Complications of Laparoscopic and Robotic Radical Prostatectomy
Jennifer K. Yates M.D., Ihor S. Sawczuk, M.D., and Ravi Munver, M.D.
INTRODUCTION
Laparoscopic radical prostatectomy (LRP) was initially described in 1997 by Schuessler and colleagues.1 Since this initial report, the application of minimally-invasive radical prostatectomy has expanded dramatically. Several technical modifications have been introduced and the procedure is currently described in detail, including both transperitoneal and extraperitoneal approaches. While offering decreased blood loss and postoperative pain as compared to the traditional open radical retropubic prostatectomy (RRP), LRP entails a steep learning curve with long operative times during the surgeon’s early experience. Pelvic laparoscopic surgery is considered challenging due to the limited working space. Unlike other laparoscopic extirpative procedures such as radical nephrectomy, laparoscopic prostatectomy is both an extirpative procedure as well as a reconstructive procedure. The need for complex suturing capability and delicate dissection for neurovascular bundle preservation add additional challenges to this procedure. These challenges of LRP created an inviting environment for the introduction of a robotic platform to facilitate laparoscopic prostate surgery.
Robot-assisted laparoscopic radical prostatectomy (RALP) was first described in 2000 by Abbou et al.2 Modification of the technique can be credited to Menon and colleagues, who have had a dramatic impact in its increasing application and acceptance in the United States.3 In its early years, RALP was only performed by a few surgeons at high volume centers. In less than a decade, the procedure is currently offered at the majority of major academic medical centers as well as in numerous smaller community hospitals.
Over the past few years, RALP has surpassed LRP and RRP in terms of total number of procedures performed annually in the United States. In Europe however, LRP remains as the more common treatment modality as compared to RALP.
The introduction and adaptation of new techniques is associated with a learning curve and inherent risks in mastering the procedure. This statement applies to both LRP and RALP. There is a clear association between complication rates and number of cases performed.4,5 Similarly, Novara et al. noted the rate of Clavien grade 3 and 4 complications to be associated with the prior number of RALP cases performed by the surgeon.6 Complications of LRP and RALP are similar in nature, with the exception of robotic equipment failure, which is an additional potential complication that is associated with RALP.
This chapter reviews the complications of LRP and RALP, including incidence, detection, prevention, and management. Studies comparing complication rates in LRP, RALP, and RRP are also reviewed, however, descriptions of complications in urologic literature are not uniform, with few authors utilizing a standardized reporting system such as the Clavien classification system.7 The Clavien classification system of surgical complications was first used to describe complications in the cholecystectomy literature, and has been adapted to other specialties as well. One of the challenges in describing complications focuses on the subjective nature of reporting of procedure specific complications. Another methodological problem in reporting complications relates to the learning curve. Some authors exclude reporting their initial complications which they attribute to their learning curve, while other authors include all complications, thus resulting in a discrepancy in complication rates between studies. These variances prevent an accurate assessment and comparison of surgical complications and argue in favor of establishing a standardized system for defining and describing urologic surgical complications.
INCIDENCE RATE OF COMPLICATIONS
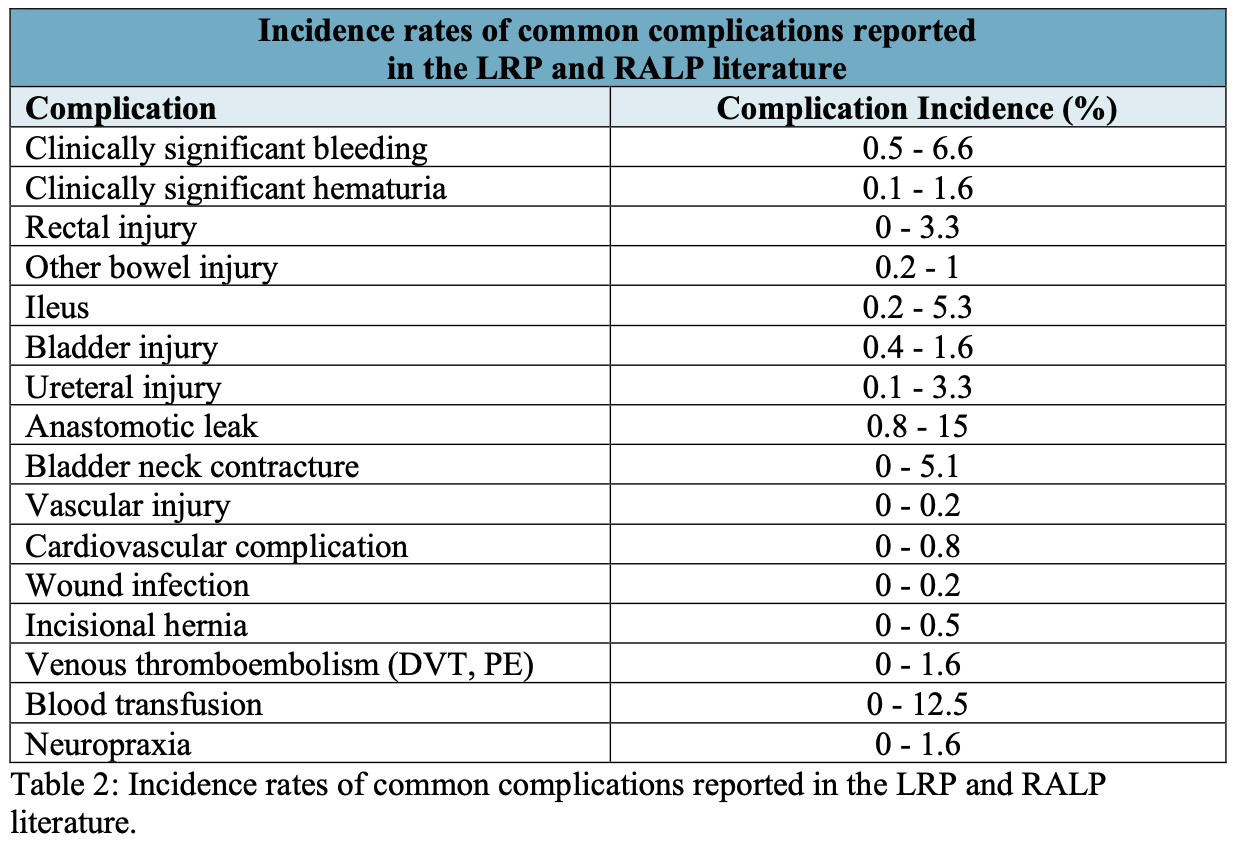
The absence of standardization in reporting surgical complications for laparoscopic and robot-assisted prostatectomy has resulted in a wide variation in the types of complications reported as well as in the overall incidence of complications. A comprehensive review of the literature of large LRP and RALP series was performed to summarize overall complication rates (Tables 1 and 2). The most common complications included perioperative bleeding or blood transfusion, bladder neck contracture, and anastomotic leakage.6,8-10
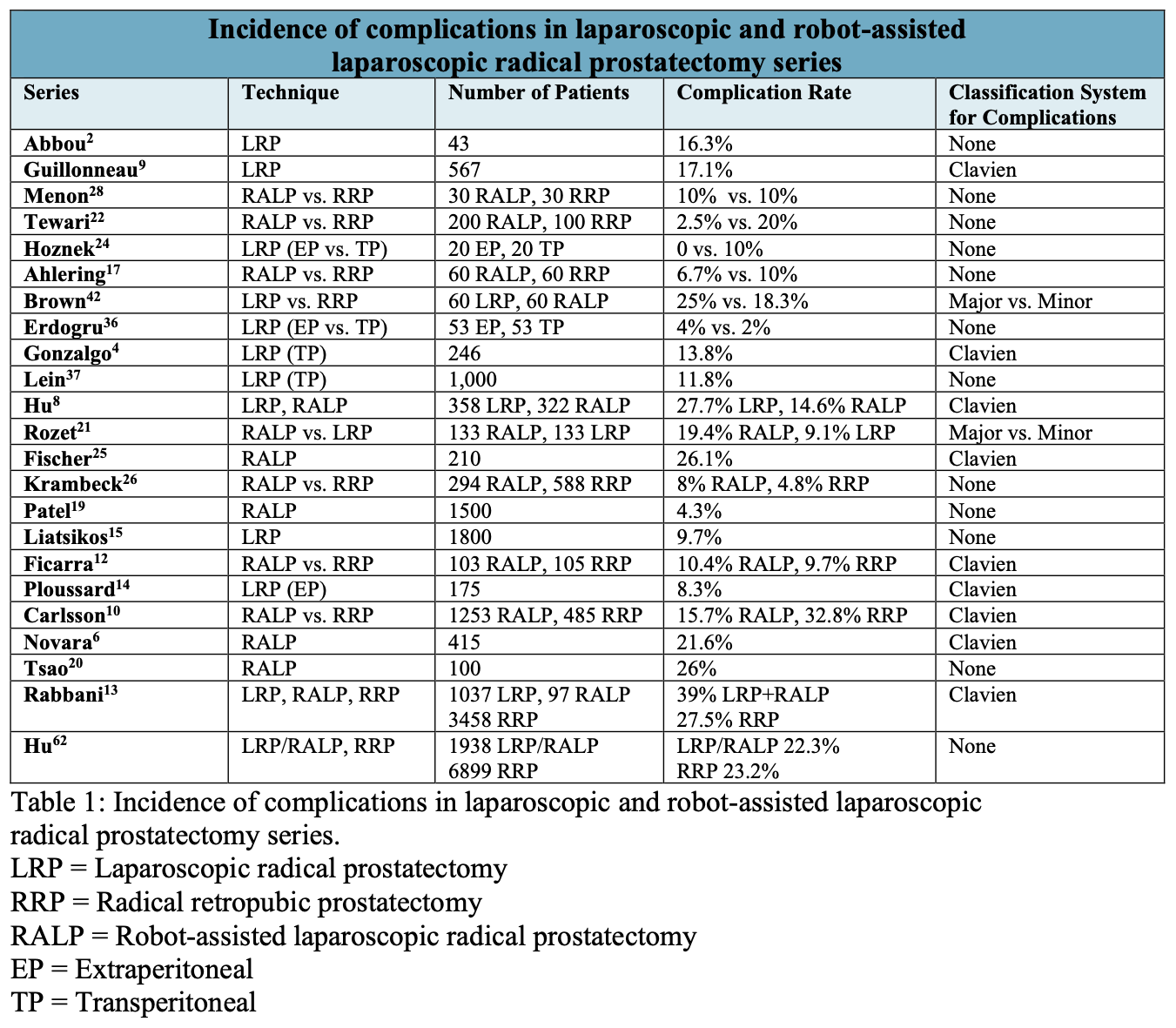
The overall complication rate for LRP ranged from 0 to 25%, while the complication rate for RALP ranged from 2.5 to 26%. It is surprising to note that a history of prior abdominal surgery or prostate radiation did not appear to affect the complication rate in one study.11 Novara et al. found that perioperative complications were more common in patients with larger prostates and in procedures performed early in a surgeon’s experience.6 Patient comorbidities and clinical characteristics of the malignancy did not affect the overall rate of complications.
