, Houthoofd S, Daenens K, Nevelsteen A. The learning curve of totally laparoscopic aortobifemoral bypass for occlusive disease. How many cases and how safe? Eur J Vasc Endovasc Surg. 2008 Jun;35(6):723-729.
- Nio D, Diks J, Bemelman WA, Wisselink W, Legemate DA. Laparoscopic vascular surgery: a systematic review. Eur J Vasc Endovasc Surg. 2007 Mar;33(3):263-271.
- Di Centa I, Coggia M, Cerceau P, et al. Total laparoscopic aortobifemoral bypass: short- and middle-term results. Ann Vasc Surg. 2008 Mar;22(2):227-232.
- Zwolak RM, Sidawy AN, Greenberg RK, Schermerhorn ML, Shackelton RJ, Siami FS. Lifeline registry of endovascular aneurysm repair: open repair surgical controls in clinical trials. J Vasc Surg. 2008 Sep;48(3):511-518.
- Coggia M, Javerliat I, Di C, I, Royer B, Kitzis M, Goeau-Brissonniere OA. Total videoscopic treatment of a type IV thoracoabdominal aneurysm. J Vasc Surg. 2005 Jan;41(1):141-145.
- Coggia M, Cerceau P, Di C, I, Javerliat I, Colacchio G, Goeau-Brissonniere O. Total laparoscopic juxtarenal abdominal aortic aneurysm repair. J Vasc Surg. 2008 Jul;48(1):37-42.
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter- Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007 Jan;45 Suppl S:S5-67.
- Rockville MD. Acute pain management: operative on medical procedures and trauma. 1993.
- Biancari F, Ylonen K, Anttila V, et al. Durability of open repair of infrarenal abdominal aortic aneurysm: a 15-year follow-up study. J Vasc Surg. 2002 Jan;35(1):87- 93.
- Johnston KW. Nonruptured abdominal aortic aneurysm: six-year follow-up results from the multicenter prospective Canadian aneurysm study. Canadian Society for Vascular Surgery Aneurysm Study Group. J Vasc Surg. 1994 Aug;20(2):163-170.
- Katz DJ, Stanley JC, Zelenock GB. Gender differences in abdominal aortic aneurysm prevalence, treatment, and outcome. J Vasc Surg. 1997 Mar;25(3):561-568.
- Lee WA, Carter JW, Upchurch G, Seeger JM, Huber TS. Perioperative outcomes after open and endovascular repair of intact abdominal aortic aneurysms in the United States during 2001. J Vasc Surg. 2004 Mar;39(3):491-496.
- Pearce WH, Parker MA, Feinglass J, Ujiki M, Manheim LM. The importance of surgeon volume and training in outcomes for vascular surgical procedures. J Vasc Surg. 1999 May;29(5):768-776.
- Chahwan S, Comerota AJ, Pigott JP, Scheuermann BW, Burrow J, Wojnarowski Elective treatment of abdominal aortic aneurysm with endovascular or open repair: the first decade. J Vasc Surg. 2007 Feb;45(2):258-262.
- Blankensteijn JD, de Jong SE, Prinssen M, et al. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2005 Jun 9;352(23):2398-2405.
- Goueffic Y, Becquemin JP, Desgranges P, Kobeiter H. Midterm survival after endovascular versus open repair of infrarenal aortic aneurysms. J Endovasc Ther. 2005 Feb;12(1):47-57.
- Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet. 2004 Sep 4;364(9437):843-848.
- Carpenter JP, Baum RA, Barker CF, et al. Durability of benefits of endovascular versus conventional abdominal aortic aneurysm repair. J Vasc Surg. 2002 Feb;35(2):222- 228.
- Henebiens M, Vahl A, Koelemay MJ. Elective surgery of abdominal aortic aneurysms in octogenarians: a systematic review. J Vasc Surg. 2008 Mar;47(3):676-681.
- Paolini D, Chahwan S, Wojnarowski D, Pigott JP, LaPorte F, Comerota AJ. Elective endovascular and open repair of abdominal aortic aneurysms in octogenarians. J Vasc Surg. 2008 May;47(5):924-927.
- Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet. 2005 Jun 25;365(9478):2187-2192.
- Hirzalla O, Emous M, Ubbink DT, Legemate D. External validation of the Glasgow Aneurysm Score to predict outcome in elective open abdominal aortic aneurysm repair. J Vasc Surg. 2006 Oct;44(4):712-716.
- Biancari F, Hobo R, Juvonen T. Glasgow Aneurysm Score predicts survival after endovascular stenting of abdominal aortic aneurysm in patients from the EUROSTAR registry. Br J Surg. 2006 Feb;93(2):191-194.
