LASER ENERGY AND MINIMALLY INVASIVE SURGERY
Raymond J. Lanzafame, MD, MBA
INTRODUCTION
Lasers have occupied the fancy of the lay public, scientists, and clinicians alike. These technologies have been applied to laparoscopic procedures in a variety of disciplines including gynecology and general surgery.1-61 An array of laser technology is available for use during laparoscopy for the incision, coagulation, or vaporization of tissue.
Currently, this includes the argon, CO2, holmium, KTP, KDP, Nd: YAG, and high-power diode laser technology. Laser technology is also available for lithotripsy. This chapter will review the laser technologies available for use in minimally invasive surgery and will discuss the relative merits and disadvantages of each.
HISTORY
Gynecologists are credited with early use of laparoscopy as a diagnostic and therapeutic procedure and for the development of video laparoscopy. Gynecologists also applied laser technology for a wide array of clinical problems, including adhesions, fertility, endometriosis and myomas.5,6,10,33,34-48
Bruhat et al37,44,47 in France and Tadir et al37,45 in Israel are credited with the initial use of the CO2 laser laparoscope during surgery in 1979 and 1981, respectively. Tadir et al45 described the development of new delivery devices for laparoscopy in 1984. Bruhat et al37,44 launched one of the first prospective and comparative studies between microsurgery and laparoscopy for the treatment of tubo-peritoneal infertility in 1980. He demonstrated that the laparoscopic method was superior. Hubert Manhes37 worked with Bruhat early on and developed several new instruments and advanced operative procedures. Mahnes is also credited with describing the conservative treatment of ectopic pregnancy via laparoscopy as early as 1973.37,38
Several other gynecologic surgeons including Martin, Kelley, and Daniel in the United States, Sutton in England, Donnez in Belgium, among others, were pioneers in their own countries in the of use advanced laparoscopic operative techniques with CO2 laser technology in gynecology.36,37,40,42,43,46,48 Laparoscopic cholecystectomy can be credited with fueling a revolution in surgical thinking and application. General surgeons initially had an intense interest in using lasers and laser technology during laparoscopic cholecystectomy. The ability to produce highly precise and controllable effects on tissues, improved hemostasis, easy adaptability to fiberoptic and minimally invasive delivery systems, and the potential to facilitate complex dissection made these devices a welcome addition to the armamentarium of the surgical laparoscopist. Nearly 80% of all cholecystectomies performed in the United States during 1992 were performed laparoscopically, with only 4% being performed with laser technology.12,16-19,32
The majority of general surgeons rapidly discarded “the laser” for electrosurgical devices and “conventional” instruments as they grappled with mastering new techniques and procedures with unfamiliar laparoscopes, video cameras, and skills, which they had never seen or used previously. The initial enthusiasm and interest in the use of lasers in minimally invasive surgery from the general surgeon’s perspective can be traced to the work of Reddick, Schultz, Saye, and McKernan.10,22,-25,29 It must be understood, however, that the advent of videoendoscopy, specific laparoscopic instrumentation for cholecystectomy, and other procedures along with the development of multiple-load endostaplers did much to simplify minimally invasive surgery for the skilled laparoscopist.
Urologists began using laser technologies via nephrostomy, transurethral and ureteroscopic routes for lithotripsy. A variety of technologies including pulsed dye, Nd: YAG, and holmium lasers were applied to calculi located in the kidney, collecting system, ureters, and urinary bladder.20,49-56,60 Malek and others pioneered the use of high- powered KTP, and more recently, KDP (ie, Greenlight) laser technology for transurethral laser prostatectomy (TULIP).20,53-55 These devices have also been applied for treatment of bladder neck contractures, transurethral resection of bladder tumors (TURBT), and other soft tissue pathologies. Endourologic procedures, such as partial nephrectomy, have also been described. Photodynamic therapy (PDT) using hematoporphyrin derivative (HPD) and aminolevulinic acid (ALA) have been used successfully for management of in situ carcinoma and early transitional cell carcinoma (TCC) of the bladder.56
Minimally invasive strategies for the management of lumbar disc disease and related disorders using Nd: YAG, KTP, and holmium laser technologies expand the reach of these technologies to neurosurgery and orthopedic surgery.57,58,60 Arthroscopic application of lasers, while described, is rarely used at the current time.
Endovenous procedures have also been developed by using various laser and nonlaser technologies.60 Future technical applications include the use of laser-induced fluorescence imaging for augmented reality surgery.59
LASER VERSUS OTHER TECHNOLOGIES
One must consider whether any laser adds anything to the laparoscopist’s armamentarium. The surgical literature is replete with articles that purport to compare lasers with electrosurgical devices for use during laparoscopic cholecystectomy and other procedures. Electrosurgical devices are much more familiar, ubiquitous in the operating room, “less expensive” from the perspective of capital equipment expenditures and for some disposables, and “faster” particularly because the surgeon is much more conversant with electrosurgical technology and its appropriate application. Other alternatives including bipolar cautery, the Harmonic scalpel, and plasma knife are becoming increasingly popular alternatives to both monopolar cautery and lasers. The skills required to use these newer technologies are also more easily acquired, because they are closely akin to the repertoire of the surgeon.
The main advantages of electrosurgical devices are their ubiquity in the operating room and the fact that no additional training or safety considerations need be implemented.
These factors, when coupled with the average surgeon’s comfort with using them, make this the technology of choice for many surgeons. Both monopolar and bipolar instrumentation is available for laparoscopic use, and the majority of “routine” laparoscopic instrumentation is insulated to permit its use with electrosurgical devices. The main disadvantages of electrosurgical devices rest on the relative imprecision of the delivery of energy to the desired target. The build-up of char and debris on the electrode surface can result in the delivery of energy to areas adjacent to the desired target rather than to the target itself. In addition, debris can cause the electrode to adhere to the tissue or drag irregularly through it. Some manufacturers of disposable electrodes have attempted to solve this problem by coating the electrode with Teflon or other polymers and with the use of ceramic materials. However, the majority of laparoscopic instrumentation is untreated. Electrosurgical devices work poorly in the presence of blood, edema, and irrigating solutions. The majority of insulated laparoscopic hand instruments have relatively large exposed electrode surfaces. A large electrode mayresult in damage to adjacent structures, particularly in close spaces. Capacitance coupling is also a potential problem. Bipolar devices and Harmonic scalpels avoid the issues of stray current injuries. However, the instrument tips can become hot during use and can damage tissues contacted after use, because they remain hot for a variable length of time after they are switched off.
Several points bear mentioning prior to the consideration of specific laser technologies. Proponents of laser technology list the high degree of precision possible with these devices and the ability to control the tissue effect at the desired target as being the main advantages of these devices. Opponents often cite the acquisition expense of laser machinery and accessories in addition to an increased operative time as the main disadvantages of lasers in general. Although these issues are often raised, few recognize that the “cost” of a technology is not necessarily correlated with the actual price of the technology and that the price has little to do with the charge or the reimbursement. It must be understood that the net or global effect of a technology may be to lower the total cost of an illness when one considers such factors as the length of hospitalization, the degree and length of disability, and the ability of the patient to return to normal productivity. Such a thesis is borne out by laparoscopic cholecystectomy relative to its open counterpart. The “cost” of the technology necessary to perform a laparoscopic cholecystectomy is higher. The overall cost to industry as the primary payer for health care is lower, given a more rapid return to the workplace.
Surgical “speed” evolves and improves and the “length of the procedure” declines after the “learning curve” and once the surgeon becomes experienced and adept at the technique and the technology used to accomplish a procedure. It should be recognized that a laser may be used for only a small portion of a procedure, and several other factors also impact the operative time.
The surgeon should have a complete working understanding of lasers, their delivery systems, and their tissue effects prior to attempting to apply them to laparoscopic or other procedures. The surgeon should attend specific hands-on laser training programs if laser education and the opportunity to use these devices during the course of an approved residency training program were not available or if the surgeon is not familiar with a particular device or delivery system. Clearly, the house officer is in the ideal position to acquire the intellectual and manual skills necessary to use lasers and other technologies properly if this opportunity is provided as a part of the residency training program.
