Complications of Robot Assisted Radical Cystectomy (RARC)
Michael E. Woods, MD, Rafael N. Nunez, MD, and Erik P. Castle, MD
INTRODUCTION
Radical cystectomy and urinary diversion remains the cornerstone for the surgical management of bladder cancer. Despite its effectiveness, the procedure is associated with significant morbidity with mortality rates approaching 2%.1 The field of urology has embraced minimally invasive surgery to decrease the impact of disease treatment on our patients, which has been clearly seen by the shift in approach to radical nephrectomy2 and radical prostatectomy.3The application of laparoscopy to radical cystectomy has followed this trend, but has experienced a much slower growth. The first laparoscopic simple cystectomy was reported in 1992 by Parra et al. describing the removal of a benign retained bladder.4 Since that publication there have been several reports of laparoscopic radical cystectomy (LRC) for malignant disease with various methods of urinary diversion.5-9 With the introduction of the daVinciTM surgical system (Intuitive Surgical, Sunnyvale, CA) the prevalence of laparoscopic radical prostatectomies has seen a sharp increase as this tool has helped overcome some the technical challenges of laparoscopic pelvic surgery.3 It did not take long for surgeons to apply the technology of the daVinci system to performing laparoscopic cystectomies. In 2003, Menon et al. published the first series of robot-assisted radical cystectomy (RARC) and urinary diversion.10 There are several technical and physiologic aspects of LRC/RARC that may potentially translate into decreased morbidity for patients. The pneumoperitoneum will decrease blood loss and there are less insensible losses due to a closed abdomen resulting decreased fluid shifts. These patients will have a smaller incision and less retractor injury to their abdominal musculature leading to decreased analgesic requirements and earlier post operative ambulation. Finally, by the creation of pneumoperitoneum and Trendelenberg positioning the bowels are displaced from the operative field by gravity alone resulting in minimal manipulation, which in theory should contribute to earlier bowel function. Despite these advantages, LRC/RARC are not immune to potential surgical complications. In and of itself, a radical cystectomy can be a technically demanding procedure with an associated morbidity that is reported in the literature to range from 28 to 64%.11-20
A minimally invasive approach may have the potential to affect the outcomes of radical cystectomy as far as perioperative complications are concerned. In a recent comparison of open radical cystectomy (ORC) to LRC there was decreased blood loss, blood transfusion, and time to oral intake in the LRC group, although overall complications were similar.21 Some early reports comparing open and robot assisted radical cystectomy, have shown a diminished complication rate of 30 to 50% in the robotic approach.20,22-23 In our own experience, perioperative complications for robot assisted radical cystectomy have decreased from 68 to 28%.24 There are some complications that are inherent to the use of laparoscopic/robotic techniques such as port placement, positioning, use of pneumoperitoneum, and instrumentation. On the other hand, there are complications that are attributable to a radical cystectomy regardless of the approach used. The purpose of this chapter is to assess the most common complications and to discuss their prevention, recognition, and management in patients undergoing LRC/RARC. Reported incidence and outcomes will be discussed while prevention and management will be specifically outlined.
CURRENT LITERATURE ON LRC/RARC
There is a plethora of published reports on robot assisted radical prostatectomy. The current available literature on RARC is not as robust but is rapidly increasing. Most reports are limited to small to moderate sized case series as well as some scattered case reports. Fifteen reports were identified accounting for a total of 294 cases. Fifty-one complications were reported (Table 1). Ileus (10 patients) followed by urinary leak (3 patients), small bowel obstruction (3 patients) and port site bleeding (3 patients) were the most common complications identified. One death was reported.25-39 In review of several large LRC series, the reported rate of complications ranged from 18-42%, with two reported deaths out of 258 patients.6,40-43 As expected, a majority of the complications were urologic and gastrointestinal in nature, but a full summery of these complications can be found in Table 1.
