Endoluminal Therapies for Gastroesophageal Reflux Disease and Obesity
András Légner, MD, Charles J. Filipi, MD
INTRODUCTION
Gastroesophageal reflux disease (GERD) is due to the failure of the gastroesophageal barrier.1,2 The disease has dramatically increased in the last 2 decades, particularly in the Western world. Nearly 40% of the North American population experiences monthly heartburn, and 18% use nonprescription medication.3,4 The optimal treatment of GERD continues to be debated.5,6 The rising number of patients having daily reflux-related symptoms and their impact on quality of life has lead to a better understanding of the pathophysiology of this condition and its appropriate management. New antisecretory medications, laparoscopic surgical techniques, and novel endoluminal devices have been introduced for the treatment of GERD. The outcomes are encouraging, but the cost of medication and surgery continues to be high.7
The prevalence of obesity has more than doubled in the last 2 decades in the United States, as 60% of adults are either overweight or obese.8 If this trend continues, more than 80% of the adult population will be overweight or obese by 2030, and all American adults will be overweight by 2048.9 The economic impact on health care costs is evident. A recent study estimated that medical expenditures attributed to obesity will double every decade. While in 1998, approximately 78 billion dollars were spent on obesity, in 2008 the overweight-associated medical expenses had reached 147 billion dollars.8
The association of chronic diseases with obesity is widely known. Guh et al10 reported 18 comorbidities attributable to obesity, such as stroke, heart and respiratory disease, certain types of cancer, and type II diabetes. Obesity, especially in the presence of diabetes, is a known risk factor for GERD, because of the elevated intragastric pressure and impaired esophageal motility caused by diabetic neuropathy of the esophagus.11-13
ANATOMICAL AND PHYSIOLOGICAL BACKGROUND OF GERD
The lower esophageal sphincter (LES) and the geometric profile of the cardia prevent GERD and are the targets of surgical and endoluminal GERD procedures. The LES is characterized by its length, relative position to the diaphragm, and pressure. A decrease in pressure, the overall length, or both pressure and overall length, or just the length of the abdominal segment of the LES predisposes to reflux, because it decreases the resistance imposed on the flow of gastric juice or bile from the higher pressure stomach. The most common cause of a permanently defective sphincter is inadequate pressure, but the efficiency of the sphincter can also be nullified by an inadequate abdominal length or an abnormally short overall length.2 In patients with severe GERD, the LES or the “high- pressure zone” is virtually nonexistent or greatly reduced, and reflux in this instance is understandable. However, the cause of reflux in milder disease with a normal lower esophageal sphincter resting pressure (LESP) is under considerable debate. It is believed that transient LES relaxations (tLESR), ie, intermittent spontaneous decreases in LESP, are responsible for reflux events.14-17 Recent electrophysiological data suggest that the relevant vagal afferent fibers terminate with specialized intraganglionic laminar endings (IGLEs). These deformity-sensitive transducers are lined in series with muscle fibers at the cardia and fundus and are believed to mediate both fundic receptive relaxation and elicitation of tLESRs.18
The normal angle of His prevents the distensive forces generated within the stomach to be transmitted to the LES, thus preventing its subsequent “unfolding.”2 As the normal geometry of the cardia disappears with increasing gastric distention, the abdominal segment of the LES becomes more exposed to the intragastric and abdominal pressure and loses its intraabdominal length. It is taken up into the stretching fundus (such as the uterine cervix during delivery). At a critical length of 1cm to 2cm, the LESP drops acutely and reflux occurs (Peters JH, personal communication).
Nissen fundoplication prevents LES shortening during gastric distension, thus minimizing GERD (Peters JH, personal communication). In light of the above-described pathophysiologic factors, endoscopic therapies should prevent reflux in one or more of the following ways: (1) alter the compliance of the cardia and prevent tLES shortening/relaxation, (2) increase baseline LES tone or, (3) increase baseline LES length.
MANAGEMENT OF GERD
The treatment of GERD is individualized, depending on the patient’s comorbidities, symptom severity, response to medication, and physiologic test results. The treatment spectrum is wide, from simple lifestyle changes to Roux-en-Y gastric bypass, and has improved greatly with the advent of antisecretories. Complete healing of esophagitis after intensive PPI therapy has been reported to be as high as 90%19,20; however, medication has no effect on the underlying anatomical defects. Acid-related symptoms can be eliminated with vigorous antisecretory therapy, but alkaline reflux may also occur causing definitive changes in the esophageal mucosa. Administration of a single daily dose of a PPI is sufficient in the majority of patients but those with more advanced disease require higher doses.21 Patients may be refractory to pharmacological therapy, and the rate of relapse reaches 100% in patients with low LES pressures.22 Thus, many patients must commit to lifelong therapy. Moreover, 50% of patients continue to exhibit low intragastric pH and objective evidence of acid regurgitation despite complete symptomatic control on PPI therapy.23
Despite the relative safety of these medications, new data have increased concern about the long-term effects and safety of antisecretory drugs.24 Patients on long-term PPI therapy encounter a higher incidence of pulmonary infection, nutritional deficiencies such as hypocalcemia, Vitamin B12 deficiency, or hypomagnesemia, and an increased rate of hip fractures.25-27 These side effects plus the high cost of antisecretories remains a significant problem.
Antireflux surgery is recommended for patients with refractory or complicated GERD and provides excellent symptom control in 85% to 90% of cases.28,29 With the advent of laparoscopic antireflux surgery (LARS), the number of antireflux operations has doubled.30 However, failure may occur and 3% to 5% of patients undergo one or more remedial operations.31,32 In addition, laparoscopic antireflux surgery requires a general anesthetic, hospitalization, postoperative lifestyle limitations for days to weeks, is expensive, and is associated with postoperative morbidity and even a mortality rate.33
Reoperative antireflux surgery is a feasible option for patients with recurrent disease, although inferior results with a higher mortality and morbidity rate as compared with primary surgery are seen.34,35 Endoluminal intervention for GERD is relatively new and still immature but is a promising option for GERD patients. Morbidities and mortality should be reduced, and repeat procedures may be easier to perform.
PHYSIOLOGY OF OBESITY
The pathophysiology of obesity is complex, because it is influenced by environmental, behavioral, genetic, endocrine, and neurotransmitter factors. Although obesity is simply a result of an imbalance between energy intake and expenditure, the various combinations of the above mentioned factors results in heterogeneous clinical manifestations of obesity. Recent research implicates environmental and social-behavioral risk factors including poor quality nutrients, chronic stress, pre- and postpartum environment, sedentary lifestyle, and exposure to chemical or pharmaceutical agents, such as antipsychotics, antidepressants, or corticosteroids.36-39
Gene-diet interactions on monozygotic twins demonstrate that the genotype has an unquestionable role on diet-related obesity.40 Recently reported genome-wide association studies have revealed several genes related to obesity risk. The insulin induced gene 2 (INSIG2) and the fat mass and obesity-associated (FTO) genes have been identified as genes having an influence on body mass.41 Although the exact number of these gene variants is unknown, it is likely that many will have a modest effect on the phenotype.42
Agents, called adipogenes, interfere with the endocrine or neuroregulatory system and influence adipogenesis and obesity. The disruption of the hypothalamic-pituitary-adrenal axis can lead to the disintegration of metabolic homeostasis and consequent weight gain.43 Hyperphagia may result from the reduced effect of the neurotransmitter dopamine, which modulates the rewarding properties of food. Wang et al44 reported that the availability of dopamine D2 receptors was decreased in obese individuals in proportion to their BMI.
The role of chemical agents in the development of obesity is well established, but a better understanding of the molecular background is crucial. As our knowledge of the pathophysiology of obesity increases, it is becoming clear that the treatment of obesity is complex and must be individualized.
MANAGEMENT OF OBESITY
Present treatment options for obesity range from lifestyle change and dieting to bariatric surgery. The efficacy of the former is variable and usually limited, because the psychological, environmental, and socioeconomic circumstances are usually not optimal. Pharmaceutical options currently available are used as an adjunct: (1) inhibitors of intestinal fat absorption, (2) sympathomimetic agents that suppress appetite, increase satiety or thermogenesis, and (3) antagonists of the endocannabinoid system.45 The use of these drugs often results in only moderate weight loss, and combination therapy is advocated for the majority of patients. For those with a BMI≥30 and if conservative measures are ineffective, bariatric surgery is the treatment of choice.46
The spectrum of bariatric surgery is wide, including adjustable gastric banding, sleeve gastrectomy, jejunoileal bypass, duodenal switch biliopancreatic bypass, and Roux-en-Y gastric bypass. The number of bariatric surgical procedures has significantly increased in the recent past. In the United States, 250 000 bariatric operations are performed annually, and it is projected to be nearly 450 000 by the year 2015.8 Approximately 80% of these procedures are performed laparoscopically.8 The complication and cost of bariatric surgery has decreased; however, surgery is available for only 1% of the morbidly obese population.47 Despite the improved results, a large proportion of patients still hesitate having operative intervention. In addition, many insurance companies do not cover bariatric surgery.
An effort to develop newer and less invasive techniques for the obese population is imperative. Endoluminal management of obesity is challenging, because it must be safe, effective, durable, and cost effective.
BACKGROUND OF ENDOLUMINAL GERD THERAPIES
The limitations of pharmaceutical and surgical therapies, in combination with the high incidence of reflux disease in the Western population, has created the need to develop a less-invasive procedure that effectively addresses the underlying problem and is devoid of the shortcomings of the surgical option.
Twenty-six years ago, the British gastroenterologist Paul Swain developed a sewing capsule attached to a flexible endoscope to perform limited surgical maneuvers in the gastrointestinal lumen.48 The idea of minimizing surgical trauma by performing operative procedures within the gastrointestinal tract provided a new perspective. As a result, in the last 20 years a spectrum of new endoscopic techniques has been developed for the treatment of GERD.49 The endoscopic antireflux procedures can be categorized into 4 groups: (1) ablation, (2) injection or implantation, (3) fixation, and (4) mucosal excision and suturing.
Ablative Technique
Stretta® Procedure
The possibility of radiofrequency ablation being used for GERD therapy was explored after successful treatment of snoring and sleep apnea.50 The Stretta procedure applies radiofrequency to the smooth muscle of the LES by 4 radially arranged titanium electrodes, resulting in muscular hypertrophy, fibrosis, and neurolysis at the level of the lower esophageal sphincter and gastric cardia. Over 3500 procedures have been performed in the United States alone. The indications have been confined to patients with early reflux disease. Although significantly better heartburn-related quality of life (HRQL) scores at 6 months and 12 months were seen in most studies, the pH normalization rate was only 30% to 40%.51-54 In a prospective, randomized, sham- controlled trial, Abdel et al55 compared the efficacy of single-application Stretta with double-application Stretta versus sham patients, and no significant difference was demonstrated. In a 3-year study,56 durability of effect was evident, but subsequently the company failed and the device was withdrawn from the market. Scar formation did result and may explain the moderate symptom improvement.
Injection/Implantation Techniques
The goal of the injection therapies is to deliver a biologically inert, injectable substance into different depths of the LES region. Submucosal injections increase the volume of the LES, and injection into the muscularis propria results in granulation and fibrous capsule formation. From the mid 1980s, several biopolymers were tested in animals and obtained FDA approval.
Enteryx®
Enteryx, an ethylene vinyl alcohol copolymer with tantalum dissolved in dimethyl sulfide, was one of the injectable agents tested. Randomized control trials demonstrated that Enteryx implantation significantly improved the HRQL scores and medication usage compared with control groups; however, improvement in LES pressure, esophagitis, and esophageal acid exposure did not occur.57,58 Enteryx was recalled by the manufacturer in 2005, after several deaths due to transluminal injection.
Gatekeeper®
The Gatekeeper Reflux Repair System was a dehydrated hydrogel prosthesis implanted into the submucosa of the cardia/LES. It hydrated to a 6-mm x 15-mm cylinder-shaped soft pliable cushion and was removable by endoscopy.59,60 In a case series of 78 patients, a significant improvement in heartburn, regurgitation, HRQL, LES pressure, and medication use at 6 months was observed, although pH normalization occurred in only 40% of patients.60,63 The retention rate of the implant was 70% at 6 months, and 15% of patients required a second treatment within 6 weeks of the primary procedure.61-63 This device has been removed from the market because of lack of efficacy.
Durasphere GR®
Durasphere is a new sterile, biocompatible injectable bulking agent composed of pyrolytic carbon-coated graphite beads containing zirconium oxide, suspended in a water-based, absorbable polysaccharide carrier gel (Figure 1). The size of the beads ranges from 90μm to 212μm to prevent migration of particals. The inert pyrolytic carbon has been widely used in implantable medical devices for the treatment of stress urinary incontinence and recently for fecal incontinence.64-66

The injection therapy is performed with the patient under conscious sedation while in a left lateral decubitus position. With a simple, 20-gauge sclerotherapy needle, the Durasphere gel is injected into the submucosa within 1cm of the Z-line. One to 3mL of solution are delivered in 4 quadrants. Injection in one area is deemed complete when the prominence reaches the midline of the esophageal lumen. Submucosal positioning is confirmed visually by bleb formation, tissue bulking, and darkening. When deeper injections are recognized, the needle is withdrawn and repositioned. The procedure is considered complete when the esophageal walls are approximated at the GEJ. Patients can be discharged within 60 minutes.
Initial short-term results are available from a single-center trial of 10 patients with uncomplicated symptomatic GERD.67 No adverse events occurred. At 1-year follow-up, the mean GERD-HRQL scores improved and esophageal pH normalization was achieved in 40%. Seventy percent of patients completely eliminated PPI or H2RA use, and 90% of patients reduced their PPI dosage by more than 50%. Five patients underwent repeat treatment.