Postgraduate continuing medical education programs are useful for those who did not have formal training elsewhere. It is imperative that the surgeon continues to develop these newly acquired skills in an ongoing, graded fashion. This requires the gradual incorporation of the use of laser technology into clinical practice by tackling the simpler procedures and tasks first, followed by more difficult problems later, after the surgeon has developed a sense of comfort with the technology. One should have a working understanding of the limits and advantages of lasers in one’s own hands. The surgeon must be aware that all lasers and delivery systems are not alike and that attention to the selection of the proper wavelength, the proper delivery system, and the proper laser parameters are central to achieving the desired clinical endpoint given the appropriate technical expertise.
The above point cannot be neglected by the surgeon. The selection of a laser device, a specific laser delivery system, or any other instrument during the course of a procedure is critical to the conduct and outcome of that procedure. The selection of instrumentation for procedures involves several variables as we have already discussed. However, the preference of the surgeon is a major determinant in this process. Preference depends on availability, skill, judgment, experience, and the sense that a particular tool “feels right” or “works well” for a particular task in the hands of a particular surgeon. One needs only to examine the back table during an operative procedure to realize that several alternatives exist for the surgeon’s execution of a particular task.
LASER CHARACTERISTICS
Several different laser wavelengths and laser delivery systems are available for use during laparoscopy. Each laser wavelength has a characteristic effect on tissue, and it is the combination of the laser tissue interaction and the selection of the appropriate delivery systems and laser parameters that determine the ultimate effects of laser use during surgery. This presumes that the surgeon has the appropriate skill and technique. Thus, it is possible to precisely select and control the degree of tissue injury during surgery.
The ability to achieve the desired effect on the target tissue is also dependent on the surgeon’s understanding about the relationship between the Irradiance or Power Density and the laser tissue interaction. Power Density represents a concentration function and is defined as:
PD = Power / π *r2 = W / cm2
The power is the selected output power of the laser given in Watts and “r” represents the radius of the beam’s spot. It can be seen that given this relationship, the spot size or beam diameter has a significant influence on the Power Density relative to a given power output of a laser. Irradiance is a more precise definition of this concentration function, because it considers the cross-sectional area of the incident laser beam and is also described as W/cm2. The length of exposure of a target tissue to the laser energy is the Fluence, measured in Joules per centimeter squared and which is defined as follows:
FLUENCE = (Power / π *r2)* (time in seconds) = W sec. / cm2 = J / cm2
The surgeon generally endeavors to use the highest power density that can be safely controlled, thereby minimizing the duration of the exposure and unwanted tissue injury as a result of conductive heating of the tissue during contact with the laser beam.
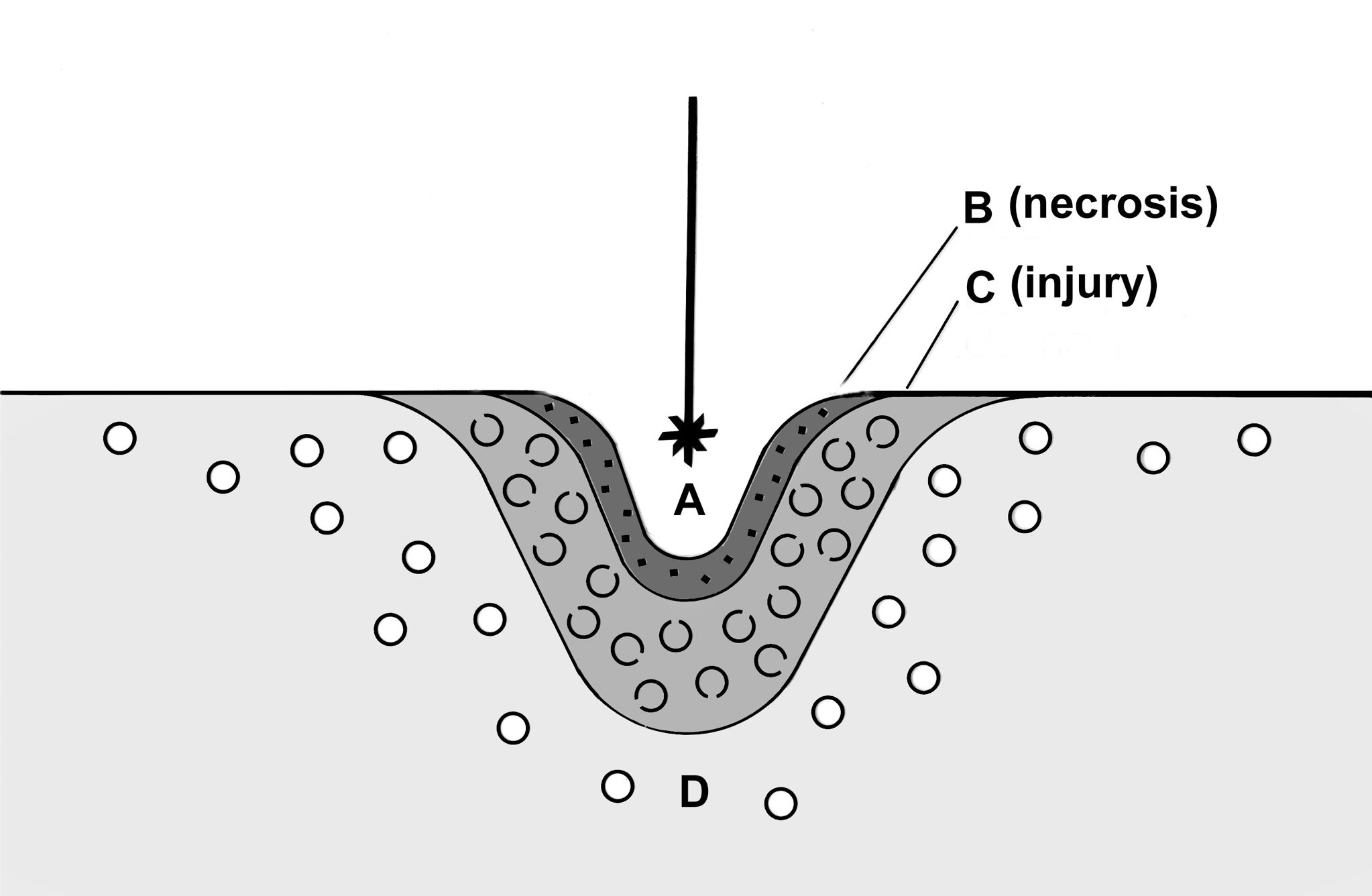
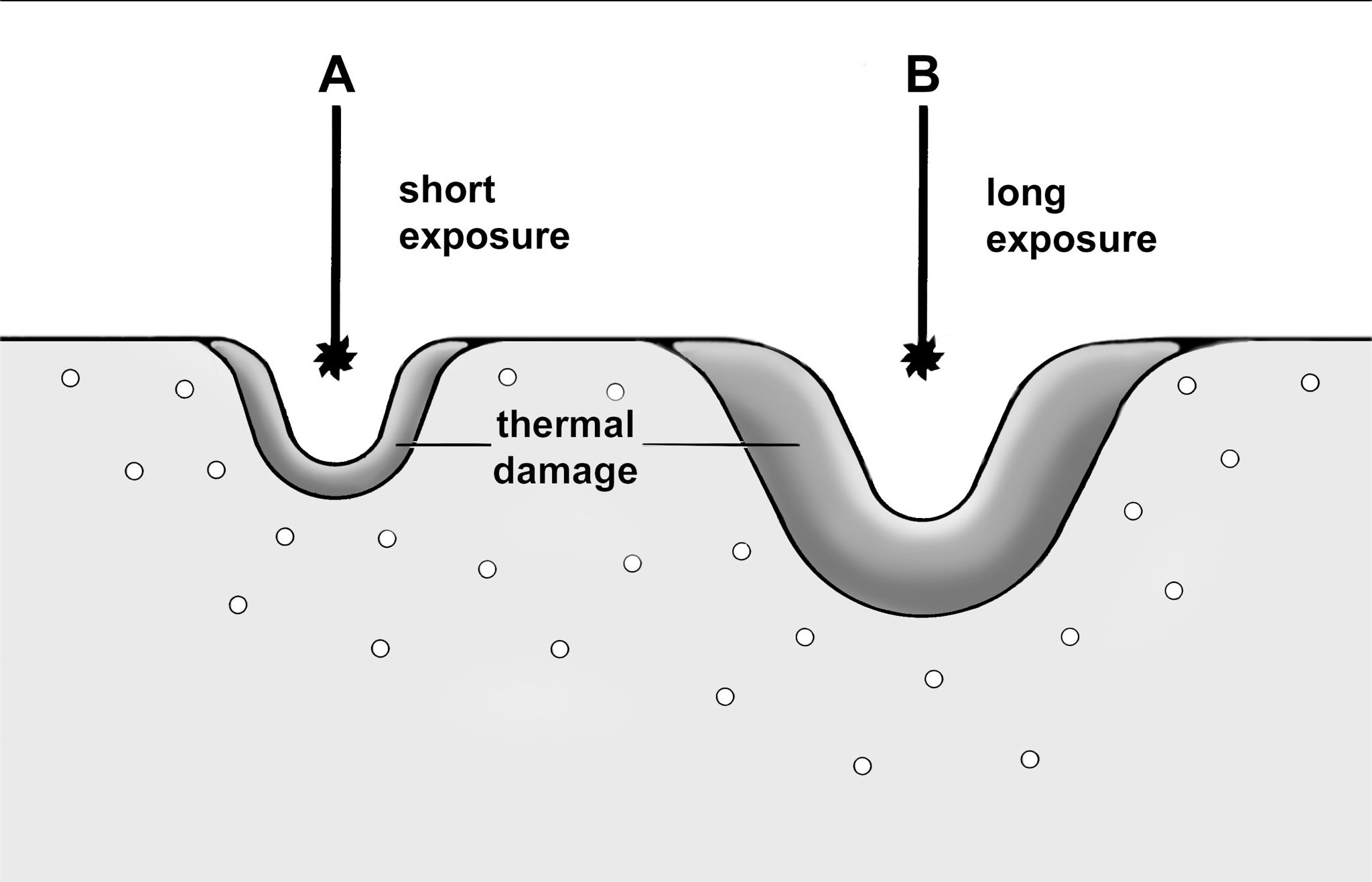
The primary result of the laser tissue interaction produces the classical histology of injury. This is demonstrated in Figure 1. The center of the wound is the zone of ablation, where tissue is vaporized or removed given a sufficiently high-power density. This is followed by a zone of irreversible injury or necrosis, which is followed by a zone of reversible injury. The effects of the length of exposure are demonstrated in Figure 2.