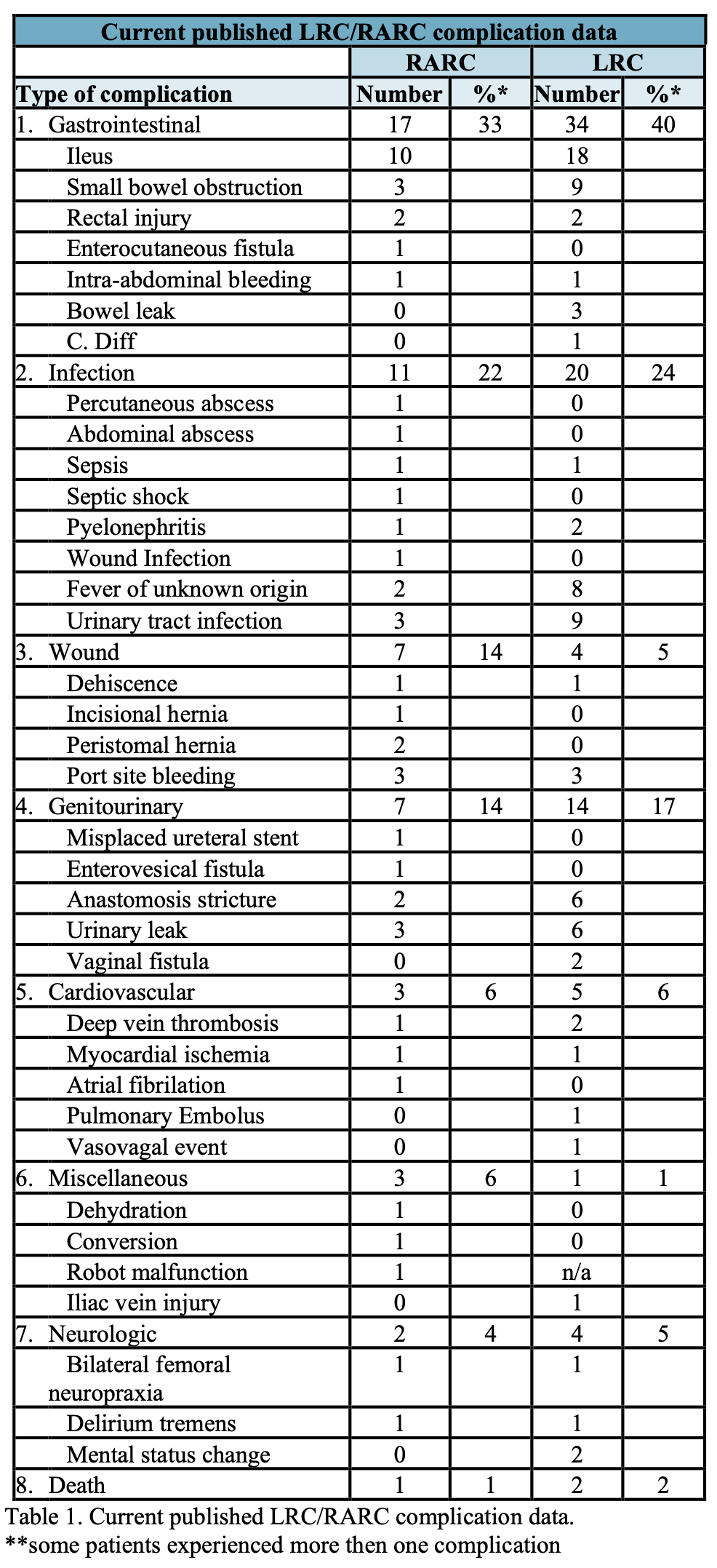
Interpreting published series on complications can be difficult regardless of approach. Complication reporting is fraught with inherent bias primarily due to how complications are defined. Unless standardized categorization is utilized such as the Clavien classification system, readers are left with rates that may vary dramatically from one series to another.14 Despite the lack of uniformity within the published literature for open and laparoscopic surgery, the current available literature provides us with the knowledge of the types of complications that may be encountered as well as the relative rates to expect post operatively. It is this knowledge that will allow surgeons to modify their technique and post surgical care thereby minimizing the risk of these complications. The full spectrum of potential complications ranging from equipment malfunction to peri- operative and post-operative complications will be covered herein this chapter.