Fixation Techniques
The endoluminal suturing techniques are based on intraluminal apposition of tissue with staples, suture fasteners, or sutures. The outcomes were somewhat encouraging initally, but better results are needed in terms of pH normalization. Below, we discuss the efficacy and safety of the available GERD tissue fixation devices.
EndoCinch® – Endoluminal Gastroplication (ELGP)
This device was originally developed by Swain48 to create a full-thickness intussusception at the gastroesophageal junction by a transoral sewing technique. Later, the technique was licensed and modified by CR BARD Endoscopic Technologies to create endoscopic plications below and at the “Z” line.
The device includes a suturing capsule, suture tags, and an anchoring system that secures the suture and cuts the strands. A short, 18-mm-outer-diameter overtube allows repeated intubations while avoiding trauma to the esophageal mucosa. The choice of sedation depends upon the patient’s condition and general health. Usually patients tolerate the procedure with conscious sedation. However, restless patients who have a class II airway require monitored anesthesia, and those with a class III or IV airway may require general anesthesia. Two to 4 plications are placed either longitudinally (one above the other), radially (next to each other), or spirally within the cardia. Each plication is formed by 2 stitches that are placed into the gastric submucosa, approximately 1cm apart.
The main steps of the procedure are as follows. After correct placement of the sewing capsule, suction is applied, the gastric wall is pulled into the hollow chamber of the capsule, and a straight needle loaded with a nonabsorbable suture and attached T tag is fired through the suctioned tissue. The system is reloaded, and a second stitch is placed adjacent to the first. The 2 stitches are pulled together and cinched by a ceramic plug and ring (Figure 2). Depending on the expertise of the operator and the number of plications intended, the procedure is completed within 40 minutes to 60 minutes.
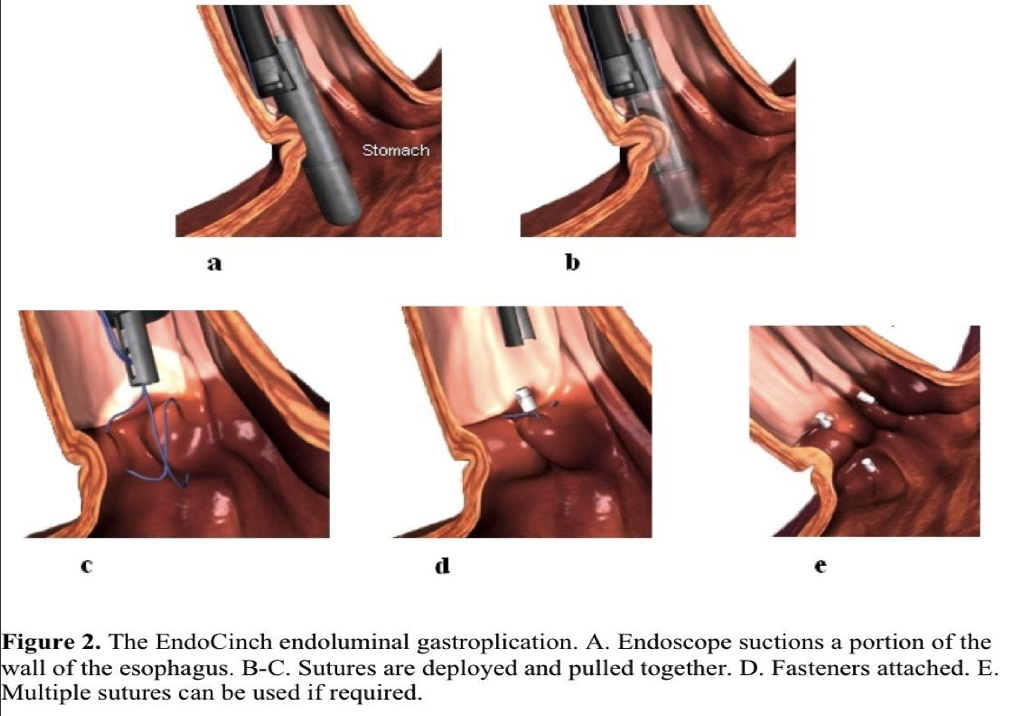
It was found that an inverted intraluminal gastroplication does not result in fusion between mucosal folds, irrespective of suture depth. A flat scar is the final outcome and appears proportional to the amount of ischemia, foreign body reaction, and suture depth.68 Application of cautery on opposing mucosal surfaces before plication has not significantly altered healing.69
Endoluminal gastroplasty was generally safe over the long term and free of serious immediate side effects. One esophageal perforation occurred that required an open thoracotomy, and another patient in the initial multicenter United States trial was found to have pneumomediastinum on CT scan after intervention. This patient was managed conservatively with antibiotics for 3 days.70 Other studies have reported minor complications, such as pharyngitis (57%), epigastrial or chest pain (15%), hypoxemia (13%), bleeding (11%), aspiration (4%), nausea and vomiting (4%), and gastric mucosal tears (4%).71,72 Airway assessment seemed particularly important, because hypoxemia and stridor on occasion did occur secondary to the overtube. Patients with class IV and III airways were at risk for the above. Patients who were obese, combative during the preliminary endoscopy, or had a class IV airway had either general anesthetic or propofol sedation with monitoring.
The effects of the EndoCinch procedure have been widely studied. The most important mechanical effect is the improvement of the gastroesophageal flap valve grade.73 The plication reduced the relaxation rate of the LES, improving the reflux symptoms and also decreased esophageal acid sensitivity.74,75 Schiefke et al76 reported improvement in mean LES length after 70 patients were treated with the EndoCinch. Liu et al77 demonstrated by ultrasound that muscular hypertrophy at the muscularis propria also occurred.
In one multicenter trial, 64 patients, all dependent on antisecretories and with 24-hour pH-proven reflux disease, were randomized between a circumferential and a linear plication configuration.78 Patients with Barrett’s esophagus, grade 3 or 4 esophagitis, large hiatal hernias, and an esophageal dysmotility disorder were excluded. No difference was found between the plication configuration groups, and postprocedure manometry and endoscopy showed no improvement in LES pressure or grade of esophagitis. A significant improvement in heartburn and regurgitation scores from baseline was found at 6-month follow-up, but the pH monitoring results showed only a 30% normalization rate. In another multicenter study, 85 symptomatic GERD patients with more advanced disease were included.79 Twenty-four-month follow-up data demonstrated durable functional improvement and a sustained reduction in antisecretory medication. Heartburn scores were reduced at both 1-year (94%) and 2-year (78%) follow-up. Likewise, PPI usage decreased with 69% of patients using <50% of their baseline medication, and 41% were completely off PPIs at 2-year follow-up. At 3 months or 6 months postprocedure, 63 patients were available for 24-hour pH monitoring. The percentage of time the pH was <4.0 was significantly reduced from a median of 9.3 to 5.8, and pH normalization occurred in 39.7% of the patients.
In a 48 patient, Japanese multicenter trial, short-term follow-up showed complete PPI cessation, complete heartburn resolution, and a resolution of esophagitis in 66% of patients.80 No esophageal pH studies were performed. The midterm results were similar.
In a European, prospective single center trial, 70 patients were treated with the EndoCinch device. Fifty-six (80%) patients were considered treatment failures, because their heartburn score did not improve or PPI medication exceeded 50% of the initial dose at 18-month follow-up.76 No significant changes in 24-hour pH monitoring or in LESP were observed.
Procedure failure is common with a reintervention rate of up to 55% within the 2 years.70,81 Schiefke et al76 reported that in 26% of cases all the sutures disappeared and only 17% of patients had all sutures in situ at 3-month follow-up. Repeat procedures can be performed safely with similar results.82 However, one study demonstrates a significant trend toward earlier onset of recurrent symptoms after repeat ELGP.83 Laparoscopic Nissen fundoplication (LNF) was feasible and effective after failed ELGP.84 Patients need to undergo upper GI endoscopy before surgery, but suture removal is not necessary. Several studies have compared ELGP with LNF and have revealed that surgical intervention offers a significantly greater reduction in medication use and greater patient satisfaction.71,85 Thus, surgery is superior to ELGP.
Bard EndoCinch plication for GERD is rarely performed now. Although symptoms improve initially, the effect is not durable, and no physiologic parameter (esophagitis, manometry, esophageal acid exposure) is altered significantly. Suture placement is superficial, and the infolded mucosa with the plication does not fuse. Although one ultrasound study86 showed smooth muscle hypertrophy underlying the plication, no proven sustainable benefits have been associated with the procedure. Technical difficulties, such as poor visualization and lack of procedure standardization, have deemed ELGP of historical interest only. The initial symptom and medication usage improvement is attributed to postprocedure edema, submucosal hemorrhage, and the sham effect.
Endoscopic Full-Thickness Plication System (NDO Plicator)
The NDO Plicator System has been developed to create and fixate gastric plications below the GEJ in the anterior cardia with serosa-to-serosa apposition. The plication is secured by a pretied, suture-based implant placed in a retroflexed manner. A 5-mm to 6- mm endoscope is inserted through the device to provide direct visualization. The system consists of a plicator instrument, a helical-shaped “corkscrew” tissue retractor, and a pretied suture insert (Figure 3.1).

The procedure is performed with the patient under intravenous propofol and midazolam sedation. A Savary guidewire is placed upon endoscope withdrawal then the Plicator is advanced directly over the guidewire into the stomach. The endoscope is inserted through the dedicated channel of the device, and the stomach is insufflated with air. Under direct endoscopic visualization, the distal end of the plicator is retroflexed, and the arms are opened. The tissue retractor is advanced into the gastric tissue 1cm below the anterior cardia. The tissue is engaged, and the retractor is pulled back to gather the tissue between the device arms. The stomach is partially desufflated, the plicator’s arms are closed, and the pledgeted sutures are deployed. The plicator is removed and the results then evaluated endoscopically (Figure 3.2). One or 2 additional transmural sutures are placed incrementally closer to the gastric cardia if necessary for maximum restructuring of the antireflux barrier. The single plication procedure is 10-minutes to 20-minutes long.87
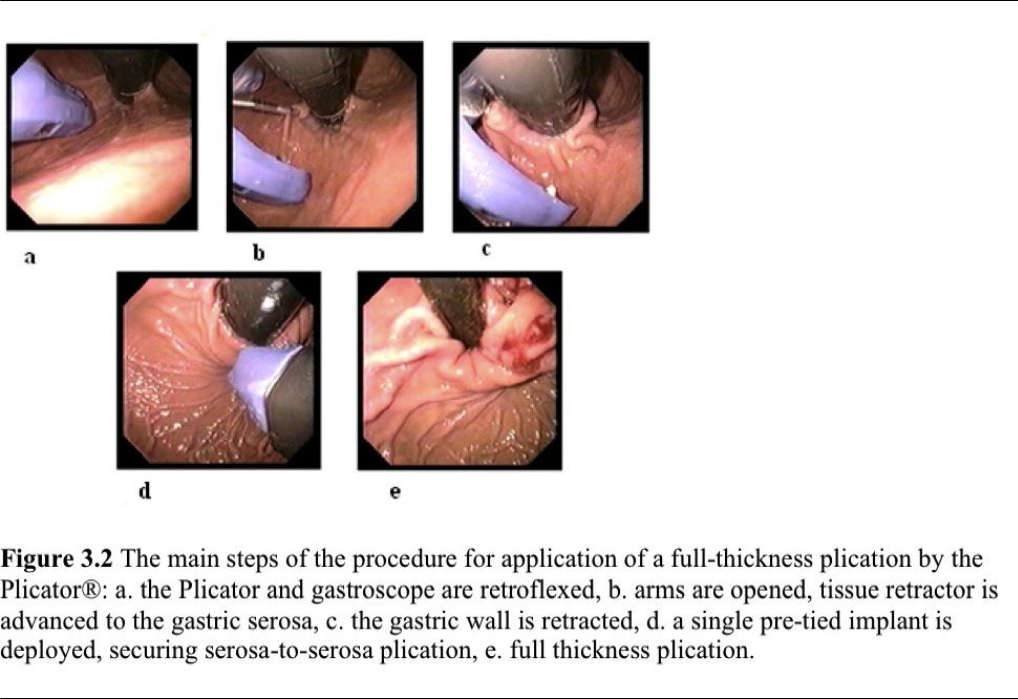
In an initial prospective multicenter trial, 64 patients with mild GERD underwent the NDO plicator procedure.87 At 6-month follow-up, significant improvement in GERD- HRQL scores (20.2 to 7.9) occurred, 65% of patients were able to discontinue all antisecretory medication, and 74% discontinued their PPI therapy. Normalization in pH score was observed in 30% of patients. One-year follow-up was completed in 57 patients. Median GERD-HRQL scores were significantly improved compared with baseline scores, but only 23% of patients were off all antisecretory medication. Forty patients (70%) reported discontinuation of PPI therapy.88 At 36-month follow-up, the GERD- HRQL scores remained significantly improved, and 50% of patients were no longer taking PPI.89 Approximately 5 years after the intervention, 33 patients were available for follow-up. Sustained reduction in the median GERD-HRQL score was seen, and in 33% of patients complete cessation of PPI therapy was achieved.89 No patients in this series required retreatment.
Similar results were reported at 6 months and 12 months in a European, prospective, multicenter study, with 41 patients.90 Significant improvement in GERD-HRQL scores and PPI therapy were seen. Daily PPI therapy was eliminated in 70% of patients. Distal esophageal acid exposure time decreased 38% compared with baseline, and the median LES pressure improved 25%. At 1-year follow-up, 75% of patients demonstrated improvement in GERD-HRQL scores, and 59% required no daily PPI therapy.91
In a sham-controlled trial, 159 patients were randomized to plication (n=78) or a sham procedure (n=81).92 Patients with chronic heartburn responsive to PPIs, abnormal esophageal pH exposure, and an LES pressure of at least 5mm Hg were included. Patients with esophagitis or HH>2cm were excluded. A significantly higher proportion of patients in a treated group achieved at least 50% GERD-HBQL score improvement. Complete cessation of PPI therapy was achieved significantly more often in the treatment group (50% vs. 24%). Reduction in esophageal acid exposure time was also significantly improved in the plication group, but distal esophageal acid normalization occurred in only 23% in the intervention group.