Several authors have begun to utilize the Clavien classification system to describe complications associated with minimally-invasive prostatectomy.4,6,8-10,12-14 Complication rates in these specific series range from 8.3% to 39%. The usefulness of the Clavien classification system was illustrated by Carlsson et al., who compared the complication rates for RRP and RALP.10 With a standardized classification system, valid comparisons are made possible. The authors found that the rate of higher-grade complications (Clavien IIIb-V) was increased with RRP (12.9%) as compared to a rate of 3.7% for RALP. Similarly, Rabbani et al utilized the Clavien system, and categorized grades I and II as ‘minor’ and grades III through V as ‘major’ complications.13 These definitions of major and minor complications can be contrasted to other studies that used these terms in an arbitrary manner without specifically defining features of each category.
ACCESS-RELATED COMPLICATIONS
Obtaining access to the intraperitoneal or extraperitoneal space for LRP or RALP is associated with specific risks based on the type of trocars used, location of access, patient anatomy, and prior abdominal surgery. In LRP or RALP, initial access is typically in the periumbilical location and is based on surgeon preference.
Bleeding related to trocar placement is the most common access-related complication. The majority of bleeding resulting from trocar placement is due to injury of small subcutaneous veins. Most of these injuries are immediately recognized upon trocar insertion or removal, and are easily controlled with the use of electrocautery. Less frequently, larger intraabdomial or pelvic vessels can be injured during laparoscopic access. Careful attention to proper laparoscopic technique can help prevent these injuries.
The most serious bleeding that can result in significant blood loss is the result of arterial injury. In one series, epigastric artery injury occurred in 3 (0.8%) of LRP cases.8 In two cases the artery was suture-repaired with placement of an extracorporeal bolster, and in one instance extension of the skin incision was required in order to obtain hemostasis. In another LRP series, the incidence of epigastric artery injury was 0.5%, of which none were detected intraoperatively.9 One patient required reoperation as a result of continued bleeding. The risk of epigastric artery injury can be reduced by remaining cognizant of the course of the vessel and by placing a fine introducer needle at the anticipated trocar location with aspirating prior to trocar placement.15 In addition, laparoscopic inspection of the abdominal wall during individual trocar insertion can serve to identify bleeding from this vessel. In order to avoid missing an epigastric arterial injury, the insufflation pressure should be reduced at the end of the procedure, and the trocars removed under direct vision to inspect for bleeding.9 In addition to suture ligation, other techniques to control inferior epigastric bleeding include using hemoclips or electrocautery. Liatsikos et al. was able to control 14 cases of inferior epigastric bleeding during extraperitoneal LRP with clips, cautery, or suture ligation of the artery.15
Bowel injury is a less common, but potential complication during laparoscopic access. Injuries are more likely to occur during transperitoneal rather than extraperitoneal access. The closed technique to access the peritoneum with a veress needle is also associated with a higher chance of injury as compared to the open Hassan technique. Use of an optical bladeless trocar can aid in safer access to the peritoneal cavity under direct vision. In patients with prior abdominal surgery, adhesions may be present, and an open access technique is advocated to minimize the chance of injury. Alternate sites for initial access may also be considered based on the type of prior surgery and location of the surgical incision. Once access is obtained, additional trocars should be placed under vision. Non- bladed fascial dilating obturators are available for laparoscopic and robotic trocars, and can be used in conjunction with careful technique to decrease the occurrence of bowel injury.
Laparoscopic access can be performed safely in the obese patient, though optimal trocar positioning requires careful planning and experience. In one series of RALP in overweight and obese patients, there was no difference in open conversion rates as a result of access issues.16 The authors noted that the trocars were generally placed further from the pubic symphysis than typical in order to achieve an appropriate angle to access the pelvis. If the instruments are unable to access the prostate apex and urethra, the insufflation pressure can be decreased to 10 mmHg and the trocars advanced.
CONVERSION TO AN OPEN PROCEDURE
Converting from a minimally invasive approach to an open procedure, in and of itself, should not be considered a complication. Instead, conversion is more likely performed in the setting of a complication. The reported rate of conversion ranges from 0% to 1.9%, although many studies exclude reporting conversions, and thus the rate may be higher.4,9,17-18 Gonzalgo reported four conversions from LRP to RRP, all of which occurred early in the series.4 Two of these four cases were due to complications including a bladder injury and a common iliac vein injury. The remaining two conversions were due to refractory hypercarbia and failure to progress through the procedure. Bhayani et al. performed a multi-institutional review of LRP conversions and noted that 46% occurred during the surgeons’ initial five cases.18 Conversion was most common during the apical and posterior prostate dissection. Twenty-six percent of the conversions occurred in the setting of a complication, including two rectal injuries, one bladder injury, and one ureteral injury. The remainder of the conversions were due to failure to progress, hypercarbia, and concerns regarding inadequate oncologic control.
In summary, conversion from minimally invasive prostatectomy to open RRP should not be reported as a complication. In the appropriate setting, judicious decision-making and open conversion can actually prevent a complication. In order to minimize the likelihood for open conversion, Bhayani et al recommend avoiding patients with a history of prior abdominal or pelvic surgery, patients that received androgen deprivation therapy, morbidly obese patients, and patients with large prostates, early in a surgeon’s experience.18
BLEEDING AND HEMORRHAGE
As addressed previously, bleeding can result from vascular injury upon obtaining access to the peritoneal or extraperitoneal space. Additional bleeding sites include the dorsal venous complex, accessory pudendal arteries, prostate pedicles, neurovascular bundles, external iliac vessels, and pelvic sidewall. The classification of bleeding as a complication of minimally-invasive prostatectomy varies by study. According to the Clavien classification of complications, bleeding requiring blood transfusion is considered a Grade II complication. The incidence of bleeding and blood transfusion ranges from 0% to 15% in the minimally-invasive prostatectomy literature.15,19-24
The inferior epigastric arteries can be injured at the time of trocar placement and is addressed in the section on access-related complications. In one series, postoperative bleeding occurred in 19 patients. In 11 patients bleeding was related to inferior epigastric artery injury, while in the remaining 8 patients, bleeding occurred from the neurovascular bundles.15 In this same series, one patient developed significant gross hematuria, requiring endoscopic clot evacuation and fulguration of a bleeding site at the bladder neck.