EQUIPMENT MALFUNCTION
When utilizing and depending on mechanical devices or instruments, the surgeon is partially at the mercy of rates of equipment malfunction. Having a “back-up” or contingency plan is crucial. To ensure proper functioning, the robotic technology must be supported by a well trained staff. They should be capable not only of quickly recognizing any equipment malfunction but also of providing an immediate and appropriate response to address problems. Fortunately, non-recoverable robot failures occur in less than 0.5% of cases as reported by Lavery et al.44
Although the exact incidence is unknown, some of the older standard robotic systems had a relatively large number of “recoverable” faults. These faults result in disengagement of the masters in the console until the fault was addressed. The disengagement was presumably to prevent inappropriate continued movement of instruments and hopefully prevented injury to the patient. Some of these faults may be attributable to the age and version of the robotic systems but may also be a product of inappropriate use and positioning. In truth, many robotic techniques were being developed and perfect arm and robot positioning may not have been completely delineated.
Prevention:
- Well trained and seasoned surgical team;
- Inspection of instruments to ensure integrity of the cable system and servos;
- Ensure that start-up procedures of the system are normal to avoid underlying system errors;
- Ensure appropriate number of “uses” are left in each instrument.
- Ensure appropriate patient positioning and port placement to minimize instrument/camera contact (this particularly important in older robotic systems)
Management:
- Stop activity that caused the malfunction;
- If the malfunction is specific to the instrument (e.g. scissors), replace with a fresh instrument;
- Robotic arm malfunction: If recoverable may be due to brief and unexpected movement. If continues, contact support services to review error codes associated with the event and identify if can be managed intraoperatively. One may consider disengaging arm and using addition “4th arm” if available to complete the procedure;
- Write down and record error codes to have for support technician.
- Undocking the instrument/arm and re-docking in a different orientation. Evaluate the “boot” of the arm or camera and determine if orientation is appropriate for the arm;
- If equipment failure is not recoverable, conversion to a laparoscopic or open approach is the ultimate management.
POSITIONING
Attention to detail when positioning a patient for LRC/RARC is extremely important in the prevention of complications. Early in the surgeon’s experience, long surgical times may be encountered and may expose patients to neurologic injury, compartment syndrome, rhabdomyolysis and ventilatory difficulties.45-48 Neurologic injuries including ulnar, brachial, femoral and peroneal neuropraxias as well as cerebral edema have also been described.45,46 For most robot assisted pelvic surgery, a low-lithotomy position with the arms tucked at the side and extreme Trendelenburg will be used. With a pure laparoscopic approach, the lithotomy portion may be omitted as that space is not needed to accommodate the robot. In almost all reports of positioning complications, the common denominator is operative time. Most often, such complications are encountered early in the learning curve when operative times are longer.30
Complications arising from positioning may present immediately in recovery room. In the case of rhabdomyolysis, the patient may complain of severe pain in a large muscle group exposed to prolonged pressure. A compartment syndrome is often encountered as well. The patient may develop brown urine and which may signify urine myoglobin. The serum creatine phosphokinase (CPK) will invariably exceed 5000 IU/dL. The treatment consists of hydration and alkalinization and needs to be instituted immediately to prevent subsequent renal failure. Early diagnosis may have a significant impact on the outcome by preventing or reducing the severity of complications from rhabdomyolysis. When suspected or risk factors are present (e.g. obesity, prolonged operating-time), measurement of the CPK level during the postoperative period is recommended. Therapy is indicated if the CPK rises >5,000. Myoglobinuric acute renal failure complicates approximately 30% of cases of rhabdomyolysis. Mortality from rhabdomyolysis can be as high as 5%.49
If a compartment syndrome is suspected, immediate orthopedic surgical consultation should be sought and compartment pressures measured. It is important to remember that peripheral pulses and capillary refill may be normal in acute compartment syndrome.