Safety of the procedure was demonstrated.90-92 Mild adverse events, such as abdominal or chest pain, were reported in 40% to 60%, pharyngitis 41%, GI disorders 17%, eructation 14%, and dysphagia in 11% of patients. Serious adverse events occurred in 13 patients: dyspnea during the procedure requiring endotracheal intubation, pneumothorax, free intraabdominal air, pneumomediastinum, gastric wall perforation, and severe abdominal or chest pain.
The endoscopic full-thickness plication system has been shown to provide a modest effect on reflux symptoms and quality of life at 5 years. However, objective measurements, such as esophageal acid exposure and improvement in esophagitis, have not been demonstrated. The company subsequently declared Chapter 11 and the intellectual property was sold.
EsophyX® – Transoral incisionless fundoplication (TIF)
The EsophyX device is used to increase the competency of the antireflux barrier by restoring the angle of His. A valve is created at the GEJ by delivering multiple full- thicknesses, nonresorbable polypropylene SerosaFuse fasteners. The end result is an anteriolateral, 200º to 300º, 3-cm to 5-cm long partial fundoplication (Figure 4).
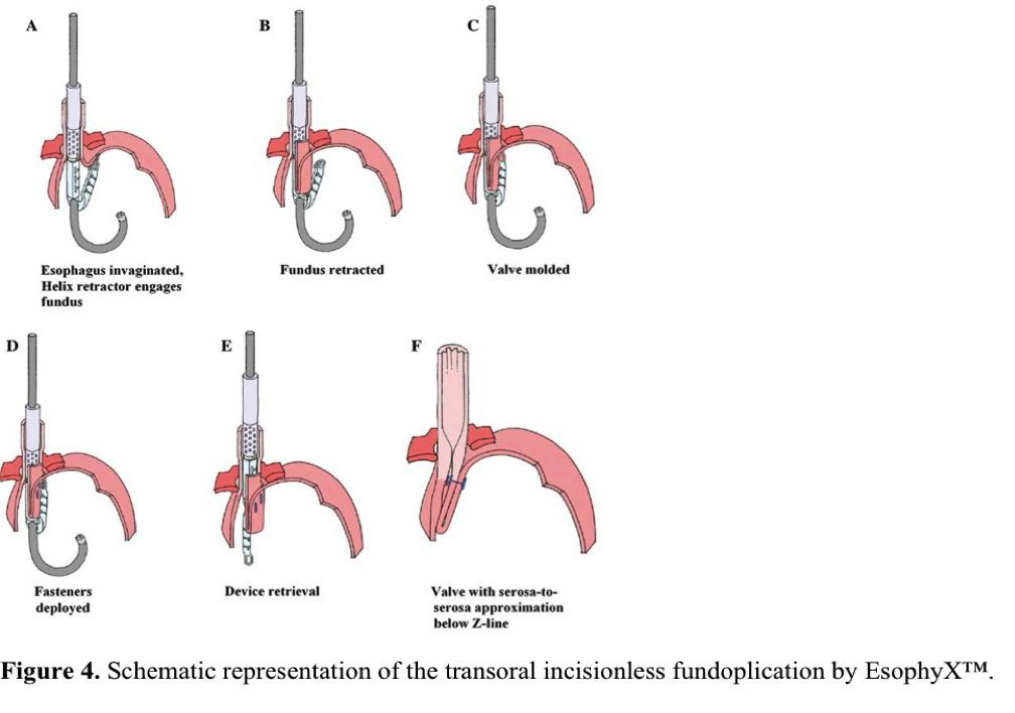
Two techniques for transoral incisionless fundoplication (TIF) have been developed. The TIF 1 results in a 220º, omega-shaped nipple type valve on the greater curvature side. The main technical steps of TIF 1 are as follows: The patient is placed under general anesthesia and turned to the left lateral decubitus position. For device manipulation, 2 physicians are required. One controls the EsophyX device, and the second manipulates the endoscope providing continuous visualization. First, upper endoscopy is performed, and the device is inserted transorally over the endoscope into the esophagus. The device then is positioned under the GEJ, and the endoscope is retroflexed. The helical tissue retractor is engaged at the opening of the GEJ, and the gastric tissue is retracted into the tissue mold. If present, a small hiatal hernia (HH) can be reduced, and the esophagus lengthened by retracting the endoscope and locating the “Z” line. Suction is applied enabling the invaginator to gently reposition the “Z” line below the diaphragm. The device is then rotated wrapping the fundus toward the lesser curvature. Under visual control, multiple, nonabsorbable polypropylene “H” fasteners are fired circumferentially, creating a double-wall thickness valve, positioning the gastric wall above the GEJ. As a result, a 3-cm to 5-cm long, 200º to 250º fundoplication iscreated.
In the TIF 2 procedure, the fastener deployment is initiated further posterior and anteriorly resulting in a 270º valve. Additional fasteners are placed 3cm to 5cm proximal to the “Z” line on the esophageal side to envelop a longer intragastric segment of the fundoplication.93 The patient takes a liquid diet during the first 2 weeks and a soft diet in the following 4 weeks.
Transoral incisionless fundoplication has been demonstrated to be safe and in the majority of cases free from serious immediate and long-term complications. However, 5 (3.8%) of 131 procedures had serious adverse events.94-69 Two esophageal perforations occurred, and 3 patients experienced significant intraluminal bleeding. The 2 perforations occurred during device insertion, one without adequate visualization and the other in a patient with a narrow hypopharynx. Both patients underwent surgical repair. Three patients had gross intraluminal bleeding requiring transfusion and short-term hospitalization. One of these patients underwent endoscopic clip application and fibrin glue injection, but the other patients required no intervention. One patient was readmitted, and a CT scan revealed free air in the upper abdomen. A Gastrografin swallow was negative, and after 3 days of antibiotics and analgesic therapy the patient was discharged. Agostoni et al97 reported development of a bilateral pneumothorax that required ICU admission for a patient who underwent the EsophyX procedure. The most common adverse events are pharyngeal irritation due to device insertion, epigastric or upper abdominal pain and nausea. Other symptoms like bloating and dysphagia have been transient.
In the first prospective clinical feasibility study, conducted by Cadiére et al,94 19 patients were enrolled. The inclusion criteria were chronic and symptomatic GERD, PPI dependence, and absence of an esophageal motility disorder. Fifty-nine percent of patients had 2-cm to 3-cm hiatal hernias, and all had esophagitis. Objective and subjective results 1 year and 2 years after the intervention were evaluated in 17 and 14 patients, respectively. At 12-month follow-up, significant improvement in the mean GERD-HRQL score (17 vs 6, P=0.002) was seen.98 Discontinuation of PPI was achieved in 82% of patients. Their pH monitoring results are confusing, because a 5.3% time below a pH of 4 rather than the standard 4% was used.
At 24 months, significant improvement in the GERD-HRQL scores was also seen (17 vs. 7. P=0.004). Complete resolution of GERD symptoms and cessation of PPI were seen in 29% of patients and partial resolution of GERD in an additional 50%. Seventy-one percent of patients needed to take daily or occasional PPIs. pH monitoring was not performed at 24 months. The fundoplication appeared durable with a median decrease in length and circumference of 25% and 5%, respectively, at 1 year, and no further change was observed at 2 years. In 2 patients, repeated treatment was necessary due to symptom recurrence after fundoplication disruption.
In a prospective European multicenter trail,95 86 patients with chronic GERD, treated with PPI on a daily basis were enrolled. Follow-up results at 1 year were available for 84 patients. The overall GERD-HRQL scores improved significantly from 24 to 7 (P<0.0001), and 75% of patients reported significant improvement in heartburn scores.
Discontinuation of PPI therapy (67%) and complete symptom resolution (75%) was achieved in the majority of patients. Postprocedural esophageal pH monitoring normalized in 37% of patients. Thirty-one percent of patients with preoperative esophagitis completely healed. The majority had grade A esophagitis. In 16 (33.3%) patients, the preoperative hiatal hernia was absent, and in an additional 60% it decreased in size. Resting pressure of the LES improved 53% (P<0.001). Patients with a Hill grade I or II valve benefited most from the procedure.
Demytteraere et al96 reported results on 26 consecutive patients in the largest North American study, after a mean follow-up of 10 months. Patients with a hiatal hernia, Barrett’s esophagus, esophageal dysmotility, or an esophageal stricture were included. The median HRQL score decreased from 22 to 10 (P<0.0007), although 68% of patients were still taking antisecretory medication, and only 32% discontinued their PPI. Three patients subsequently required surgical intervention due to treatment failures, and 2 had major bleeding with consequent blood transfusion. No pH monitoring was performed.
Testoni et al99 reported that the total number of reflux episodes changed significantly, but no change in DeMeester scores was seen in 20 consecutive patients who underwent the TIF 2 procedure. PPI usage and GERD-HRQL scores did improve.
EsophyX-TIF has been demonstrated to be a relatively safe and a moderately effective treatment for GERD. Procedure modification has been necessary and satisfactory pH normalization rates are still awaited. A sham-controlled European study is being initiated, because some insurance carriers are still labeling this procedure experimental.
Medigus SRS® Endoscopy System
The Medigus SRS system is a special endoscope that combines a surgical stapler and 2 video cameras to create a 180º fundoplication (Figure 5.1, Figure 5.2). A serosa-to- serosa apposition is created between the esophageal and proximal gastric wall. The distal tip is 15mm in diameter and is passed into the stomach and then retroflexed. The tip of the endoscope flexes to the endoscope’s rigid 6-cm long segment, which contains a miniature camera and a rechargeable stapler. The tip of the endoscope functions as the stapler anvil. Ultrasonic sights are integrated into the tip of the device for accurate positioning. After the stapler segment is positioned 2cm to 3cm above the GEJ, the tip of the endoscope is completely retroflexed, and esophageal and gastric tissue is compressed. Ultrasound is used to ensure that the target tissues are captured, the alignment is correct, and the tissue thickness is satisfactory for staple deployment. Then 2 tiny screws from the tip of the device are passed through the tissue into the shaft to hold the anvil and stapler in apposition. The staples are fired, the screws are withdrawn, and the endoscope is removed. A new cartridge is loaded, and the procedure is repeated.
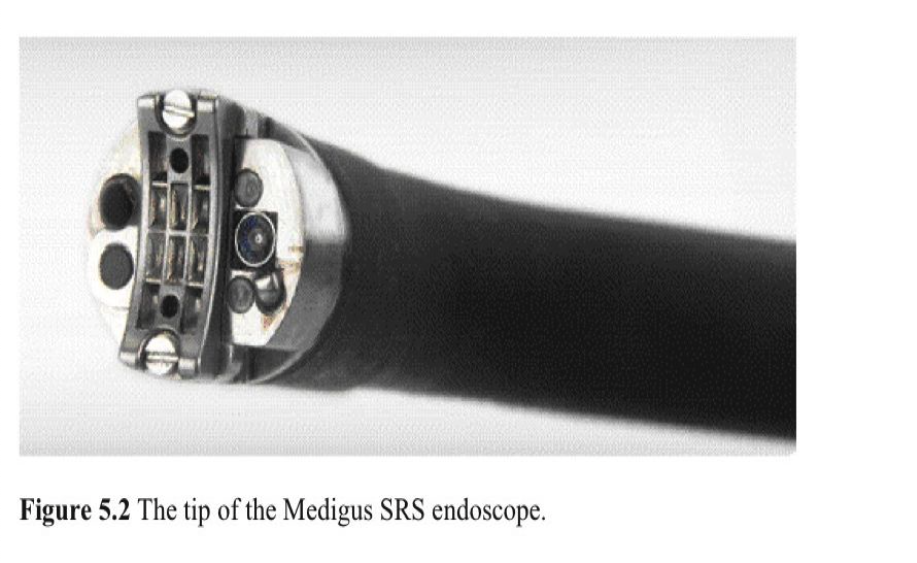
In the initial feasibility/safety study,100 12 pigs underwent a Medigus SRS fundoplication. There were no intraoperative or postoperative complications. All fundoplications were intact, and the stapled area was well healed at 2 weeks, 4 weeks, and 8 weeks postprocedure. A multicenter international trial has been initiated.
