Laparoscopic Physiologic Perturbations: Implications for At-Risk Patients
Michael Dunham, MD, Amy E. Hutchinson, MD, Michael S. Kavic, MD
INTRODUCTION
The use of carbon dioxide (CD) pneumoperitoneum (CDP) in the vast majority of general surgery, gynecological, and urological cases is safe. It is readily available and cost effective. CD gas is rapidly absorbed in blood and peritoneum, colorless, does not support combustion, and generally has few systemic side effects in healthy patients.
Specifically, CD is relatively benign because most of the gas in the blood is bound to terminal amino groups of hemoglobin or converted to bicarbonate. Although a small portion of CD exists as a gas in the blood, pulmonary exhalation is generally effective. The majority of CD combines with water in red blood cells to form carbonic acid that subsequently dissociates into hydrogen and bicarbonate. The hydrogen ion combines with hemoglobin, and bicarbonate diffuses into the plasma. Functionally, there is an extensive buffering capacity of blood and tissue for CD, and there is rapid secretion of CD by the lungs. For these reasons, CD is widely accepted for the establishment and maintenance of pneumoperitoneum during laparoscopic surgery. Many thousands of cases occur worldwide each day with safety and efficacy.
Other agents have been tested for pneumoperitoneum, but to date, appear to be inferior. Nitrogen gas is not very soluble in blood and can cause gas embolism. Nitrous oxide is combustible and has been associated with sudden cardiac arrest. Helium is inert, but very poorly absorbed from the abdominal cavity. Helium, because of its low solubility in blood, has a high risk of embolism. Argon is also poorly soluble in blood and has a high risk of embolism. Argon is slowly absorbed and can remain in a closed space such as the peritoneal cavity for weeks. Oxygen and air while inexpensive, colorless, and easily absorbed, eagerly support combustion and preclude the use of many energy devices during laparoscopic interventions.
Although CDP seems a safe altered physical state, as attested by extensive nationwide and worldwide use, it is a complex, pathophysiologic condition with significant physiologic effects. The physiologic effects of CDP are principally due to raised intraabdominal pressure (IAP) and, possibly, hypercarbia. Of import, CDP induces changes in homeostatic systems that may be deleterious in compromised patients. These changes are typically insignificant in healthy patients; however, they may have severe consequences in those with at-risk or major pre-existing medical conditions. This chapter presents literature evidence describing CDP physiologic perturbations, patients at-risk for perioperative morbidity and mortality, recommended monitoring, and management of intraoperative physiologic instabilities.
CDP PHYSIOLOGIC PERTURBATIONS
Systemic Blood Pressure
Of 51 human investigations providing CDP systemic blood pressure results relative to postanesthesia induction values, 44 demonstrate an increase1-44 and 7 show no increase.45-51 Isolated episodes of hypotension can occur and include gas embolism52,53 and tension pneumothorax.54 The above observations indicate that an increase in systemic blood pressure is likely to occur during CDP. However, hypotension is not common and usually signals serious clinical instability.
Heart Rate
Of 45 human investigations providing CDP heart rate results relative to postanesthesia induction values, 15 demonstrate an increase5-7,10,17,21,25,30,34,38,43,45,49,51,55 and 30 show no increase.1,4,9,11-14,16,23,29,32,35-37,39,40,42,44,46-48,50,51,56-62 Literature descriptions of dysrhythmias40,63,64 and cardiac arrest53,56 exist. The literature suggests that an increase in heart rate is relatively common and that cardiac dysrhythmias or cardiac arrest may occur, but are unlikely.
Cardiac Output
Of 42 human investigations providing CDP cardiac output results relative to postanesthesia induction values, 23 demonstrate a decrease4,5,8,9,13,14,16,25,29,31,32,36,37,42,43,49,50,56,60,62,65-67 and 19 show an increase or no change.1,6,7,10-12,21,23,24,34,38,44,48,58,59,61,68-70 One can conclude that a decrease in cardiac output is likely to occur during CDP.
Stroke Volume
Of 12 human investigations providing CDP stroke volume results relative to postanesthesia induction values, 9 demonstrate a decrease,4,7,8,14,33,43,49,56,69 and 3 show no change.25,42,68 The literature implies that a decrease in stroke volume is likely to occur during CDP.
Ejection Fraction
Of 7 human investigations providing CDP cardiac ejection fraction results relative to postanesthesia induction values, 4 demonstrate a decrease,38,42,49,57 and 3 show no change.3,10,44 The evidence suggests that a decrease in ejection fraction is likely to occur during CDP.
Systemic Vascular Resistance
Of 19 human investigations providing CDP systemic vascular resistance results relative to postanesthesia induction values, 18 demonstrate an increase,1,4,9,11,16,23,25,29,31-33,36,37,41,42,50,59,66 and 1 shows no increase.34 Five human investigations provide CDP left ventricular work results relative to postanesthesia induction values2,3,9,35,42 and demonstrate an increase in left ventricular work. The literature indicates that an increase in systemic vascular resistance usually occurs with CDP, and an increase in left ventricular stroke work is likely.
Cardiac Preload
Of 23 human investigations providing CDP central venous pressure results relative to postanesthesia induction values, 22 demonstrate an increase,1,7,13,14,17,21-23,25,27,30,35-37,42,44,55,59-61,65,71 and 1 demonstrates no increase.45Of 21 human investigations providing CDP pulmonary artery occlusion pressure results relative to postanesthesia induction values, 19 demonstrate an increase1,7,9,12-14,23,24,27,35-37,42,44,44,60,61,65,71 and 2 show no increase.59,70 Of 7 human investigations providing CDP cardiac preload volume results relative to postanesthesia induction values, 3 demonstrate no change,12,34,58 2 show an increase,38,57 and 2 reveal a decrease.67,69 The evidence indicates that an increase in CVP and PAOP usually occurs with CDP. However, cardiac preload volume may increase, decrease, or remain stable during CDP. It is likely that CVP and PAOP values do not reliably indicate cardiac preload volume status.
Pulmonary Vascular Resistance
Of 13 human investigations providing CDP pulmonary artery pressure results relative to postanesthesia induction values, 10 demonstrate an increase,1,7,12-14,23,25,35,42,61 and 3 show no increase.48,70,72 Of 7 human investigations providing CDP pulmonary vascular resistance results relative to postanesthesia induction values, 3 demonstrate an increase,9,16,37 and 4 show no increase.10,23,36,59 The literature suggests that an increase in pulmonary artery pressure is likely to occur with CDP and may be associated with an increase in pulmonary vascular resistance.
Systemic Oxygen Delivery
Of 2 human investigations providing CDP lactate results relative to postanesthesia induction values, 1 demonstrates an increase,37 and 1 shows no increase.73 Of 5 human investigations providing CDP venous oxygen saturation (SvO2) results relative to postanesthesia induction values, 3 demonstrate no change,37,44,59 1 shows a decrease,29and 1 reveals an increase.34 This evidence indicates that an increase in lactate or decrease in SvO2 may occur during CDP, indicating inadequate systemic oxygen delivery.
PaCO2
Forty-four human studies describe PaCO2 monitoring during CDP.1,9,11,12,15,17,18,20,21,23,25,34-37,42,44-49,55,59,66,70,73-89 Forty-five human studies document end-tidal CO2 (PetCO2) monitoring during CDP.1,3,4,6,8,9,11,14,15,17,20,23,25,28,35-37,40,42-44,48,50,55,57,59,66,73-75,79,80,82-84,86,90-97 Of 39 human investigations providing CDP PaCO2 results relative to postanesthesia induction values, 27 demonstrate an increase in PaCO2 with CDP,1,9,15,20,21,23,25,34,35,42,44,46,47,49,55,59,70,73,74,76,77,79,80,84-86,89 and 12 show no change in PaCO2.12,18,36,37,39,45,48,78,80-82,85 Of 29 human investigations providing CDP PetCO2 results relative to postanesthesia induction values, 22 demonstrate an increase in PetCO2 with CDP,3,9,20,23,28,30,43,44,50,55,73,79,83,84,86,90-92,92,94-97 and 7 show no change in PetCO2.1,4,6,40,42,48,57
Nine human investigations provide CD exhalation volume data during CDP, with all reports demonstrating an increase in CD exhalation volume.11,50,75,77,78,89,92,92,98 Of 12 human investigations providing CDP dead space results relative to postanesthesia induction values, 4 demonstrate an increase in dead space with CDP,74,80,87,99 and 8 show no change.11,20,34,75,77,82,86,89
Of 29 human investigations that describe in methodology sections whether there is an increase in minute ventilation volume, 23 indicate an increase in minute ventilation volume,4,6,9,11,14,17,18,20,23,25,36,37,44-46,59,74,75,78,82,90,93,97 but 6 note no increase.39,42,46,49,76,100
Of 15 human investigations providing CDP PaCO2 results relative to postanesthesia induction values after increasing minute ventilation volume, 8 demonstrate an increase with CDP,9,15,20,23,25,44,59,74 and 7 show no change.36,37,45,78,81,82,85 Six human investigations demonstrate an increase in CDP PetCO2 results relative to postanesthesia induction values, despite increasing minute ventilation volume.9,20,23,44,90,97
The following conclusions emanate from the above literature. PaCO2 and PetCO2 monitoring are common during CDP. Hypercarbia, relative to postanesthesia induction, is likely to occur during CDP. The increase in exhaled CD occurring with CDP indicates that the insufflated CD enters systemic and pulmonary circulation. However, effects on intracellular pH and CD partial pressures are uncertain. The finding that increases in pulmonary or cardiac dead space can occur during CDP creates caution that PetCO2 is not always a reliable surrogate for PaCO2. Increasing minute ventilation volume during CDP is common practice. The documentation of hypercarbia despite increases in minute ventilation volume suggests that there is hypoventilation, relative to CD production, or an increase in dead space, or both of these.
pH
Of 19 human investigations providing CDP pH results relative to postanesthesia induction values, 11 demonstrate a decrease in pH,9,20,21,35,46,47,49,70,74,80,85 and 8 show no change.18,36,39,45,48,73,80,85 Five human investigations indicating that minute ventilation volume is increased provide a comparison of pH during CDP relative to postanesthesia induction values.20,36,45,74,85 pH decreases in 2 of the 5 studies, despite increasing minute ventilation volume.20,74 This literature indicates that a decrease in blood pH is likely to occur during CDP, even if there is an increase in minute ventilation volume.
PaO2
Of 21 human investigations providing CDP hypoxemia results relative to postanesthesia induction values, 19 demonstrate no hypoxemia with CDP,1,12,17,34,35,37,39,40,42,44,46,47,59,78,80-82,86,89 and 2 show that hypoxemia develops.11,17 One human investigation measuring pulmonary shunt during CDP finds no change relative to postanesthesia induction.59 According to the literature, isolated cases of hypoxemia from pneumothorax (simple or tension) or gas embolism can occur.54,101,102 The evidence suggests that hypoxemia is unlikely to transpire during CDP; however, isolated episodes occur and can be clinically detrimental.
Pulmonary Pressure and Volume
Twenty-one human1,3,9,12,28,30,35,39,44-46,59,75,76,83,90,97,100,103-105 and 5 animal106-110 investigations document increases in airway pressures with CDP. Of 20 human investigations providing CDP lung compliance results relative to postanesthesia induction values, 19 demonstrate a decrease in lung compliance with CDP,17,19,28,39,46,50,74,76,79,86,91,99,100,104,105,111-114 and one shows no change.58 Two human investigations document CDP functional residual capacity and find a decrease relative to postanesthesia induction values.86,114 The literature shows that lung volume and compliance decreases typically occur during CDP, hypoxemia is not common, and the impact on pulmonary dead space is uncertain.
Bicarbonate
Of 7 human investigations providing CDP bicarbonate results relative to postanesthesia induction values, 2 demonstrate a decrease in bicarbonate with CDP,49,70 and 5 show no change.20,39,45-47 The literature indicates that metabolic acidosis may occur during CDP; however, the relationship with PaCO2 and pH has not been well described.
Oxygen Consumption
Six human studies document no change in oxygen consumption with CDP.37,59,74,75,92,98 This suggests that, in general, there is little expectation that systemic oxygen consumption will change during CDP.
Renal Physiology
Of investigations evaluating urinary output during laparoscopy, 12 human17,18,76,88,115-122 and 9 animal123-131 studies document oliguria. From investigations evaluating renal function during laparoscopy, 6 human49,70,115,117,120,132 and 5 animal125,126,128,129,133 studies find a decrease. Of investigations evaluating renal blood flow during laparoscopy, 3 human115,117,132 and 12 animal123,125-127,129-131,133-137 studies note a decrease. The literature indicates that laparoscopy can induce oliguria, decrease renal function, and impair renal blood flow.
Gastrointestinal Physiology
Only a few investigations study abdominal aortic blood flow during laparoscopy. One human study shows an increase,55 whereas one animal report describes a decrease138 in flow. A single animal study evaluates inferior vena cava flow during laparoscopy and demonstrates a decrease.139 One human study140 and 7 animal investigations124,127,141-145 show an increase in inferior vena cava pressure with laparoscopy. Another human investigation documents no increase in superior vena cava pressure.140 Of relevance, multiple human and animal studies demonstrate an increase in femoral venous pressures19,107,136,139,146-149 and a decrease in femoral venous flow146,147,150-154 during laparoscopy.
With laparoscopy, 2 human investigations155,156 and one animal study157 show decreases in gastric blood flow. A single human study18 and one animal investigation158 demonstrate gastric ischemia, whereas one human report notes no ischemia.73 During laparoscopy, several human and animal investigations demonstrate decreases in mesenteric blood flow141,156,159-161 and the development of small bowel ischemia.138,141,159,161,162 Of clinical relevance, there are 6 human reports163-168 and one animal study159 describing small bowel infarction following laparoscopy.
The literature indicates that there are regional alterations in macro- and micro-circulatory mesenteric dynamics during laparoscopic surgery. Gastrointestinal ischemia and infarction can occur with laparoscopic-induced alterations in abdominal circulation.
Hepatic Physiology
The animal literature documents that laparoscopy increases hepatic venous138 and portal venous127,138,141 pressures. Multiple investigations study hepatic blood flow before and during laparoscopy. Two human studies155,169 and 6 animal reports127,135,170-173 document decreases in hepatic blood flow. In contrast, one animal study indicates that hepatic blood flow does not change,174 whereas, another shows an increase in flow.175 Four animal studies show a decrease in portal venous flow,138,160,170,176 whereas one investigation indicates that flow does not change.175
Nine human studies document increases in liver enzymes following laparoscopic surgery.73,162,177-183 One of these studies demonstrates that laparoscopy increases prothrombin time.178 The literature indicates that laparoscopy is likely to compromise hepatic blood flow and may impede liver function.
Endocrine Physiology
Multiple investigations demonstrate increases in plasma vasoconstrictor hormones during CDP. Hormonal changes include antidiuretic hormone (vasopressin),17,37,41,51,76,93,116,184-187 aldosterone,26 rennin,17,26,37,116 ACTH,93,188 cortisol,26,37,93,188-191 adrenaline,17,26,37,76,93,188,189 and noradrenaline.17,23,26,37,76,93,116,188,189 Of human investigations documenting serum glucose during laparoscopy, 2 find an increase;93,188 however, one notes no increase190 in values. Two human studies evaluating plasma insulin show an increase during laparoscopy.93,190 Literature findings document that laparoscopic surgery induces an increase in vasoconstrictor hormones and can create hyperglycemicstress.
Central Nervous System Physiology
Of investigations evaluating cerebral blood flow during CDP, 2 human studies4,84 and one animal123 report document an increase. However, one human investigation finds no increase in cerebral blood flow.65 Of investigations evaluating intracranial pressure during CDP, 2 human studies192,193 and 5 animal reports110,143,145,194,195 find anincrease.
Although one animal investigation indicates that CDP increases intracranial pressure, jugular venous saturation is without change.110 According to the literature, CDP is likely to increase cerebral blood flow and intracranial pressure. Such increases primarily relate to elevation in IAP, yet hypercarbia may intensify this effect.
CDP PATHOPHYSIOLOGIC CONSIDERATIONS
Precise pathophysiologic alterations during CDP are somewhat uncertain. However, the literature-based physiologic perturbations in the previous section are edifying. By combining accepted principles of critical care pathophysiology196 with documented CDP physiologic alterations, we describe some of the potential mechanisms that may alter physiology.