Pain out of proportion is the hallmark sign and likely due to neuropathy of the sensory nerve in that compartment. Therefore the surgeon should not take comfort in observing normal pulses if there is severe pain. Compartment syndrome can have severe consequences including loss of limb and death making immediate recognition key to its management. There have been anecdotal reports of compartment syndromes being identified in the upper extremities due to aggressive arm tucking and subsequent peripheral IV infiltration. It is important for the anesthesia team to ensure proper functioning of all IV infusions and periodically check the arms under the drapes if the operation is lasting more than four to six hours.
Early in the robotic experience, some surgeons used shoulder sleds due to the extreme Trendelenburg position. The problem with these devices is the possibility of shoulder impingement and pain along the superior aspect of the shoulder and acromium process. Use of foam and padding, arm tucking and a low lithotomy position prevents slippage or significant movement of the patient. The areas to be sure are adequately padded include the popliteal fossa, peroneal surface, arms, wrists, back, buttocks, and the head and neck.
Prevention:
- Adequate padding of the legs, arms, hands, back, buttocks, head and neck;
- Ergonomic orientation of the wrists, arms and legs;
- Keep operative times to a minimum and consider open conversion early in the learning curve if operative times are excessive;
- Periodic examination of the arms and wrists for long cases (>4hrs);
- Try to place peripheral IVs away from potential pressure points.
Management:
- Early identification is a key to minimizing poor outcomes;
- Early orthopedic surgical consultation if patient complains of pain out of proportion in a limb or location away from the surgical site;
- Measurement of compartment pressures if compartment syndrome is suspected;
- Measure serum creatine phosphokinase (CPK) and urine myoglobin if rhabdomyolysis is suspected;
- IV fluid hydration and alkalinization if myoglobinuria is identified.
CARDIOPULMONARY COMPLICATIONS
Although cardiopulmonary complications are inherent to any major surgical procedure, it is deserves mention here due to the cardiopulmonary effects of the pneumoperitoneum.
The most common arrhythmia is sinus tachycardia; bradyarrhythmias (e.g., atrioventricular dissociation, nodal rhythm, sinus bradycardia) may also develop independently or in combination with tachycardia during the same procedure.50 Conditions leading to development of arrhythmias are CO2 insufflation, hypercapnia, and increased vagal tone owing to traction on pelvic or peritoneal structures, Trendelenburg position, anesthetic drugs (especially halothane in combination with spontaneous ventilation), preoperative patient anxiety, endotracheal intubation, and gas embolism.51 The role of the anesthesiologist throughout LRC/RARC is of paramount importance.
Continuous monitoring of cardiovascular and pulmonary parameters is essential. Invasive cardiac monitoring may be instituted in patients with heart disease (using a Swan-Ganz catheter) or in high-risk (i.e., ASA 3 or 4) patients when prolonged and complicated laparoscopic procedures are expected, especially since a central venous pressure line may not be as reliable in laparoscopic procedures as in open procedures.
Because hypercapnia is one of the most common underlying causes of cardiac arrhythmias, it is essential to monitor and control this problem. Overall, hypercapnia can be corrected by adjustment of ventilatory rate, tidal volume, use of positive end- expiratory pressure as needed, and reduction of intra-abdominal pressure. Exsufflation of the pneumoperitoneum for 5 to 10 minutes may allow the anesthesiologist to “catch up” and correct the hypercapnia; the pneumoperitoneum may then be reinitiated at a lower pressure (5 to 10 mm Hg).50,51
In the event of any cardiac compromise, the surgeon should immediately exsufflate the abdomen. If cardiac arrest is encountered, one should provide cardiac massage (compressions) while the anesthesiologist administers 100% oxygen and appropriate drug therapy. If a CO2 embolus is suspected, additional maneuvers, such as turning the patient to a left lateral decubitus position and attempting to aspirate the embolus, can be attempted.