This figure illustrates the fact that minimizing the duration of laser exposure will optimize the tissue effects for most applications.
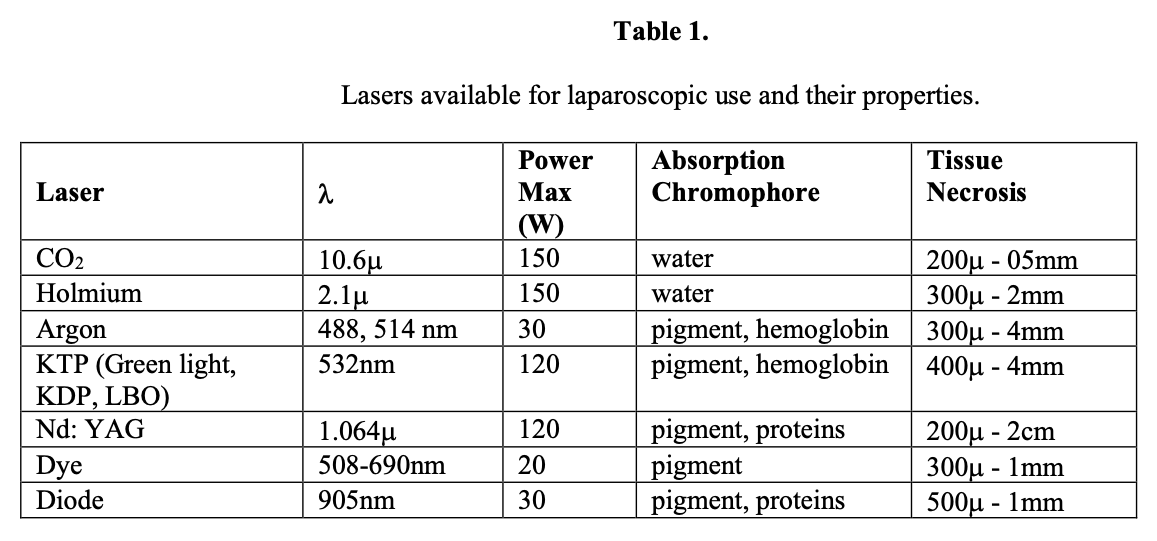
The types of laser technology available for endoscopic use are listed in Table 1. We will now discuss these laser wavelengths in more detail and consider the applications and shortfalls for each.
CO2 LASER
CO2 lasers have been used extensively for an array of gynecologic laparoscopic applications but have been rarely utilized for laparoscopic cholecystectomy and other minimally invasive surgical procedures. The energy of the CO2 laser (wavelength=10 600nm) is in the far-infrared portion of the electromagnetic spectrum. This wavelength is intensely absorbed by cellular water. This property results in “superficial” injury to tissues and enables the sealing of blood vessels and lymphatics that are up to 0.5mm to 1.0mm in diameter. The potential for inadvertent injury to deeper structures is minimal. The zone of necrosis is approximately 100µ to 300m, when the CO2 laser is used at appropriate fluences in a cutting mode. This most closely resembles the histology of an incision created by the scalpel. This wavelength is absorbed independently of the color of the tissue. Thus, the clinical effect seen in soft tissues is relative to the water content of the target tissue. The local infiltration of tissue with saline or anesthetic solutions will protect or insulate them from injury by the laser beam until the fluid has been vaporized. This laser is the most efficient modality available for ablation or vaporization of large volumes of tissue, such as tumor nodules or endometriomas.
The CO2 laser wavelength is carried via hollow tubes, waveguides, and mirrors. Conventional fiberoptics are not currently available for clinical use. The laparoscopic use of this wavelength is possible with the use of a focusing cube and an operative laparoscope or with a variety of waveguides designed for multi-puncture laparoscopic applications. The focusing cube permits the use of the CO2 laser in a free beam mode for cutting, vaporization, and coagulation of tissue. The focusing cube also is capable of transmitting an aiming beam. This feature makes it easier for the surgeon to direct the laser energy to the desired target. A variety of procedures, such as myomectomy, partial oophorectomy, resection and ablation of endometriomas, adhesiolysis, and even cholecystectomy have been accomplished successfully with this delivery system.
Cholecystectomy requires a McKernan-type approach. The successful use of this approach requires knowledge and facility with the operative laparoscope and the surgeon’s ability to visualize the desired target and maneuver a micromanipulator or joystick. The surgeon can alter the tissue effect by focusing or defocusing the laser beam as well as varying the laser wattage selected. Laser waveguides are hollow tubes with mirror-like surfaces that reflect the CO2 wavelength. Waveguides are available in both rigid and flexible versions and can be used to achieve a spot size (ie, burn or incision) that is in the range of 0.8mm to 2.2mm. As a general principle, the waveguide is used in a noncontact fashion, particularly because tissue contact can obstruct the waveguide and liquid can be drawn into the hollow waveguide by capillary action. The result of these events is the irreversible destruction of the waveguide. Recent developments include the Omniguide, which is a small-diameter solid chalcogenide glass waveguide and which is currently being used in otolaryngology and neurosurgical applications.