Cardiovascular System
The literature indicates that CDP typically increases systemic blood pressure, systemic vascular resistance, and vasoconstrictor hormones. Additional evidence suggests that these vasoconstrictor hormones may cause elevations in systemic blood pressure and vascular resistance.197-200 Further, the literature shows that with CDP cardiac output, stroke volume, and ejection fraction commonly decrease. It is probable that these findings follow the increases in systemic blood pressure and vascular resistance. Decrements in cardiac preload volume may occur and can contribute to decreases in cardiac output and stroke volume. Additionally, the increase in pulmonary artery pressures and pulmonary vascular resistance may overload the right ventricle, creating right ventricular distention, shifting the interventricular septum thus impeding left ventricular function.
Many of the cardiovascular changes occurring with CDP also exist with CD exposure. The literature indicates that CD exposure leads to increases in sympathetic activity,201-203 adrenaline,202,204 and noradrenaline.202,204 Further, CD exposure can induce vasodilator202-204 and vasoconstrictor201-206 effects. CD exposure also has a number of cardiac effects.
An increase in heart rate202-204,206 and dysrhythmias203,204,206 can occur. Cardiac contractility may decrease202-204,207 or increase.205 Likewise, cardiac output can increase201-204,206 or decrease.208 Additionally, increases in myocardial oxygen consumption202 and inverted T waves or depressed ST segments206 may occur. Because many of the same cardiovascular physiologic alterations occurring with CD exposure also manifest during CDP, it is tempting to postulate that cardiovascular perturbations during CDP may be due to systemic CD exposure.
A number of investigations compare CD and non-CD pneumoperitoneum cardiovascular parameter values with postanesthesia induction values. One human study47 and 4 porcine models 107,127,173,209 provide relevant data. There are essentially no differences between the CD and non-CD pneumoperitoneum groups relative to systemic blood pressure, cardiac output, stroke volume, heart rate, systemic or pulmonary vascular resistance, pulmonary artery pressure, pulmonary artery occlusion pressure, central venous pressure, inferior vena cava or femoral venous pressure, bicarbonate (metabolic acidosis), portal venous pressure, or hepatic blood flow. Multiple, large animal, CDP models demonstrate a decrease in renal blood flow.123,125-127,134,135 Apropos, Rosin136 also observes a decrease in renal blood flow with nitrogen pneumoperitoneum in a large animal model. These data indicate that the overwhelming majority of cardiovascular physiologic observations do not differ between CD and non-CD pneumoperitoneum. Such findings strongly indicate that CD, per se, does not influence cardiovascular physiologic perturbations; thus, changes principally relate to increases in IAP.
Multiple studies providing both PaCO2 and hemodynamic data offer insight into the potential role of hypercarbia on cardiovascular physiologic perturbations. Thirteen human investigations demonstrating an increase in PaCO2 with CDP also provide BP data.9,15,20,21,23,25,34,35,42,44,46,47,49 Of these 13 investigations, 10 (76.9%) demonstrate an increase in BP during CDP.9,15,20,21,23,25,34,35,42,44 Seven human investigations demonstrating no increase in PaCO2 with CDP also display BP data.12,18,36,37,39,45,48 Of these 7 investigations, 5 (71.4%) have an increase in BP during CDP.12,18,36,37,39 These observations indicate that hypercarbia during CDP does not increase the likelihood of developing an increase in BP. Such findings suggest that an increase in BP is primarily due to the increase in IAP.
Nine human investigations demonstrating an increase in PaCO2 with CDP also provide cardiac output data.1,21,23,25,42,44,49,59,70 Of the 9 investigations, 3 (33.3%) have a decrease in cardiac output during CDP.25,42,49Four human investigations demonstrating no increase in PaCO2 with CDP also give cardiac output data.12,36,37,48 Of these 4 investigations, 2 (50.0%) have a decrease in cardiac output during CDP.36,37 These data indicate that hypercarbia during CDP does not increase the likelihood of developing a decrease in cardiac output. This information suggests that a decrease in cardiac output is primarily due to increases in IAP.
Seven human investigations demonstrating an increase in PaCO2 with CDP also present systemic vascular resistance data.1,9,23,25,34,42,59 Of the 7 investigations, 6 (85.7%) report an increase in systemic vascular resistance during CDP.1,9,23,25,42,59T wo human investigations demonstrating no increase in PaCO2 with CDP provide systemic vascular resistance data.36,37 Of these 2 investigations, both (100.0%) show an increase in systemic vascular resistance during CDP. Such observations indicate that hypercarbia during CDP does not increase the likelihood of developing an increase in systemic vascular resistance. These findings suggest that an increase in systemic vascular resistance is primarily due to an increase in IAP. The above literature findings strongly indicate that increases in IAP, not CD exposure, are primarily responsible for cardiovascular physiologic perturbations during CDP.
Although hypertension is typical with CDP, hypotension can also occur, particularly with IAP ≥20mm Hg. In this instance, hypotension may be due to compression of the inferior vena cava impeding venous return that causes a reduction in cardiac output and blood pressure. Intermittent positive pressure ventilation, by causing increased intrathoracic pressure, can also impair venous return and cardiac output, particularly if positive end- expiratory pressures are applied.58,109,210,211 Dysrhythmias can occur with initiation of pneumoperitoneum. The reported incidence is between 14% and 27%.212 Sinus tachycardia and ventricular extrasystoles may be due to catecholamine release and bradydysrhythmias (sinus bradycardia, nodal rhythm, atrio-ventricular dissociation, asystole) may stem from vagal-mediated cardiovascular reflexes, instigated by rapid stretching of the peritoneum during establishment of pneumoperitoneum.63 Reported cardiac arrest rate estimates are 2 times to 20 times per 100 000 laparoscopic cases.212 Two potential causes of cardiac arrest include profound vasovagal response to rapid peritoneal stretch and gas embolism.
Patients undergoing CDP may develop gas embolism that can manifest as hemodynamic instability or cardiac arrest. This life threatening, dramatic complication usually results from direct injection of gas into the venous system during initial insufflation through a Veress needle.213 It may also occur with the opening of a large venous sinus, as with hepatic resection, where CD gains access into the venous circulation. The reported gas embolism incidence is 0.002% to 0.02%. If the gas embolism is large enough, the gas bubble can block right heart outflow and pulmonary vasculature producing varying degrees of circulatory collapse.
Respiratory System
Increases in IAP with CDP restrict diaphragmatic mobility and, by causing cephalad displacement of the diaphragm, reduce lung volumes. This leads to an increase in peak and plateau airway pressures and associated decreases in pulmonary compliance, inspiratory tidal volume, and functional residual capacity. The Trendelenburg position can further restrict diaphragmatic motion and alter mechanical ventilation.
The literature documents that hypercarbia is common during CDP. Hypercarbia, in general, emanates from increased CD production, increased dead space, or hypoventilation. The evidence indicates that an increase in systemic CD is typical with CDP, whereas, increases in dead space or hypoventilation are variable, but not rare. An increase in dead space, manifested by a PaCO2-to-PetCO2 gradient (PaCO2 minus PetCO2) >5mm Hg, is likely due to lung overventilation, decreased cardiac output, or pulmonary embolism (thrombotic or gas). A decrease in blood pH during CDP is more likely due to hypercarbia; however, metabolic acidosis can occur and may represent inadequate tissue oxygen delivery (increased lactate).
Hypoxemia can occur during CDP and is likely secondary to lung compression and a reduction in functional residual capacity. Hypercarbia, due to variable causes, may also lead to hypoxemia. If a gas embolism is large enough, the gas bubble can block right heart outflow and pulmonary vasculature, producing varying degrees of hypoxemia or hypercarbia.
Renal System
The literature documents that a decrease in renal blood flow, urinary output, and renal function is likely during CDP. It is likely the decrease in renal blood flow relates to a regional effect, increased IAP; however, a systemic cause, decreased cardiac output, stroke volume, or ejection fraction, is also plausible. Some investigations in large animals suggest that abdominal hypertension is the primary problem.133,214 However, others indicate that a decrement in cardiac output and stroke volume is a substantial factor.125,215 It is tempting to assume that the reduction in urinary output and renal function are due to the reduction in renal blood flow. However, the precise pathophysiology is unclear and multiple factors are likely present. Increases in antidiuretic hormone (vasopressin), aldosterone, and rennin occur during CDP and may be relevant mediators of the renal dysfunction.
Gastrointestinal System
The literature review indicates that gastric and mesenteric insufficiency and ischemia can occur during CDP. Documented decreases in blood flow during CDP include aortic, inferior vena cava, gastric, and mesenteric vessels. Evidence for increases in venous pressure exists for the inferior vena cava, the portal vein, and hepatic veins. The latter findings indicate that increases in IAP likely lead to gastric and mesenteric insufficiency and ischemia. However, a systemic cause, such as decreased cardiac output, stroke volume, or ejection fraction, is also feasible. Mechanical compression of the splanchnic capillary beds likely causes an increase in visceral vascular resistance.159,174,216 Increased vascular resistance can lead to vasoconstriction potentially by neural, hormonal, or intrinsic mechanisms. Blood vessel flow is proportional to the fourth power of its radius, and even a small decrease in vessel diameter can lead to a significant increase in vascular resistance and decrease in flow.159
Liver
The literature shows that hepatic dysfunction can occur with CDP. There is also evidence that hepatic and portal venous pressures increase and flow decreases. It seems likely that hepatic functional alterations relate to these changes in blood pressure and flow.
However, a systemic cause, such as decreased cardiac output, stroke volume, or ejection fraction, may also be contributory.
Endocrine System
The evidence seems clear that CDP elicits substantial stress hormonal response, similar to laparotomy. The precise pathophysiology is uncertain, but likely stems from surgical stress and regional pressure and flow alterations due to increases in abdominal pressure. One should also consider that systemic hormonal stress responses might also be a result of decreases in cardiac output, stroke volume, or ejection fraction.
Central Nervous System
The literature indicates that CDP can increase cerebral blood flow and intracranial pressure. To understand intracranial pathophysiology, it is instructive to recall that the skull essentially contains 3 components: parenchyma tissue, cerebrospinal fluid, and arterial and venous blood. An increment in volume of any one of these components can cause an increase in intracranial pressure. Right atrial pressure typically increases as IAP and intrathoracic pressures increase during CDP, thus expanding intracranial blood volume, with risk for increasing intracranial pressure. A decrease in cerebrospinal fluid absorption during pneumoperitoneum may also increase intracranial CSF and raise intracranial pressure. Several large animal investigations provide insight as to the cause of increasing intracranial pressure during laparoscopy. Investigators show that abdominal balloon inflation217 or nitrogen pneumoperitoneum136 increase intracranial pressure. One study finds that increased intracranial pressure occurs with CDP; however, hypercarbia is not present.110 Schob195 induces pneumoperitoneum separately with CD, nitrous oxide, and helium. Insufflation of each gas raises intracranial pressure; however, the increase is greater with CD compared with the other gases. These investigations suggest that increases in IAP principally account for increases in intracranial pressure; however, CDP or hypercarbia may be an exacerbating factor.
AT-RISK LAPAROSCOPIC PATIENTS
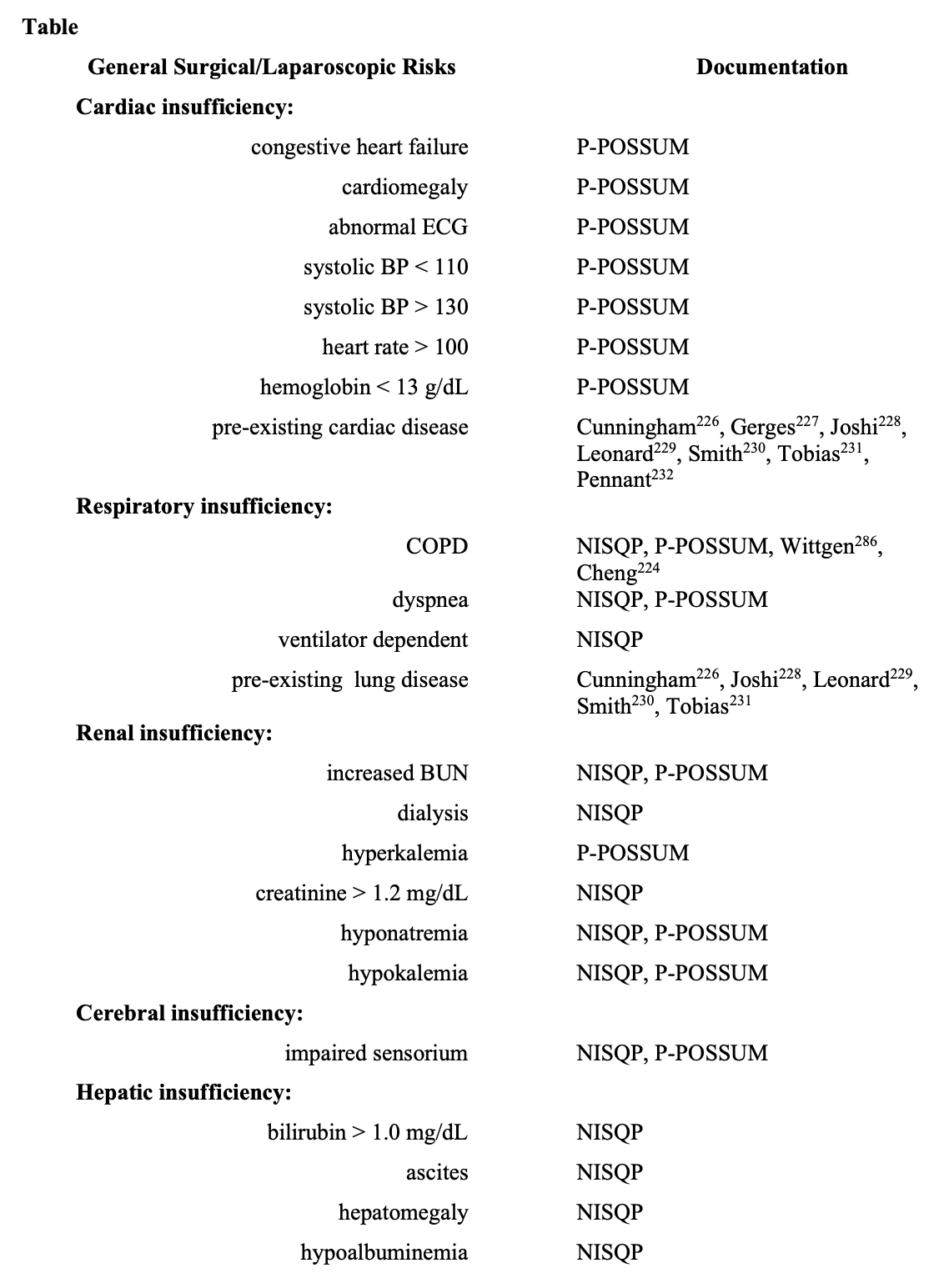
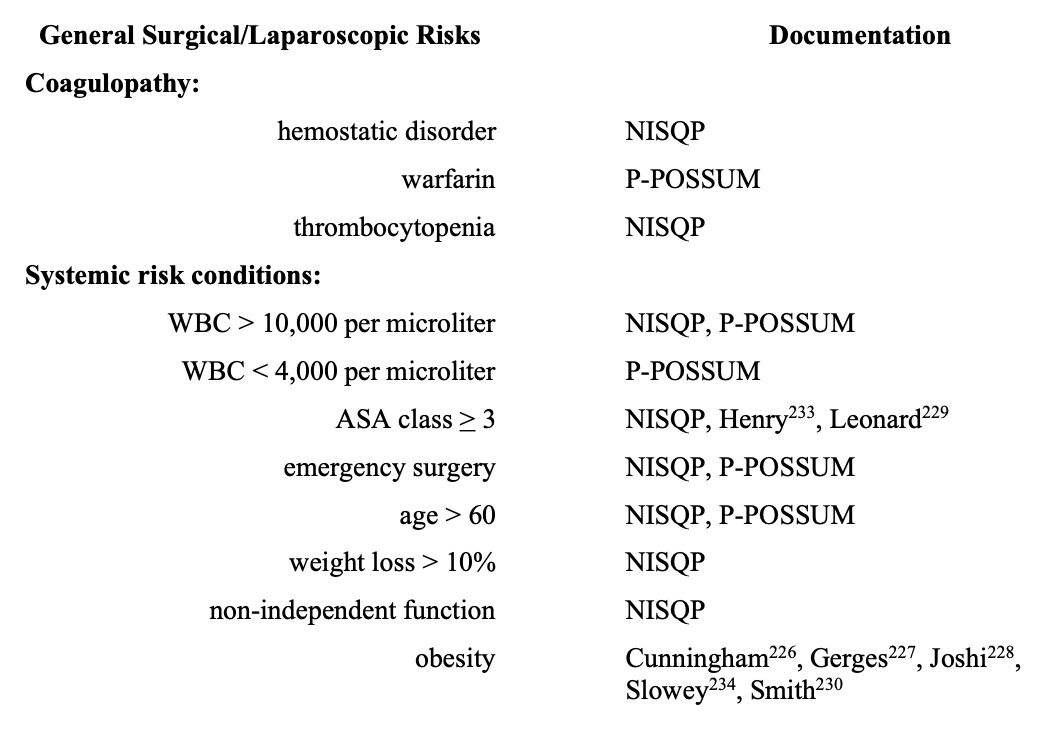
Several risk-scoring systems elucidate adult patient characteristics with an increase in perioperative morbidity and mortality.218 To depict general surgical (laparoscopic) patients with increased risk, elements of National Surgical Quality Improvement Program (NISQP),218-220 Portsmouth-Physiological and Operative Severity Score for EnUmeration of Mortality and Morbidity (P-POSSUM),218,221 and American Society of Anesthesiology classification (ASA),218,220,222 are in the Table. The literature indicates that POSSUM and P-POSSUM scores are useful indicators of morbidity in elderly patients undergoing laparoscopic surgery.223-225 Endorsement of several risks in the Table emanate from the laparoscopic anesthesia literature.226-234
MANAGEMENT OF INTRAOPERATIVE PHYSIOLOGIC INSTABILITY IN AT-RISK PATIENTS
Laparoscopic surgery is embraced by multiple specialties and involves an expanding patient population that includes pediatric, obstetric, geriatric, and at-risk individuals with significant underlying medical conditions. Perioperative risk for laparoscopic surgery, as with all surgery, is multifactorial. The interaction of anesthesia-patient-surgery specific factors all influence intraoperative and postoperative outcomes. The experience and skill of the anesthesiologist and the surgeon may be as important to outcome as the patient’s coexisting disease. Anesthesia for laparoscopic surgery with the physiologic changes induced by pneumoperitoneum, extremes of patient positioning, inherent difficulty in measuring blood loss, and the unexpected visceral or vascular injury, can itself be at risk.233 The unique physiologic perturbations associated with laparoscopic surgery combined with a patient’s underlying medical conditions raise the question of does the risk of surgery outweigh its benefits, and do the imposed physiologic effects of increased IAP outweigh the benefits of laparoscopy versus an open procedure.