Several series have compared the incidence of bleeding between RRP, LRP, and RALP. Rozet et al compared patients undergoing extraperitoneal RALP and extraperitoneal LRP and found the transfusion rate to be higher for the RALP group, with rates of 9.8% and 3%, respectively.21 However, other series report lower transfusion rates with RALP.8,25 Rassweiler et al. reviewed the literature and compared outcomes of RRP and LRP.23 They found transfusion rates and blood loss to be lower for LRP in most series. Similarly, the transfusion rate was higher in RRP patients in a series comparing this procedure to RALP.26
The diagnosis and management of inferior epigastric arterial bleeding and external iliac vessel bleeding is addressed in more depth in other sections of this chapter. Bleeding from the accessory pudendal vessels and the pelvic sidewall can be avoided by careful attention to pelvic anatomy and minimizing instrument movements outside the field of view. Oftentimes, accessory pudendal vessels can be preserved by lateral mobilization of the vessels toward the pelvic sidewall. Judicious application of surgical clips and bipolar cautery can generally control excessive bleeding from these sites. Bleeding from the dorsal venous complex can be controlled with the placement of additional sutures. Other measures to control bleeding include the use of bipolar cautery, clips, or vascular stapling devices. The prostatic vascular pedicles are generally controlled with clips. In the event that the clips dislodge, significant hemorrhage can be encountered. Hemostatic control can be established by replacement of clips, suture ligation, or use of bipolar electrocautery. Bleeding from the neurovascular tissues can be addressed with surgical clips, as cautery use in this area must be minimized to avoid thermal energy spread that can potentially damage the nerurovascular bundles.
Management of postoperative bleeding is dependent on the site and degree of bleeding. On occasion, the source of bleeding cannot be identified. Guillonneau et al reported that the cause of bleeding was not identified in two of five patients that underwent postoperative re-exploration for bleeding.27 The chance of postoperative bleeding can be minimized by meticulously inspecting the surgical field at the completion of the procedure with the pneumoretroperitoneum pressure lowered to 5 mmHg. This maneuver can potentially reveal a bleeding source that may have been temporarily tamponaded by the intraabdominal pressure associated with the pneumoperitoneum.
Patients with clinical evidence of persistent postoperative bleeding that is not responsive to blood transfusion may require re-exploration. There is limited data in the literature that favors the approach to re-exploration, whether laparoscopic or open. Recently, reports on laparoscopic exploration, hematoma evacuation, and hemostasis have proven successful, obviating the need for a large open incision. Hoznek et al. described one case of inferior epigastric arterial bleeding on the second postoperative day that was managed laparoscopically.24 Liatsikos et al reported a 1.1% rate of bleeding, with 13 patients undergoing laparoscopic exploration and 6 patients undergoing open exploration.15 Guillonneau et al. attempted laparoscopic re-exploration for bleeding in five patients.9 Laparoscopic control of the bleeding was successful in one patient, but in four patients the procedure was converted to an open approach due to difficulty in evacuating the coagulated blood with laparoscopic instruments. In conclusion, it appears reasonable to consider a laparoscopic approach for re-exploration, with conversion to an open procedure as necessary.
RECTAL INJURY
The incidence of rectal injury ranges from 0 to 2.5% in the LRP and RALP literature.4,8,9,19,28 One series compared the incidence of rectal injury in their LRP and RALP experiences and found the incidence to be higher in the LRP group at 2.5%, with no rectal injuries in the RARP group.8 Another series comparing RRP and RALP found the incidence to be slightly lower in the RALP group at 0.15% compared to 1.6% in the RRP group.10
Based on the close proximity of the rectum to the prostate, rectal injury can occur at any point during the posterior dissection, but is most likely near the apex of the prostate.15 Careful and meticulous dissection of the posterior prostate apex can serve to minimize the incidence of rectal injury. If encountered, small rectal injuries without significant contamination of fecal material can be repaired laparoscopically with or without robotic assistance. Preoperative oral mechanical bowel preparation and a cleansing enema can minimize the potential for contamination. Initially, the surgical field can be copiously irrigated with saline or an antibiotic irrigant. An intrarectal bougie can serve to identify the edges of the defect, and a two or three-layer closure is performed with absorbable sutures for the rectal mucosa and serosa and absorbable or nonabsorbable sutures for the perirectal tissues. The integrity of the repair is evaluated by immersing the repair with irrigant and injecting air into the rectum to evaluate for air bubbles that would indicate persistence of a defect. Omental interposition can be performed to potentially reduce the rate of rectourethral fistula formation. Extra attention should be applied to the urethrovesical anastomosis in order to prevent an anastomotic leak that can increase the chances of development of a rectourethral fistula. A surgical drain is placed in the space of Retzius. Patients are managed with perioperative antibiotics until Foley catheter removal and a low-residue diet for 3-5 days.
Guillonneau reported 8 rectal injuries in his series of LRPs, of which 7 occurred during the apical dissection.9 Most rectal injuries reported in the literature were identified intraoperatively.8,9 Rectal injury is more common in the early portion of a surgeon’s learning curve. In the experience of Patel and colleagues, the incidence of rectal injury was 0.13%, with two rectal injuries occurring in the first 25 cases.19 Failure to recognize injury to the rectum can lead to significant morbidity. In a LRP series reported by Hu et al., rectal injury was recognized intraoperatively and successfully repaired in 7 patients.9 In an additional 3 patients in whom rectal injury was unrecognized, all patients developed rectourethral fistulas postoperatively. In a series comparing RALP and RRP, Carlsson et al. reported that rectal injury was not identified intraoperatively in one patient each group.10 Both patients required temporary colostomy two days postoperatively with delayed closure. In most cases, immediate recognition of a rectal injury can result in primary repair, which can potentially avoid the complication of rectourethral fistula and obviate the need for bowel diversion.
In a review of the Montsouris experience, which included 1000 LRP cases, the management and outcomes of 13 patients with rectal injury was described.27 Of these, 11 (85%) injuries were identified and repaired intraoperatively. Injury occurred in 10 of the 11 patients during dissection of the prostate apex at its posterior aspect, with one injury occurring during wide excision at the lateral aspect of the prostate. All 11 injuries were repaired primarily without open conversion or bowel diversion. Nine patients had an uncomplicated postoperative course. Two patients developed fever and abdominal pain on the third and fourth postoperative days and underwent exploratory laparotomy. One rectal injury was repaired primarily and the other required a diverting colostomy. Of the two rectal injuries that were missed intraoperatively, the diagnosis was made on the basis of peritonitis a few days postoperatively. Both patients underwent diverting colostomy without closure of the rectal injury, of which one ultimately developed a rectourethral fistula that was subsequently repaired.
Guillonneau et al. reported a complication rate of 18.2% in patients in whom the rectal injury was immediately diagnosed and repaired.27 Of interest, the authors found that the two complications occurred in patients in which a one-layer closure was performed, and thus advocate a multi-layer closure. In twelve of the thirteen patients with rectal injury, a non-nerve-sparing procedure was performed. The authors propose that the higher rate of rectal injury may be due to a plane of dissection that is closer to the rectum and recommend utilizing an ‘intrarectal’ device such as a bougie to assist in identifying the rectum in order to prevent injury.
Another series of LRP described an 8% incidence of rectal injury during 110 extraperitoneal laparoscopic radical prostatectomies.29 Seven of the 9 rectal injuries occurred in the first 50 patients, further suggesting the impact of the learning curve on rectal injury. Six of the 9 injuries (66%) were diagnosed and repaired intraoperatively, however three patients still developed a rectourethral fistula. Of the 3 patients without intraoperative detection of the rectal injury, one presented with Foley catheter balloon protruding from the rectum, and another presented with pneumaturia, and the third patient developed peritonitis requiring exploratory laparotomy. At the time of exploration a rectal injury was not detected and the patient developed a rectourethral fistula 6 weeks postoperatively. The site of injury in 6 of the patients was the prostate apex and may have been related to the authors’ use of monopolar scissors to incise the rectourethralis. Three of the injuries occurred during division of the prostate pedicles using a bipolar vessel- sealing device. This series underscores the importance of identifying a rectal injury intraoperatively to reduce patient morbidity.
Patients undergoing salvage prostatectomy after radiation failure are considered at higher-risk for rectal injury based on the RRP literature. A small series of 4 patients undergoing salvage RALP experienced no intraoperative or postoperative complications.30 A larger series of 18 patients undergoing RALP after radiation therapy reported no rectal injuries.31 Additional studies are necessary to determine whether RALP or LRP offer advantages over traditional RRP in the post-radiation setting.
Renal transplant patients were reported to have a higher rate of rectal injury in a series of 9 patients undergoing extraperitoneal LRP.32 The authors compared patients with and without a history of renal transplant undergoing LRP at their institution and found the overall incidence of rectal injury to be 1.8% as compared to 22.2% (n=2) in renal transplant patients. The injuries in the renal transplant group occurred during dissection of the neurovascular bundles and were both identified intraoperatively. One of the renal transplant patients developed a postoperative rectourethral fistula. This population of patients represents a challenging cohort in the setting of rectourethral fistula, as their immunosuppressed status can increase the risk of infectious complications. Close collaboration with the organ transplant team is warranted when treating these patients.