Mucosal Excision and Suturing
SafeStitch Medical Gastroplasty System (SGS)
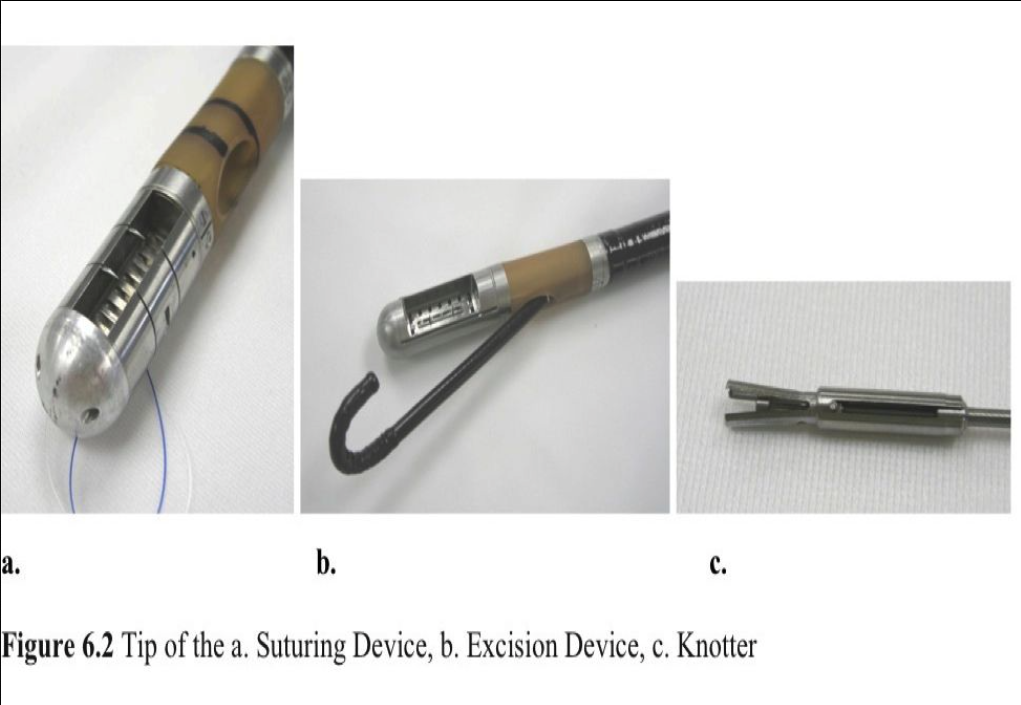
The SGS is designed to excise proximal gastric mucosa at the GEJ and suture together opposing excision beds by using full-thickness 2-0 Prolene sutures. Reduction of the volume of the GEJ and a muscle-to-muscle apposition with resultant scar tissue formation creates the antireflux effect. The SGS includes 3 flexible endoscopic devices: the Excision Device, the Suturing Device, and a Knotter (Figure 6.1).

The Excision and Suturing devices are similar in construction, each having an operational distal tip, flexible transition piece, 60-Fr flexible insertion tube, and a handle. The trough of the Excision Device contains vertical needles for tissue injection, suction ports, and a horizontal blade for mucosal excision (Figure 6.2 a.). The Suturing Device operates 2 circular needles, each connected to a separate 2.0 Prolene suture (Figure 6.2 b). The Knotter is 3mm in diameter and secures the sutures by delivering plastic anchors under direct endoscopic visualization (Figure 6.2 c.).
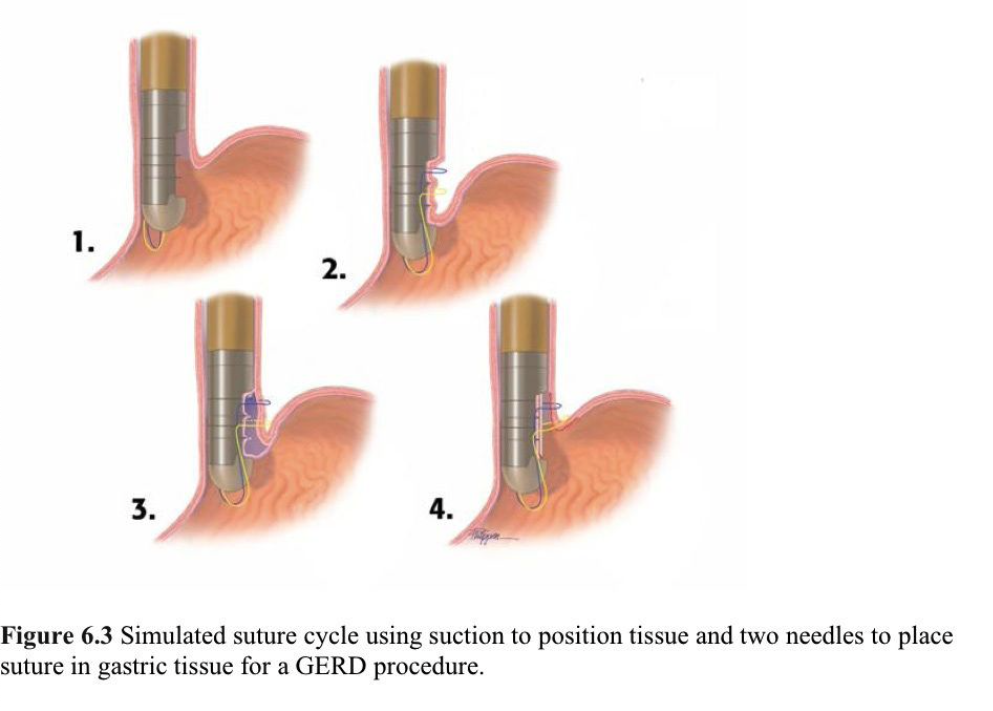
With the patient in the left lateral decubitus position under intravenous propofol sedation, a Savary guidewire is placed, and the Excision Device is advanced over the guidewire into the proximal stomach. A 5.5-mm to 6.5-mm endoscope is inserted through a dedicated channel of the device, and the stomach is insufflated with air. The endoscope is retroflexed to visualize the operating field and the tip of the device. The suction trough is positioned against the proximal gastric mucosa just below the GEJ, suction is applied, and the mucosa is captured. A 1:100 000 adrenaline solution is injected into the submucosa, creating a wide target zone for the excision blade. The mucosa is excised and removed by an endoscope forceps. The procedure is repeated both on the anterior and the posterior wall of the stomach, and the Excision Device is withdrawn.
The Suturing Device is inserted, and the endoscope is advanced into position. The proximal parts of 3 excision beds are captured in succession by the 2 circular needles that rotate 360º through the gastric wall. The device is then withdrawn. The Knotter is inserted under direct visualization, and individual sutures sets are tied and cut in a single action (Figure 6.3).
Results from ex-vivo and in-vivo animal experiments are available.101 In ex-vivo experiments focusing on excision capsule trough size and knife blade depth, reliable mucosal excision has been proven (n=114). The injection needle positions allowed consistent submucosal injection and successful excision overlap in 99.1% of 124 experiments. Needle actuation reliability of the Suturing Device was 96.7% in 7 in-vivo porcine experiments. In-vivo baboon 8-week survival experiments were conducted in 12 animals. Suture needle actuation was 96.7% reliable, but one stomach wall perforation 0.9 % (1/114) occurred. The perforation was successfully closed endoluminally using the device, and the animal was kept alive without incident. Postexcision bleeding was noted in 1 of 12 survival animals with a single melanotic stool being passed. These preliminary results demonstrate reasonable instrument reliability and procedural safety.
BACKGROUND OF ENDOLUMINAL OBESITY THERAPIES
The increasing need for bariatric surgery and the initial feasibility of endoluminal therapy for GERD has stimulated interest in the endoscopic management of obese patients. The approach is in its infancy but holds great promise for providing presurgical weight loss, postsurgical revisions, and even primary surgery. The current and emerging endoscopic devices for obesity are numerous and can be categorized as (1) space occupying devices, (2) transoral endoluminal stapling or suturing devices, (3) prosthetic gastric sleeves, and (4) miscellaneous.
Space Occupying Devices
BioEnterics Intragastric Balloon (BIB) system
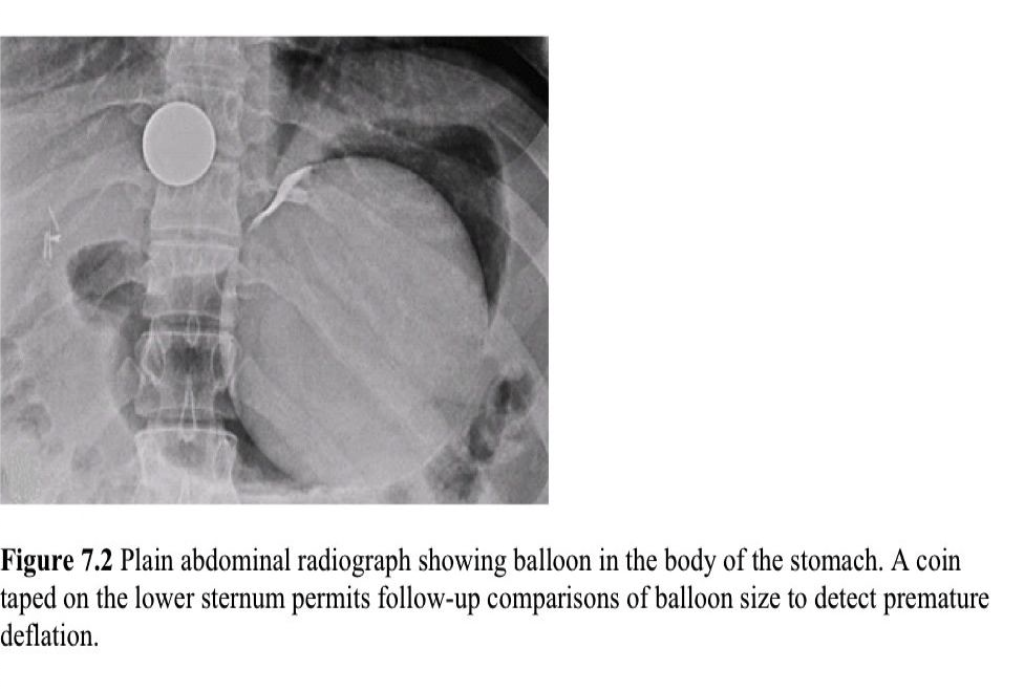
The BIB is a spherical, smooth silicone device filled with saline and has an adjustable volume (400mL to 700mL) (Figure 7.1). Tests for Helicobacter pylori are necessary prior to the procedure. The placement of the device is performed with the patient under conscious sedation. After the balloon is placed in the fundus, it is inflated under direct endoscopic visualization with normal saline and 10% methylene blue (Figure 7.2). The procedural time is 10 minutes to 15 minutes in experienced hands. The patient is then advised to take a full liquid diet for 3 weeks, progress to half-solid food for one week then continue with regular meals. The device is usually removed at 6 months.

The BIB has multiple morbidities that include GERD and consequent esophagitis in 50% of cases, and nausea or vomiting in the first week that usually responds to medication.102,103 Early removal is required in 2.8% of cases mainly due to intolerance of the device.104 Serious adverse events, such as gastric perforations resulting in death, small bowel obstruction from a collapsed balloon, and cardiac arrest have been reported.102,105 The overall complication rate in a Japanese study was 9.5%.106
In a short-term, double-blind, randomized controlled, crossover trial in morbidly obese patients, Genco et al107 found that weight loss was significantly higher (15±6kg, BMI 5.8±0.5kg/m2, P<0.001) in patients with BIB compared with a sham procedure. Other sham-controlled trials did not show significant weight loss from the BIB procedure.108,109 The study of Göttig et al102,103 in extremely obese patients revealed a significant (P<0.001) decrease both in body weight (26.3±15.2kg) and BMI (8.7±5.1 kg/m2) after 6 months. Improvement of comorbidities was also noted. The studies have shown that the greatest weight loss occurs in the first 3 months, and patients who lost more than 6.5kg in this period are more likely to have lower body weights at 6, 12, and 24 months.109
The BIB system is generally safe. It may be an acceptable option for super obese patients who are referred for bariatric surgery. However, there is no widely accepted evidence for BIB as a primary intervention, as the device can remain in place for only 6 month. The device has not been approved by the FDA, but US clinical trials are ongoing.
Heliosphere Bag® (HB)
The HB is an air-filled balloon loaded by a simplified inflation system. With the patient under general anesthesia or conscious sedation, the bag is delivered into the stomach and inflated with 500mL to 800mL of air. The positioning time is 10 minutes to 15 minutes. The device is left in place for 6 months.
A preliminary study showed satisfactory weight loss (17.5±16.2kg, %EWL 29.1±20.1), but a spontaneous deflation rate of 40% was noted.110 Two prospective multicenter studies also demonstrated weight loss. In one,111 83% of patients reported a mean 7kg (range, 1 to 29) weight loss after 7 months, and in the other study112 the mean percentage body weight loss was 9.3% (range, 3 to 20) at 4 months postprocedure. Thirty percent of patients maintained >10% weight lost after 20 months. The results of the HB are equivalent to results with other balloon devices.
Similed Gastric Balloon (SGB)
The SGB is a novel, space-occupying device delivered under direct endoscopic visualization. The balloon is rolled up inside a thin silicon sheath, with the end anchored to the tip of the endoscope by a snare. Then the device is smoothly inserted into the gastric fundus, and the SGB is filled with 700mL of saline, Iopamiron contrast and methylene blue (Figure 8). During removal, it is emptied and pulled out through an overtube. Both placement and removal require 10 minutes to 15 minutes.
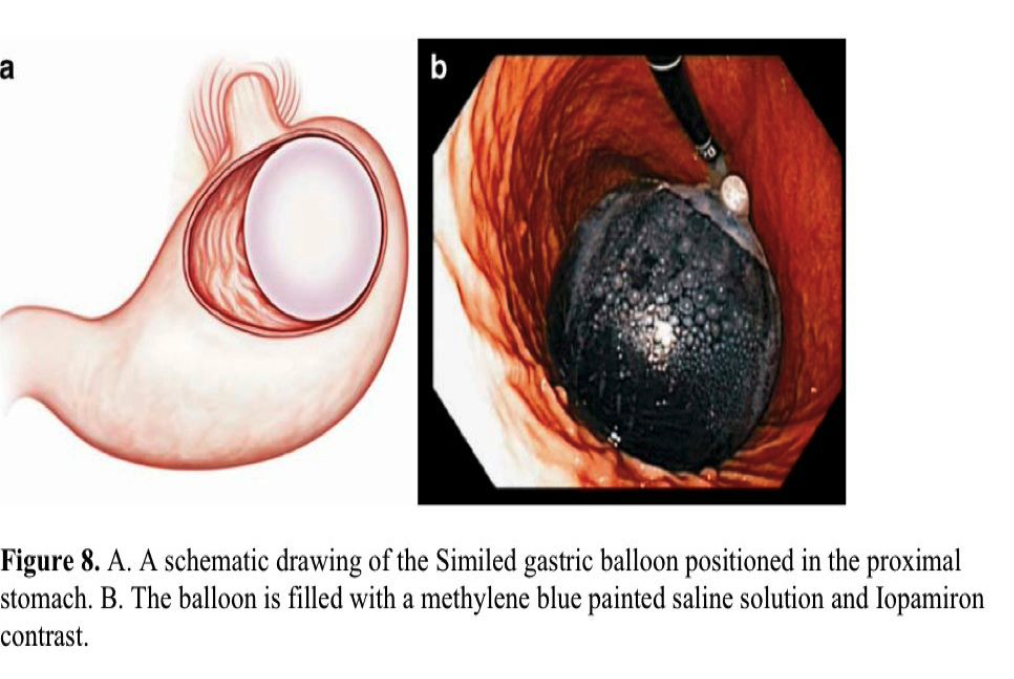
Preliminary results (n=52) demonstrated safety and modest weight loss.113 All procedures were performed on an outpatient basis. The only complications were episodes of nausea, vomiting, and epigastric pain. In 21% of patients, early device removal was necessary because of severe epigastric pain. After 6 months, significant improvement in mean weight loss (11.3 kg), in BMI loss (3.9 kg/m2), and in % of EWL (34.6%) occurred. Further investigations are planned.