Prevention:
- Limit intra-abdominal pressures to 15 mm Hg and in some cases to 10 mm Hg in patients with COPD;
- Avoidance of certain anesthetic agents and combinations (e.g., halothane and spontaneous ventilation) has been reported as well as premedication with atropine may prevent excessive vagal stimulation.52
Management:
- Immediate exsufflation of the pneumoperitoneum;
- Follow appropriate cardiac and resuscitation measures
UNIDENTIFIED BOWEL INJURY
Unidentified bowel injury is an inherent risk of minimally invasive surgery and is commonly due to “blind” passage of instruments as well as thermal spread of energy.
Specific mention of this potential complication is important due to the significant morbidity associated with delayed identification. Bowel injuries are generally categorized into those resulting from thermal energy and those resulting from direct puncture.
ELECTROSURGICAL ETIOLOGY
Electrical and thermal burns to patient are usually predictable and preventable. Postoperatively, the patient with unrecognized bowel trauma may not develop fever, nausea, or signs of peritonitis for many days.52 Accordingly, a bowel injury must be ruled out for any patient who develops unexplained fever and has signs of peritonitis.
Laparoscopic surgery affords patients decreased post operative pain, therefore any patient with abdominal pain greater than expected should raise the suspicion for an acute abdomen. The most sensitive test is an abdominal CT scan with oral contrast accompanied by delayed films, usually 6 or more hours after the initial oral contrast load. Laboratory values may be remarkable for leukocytosis with an associated left shift, but in some patients, a normal or even low leukocyte count is encountered, making the “left shift” a more reliable sign than the absolute white cell count.53
Thermal injury caused by monopolar cautery often results in tissue damage that extends beyond the visible area of necrosis. With this in mind, the surgeon should perform a bowel resection with a safety margin of on either side before completing an anastomosis. General surgical consultation should be sought in this setting.
Thermal injury caused by bipolar cautery is more confined to the visible area of damage. These injuries only occur due to direct firing of the instrument on the bowel. If the injury is small, it can be managed by simple excision of the defect and closure of the bowel wall. Bipolar injuries that involve more than half of the circumference of the bowel should be treated by excision of the affected segment.54
MECHANICAL ETIOLOGY
Mechanical injury involving the bowel is usually secondary to trocar insertion or operative trauma. Inadvertent mechanical damage can be caused by a wide variety of sharp and blunt instruments (e.g., graspers, scissors, retractors). This type of injury is usually visible to the surgeon and repaired intraoperatively. Given its localized nature, bowel resection is rarely necessary. If the injury is missed during the procedure, then postoperatively symptoms (fever, nausea, ileus, and peritonitis) develop much earlier than with an electrosurgical injury. Diagnosis is confirmed by an abdominal CT scan with oral contrast material. This type of injury should be managed with immediate return to the operating room to correct the problem by local excision or resection of bowel with subsequent reanastomosis and copious irrigation of the abdomen. Once again, general surgical consultation is recommended in this setting.
Prevention:
- Ensure appropriate insulation and integrity of instrumentation;
- Avoid blind passage of instrumentation whenever possible;
- Assistants should take great care in passing sharp instruments into surgical field without direct visualization;
Management:
- CT of the abdomen and pelvis with oral contrast should be obtained in any patient with suspected bowel injury or signs of peritonitis;
- Immediate open repair and resection may be needed to avoid continued leakage of bowel contents;
- In rare cases, percutaneous drainage may be used to create a controlled leak resulting in a fistula. Usually in cases of very delayed presentation;
- General surgical consultation recommended in all cases.
VASCULAR INJURY
Fortunately, direct vascular injury during laparoscopic dissection is a rare event. The use of blunt trocars, small instrumentation, limitations on surgical speed, and magnification of the surgical field combine to decrease this potential problem. Vascular injury can include abdominal wall vessels such as the inferior epigastric, intraperitoneal, or retroperitoneal vasculature.