In summary, rectal injury during LRP or RALP may be more likely during the learning curve. Intraoperative identification with meticulous closure is critical to avoid additional morbidity. While there is not a standardized technique for repair, a multi layer closure, omental interposition, and a watertight urethrovesical anastomosis may decrease the risk of failure and subsequent fistula formation.
OTHER BOWEL INJURY
Injury to other segments of bowel can occur during laparoscopic access and is addressed in the access-related complications section. Bowel injuries are more likely to occur during transperitoneal procedures, however they can occur in extraperitoneal procedures as well, in cases where the bowel is adherent to the peritoneum and more susceptible to injury. During the prostatectomy procedure, bowel injury can be minimized by insertion of laparoscopic or robotic instruments under direct vision. Instrument maneuvers that occur outside of the operative field of view are more prone to causing this complication. Surgeons must be cognizant that a suction-irrigation device can conduct an electrical current if it comes into contact with an instrument that utilizes monopolar energy. Ifthere is concern for a bowel injury intraoperatively, general surgical consultation should be considered.
Guillonneau et al. reported a 0.93% incidence of bowel injury during LRP. The sigmoid colon was injured in one patient and the ileum injured in two patients.9 None of the injuries were identified intraoperatively. The sigmoid injury presented as peritonitis 3 days postoperatively and required temporary colostomy. Both of the patients with ileal injuries presented with peritonitis, with one patient undergoing primary repair of the injury and the other patient receiving a temporary ileostomy.
In another series of LRP and RALP, 3 injuries were unrecognized intraoperatively.8 One patient had undergone concurrent LRP and lysis of adhesions, and presented with an enterocutaneous fistula on the third postoperative day. One patient had an ileal injury presenting with fascial dehiscence 10 days after RALP. The third patient had a colonic injury requiring exploratory laparotomy with cecectomy and ileostomy on the tenth postoperative day. The authors note that all injuries were associated with ileus as the initial finding. Interestingly, these patients did not present with the classic findings of focal port site pain, leucopenia, or diarrhea, which has previously been reported in patients with bowel injury following laparoscopic surgery.33
Intraabdominal adhesions can form in patients who have undergone prior transperitoneal abdominal surgery, thus potentially increasing the risk of bowel injury. Prior abdominal surgery can increase the likelihood of conversion to an open procedure.34 A thorough history should be obtained from the patient to ascertain the details of prior surgeries. The patient’s abdomen should be examined and the location of the prior incisions noted. The risk of adhesions appears greater in patients with a midline incision, a greater number of prior abdominal surgeries, and the presence of bleeding or intraabdominal infection at the time of the initial surgery.35 In this group of patients, prevention of bowel injury requires judicious planning of the access site. For instance, placing the initial trocar away from a prior midline incision may be considered a safer maneuver. An open technique for trocar placement rather than the use of a Veress needle can help identify underlying adhesions. Once access is obtained, the peritoneal cavity should be carefully inspected for adhesions and for bowel injury. Adhesiolysis can be performed as necessary using standard laparoscopic instruments or robotic instruments. Electrocautery use should be minimized during adhesiolysis to avoid inadvertent bowel injury from transmitted energy. The projected course of the laparoscopic or robotic instruments should be assessed and further adhesiolysis performed to avoid injury from passage of instruments. Once adhesiolysis is complete, the remainder of the procedure can be safely performed. Before removing the trocars at the completion of the procedure, the bowel should be inspected again to evaluate for injury.
PROLONGED ILEUS
The definition of prolonged ileus varies among studies, and in certain studies the duration of delayed bowel function is not disclosed. The incidence of ileus in the LRP and RALP literature ranges from 0 to 5%. In one LRP series utilizing the Clavien system, the incidence of ileus was 3.3%.4 In this series, ileus was the most common Grade II complication and the most common overall complication. Prolonged ileus after minimally-invasive prostatectomy can have multiple etiologies. Minimal mobilization of the bowel from the pelvis has been noted to cause a temporary ileus, while more extensive bowel mobilization and adhesiolysis increases the risk. In addition, anesthesia, postoperative narcotic analgesic medications, anticholinergic medication, and decreased mobility may contribute to delayed return of bowel function.
Prolonged ileus can also be related to urinary extravasation from the urethrovesical anastomosis. In a comparison of transperitoneal and extraperitoneal LRP, prolonged ileus was associated with urinary extravasation in the transperitoneal LRP group alone.36 Hu reported the incidence of ileus “delaying discharge” in 5.4% of LRP patients and 2.8% of RALP patients.8 Nine of the 19 LRP patients and 4 of the 9 RALP patients with prolonged ileus were found to have concurrent urinary anastomotic leaks, supporting the link between urinary extravasation and ileus. Postoperative bleeding may also be associated with development of postoperative ileus, as two of the 19 LRP patients in the series had clinically significant postoperative bleeding.
Prevention of a prolonged ileus can be approached intraoperatively and postoperatively. Handling of the bowel should be minimized when possible, and at the end of the procedure any blood or urine that has accumulated should be evacuated from the abdominal cavity and pelvis. Postoperatively, patients should be encouraged to begin ambulating as soon as is feasible. Narcotics and anticholinergics can play a role in a prolonged ileus and should be minimized. Management of the postoperative ileus includes limiting diet, intravenous fluid support, and nasogastric tube placement in some cases. While the course of a prolonged ileus is unpredictable, failure to resolve in several days should prompt the surgeon to pursue additional imaging studies to evaluate for other etiologies.
BLADDER AND URETERAL INJURY
Injury to the urinary bladder during LRP and RALP is relatively infrequent, occurring in 0.4-1.4% of patients.9,15,39 Bladder injury is most likely to occur during development of the retroperitoneal space and initial mobilization of the bladder. Failure to identify the correct plane of dissection can result in an injury to the bladder depending on the approach, whether via an anterior or posterior approach. This is often recognized early in the operative course due to the development of urinary ascites. Bladder injury can also occur during the bladder neck dissection, specifically while dissecting the posterior bladder neck. Injury to the posterior wall of the bladder can occur if an incorrect plane is entered while searching for the vas deferens and seminal vesicles.
Liatsikos et al. reviewed their series of extraperitoneal LRP and found that all three bladder injuries in their series were in the setting of prior inguinal hernia repair with mesh. In a transperitoneal series, bladder injury occurred in 1.38% of patients, with all injuries occurring during dissection of the retroperitoneal space.9 Bladder injuries that occur during initial mobilization of the bladder can be avoided by remaining in the relatively avascular plane between the bladder and the anterior abdominal wall. Returning to the midline to regain access to this fibroareolar tissue can help reorient the surgeon to the correct plane of dissection. Injuries that occur during the posterior bladder neck dissection can be avoided by remaining cognizant of the bladder wall thickness. A relatively thin posterior bladder neck as compared to the remainder of the bladder neck usually indicates that an incorrect plane has been entered and increases the risks of injury to the posterior bladder wall and ureters. Bladder injuries are usually noted intraoperatively due to the development of urinary extravasation. These injuries should be repaired with a multiple layer closure using absorbable suture. If the posterior wall of the bladder is thinned and injured, the ureteral orifices should be identified to exclude a concomitant ureteral injury. Administration of intravenous indigo carmine can aid in identification of the ureteral orifices if they are not readily visible. Foley catheter drainage to allow healing is a standard protocol for any radical prostatectomy and applies to bladder injury as well.
Ureteral injury is an uncommon complication, although is more likely to occur in the setting of a large prostate, prior history of prostatitis, a large median lobe, prior prostate procedures, and extended pelvic lymph node dissection. Ureteral injury can occur both to the intravesical and extravesical ureter. Intravesical injury occurs during dissection of the posterior bladder neck. Aberrant anatomy or proximal dissection of the bladder neck can result in injury to the ureteral orifices. Extravesical ureteral injury can occur during lateral dissection of the peritoneal reflection to develop the extraperitoneal space. In addition, the ureter can be injured as it can be mistaken for the vas deferens. Less commonly, the ureter can be injured during pelvic lymph node dissection (PLND).
Guillonneau et al. reported a ureteral injury rate of 0.7% during LRP.9 In one patient, the ureter was sectioned during posterior dissection of the vesiculo-deferential junction and was immediately repaired. In two patients, injury occurred during lateral dissection of the peritoneum and was discovered later in the postoperative course due to urinary ascites.