Expandable Polymer Pill
More recently, the gastric retention technologies group of BaroNova Therapeutics, Inc. has developed an expandable polymer pill that takes up space in the stomach. In theory, this pill is able to degrade and pass through the gastrointestinal system and can be taken at regular intervals based on response.114 This technology has not yet been clinically tested.
Endoluminal Incisionless Stapling or Suturing Devices
Bard EndoCinch and RESTORe (RS2) Suturing Systems
The EndoCinch device was originally developed for the treatment of GERD, but long- term results failed to demonstrate objective efficacy. The device is now used for reduction of dilated gastrojejunostomies after failed RNY gastric bypass. Ryou et al115 reported the results of 151 patients who underwent 227 placements of interrupted sutures with tissue cauterization at the dilated (>2cm) GJ anastomosis. The mean stoma size reduction was 67% using an average of 4.2 stitches. At 1-year follow-up, 29.9% EWL occurred, and 65% of patients stabilized their weight.
A modified EndoCinch obesity procedure was introduced to reduce gastric volume. Results from a single-center, uncontrolled study in a severely obese adult population are available.116 Most endoscopists with EndoCinch experience discount this study, and it is mentioned for historical interest only.
The new RS2 Bard device is designed for transoral gastric volume reduction. The platform is similar to the EndoCinch antireflux device, but it has improved durability and is easier to use. The device sutures the anterior and posterior gastric fundus and body using a series of 2 or 3 stitches in a “quilting” pattern. This approach prevents the stomach from relaxing to receive food.
A nonrandomized, multicenter feasibility study (TRIM trial) with 18 patients (BMI ranging from 30kg/m2 to 45kg/m2) showed at 6-months postprocedure that 67% of patients achieved a mean weight loss of 27.9 pounds (an average of 30.4% EWL).117 At 9 months, the mean weight loss was 36.5 pounds, and the mean %EWL was 34.4% in 33% of patients.
The results of this method are promising and may represent a treatment option for obesity. However, the data is limited. Further randomized, sham-controlled multicenter trials are required to demonstrate long-term efficacy.
USGI Medical Incisionless Operating Platform (IOP)
This surgical platform is used for transluminal, endoluminal, or single-incision surgery. For bariatric patients, it is intended for either primary or revisional procedures. The device includes the flexible TransPort Multi-lumen Platform, the g-ProxGrasping/Tissue Approximation Device, g-Cath Tissue Anchor Delivery Catheters, and a variety of endosurgical tissue graspers. The IOP contains 4 channels, one for a flexible 4.9-mm endoscope for visualization and the other 3 for tissue manipulation. The specially designed Expandable Tissue Anchors provide durable tissue approximation, because the holding force is widely distributed.
The device first was used to reduce the stomach pouch or stoma in Roux-en-Y gastric bypass patients who regained weight. The delivered anchors secure tissue folds in the pouch and around the gastrojejunostomy118 (Figure 9). In a single-center short-term study, 20 patients with a dilated pouch and stoma were enrolled.119 Seventeen cases were considered successful. On average, 5 plications were placed in the pouch and 3.4 at the stoma. A 65% reduction in the mean stoma diameter and 36% in pouch volume was achieved. The mean weight loss of successfully treated patients was 8.8kg at 3 months. No major complications were encountered. Abdominal bloating and a sore throat for several days were the most frequently reported adverse events.
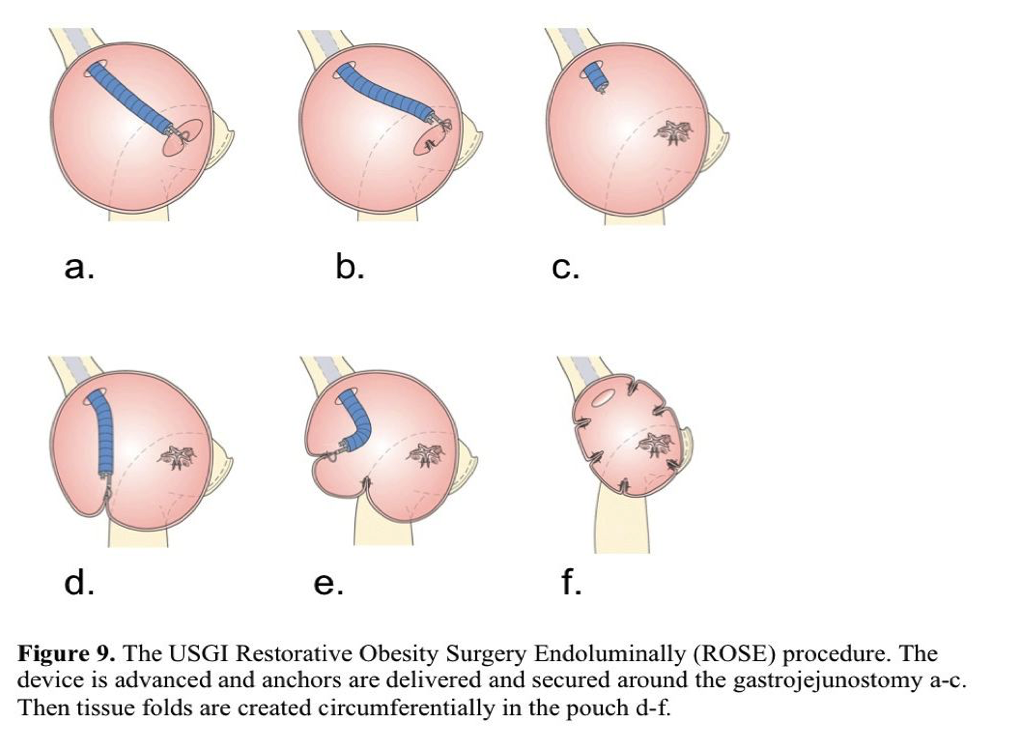
Horgan et al120 reported results of 112 patients who underwent the restorative procedure. The mean stoma diameter was reduced by 50% and the pouch length by 44% when an average of 6 anchors was placed. At 6 months postprocedure, 96 patients lost 32% of the weight regained after bariatric surgery. One-year follow-up was available for the first 19 patients, and endoscopies showed that the anchors remained in place and the tissue folds were present. Most patients reported minimal or no pain. Application of the IOP platform for revisional bariatric surgery appears to be safe. Studies on long-term procedure durability and evaluation of the device for primary obesity surgery are planned.
EndoGastric Solutions StomaphyX®
The StomaphyX is a single-use device, designed to create large gastric tissue folds (Figure 10). The procedure is performed under direct visualization using an adult endoscope placed through the device’s dedicated channel. The device is placed in the correct position, suction is applied, and tissue is drawn into the distal part of the device. Nonresorbable polypropylene fasteners are then delivered through the tissue fold. To reduce volume, 5 folds to 6 folds are created circumferentially at multiple levels in the stomach.
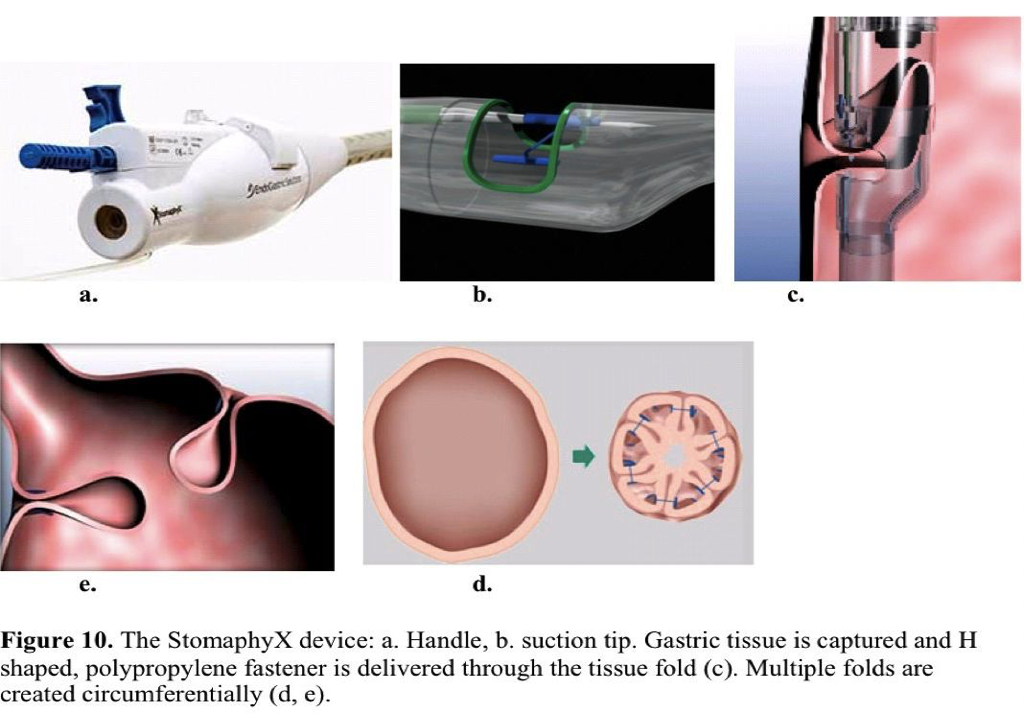
Revisional bariatric procedure preliminary results from a single-center trial are available. Enrolled in the study were 39 patients with an average preprocedure excess body weight of 51.1kg. No major complications occurred. The mean excess body weight loss at 1-year follow-up was 20%.121 Successful repairs of gastric pouch leaks with the StomaphyX device also have been reported.122 Results on the StomaphyX device are limited. In July 2009, a US randomized sham-controlled clinical trial for revisional bariatric surgery was initiated.
Transoral Gastroplasty (TOGA) System
The TOGA system is an endoscopic stapling device designed to place a staple line parallel to the lesser curvature to create a gastric sleeve. The procedure is performed under direct visualization, because a gastroscope can be advanced through the device and retroflexed. An extendable wire sail impacts the greater curvature to prevent remote tissue capture. The stapler then engages the anterior and posterior gastric walls by applying suction. After staple application, the stapler is withdrawn and reloaded. The second staple row extends the sleeve distally creating an 8-cm long x 2-cm wide sleeve. A TOGA restrictor is then used to clamp and staple gastric folds together to reduce the sleeve outlet size (Figure 11).
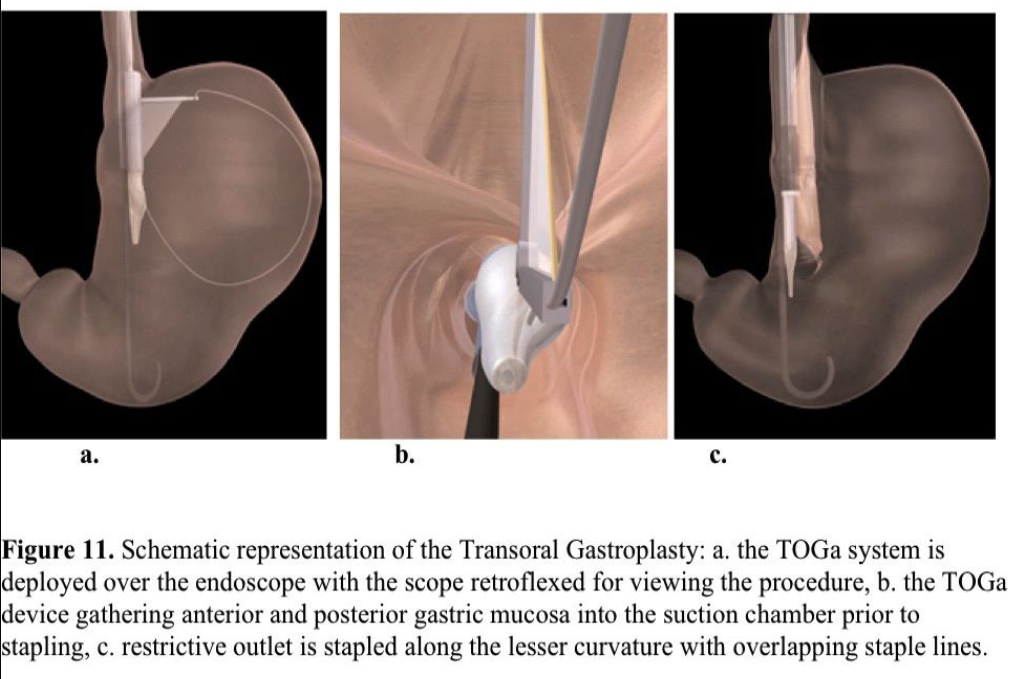
A total of 32 morbidly obese patients were enrolled in 2 studies.123,124 The follow-up period was 6 months. The excess weight loss was 24.4% with the first-generation and 46% with the second-generation device. The BMI decreased from 43.3 to 38.5 and from 41.6 to 33.1, respectively. No major complications occurred. The most common adverse events were transient pain, nausea, and dysphagia. With the second-generation device, 82% of the staple lines were found to be intact at the 6-month follow-up.