Management of an injury to the inferior epigastric vessels includes: direct identification and clipping or cutting down to the vessel and ligating it. In most cases, identification of the injury occurs when blood is seen dripping around a port. In these cases, the surgeon can put a large “figure-of-eight” suture through and through the abdomen to compress the vessels. The surgeon can then complete the procedure and address the injury at the completion of the case. The suture is unlikely to be adequate in the long run and the authors suggest that one should cut down on the port site and formally ligate the vessels to avoid the delayed development of a rectus sheath hematoma or even intraperitoneal hemorrhage.
In almost all cases of radical cystectomy for bladder cancer, a pelvic lymphadenectomy is performed. It is during this portion of the operation when a vascular injury may occur.
This is particularly the case when gross, bulky lymphadenopathy is encountered. When a vascular injury occurs, the surgeon can undertake several steps to resolve the bleeding and these are dictated by whether an artery or vein is injured. In most all cases of venous injury, control can be achieved via the laparoscopic/robotic approach even with injury to larger vessels such as the iliac veins or vena cava. First, the pneumoperitoneum pressure can be raised to 20 mm Hg, thereby slowing or stopping any venous bleeding. If the injury is small, it may respond to simple tamponade and formal repair may not be necessary. Injuries to larger vessels and large venotomies should be repaired with a 4-0 or 5-0 vascular suture. The first step is simply to apply pressure to the opening. With five minutes of continuous pressure, a suitable platelet plug will form and allow for suture repair of most venotomies, including the vena cava. In addition, a hemostatic patch, fibrin glue or other hemostatic agents can be used.
In cases of small arteriotomies or small artery avulsion, clipping or suture ligation may be possible. In most cases of significant arterial injury, laparoscopic control is not possible and immediate conversion to an open approach with rapid vascular control is recommended. Open conversion should not be avoided or thought of as failure, but instead should be considered good judgment. Vascular surgical consultation may be required. Fortunately, this scenario is rare as the arterial vessels are resilient and more forgiving than the venous structures.
Prevention:
- Be aware of the vascular anatomy of the pelvis;
- Sharp dissection along the pelvic vessels when bulky lymphadenopathy is encountered;
- Directly visualize all movements along the pelvic vessels.
Management:
- Have a laparoscopic vascular tray including laparoscopic bulldogs and clamps as well as vascular suture close by when encountering difficult anatomy;
- Intracorporeal repair and suturing should be attempted on venous injuries;
- Immediate open conversion and vascular control is recommended if significant hemorrhage is encountered.
POSTOPERATIVE ILEUS
Post-operative ileus (POI) is the most common reported postoperative complication after open radical cystectomy. It is often defined as delay of return of bowel function greater than four days.55,56 Although laparoscopic surgery theoretically minimizes peritoneal irritation and surgical manipulation of the abdominal contents, POI is still the most common complication following LRC/RARC. Delayed return of gastrointestinal motility may result in pain, abdominal distension, nausea and vomiting. In addition, POI often results in an increased length of hospital stay.55 Different factors have been postulated to contribute to the induction and maintenance of an ileus; however the etiology is not completely known. Urine leak following urinary diversion is often a contributing factor to POI.56
Recent literature has described some novel measures and modifications to traditional preoperative management to prevent or diminished POI. Maffezzini et al. analyzed the elimination of mechanical bowel preparation and fasting before surgery with an early nutritional support, combining both parenteral and enteral routes. Donat et al. analyzed the use of intravenous metoclopramide combined with early nasogastric tube removal. All have shown to varying degrees a positive impact on diminishing the time to recover of bowel activity. However, they have not been widely adopted.
It has been the authors’ experience that the robotic approach has shortened the length of POI and in many cases eliminated it entirely. We also feel the elimination of mechanical and antibiotic bowel preparation is beneficial as well. Patients are likely to be less volume depleted the day of surgery and require less aggressive fluid resuscitation intra operatively. In some cases, however, there is nothing that can be done to prevent the onset of POI and it is simply a function of a bowel anastomosis and creation of a urinary diversion. Some people have postulated that “urine in the wound” whether through microscopic leaks of the ureteroenteric anastomosis or reabsorption via the intestinal segment may play roles in the development of POI.