Treatment included placement of a ureteral stent in one patient and ureteral reimplantation in the second patient. In another patient, injury occurred in the setting of the patient having undergone prior transurethral resection of the prostate. Ureteral obstruction was caused by close proximity of the anastomosis to the ureteral orifice. The patient underwent laparoscopic revision of the anastomosis with no long-term sequelae. Hu et al also reported successful laparoscopic ureteral reimplantation after intraoperative injury in two patients.8 Postoperative edema may occasionally cause ureteral obstruction if the ureteral orifices are in close proximity to the anastomosis. Gonzalgo reported one such case, which was treated with percutaneous nephrostomy placement.4 Cystoscopy had demonstrated the ureteral orifice was very close to the urethrovesical anastomosis.
After the postoperative edema subsided the percutaneous nephrostomy tube was removed.
Teber reviewed the minimally invasive prostatectomy literature for ureteral injury and also reported their institution’s experience.38 During the study period, 3 ureteral injuries occurred during LRP performed by 3 different surgeons. Not surprisingly, all three injuries occurred during the initial 100 cases in these surgeons’ experiences. Two injuries occurred during posterior dissection of the bladder neck and seminal vesicles, and one occurred during an extended pelvic lymph node dissection. All three injuries were identified and repaired at the time of prostatectomy. Two of the injuries were complete transections and were repaired via a laparoscopic Lich-Gregoir reimplant technique.
Minimal mobilization was necessary to gain adequate ureteral length. The third injury was a partial transaction and was repaired with interrupted sutures. There was no evidence of reflux or hydronephrosis at one-year follow-up in these patients. Robot- assisted ureteral reimplantation has similarly been described and can be performed in patients undergoing RALP.39
Prevention of ureteral injury is best accomplished by carefully identifying anatomic landmarks. To avoid confusing the ureter with the vas deferens, the vas deferens can be traced along its course from the lateral peritoneal reflection to the ampulla and seminal vesicles. Some authors recommend placement of ureteral catheters for patients at risk for ureteral injury.4 El Douaihy et al. described a technique of double pigtail stent placement at the time of anastomosis construction during RALP.41 These authors describe passing a guidewire and stent through an assistant trocar and utilize robotic assistance to position the stents. They have utilized this technique in 30 patients with large median lobes, and note that this maneuver adds approximately ten minutes to the procedure. In addition, preoperative or intraoperative cystoscopic examination can be utilized to delineate the bladder neck and ureteral orifice anatomy in patients with large median lobes or prior prostate procedures.
COMPLICATIONS OF THE ANASTOMOSIS
Anastomotic complications include prolonged urinary leakage, bladder neck contracture, and clip migration. Prolonged urinary leakage as a complication is inadequately reported in the literature, and defined variably as prolonged drain output, urinary extravasation on a postoperative cystogram, or elevated surgical drain creatinine level. The incidence of prolonged urinary leakage ranges from 0.67 to 10%.8,9,15,19-20,26,42
Several factors may be associated with an increased incidence of postoperative anastomotic leakage, although these factors are not validated in large series. Jaffe et al. found the overall complication rate to be higher in patients who had undergone prior transurethral resection of the prostate (TURP) when compared to patients without prior TURP history.43 In comparing minimally-invasive prostatectomy to RRP outcomes, one study found the rate of anastomotic leakage to be higher in the RALP group (9.1%) than the RRP group (2.5%).26 The type of anastomotic reconstruction may also play a role in the incidence of anastomotic leaks. Abbou reported their early series of LRP, including 43 patients in which the authors utilized an interrupted anastomotic suture technique for the first ten patients and a running anastomosis for the last 33 patients.2 Four patients experienced an anastomotic leak that necessitated open revision in three patients and laparoscopic revision in one patient. As a result, the authors changed their anastomotic technique from an interrupted to a running anastomosis.
The symptoms associated with an anastomotic leak can vary based on whether the procedure is performed via a transperitoneal or extraperitoneal approach. The transperitoneal approach allows extravasated urine to contact peritoneal surfaces, which can present as irritation and abdominal pain. In one series comparing transperitoneal and extraperitoneal LRP, one patient in the transperitoneal group experienced a symptomatic leak. The patient was readmitted with abdominal pain that resolved with Foley catheter placement.24 Remzi et al compared patients undergoing extraperitoneal and transperitoneal LRP with patients undergoing RRP.44 They found that urinary leakage was associated with increased postoperative pain in all three groups, but to a greater degree in the transperitoneal LRP group. There was only one case of a prolonged ileus in the cohort, occurring in a transperitoneal LRP patient with urinary leakage after Foley removal.
Prevention of urinary leaks is best accomplished by creating a watertight anastomosis. Once the anastomosis is complete, the sutures should be carefully tightened as the posterior bladder neck anastomotic suture can loosen and create a gap. The urethral tissue should be handled gently as crush injury can lead to tissue ischemia and anastomotic breakdown. Meticulous hemostasis is also important as a pelvic hematoma can disrupt a previously intact anastomosis.
Management of an anastomotic leak varies based on several factors, including time since surgery, degree of leakage, and patient symptomatology. Many urine leaks will resolve with maintenance of the Foley catheter and surgical drain. Some authors advocate the use of a running anastomotic suture rather than an interrupted technique.20 One series describes a more aggressive approach to anastomotic leakage. Liatsikos et al describes a 0.67% incidence of ‘major’ leaks and 1.8% incidence of ‘minor’ leaks in their extraperitoneal LRP experience.15 They recommend intervention with laparoscopic revision of the anastomosis if the drain output exceeds the Foley catheter output for greater than 48 hours. In their series, three patients that underwent revision of the anastomosis had an unremarkable postoperative course.
In another series of 57 patients, the rate of anastomotic leakage was 10%.9 Forty-six of these patients experienced primary anastomotic leakage, defined as persistent urine in the surgical drain for six or more days after surgery. Of these patients, 43 demonstrated spontaneous resolution with prolonged catheterization for a mean of 12 days and concurrent use of a surgical drain. One patient required laparoscopic revision of the anastomosis on the eighth postoperative day for persistent high-volume leakage, and 2 patients required percutaneous aspiration. In 11 cases of secondary leakage, defined as leakage after the Foley was removed, all resolved with additional Foley catheter drainage.
For a persistent anastomotic leak, one group described the use of a nephroureteral stent to divert the urine away from the anastomosis thereby allowing it to heal appropriately.45 In this patient, a postoperative cystogram demonstrated extravasation of contrast from the anastomosis. A percutaneous drain was replaced to drain the urinary ascites, though the majority of the urine continued to drain from the drain site rather than the Foley. A right nephroureteral stent was placed and remained to intermittent suction. After 48 hours, the urinary extravasation had resolved, the stent was capped, and subsequently removed.
Another anastomotic complication reported in the minimally invasive prostatectomy literature involved hemoclip migration. In an effort to minimize the use of thermal energy for nerve-sparing procedures, the placement of titanium, and more commonly polymer clips, on the prostatic pedicles and perineural tissues is utilized. Mora et al. described a patient who voided a calcified polymer clip five weeks after LRP.46 During the procedure, three polymer clips had been placed adjacent to the right neurovascular bundle and a running water-tight anastomosis had been performed. Banks et al. described a similar clinical scenario, but one in which the patient developed urinary urgency 10 months postoperatively.47 A computed tomography scan indicated a 1-centimeter calcification at the bladder neck, and cystoscopic examination revealed a stone surrounding the clip in this location. The stone and clip were successfully extracted cystoscopically without event. Gonzalgo et al. reported erosion of polymer clips in two patients (0.8%) requiring cystoscopic removal.4 There are no clearly identifiable risk factors for clip migration. Judicious placement of clips away from the anastomosis may help to decrease the incidence of this complication.
The incidence of bladder neck contracture (BNC) is not well defined in many studies, as follow-up is relatively short. In those series reporting BNC, the incidence ranges from 0.13% to 3%.4,8,10,19-20,37 One series compared the incidence of BNC in RRP and RALP and found the rate to be higher in the RRP group at 4.6% and 1.2%, respectively.26
Another study found similar results, with rates in RALP and RRP of 0.2% and 4.5%, respectively.10 Hu et al. compared LRP and RALP and found the incidence of BNC to be higher in the LRP group, with rates of 1.4% and 0.3%, respectively.8 Msezane et al reviewed over 400 RALP cases at their institution and found the rate of BNC to be 1.1%.48 The mean time to presentation after surgery was 4.8 months, with a range from 3 to 12 months. The only factor associated with development of BNC was a significantly longer operative time when compared to patients without BNC. The low rate of BNC in RALP compared to RRP may be attributed to less tissue handing with robotic instruments, better visualization, and more widespread use of a running anastomosis.
Bladder neck contracture incidence can be minimized by preserving healthy urethral and bladder tissue. The anastomosis should be approached as a plastic reconstructive procedure, with gentle handling of the tissues. The surgeon may be unaware of the force applied to the tissues by robotic instruments due to lack of tactile feedback, and therefore direct grasping of the urethra should be avoided. During transection of the urethra and division of the periurethral tissues, thermal energy should be minimized to preserve the blood supply to the tissues. As surgical clip migration can be a factor in the development of BNC, judicious use of the clips away from the anastomosis is important. The management of the BNC is similar to that for RRP patients, with transurethral dilation or incision of the fibrotic tissue.