Failure may occur. Closset et al125 reported 4 patients who had undergone the TOGA procedure, but 1-year postprocedure they were considered for revisional surgery because of unsatisfactory weight loss or weight regain. All of them underwent uneventful RNY gastric bypasses.126 The procedure is designed to be restrictive, but the literature reports an average outlet size of 12mm to 20mm and the recommended size is 10mm.
The TOGA System seems to be a safe restrictive endoluminal procedure. The short-term results do not allow us to determine the durability and real efficacy of the procedure but provide the rationale for a large, randomized sham-controlled trial. The latter has been terminated due to lack of efficacy.
SafeStitch Medical Gastroplasty System
The SafeStitch devices have successfully created a gastric internal restrictive outlet in canine models. Clinical trials are planned.
Prosthetic Gastric Sleeves
Prosthetic gastric sleeves are tube-like devices delivered and secured endoscopically in the proximal GI tract to restrict absorption of nutrients in the small intestine and/or exclude the stomach from the digestive process. The procedure is minimally invasive and does not permanently alter the anatomy of the GI tract. Its potential disadvantages are difficulty in securing the device with consequent migration and small bowel obstruction and erosion.
Valen Tx
The Valen Tx gastric sleeve is designed to mimic the effect of the Roux-en-Y gastric bypass. The device is attached by transmural anchors to the GEJ, and extends through the stomach and into the distal duodenum or proximal jejunum. The length of the sleeve is variable depending on the therapeutic goal.
In the first human clinical trial, 12 patients were enrolled with a BMI ranging from 35 to 50.126 The device was delivered safely and anchored successfully to the GEJ. The implantation period was 12 weeks, and no spontaneous anchor detachments occurred. The mean excess weight loss was 46% at 3 months postprocedure. No complications occurred during the study period or at removal.
GI Dynamics- Endobarrier (duodenal-jejunal bypass sleeve)
The Endobarrier is a 60-cm long, flexible tube composed of a nutrient-impermeable fluoropolymer that is deployed in the duodenal bulb and extends to the jejunum (Figure 12). The device prevents nutrient absorption and mixing with digestive enzymes, mimicking one of the components of the RNY gastric bypass. A self-expandable crown- shaped, nitinol anchor holds the proximal orifice open and in place. Safety and feasibility have been demonstrated.120-129
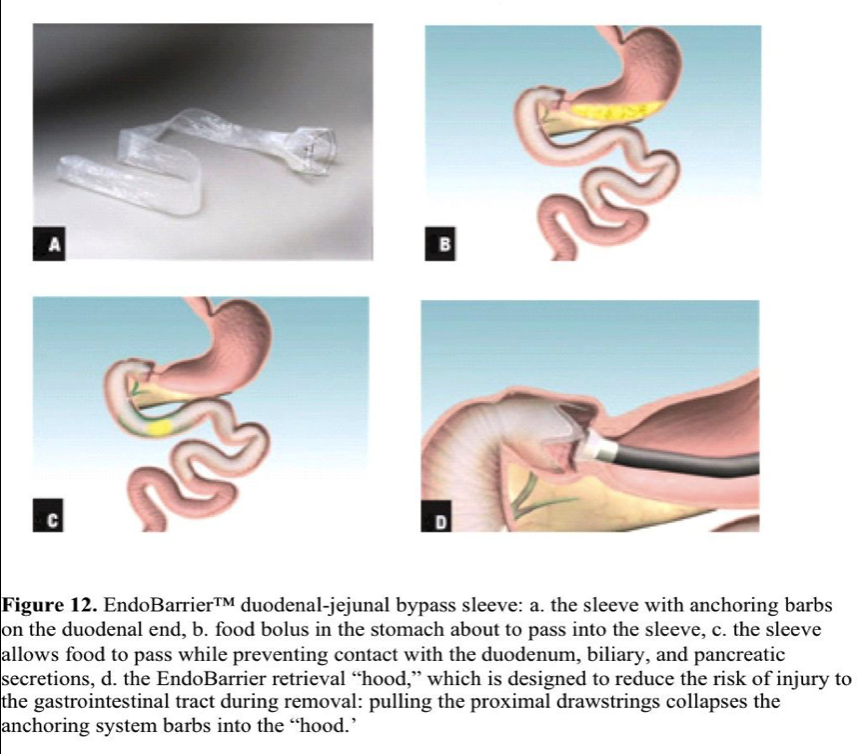
In a first single-center human trial, 12 patients with a mean BMI of 43kg/m2 were enrolled.130 The study interval was 12 weeks. All the devices were delivered and removed safely. In 2 patients, early removal was necessary due to poor placement. Adverse events, such as nausea, vomiting, or abdominal pain, frequently occurred within 2 weeks of implantation. Implant site inflammation was encountered in all patients. The mean excess weight loss was 23.6%. The fasting glycemia level in 4 diabetic patients improved and the hemoglobin A1c improved in 3. A blinded, randomized prospective trial has been initiated to evaluate the safety and feasibility of the device.
The device has been recently modified. A membrane with a small central orifice is attached distally to the nitinol ring providing an adjustable restriction at the outlet of the stomach to improve the therapeutic effect. Efficacy of the new flow restrictor was evaluated in a single-center study involving 10 morbidly obese patients. At 12 weeks, there was a mean excess weight loss of 39.6%. Mild to moderate abdominal pain, nausea, and vomiting were encountered. Further clinical investigation to evaluate the efficacy of the device for treatment of type 2 diabetes has been intended.
Other Therapies
Electrical Stimulation
The bariatric effect of electrical stimulation therapy is based on a series of low-energy electrical impulses delivered to the smooth muscle of the stomach intended to create a feeling of fullness. Laparoscopically delivered devices have been placed, and an endoscopically delivered gastric pacemaker is in development. Natural orifice surgery may further support the wider applicability of electrical gastric stimulation.
Radiofrequency Ablation
Localized tissue ablation using radiofrequency may have beneficial effects on weight loss. Radiofrequency ablation of the gastric antrum and pylorus may cause structural and functional changes that lead to decreased appetite and consequent weight loss. The nObese device developed by Silhouette Medical, Inc., is endoscopically delivered and positioned in the stomach. For 3 minutes to 5 minutes, energy is applied for ablation. The theoretical treatment effects are as follows: (1) decreased appetite presumably due to mucosal lining changes resulting in reduced hormone and HCl release, (2) satiety due to gastric volume reduction and elasticity, and (3) a decrease in hunger by delaying gastric emptying and reduction of Gherlin. The device is in its preclinical phase.
Lessons Learned
Endoluminal therapy for obesity and GERD is in its infancy but it holds promise. However, there is insufficient evidence at present to establish their real efficacy, particularly in the long-term. Only objective outcomes, such as pH normalization (Table 1) and excess body weight loss at one year (Table 2), are satisfactory parameters.
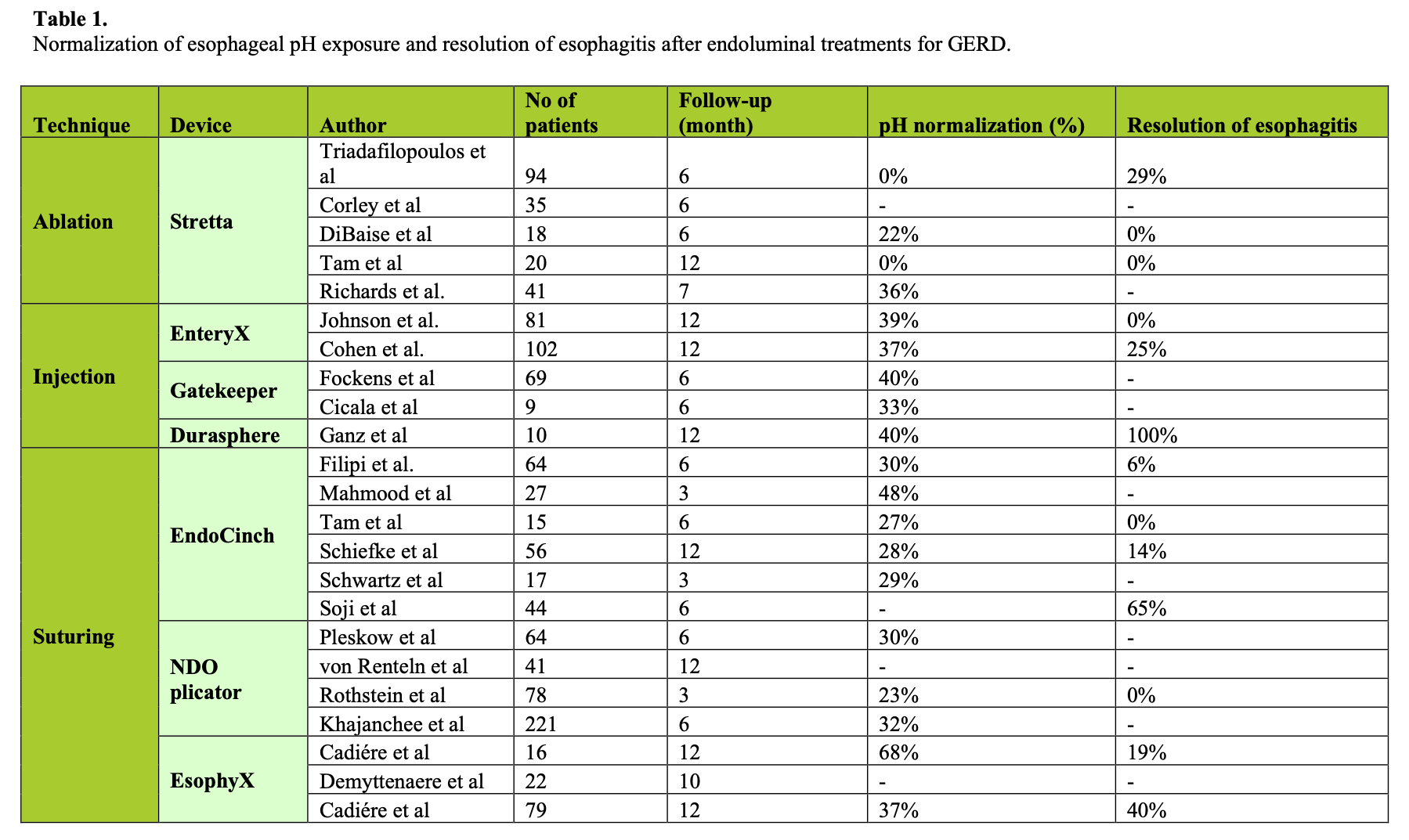
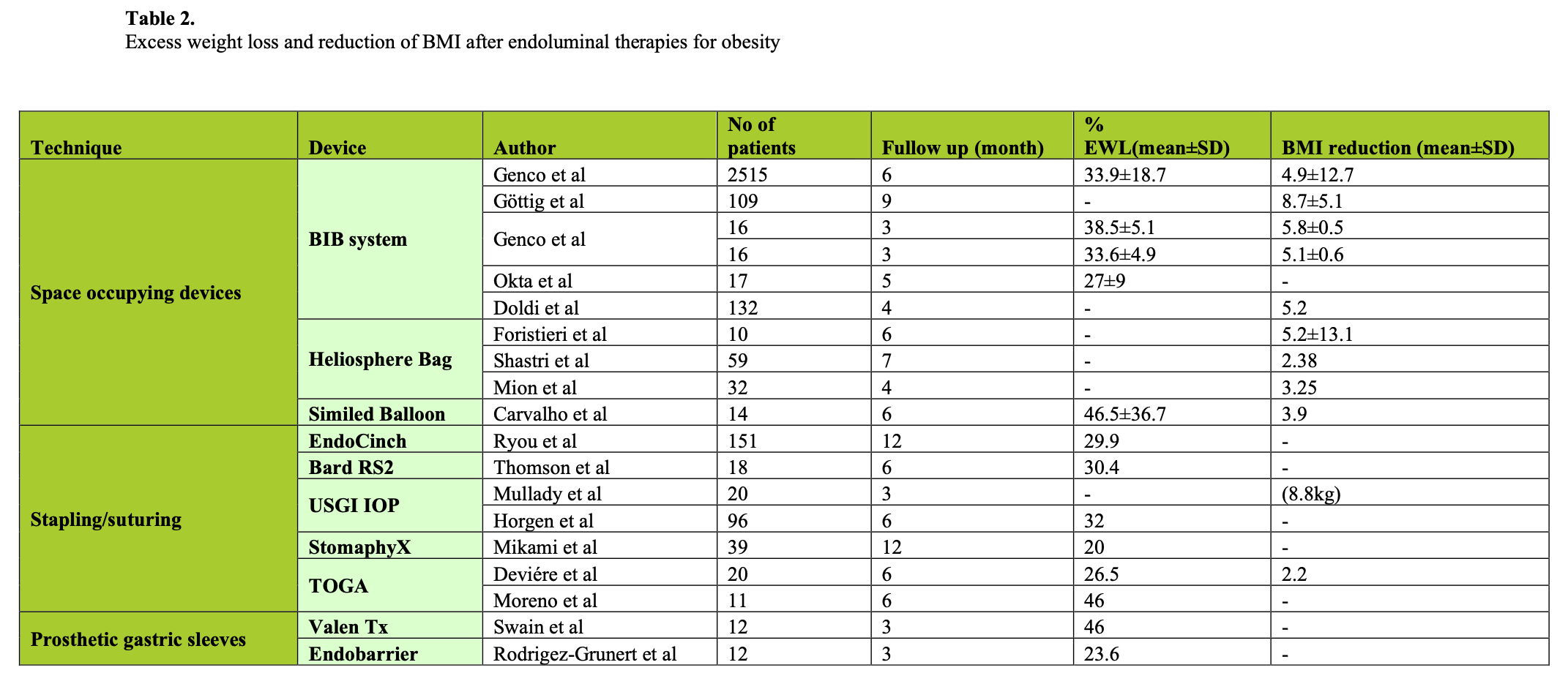
GERD symptom improvement and medication usage have been surprising in sham studies with a higher rate of success in this group than anticipated, negating these parameters by and large for GERD. We do not have any sham endoluminal studies for obesity yet, but it is apparent in at least 2 studies that the study effect is active. That is, patients are doing well with their prescribed diet, and yet the restriction planned is not present. Studies on obesity that control the diet and study effect better are needed.