There are many risk assessment tools to identify an at-risk surgical patient in the perioperative period with varied predictive results. Regardless, little clinical data are available showing that any of the risk assessment tools influence management of an at- risk patient or that advance knowledge of the risk score changes the risk outcome.235 Perhaps, the greatest utility of the risk-assessment evaluation is that it encourages preoperative dialogue between the practitioners involved in the care of the patient deemed at risk. In general, though, as the ASA level of the patient increases so will the level of intraoperative and postoperative monitoring.
Primary intraoperative objectives include customization of an anesthetic management plan based on preoperative risk assessment and the intended operative procedure, use of appropriate monitoring, and intraoperative vigilance for potential complications.
Recommendations include an IAP 12mm Hg to 15mm Hg or lower226,227 and slow inflation of the abdomen in at-risk patients.226,233 Balanced general anesthesia (opioids, inhalational agents, and neuromuscular blockade) with endotracheal intubation and controlled ventilation is the typical procedure for most laparoscopic, nonobstetric patients.226,229-233,236-242 Hyperventilation is common, with the intent to normalize CO2 levels.4,6,9,11,14,17,18,20,23,25,36,37,44-46,59,74,75,78,82,90,93,97,229,239 However, the precise value of this strategy is uncertain. Regional anesthesia with epidural, spinal anesthesia, or both epidural and spinal anesthesia, may occasionally be appropriate in select at-risk patients.227,229-231,233,236-238,243
INTRAOPERATIVE MONITORING
Basic Monitoring
The American Society of Anesthesiologists has published Standards for Basic Anesthetic Monitoring amended last in 2005. Multiple laparoscopic anesthesia literature reviews endorse these basic monitoring techniques, as described in the following section.227,229,232,233,236,238,241,242 Basic anesthetic monitoring requires that during anesthesia the patient’s oxygenation, ventilation, circulation, and temperature be continually monitored and appropriate alarm settings utilized. The patient’s level of oxygenation is monitored with an inspired oxygen analyzer, pulse oximetry, and clinical observation. It is essential to ensure adequate ventilation during a general anesthetic by monitoring expired gas volumes and using a disconnection detector on the anesthesia circuit. One should continually analyze PetCO2 by numeric value and capnography. Peak airway pressure on the anesthesia circuit is monitored to follow changes in chest wall, lung parenchyma, airway, and circuit compliance. Clinical observation including auscultation of ventilation should be maintained or accessible. Assessment of the patient’s circulation is by ECG and a means of blood pressure monitoring using a blood pressure cuff or direct intraarterial measurement. ST segment evaluation and 5-lead ECG are useful for the patient at risk for cardiac ischemia. Continuous heart rate monitoring occurs with palpation of the pulse, auscultation of heart sounds, intraarterial pressure tracing, pulse plethysmography, or oximetry. Continually monitor the patient’s temperature and use warming devices, as needed. Although typically only a small area of the patient has exposure to ambient air during laparoscopic surgery, the temperature and humidity of the insufflation gas may be clinically significant.244Urine output is not an ASA basic monitor, but is important during laparoscopic surgery. Because of the potential for altered renal blood flow or for bladder injury during pelvic laparoscopy, we recommend inserting a Foley catheter and monitoring urine output. Monitor the potential for patient intraoperative awareness using a bispectral index (BIS) device. Other recommendations include the utilization of a nerve stimulator when muscle relaxants are given and quantifying the inspired and expired concentration of volatile anesthetics.
Continuous IAP monitoring, documentation, and re-assessment by the surgeon and anesthesiologist are essential to optimize care of the at-risk patient.
Advanced Monitoring
Decisions regarding intraoperative advanced monitoring depend on pre-existing medical conditions of the patient, the anticipated surgery with knowledge of positioning requirements, and the experience of the surgeon as well as the anesthesiologist. Advanced monitoring and management techniques include, in addition to basic monitoring, (1) arterial catheterization for continuous systemic blood pressure assessment and intermittent arterial blood gas analysis to document PaO2, pH, base deficit, PaCO2, and PaCO2-to-PetCO2 gradient (PaCO2 minus PetCO2); (2) esophageal stethoscope or precordial Doppler to detect gas embolism; (3) central venous catheterization; and (4) cardiac output monitoring. The PaCO2-to-PetCO2 gradient is useful to assess the magnitude of physiologic dead space.245-248 A PaCO2-to-PetCO2 gradient >5mm Hg suggests that physiologic dead space is increased. This implies that there is an increase in the ventilation-to-perfusion ratio such that ventilation occurs, but there is a reduction or absence of perfusion. Relevant clinical conditions include (1) alveolar overventilation with pulmonary capillary compression, (2) decreased cardiac output, (3) cardiac arrest, and (4) pulmonary embolism (gas or thrombotic). Multiple experts recommend arterial catheterization for pre-existing cardiopulmonary diseases,226-229,231,232,236,238 intraoperative hypoxemia,227 high airway pressures,227 intraoperative hemodynamic instability,238 obesity,238 and ASA III or IV patients.229,233 It seems reasonable that virtually any at-risk laparoscopic patient should undergo arterial catheterization with systemic blood pressure and ABG monitoring. A review of laparoscopic anesthesia review articles provide a recommendation for cardiac output monitoring when patients have pre-existing cardiac disease232,233,236 or are ASA class III or IV.229,233 Extremes of positioning and insufflation of intraperitoneal gas can cause rapid and deleterious swings in hemodynamics, especially in the patient with compromised cardiac function.249
Direct intraarterial blood pressure monitoring allows for rapid recognition and treatment of any cardiopulmonary instability. Indications for intraarterial cannulation include anticipated need for continuous real time blood pressure monitoring, repeated blood sampling, and failure of or difficulty with indirect measurement of blood pressure.
Repeated ABGs may be necessary in those patients with borderline oxygenation or ineffective ventilation and elimination of CO2. The waveform generated by direct intraarterial pressure monitoring can also provide additional diagnostic information regarding the patient’s volume status, potential for valvulopathy, and systolic left ventricular function.250,251 Newer technologies such as arterial pressure-based cardiac output systems may allow for trending of cardiac function without the invasiveness and associated risks of central venous access or a pulmonary artery catheter.252 Marik251 documents in a systematic literature review of mechanically ventilated patients that dynamic measures (pulse pressure variation, stroke volume variation, systolic pressure variation), compared with static measures (central venous pressure, end-diastolic volume index, end-diastolic area index), are more predictive of cardiac index fluid-bolus responsiveness. Advanced hemodynamic monitoring may be added intraoperatively for persistent or unexplained hemodynamic instability, hypoxemia, increased airway pressures, or problematic hypercarbia.
As the patient’s preoperative status or intraoperative conditions dictate, consideration can be given to invasive measures of central venous pressure, cardiac output with a pulmonary artery catheter, and determination of mixed venous oxygen saturation.
Evaluation of cardiac filling and cardiac output will help guide therapy in the patient with significant cardiac disease. It is important to remember that extremes of positioning and changes in IAP will be reflected in intrathoracic pressure changes making the absolute values generated by a central venous pressure catheter or pulmonary artery catheter unreliable. It is important to note that several laparoscopic anesthesia articles describe, in detail, monitoring strategies, yet none of these include central venous pressure monitoring.226,229,232,233,236,238,241 The likely reason is that CDP typically increases central venous pressure, and central venous pressure is not synonymous with preload volume.
Osman253 demonstrates that central venous and pulmonary artery occlusion pressures are poor predictors of fluid responsiveness in hemodynamically unstable patients.
Specifically, patients with low pressures often do not increase cardiac index with a fluid bolus, whereas those with normal or elevated pressures commonly have an increase in cardiac index with a volume challenge. The trend in these values and measurement of cardiac output using thermodilution via the PA catheter, though, can guide therapy and interventions.233 Central access to the circulation is also useful in the administration of vasopressors or inotropic agents or in the resuscitation of a venous air embolism. The use of a pulmonary artery catheter intraoperatively, though, is not without controversy or risk. Transesophageal echocardiography is a reliable and minimally invasive intraoperative monitor of cardiac function and volume status, a diagnostic tool for detection of venous air embolism, and a resuscitation aid.232,233,254 When used either as a planned intraoperative monitor of cardiac function or as an emergent guide to resuscitation or therapy, transesophageal echocardiography is beneficial in 40% to 80% of patients.255,256 In greater than one-third of patients, intraoperative transesophageal echocardiography has an association with a change in medical therapy and, in 25%, with a change in surgical procedure.257 The high cost of equipment and necessary operator skill limits its usefulness in all centers that perform laparoscopic surgery. However, as the utility of intraoperative and intensive care unit transesophageal echocardiography is recognized, pathways for teaching and certifying basic perioperative echocardiography are being developed. The use of transthoracic impedance cardiography may be useful during CDP.236
PROBLEM ORIENTED MANAGEMENT FOR PHYSIOLOGIC INSTABILITIES
As our patient population ages and the list of comorbid conditions lengthens, intraoperative events, those anticipated and those unexpected, occur. However, the physiologic perturbations associated with CO2 gas insufflation and the extremes of positioning add a new dimension to the problems that may be encountered during laparoscopic surgery. A problem- oriented approach to some of the more common intraoperative instabilities will be discussed below. Should physiologic instability ensue, in addition to specific interventions, always consider the potential benefit of abdominal gas deflation or lowering of IAP4,5,13,17,19,29,30,34,38,40,49,61,62,68,70,76,83,84,117,119,193,226-229,232-234,238,242,258,259 or conversion to open laparotomy.4,60,84,126,128,135,227-229,231-233,259
Hypotension
Hypotension is the second most common intraoperative complication,260 but hypotension in perioperative medical literature is ill-defined.261 A 20% to 30% decrease from baseline blood pressure in healthy ASA I and II patients is believed to be well tolerated.261 The definition of hypotension and its tolerance in ASA III to IV patients and the elderly remains unspecified with no consensus reported in the literature. Despite being ill- defined, Monk262 found that intraoperative hypotension is a significant, independent predictor of increased 1-year mortality after noncardiac surgery. Reich263 studied the predictors of hypotension after induction of general anesthesia and found patients that were ASA III to IV (higher risk patients), had a baseline MAP<70mm Hg, were age >50 years old, and had propofol used as the induction agent with increased doses of fentanyl were at increased risk.
Several common causes of intraoperative hypotension exist226,233,237,238: hypovolemia,38,226,228-234,236,241 hemorrhage,226,228,229,234,237,242 ventricular failure,228,237 preexisting cardiac disease,228,229 anesthetic agents,226,228,234,237,238,240 adjunct medications, excessive IAP,226,228,229,231,232,234,237,238 PEEP,58,211,233,264,265 increased systemic vascular resistance,231,241,242 severe hypercarbia,226,228,229,232 decreasing surgical stimulation, septicemia, and adrenal insufficiency. Prolonged preoperative fasting, bowel preparations, emesis, and gastric suctioning all contribute to intravascular volume depletion on presentation to the operating room. Acute hemorrhage, insensible losses, compression of the inferior vena cava or other large intraabdominal vessels by the surgeon or placement of packs or retractors can decrease venous return to the heart.
Abrupt changes in patient position such as reverse Trendelenburg aggravate any cause of intravascular volume deficit. Steep Trendelenburg, by causing cephalad movement of the abdominal contents, and increased IAP with CDP may increase intrathoracic pressure and decrease cardiac preload, potentially reducing cardiac output and blood pressure. Other causes of hypotension include cardiac tamponade,228,234 dysrhythmias,228,234 tension pneumothorax,54,226-229,237,266 pneumopericardium or pneumomediastinum,228,229 and vasovagal reaction.228,232,234
Anesthetic agents can be both vasodilators and myocardial depressants.226,228,234,237,238,240 Myocardial ischemia will affect cardiac output and the patient’s response to the anesthetics and the surgical procedure. Adjunct medications, such as antihypertensive agents, neuromuscular blocking drugs, and histamine-releasing medications, can contribute to intraoperative hypotension. Periods of decreased surgical stimulation can also lead to hypotension. Unrecognized adrenal insufficiency and septicemia, pre-existing or an acute response to a bacterial load, can also cause profound intraoperative hypotension.
General intraoperative evaluation and treatment of hypotension involves first to efficiently assure the accuracy of blood pressure measurement, then assess the most likely causes of hypotension and begin treatment. Kheterpal267 found that at-risk surgical patients who experience an adverse cardiac event after surgery are more likely to have had experienced a 10-minute period of intraoperative hypotension, defined as a mean arterial pressure <50mm Hg or a 40% decrease in mean arterial pressure from the baseline level. Direct clinical observation of the patient is the anesthesiologist’s best assessment tool. However, because of limitations inherent in laparoscopic surgery, this may be difficult and less than satisfactory. Adequate oxygenation and ventilation, however, must be assured. Scan for changes in peak airway pressures and end-expiratory pressure to help eliminate increases in intrathoracic pressure as the cause of hypotension. If positive end-expiratory pressure, prescribed or dynamic hyperinflation (auto-positive end-expiratory pressure), impedes venous return, a quick disconnect from the breathing circuit and positive end-expiratory pressure reduction may restore blood pressure.58,211,233,264,265 Then make ventilation adjustments. Scan of the ECG and ST- segment analysis will reveal dysrhythmias or the possibility of myocardial ischemia. If no cause of hypotension is immediately evident, a fluid bolus is prudent taking into consideration the patient’s preexisting medical conditions, cardiac function, and fluid deficit calculations.13,232-234,237,242 The level of anesthesia is decreased and any offending vasodilators or negative inotropic agents are discontinued.226,228,234,237,238,240
Concerns specific to laparoscopic surgery include extremes of positioning233 and effects of increased IAP.226,228,229,231,232,234,237,238 Fluid bolus prior to insufflation and positioning helps to attenuate the affects of increased IAP.228 The expected hypertensive response to continued CO2 peritoneum makes hypotension unresponsive to fluid bolus concerning. Placing a hypovolemic patient in the head-up position may lead to a decrease in blood pressure and cardiac output.3,6,12,16,46,57,226,227,229,230,232-234,237-241,258 Moving a head-up patient to a supine position or lowering the head of a supine patient may improve cardiac output.38,226,228-234,236,241,258 However, this maneuver may not enhance blood pressure and cardiac output.57 A patient with preexisting cardiac disease may be unable to compensate for increased afterload, decreased preload, or elevated heart rate. Hypercarbia-induced direct and indirect sympathetic stimulation may not be well tolerated in the cardiac- challenged patient. Acute blood loss anemia or vascular injury with the inherent difficulties during laparoscopy to control bleeding are causes for hypotension that the anesthesia team must treat quickly with fluid and, if needed, blood administration.226,228,229,234,237,242 Because most laparoscopic surgical patients do not undergo a type and cross-match procedure, rapid acquisition of patient-specific blood is not usually feasible. CDP typically increases central venous and pulmonary artery occlusion pressures. Accordingly, they are not a reliable indication of cardiac preload volume status.226,233,241,258 Opening the abdomen may be necessary to rapidly and effectively control abdominal bleeding. Visceral injury can complicate the intraoperative course or be a source of sepsis and postoperative hypotension.