The successful use of this laser for dissection and hemostasis requires that the surgeon be adept and expert with the laser, as this will affect the ability to dissect tissues and achieve an adequate degree of hemostasis. Both the focusing cube and waveguide systems require a direct line of sight or the use of angled mirrors. This further complicates the maneuverability of these devices more so than fiber capable lasers and conventional instruments. Both configurations require flowing gas to cool the system and to prevent vaporized tissue plume from being thrown into the device. The most frequently used purge gases are argon and carbon dioxide. High CO2 gas flow rates can actually absorb the laser energy and reduce its efficiency (ie, the transmission of energy from the laser to the tissue). Therefore, lower flow rates (ie, 1L/min) are suggested. Some laser systems are equipped with a nitrogen purge gas system. The surgeon should NOT use nitrogen during laparoscopy, because its absorption from the peritoneum can cause “the bends.”
The optimal use of the CO2 laser for laparoscopic or open use is achieved when the beam is oriented perpendicular to the desired target. Hemostasis is enhanced by tissue compression, the use of epinephrine-containing local anesthetic solutions and the ability of the operator to recognize the presence of a vessel prior to its division. Under these conditions, the surgeon defocuses the laser (ie, moves the handpiece, waveguide, or operating laparoscope farther away from the target) and then applies short bursts of energy to the vessel in the area to be divided. This maneuver heats and coagulates the vessel, thereby enabling its division by the focused beam. The surgeon should use the highest power setting with which he or she is comfortable, because this will enable more efficient cutting, better hemostasis, and less thermal injury to the wound edges by minimizing conductive and radiative heat loss into the wound. Intermittent evacuation of the vaporized tissue plume or the use of a recirculating filtration system assure a clear field of view and prevent absorption of toxic products of combustion by the patient. This problem is identical in magnitude and toxicity to the vaporized tissue plume created by any electrosurgical, thermal, or laser source. Similarly the “smoke” should not be vented into the operating room, because it is considered hazardous for OR personnel.
OSHA/NIOSH has written regulations that require that OR staff be protected from vaporized tissue plume regardless of its source.
ARGON LASER
The argon laser has been used extensively for gynecologic laparoscopic procedures in the past.10,33 It has largely been replaced by other technologies today. This laser produces light in the visible portion of the spectrum. This laser actually produces 6 lines (wavelengths). However, the majority of the laser output is in the blue-green spectrum (wavelength = 488 514nm). This energy is intensely absorbed by hemoglobin and melanin, although other exogenous chromophores will absorb these wavelengths efficiently. Visible light wavelengths can be passed through water, enabling the argon laser to be used in aqueous environments, such as the bladder, during hysteroscopy, arthroscopy, and in the presence of irrigating fluids. This property enables the surgeon to photocoagulate a bleeding area while irrigating to locate the source of the bleeding.
Both free-beam and conventional fiberoptic applications are utilized during operative laparoscopy. A Microslad unit may be coupled with the operative laparoscope. A gimbaled mirror and joystick allow the surgeon to maneuver the beam in the surgical field. The fiber can be used in both a contact and noncontact mode.
Argon laser light penetrates and scatters in tissues. The resultant damage can be as much as 6mm. When used in an incisional mode, the speed of incision and the degree of hemostasis are adequate. Blood vessels on the order of 2mm in diameter can be divided and coagulated with this wavelength. Although some authors10,13,16-20,25 have reported successful hemostasis with vessels as large as 3mm to 4mm in diameter, delayed rebleeding may occur. Therefore, the surgeon would do well to use ligature and hemo- clip methods for hemostasis in these instances. The etiology of the delayed bleeding is necrosis of photocoagulated tissue and resultant tissue slough. This condition also occurs after use of the Nd: YAG laser in a free beam mode on similar tissues.
The contact or fiber-optic method is much more easily mastered than is the free-beam approach, because the surgeon has direct tactile feedback from the tissue. The speed of incision and the degree of hemostasis are adequate, and the more selective absorption of the wavelength in hemoglobin enables the surgeon to photocoagulate vessels before their division by bringing the fiber away from the tissue surface. This maneuver is similar to defocusing the free beam. The defocused mode is used to vaporize endometriomas. Some manufacturers produce a variety of sculpted fibers and metal-jacketed fiber delivery systems. These fibers are constructed to be more durable and work “more like a scalpel” due to absorption of some of the laser energy in the fiber resulting in heating of the fiber. This produces an optically driven cautery effect. So-called bare or urologic fibers are easily used and are cleaved and stripped as the fiber end degrades with use. Optimal cutting occurs by using the tip of the fiber either end-on or oblique to the plane of the dissection.
Because these wavelengths are color dependent, the surgeon should note that white or lightly colored tissue, such as meniscus and tumor implants, will not cut efficiently and will not be vaporized (ablated) unless they are first painted with India ink, indigo carmine dye, or another exogenous chromophore. A droplet of blood placed on the surface is sometimes effective for this purpose. Blackened or ebonized instruments and the use of optical backstops are required to prevent beam reflection and iatrogenic injury.
One of the main drawbacks of the laparoscopic use of the argon laser is the camera/eye safety filter. The eye and camera filters must block the 6 lines (ie, wavelengths) produced by the laser. These filters are usually a deep orange color and absorb 30% to 60% of the visible spectrum. As a result, the color balance of the image is distorted, and the need for a high-powered light source is critical to the surgeons’ ability to visualize the operative field. Many laser systems have intermittent shutter mechanisms that place the filter in the visual field only while the laser is actually being fired. The surgeon must be an expert at the local anatomy and the details of the procedure prior to attempting to work with this laser. This laser system is rarely used today due to the availability of KTP and KDP laser systems, which are much less cumbersome to use.
Nd: YAG LASER
The neodymium YAG laser produces near infrared light at a wavelength of 1060nm. This wavelength is carried via conventional fiberoptics, and, like visible light lasers, the energy will be transmitted through water. The energy can be applied to tissues with a wide array of delivery systems including cleaved bare fibers (ie, urologic fibers), polished GI fibers, sapphire tips (ie, the delivery device that is marketed as the Contact Laser), sculpted fiber (eg, Microcontact tip and various other proprietary versions of this technology), as well as free beam via a micromanipulator or Microslad unit.1,3,5,6,12,16-20,30 The energy of the Nd: YAG laser is intensely absorbed by tissue protein and chromophores and is highly scattered in tissue. These properties result in deep penetration of the energy and much greater damage below the tissue than can be appreciated at the surface. This makes noncontact (ie, GI fiber, free beam) and bare-fiber applications of the Nd: YAG laser extremely dangerous unless the surgeon has a thorough understanding of the laser-tissue interaction and orients the beam in a direction that would reduce the likelihood of damaging nearby structures. Specialized angled delivery or ADD fibers have been developed for use in photocoagulation of the prostate. These devices project the beam at right angles to the long axis of the probe, thereby allowing the prostatic tissue to be photocoagulated or vaporized. These applications require knowledge of the anatomy and tissue effects. The surgeon orients the laser output toward the 10:00 and 12:00 positions and will fire the laser at a preset energy for a specified length of time based on the volume of tissue to be photocoagulated.