Regardless of the cause, the management is most often conservative, but in some cases gastric decompression is needed. It is important to rule out any electrolyte abnormalities such as hypokalemia, hypomagnesemia, hypercalcemia, and hypocalcemia. Most importantly, the surgeon must rule out a partial or complete small bowel obstruction. A CT scan of the abdomen and pelvis with oral contrast should be ordered if a bowel obstruction is suspected. In cases of severe or prolonged ileus, a urine leak may be the cause and drain fluid, if available, should be evaluated for creatinine. Finally, drainage of any intrabdominal fluid collection may be necessary in prolonged ileus if infection, lymphocele, or urinoma is suspected.
Prevention:
- Consider elimination of mechanical and antibiotic bowel preparation;
- Early ambulation in the postoperative setting, same day if possible;
- Maintain normal electrolyte levels.
Management:
- Rule out any potential bowel obstruction with a CT scan of the abdomen and pelvis with oral contrast;
- Nasogastric tube placement for decompression in cases of severe discomfort, airway compromise, or extremely prolonged ileus;
- Percutaneous drainage of any intra-abdominal fluid collection felt to be a potential source such as a lymphocele, ileus, or abscess.
- Appropriate evaluation to rule out a urine leak
LYMPHOCELE AND LYMPH LEAK
Lymphocele formation is commonly associated with pelvic procedures, particularly pelvic lymph node dissection (2 to 9%).57 A lymphocele may take weeks to develop and may occur despite a transperitoneal approach. Presentation often includes lower extremity edema secondary to local compression of the pelvic vasculature, pain, or infection. In rare cases, the presenting findings may be venous thrombosis and pulmonary embolism. Lymphoceles are readily diagnosed by CT.
There are numerous options for treatment and management. In the asymptomatic patient, observation may be elected in the absence of any evidence of infection or vascular compromise. In the symptomatic patient, treatment is either immediate percutaneous drainage or intraperitonealization which can be performed via a laparoscopic approach. Sclerosant therapy can also be used to treat lymphoceles. During intraperitonealization omentum can be placed into the opening made in the lymphocele to prevent recurrence. Prevention of lymphocele formation requires close attention to clipping suspected lymphatic structures.
Prolonged lymph leak has been observed by the authors in patients undergoing RARC with extended lymphadenectomy. Male patients may complain of fluid leaking from the native urethra and feel as if they are voiding. Female patients may complain of leakage from the vagina requiring pad usage. Although disconcerting, this is self limiting, but may take several weeks to resolve in some cases. We feel that this may be due to the extensive nature of the lymphadenectomy as well as the use of cautery for much of the dissection.
Prevention:
- Use of meticulous clipping at proximal and distal borders of lymphadenectomy;
- Avoid subcutaneous heparin in the lower extremities;
- Optimize nutritional status of the patient.
Management:
- Percutaneous drainage with ultrasound or CT guidance is often the initial step in the management of symptomatic lymphocele;
- Sclerosing therapy;
- Intraperitonealization of the lymphocele by creating a surgical window;
- Lymph leaks via the urethra or vagina are self limiting and need no intervention other than improving nutritional status.
DEEP VENOUS THROMBOSIS
Signs of deep venous thrombosis include localized calf tenderness with associated swelling. However, many patients with postoperative deep venous thrombosis have a subclinical course. The most common clinical scenario is detection of a deep venous thrombosis only after a patient has developed a pulmonary embolus. Treatment is immediate anticoagulation. In patients with a pulmonary embolus who are not candidates for anticoagulation, a caval filter can be placed. In many cases a retrievable filter can be placed if only temporary management is anticipated.