Mucosa to mucosa apposition will not last regardless of the fixation method. An additional technique or device that promotes profound healing similar to an anastomosis is necessary in the stomach, because it is a dynamic organ that also can be filled rapidly to capacity. External bolsters for sutures or T tags will not suffice, and staples alone will not be durable.
Space occupying devices for obesity are unlikely to work in the long-term but may be helpful for preparing the super obese for surgical intervention. An expanding deflating pill is an intriguing idea, and the progress with the concept is awaited.
Address correspondence to: Charles J. Filipi, MD, Professor of Surgery, Department of Surgery, Suite 3740, Creighton University School of Medicine, 601 N. 30th Street, Omaha, Nebraska 68131, USA. Telephone: (402) 280-4213, Fax: (402) 280-4278, E-mail: cjfilipi@creighton.edu
References
- Orlando RC. Pathophysiology of gastroesophageal reflux disease. J Clin Gastroenterol. 2008 May-Jun;42(5):584-588.
- DeMeester TR, Peters JH, Bremner CG, et al. Biology of gastro-esophageal reflux disease: Pathophysiology relating to medical and surgical treatment. Annu Rev Med. 1999;50:469.
- Richter JE. The many manifestations of gastroesophageal reflux disease: presentation, evaluation and treatment. Gastroenterol Clin North Am. 2007;36:577-599.
- Dent J, El-Serag HB, Wallander M-A, Johansson S. Epidemiology of gastroesophageal reflux disease: A systematic review. Gut. 2005;54:710-717.
- Richter JE. Let the patient beware: the evolving truth about laparoscopic antireflux surgery. Am J Med. 2003 Jan;114(1):71-73.
- Smith DC. Antireflux surgery. Surg Clin N Am. 2008;88:943-958.
- Metz DC, Inadomi JM, Howden CW, van Zanten SJ, Bytzer P. On-demand therapy for gastroesophageal reflux disease. Am J Gastroenterol. 2007 Mar;102(3):642-653.
- Taylor A. New Developments in obesity intervention. Medtech Insight (obesity). 2009;11(9):40-51.
- Wang Y, Beydoun MA, Liang L, et al. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity. 2008;16:2323-2330.
- Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88.
- Friedenberg FK, Zanthopoulos M, Foster GD, Richter JE. The association between gastroesophageal reflux disease and obesity. Am J Gastroenterol. 2008 Aug:103(8);2111- 2122.
- Kinekawa F, Kubo F, Matsuda K, et al. Esophageal function worsens with long duration of diabetes. J Gastroenterol. 2008;43:338-344.
- Faraj J. Melander O, Sundkvist G, et al. Oesophageal dysmotility, delayed gastric emptying and gastrointestinal symptoms in patients with diabetes mellitus. Diabetic Medicine. 2007;24:1235-1239.
- Liebermann MD, Allgower M, Schmid P, et al. Muscular equivalent of the lower esophageal sphincter. Gastroenterology. 1979;76:31.
- Massey BT. Potential control of gastroesophageal reflux by local modulation of transient lower esophageal sphincter relaxations. Am J Med. 2001;3:186S.
- Hirsch DP, Mathus-Vliegen EM, Dagli U, et al. Effect of prolonged gastric distention on lower esophageal sphincter function and gastroesophageal reflux. Am J Gastroenterol. 2003;98(8):1696.
- Kahrilas PJ. GERD pathogenesis, pathophysiology and clinical manifestations. Cleve Clin J Med. 2003;70Suppl:S4.
- Kahrilas PJ. Radiofrequency therapy of the lower esophageal sphincter for the treatment of GERD. Gastrointest Endosc. 2003;57(6):723.
- Chiba N, De Gara CJ, Wilkinson JM, et al. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta-analysis. Gastroenterology. 1997;112:1798-810.
- Vigneri S, Termini R, Leandro G, et al. A comparison of five maintenance therapies for reflux esophagitis. N Engl J Med. 1995;333:1106-1110.
- Fass R. Proton pump inhibitor failure- what are the therapeutic options? Am J Gastroenterol. 2009 Mar;104(Suppl 2):S33-S38.
- Liebermann DA. Medical therapy for chronic reflux esophagitis: A long-term follow- up. Arch Int Med. 1987;147:1717.
- Liu JJ, Saltzman JR. Refractory gastro-esophageal reflux disease: diagnosis and management. Drugs. 2009 Oct 1;69(14):1935-1944.
- Ali T, Roberts DN, Tierny WM. Long-term safety concerns with proton pump inhibitors. Am J Med. 2009 Oct;33(10):2034-2038.
- Laheij RJ, Sturkenboom MC, Hassing RJ, et al. Risk of community acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004 Oct 27;292(16):1955- 1960.
- Dial MS. Proton pump inhibitor use and enteric functions. Am J Gastroenterol. 2009 Mar;104(Suppl 2):S10-S16.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006 Dec 27;296(24):2947-2953.
- Pace F, Tonini M, Pallotta S, et al. Systematic review: maintenance treatment of gastro-esophageal reflux disease with proton pump inhibitors taken “on-demand.” Aliment Pharmacol Ther. 2007;26:195-204.
- Richter JE. Gastroesophageal reflux disease. Best Pract Clin Gastroenterol. 2007;21(4):609-631.
- Finlayson SR, Birkmayer JD, Laycock WS. Trends in surgery for gastroesophageal reflux disease: the effect of laparoscopic surgery on utilization. Surgery. 2003;133:147- 153.
- Carlson MA, Frantzides CT. Complications and results of primary minimally invasive antireflux procedures: review of 10735 reported cases. J Am Coll Surg. 2001;193(4):428-439.
- Dallemegne B, Weerts J, Markiewicz S, et al. Clinical results of laparoscopic fundoplication at ten years after surgery. Surg Endosc. 2006 Jan;20(1):159-165.
- Valera JE, Hinojosa MW, Nguyen NT. Laparoscopic improves perioperative outcomes of antireflux surgery at US academic centers. Am J Surg. 2008 Dec;196(6):989- 993.
- Furnée EJ, Draaisma WA, Broeders IA, Gooszen HG. Surgical reintervention after failed antireflux surgery: a systematic review of the literature. J Gastroenterol Surg. 2009 Aug;13(8):1539-1549.
- Smith CD, McClusky DA, Rajad MA, et al. When fundoplication fails. Redo? Ann Surg. 2005;241(6):861-871.
- Grün F, Blumberg B. Endocrine disrupters as obesogens. Mol Cell Endocrinol. 2009;304:19-29.
- Farooqi IS, O’Rahilly s. Monogenic human obesity syndromes. Recent Prog Horm Res. 2004;59:409-424.
- Martinez-Hernandez A, Enriquez L, Moreno-Moreno MJ, Marti A. Genetics of obesity. Public Health Nutr. 2007;10:1138-1144.
- Trasande L, Cronk C, Durkin M, et al. Environment and obesity in the National Children’s Study. Environ Health Perspec. 2009;117(2):159-166. Epub 2008 Sept 12. Review.
- Loos RJ, Rankinen T. Gene-diet interactions on body weight changes. J Am Diet Assoc. 2005 May;105(5 Suppl 1):S29-S34.
- Herbert A, Gerry NP, McQueen NM. A common genetic variant with adult and childhood obesity. Science. 2006 Apr 14;312(5771):279-283.
- Shuldiner AR. Obesity genes and gene-environment-behavior interactions: recommendations for a way forward. Obesity (Silver Spring). 2008 December;16(Suppl 3):S79-S81.
- Bose M, Olivan B, Laferrere B. Stress and obesity: the role of the hypothalamic- pituitary-adrenal axis in metabolic disease. Curr Opin Endocrinol Diabates Obes. 2009 Oct;16(5):340-346.
- Wang G-J, Volkow ND, Logan J, et al. Brain dopamine and obesity. Lancet. 2001;357:354-357.
- Padwal RS, Majumdar SR. Drug therapy for obesity: orlistat, sibutramine and rimonabant. Lancet. 2007 Jan 6;369(9555):71-77. Review.
- Dixon JB. Referral for a bariatric surgical consultation: it is time to set a standard of care. Obes Surg. 2009;19:641-644.
- Zaho Y, Encinosa W. Bariatric surgery utilization and outcomes in 1998 and 2004: statistical brief no. 23. Rockville (MD): Agency of Healthcare Research and Quality; 2007. Retrieved April 17, 2010. Available at: http://www.hcup- us.ahrq.gov/reports/statbriefs/sb23.pdf.
- Swain CP, Mills TN. An endoscopic sewing machine. Gastrointest Endosc. 1986;32:36-38.
- Schauer P, Chand B, Brethauer S. New applications for endoscopy: the emerging field of endoluminal and transgastric bariatric surgery. Surg Endsoc. 2007;21:347-356.
- Loube D. Radiofrequency ablation for sleep-disordered breathing. Chest. 1998 May;113(5):1151-1152.
- Triadafilopoulos G, DiBaise JK, Nostrant TT, et al. The Stretta procedures for the treatment of GERD: 6 and 12 month follow-up of the U.S. open label trial. Gastrointest Endosc. 2002;55(2):149.
- Corley DA, Katz P, Wo JM, et al. Improvement of gastroesophageal reflux symptoms after radiofrequency energy: a randomized, sham-controlled trial. Gastroenterology. 2003;125(3):668.
- DiBaise JK, Brand RE, Quigley EMM. Endoluminal delivery of radiofrequency energy to the gastroesophageal junction in uncomplicated GERD: efficacy and potential mechanism of action. Am J Gastroenterol. 2002;97:833-842.
- Tam WCE, Schoeman MN, Zhang Q, et al. Delivery of radiofrequency energy to the lower oesophageal sphincter and gastric cardia inhibits transient lower oesophageal sphincter relaxations and gastro-oesophageal reflux in patients with reflux disease. Gut. 2003;52:479-485.
- Abdel Aziz AM, El-Khayat HR, Sadek A, et al. A prospective randomized trial of sham, single-dose Stretta, and double-dose Stretta for the treatment of gastroesophageal reflux disease. Surg Endosc. 2009 Sep 3. [Epub ahead of print].
- Noar MD, Sahar Lotfi-Erman. Sustained improvement in symptoms of GERD and antisecretory drug use: 4-year follow-up of the Stretta procedure. Gastrointest Endosc. 2007;65(3):367-372.
- Deviere J, Pastorelli A, Louis H, et al. Endoscopic implantation of a biopolymer in the lower esophageal sphincter for gastroesophageal reflux: a pilot study. Gastrointest Endosc. 2002;55(3):335.
- Cohen LB, Johnson DA, Ganz RA, et al. Enteryx implantation for GERD: expanded multicenter trial results and interim postapproval follow-up to 24 months. Gastrointest Endosc. 2005;61(6):650-658.
- Fockens P, Bruno M, Boeckxstaens G, et al. Endoscopic removal of the Gatekeeper system prosthesis. Gastrointest Endosc. 2002;55(5):260.
- Cicala M, Gabbrielli A, Emerenziani S, Guarino MPL, et al. Effect of endoscopic augmentation of the lower oesophageal sphincter (Gatekeeper reflux repair system) on intraoesophageal dynamic characteristics of acid reflux. Gut. 2005;54:183-186.
- Fockens P, Boeckxstaens G, Gabbrielli A, et al. Endoscopic augmentation of the lower esophageal sphincter for GERD: final results of a European Multicenter Study of the Gatekeeper System. Gastrointest Endosc.2003;59(5):AB242.
- Fockens P. Gatekeeper reflux repair system: technique, pre-clinical and clinical experience. Gastrointest Endosc Clin N Am. 2003;13(1):179.
- Fockens P, Bruno MJ, Gabrielli A, et al. Endoscopic augmentation of the lower esophageal sphincter for the treatment gastroesophageal reflux disease: multicenter study of the gatekeeper reflux repair system. Endoscopy. 2004;36:682-689.
- Beavan A. Material properties and applications of pyrolite carbon. Mater Eng. 1990;31:1-5.
- Lightner D, Calvosa C, Anderson R, et al. A new injectable bulking agent for the treatment of stress urinary incontinence: results of a multicenter, randomized, controlled double-blind study of Durasphere. Urology. 2001;58:12-15.
- Davis K, Kumar D, Poloniecki J. Preliminary evaluation of an injectable anal sphincter bulking agent (Durasphere) in the management of fecal incontinence. Aliment Pharmacol Ther. 2003;18:237-243.
- Ganz RA, Fallon E, Wittchow T, Klein D. A new injectable agent for the treatment of GERD: results of the Durasphere pilot trial. Gastrointest Endosc. 2009 Feb;69(2):318- 323.
- Mosler P, Adbel Aziz AM, Hieston K, Filipi C, Lehman G. Evaluation of supplemental cautery during endoluminal gastroplication for the treatment of gastroesophageal reflux disease. Surg Endoscopy. 2009;22:2158-63.