The Nd: YAG laser is a poor cutting instrument when it is used in a noncontact mode. The development of sapphire tips and sculpted fiber technologies facilitate use of this laser in contact with tissue. Free-beam type applications can result in damage to 1cm to 2cm of liver tissue and the photocoagulation of vessels up to 4mm in diameter. However, the sapphire tip technology is a combination of a combined thermal and optical interaction with tissue. Much of the Nd: YAG energy is absorbed by the sapphire or fiber tip and converted to heat. The result is to produce optically driven cautery. The temperature of the tip can be tightly regulated for some applications. These instruments improve the cutting ability of the laser, but the tissue damage and the extent of coagulation are reduced dramatically. The histology of these devices is quite similar to the results produced by electrosurgical devices. Since their main tissue interaction is thermal cautery, the rate of incision and the degree of hemostasis can be reduced when these devices are used in the presence of irrigating fluids or in the aqueous environment of the bladder or joint space. The surgeon adjusts the laser parameters accordingly to achieve the desired effect. Sapphire tips are fragile and are expensive in comparison with other delivery systems. They remain hot for a short while after the laser has been turned off, which can cause damage or adherence to adjacent structures upon accidental contact. The sapphire tip may become disconnected from the fiber while an operation is underway and may be lost. When these devices are used in vascular and aqueous conditions, such as the bladder, prostate, or uterus, the fibers must NEVER be cooled with air or gas, because embolism of gas has proven fatal. Fiber cooling with saline or other irrigating fluids is quite safe however. Sapphire tip technology is infrequently used today.
Sculpted fibers were developed with many of the same properties as the sapphire tip, but without the liability of the tip remaining hot for an appreciable period of time after lasing has ceased and with less fragility of the tip. Some types of sculpted fibers transmit a sufficient quantity of laser energy to permit the coagulation of bleeders by using the fiber in a noncontact mode. Sculpted fibers are a good compromise for many laparoscopic applications. However, many of the currently available fiber designs are much stiffer than conventional fibers, which make them somewhat less pliable and somewhat more fragile than conventional silica-silica and quartz fibers.
Surgery with sapphire tips and sculpted fibers is facilitated by using them at an oblique angle relative to the plane of dissection. This technique enhances hemostasis by taking advantage of the heated mass of sapphire (or fiber tip), which coagulates the tissues prior to their division by the much hotter tip portion. Both the sapphire tip and sculpted fiber technologies are used infrequently today, with most current use being associated with bare fiber techniques.
Use of the bare fiber for dissection is practiced safely by experienced surgeons. However, the surgeon must have an intimate understanding of anatomy and should orient the fiber in a plane that is tangential to the line of incision to limit the forward scattering of the energy into the tissues. The use of ebonized instruments and optical backstops is mandatory.
Hemostasis with the YAG laser is best accomplished by using the laser (fiber, beam) in a defocused mode and delivering short bursts of energy to the area and its immediate periphery. It is generally better to deliver a few pulses and then wait for a few minutes to observe the area rather than attempting to lase continuously, because the latter practice will increase tissue damage unnecessarily and may actually result in the vaporization of the clot and re-bleeding.
The Nd: YAG laser has been used for the vaporization (ablation) of tumors. Typically, the surgeon will orient the beam parallel to the long axis of hollow viscera to avoid iatrogenic injury or perforation. Most of these procedures are performed in stages. In other words, the tumor is treated, allowed to slough, and then is treated as needed in subsequent sessions after debridement or natural sloughing of the photocoagulated tissue. The YAG wavelength is actually a poor ablating wavelength, particularly compared with the CO2, KTP, or holmium wavelengths whose rates of ablation are significantly faster. Lightly colored tissues require substantially more energy to initiate tissue ablation.
Ablation does not proceed until an area becomes desiccated and/or carbonized. The carbon then absorbs the laser energy and “catalyzes” the ablation of the subjacent tissue. Painting the tissue with India ink, Methylene blue, blood, or other chromophores makes the process much more efficient and safer by dramatically reducing the total amount of energy required to photoablate a given volume of tissue.
KTP LASER
The KTP laser is a frequency-doubled YAG laser that produces pure lime green light at a wavelength of 532nm. The 532nm wavelength is also available using crystals other than potassium-titanyl-phosphate, such as KDP and LBO. The Combolaser version was produced by Laserscope (San Jose, CA) and is capable of delivering both the 532nmKTP wavelength and the 1060nm, YAG wavelength. The surgeon can switch between these wavelengths depending on the needs of the procedure. The KTP wavelength is intensely absorbed by hemoglobin and melanin. Its absorption by hemoglobin is quite efficient, because it is very near the hemoglobin absorption peak at 540nm. This wavelength is easily transmitted through water and is carried via conventional fiberoptics. Typically, a cleaved and stripped bare fiber is used with a suction-irrigation instrument. Free-beam applications are possible with a micromanipulator or Microslad. The KTP laser is capable of incision, coagulation, and vaporization of tissue. This wavelength is much more efficient than the argon laser for these functions and surpasses the YAG laser as regards cutting and vaporization functions. These properties make this laser quite versatile for laparoscopic procedures. Vessels of up to 2mm in diameter are easily coagulated. The hemoglobin selectivity of this wavelength enables the surgeon to defocus the beam (ie, move the fiber away from the tissue surface) and preferentially coagulate a vessel before its division. Bleeders, such as those encountered in the gallbladder bed during cholecystectomy, are dealt with by irrigating the area with saline, using the laser in a defocused mode, and delivering short bursts of energy. Persistent bleeding can be dealt with by switching to the Nd: YAG laser output, if a dual wavelength laser is available. As with the argon and Nd: YAG lasers, the KTP laser requires eye safety filters, camera filters, and optical backstops along with ebonized instruments to prevent damage from stray light or beam reflection hazards. Since the KTP laser produces a single line of visible light, interference filters are available. Interference filters protect the camera and the eyes from injury by blocking the transmission of the 532nm and 1060nm wavelengths. This causes little if any color distortion. The CCD camera and monitor are easily adjusted to compensate for the filtered color. The tip of the fiber is the portion emitting laser energy and is therefore the part that incises and/or coagulates the tissue. Therefore, the surgeon must position the fiber either perpendicularly or obliquely relative to the plane of dissection.
A high-power KTP laser system capable of delivering 80 Watts to tissue has recently been developed for laser ablation of the prostate. This laser, which is marketed as the Niagara Laser, uses ADD fibers to vaporize prostatic tissue and produces an effect similar to a traditional TURP, with significantly reduced perioperative bleeding and with shorter operative times relative to its counterpart. The KDP laser is marketed as the Greenlight laser. It produces up to 120 Watts of output at 532nm. The present technology is being used for urologic applications and is most used for TURP and TULIP procedures. It is likely that further developments of these technologies will be spun off to other endoscopic and laparoscopic applications in the future.
HOLMIUM LASER
The holmium laser produces infrared light at a wavelength of 2100nm. This wavelength is intensely absorbed by water. The holmium laser wavelength can be carried via conventional fiberoptics unlike the CO2 wavelength. The fiber is usually encased in a metallic sheath for single puncture use or for use in combination with a suction-irrigation probe. This delivery system is quite durable and tends to be “self-cleaning.” The highly efficient absorption of this wavelength by water permits cutting and ablation of bone and cartilage. Despite its water absorption, this laser can be used in an aqueous environment due to the development of a cavitation bubble between the fiber and the tissue. This “Moses effect” transmits the laser energy to the tissue. Current systems can achieve outputs of as much as 150 Watts. The laser output is pulsed and high fluences may produce significant splattering and sputtering of tissue from the target area. This can coat the laparoscope and obscure the view. This problem can be minimized by selecting an appropriate power output and repetition rate as well as viewing the surgical site at a slightly greater distance than one would normally use with other modalities.
The incisional and ablative speed of this laser is somewhat slower than many of the other wavelengths available for laparoscopic use, particularly at lower fluences. However, this is offset by the ease of use and durability of the fiberoptics. The zone of coagulation is similar to that seen with electrosurgical devices and sculpted fibers. This laser is the most efficient wavelength for meniscectomy and percutaneous laser disc decompression (PLDD).