- Nesi F, Leo E, Biancari F, et al. Preoperative risk stratification in patients undergoing elective infrarenal aortic aneurysm surgery: evaluation of five risk scoring methods. Eur J Vasc Endovasc Surg. 2004 Jul;28(1):52-58.
- Di Centa I, Coggia M, Cochennec F, Javerliat I, Alfonsi P, Goeau-Brissonniere O. Total laparoscopic repair of abdominal aortic aneurysm with short proximal necks. Ann Vasc Surg. 2009 Jan;23(1):43-48.
- Prinssen M, Buskens E, de Jong SE, et al. Cost-effectiveness of conventional and endovascular repair of abdominal aortic aneurysms: results of a randomized trial. J Vasc Surg. 2007 Nov;46(5):883-890.
- Hayter CL, Bradshaw SR, Allen RJ, Guduguntla M, Hardman DT. Follow-up costs increase the cost disparity between endovascular and open abdominal aortic aneurysm repair. J Vasc Surg. 2005 Nov;42(5):912-918.
- Noll RE, Jr., Tonnessen BH, Mannava K, Money SR, Sternbergh WC, III. Long-term postplacement cost after endovascular aneurysm repair. J Vasc Surg. 2007 Jul;46(1):9-15.
- Coggia M, Bourriez A, Javerliat I, Goeau-Brissonniere O. Totally laparoscopic aortobifemoral bypass: a new and simplified approach. Eur J Vasc Endovasc Surg. 2002 Sep;24(3):274-275.
- Lacroix H, Nevelsteen A, Suy R. Aorto-bi-femoral bypass for aorto-iliac occlusive disease using a videoscopic assited retroperitoneal approach: a preliminary report. Acta Chir Belg. 1999;99:241-244.
- Alimi YS, Di Molfetta L, Hartung O, et al. Laparoscopy-assisted abdominal aneurysm endoaneurysmorraphy: Early and mid-term results. J Vasc Surg. 2003;37:744- 749.
- Da Silva L, Kolvenbach R, Pinter The feasibility of hand-assisted laparoscopic aortic bypass using a low transverse incision. Surg Endosc. 2002;16:173-176.
- Kolvenbach R, Deling O, Schwierz E, Landers B. Reducing the operative trauma in aortoiliac reconstructions: a prospective study to evaluate the role of video-assisted vascular surgery. Eur J Vasc Endovasc Surg. 1998 Jun;15(6):483-488.
- Stadler P, Matous P, Vitasek P, Spacek M. Robot-assisted aortoiliac reconstruction: A review of 30 cases. J Vasc Surg. 2006 Nov;44(5):915-919.
- Alimi YS, Hartung O, Valerio N, Juhan C. Laparoscopic aortoiliac surgery for aneurysm and occlusive disease: When should a minilaparotomy be performed? J Vasc Surg. 2001;33(3):469-475.
- Thaveau F, Dion YM, Warnier de Wailly G, et al. Early transient hydronephrosis after laparoscopic aortobifemoral bypass grafting. J Vasc Surg. 2003 Sep;38(3):603-608.
- Alimi YS. Laparoscopic aortic surgery: recent development in instrumentation. Acta Chir Belg. 2004 Oct;104(5):505-512.
- Edoga JK, Asgarian K, Singh D, et al. Laparoscopic surgery for abdominal aortic aneurysms. Technical elements of the procedure and a preliminary report of the first 22 patients. Surg Endosc. 1998 Aug;12(8):1064-1072.
- Romachou F. Les appareils soviétiques chirurgicaux de suture. Presse Med. 1964;(72):277-282.
- Nakayama K, Tamiya T, Yamamoto K. A simple new apparatus for small vessels anastomosis. Surgery. 1962;(52):918-922.
- Zeebregts CJ, Heijmen RH, van den Dungen JJ, van SR. Non-suture methods of vascular anastomosis. Br J Surg. 2003 Mar;90(3):261-271.
- Millon A, Boufi M, Garitey V, et al. Evaluation of a new vascular suture system for aortic laparoscopic surgery: an experimental study on pigs and cadavers. Eur J Vasc Endovasc Surg. 2008 Jun;35(6):730-736.
- Nakano Y, Hori Y, Sato A, et al. Evaluation of a Poly(l-lactic acid) stent for sutureless vascular anastomosis. Ann Vasc Surg. 2008 Sep 5.
- Scarcello E, Triggiani G, Arispici M, et al. Experimental study of a new vascular anastomotic technique in a swine model: short and mid-term results. Ann Vasc Surg. 2007 May;21(3):346-331.