In the treatment of intraoperative hypotension, incremental doses of vasopressors and inotropes may be necessary, but their use should not be excessive.36,161,228 If hypotension persists despite reasonable measures, the position of the patient should be leveled after notification of the surgeon. Any surgical packs or surgical interference with venous return should be released. If hypotension persists, the abdominal pressure should be released. If hypotension resolves sufficiently to continue the laparoscopic procedure, reinsufflation can proceed with adjustments to the rate of insufflation or intraabdominal pressure achieved. Cunningham recommended intraabdominal pressures not to exceed 15mm Hg, but this may still exceed the patient’s physiologic tolerance. When hypotension persists or questions arise as to the volume status or cardiac function of the patient, invasive monitoring devices including TEE should be considered. Surgery should be completed as safely and expeditiously as possible, which may include conversion to open procedure.
Clinically significant venous air embolism occurs in 0.002% to 0.08% of laparoscopic cases. When transesophageal echocardiography was used, venous air embolism was detected in 68% of all laparoscopic cholecystectomy patients268 and 100% of all laparoscopic abdominal hysterectomies.269 It occurs secondary to direct intravascular gas insufflations or a tear in an abdominal wall or peritoneal vessel. The lethal dose of CO2 is 5 times that of air, making CO2 a safer choice for gas insufflations. Clinically significant venous air embolism has a plethora of manifestations.227-229,232-234,236,237,242 They include presentation with profound hypotension, cyanosis, or dysrhythmias progressing to asystole. Typically, there is a sudden increase in PetCO2 followed by a rapid decline due to cardiovascular collapse and reduced pulmonary blood flow. A “mill-wheel” murmur may be detected on cardiac auscultation before cardiac arrest. When venous air embolism is suspected, multiple interventions need rapid consideration and implementation.227-229,232-234,236,237,242 Initial measures are to discontinue the operation, immediately deflate the abdomen, and stop anesthetic agents. The patient should be placed in left lateral decubitus with head down to facilitate gas rising to the apex of the right ventricle and to decrease the amount of gas entering the pulmonary artery. The patient is given 100% oxygen with gentle hyperventilation for rapid CO2 removal. Placement of a transesophageal echocardiographic probe will usually show air in the venous system. A central venous catheter may be considered for aspiration of gas although rapid diffusion of CO2 occasionally makes this unnecessary. As in any case of cardiovascular collapse, ACLS protocol should be followed and modified for intraoperative care, as necessary.270 Chest compressions, if indicated by ACLS, may break the intracardiac CO2 ‘lock.’
Other examples of inadvertent extraperitoneal gas insufflation include subcutaneous emphysema, pneumothorax, pneumomediastinum, and pneumopericardium.
Subcutaneous emphysema occurs in 0.4% to 2% of laparoscopic procedures. It is caused by insufflated gas dissecting into tissue plains around the trocar site. Subcutaneous emphysema can be associated with increased airway pressures or increased PetCO2 after equilibrium is achieved or beyond that which is normally expected. Respiratory acidosis may become marked. Other than discontinuing any N2O used, no treatment is generally necessary. Subcutaneous emphysema of the neck and face can result in gas tracking to the thorax and mediastinum, resulting in pneumothorax or pneumomediastinum.
Subcutaneous emphysema generally resolves in 30 minutes to 60 minutes after abdominal deflation.
Pneumothorax occurs from a tear in the visceral peritoneum, breach of the parietal pleura during dissection around the esophagus, a congenital defect in the diaphragm, spontaneous rupture of preexisting emphysematous bulla, or extension of subcutaneous emphysema. Typically, a pneumothorax related to barotrauma is more serious and more likely to require chest tube insertion for resolution. Pneumothorax under tension will lead to increases in peak airway pressures, decrease in SpO2 and, if severe, cause profound hypotension and cardiac arrest. Clinically, pneumothorax causes a sudden decrease in pulmonary compliance, increased peak airway pressures with an increase in PetCO2, unchanged or decreased SpO2, and absence of breath sounds on the affected side.
Endobronchial intubation, which can occur during laparoscopic surgery due to cephalad movement of the carina from the diaphragm, can be ruled-out by fiberoptic bronchoscopy, intraoperative fluoroscopy, or chest X-ray. Once pneumothorax is suspected, N2O is discontinued. Treatment of pneumothorax depends on the patient’s clinical condition. Asymptomatic pneumothorax may resolve after release of pneumoperitoneum and require no specific treatment. Extensive pneumothorax or pneumothorax with hemodynamic compromise will require immediate release of pneumoperitoneum and insertion of a chest tube if instability persists.54,226-229,237,266 Pneumothorax may only be recognized postoperatively when the patient complains of shortness of breath or pain or exhibits respiratory distress not related to residual muscle relaxation. This patient is treated with 100% oxygen to enhance CO2 elimination.
If subcutaneous emphysema in the cervical region extends into the thorax and mediastinum, a pneumomediastinum or pneumopericardium may develop. CO2 may also be forced around the inferior vena cava and tract through a defect in the membranous portion of the diaphragm. N2O is discontinued. Pneumothorax may be asymptomatic or may require release of the CDP. If the patient remains unstable after the release of pneumoperitoneum, hemodynamic support is required until resolution. Symptoms of pneumopericardium may mimic pericardial tamponade but, fortunately, usually dissipate with resolution of pneumoperitoneum.
Hypertension
CDP typically induces relative systemic hypertension via vasoconstrictor hormonal release. Further, intraoperative hypertension is commonly a response to stimulation that results in release of endogenous catecholamines. Preexisting hypertension may be undiagnosed, poorly controlled, or the result of a missed dose of antihypertensive medication. Whenever intraoperative hypertension occurs and does not respond quickly to deepening the levels of anesthesia, a search for other causes must occur. Intraoperative hypertension may be iatrogenic, the result of medications given or excessive fluid administration. It may also signal hypoxemia, hypercarbia, hypovolemia, malignant hyperthermia, increased intracranial pressure, bladder distension, or prolonged tourniquet use. During laparoscopic surgery, the increased IAP raises systemic vascular resistance by direct compression of the abdominal aorta and increased venous resistance. Afterload is also increased by release of catecholamines, vasopressin, and increased rennin- angiotensin activity. MAP is usually maintained or increased because the increase in systemic vascular resistance is greater than the reduction in cardiac output. Hypercarbia also causes direct and indirect sympathetic stimulation. Commonly, hemodynamic changes return to preinsufflation values 30 minutes after the start of controlled pneumoperitoneum. Treatment of intraoperative hypertension is to assure adequate depth of anesthesia,230,234 oxygenation, ventilation, and cardiac preload volume status. The above-listed causes must be investigated and ruled out. Once done, treatment may consist of antihypertensive agents titrated to within the patient’s baseline MAP.234 Nitroglycerin,36,228clonidine,37,271 and esmolol272 may be useful agents for managing hypertension during laparoscopic surgery.
Dysrhythmias
The most common intraoperative physiologic aberration is dysrhythmia.260 The majority of dysrhythmias are benign. The sudden appearance of any abnormal rhythm should prompt investigation for precipitating events. The importance of any dysrhythmia depends on the patient’s medical condition, the hemodynamic effects, and the specific rhythm disturbance.
Sinus tachycardia is the most commonly seen dysrhythmia during anesthesia. Other tachydysrhythmias occurring during laparoscopy include atrial and other supraventricular ectopic beats, premature ventricular contractions, ventricular tachycardia, and ventricular fibrillation. Benign tachydysrhythmias such as sinus tachycardia may be a normal response to hypotension or hypovolemia. There may be an insufficient level of anesthesia or analgesia, causing an increased circulation of catecholamines. Tachycardia may be the result of hypoxemia, hypercarbia,226,229,232,237,241 fever, malignant hyperthermia, gas embolism,227,228,233,234,237 hypovolemia,234 pain,234,241 or the head-up position.6 Endocrine disorders, such as pheochromocytoma, thyrotoxicosis, or adrenal insufficiency, may be heralded by sinus tachycardia. Myocardial dysfunction including ischemia, infarction, and failure may have an associated tachycardia. Pulmonary emboli can cause tachycardia, as can medications including anesthetic agents. The treatment of sinus tachycardia focuses on identification and treatment of its underlying cause. However, it should be remembered that sustained intraoperative tachycardia, heart rate >100, is a risk factor for adverse cardiac events after general surgery. Intervention options include fluid infusion for hypovolemia,234 increasing anesthesia for pain,234,241 esmolol,273 clonidine,271 and abdominal deflation with slow inflation in the supine position.227 Atrial tachydysrhythmias, such as atrial fibrillation and atrial flutter, may be preexisting and well tolerated. If the rate becomes uncontrolled or its appearance is unexpected, underlying causes must be sought and the heart rate controlled. Look for and treat evidence of myocardial ischemia, electrolyte abnormalities, acid-base imbalances, in addition to aberrations in oxygenation and ventilation. If hemodynamic instability occurs, ACLS protocols should be followed. Any persistent hemodynamic instabilities need to be communicated to the surgeon, necessitating completion of surgery in an expeditious manner.
Bradyarrhythmias, unless associated with hemodynamic instability, usually do not require treatment. These may include sinus bradycardia, wandering atrial pacemaker, junction rhythm, atrioventricular heart block, and asystole. The sudden appearance of bradycardia should prompt a search for underlying causes. Bradycardia can be a response to surgical stimulation. Specifically during laparoscopy, it can be a response to sudden peritoneal stretch.226,227,234,241,242 Head-down positioning may lead to an increase in central venous pressure and cardiac output. The baroreceptor reflex adjusts to this pressure increase by causing vasodilatation and bradycardia.234Medications including anesthetic agents and narcotics can cause bradycardia, as can hypercarbia and respiratory acidosis234 or gas embolism.227,228,234,242 Ischemic heart disease and increased intracranial pressure can both show alterations in heart rate. Treatment of bradycardia begins with stopping the stimulation and investigating underlying causes. Abdominal gas deflation and subsequent slow re-inflation226,227,234,241,242 in the supine position227 may be palliative. Anticholinergic agents (atropine, glycopyrrolate) may be useful and at any time hemodynamic instability exists,228,234 the ACLS protocol modified for the OR should be followed.
Cardiac Arrest (Cardiovascular Collapse)
Cardiac arrest in the operating room is fortunately a rare occurrence. However, cardiovascular collapse can occur in the previously healthy as well as the at-risk patient during laparoscopic surgery. Etiological factors include those already discussed in the Hypotension and Dysrhythmias sections. Vasovagal response to peritoneal stimulation can lead to various degrees of heart block including asystole. Termination of peritoneal stimulation usually restores normal cardiac conduction. Lessening the amount or rate of peritoneal stimulation typically attenuates the response. Consideration can also be given to the administration of a vagolytic agent before further peritoneal stimulation is attempted. The myocardium can be sensitized to catastrophic dysrhythmias by inhalational anesthetics and hypercarbia.210 Newer inhalational agents in use today are less arrhythmogenic than their predecessors but vigilance is always required during administration of any anesthetic. Any of the reasons for intraoperative hypotension during laparoscopic surgery can progress to cardiovascular collapse in its extremes.
Reverse Trendelenburg, inferior vena cava compression, effects of high-insufflation pressure, profound hypovolemia, and acute blood loss all may decrease venous return sufficiently to produce cardiovascular collapse and the need for immediate resuscitation. Inadvertent extraperitoneal gas insufflation, including venous air embolism, pneumothorax, and pneumomediastinum, can lead to hemodynamic collapse.
Cardiac arrest, during laparoscopic surgery, necessitates the surgical procedure be stopped and pneumoperitoneum released. Interventions described in the Hypotension and Dysrhythmias sections are promptly considered. The patient should be ventilated with 100% oxygen while a rapid clinical evaluation is conducted. Room lights should be turned on and the patient leveled. Remove drapes and inspect the patient, including auscultation of the heart and lung sounds. Monitor PetCO2 to determine the effectiveness of cardiac output.274-276 A PetCO2 value <20mm Hg suggests that cardiac output is negligible. Administer fluids and initiate the ACLS protocol. Consideration should be given to placement of transesophageal echocardiography probe while treatment is underway. If large volume blood loss has occurred, the abdomen should be opened and bleeding controlled while blood is administered. Consideration should be given to rapid, modest hypothermia. Electrolytes and blood gas evaluation should be done. The differential diagnosis of cardiac arrest includes dysrhythmias, ischemia, and left or right heart failure. Right heart failure may be due to extracardiac causes, such as pulmonary emboli or venous air embolism. Other causes of cardiac failure include cardiac tamponade including pneumopericardium, tension pneumothorax, electrolyte or acid-base abnormalities, and absent venous return. Profound hypovolemia, acute blood loss, and mechanically impeded venous return greatly diminish preload and cardiac output.
Respiratory failure or severe hypoxemia can result from tension pneumothorax, cardiac failure, and failure of ventilation including endobronchial intubation and barotrauma. Pulmonary emboli or direct lung injury can result in failure of ventilation leading to cardiorespiratory arrest. Neurologic failure from increased intracranial pressure, vascular insufficiency, or embolic stroke can precipitate cardiac arrest.
Hypercapnia
Changes in respiratory mechanics are anticipated effects of general anesthesia and patient positioning during surgery, even in the healthiest of patients. Due to the effects of positioning and CDP, patients with significant cardiopulmonary disease are at particular risk for hypoxemia and exaggerated hypercapnia.
Hypercarbia occurs during an anesthetic when there is an imbalance between the production of CO2 and its elimination. Increased systemic absorption of CO2 occurs with CDP.11,50,75,77,78,89,92,92,98 Production of CO2 by the patient is also increased by fever, malignant hyperthermia,228 sepsis, or thyrotoxicosis. Iatrogenic sources of carbon dioxide include re-breathing carbon dioxide in the anesthesia circuit and insufflation of exogenous CO2. Elimination of CO2 can be impaired by inadequate ventilation,226,227 ventilator circuit disconnection,228 or increased dead space.74,80,87,99,228 The head-down position39,111,112,226,238,240,241,277 and increased IAP228,234 predispose to hypoventilation. The head-down position may also increase dead space.241 Other factors increasing the likelihood of hypercarbia include gas embolism,228,231,242 subcutaneous CO2 insufflation,228,229 pneumothorax,229 and patients with ASA class III or IV status258 or pre- existing cardiopulmonary disease.80,226,228 Hypertension, tachycardia, and other dysrhythmias usually do not appear until the PaCO2 is markedly elevated. Hypercarbia activates the sympathetic nervous system, increasing blood pressure, heart rate, myocardial contractility, and dysrhythmias. Hypercarbia may be particularly dysrhythmogenic when combined with hypoxemia. Moderate to severe respiratory acidosis can act as a direct myocardial depressant and cause pulmonary vasoconstriction that may worsen right heart failure in those with preexisting pulmonary hypertension.202
There may be an imbalance between production, insufflation, and elimination of CO2 during CDP. An increase in PaCO2-to-PetCO2 gradient during laparoscopy occurs as underperfused alveoli are ventilated, increasing physiologic dead space. This happens because of patient positioning, increased intrathoracic pressure, decreasing cardiac output, and pulmonary blood flow, and ablation of hypoxic pulmonary vasoconstriction by anesthetic agents or vasodilators. With increased dead space, PetCO2 is relatively normal, yet PaCO2 can be substantially elevated.74,80,87,99,228,229,241,258 Thus, it is important to obtain PaCO2 values and compare them with PetCO2 as a routine for at-risk patients and during any period of physiologic instability. During laparoscopy, there is transperitoneal absorption of CO2 as well as mechanical impairment of diaphragmatic and intercostal muscles from the pneumoperitoneum.249 More CO2 is absorbed from extraperitoneal insufflation than intraperitoneal insufflation.278 Extraperitoneal insufflation therefore leads to higher PaCO2 values. Treatment of intraoperative hypercapnia involves provision of adequate minute ventilation, mitigating pulmonary overventilation, and ensuring that the anesthesia circuit is intact. Look for and treat causes of hypermetabolism. During laparoscopy with CO2 insufflation, most patients will require an increased minute ventilation of 15% to 30% to maintain a PetCO2 of approximately 35mm Hg.227,228,230,232,233,237,238,240-242,258 Increasing respiratory rate or tidal volume may not improve hypercarbia, if dead space is increased.113 In patients with COPD, spontaneous pneumothorax, or bullous emphysema, the respiratory rate rather than the tidal volume should be increased.95 In those same patients, though, enough time for exhalation must be given to avoid auto-peep. A sudden decrease in PetCO2 occurs intraoperatively when there is obstruction to airflow or in the sample tubing, when the patient has been extubated or there is a circuit leak or disconnect. Sudden decrease in PetCO2 also occurs with cardiac arrest or venous air or pulmonary embolism.