DIODE LASER TECHNOLOGY
High-powered diode laser systems are becoming available for clinical use. The promise of these systems is their compact size, easy portability, and the potential for lower capital and maintenance costs. The Diomed laser (Diomed Inc., The Woodlands, TX) was the first such unit approved for surgical use. This laser produces near-infrared light at805nm. The wavelength is carried by conventional fiberoptics and is most frequently applied with sculpted fiber technology. Wound histology and the incisional speed of this device are quite similar to that observed with the Nd: YAG laser. It is likely that other diode laser devices will become available in the future. Eye safety and intraoperative measures to prevent iatrogenic injury are similar to those described for the Nd: YAG laser. However, this wavelength scatters less in tissue, and therefore the depth of penetration of the free beam is shallower than the free beam YAG laser.
LITHOTRIPSY DEVICES
During the early years of laparoscopic cholecystectomy, the surgeon had a limited number of options as regards management of the patient with choledocholithiasis. As surgeons became more comfortable with minimally invasive surgical techniques and as smaller diameter fiberscopes and glide-wires became available, it became possible to perform common duct exploration and stone extraction laparoscopically.4,6,24
Stone extraction with baskets and forceps proceeds in a manner that is similar to that of open common duct exploration. However, these procedures can be difficult in the presence of large stones or stones that have become impacted at the ampulla. These patients can be successfully managed with a variety of lithotripsy techniques. Laser- and nonlaser-based technologies have both been applied to laparoscopic common duct exploration and lithotripsy. Electrohydraulic or spark gap lithotripsy devices were developed several years ago along with the development of flexible choledochoscopy.
These devices relied on a piezoelectric or spark-gap device implanted at the distal end of the catheter to generate shock waves that would then fragment the calculus. These catheters were relatively inexpensive compared with other devices. However, they were relatively large in diameter, which made them impractical for use with small-diameter flexible fiber choledochoscopes.
Laser-based lithotripsy devices are available for laparoscopic and urethroscopic use. A variety of wavelengths have been tested including pulsed dye lasers, alexandrite, holmium, and excimer lasers. Of these, the pulsed dye laser, alexandrite lasers, and holmium laser devices are available for clinical use at the present time. These lasers generate photoacoustic shock waves at the surface of the calculus at the point of the laser’s impact. This jackhammers and disintegrates the stone into small particles that can be flushed from the duct. A cleaved fiber is placed in contact with the stone, and a cavitation bubble develops as the laser is fired. Absorption of laser energy at the proper fluence and duty cycle causes the bubble to vibrate and fragment the stone. These devices are quite simple to use. They can be applied under direct vision with a fiberscope, or they can be placed percutaneously or threaded into the common bile duct or ureter “blindly.” A characteristic cracking or snapping sound is audible as the stone is contacted and as fragmentation occurs. Matching of the wavelengths and the relatively low fluences required for this photoacoustic effort enables destruction of the calculus without damage to the tissues of the common duct or ureter.
PRACTICAL TIPS FOR LASER USE
We have considered the various laser technologies and delivery systems available for laparoscopic use. This section will discuss various practical tips to optimize the clinical results from these devices.
It must again be emphasized that the surgeon should have an intimate understanding of the details of the procedure as well as the laser technology and delivery systems selected for use. It is preferable to learn the procedure and become comfortable with it after having successfully accomplishing it using so-called “conventional” devices priorto attempting it with laser technologies. The surgeon should practice with the laser devices as much as possible before using them clinically. A thorough understanding of tissue effects and the ability to assemble and troubleshoot the instrumentation is critical. It is helpful to work with the instrumentation in a pelvic trainer and then gradually introduce laser technology into clinical procedures.
Trocar placement should be well thought out and should be based on the needs of the procedure as well as the habitus of the patient. As a general principle, the operative port should be positioned such that the laser fiber (and other instruments) can easily reach the intended surgical site end-on. This is particularly important for direct fiber systems, such as bare fibers for KTP, holmium, Nd: YAG, or argon lasers or waveguides for CO2 lasers. Sculpted fibers and contact tips (sapphire tips) cut and coagulate optimally when they can be dragged obliquely across the tissues rather than using them end-on.
Therefore, the trocar placement may need to be modified for the specific laser and delivery system that is to be utilized. These principles are graphically demonstrated in Figure 3.
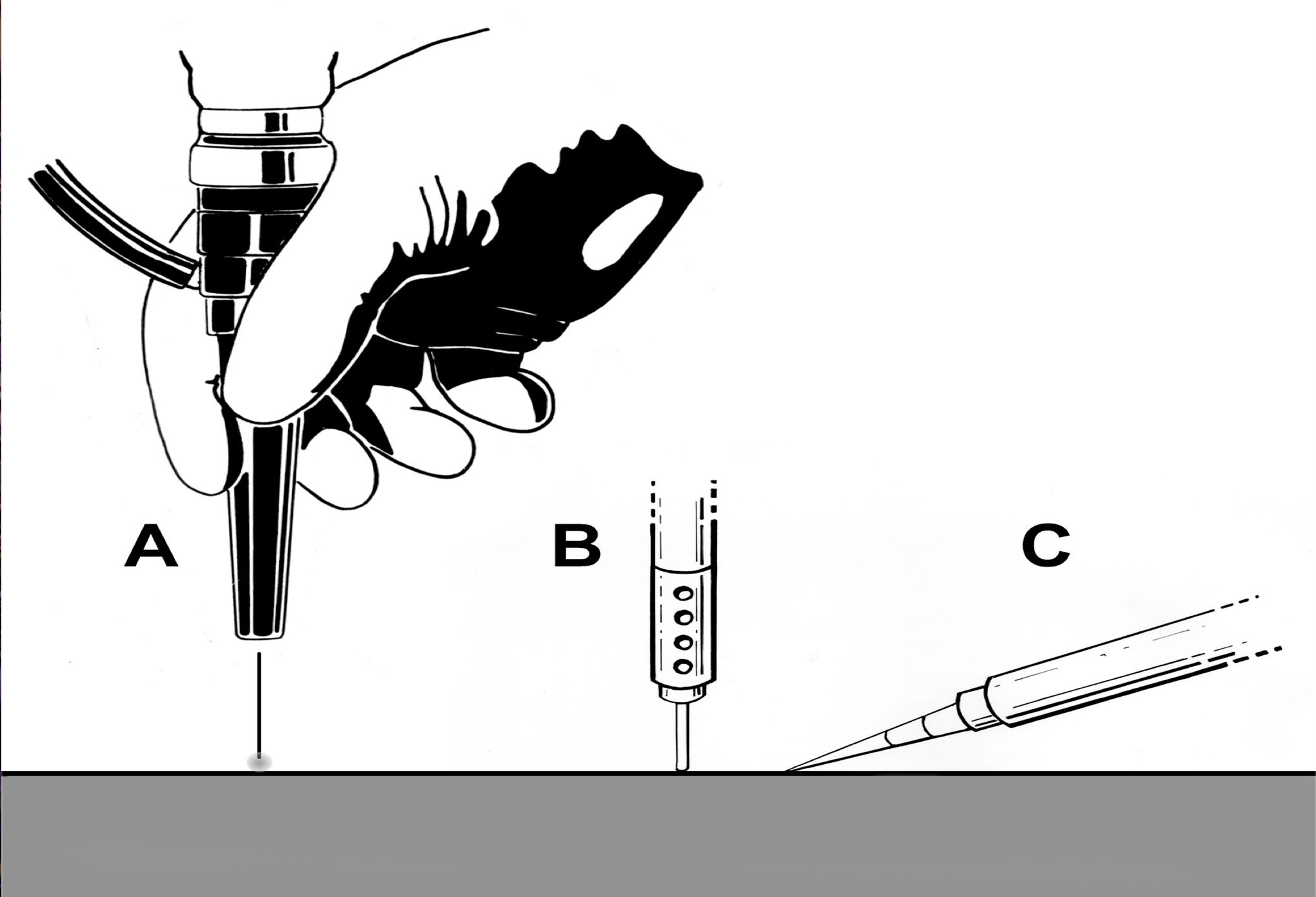
The assistant surgeon should provide steady countertraction to distract the line of incision, facilitate exposure, and optimize the efficiency and efficacy of laser use. The tissues should be incised in fluid, complete strokes as this too will increase the efficiency of the dissection and enable the dissection to proceed with better hemostasis. Staccato and repetitive passage of the laser fiber over the same area tends to produce a more irregular incision and frequently causes bleeding as vessels become injured at multiple points in the irregular wound.