ROBOTIC SYSTEM FAILURE
Device failure is a complication that relates primarily to the robotic approach to minimally invasive prostatectomy. The most comprehensive review of device failures at a single institution is provided by Zorn et al.9 The authors review over 700 cases at their institution, which utilized one robotic unit during the study period. Four cases (0.5%) were aborted due to system failure, with all aborted cases occurring before the patient entered the operating suite. Errors included one failure to power-up the system and three cases of optical malfunction. There were three other cases of robotic platform-related errors causing a surgeon handicap. These included loss of 3-dimensional vision and robotic arm failure during the anastomosis. In these three cases the surgeon was able to complete the procedure without conversion to an open procedure.
The authors reviewed the literature of robotic platform failures, including a 2% delayed start due to software failure and 0.5% conversion rate to conventional LRP due to a mechanical failure reported by Eichel et al.50 In another series, a 4.6% case abortion rate was reported by Kozlowski during the first 130 RALP’s at their institution, of which four of the six cases aborted were done so prior to the induction of anesthesia.51 Lavery et al. performed a multi-institutional study and found a unrecoverable failure rate of 0.4%.52 Of the 34 failures, 24 of the procedures were cancelled before the procedure was initiated, two cases were converted to pure LRP, and eight procedures were converted to RRP. Based on their experience and reviewing the literature, Zorn and colleagues advocate for a hospital policy that mandates the robotic system be calibrated and fully functional prior to the induction of anesthesia.
In the case of a robotic unit malfunction, an experienced team that is able to assess and rectify the problem is invaluable. Prevention of equipment failure can be accomplished by booting and checking the system before the patient enters the surgical suite. Error alerts should be addressed immediately. Duplicate instruments should be available in the event that an instrument expires or fails. In institutions with more than one robot, the malfunctioning robot can be replaced, with careful undocking of all robotic arms before shutting down the system or moving the equipment. In the event of unrecoverable equipment failure during the procedure, the surgical team may elect to convert to either a pure laparoscopic or open approach. If the dorsal venous complex and bladder neck have not been divided, the option exists to end the procedure and reattempt at a later date after the device has been repaired. Most importantly, knowledgeable operating room staff that is able to troubleshoot is critical to a robotic program’s success.
PELVIC LYMPH NODE DISSECTION-RELATED COMPLICATIONS
The close proximity of the pelvic lymph nodes to the external iliac vessels and the obturator nerve places these structures at risk for injury. In addition, the ureter can be injured during extended pelvic lymph node dissection (PLND) as mentioned previously. One series reported an obturator nerve transection during LRP and PLND.8 The nerve sheath was primarily reapproximated and the patient had full return of adductor function at one month postoperatively. Safi et al. reported the transection of a tortuous external iliac artery during LRP and PLND.53 Bleeding from the transected arterial ends was reasonably controlled, thus allowing an end-to-end anastomosis to be performed laparoscopically without long-term sequelae.
Injury to vascular structures and nerves can be avoided by remaining cognizant of the pelvic anatomy. Major structures should be clearly identified before placement of surgical clips or delivery of thermal energy. In the event of vascular injury, pure laparoscopic technique or robotic assistance can be used to suture the vessel of interest. Laparoscopic vascular clamps should be available to obtain control of the ends of the vessel to optimize visualization. For bleeding sites that are not amenable to being sutured, cautious application of thermal energy, clips, or hemostatic agents can be suitable alternatives.
Few studies report the incidence of lymphoceles in the LRP and RALP literature. This may be due to the relatively low incidence or perhaps the means by which the diagnosis is established. When computed tomography (CT) imaging is utilized, the reported incidence can be as high as 61% in RRP patients and 37% in LRP patients.54 Upon measuring the lymphocele size, RRP patients had a 27% incidence of large lymphoceles compared to 8% in LRP patients. Clinically significant lymphoceles were noted in 2.3% of RRP patients and in none of the LRP patients. Another comparison of robotic and open PLND found similar rates of clinically significant lymphoceles, including 2% of RALP patients and 2.5% of RRP patients.55 In two large series of transperitoneal LRP and RALP patients, the incidence of lymphocele was 0.15% and 0.13% respectively.9,19 In a series of extraperitoneal LRP, the incidence of clinically significant lymphoceles was 2.5%.
In cases of transperitoneal prostatectomy, lymphatic fluid that would otherwise form a lymphocele can be absorbed by the peritoneal lining. Lymphoceles can be prevented by careful control of lymphatic channels with cautery, clips, or suture. It may be more difficult to identify lymphatic leaks during laparoscopic PLND due to the intraabdominal pressure exerted by the pneumoperitoneum. Surgical clips and judicious use of thermal energy are usually sufficient in securing small lymphatic channels to prevent this complication.
Management of a lymphocele depends on the symptoms and overall presentation. Incidental lymphoceles may be detected on imaging studies performed for another indication. These lymphoceles may be conservatively managed due to the risk of introducing infection. Symptomatic lymphoceles causing symptoms such as abdominal or pelvic pain, lower extremity swelling, nausea, or other related complaints, should be drained. Lower extremity swelling must be regarded with caution, as these patients are at risk for lower extremity deep vein thrombosis. Percutaneous drainage can be performed by a radiologist under image guidance. The drain should be left in place until the output decreases significantly, at which point reimaging can be performed. The drain can be removed if the imaging study shows resolution of the fluid collection. Some lymphoceles may require multiple drainage attempts, in which case a sclerosing agent may be used in non-infected lymphoceles. Refractory non-infected lymphoceles can be treated with laparoscopic unroofing, with the peritoneal cavity serving as a natural absorptive surface to prevent fluid reaccumulation. Infected lymphoceles can be drained percutaneously, however a sclerosing agent is contraindicated. Refractory lymphoceles in the setting of infection should be treated with open drainage.
ANESTHESI-RELATED COMPLICATIONS
Similar to other laparoscopic procedures, minimally invasive prostatectomy carries risks of cardiovascular, pulmonary, and other anesthetic-related risks. Unique to LRP and RALP are the risks associated with patient positioning in the steep Trendelenburg position. Care must be taken to protect all pressure points and prevent excessive tension to avoid neuromuscular injury. The lower extremities are placed in stirrups during RALP and must be carefully positioned to avoid excessive flexion and abduction of the hips.
A unique complication reported by Hoda et al in the LRP literature is three separate incidences of cardiac arrest.56 The authors reviewed their extraperitoneal LRP database and found this complication to occur in 0.7 of 100 patients. All three of the patients were free of significant major comorbidities preoperatively. Bradycardia was noted during inflation of the balloon trocar which progressed to cardiac arrest. Patients were resuscitated successfully and the remainder of their postoperative course was unremarkable. The authors attribute this complication to a vagal reaction that was caused by compression of the intraabdominal great vessels.
Judicious screening of patients for minimally invasive prostatectomy may help minimize anesthesia-related complications. To prevent injury related to positioning, the surgeon and anesthesiologist should jointly participate in the positioning of the patient. The arms should be tucked by the patient’s side with the hands in a neutral position. Padding can be used at all pressure points. Placement in the steep Trendelenburg position can be tested before commencing with the procedure to ensure that the position is tolerated by the patient.
Despite adequate preoperative preparation and planning, some of adverse anesthetic events remain unpredictable. An anesthesia team that is experienced in minimally- invasive surgery is beneficial, and communication between the surgeon and the anesthesiologist cannot be overemphasized.
WOUND-RELATED COMPLICATIONS
Wound infections and incisional hernias can occur after any laparoscopic or open surgery. The incidence of wound infection for LRP and RALP ranges from 0.26% to 1.2%, while the incidence of incisional hernias ranges from 0.2% to 3%. Wound infection may be less common with minimally-invasive prostatectomy compared to RRP. Carlsson et al. compared the incidence of wound infection in RRP and RALP patients, and found the incidence to be higher in the RRP group at 5.9% versus 0.2% of RALP patients experiencing this complication.10
Technical and host factors can contribute to the development of trocar site hernias, including trocar size and type, tissue ischemia, trocar site (notably a higher risk associated with an umbilical location), extension of the trocar site for specimen extraction, infection, diabetes, and obesity. It is recommended that bladed trocar sites 10 mm or greater in size be closed to decrease the incidence of incisional hernias, though hernias have been reported at 5 mm and 8 mm trocar sites as well.57 Bladeless radially expanding trocars were introduced in an attempt to obviate the need to close the trocar sites while minimizing the incidence of hernias. Many surgeons do not attempt closure of bladeless trocar sites. However, a series of laparoscopic urologic oncology procedures performed at a single institution over a three-year period suggested that the incidence of incisional hernias associated with the use of bladeless trocars was higher than previously thought.58 In this series, which included 441 patients undergoing RALP and 141 patients undergoing LRP, the incidence of trocar site hernias after the use of a bladeless 12 mm trocar was 0.66%. Four of the seven patients with hernias presented with intrafascial hernias, in which intraabdominal contents herniated between the transversalis and internal oblique fascia levels, with the external oblique fascia remaining intact. This study suggests that incisional closure may be warranted when using 12 mm bladeless radially expanding trocars.