Endobronchial intubation may occur during abdominal gas insufflation as the lungs and mediastinum undergo cephalad displacement. Endobronchial intubation may be signaled by a decrease in PetCO2. Placing the patient in a head-up position may mitigate hypercarbia46,230,233; however, this is not a universal response.277
Postpneumoperitoneum absorbed CO2 is rapidly eliminated from the patient because of its high solubility and diffusibility. Generally PaCO2 levels return to baseline within one hour.98 Because more CO2 is absorbed from extraperitoneal insufflations or especially prolonged procedures, postanesthesia PetCO2 levels may be higher and take longer to resolve than normally.279 Until absorbed CO2 is eliminated, the respiratory rate and PetCO2 of patients breathing spontaneously during postanesthesia recovery will be higher than after open procedures. For the patients with compromised cardiopulmonary reserve, this prolonged hypercapnia may exceed their ability to compensate. If alveolar ventilation is impaired or cardiopulmonary status of the patient is compromised, postoperative hypercarbia and respiratory acidosis may persist leading to respiratory failure.80
Hypoxemia
General causes of hypoxemia during anesthesia include decrease in inspired oxygen concentration, accidental disconnection of the breathing circuit, and hypoventilation. Hypoventilation and hypercarbia are known adversities with CDP.228,258 Ventilation-to- perfusion mismatch is the most common cause of intraoperative hypoxemia. During administration of any general anesthetic, a decrease in functional residual capacity occurs. Multiple investigators show that CDP reduces functional residual capacity228,230,232,233,238-240,242,258 and can create hypoxemia.11,17Head-down positioning with CDP further decreased functional residual capacity.226,227,229,230,232,236,240-242,258 Diffusion abnormalities such as aspiration226,228,231,234,258 or pulmonary edema233 may lead to hypoxemia during the course of surgery, as well as any time there is a decrease in mixed venous oxygen saturation. Other causes of hypoxemia during CDP include gas embolism,228,229,231,242,258 pneumothorax,54,101,102,226-229,231,233,258,266,280 pre-existing cardiopulmonary disease,226,228,233,258 morbid obesity,226,228,258 endobronchial intubation,226,228,231,258 and reduced cardiac output.226,228,238,258 Right main stem bronchial intubation can occur when the diaphragm and mediastinum undergo a cephalad shift with abdominal insufflation.242
Treatment begins with administration of 100% oxygen233 and confirmation that adequate oxygen and tidal volumes are delivered to the patient. Controlled mechanical ventilation is recommended.237 The lungs should be auscultated and breath sounds evaluated for symmetry and quality. Clinical evaluation should include observation of chest rise and fall. A decrease in unilateral breath sounds or chest movement or a rise in airway pressures may indicate endobronchial intubation or pneumothorax.54,101,102,226-229,231,233,258,266,280 This may be differentiated by a quick fiberoptic bronchoscopy or intraoperative fluoroscopy or chest X-ray. Severe hypoxia or hemodynamic instability should prompt rapid insertion of a chest tube. Specific treatment for bronchospasm or pulmonary edema is necessary, when present. Evaluate capnography and capnogram for airway or circuit obstruction, bronchospasm, ventilator disconnection, or sudden increase or decrease in values. Hand ventilation will allow the experienced practitioner to evaluate patient and circuit compliance. If hemodynamic parameters are compromised, resuscitate accordingly. Consider invasive monitoring and transesophageal echocardiography. If assessment of the patient rules out hemodynamic causes of decreased mixed venous oxygen and subsequent hypoxemia, adjust ventilation including application of positive end-expiratory pressure to better meet the needs of the patient and to decrease what may be intraoperative atelectasis. Laparoscopic patients are at increased risk of intraoperative atelectasis. Head-down positioning leads to further cephalad displacement of the diaphragm and further decrease in functional residual capacity above that induced by general anesthesia. Patient movement from the head-down to the supine position or from the supine to the head-up position may improve oxygenation.226,227,229,230,232,236,240-242,258 Odeberg and Sollevi demonstrate in a 1995 study59 that head-up positioning decreases elevated central venous and pulmonary artery occlusive pressures in contradistinction to his 1994 study that shows no decrement.27 Henny and Hofland233 advocate avoidance of any extreme positioning of patients with a history of congestive heart failure. Increased IAP also decreases functional residual capacity, and in the obese patient this may approach or exceed closing lung volumes to a greater extent. Treatment consists of adjusting tidal volume, recruitment procedures,281 application of positive end-expiratory pressure,231-233,242,282 pressure cycled modes of ventilation, and increasing inspiratory-to- expiratory ratio, as tolerated. Careful positive end-expiratory pressure titration is important, because positive end-expiratory pressure can decrease cardiac preload volume,58 reduce cardiac output,231-233,242 and induce hemodynamic instability.109,211,264,265,282 If refractory hypoxemia or hypercapnia occur, release the pneumoperitoneum and reinflate as tolerated with lower pressure. If hypoxemia or hypercapnia reoccurs, the abdomen may need to be opened.
Splanchnic and Renal Hypoperfusion
Anesthetic agents, intraoperative events, perioperative bowel preparations, NPO status, and decrease in cardiac output or MAP adversely affect intraoperative renal perfusion and urine output. Laparoscopic surgery adds to changes in renal perfusion by the presence of pneumoperitoneum and extremes of positioning. Cool, room-temperature CO2 may decrease not only core temperature, but urine output as well. Warmed or body- temperature insufflation may cause local renal vasodilatation and may be beneficial to patients with marginal renal function.283 The best way to maintain renal and splanchnic perfusion is to ensure adequate intravascular volume. Regardless, transient oliguria is common. The patient’s NPO fluid deficit and maintenance fluid requirements should be replaced. The initial decrease in venous return caused by the positive IAP may be overcome by judicious fluid administration, without creating hypervolemia. Insensible losses and third space fluid sequestration are at a minimum and any irrigant used that is not suctioned from the abdominal cavity needs to be added to the total patient fluid intake. An IAP of 8mm Hg to 10mm Hg is likely to benefit patients with pre-existing renal, splanchnic, or hepatic pathology.138,156,233,258 Because the head-up position decreases hepatic blood flow and worsens renal function, it is avoided, when possible, in patients with hepatic insufficiency or renal insufficiency.175,258,284 Patients with renal insufficiency or oliguria likely benefit from fluid infusion.125,233 Low dose dopamine120 and esmolol272 may also reduce oliguria. Humidification of insufflated CO2 also serves to decrease insensible losses. After release of the pneumoperitoneum, diuresis usually begins and renal function typically returns to baseline.
Increased Intracranial Pressure
Cerebral vasodilation from hypercapnia,227,228,232,241,258 increased systemic vascular resistance, head-down positioning,143,227,229,232,233,236,258,285 and increasing IAP229,232,241,258 can each increase intracranial pressure and decrease cerebral perfusion pressure. CDP commonly decreases cardiac output and may decrease cerebral perfusion pressure.229,232,241,258 CDP also increases cerebral blood flow with a concomitant increase in intracranial pressure.84,229 Laparoscopic surgery is therefore inadvisable in patients with decreased intracranial compliance. A patient with ventriculoperitoneal or peritoneal- jugular shunt must have the shunt closed before pneumoperitoneum is induced. Signs of increased intracranial pressure include bradycardia-to-asystole, systemic hypertension, EKG changes, accelerated cardiac rhythms, and hypotension-to-cardiovascular collapse. Manifestations of intracranial hypertension also include increased intracranial pressure, altered pupils, and decreased BIS. Adjunct therapy aimed at lowering the intracranial pressure and improving the cerebral perfusion pressure include mild hyperventilation, assuring a deeper level of anesthesia without compromising mean arterial pressure, and judicious use of loop diuretics, mannitol, or hypertonic saline, as tolerated. Because the head-down position increases intracranial pressure, many advocate the head-up position for patients at-risk during CDP.227,229,232,233,236,258 However, investigators have documented either no reduction in intracranial pressure143 or only partial reduction193 with the head-up position. Opinions and investigations indicate that hyperventilation may not lower intracranial pressure during CDP.145,258 Intracranial pressure monitoring during laparoscopic surgery is recommended for patients with altered intracranial compliance.192,193 Some experts consider that CDP is relatively contraindicated in patients with altered intracranial compliance.231,232,241,258 With evidence of intracranial hypertension, lower the IAP, deflate the abdomen, or convert to an open procedure, if instability persists.4,84,193,233,258
References
- Andersson L, Lindberg G, Bringman S, Ramel S, Anderberg B, Odeberg-Wernerman S. Pneumoperitoneum versus abdominal wall lift: effects on central haemodynamics and intrathoracic pressure during laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 2003;47:838-846.
- Branche PE, Duperret SL, Sagnard PE, Boulez JL, Petit PL, Viale JP. Left ventricular loading modifications induced by pneumoperitoneum: a time course echocardiographic study. Anesth Analg. 1998;86:482-487.
- Cunningham AJ, Turner J, Rosenbaum S, Rafferty T. Transoesophageal echocardiographic assessment of haemodynamic function during laparoscopic cholecystectomy. Br J Anaesth. 1993;70:621-625.
- De Cosmo G, Iannace E, Primieri P, et al. Changes in cerebral hemodynamics during laparoscopic cholecystectomy. Neurol Res. 1999;21:658-660.
- Dexter SP, Vucevic M, Gibson J, McMahon MJ. Hemodynamic consequences of high- and low-pressure capnoperitoneum during laparoscopic cholecystectomy. Surg Endosc. 1999;13:376-381.
- Dorsay DA, Greene FL, Baysinger CL. Hemodynamic changes during laparoscopic cholecystectomy monitored with transesophageal echocardiography. Surg Endosc. 1995;9:128-133.
- Dumont L, Mattys M, Mardirosoff C, Picard V, Alle JL, Massaut J. Hemodynamic changes during laparoscopic gastroplasty in morbidly obese patients. Obes Surg. 1997;7:326-331.
- Elliott S, Savill P, Eckersall S. Cardiovascular changes during laparoscopic cholecystectomy: a study using transoesophageal Doppler monitoring. Eur J Anaesthesiol. 1998;15:50-55.
- Feig BW, Berger DH, Dougherty TB, et al. Pharmacologic intervention can reestablish baseline hemodynamic parameters during laparoscopy. 1994;116:733-739.
- Fried M, Krska Z, Danzig V. Does the laparoscopic approach significantly affect cardiac functions in laparoscopic surgery? Pilot study in non-obese and morbidly obese patients. Obes Surg. 2001;11:293-296.
- Girardis M, Da Broi U, Antonutto G, Pasetto A. The effect of laparoscopic cholecystectomy on cardiovascular function and pulmonary gas exchange. Anesth Analg. 1996;83:134-140.
- Hachenberg T, Ebel C, Czorny M, Thomas H, Wendt M. Intrathoracic and pulmonary blood volume during CO2-pneumoperitoneum in humans. Acta Anaesthesiol Scand. 1998;42:794-798.
- Hirvonen EA, Poikolainen EO, Paakkonen ME, Nuutinen LS. The adverse hemodynamic effects of anesthesia, head-up tilt, and carbon dioxide pneumoperitoneum during laparoscopic cholecystectomy. Surg Endosc.2000;14:272-277.
- Hirvonen EA, Nuutinen LS, Kauko M. Hemodynamic changes due to Trendelenburg positioning and pneumoperitoneum during laparoscopic hysterectomy. Acta Anaesthesiol Scand. 1995;39:949-955.
- Huang SJ, Lee CY, Yeh FC, Chang CL. Hypercarbia is not the determinant factor of systemic arterial hypertension during carboperitoneum in laparoscopy. Ma Zui Xue Za Zhi. 1991;29:592-595.
- Joris JL, Noirot DP, Legrand MJ, Jacquet NJ, Lamy ML. Hemodynamic changes during laparoscopic cholecystectomy. Anesth Analg. 1993;76:1067-1071.
- Koivusalo AM, Kellokumpu I, Scheinin M, Tikkanen I, Makisalo H, Lindgren L. A comparison of gasless mechanical and conventional carbon dioxide pneumoperitoneum methods for laparoscopic cholecystectomy. Anesth Analg. 1998;86:153-158.
- Koivusalo AM, Kellokumpu I, Ristkari S, Lindgren L. Splanchnic and renal deterioration during and after laparoscopic cholecystectomy: a comparison of the carbon dioxide pneumoperitoneum and the abdominal wall lift method. Anesth Analg. 1997;85:886-891.
- Lindgren L, Koivusalo AM, Kellokumpu Conventional pneumoperitoneum compared with abdominal wall lift for laparoscopic cholecystectomy. Br J Anaesth. 1995;75:567-572.
- Liu SY, Leighton T, Davis I, Klein S, Lippmann M, Bongard F. Prospective analysis of cardiopulmonary responses to laparoscopic cholecystectomy. J Laparoendosc Surg. 1991;1:241-246.
- Marshall RL, Jebson PJ, Davie IT, Scott DB. Circulatory effects of carbon dioxide insufflation of the peritoneal cavity for laparoscopy. Br J Anaesth. 1972;44:680-684.
- McLaughlin JG, Scheeres DE, Dean RJ, Bonnell BW. The adverse hemodynamic effects of laparoscopic cholecystectomy. Surg Endosc. 1995;9:121-124.
- Myre K, Rostrup M, Buanes T, Stokland O. Plasma catecholamines and haemodynamic changes during pneumoperitoneum. Acta Anaesthesiol Scand. 1998;42:343-347.
- Myre K, Buanes T, Smith G, Stokland O. Simultaneous hemodynamic and echocardiographic changes during abdominal gas insufflation. Surg Laparosc Endosc. 1997;7:415-419.
- Nguyen NT, Ho HS, Fleming NW et al. Cardiac function during laparoscopic vs open gastric bypass. Surg Endosc. 2002;16:78-83.
- O’Leary E, Hubbard K, Tormey W, Cunningham AJ. Laparoscopic cholecystectomy: haemodynamic and neuroendocrine responses after pneumoperitoneum and changes in position. Br J Anaesth. 1996;76:640-644.
- Odeberg S, Ljungqvist O, Svenberg T, et al. Haemodynamic effects of pneumoperitoneum and the influence of posture during anaesthesia for laparoscopic surgery. Acta Anaesthesiol Scand. 1994;38:276-283.
- Rauh R, Hemmerling TM, Rist M, Jacobi KE. Influence of pneumoperitoneum and patient positioning on respiratory system compliance. J Clin Anesth. 2001;13:361-365.
- Safran D, Sgambati S, Orlando R, III. Laparoscopy in high-risk cardiac patients. Surg Gynecol Obstet. 1993;176:548-554.
- Schleifer W, Bissinger U, Guggenberger H, Heuser D. Variance of cardiorespiratory parameters during gynaecological surgery with CO2-pneumoperitoneum. Endosc Surg Allied Technol. 1995;3:167-170.
- Torrielli R, Cesarini M, Winnock S, Cabiro C, Mene JM. [Hemodynamic changes during celioscopy: a study carried out using thoracic electric bioimpedance] Modifications hemodynamiques durant la coelioscopie: etude menee par bioimpedance electrique thoracique. Can J Anaesth. 1990;37:46-51.
- Westerband A, Van De WJ, Amzallag M, et al. Cardiovascular changes during laparoscopic cholecystectomy. Surg Gynecol Obstet. 1992;175:535-538.