Typically, the fiber-capable lasers are applied by placing the fiber into a suction- irrigation cannula. The fiber should be positioned such that it is easily visualized and such that the proposed line of incision is not obstructed by the suction irrigator. An optimal distance is often 1cm to 2cm for fiber extension. This permits visualization and maneuverability of the fiber and the surgical site without the fiber being too floppy as a result of having too much fiber length protruding from the suction irrigator. It is also critical that the fiber be retracted completely within the instrument when the instrument is being removed or inserted into the abdomen (or other site). This maneuver prevents fiber breakage or iatrogenic injury. The foot pedal for the laser and the electrosurgical device should be within easy reach while the monitor is viewed and the instrumentation is manipulated. Ideally, the surgeon should only have access to one pedal at one time to prevent inadvertent triggering of the wrong device.
Several devices are available that bend or angulate the bare fiber and thereby permit the surgeon to optimize the position of the fiber relative to the topography of the dissection.
Again, the fiber should be permitted to enhance visualization and minimize fiber breakage.
The surgeon should learn to use a light touch when using laser fibers. The rate of movement of the fiber should be deliberately slow enough to allow the tissue to be cut through completely prior to advancing the fiber. The fiber should not be visibly bowed as this indicates undue pressure or too deep a placement of the fiber into the wound. These conditions reduce efficiency and increase the likelihood of fiber breakage.
Sapphire tips and sculpted fibers should be used in a similar fashion to the method suggested for bare fibers. However, the tip or probe should be oriented more obliquely or tangential to the line of incision. This optimizes the coagulative effect of the laser and facilitates the dissection.
LASER INJURY
We have discussed the various wavelengths available for use during minimally invasive procedures and have considered some of the techniques to prevent injury. The surgeon would do well to understand and implement safety procedures as recommended by the ANSI Standard (ANSI Z136.3, 2005) and other appropriate regulatory bodies.
Implementation of these guidelines will prevent injury to patients and personnel.
The primary risk of injury during laparoscopy is to the patient’s intraabdominal and pelvic structures due to the closed nature of the surgery and the proximity of adjacent structures. Several methods have been developed to minimize the risk of potential injuries due to reflection of energy off of surgical instruments. These methods are designed to scatter the beam or absorb the incident laser energy. It should be remembered that the CO2 laser wavelength is color independent. Therefore, ebonized surfaces are not helpful in this case. Instruments should have brushed beaded or sand-blasted surfaces.
Titanium is preferable as a back stop material. The use of ebonized surfaces is helpful in the case of visible light and near infrared lasers. However, the surgeon must remember that these instruments will become hot as they are absorbing the laser’s energy.
Therefore, inadvertent contact with adjacent structures must be carefully avoided to prevent secondary burns.
The surgeon should orient the laser fiber and beam such that the possibility of past- pointing is avoided. This too can result in damage to nearby structures, particularly if backstops are not in use during the dissection. Accidental injuries from inadvertent activation of the laser foot pedal can also occur. These potential problems are best avoided by placing only a single foot pedal in the surgeon’s proximity and by placing the laser in stand-by mode when it is not in use.
The bowel and bladder should always be checked for perforation injuries or potential burns, particularly after extensive dissections or vaporizational procedures. Such a practice is prudent irrespective of the technology that has been used during the conduct of the case. Several strategies have been used including filling the area with irrigation fluid, insufflating the bowel with air, and/or the instillation of Betadine, methylene blue, indigo carmine, or other dyes and observing the tissue for any leaks or staining. Leaks or suspected areas of injury should be oversewn or closed using good surgical technique.
Any site of stray burn or contact with a heated instrument should be inspected carefully and should be handled as if it were a frank perforation. This is particularly important when using the Nd: YAG laser, because the degree of damage is grossly underestimated by visualization of the surface.
As should be the case with any minimally invasive procedure, the patient that has symptoms beyond those expected for the procedure, or the patient with ileus or “doing poorly” postoperatively should be suspected of having an iatrogenic injury and should be managed accordingly.
SUMMARY
This chapter has discussed the tissue effects and delivery systems available for laser utilization during minimally invasive surgical procedures. These versatile devices have many justifiable uses during surgery. The surgeon should have a thorough understanding of the procedure to be performed as well as the laser device, its delivery systems, and safety considerations. Practice and continued use of these devices will lead to improved outcomes.
References
- American National Standard for Safe Use of Lasers in Medical Facilities (ANSI Z136.3-2005). Orlando: The Laser Institute of America; 2005: 82.
- Bakri YN, Sundin T, Mansi M. Ureteral injury secondary to laparoscopic CO2 laser. Acta Obstet Gynecol Scand. 1994;73:665-667.
- Carroll B, Chandra M, Papaioannou T, et al. Biliary lithotripsy as an adjunct to laparoscopic common bile duct stone extraction. Surg Endosc. 1993;74:356-359.
- Corbitt JD JR. Laparoscopic cholecystectomy: Laser versus electrocautery. Surg Laparosc Endosc. 1991;1(4):268-269.
- Crowgey SR, Adamson GD. Endoscopic energy: laser. In: Adamson GD, Martin DC, eds. Endoscopic Management of Gynecologic Disease. Philadelphia: Lippincott-Raven Publishers; 1996:27-41.
- Diamond MP, Daniell JF, Feste J, et al. Initial report of the carbon dioxide laser laparoscopy study group: complications. J Gynecol Surg. 1989;5:269-272.
- Grainger DA, Soderstrom RM, Schiff SF, Glickman MD, DeCherney AH, Diamond MP. Ureteral injuries at laparoscopy: insights into diagnosis management, and prevention. Obstet Gynecol. 1990;75-839-843.
- Hersman MJ, Rosin RD. Laparoscopic laser cholecystectomy: our first 200 patients. Ann R Coll Surg Engl. 1992;74(4):242-247.
- Hinshaw JR, Daykhovsky L, Glantz G, et al. Current controversies in laparoscopic cholecystectomy: A roundtable discussion. J Laparoendosc Surg. 1990;1(1):17-29.
- Hulka JF, Reich H. Textbook of Laparoscopy. 3rd ed. Philadelphia: WB Saunders Co.; 1998: 548.
- Hunter JG. Exposure, dissection, and laser versus electrosurgery in laparoscopic cholecystectomy. Am J Surg. 1993;165(4):492-496.
- Kim AK, Adamson GH. Laparoscopic laser injury. In: Kavic MS, Levinson CJ, Wetter PA, eds. Prevention and Management of Laparoscopic Surgical Complications. Miami: Society of Laparoendoscopic Surgeons; 1999:21-28.
- Kollmorgan TA, Malek RA, Barrett DM. Laser prostatectomy: two and a half years’ experience with aggressive multifocal therapy. Urology. 1996;48:217-222.
- Kuntzman TA, Malek RA, Barrett DM. High-power potassium titanyl phosphate laser vaporization prostatectomy. Mayo Clin Proc. 1998;73:798-801.
- Lanzafame RJ. Applications of lasers in laparoscopic cholecystectomy. J Laparoendosc Surg. 1990;1(1):33-36.
- Lanzafame RJ. Applications of laser in laparoscopic cholecystectomy: technical considerations and future directions. SPIE. 1991;1421:189-196.
- Lanzafame RJ, Brien T, Rogers DW, et al. Comparison of sapphire contact scalpels for surgery. Lasers Surg Med. 1990;2(Suppl):9.