- Feitoza AB, Gostout CJ, Rajan E, et al. Understanding endoluminal gastroplications: a histopathologic analysis of intraluminal suture plications. Gastrointest Endosc. 2003;57(7):868.
- Filipi CJ, Lehman GA, Rothstein RI, et al. Transoral, flexible endoscopic suturing for treatment of GERD: a multicenter trial. Gastrointest Endosc. 2001;53(4):416.
- Chadalavada R, Lin E, Swafford V, Sedghi S, Smith CD. Comparative results of endoluminal gastroplasty and laparoscopic antireflux surgery for treatment of Surg Endosc. 2004 Feb;18(2):261-265. Epub 2003 Dec 29.
- Mahmood Z, Byrne PJ, McMahon BP, et al. Comparison of transesophageal endoscopic plication (TEP) with laparoscopic Nissen fundoplication (LNF) in the treatment of uncomplicated reflux disease. Am J Gastroenterol. 2006 Mar;101(3):431- 436.
- Hill LD, Kozarek RA. The gastroesophageal flap valve. J Clin Gastroenterol. 1999 Apr;28(3):194-197.
- Tam WC, Holloway RH, Dent J, Rigda R, Schoeman MN. Impact of endoscopic suturing of the gastroesophageal junction on lower esophageal sphincter function and gastroesophageal reflux in patients with reflux disease. Am J Gastroenterol. 2004 Feb;99(2):195-202.
- Wenzel G, Kuhlbusch R, Heise J, Frieling T. Relief of reflux symptoms after endoscopic gastroplication may be associated with reduced esophageal Acid sensitivity: pilot study. Endoscopy. 2005 Mar;37(3):236-239.
- Schiefke I, Zabel-Langhennig A, Neumann S et al. Long-term failure of endoscopic gastroplication (EndoCinch). Gut. 2005 Jun;54(6):752-758.
- Liu JJ, Glickman JN, Carr-Locke DL, Brooks DC, Saltzman JR. Gastroesophageal junction smooth muscle remodeling after endoluminal gastroplication. Am J Gastroenterol. 2004;99:1895.
- Schwartz MP, Wellink H, Gooszen HG, et al. Endoscopic gastroplication for the treatment of gastro-oesophageal reflux disease: a randomized, sham-controlled trial. Gut. 2007 Jan;56(1):20-8. Epub 2006 Jun 8.
- Chen YK, Raijman I, Ben-Menachem T, et al. Long-term outcomes of endoluminal gastroplication: a U.S. multicenter trail. Gastrointest Endosc. 2005;61(6):659-667.
- Ozawa S, Kumai K, Higuchi K, et al. Short-term and long-term outcome of endoluminal gastroplication for the treatment of GERD: the first multicenter trial in Japan. J Gastroenterol. 2009;44:675-684.
- Arts J, Lerut T, Rutgeerts P, et al. A One-year follow-up study of endoluminal gastroplication (Endocinch) in GERD patients refractory to proton pump inhibitor therapy. Dig Dis Sci. 2005;50(2):351-356.
- Paulssen EJ, Lindsetmo RO. Long-term outcome of endoluminal gastroplication in the treatment of gastro-oesophageal reflux disease: effect of a second procedure. Scand J Gastroenterol. 2008 Jan;43(1):5-12.
- Menachem T, Chen Y, Raijman I, et al. Symptom recurrence after endoluminal gastroplication for GERD: Comparison of initial versus repeat ELGP. [abstract M1737] Gastrointest Endosc. 2003;57(5):130.
- Velanovich V, Ben-Menachem T. Laparoscopic Nissen fundoplication after failed endoscopic gastroplication. J Laparoendosc Adv Surg Tech A. 2002;12(5):305.
- Velanovich V, Ben-Menachem T, Goel S. Case-control comparison of endoscopic gastroplication with laparoscopic fundoplication in the treatment of gastroesophageal reflux disease. Surg Laparosc Endosc Percutan Tech. 2002;12:219.
- Liu JJ, Glickman JN, Carr-Locke DL, Brooks DC, Saltzman JR. Gastroesophageal junction smooth muscle remodeling after endoluminal gastroplication. Am J Gastroenterol. 2004;99:1895.
- Pleskow D, Rothstein R, Lo S, et al. Endoscopic full-thickness plication for the treatment of GERD: a multicenter trial. Gastrointest Endosc. 2004 Feb;59(2):163-171.
- Pleskow D, Rothstein R, Lo S, et al. Endoscopic full-thickness plication for the treatment of GERD: 12-month follow-up for the North American open-label trial. Gastrointest Endosc. 2005 May;61(6):643-649.
- Pleskow D, Rothstein R, Lo S, et al. Endoscopic full-thickness plication for the treatment of GERD: Five-year long-term multicenter results. Surg Endosc. 2008;22:326- 332.
- von Renteln D, Schiefke I, Fuchs K-H, et al. Endoscopic full-thickness plications for the treatment of GERD by application of multiple Plicator implants: a multicenter study. Gastrointest Endosc. 2008;68:833-844.
- von Renteln D, Schiefke I, Fuchs KH, et al. Endoscopic full-thickness plications for the treatment of GERD by application of multiple Plicator implants: 12-month multicenter study results. Surg Endosc. 2009;23:1866-1875.
- Rothstein R, Filipi C, Caca K, et al. Endoscopic full-thickness plication for the treatment of gastroesophageal reflux disease: a randomized, sham-controlled trial. Gastroenterology. 2006;131:704-712.
- Cadière GB, Rajan A, Rqibate M, et al. Endoluminal fundoplication (ELF)— evolution of EsophyX, a new surgical device for transoral surgery. Minim Invasive Ther Allied Technol. 2006;131:704-712.
- Cadière GB, Rajan A, Germay O, et al. Endoluminal fundoplication by a transoral device for the treatment of GERD: A feasibility study. Surg Endosc. 2008;22:333-342.
- Cadière G-B, Buset M, Muls V, et al. Antireflux transoral incisionless fundoplication using EsophyX: 12-month results of a prospective multicenter study. World J Surg. 2008;32:1676-1688.
- Demytteraere SV, Bergman S, Pham T, et al. Transoral incisionless fundoplicatoin for gastroesophageal reflux disease in an unselected patient population. Surg Endosc. 2010 Apr;24(4):84-858.
- Agostoni M, Boemo C. Bilateral pneumothorax during transoral incisionless fundoplication. Eur J Anaesthesiol. 2010 Feb;27(2):216-217.
- Cadière G-B, Van Sante N, Graves JE, et al. Two-year results of a feasibility study on antireflux transoral incisionless fundoplication using EsophyX. Surg Endosc. 2009;23:957-964.
- Testoni PA, Corsetti M, Di Pietro S, et al. Effect of transoral incisionless fundoplication on symptoms, PPI use, and pH-impedance refluxes of GERD World J Surg. 2010; Apr;34(4):750-757.
- Kauer WKH, Roy-Shapira A, Watson D, et al. Preclinical trial of a modified gastroscope that performs a true anterior fundoplication for the endoluminal treatment of gastroesophageal reflux disease. Surg Endosc. 2009;23:2728-2731.
- Filipi CJ, Stadlhuber RJ. Initial experience with new intraluminal devices for GERD Barrett’s esophagus and obesity. J Gastrointest Surg. 2010 Feb;14 Suppl 1;S121- S126. Epub 2009 Sep 24.
- Genco A, Bruni T, Doldi SB, et al. BioEnterics Intragastric Balloon: The Italian experience with 2,515 patients. Obes Surg. 2005 Sep;15(8):1161-1164.
- Göttig S, Daskalakis M, Weiner S, et al. Analysis of safety and efficacy of intragastric balloon in extremely obese patients. Obes Surg. 2009;19:677-683.
- Imaz I, Martinez-Cervell C, Garcia-Alvarez EE, et al. Safety and effectiveness of the intragastric balloon for obesity. A meta-analysis. Obes Surg. 2008 Jul;18(7):841-846. Epub 2008 May 6.
- Cubattoli L, Barneschi C, Mastrocinque E, et al. Cardiac arrest after intragastric balloon insertion in a super-obese patient. Obes Surg. 2009;19:253-256.
- Ohta M, Kitano S, Kai S, et al. Initial Japanese experience with intragastric balloon placement. Obes Surg. 2009;19:791-795.
- Genco A, Cipriano M, Bacci V, et al. BioEntericss Intragastric Balloon (BIBs): a short-term, double-blind, randomised, controlled, crossover study on weight reduction in morbidly obese patients. Int J Obes. 2006;30:129-133.
- Martinez-Brocca MA, Belda O, Parejo J. Intragastric balloon-induced satiety is ot mediated by modification in fasting or postprandial plasma ghrelin levels in morbid obesity. Obes Surg. 2007;17:649-657.
- Mathus-Vliegen EM, Tytgat GN. Intragastric balloon for treatment-resistant obesity: safety, tolerance, and efficacy of 1-year balloon treatment followed by a 1-year balloon-free follow-up. Gastrointest Endosc.2005;61:635-637.
- Forestieri P, De Palma GD, Formato A, et al. Helosphere® Bag in the treatment of severe obesity: preliminary experience. Obes Surg. 2006;16:635-637.
- Shastri Y, Haass S, Martin U, et al. Multicenter Study using air filled stomach balloon as a valid option for morbid obesity. Gastrointest Endosc. 2007;65:AB281.
- Mion F, Gincul R, Roman S, et al. Tolerance and efficacy of an air-filled balloon in non-morbidly obese patients: results of a prospective multicenter study. Obes Surg. 2007 Jun;17(6):764-769.
- Carvalho G, Barros C, Okazaki M, et al. An improved intragastric balloon procedure using a new balloon: preliminary analysis of safety and efficiency. Obes Surg. 2009;19:237-242.
- Malik A, Mellinger JD, Hazey JW, et al. Endoluminal and transluminal surgery: current status and future. Possibilities. Surg Endosc. 2006;20:1179-1192.
- Ryou M, Wu B, Lautz DB, et al. Transoral sutured revision of dilated gastrojejunostomy to treat weight regain in Roux-en-Y gastric bypass patients: a retrospective study of 227 procedures with mean 1 year follow up. [abstract 258] Gastrointest Endosc. 2009;69(5):
- Fogel R, De Fogel J, Bonilla Y, et al. Clinical experience of transoral suturing for an endoluminal vertical gastroplasty: 1-year follow-up in 64 patients. Gastrointest Endosc. 2008;68:51-58.
- Thompson CC, Brethauer SA, Chand B, et al. Transoral gastric volume reduction as an intervention for weight management (TRIM) Multicenter Feasibility Study: a report of early outcomes, [abstract] Digestive Disease Week. 2009:M1259. Available at: http://download.abstractcentral.com/DDW2009/myddw2009/M1259.html
- Herron DM, Birkett DH, Thompson CC, et al. Gastric bypass pouch and stoma reduction using a transoral endoscopic anchor placement system: feasibility study. Surg Endosc. 2008 Apr;22(4):1033-1039.
- Mullady DK, Lautz DB, Thompson CC. Treatment of weight regain after gastric bypass surgery when using a new endoscopic platform: initial experience and early outcomes. Gastrointest Endosc. 2009 Sep;70(3):440-444.
- Horgan S, Jacobsen G, Weiss GD, et al. Incisionless revision of post Roux-en-Y bypass stomal and pouch dilatation: multi-center registry results. [abstract PL-313] Surg for Obes and Related Dis. 2009 Suppl May-June;5(3):S22.
- Mikami D, Needelman B, Narula V, et al. Natural orifice surgery: initial US experience utilizing the StomaphyX™ device to reduce gastric pouches after Roux-en-Y gastric bypass. Surg Endosc. 2010 Jan;24(1):223-228.
- Overcash WT. Natural orifice surgery (NOS) using StomaphyX™ for repair of gastric leaks after bariatric revisions. Obes Surg. 2008 Apr;18:882-885.
- Diviere J, Ojeda G, Caldes V, Herrera LC, et al. Safety, feasibility and weight loss after transoral gastroplasty: first human multicenter study. Surg Endosc. 2008;22:589- 598.
- Morena C, Closset J, Dugardeyn S, et al. Transoral gastroplasty is safe, feasible, and induces significant weight loss in morbidly obese patients: results of the second human pilot study. Endoscopy. 2008 May;40(5):406-4123. Erratum in: Endoscopy 2008 Jun;40(6):537.
- Closset J, Germanova D, Loi P, Mehdi A, Moreno C, Devière J. Laparoscopic gastric bypass as a revision procedure after transoral gastroplasty. Obes Surg. 2009 Dec 10 [Epub ahead of print].
- Swain P, Rumbaut RA, Gonzalez LT, et al. First clinical experience with a novel endoscopic device that mimics the Roux-en-y gastric bypass procedure. [abstract S1499 retrieved August 2, 2010] Digestive Disease Week 2009. Available at: http://download.abstractcentral.com/DDW2009/myddw2009/S1499.html
- Aguirre V, Stylopoulos, Grinbaum R, et al. An endoluminal sleeve induces substantial weight loss and normalizes glucose homeostasis in rats with diet-induced obesity. Obesity. 2008;16:2585-2592.
- Tarnoff M, Shikora S, Lembo A. Acute technical feasibility of an endoscopic duodenal-jejunal bypass sleeve in a porcine model: a potentially novel treatment for obesity and type 2 diabetes. Surg Endosc. 2008;22:772-776.
- Tarnoff M, Shikora S, Lembo A, et al. Chronic in vivo experience with an endoscopically delivered and retrieved duodenal-jejunal bypass sleeve in a porcine model. Surg Endosc. 2008;22:1023-1028.
- Rodriguez-Grunert L, Neto MPG, Alamo M, et al. First human experience with endoscopically delivered and retrieved duodenal-jejunal bypass sleeve. Surg Obes Relat Dis. 2008;4:55-59.