- Critchley LA, Critchley JA, Gin T. Haemodynamic changes in patients undergoing laparoscopic cholecystectomy: measurement by transthoracic electrical bioimpedance. Br J Anaesth. 1993;70:681-683.
- Dhoste K, Lacoste L, Karayan J, Lehuede MS, Thomas D, Fusciardi J. Haemodynamic and ventilatory changes during laparoscopic cholecystectomy in elderly ASA III patients. Can J Anaesth. 1996;43:783-788.
- Fujise K, Shingu K, Matsumoto S, Nagata A, Mikami O, Matsuda T. The effects of the lateral position on cardiopulmonary function during laparoscopic urological Anesth Analg. 1998;87:925-930.
- Hein HA, Joshi GP, Ramsay MA, et al. Hemodynamic changes during laparoscopic cholecystectomy in patients with severe cardiac disease. J Clin Anesth. 1997;9:261-265.
- Joris JL, Chiche JD, Canivet JL, Jacquet NJ, Legros JJ, Lamy ML. Hemodynamic changes induced by laparoscopy and their endocrine correlates: effects of clonidine. J Am Coll Cardiol. 1998;32:1389-1396.
- Larsen JF, Svendsen FM, Pedersen V. Randomized clinical trial of the effect of pneumoperitoneum on cardiac function and haemodynamics during laparoscopic cholecystectomy. Br J Surg. 2004;91:848-854.
- Salihoglu Z, Demiroluk S, Cakmakkaya S, Gorgun E, Kose Y. Influence of the patient positioning on respiratory mechanics during pneumoperitoneum. Middle East J Anesthesiol. 2002;16:521-528.
- Uemura N, Nomura M, Inoue S, et al. Changes in hemodynamics and autonomic nervous activity in patients undergoing laparoscopic cholecystectomy: differences between the pneumoperitoneum and abdominal wall-lifting method. 2002;34:643-650.
- Walder AD, Aitkenhead AR. Role of vasopressin in the haemodynamic response to laparoscopic cholecystectomy. Br J Anaesth. 1997;78:264-266.
- Harris SN, Ballantyne GH, Luther MA, Perrino AC, Jr. Alterations of cardiovascular performance during laparoscopic colectomy: a combined hemodynamic and echocardiographic analysis. Anesth Analg. 1996;83:482-487.
- Lenz RJ, Thomas TA, Wilkins DG. Cardiovascular changes during laparoscopy. Studies of stroke volume and cardiac output using impedance cardiography. 1976;31:4-12.
- Gannedahl P, Odeberg S, Brodin LA, Sollevi A. Effects of posture and pneumoperitoneum during anaesthesia on the indices of left ventricular filling. Acta Anaesthesiol Scand. 1996;40:160-166.
- Meininger D, Byhahn C, Bueck M, et al. Effects of prolonged pneumoperitoneum on hemodynamics and acid-base balance during totally endoscopic robot-assisted radical prostatectomies. World J Surg. 2002;26:1423-1427.
- Salihoglu Z, Demiroluk S, Dikmen Y. Respiratory mechanics in morbid obese patients with chronic obstructive pulmonary disease and hypertension during pneumoperitoneum. Eur J Anaesthesiol. 2003;20:658-661.
- Magno R, Medegard A, Bengtsson R, Tronstad SE. Acid-base balance during laparoscopy. The effects of intraperitoneal insufflation of carbon dioxide and nitrous oxide on acid-base balance during controlled ventilation. Acta Obstet Gynecol Scand. 1979;58:81-85.
- D’Ugo D, Persiani R, Pennestri F, et al. Transesophageal echocardiographic assessment of hemodynamic function during laparoscopic cholecystectomy in healthy patients. Surg Endosc. 2000;14:120-122.
- Ninomiya K, Kitano S, Yoshida T, Bandoh T, Baatar D, Matsumoto T. Comparison of pneumoperitoneum and abdominal wall lifting as to hemodynamics and surgical stress response during laparoscopic cholecystectomy. Surg Endosc. 1998;12:124-128.
- Johannsen G, Andersen M, Juhl B. The effect of general anaesthesia on the haemodynamic events during laparoscopy with CO2-insufflation. Acta Anaesthesiol Scand. 1989;33:132-136.
- Youssef MA, Saleh Al-Mulhim A. Effects of different anesthetic techniques on antidiuretic hormone secretion during laparoscopic cholecystectomy. Surg Endosc. 2007;21:1543-1548.
- de Plater RM, Jones IS. Non-fatal carbon dioxide embolism during laparoscopy. Anaesth Intensive Care. 1989;17:359-361.
- Duncan C. Carbon dioxide embolism during laparoscopy: a case report. AANA J. 1992;60:139-144.
- Dawson R, Ferguson CJ. Life-threatening tension pneumothorax during laparoscopic cholecystectomy. Surg Laparosc Endosc. 1997;7:271-272.
- Schulze S, Lyng KM, Bugge K, et al. Cardiovascular and respiratory changes and convalescence in laparoscopic colonic surgery: comparison between carbon dioxide pneumoperitoneum and gasless laparoscopy. Arch Surg. 1999;134:1112-1118.
- McKenzie R, Wadhwa RK, Bedger RC. Noninvasive measurement of cardiac output during laparoscopy. J Reprod Med. 1980;24:247-250.
- Rist M, Hemmerling TM, Rauh R, Siebzehnrubl E, Jacobi KE. Influence of pneumoperitoneum and patient positioning on preload and splanchnic blood volume in laparoscopic surgery of the lower abdomen. J Clin Anesth.2001;13:244-249.
- Kraut EJ, Anderson JT, Safwat A, Barbosa R, Wolfe BM. Impairment of cardiac performance by laparoscopy in patients receiving positive end-expiratory pressure. Arch Surg. 1999;134:76-80.
- Odeberg S, Sollevi A. Pneumoperitoneum for laparoscopic surgery does not increase venous admixture. Eur J Anaesthesiol. 1995;12:541-548.
- Galizia G, Prizio G, Lieto E, et al. Hemodynamic and pulmonary changes during open, carbon dioxide pneumoperitoneum and abdominal wall-lifting cholecystectomy. A prospective, randomized study. Surg Endosc.2001;15:477-483.
- Uchikoshi F, Kamiike W, Iwase K, et al. Laparoscopic cholecystectomy in patients with cardiac disease: hemodynamic advantage of the abdominal wall retraction Surg Laparosc Endosc. 1997;7:196-201.
- Alijani A, Hanna GB, Cuschieri A. Abdominal wall lift versus positive-pressure capnoperitoneum for laparoscopic cholecystectomy: randomized controlled trial. Ann Surg. 2004;239:388-394.
- Myles PS. Bradyarrhythmias and laparoscopy: a prospective study of heart rate changes with laparoscopy. Aust N Z J Obstet Gynaecol. 1991;31:171-173.
- Scott DB, Julian DG. Observations on cardiac arrythmias during laparoscopy. Br Med J. 1972;1:411-413.
- Kirkinen P, Hirvonen E, Kauko M, Purhonen S, Nuutinen Intracranial blood flow during laparoscopic hysterectomy. Acta Obstet Gynecol Scand. 1995;74:71-74.
- Mann C, Boccara G, Pouzeratte Y, Navarro F, Domergue J, Colson P. [Hemodynamic monitoring using esophageal Doppler ultrasonography during laparoscopic cholecystectomy] Monitorage hemodynamique par Doppler oesophagien lors de la cholecystectomie par coelioscopie. Can J Anaesth. 1999;46:15-20.
- Zuckerman RS, Heneghan S. The duration of hemodynamic depression during laparoscopic cholecystectomy. Surg Endosc. 2002;16:1233-1236.
- Matteucci C, Bagarani M. [The transesophageal echocardiographic evaluation of the hemodynamic effects of the pneumoperitoneum in laparoscopic hemicolectomy] Valutazione con ecocardiografia transesofagea degli effetti emodinamici del pneumoperitoneo nell’emicolectomia per via laparoscopica. G Ital Cardiol. 1999;29:424- 430.
- Zuckerman R, Gold M, Jenkins P, Rauscher LA, Jones M, Heneghan S. The effects of pneumoperitoneum and patient position on hemodynamics during laparoscopic cholecystectomy. Surg Endosc. 2001;15:562-565.
- Kubota K, Kajiura N, Teruya M, et al. Alterations in respiratory function and hemodynamics during laparoscopic cholecystectomy under pneumoperitoneum. Surg Endosc. 1993;7:500-504.
- Iwase K, Kamiike W, Uchikoshi F, Takenaka H, Miyata M, Matsuda H. Effect of pneumoperitoneum on interatrial pressure gradient during laparoscopic World J Surg. 1996;20:234-237.
- Greif WM, Forse RA. Cardiopulmonary effects of the laparoscopic pneumoperitoneum in a porcine model of adult respiratory distress syndrome. Am J Surg. 1999;177:216-221.
- Thaler W, Frey L, Marzoli GP, Messmer K. Assessment of splanchnic tissue oxygenation by gastric tonometry in patients undergoing laparoscopic and open cholecystectomy. Br J Surg. 1996;83:620-624.
- Hirvonen EA, Nuutinen LS, Kauko M. Ventilatory effects, blood gas changes, and oxygen consumption during laparoscopic hysterectomy. Anesth Analg. 1995;80:961-966.
- Luiz T, Huber T, Hartung HJ. [Ventilatory changes during laparoscopic cholecystectomy] Veranderungen der Ventilation wahrend laparoskopischer Cholezystektomie. 1992;41:520-526.
- Ogihara Y, Isshiki A, Kindscher JD, Goto H. Abdominal wall lift versus carbon dioxide insufflation for laparoscopic resection of ovarian tumors. J Clin Anesth. 1999;11:406-412.
- Puri GD, Singh H. Ventilatory effects of laparoscopy under general anaesthesia. Br J Anaesth. 1992;68:211-213.
- Tan PL, Lee TL, Tweed WA. Carbon dioxide absorption and gas exchange during pelvic laparoscopy. Can J Anaesth. 1992;39:677-681.
- Versichelen L, Serreyn R, Rolly G, Vanderkerckhove D. Physiopathologic changes during anesthesia administration for gynecologic laparoscopy. J Reprod Med. 1984;29:697-700.
- Wittgen CM, Andrus CH, Fitzgerald SD, Baudendistel LJ, Dahms TE, Kaminski Analysis of the hemodynamic and ventilatory effects of laparoscopic cholecystectomy. Arch Surg. 1991;126:997-1000.
- Baratz RA, Karis JH. Blood gas studies during laparoscopy under general anesthesia. Anesthesiology. 1969;30:463-464.
- Brampton WJ, Watson RJ. Arterial to end-tidal carbon dioxide tension difference during laparoscopy. Magnitude and effect of anaesthetic technique. 1990;45:210-214.
- De Waal EE, Kalkman CJ. Haemodynamic changes during low-pressure carbon dioxide pneumoperitoneum in young children. Paediatr Anaesth. 2003;13:18-25.
- Fujii Y, Tanaka H, Tsuruoka S, Toyooka H, Amaha K. Middle cerebral arterial blood flow velocity increases during laparoscopic cholecystectomy. Anesth Analg. 1994;78:80- 83.
- Makinen MT, Heinonen PO, Klemola UM, Yli-Hankala A. Gastric air tonometry during laparoscopic cholecystectomy: a comparison of two PaCO2 levels. Can J Anaesth. 2001;48:121-128.
- Pelosi P, Foti G, Cereda M, Vicardi P, Gattinoni Effects of carbon dioxide insufflation for laparoscopic cholecystectomy on the respiratory system. Anaesthesia. 1996;51:744-749.
- Wahba RW, Mamazza J. Ventilatory requirements during laparoscopic cholecystectomy. Can J Anaesth. 1993;40:206-210.
- Wakizaka Y, Sano S, Koike Y, Nakanishi Y, Uchino J. [Changes of arterial CO2 (PaCO2) and urine output by carbon dioxide insufflation of the peritoneal cavity during laparoscopic cholecystectomy]. Nippon Geka Gakkai Zasshi. 1994;95:336-342.
- Ishikawa S, Makita K, Sawa T, Toyooka H, Amaha K. Ventilatory effects of laparoscopic cholecystectomy under general anesthesia. J Anesth. 1997;11:179-183.
- Hsing CH, Hseu SS, Tsai SK, et al. The physiological effect of CO2 pneumoperitoneum in pediatric laparoscopy. Acta Anaesthesiol Sin. 1995;33:1-6.
- Kendall AP, Bhatt S, Oh TE. Pulmonary consequences of carbon dioxide insufflation for laparoscopic cholecystectomies. 1995;50:286-289.
- Mullett CE, Viale JP, Sagnard PE, et al. Pulmonary CO2 elimination during surgical procedures using intra- or extraperitoneal CO2 insufflation. Anesth Analg. 1993;76:622- 626.
- Ortega AE, Peters JH, Incarbone R, et al. A prospective randomized comparison of the metabolic and stress hormonal responses of laparoscopic and open cholecystectomy. J Am Coll Surg. 1996;183:249-256.
- Baraka A, Jabbour S, Hammoud R, et al. End-tidal carbon dioxide tension during laparoscopic cholecystectomy. Correlation with the baseline value prior to carbon dioxide insufflation. 1994;49:304-306.
- Hsieh CH. Laparoscopic cholecystectomy for patients with chronic obstructive pulmonary disease. J Laparoendosc Adv Surg Tech A. 2003;13:5-9.
- Sfez M, Guerard A, Desruelle P. Cardiorespiratory changes during laparoscopic fundoplication in children. Paediatr Anaesth. 1995;5:89-95.
- Tobias JD, Holcomb GW, III, Brock JW, III, Deshpande JK, Lowe S, Morgan WM, III. Cardiorespiratory changes in children during laparoscopy. J Pediatr Surg. 1995;30:33-36.
- Kazama T, Ikeda K, Kato T, Kikura M. Carbon dioxide output in laparoscopic cholecystectomy. Br J Anaesth. 1996;76:530-535.
- Wahba RW, Beique F, Kleiman SJ. Cardiopulmonary function and laparoscopic cholecystectomy. Can J Anaesth. 1995;42:51-63.
- Bardoczky GI, Engelman E, Levarlet M, Simon P. Ventilatory effects of pneumoperitoneum monitored with continuous spirometry. 1993;48:309- 311.
- Ferzli GS, Kiel T, Hurwitz JB, et al. Pneumothorax as a complication of laparoscopic inguinal hernia repair. Surg Endosc. 1997;11:152-153.
- Gabbott DA, Dunkley AB, Roberts FL. Carbon dioxide pneumothorax occurring during laparoscopic cholecystectomy. 1992;47:587-588.
- Carry PY, Gallet D, Francois Y, et al. Respiratory mechanics during laparoscopic cholecystectomy: the effects of the abdominal wall lift. Anesth Analg. 1998;87:1393- 1397.
- Makinen MT, Yli-Hankala A. The effect of laparoscopic cholecystectomy on respiratory compliance as determined by continuous spirometry. J Clin Anesth. 1996;8:119-122.
- Garcia-Perez M, Belda F, Lla J, et al. [Changes in chest wall and lung compliance during laparoscopic cholecystectomy] Cambios de la compliancia toracica y pulmonar durante la colecistectomia laparoscopica. Rev Esp Anestesiol Reanim. 2001;48:171-175.
- Williams MD, Murr PC. Laparoscopic insufflation of the abdomen depresses cardiopulmonary function. Surg Endosc. 1993;7:12-16.
- Junghans T, Bohm B, Grundel K, Schwenk W. Effects of pneumoperitoneum with carbon dioxide, argon, or helium on hemodynamic and respiratory function. Arch Surg. 1997;132:272-278.
- Windberger U, Siegl H, Woisetschlager R, Schrenk P, Podesser B, Losert U. Hemodynamic changes during prolonged laparoscopic surgery. Eur Surg Res. 1994;26:1- 9.
- Luz CM, Polarz H, Bohrer H, Hundt G, Dorsam J, Martin E. Hemodynamic and respiratory effects of pneumoperitoneum and PEEP during laparoscopic pelvic lymphadenectomy in dogs. Surg Endosc. 1994;8:25-27.
- Moncure M, Salem R, Moncure K, et al. Central nervous system metabolic and physiologic effects of laparoscopy. Am Surg. 1999;65:168-172.
- Fahy BG, Barnas GM, Flowers JL, Nagle SE, Njoku MJ. The effects of increased abdominal pressure on lung and chest wall mechanics during laparoscopic surgery. Anesth Analg. 1995;81:744-750.