- Yoffe B, Vaysbeyn I, Urin Y, et al. Experimental study of a novel suture-less aortic anastomotic device. Eur J Vasc Endovasc Surg. 2007 Jul;34(1):79-86.
- Elkouri S, Noel AA, Gloviczki P, et al. Stapled aortic anastomoses: a minimally invasive, feasible alternative to videoscopic aortic suturing? Vasc Endovasc Surg. 2004 Jul;38(4):321-330.
- Shifrin EG, Moore WS, Bell PR, Kolvenbach R, Daniline EI. Intravascular stapler for “open” aortic surgery: preliminary results. Eur J Vasc Endovasc Surg. 2007 Apr;33(4):408-411.
- Kolvenbach R, Shiffrin E, Schwierz E, Wassiljew S, Caggianos C. Evaluation of an aortic stapler for an open aortic anastomosis. J Cardiovasc Surg (Torino). 2007 Oct;48(5):659-665.
- Calles-Vazquez MC, Viguera FJ, Crisostomo V, Uson-Gargallo J. Vascular closure stapler clip anastomosis decreases aortic cross-clamping time compared to interrupted nonabsorbable and running absorbable sutures in growing pigs. Ann Vasc Surg. 2006 Jan;20(1):35-41.
- Barbera L, Mumme A, Metin S, Zumtobel V, Kemen M. Operative results and outcome of twenty-four totally laparoscopic vascular procedures for aortoiliac occlusive disease. J Vasc Surg. 1998;28(1):136-142.
- Dion YM, Griselli F, Douville Y, Langis P. Early and mid-term results of totally laparoscopic surgery for aortoiliac disease. Surg Laparosc Endosc Percutan Tech. 2004 Dec;14(6):329-334.
- Olinde AJ, McNeil JW, Sam II A, Hebert SA, Frusha JD. Totally laparoscopic aortobifemoral bypass: a review of 22 cases. J Vasc Surg. 2005 July;42(1):27-34.
- Rouers A, Meurisse N, Lavigne JP, et al. Potential benefits of laparoscopic aorto- bifemoral bypass surgery. Acta Chir Belg. 2005;105:610-615.
- Cau J, Ricco JB, Marchand C, et al. Total laparoscopic aortic repair for occlusive and aneurysmal disease: first 95 cases. Eur J Vasc Endovasc Surg. 2006;31:567-574.
- Dooner J, Lee S, Griswold W, Kuechler P. Laparoscopic aortic reconstruction: early experience. Am J Surg. 2006;191:691-695.
- Fourneau I, Mariën I, Remy P, et al. Conversion during laparoscopic aortobifemoral bypass: a failure? Eur J Vasc Endovasc Surg. 2009;xx:1-7.
- Alimi YS, Giovanni DC, Hartung O, et al. Laparoscopy-assisted reconstruction to treat severe aortoiliac occlusive disease: early and midterm results. J Vasc Surg. 2004:39 (4):777-783.
- Debing E, Vanhulle A, Ven den Brande P. Laparoscopic hand-assisted abdominal aortic surgery for aneurismal and occlusive disease: a one-year clinical experience. Acta Chir Belg. 2003;103:203-207.
- Wijtenburg E, Remy Ph, D’hont Ch, Vindevogel Ch, Blampain JP, Massin H. Hand- assisted laparoscopic aortoiliac surgery: preliminary report of 25 cases. Acta Chir Belg. 2003;103:493-496.
- Fourneau I, Daenens K, Nevelsteen A. Hand-assisted laparoscopic aortobifemoral bypass for occlusive disease. Early and midterm results. Eur J Vasc Endovasc Surg. 2005;30(5):489-493.
- Castronuovo JJ, James KV, Resnikoff M, McLean ER, Edoga JK. Laparoscopy- assisted abdominal aortic aneurysmectomy. J Vasc Surg. 2000;32(2):224-233.
- Coggia M, Di Centa I, Javerliat I, Alfonsi P, Kitzis M, Goëau-Brissonnière A. Total laparoscopic abdominal aortic aneurysms repair. J Cardiovasc Surg. 2005; 46:407-414.
- Kline RG, D’Angelo AJ, Chen MHM, Halpern VJ, Cohen JR. Laparoscopically assisted abdominal aortic aneurysm repair: first 20 cases. J Vasc Surg. 1998; 27(1):81-88.
- Ferrari M, Adami D, Berchiolli F, Del Corso A, Pietrabissa A. Laparoscopic-assisted treatment of abdominal aortic aneurysm requiring suprarenal cross-clamping. J Vasc Surg. 2009;50(5):1006-1011.