- Lanzafame RJ. Laser utilization in minimally invasive surgery: applications and pitfalls. In: Lanzafame RJ (ed). Prevention and Management of Complications in Minimally Invasive Surgery. New York: Igaku-Shoin;1996:30-42.
- Laycock WS, Hunter JG. Electrosurgery and laser application. In: MacFayden BV, Ponsky JL, eds. Operative Laparoscopy and Thoracoscopy. Philadelphia: Lippincott- Raven; 1996: 79-91.
- Malek PA, Kuntzman RS, Barrett DM. High power potassium-titanyl phosphate laser vaporization prostatectomy. J Urol. 2000;163:1730-1733.
- Norderlon BM, Hobday KA, Hunter JG. Laser vs. electrosurgery in laparoscopic cholecystectomy. A prospective randomized trial. Arch Surg. 1993;128(2):33-36.
- Reddick EJ, Olsen DO. Laparoscopic laser cholecystectomy. A comparison with mini-lap cholecystectomy. Surg Endosc. 1989;3(3):131-133.
- Reddick EJ, Baird D. Daniel J, et al. Laparoscopic laser cholecystectomy. Ann Chir Gynaecol. 1990;79(4):189-191.
- Reddick EJ, Olsen D, Alexander W, et al. Laparoscopic laser cholecystectomy and choledocholithiasis. Surg Endosc. 1990;4(3):133-134.
- Schultz LS, Hickok DF, Garber JN, et al. The use of lasers in general surgery. A preliminary assessment. Minn Med. 1987;70(8):439-442.
- Schwartz RO. Complications of laparoscopic hysterectomy. Obstet Gynecol. 1993; 81:1022-1024.
- Smith EB. Complications of laparoscopic cholecystectomy. J Natl Med Assoc. 1992;84(10):880-882.
- Soderstrom RM. Bowel injury litigation after laparoscopy. J Am Assoc Gynecol Laparosc. 1993;1:74-77.
- Spaw AT, Reddick EJ, Olsen DO. Laparoscopic laser cholecystectomy: Analysis of 500 procedures. Surg Laparosc Endosc. 1991;1(1):2-7.
- Steger AC, Moore KM, Hira N. Contact laser or conventional cholecystectomy: a controlled trial. Br J Surg. 1988;75(3):223-225.
- Unger SW, Edelman DS, Scott JS, et al. Laparoscopic treatment of acute cholecystitis. Surg Laparosc Endosc. 1991;1(1):14-16.
- Williams IM, Lewis DK, Shandall AA, et al. Laparoscopic cholecystectomy: laser or electrosurgery. J R Coll Surg Edinb. 1994;39(6):348-349.
- Keye WR. KTP and argon laser laparoscopy. Obstet Gynecol Clin N Am. 1991;18(3): 605-611.
- Jones KD, Sutton CJD. Recurrence of chocolate cysts after laparoscopic ablation. J Am Assoc Gynecol Laparosc. 2002;9(3): 315-320.
- Lane GE, Lathrop JC. Comparison of results of KTP/532 laser versus monopolar electrosurgical dissection in laparoscopic cholecystectomy. J Laparoendosc Surg. 1993;3(3):209-214.
- Daniell JF, Miller W. Polycystic ovaries treated by laparoscopic laser vaporization. Fertil Steril. 1989;51(2):232-236.
- Nezhat C. History of Laparoscopy. 2009;ch22, ch23. Available at: sls.org
- Mage G, Canis M, Pouly JL, Manhes H, Wattiez A, Bruhat MA. CO2 Laserlaparoscopy: a ten year experience. Eur J Obstet Gynecol Reprod Biol. 1988;28(2):120- 123.
- Nezhat C, Crowgy SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy and videolaseroscopy. Contrib Gynecol Obstet. 1987;16:303-312.
- Donnez J. CO2 laser laparoscopy in infertile women with endometriosis and womenwith adnexal adhesions. Fertil Steril. 48(3):390-4, 1987.
- Tulandi T. Adhesion reformation after reproductive surgery with and without the carbon dioxide laser. Fertil Steril. 1987;47(4):704-706.
- Martin DC. CO2 laser laparoscopy for endometriosis associated with infertility. J Reprod Med. 1986;31(12):1089-1094.
- Daniell JF, Herbert CM. A new second-puncture probe for CO2 laser laparoscopy. J Reprod Med. 1985;30(2):89-92.
- Bruhat MA, Mage G. The use of CO2 laser in tubal surgery. [French]. Nouvelle Presse Medicale. 1980;9(1):47-48.
- Tadir Y, Kaplan I, Zuckerman Z, Edelstein T, Ovadia J. New instrumentation and technique for laparoscopic carbon dioxide laser operations: a preliminary report. Obstet Gynecol. 1984;63(4):582-585.
- Riedel HH, Semm K. An initial comparison of coagulation techniques of sterilization. J Reprod Med 1982;27(5):261-267.
- Bruhat MA, Mage G, Jacquetin B, Pouly JL, Ropert J. Carbon dioxide laser and gynaecological surgery. [French]. J Gynecol Obstet Biol Reprod. 1979;8(8):739-744.
- Diamond MP, Daniell JF, Martin DC, Feste J, Vaughn WK, McLaughlin DS. Tubal patency and pelvic adhesions at early second-look laparoscopy following intraabdominal use of the carbon dioxide laser: initial report of the intraabdominal laser study group. Fertil Steril. 1984;42(5):717-723.
- Kutikov A, Vanarsdalen KN, Gershman B, et al. Enucleation of renal cell carcinoma with ablation of the tumour base. BJU International. 2008;102(6):688-691.
- Honeck P, Wendt-Nordahl G, Bolenz C, et al. Hemostatic properties of four devices for partial nephrectomy: a comparative ex vivo study. J Endourol. 2008;22(5):1071- 1076.
- Brock JW 3rd, Holcomb GW 3rd, Morgan WM 3rd. The use of laparoscopy in the management of the nonpalpable testis. J Laparoendosc Surg. 6 Suppl 1996;1:S35-S39.
- Malek RS, Kuntzman RS, Barrett DM. Photoselective potassium-titanyl-phosphate laser vaporization of the benign obstructive prostate: observations on long-term outcomes. J Urol. 2005;174(4 Pt 1):1344-1348.
- Te AE, Malloy TR, Stein BS, Ulchaker JC, et al. Photoselective vaporization of the prostate for the treatment of benign prostatic hyperplasia: 12-month results from the first United States multicenter prospective trial. J Urol. 2004;172(4 Pt 1):1404-1408.
- Afshar K, McLorie G, Papanikolaou F, et al. Outcome of small residual stone fragments following shock wave lithotripsy in children. J Urol. 2004;172(4 Pt 2):1600- 1603.
- Hai MA, Malek RS. Photoselective vaporization of the prostate: initial experience with a new 80 W KTP laser for the treatment of benign prostatic hyperplasia. J Endourol. 2003;17(2):93-96.
- Lee J, Gianduzzo TR. Advances in laser technology in urology. Urol Clin N Amer. 2009;36(2):189-198.
- Beldzinski P, Dzierzanowski J, Sloniewski P. Minimally invasive surgical techniques for the treatment of lumbar disc disease. Ortoped Traumatol Rehabil. 2004;6(3):308-313.
- von Jako RA, Cselik Z. Percutaneous laser discectomy guided with stereotactic computer-assisted surgical navigation. Lasers Surg Med. 2009;41(1):42-51.
- Noonan DP, Elson DS, Mylonas GP, Darzi A, Yang GZ. Laser-induced fluorescence and reflected white light imaging for robot-assisted MIS. IEEE Transactions on Biomed Eng. 2009;56(3):889-892.
- Reijnen MM, Disselhoff BC, Zeebregts CJ. Varicose vein surgery and endovenous laser therapy. Surg Tech Internatl. 2007;16:167-174.
- Anonymous. Holmium:YAG surgical lasers. Health Devices. 1995;24(3):92-122.