- Makinen MT, Yli-Hankala A. Respiratory compliance during laparoscopic hiatal and inguinal hernia repair. Can J Anaesth. 1998;45:865-870.
- Sprung J, Whalley DG, Falcone T, Wilks W, Navratil JE, Bourke DL. The effects of tidal volume and respiratory rate on oxygenation and respiratory mechanics during laparoscopy in morbidly obese patients. Anesth Analg. 2003;97:268-274, table.
- Drummond GB, Martin LV. Pressure-volume relationships in the lung during laparoscopy. Br J Anaesth. 1978;50:261-270.
- Iwase K, Takenaka H, Ishizaka T, Ohata T, Oshima S, Sakaguchi K. Serial changes in renal function during laparoscopic cholecystectomy. Eur Surg Res. 1993;25:203-212.
- Koivusalo AM, Kellokumpu I, Scheinin M, Tikkanen I, Halme L, Lindgren L. Randomized comparison of the neuroendocrine response to laparoscopic cholecystectomy using either conventional or abdominal wall lift techniques. Br J Surg. 1996;83:1532-1536.
- Miki Y, Iwase K, Kamiike W, et al. Laparoscopic cholecystectomy and time-course changes in renal function. The effect of the retraction method on renal function. Surg Endosc. 1997;11:838-841.
- Nguyen NT, Perez RV, Fleming N, Rivers R, Wolfe BM. Effect of prolonged pneumoperitoneum on intraoperative urine output during laparoscopic gastric bypass. J Am Coll Surg. 2002;195:476-483.
- Nishio S, Takeda H, Yokoyama M. Changes in urinary output during laparoscopic adrenalectomy. BJU Int. 1999;83:944-947.
- Perez J, Taura P, Rueda J, et al. Role of dopamine in renal dysfunction during laparoscopic surgery. Surg Endosc. 2002;16:1297-1301.
- Chang DT, Kirsch AJ, Sawczuk IS. Oliguria during laparoscopic surgery. J Endourol. 1994;8:349-352.
- Dunn MD, McDougall EM. Renal physiology. Laparoscopic considerations. Urol Clin North Am. 2000;27:609-614.
- Chiu AW, Chang LS, Birkett DH, Babayan RK. The impact of pneumoperitoneum, pneumoretroperitoneum, and gasless laparoscopy on the systemic and renal hemodynamics. J Am Coll Surg. 1995;181:397-406.
- Horvath KD, Whelan RL, Lier B, et al. The effects of elevated intraabdominal pressure, hypercarbia, and positioning on the hemodynamic responses to laparoscopic colectomy in pigs. Surg Endosc. 1998;12:107-114.
- London ET, Ho HS, Neuhaus AM, Wolfe BM, Rudich SM, Perez RV. Effect of intravascular volume expansion on renal function during prolonged CO2 pneumoperitoneum. Ann Surg. 2000;231:195-201.
- McDougall EM, Monk TG, Wolf JS, Jr. et al. The effect of prolonged pneumoperitoneum on renal function in an animal model. J Am Coll Surg. 1996;182:317- 328.
- Shuto K, Kitano S, Yoshida T, Bandoh T, Mitarai Y, Kobayashi M. Hemodynamic and arterial blood gas changes during carbon dioxide and helium pneumoperitoneum in pigs. Surg Endosc. 1995;9:1173-1178.
- Chiu AW, Chang LS, Birkett DH, Babayan RK. Changes in urinary output and electrolytes during gaseous and gasless laparoscopy. Urol Res. 1996;24:361-366.
- Cisek LJ, Gobet RM, Peters CA. Pneumoperitoneum produces reversible renal dysfunction in animals with normal and chronically reduced renal function. J Endourol. 1998;12:95-100.
- Lindberg F, Bergqvist D, Bjorck M, Rasmussen I. Renal hemodynamics during carbon dioxide pneumoperitoneum: an experimental study in pigs. Surg Endosc. 2003;17:480-484.
- McDougall EM, Bennett HF, Monk TG, et al. Functional MR imaging of the porcine kidney: physiologic changes of prolonged pneumoperitoneum. 1997;1:29- 35.
- Demyttenaere S, Feldman LS, Fried GM. Effect of pneumoperitoneum on renal perfusion and function: a systematic review. Surg Endosc. 2007;21:152-160.
- Harman PK, Kron IL, McLachlan HD, Freedlender AE, Nolan SP. Elevated intra- abdominal pressure and renal function. Ann Surg. 1982;196:594-597.
- Are C, Kutka M, Talamini M, et al. Effect of laparoscopic antireflux surgery upon renal blood flow. Am J Surg. 2002;183:419-423.
- Hashikura Y, Kawasaki S, Munakata Y, Hashimoto S, Hayashi K, Makuuchi Effects of peritoneal insufflation on hepatic and renal blood flow. Surg Endosc. 1994;8:759-761.
- Rosin D, Brasesco O, Varela J, et al. Low-pressure laparoscopy may ameliorate intracranial hypertension and renal hypoperfusion. J Laparoendosc Adv Surg Tech A. 2002;12:15-19.
- Chiu AW, Chang LS, Birkett DH, Babayan RK. A porcine model for renal hemodynamic study during laparoscopy. J Surg Res. 1996;60:61-68.
- Windberger UB, Auer R, Keplinger F, et al. The role of intra-abdominal pressure on splanchnic and pulmonary hemodynamic and metabolic changes during carbon dioxide pneumoperitoneum. Gastrointest Endosc. 1999;49:84-91.
- Ivankovich AD, Miletich DJ, Albrecht RF, Heyman HJ, Bonnet RF. Cardiovascular effects of intraperitoneal insufflation with carbon dioxide and nitrous oxide in the dog. 1975;42:281-287.
- Iwase K, Takao T, Watanabe H, et al. Intra-abdominal venous pressure during laparoscopic cholecystectomy. HPB Surg. 1994;8:13-17.
- Kotzampassi K, Kapanidis N, Kazamias P, Eleftheriadis E. Hemodynamic events in the peritoneal environment during pneumoperitoneum in dogs. Surg Endosc. 1993;7:494- 499.
- Liem T, Applebaum H, Herzberger B. Hemodynamic and ventilatory effects of abdominal CO2 insufflation at various pressures in the young swine. J Pediatr Surg. 1994;29:966-969.
- Halverson A, Buchanan R, Jacobs L, et al. Evaluation of mechanism of increased intracranial pressure with insufflation. Surg Endosc. 1998;12:266-269.
- Ho HS, Gunther RA, Wolfe BM. Intraperitoneal carbon dioxide insufflation and cardiopulmonary functions. Laparoscopic cholecystectomy in pigs. Arch Surg. 1992;127:928-932.
- Rosenthal RJ, Friedman RL, Chidambaram A, et al. Effects of hyperventilation and hypoventilation on PaCO2 and intracranial pressure during acute elevations of intraabdominal pressure with CO2 pneumoperitoneum: large animal observations. J Am Coll Surg. 1998;187:32-38.
- Beebe DS, McNevin MP, Crain JM, et al. Evidence of venous stasis after abdominal insufflation for laparoscopic cholecystectomy. Surg Gynecol Obstet. 1993;176:443-447.
- Goodale RL, Beebe DS, McNevin MP, et al. Hemodynamic, respiratory, and metabolic effects of laparoscopic cholecystectomy. Am J Surg. 1993;166:533-537.
- Sobolewski AP, Deshmukh RM, Brunson BL, et al. Venous hemodynamic changes during laparoscopic cholecystectomy. J Laparoendosc Surg. 1995;5:363-369.
- Duke T, Steinacher SL, Remedios AM. Cardiopulmonary effects of using carbon dioxide for laparoscopic surgery in dogs. Vet Surg. 1996;25:77-82.
- Ido K, Suzuki T, Kimura K, et al. Lower-extremity venous stasis during laparoscopic cholecystectomy as assessed using color Doppler ultrasound. Surg Endosc. 1995;9:310-313.
- Millard JA, Hill BB, Cook PS, Fenoglio ME, Stahlgren LH. Intermittent sequential pneumatic compression in prevention of venous stasis associated with pneumoperitoneum during laparoscopic cholecystectomy. Arch Surg. 1993;128:914-918.
- Nguyen NT, Cronan M, Braley S, Rivers R, Wolfe BM. Duplex ultrasound assessment of femoral venous flow during laparoscopic and open gastric bypass. Surg Endosc. 2003;17:285-290.
- Schwenk W, Bohm B, Fugener A, Muller JM. Intermittent pneumatic sequential compression (ISC) of the lower extremities prevents venous stasis during laparoscopic cholecystectomy. A prospective randomized study. Surg Endosc. 1998;12:7-11.
- Jorgensen JO, Gillies RB, Lalak NJ, Hunt DR. Lower limb venous hemodynamics during laparoscopy: an animal study. Surg Laparosc Endosc. 1994;4:32-35.
- Eleftheriadis E, Kotzampassi K, Botsios D, Tzartinoglou E, Farmakis H, Dadoukis J. Splanchnic ischemia during laparoscopic cholecystectomy. Surg Endosc. 1996;10:324- 326.
- Schilling MK, Redaelli C, Krahenbuhl L, Signer C, Buchler MW. Splanchnic microcirculatory changes during CO2 laparoscopy. J Am Coll Surg. 1997;184:378-382.
- Yavuz Y, Ronning K, Lyng O, Marvik R, Gronbech JE. Effect of increased intraabdominal pressure on cardiac output and tissue blood flow assessed by color- labeled microspheres in the pig. Surg Endosc.2001;15:149-155.
- Knolmayer TJ, Bowyer MW, Egan JC, Asbun HJ. The effects of pneumoperitoneum on gastric blood flow and traditional hemodynamic Surg Endosc. 1998;12:115-118.
- Diebel LN, Dulchavsky SA, Wilson RF. Effect of increased intra-abdominal pressure on mesenteric arterial and intestinal mucosal blood flow. J Trauma. 1992;33:45- 48.
- Ishizaki Y, Bandai Y, Shimomura K, Abe H, Ohtomo Y, Idezuki Y. Safe intraabdominal pressure of carbon dioxide pneumoperitoneum during laparoscopic surgery. 1993;114:549-554.
- Agusti M, Elizalde JI, Adalia R, Cifuentes A, Fontanals J, Taura P. Dobutamine restores intestinal mucosal blood flow in a porcine model of intra-abdominal hyperpressure. Crit Care Med. 2000;28:467-472.
- Andrei VE, Schein M, Wise Small bowel ischemia following laparoscopic cholecystectomy. Dig Surg. 1999;16:522-524.
- Hebebrand D, Menningen R, Sommer H, Roehr SP, Troidl H. Small-bowel necrosis following laparoscopic cholecystectomy: a clinically relevant complication? 1995;27:281.
- Jaffe V, Russell RC. Fatal intestinal ischaemia following laparoscopic cholecystectomy. Br J Surg. 1994;81:1827-1828.
- Klugewitz K, Rehermann B, Seifert U, et al. A rare case of bloody diarrhea: thrombosis of the V. mesenterica inferior following laparoscopic cholecystectomy. Z Gastroenterol. 1998;36:35-39.
- Leduc LJ, Mitchell A. Intestinal ischemia after laparoscopic cholecystectomy. JSLS. 2006;10:236-238.
- Paul A, Troidl H, Peters S, Stuttmann R. Fatal intestinal ischaemia following laparoscopic cholecystectomy. Br J Surg. 1994;81:1207.
- Schorr RT. Laparoscopic upper abdominal operations and mesenteric infarction. J Laparoendosc Surg. 1995;5:389-392.
- Sato K, Kawamura T, Wakusawa R. Hepatic blood flow and function in elderly patients undergoing laparoscopic cholecystectomy. Anesth Analg. 2000;90:1198-1202.
- Diebel LN, Wilson RF, Dulchavsky SA, Saxe J. Effect of increased intra-abdominal pressure on hepatic arterial, portal venous, and hepatic microcirculatory blood flow. J Trauma. 1992;33:279-282.
- Tunon MJ, Gonzalez P, Jorquera F, Llorente A, Gonzalo-Orden M, Gonzalez- Gallego J. Liver blood flow changes during laparoscopic surgery in pigs. A study of hepatic indocyanine green removal. Surg Endosc. 1999;13:668-672.
- Ali NA, Eubanks WS, Stamler JS et al. A method to attenuate pneumoperitoneum- induced reductions in splanchnic blood flow. Ann Surg. 2005;241:256-261.
- Sala-Blanch X, Fontanals J, Martinez-Palli G, et al. Effects of carbon dioxide vs helium pneumoperitoneum on hepatic blood flow. Surg Endosc. 1998;12:1121-1125.
- Ishizaki Y, Bandai Y, Shimomura K, Abe H, Ohtomo Y, Idezuki Y. Changes in splanchnic blood flow and cardiovascular effects following peritoneal insufflation of carbon dioxide. Surg Endosc. 1993;7:420-423.
- Klopfenstein CE, Morel DR, Clergue F, Pastor CM. Effects of abdominal CO2 insufflation and changes of position on hepatic blood flow in anesthetized pigs. Am J Physiol. 1998;275:H900-H905.
- Takagi S. Hepatic and portal vein blood flow during carbon dioxide pneumoperitoneum for laparoscopic hepatectomy. Surg Endosc. 1998;12:427-431.
- Hasukic S, Kosuta D, Muminhodzic K. Comparison of postoperative hepatic function between laparoscopic and open cholecystectomy. Med Princ Pract. 2005;14:147-150.
- Morino M, Giraudo G, Festa V. Alterations in hepatic function during laparoscopic surgery. An experimental clinical study. Surg Endosc. 1998;12:968-972.
- Nguyen NT, Braley S, Fleming NW, Lambourne L, Rivers R, Wolfe BM. Comparison of postoperative hepatic function after laparoscopic versus open gastric bypass. Am J Surg. 2003;186:40-44.
- Saber AA, Laraja RD, Nalbandian HI, Pablos-Mendez A, Hanna K. Changes in liver function tests after laparoscopic cholecystectomy: not so rare, not always Am Surg. 2000;66:699-702.
- Sakorafas G, Anagnostopoulos G, Stafyla V, et al. Elevation of serum liver enzymes after laparoscopic cholecystectomy. N Z Med J. 2005;118:U1317.
- Kotake Y, Takeda J, Matsumoto M, Tagawa M, Kikuchi H. Subclinical hepatic dysfunction in laparoscopic cholecystectomy and laparoscopic colectomy. Br J Anaesth. 2001;87:774-777.
- Tan M, Xu FF, Peng JS, et al. Changes in the level of serum liver enzymes after laparoscopic surgery. World J Gastroenterol. 2003;9:364-367.
- Hazebroek EJ, de Vos tot Nederveen Cappel, Gommers D, et al. Antidiuretic hormone release during laparoscopic donor nephrectomy. Arch Surg. 2002;137:600-604.
- Melville RJ, Frizis HI, Forsling ML, LeQuesne LP. The stimulus for vasopressin release during laparoscopy. Surg Gynecol Obstet. 1985;161:253-256.
- Solis Herruzo JA, Castellano G, Larrodera L, et al. Plasma arginine vasopressin concentration during laparoscopy. 1989;36:499-503.
- Viinamki O, Punnonen R. Vasopressin release during laparoscopy: role of increased intra-abdominal pressure. 1982;1:175-176.
- Glaser F, Sannwald GA, Buhr HJ, et al. General stress response to conventional and laparoscopic cholecystectomy. Ann Surg. 1995;221:372-380.
- Hirvonen EA, Nuutinen LS, Vuolteenaho O. Hormonal responses and cardiac filling pressures in head-up or head-down position and pneumoperitoneum in patients undergoing operative laparoscopy. Br J Anaesth.1997;78:128-133.
- Larsen JF, Ejstrud P, Svendsen F, Pedersen V, Redke F. Systemic response in patients undergoing laparoscopic cholecystectomy using gasless or carbon dioxide pneumoperitoneum: a randomized study. J Gastrointest Surg. 2002;6:582-586.
- McMahon AJ, O’Dwyer PJ, Cruikshank AM, et al. Comparison of metabolic responses to laparoscopic and minilaparotomy cholecystectomy. Br J Surg. 1993;80:1255-1258.
- Uzzo RG, Bilsky M, Mininberg DT, Poppas DP. Laparoscopic surgery in children with ventriculoperitoneal shunts: effect of pneumoperitoneum on intracranial pressure– preliminary experience. 1997;49:753-757.