To minimize the incidence of incisional hernias, the fascia of the periumbilical extraction incision should be securely approximated in a running or interrupted fashion. While the other trocar sites are not typically closed, the fascia should be assessed to verify the absence of a significant defect. Postoperatively, patients should be maintained on abowel regimen that avoids excessive straining that could compromise the fascial closure. In addition, patients should be advised to avoid strenuous activity for several weeks.
Wound infection may be less common in minimally invasive prostatectomy as compared to open RRP due to the presence of smaller incisions and less manipulation of the subcutaneous tissues. In order to minimize the risk of infection, the American Urologic Association (AUA) guidelines recommend that patients receive a dose of perioperative antibiotics prior to surgical incision. The periumbilical extraction should be irrigated and closed in layers in order to eliminate spaces that may be prone to develop infection.
Sterile technique in the application of dressings should be observed as with any laparoscopic surgery.
VENOUS THROMBOEMBOLIS
The incidence of venous thromboembolism (VTE) in the minimally invasive prostatectomy literature ranges from 0 to 1%. Venous thromboembolism events include deep vein thrombosis (DVT) and pulmonary embolism (PE), which are separate and diagnosable entities. A number of risk factors have been identified that may contribute to post-prostatectomy VTE. Guillonneau et al reported a DVT incidence of 0.3% and noted that in each instance, the patients required bed rest in the setting of another complication.9 In perhaps the largest review of VTE incidence associated with minimally-invasive prostatectomy, Secin et al compiled data from multiple institutions and found the VTE incidence to be 0.5%.59 In a review of the literature, the authors found that the highest risk factors for VTE include tobacco smoking, lengthy operative time, return to the operating suite for re-exploration, and long hospital stay. Finally, minimally invasive prostatectomy may actually carry a lower VTE risk compared to RRP. Carlsson et al noted a VTE rate of 0.1% in patients undergoing RALP versus 1% in the RRP patient population.10
A high index of suspicion is crucial for diagnosing VTE. While prior literature has suggested that calf and superficial DVT rarely progress to PE, Secin et al. found that two of five patients with PE presented with calf or greater saphenous vein DVT. The median time from surgery to diagnosis of DVT was ten days. Patients should be counseled on the importance of ambulation and the risks for developing VTE following discharge from the hospital.
The use of heparin as prophylaxis for VTE is controversial. Secin et al. reported increased intraoperative blood loss, longer hospital stays, and increased transfusion rates in patients receiving preoperative subcutaneous heparin.59 The authors acknowledge the methodological limitations of the multi-institutional study and conclude that the use of heparin may be justified in higher risk patients.
Several precautionary measures may reduce the incidence of VTE. Lower extremity sequential compression devices (SCDs) can be placed prior to the induction of anesthesia and maintained throughout the patient’s hospital stay except during ambulation. Early ambulation should be emphasized a protocol on a designated surgical floor can assist in achieving this goal. Selective use of heparin or enoxaparin should be based on a patient’s risk of VTE and surgeon preference. While in the hospital, patients should be monitored for signs and symptoms of VTE, including calf swelling or pain, unexplained tachycardia, and oxygen desaturation. These findings should be aggressively evaluated with the use of Doppler ultrasound or computed tomography imaging of the chest as appropriate. Upon discharge, patients should be instructed on daily ambulation and on identifying signs or symptoms concerning for VTE.
MINIMALLY INVASIVE PROSTATECTOMY IN THE OBESE PATIENT
Several studies have addressed complication rates in the obese patient with a body mass index (BMI) of 30 kg/m2 or greater. Ahlering et al. reported a significantly increased complication rate of 26.3% in obese patients undergoing RALP compared to 4.9% in nonobese patients.60 The complications in obese patients were also more severe, including VTE events and anastomotic disruptions. Mikhail et al studied both overweight (BMI 25-30 kg/m2) and obese patients, and found no difference in complication or conversion rates.16 However, obese patients had a greater estimated blood loss compared to nonobese patients. Finally, Khaira et al. found complication rates of 13.6% and 20%, respectively, in nonobese and obese patients undergoing RALP, though this difference was not statistically significant.61 The complication rate between obese and nonobese patients in the first 100 cases did not differ in their experience.
For obese patients that are offered minimally invasive prostatectomy, precautions should be observed to minimize the risk of complications. Patient positioning is similar to that in nonobese patients, however modifications may need to be made to accommodate the patient. For instance, tucking the arms may be difficult due to the operating table width, in which case carefully positioned arm boards may be needed. During LRP and RALP, the steep Trendelenburg position may impede respiratory effort to a greater degree than in nonobese patients, and communication with the anesthesia team is important. Longer trocars should be available, and careful attention to the placement of the trocars is essential for the instruments to comfortably access the pelvis.
CONCLUSION
Minimally invasive radical prostatectomy has become a well-accepted treatment modality for localized prostate cancer. After transcending the learning curve, LRP and RALP may potentially be associated with lower complication rates as compared to RRP. As demonstrated in this chapter, it is often difficult to critically analyze complication data in the absence of a standardized classification system of surgical complications. As more data with longer follow-up is available, the complication profile of LRP and RALP will be further elucidated and allow more meaningful comparisons to the RRP literature.
References
- Schuessler WW, Schulam PG, Clayman RV, et al. Laparoscopic radical prostatectomy: Initial short-term experience. Urology. 1997; 50:854-857.
- Abbou CC, Salomon L, Hoznek A, et al. Laparoscopic radical prostatectomy: Preliminary results. Urology. 2000; 55:630-634.
- Tewari A, Peabody J, Sarle R, et al. Technique of da Vinci robot-assisted anatomic radical prostatectomy. Urology. 2002; 60: 569-572.
- Gonzalgo ML, Pavlovich CP, Trock BJ, et al. Classification and trends of perioperative morbidities following laparoscopic radical prostatectomy. J Urol. 2005; 174:135-139.
- Frede T, Erdogru T, Zukosky D, et al. Comparison of training modalities for performing laparoscopic radical prostatectomy: Experience with 1,000 patients. J Urol. 2005; 174:673-678.
- Novara G, Ficarra V, D’Elia C, et al. Prospective evaluation with standardized criteria for postoperative complications after robotic-assisted laparoscopic radical prostatectomy. Eur Urol. 2009;epub ahead of print.
- Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery, 1992; 111:518.
- Hu JC, Nelson RA, Wilson TG, et al. Perioperative complications of laparoscopic robotic assisted laparoscopic radical prostatectomy. J Urol. 2006; 175:541-546.
- Guillonneau B, Rozet F, Cathelineau X, et al. Perioperative complications of laparoscopic radical prostatectomy: The Montsouris 3-year experience. J Urol 2002; 167:51-56.
- Carlsson S, Nilsson AE, Schumacher MC, et al. Surgery-related complications in 1253 robot-assisted and 485 open retropubic radical prostatectomies at the Karolinska University Hospital, Sweden. Urology. 2009; epub.
- Martin AD, Desai PJ, Nunez RN, et al. Does a history of previous surgery or radiation to the prostate affect outcomes of robot-assisted radical prostatectomy? BJU Int. 2008; 103:1696-8.
- Ficarra V, Novara G, Artibani W, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: A systematic review and cumulative analysis of comparative studies. Eur Urol. 2009; 55: 1037-1063.
- Rabbani F, Yunis LH, Pinochet R, et al. Comprehensive standardized report of complications of retropubic and laparoscopic radical prostatectomy. Eur Urol. 2009; epub ahead of print.
- Ploussard G, Xylinas E, Salomon L, et al. Robot-assisted extraperitoneal laparoscopic radical prostatectomy: experience in a high-volume laparoscopy reference centre. BJU Int. 2009; epub ahead of print.
- Liatsikos E, Rabenalt R, Burchardt M, et al. Prevention and management of perioperative complications in laparoscopic and endoscopic radical prostatectomy. World J Urol. 2008; 26:571-80.
- Mikhail AA, Stockton BR, Orvieto MA, et al. Robotic-assisted laparoscopic prostatectomy in overweight and obese patients. Urology. 2006;67:774-779
- Ahlering TE, Woo D, Eichel L, et al. Robot-assisted versus open radical prostatectomy: A comparison of one surgeon’s outcomes. Urology. 2004; 63:819-22.