- Irgau I, Koyfman Y, Tikellis JI. Elective intraoperative intracranial pressure monitoring during laparoscopic cholecystectomy. Arch Surg. 1995;130:1011-1013.
- Josephs LG, Este-McDonald JR, Birkett DH, Hirsch EF. Diagnostic laparoscopy increases intracranial pressure. J Trauma. 1994;36:815-818.
- Schob OM, Allen DC, Benzel E, et al. A comparison of the pathophysiologic effects of carbon dioxide, nitrous oxide, and helium pneumoperitoneum on intracranial pressure. Am J Surg. 1996;172:248-253.
- Hall JB, Schmidt GA. Principles of Critical Care. McGraw-Hill Companies; 2005.
- Gomez-Sanchez CE, Gomez-Sanchez EP, Yamakita N. Endocrine causes of hypertension. Semin Nephrol. 1995;15:106-115.
- Hall WD. Resistant hypertension, secondary hypertension, and hypertensive crises. Cardiol Clin. 2002;20:281-289.
- Taler SJ. Secondary causes of hypertension. Prim Care. 2008;35:489-500, vi.
- Sica DA. Endocrine causes of secondary hypertension. J Clin Hypertens (Greenwich). 2008;10:534-540.
- Gregory GA, Eger EI, Smith NT, Cullen BF. The cardiovascular effects of carbon dioxide in man awake and during diethyl ether anesthesia. 1974;40:301- 304.
- Rasmussen JP, Dauchot PJ, DePalma RG, et al. Cardiac function and hypercarbia. Arch Surg. 1978;113:1196-1200.
- Feihl F, Perret C. Permissive hypercapnia. How permissive should we be? Am J Respir Crit Care Med.1994;150:1722-1737.
- Naude GP, Bongard FS. Helium insufflation in laparoscopic surgery. Endosc Surg Allied Technol. 1995;3:183-186.
- Cullen DJ, Eger EI. Cardiovascular effects of carbon dioxide in man. Anesthesiology. 1974;41:345-349.
- Langford NJ. Carbon dioxide poisoning. Toxicol Rev. 2005;24:229-235.
- Orchard CH, Kentish JC. Effects of changes of pH on the contractile function of cardiac muscle. Am J Physiol. 1990;258:C967-C981.
- Sexton J, Mueller K, Elliott A, Gerzer D, Strohl KP. Low level CO2 effects on pulmonary function in humans. Aviat Space Environ Med. 1998;69:387-390.
- Leighton TA, Liu SY, Bongard FS. Comparative cardiopulmonary effects of carbon dioxide versus helium pneumoperitoneum. 1993;113:527-531.
- Gutt CN, Oniu T, Mehrabi A, et al. Circulatory and respiratory complications of carbon dioxide insufflation. Dig Surg. 2004;21:95-105.
- Moffa SM, Quinn JV, Slotman GJ. Hemodynamic effects of carbon dioxide pneumoperitoneum during mechanical ventilation and positive end-expiratory pressure. J Trauma. 1993;35:613-617.
- Magrina JF. Complications of laparoscopic surgery. Clin Obstet Gynecol. 2002;45:469-480.
- Wolf JS, Jr., Stoller ML. The physiology of laparoscopy: basic principles, complications and other considerations. J Urol. 1994;152:294-302.
- Chiu AW, Azadzoi KM, Hatzichristou DG, Siroky MB, Krane RJ, Babayan Effects of intra-abdominal pressure on renal tissue perfusion during laparoscopy. J Endourol. 1994;8:99-103.
- Demyttenaere SV, Feldman LS, Bergman S, et al. Does aggressive hydration reverse the effects of pneumoperitoneum on renal perfusion? Surg Endosc. 2006;20:274- 280.
- Baxter JN, O’Dwyer PJ. Pathophysiology of laparoscopy. Br J Surg. 1995;82:1-2.
- Bloomfield GL, Ridings PC, Blocher CR, Marmarou A, Sugerman HJ. Effects of increased intra-abdominal pressure upon intracranial and cerebral perfusion pressure before and after volume expansion. J Trauma. 1996;40:936-941.
- Chandra A, Mangam S, Marzouk D. A review of risk scoring systems utilised in patients undergoing gastrointestinal surgery. J Gastrointest Surg. 2009;13:1529-1538.
- Napolitano LM, Bass BL. Risk-adjusted outcomes and perioperative care. Surg Clin North Am. 2005;85:1341-6, xiv.
- Khuri SF, Daley J, Henderson W, et al. Risk adjustment of the postoperative mortality rate for the comparative assessment of the quality of surgical care: results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg. 1997;185:315-327.
- Can MF, Yagci G, Tufan T, Ozturk E, Zeybek N, Cetiner S. Can SAPS II predict operative mortality more accurately than POSSUM and P-POSSUM in patients with colorectal carcinoma undergoing resection? World J Surg. 2008;32:589-595.
- Wolters U, Wolf T, Stutzer H, Schroder T. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth. 1996;77:217-222.
- Ceulemans R, Al Ahdab N, Leroy J, et al. Safe laparoscopic surgery in the elderly. Am J Surg. 2004;187:323-327.
- Cheng SP, Chang YC, Liu CL, et al. Factors associated with prolonged stay after laparoscopic cholecystectomy in elderly patients. Surg Endosc. 2008;22:1283-1289.
- Tambyraja AL, Kumar S, Nixon SJ. POSSUM scoring for laparoscopic cholecystectomy in the elderly. ANZ J Surg. 2005;75:550-552.
- Cunningham AJ. Anesthetic implications of laparoscopic surgery. Yale J Biol Med. 1998;71:551-578.
- Gerges FJ, Kanazi GE, Jabbour-Khoury SI. Anesthesia for laparoscopy: a review. J Clin Anesth. 2006;18:67-78.
- Joshi GP. Complications of laparoscopy. Anesthesiol Clin North America. 2001;19:89-105.
- Leonard IE, Cunningham AJ. Anaesthetic considerations for laparoscopic cholecystectomy. Best Pract Res Clin Anaesthesiol. 2002;16:1-20.
- Smith Anesthesia for laparoscopy with emphasis on outpatient laparoscopy. Anesthesiol Clin North America. 2001;19:21-41.
- Tobias JD. Anesthetic considerations for laparoscopy in children. Semin Laparosc Surg. 1998;5:60-66.
- Pennant JH. Anesthesia for laparoscopy in the pediatric patient. Anesthesiol Clin North America. 2001;19:69-88.
- Henny CP, Hofland J. Laparoscopic surgery: pitfalls due to anesthesia, positioning, and pneumoperitoneum. Surg Endosc. 2005;19:1163-1171.
- Slowey KB, Slowey MJ, Cato J. Cardiac complications of laparoscopy: anesthetic implications. 1996;7:9-13.
- Galland RB. Severity scores in surgery: what for and who needs them? Langenbecks Arch Surg. 2002;387:59-62.
- Coskun F. Anesthesia for gynecologic laparoscopy. J Am Assoc Gynecol Laparosc. 1999;6:245-258.
- Hanley ES. Anesthesia for laparoscopic surgery. Surg Clin North Am. 1992;72:1013-1019.
- Takrouri MS. Anesthesia for laparoscopic general surgery. A special review. Middle East J Anesthesiol. 1999;15:39-62.
- Kuczkowski KM. Laparoscopic procedures during pregnancy and the risks of anesthesia: what does an obstetrician need to know? Arch Gynecol Obstet. 2007;276:201- 209.
- Steinbrook RA, Brooks DC, Datta S. Laparoscopic cholecystectomy during pregnancy. Review of anesthetic management, surgical considerations. Surg Endosc. 1996;10:511-515.
- Wedgewood J, Doyle E. Anaesthesia and laparoscopic surgery in children. Paediatr Anaesth. 2001;11:391-399.
- Petrat G, Weyandt D, Klein U. Anesthetic considerations in pediatric laparoscopic and thoracoscopic surgery. Eur J Pediatr Surg. 1999;9:282-285.
- Collins LM, Vaghadia H. Regional anesthesia for laparoscopy. Anesthesiol Clin North America.2001;19:43-55.
- Ott DE. Desertification of the peritoneum by thin-film evaporation during laparoscopy. JSLS. 2003;7:189-195.
- Yamanaka MK, Sue DY. Comparison of arterial-end-tidal PCO2 difference and dead space/tidal volume ratio in respiratory failure. 1987;92:832-835.
- Casati A, Salvo I, Torri G, Calderini E. Arterial to end-tidal carbon dioxide gradient and physiological dead space monitoring during general anaesthesia: effects of patients’ position. Minerva Anestesiol. 1997;63:177-182.
- Hardman JG, Aitkenhead AR. Estimation of alveolar deadspace fraction using arterial and end-tidal CO2: a factor analysis using a physiological simulation. Anaesth Intensive Care. 1999;27:452-458.
- Hardman JG, Aitkenhead AR. Estimating alveolar dead space from the arterial to end-tidal CO(2) gradient: a modeling analysis. Anesth Analg. 2003;97:1846-1851.
- Sharma KC, Brandstetter RD, Brensilver JM, Jung LD. Cardiopulmonary physiology and pathophysiology as a consequence of laparoscopic surgery. 1996;110:810-815.
- Michard F. Changes in arterial pressure during mechanical ventilation. Anesthesiology. 2005;103:419-428.
- Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37:2642-2647.
- Mayer J, Boldt J, Poland R, Peterson A, Manecke GR, Jr. Continuous arterial pressure waveform-based cardiac output using the FloTrac/Vigileo: a review and meta- analysis. J Cardiothorac Vasc Anesth. 2009;23:401-406.
- Osman D, Ridel C, Ray P, et al. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med. 2007;35:64-68.
- Memtsoudis SG, Rosenberger P, Loffler M, et al. The usefulness of transesophageal echocardiography during intraoperative cardiac arrest in noncardiac surgery. Anesth Analg. 2006;102:1653-1657.
- Suriani RJ, Neustein S, Shore-Lesserson L, Konstadt S. Intraoperative transesophageal echocardiography during noncardiac surgery. J Cardiothorac Vasc Anesth. 1998;12:274-280.
- Denault AY, Couture P, McKenty S, et al. Perioperative use of transesophageal echocardiography by anesthesiologists: impact in noncardiac surgery and in the intensive care unit. Can J Anaesth. 2002;49:287-293.
- Shernan, S. Utility of perioperative transesophageal echocardiography in non- cardiac surgery. 2008. American Society of Anesthesiologists Annual Review Course Meeting.
- O’Malley C, Cunningham AJ. Physiologic changes during laparoscopy. Anesthesiol Clin North America. 2001;19:1-19.
- Cunningham AJ. Laparoscopic surgery–anesthetic implications. Surg Endosc. 1994;8:1272-1284.
- Cohen MM, Duncan PG, Pope WD, Wolkenstein C. A survey of 112,000 anaesthetics at one teaching hospital (1975-83). Can Anaesth Soc J. 1986;33:22-31.
- Bijker JB, van Klei WA, Kappen TH, van Wolfswinkel L, Moons KG, Kalkman CJ. Incidence of intraoperative hypotension as a function of the chosen definition: literature definitions applied to a retrospective cohort using automated data collection. 2007;107:213-220.
- Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic management and one-year mortality after noncardiac surgery. Anesth Analg. 2005;100:4-10.
- Reich DL, Hossain S, Krol M, et al. Predictors of hypotension after induction of general anesthesia. Anesth Analg. 2005;101:622-8, table.
- Woolley DS, Puglisi RN, Bilgrami S, Quinn JV, Slotman GJ. Comparison of the hemodynamic effects of gasless abdominal distention and CO2 pneumoperitoneum during incremental positive end-expiratory pressure. J Surg Res. 1995;58:75-80.
- Burchard KW, Ciombor DM, McLeod MK, Slothman GJ, Gann DS. Positive end expiratory pressure with increased intra-abdominal pressure. Surg Gynecol Obstet. 1985;161:313-318.
- Whiston RJ, Eggers KA, Morris RW, Stamatakis JD. Tension pneumothorax during laparoscopic cholecystectomy. Br J Surg. 1991;78:1325.
- Kheterpal S, O’Reilly M, Englesbe MJ, et al. Preoperative and intraoperative predictors of cardiac adverse events after general, vascular, and urological Anesthesiology. 2009;110:58-66.
- Derouin M, Couture P, Boudreault D, Girard D, Gravel D. Detection of gas embolism by transesophageal echocardiography during laparoscopic Anesth Analg. 1996;82:119-124.
- Kim CS, Kim JY, Kwon JY, et al. Venous air embolism during total laparoscopic hysterectomy: comparison to total abdominal hysterectomy. 2009;111:50-54.
- Gabrielli A, O’Connor MF, Maccioli GA. Anesthesia Advanced Circulatory Life Support (American Society of Anesthesiologists and American Society of Critical Care Anesthesiologists). 2008.
- Laisalmi M, Koivusalo AM, Valta P, Tikkanen I, Lindgren L. Clonidine provides opioid-sparing effect, stable hemodynamics, and renal integrity during laparoscopic cholecystectomy. Surg Endosc. 2001;15:1331-1335.
- Koivusalo AM, Scheinin M, Tikkanen I, et al. Effects of esmolol on haemodynamic response to CO2 pneumoperitoneum for laparoscopic surgery. Acta Anaesthesiol Scand. 1998;42:510-517.
- Coloma M, Chiu JW, White PF, Armbruster SC. The use of esmolol as an alternative to remifentanil during desflurane anesthesia for fast-track outpatient gynecologic laparoscopic surgery. Anesth Analg. 2001;92:352-357.
- Grmec S, Lah K, Tusek-Bunc K. Difference in end-tidal CO2 between asphyxia cardiac arrest and ventricular fibrillation/pulseless ventricular tachycardia cardiac arrest in the prehospital setting. Crit Care. 2003;7:R139-R144.
- Yoshida T, Watanabe M, Murakami M, Furukawa H, Nakahara H. End-tidal carbon dioxide monitoring indicates recovery from cardiogenic shock in patients receiving percutaneous cardiopulmonary support. J Artif Organs. 2005;8:63-66.
- Moon SW, Lee SW, Choi SH, Hong YS, Kim SJ, Kim NH. Arterial minus end-tidal CO2 as a prognostic factor of hospital survival in patients resuscitated from cardiac arrest. 2007;72:219-225.
- Sprung J, Whalley DG, Falcone T, Warner DO, Hubmayr RD, Hammel J. The impact of morbid obesity, pneumoperitoneum, and posture on respiratory system mechanics and oxygenation during laparoscopy. Anesth Analg. 2002;94:1345-1350.
- Demiroluk S, Salihoglu Z, Bakan M, Bozkurt P. Effects of intraperitoneal and extraperitoneal carbon dioxide insufflation on blood gases during the perioperative period. J Laparoendosc Adv Surg Tech A. 2004;14:219-222.
- Liu SY, Bongard FS. Cardiopulmonary pathophysiology of laparoscopy and pneumoperitoneum. In: While RA, Klein SR, eds. Endoscopic surgery. Louis: Mosby Year-Book Publishers; 1991:159-169.
- Leong LM, Ali A. Carbon dioxide pneumothorax during laparoscopic fundoplication. 2003;58:97.
- Pang CK, Yap J, Chen PP. The effect of an alveolar recruitment strategy on oxygenation during laparascopic cholecystectomy. Anaesth Intensive Care. 2003;31:176- 180.
- Loeckinger A, Kleinsasser A, Hoermann C, Gassner M, Keller C, Lindner Inert gas exchange during pneumoperitoneum at incremental values of positive end- expiratory pressure. Anesth Analg. 2000;90:466-471.
- Backlund M, Kellokumpu I, Scheinin T, von Smitten K, Tikkanen I, Lindgren L. Effect of temperature of insufflated CO2 during and after prolonged laparoscopic surgery. Surg Endosc. 1998;12:1126-1130.
- Junghans T, Bohm B, Grundel K, Schwenk W, Muller JM. Does pneumoperitoneum with different gases, body positions, and intraperitoneal pressures influence renal and hepatic blood flow? 1997;121:206-211.
- Rosenthal RJ, Hiatt JR, Phillips EH, Hewitt W, Demetriou AA, Grode M. Intracranial pressure. Effects of pneumoperitoneum in a large-animal model. Surg Endosc. 1997;11:376-380.
- Wittgen CM, Naunheim KS, Andrus CH, Kaminski DL. Preoperative pulmonary function evaluation for laparoscopic cholecystectomy. Arch Surg. 1993;128:880-885.