- Bhayani SB, Pavlovich CP, Strup SE, et al. Laparoscopic radical prostatectomy: A multi-institutional study of conversion to open surgery. Urology. 2004; 63:99-102.
- Patel V, Palmer KJ, Coughlin G, et al. Robot-assisted laparoscopic radical prostatectomy: Perioperative outcomes of 1500 cases. J Endourol. 2008; 22:2299-2305.
- Tsao AK, Smaldone MD, Averch TD, et al. Robot-assisted laparoscopic prostatectomy: The first 100 patients – Improving patient safety and outcomes. J Endourol. 2009; 23:481-484.
- Rozet F, Jaffe J, Braud G, et al. A direct comparison of robotic assisted versus pure laparoscopic radical prostatectomy: A single institution experience. J Urol. 2007; 178:478-82.
- Tewari A, Srivasatava A, Menon M. A prospective comparison of radical retropubic and robot-assisted prostatectomy: experience in one institution. BJU Int. 2003; 92:205- 210.
- Rassweiler J, Hruza M, Teber D, et al. Laparoscopic and robotic assisted radical prostatectomy – critical analysis of the results. European Urology. 2006; 49:612-24.
- Hoznek A, Antiphon P, Borkowski T, et al. Assessment of surgical technique and perioperative morbidity associated with extraperitoneal versus transperitoneal laparoscopic radical prostatectomy. Urology. 2003; 61:617-622.
- Fischer B, Engel N, Fehr J-L, et al. Complications of robotic assisted radical prostatectomy. World J Urol. 2008; 26: 595-602.
- Krambeck AE, DiMarco DS, Rangel LJ, et al. Radical prostatectomy for prostatic adenocarcinoma: A matched comparison of open retropubic and robot-assisted techniques. BJU Int. 2008; 103:448-453.
- Guillonneau B, Gupta R, Fettouh HE, et al. Laparoscopic management of rectal injury during laparoscopic radical prostatectomy. J Urol. 2008; 169:1694-96.
- Menon M, Tewari A, Baize B, et al. Prospective comparison of radical retropubic prostatectomy and robot-assisted anatomic prostatectomy: The Vattikuti Urology Institute experience. Urology. 2002; 60:864-868.
- Castillo OA, Bodden E, Vitagliano G. Management of rectal injury during laparoscopic radical prostatectomy. Int Braz J Urol. 2006; 32(4):428-433.
- Kaouk JH, Hafron J, Goel R, et al. Robotic salvage retropubic prostatectomy after radiation/brachytherapy: initial results. BJU Int. 2008; 93-96.
- Eandi JA, Link BA, Nelson RA, et al. Robotic assisted laparoscopic salvage prostatectomy for radiation resistant prostate cancer. J Urol. 2010; 183:133-137.
- Robert G, Elkentaoui H, Pasticier G, et al. Laparoscopic radical prostatectomy in renal transplant patients. Urology. 2009; 74:683-687.
- Bishoff JT, Allaf M, Kirkels W, et al. Laparoscopic bowel injury: Incidence and clinical presentation. J Urol. 1999; 161:887-890.
- Richstone L, Seideman C, Baldinger L, et al. Conversion during laparoscopic surgery: Frequency, indications, and risk factors. J Urol. 2008; 180:855-859.
- Van Goor H. Consequences and complications of peritoneal adhesions. Colorectal Dis. 2007; 2:25-34.
- Erdogru T, Teber D, Frede T, et al. Comparison of transperitoneal and extraperitoneal laparoscopic radical prostatectomy using match-pair analysis. Eur Urol. 2004; 46:312-320.
- Lein M, Stibane I, Mansour R, et al. Complications, urinary continence, and oncologic outcome of 1000 laparoscopic transperitoneal radical prostatectomies – Experience at the Charite Hospital Berlin, Campus Mitte. European Urology. 2006; 50:1278-1284.
- Teber D, Gozen AS, Cresswell J, et al. Prevention and management of ureteral injuries occurring during laparoscopic radical prostatectomy: the Heilbronn experience and a review of the literature. World J Urol. 2009;27:613-618.
- Singh I, Kader K, Hemal AK. Robotic distal ureterectomy with reimplantation in malignancy: technical nuances. Can J Urol. 2009; 16:4671-4676.
- Guillonneau B and Sulser T; 2005, Laparoscopic radical prostatectomy. In: Moore RP, Bishoff JT, Loening S, Docimo SG (eds) Minimal invasive urologic surgery. Taylor and Francis, New York. 637-651.
- El Douaihy Y, Tan GY, Dorsey PJ, et al. Double-pigtail stenting of the ureters: Technique for securing the ureteral orifices during robot-assisted radical prostatectomy for large median lobes. J Endourol. 2009; 23:1975-1977.
- Brown JA, Garlitz C, Gomella LG, et al. Perioperative morbidity of laparoscopic radical prostatectomy compared with open radical retropubic prostatectomy. Urol Oncol. 2004; 22:102-106.
- Jaffe J, Stakhovsky O, Cathelineau X, et al. Surgical outcomes for men undergoing laparoscopic radical prostatectomy after transurethral resection of the prostate. J Urol. 2007; 178:483-487.
- Remzi M, Klingler HC, Tinzl MV, et al. Morbidity of laparoscopic extraperitoneal versus transperitoneal radical prostatectomy versus open retropubic radical prostatectomy. European Urology. 2005; 48:83-89.
- Shah G, Vogel F, Moinzadeh A. Nephroureteral stent on suction for urethrovesical anastomotic leak after robot-assisted laparoscopic radical prostatectomy. Urology. 2009; 73:1375-6.
- Mora ER, Gali OB, Garin JAL, et al. Intravesical migration and spontaneous expulsion of a hem-o-lok polymer ligating clip after laparoscopic radical prostatectomy. Urology. 2009; epub ahead of print.
- Banks EB, Ramani A, Monga M. Intravesical Weck clip migration after laparoscopic radical prostatectomy. Urology. 2008; 71(2):351.e3-351.e4.
- Msezane LP, Reynolds WS, Gofrit ON, et al. Bladder neck contracture after robot- assisted laparoscopic radical prostatectomy: Evaluation of the incidence, risk factors, and impact on urinary function. J Endourol. 2008; 22:377-383.
- Zorn KC, Gofrit ON, Orvieto MA, et al. Da VinciTM robot error and failure rates: Single institution experience on a single three-arm robot unit of more than 700 consecutive robot-assisted laparoscopic radical prostatectomies. J Endourol. 2007; 21(11):1341-1344.
- Eichel L, Ahlering TE, Clayman RV. Robotics in urologic surgery: Risks and benefits. AUA Update Series. 2005; 24(lesson 13):106-111.
- Kozlowski PM, Porter CR, Corman JM. Mechanical failure rate of DaVinciTM robotic system: Implications for pre-operative patient counseling (Abstract 1159). J Urol. 2006; 175(suppl):s372-s373.
- Lavery HJ, Thaly R, Albala D, et al. Robotic equipment malfunction during robotic prostatectomy: A multi-institutional study. J Endourol. 2008; 22:2165-2168.
- Safi KC, Teber D, Moazen M, et al. Laparoscopic repair of external iliac-artery transaction during laparoscopic radical prostatectomy. J Endourol. 2006; 20(4):237-239.
- Solberg A, Angelsen A, Bergan U, et al. Frequency of lymphoceles after open and laparoscopic pelvic lymph node dissection in patients with prostate cancer. Scand J Urol Nephrol. 2003; 37(3):218-21.
- Zorn KC, Katz MH, Bernstein A, et al. Pelvic lymphadenectomy during robot- assisted radical prostatectomy: Assessing nodal yield, perioperative outcomes, and complications. Urology. 2009; 74:296-302.
- Hoda MR, Friedrichs M, Kummel C, et al. Asystolic cardiac arrest during balloon insufflation for endoscopic extraperitoneal radical prostatectomy. J Endourol. 2009; 23:329-331.
- Tonouchi H, Ohmori Y, Kobayashi M, et al. Trocar site hernia. Arch Surg. 2004; 139:1248-1256.
- Chiong E, Hegarty PK, Davis JW, et al. Port site hernias occurring after the use of bladeless radially expanding trocars. Urology. 2009; epub ahead of print.
- Secin FP, Jiborn T, Bjartell AS, et al. Multi-institutional study of symptomatic deep venous thrombosis and pulmonary embolism in prostate cancer patients undergoing laparoscopic or robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2008; 53:134-145.
- Ahlering TE, Eichel L, Edwards R, et al. Impact of obesity on clinical outcomes in robotic prostatectomy. Urology. 2005; 65:740-744.
- Khaira HS, Bruyere F, O’Malley PJ, et al. Does obesity influence the operative course or complications of robot-assisted laparoscopic prostatectomy? BJU Int. 2006; 98:1275-1278.
- Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive open radical prostatectomy. JAMA. 2009; 302:1557-1564.