Deep venous thrombosis can be avoided by utilizing standard measures such as pneumatic sequential compression devices and/or mini-dose heparin and early postoperative ambulation. Currently the American College of Chest Physicians recommends either pneumatic compression stockings or heparin for all major pelvic urologic procedures.58 Pneumatic compression stockings should be placed preoperatively and continued for 48 to 72 hours postoperatively or until ambulatory. Some surgeons use low molecular weight heparin (Lovenox®) in addition to stockings in high risk patients. The laparoscopic/robotic approach may be of particular advantage in this regard by allowing earlier ambulation.
Prevention:
- Early ambulation;
- Use of lower extremity stockings or sequential compression devices initially in the pre-operative holding area throughout the first 48 to 72 hours;
- Use of unfractionated or low molecular weight heparin in prophylactic doses in addition to lower extremity measures in high risk patients.
Management:
- Systemic anticoagualtion in almost all cases for at least three to six months;
- Placement of an inferior vena caval filter in cases when anticoagulation is contraindicated.
COMPLICATIONS OF URINARY DIVERSION AND URINE LEAK
Whether an operation is performed open, laparoscopically, or robotically, there are complications that are inherent to the creation of the urinary diversion. These complications include problems at the bowel anastomosis such as a bowel leak, or complications of the diversion itself, including urine leak and ureteroenteric strictures. In addition to mechanical problems, patients with urinary diversions may suffer electrolyte and acid-base abnormalities related to urine reabsorption. The most common disturbance is a hyperchloremic, metabolic acidosis associated with the use of ileum. In most cases it is mild not requiring treatment, but it may require systemic alkalinization if bone resorption or fatigue are associated with the syndrome. Other disturbances may arise with the use of gastric or jejunal segments. Furthermore, absorption problems such as B12 deficiency are known complications of the use of intestinal segments for urinary diversion. The full range of complications and management of these potential complications are beyond the scope of this chapter but are well established in the urologic literature.59 As most of the urinary diversions reported in the literature employ extracorporeal creation of the diversion, the same open surgical principles for bowel anastomotic and ureteroenteric anstomotic techniques should be used. The surgeon performing minimally invasive radical cystectomy with urinary diversion should be aware of these potential complications, be able to manage them, and fully inform the potential patient of these outcomes.
Prevention:
- Use of standard open surgical principles of tissue handling, suturing and techniques of anastomosis
- Appropriate drainage with ureteral and diversion catheters when appropriate;
- Appropriate use of surgical drains;
- Watertight closure of all suture lines and anastomosis’.
Management:
- Percutaneous drainage of any uncontrolled leak;
- Maximal drainage with urethral or transcutaneous catheters when appropriate;
- Percutaneous nephrostomy tube placement when an uncontrolled leak persists.
SUMMARY
Minimally invasive radical cystectomy continues to increase in application and acceptance, particularly with the development of robotic surgery. While the benefits of the LRC/RARC are apparent, it is important for any surgeon performing these procedures to be well versed in the associated complications. It is also important for the surgeon to be aware of the standard complications associated with open pelvic surgery and urinary diversion. With knowledge in the prevention and management of all potential complications, LRC/RARC will be successfully added to the armamentarium in the management of bladder cancer.
References
- Lowrance WT, Rumohr JA, Chang SS, Clark PE, Smith JA, Jr., Cookson MS. Contemporary open radical cystectomy: analysis of perioperative outcomes. J Urol. 2008;179:1313-1318.
- Portis AJ, Yan Y, Landman J, et al. Long-term followup after laparoscopic radical nephrectomy. J Urol. 2002;167:1257-1262.
- Badani KK, Kaul S, Menon M. Evolution of robotic radical prostatectomy: assessment after 2766 procedures. Cancer. 2007;110:1951-1958.
- Parra RO, Andrus CH, Jones JP, Boullier JA. Laparoscopic cystectomy: initial report on a new treatment for the retained bladder. J Urol. 1992;148:1140-1144.
- Hemal AK, Kolla SB, Wadhwa P, Dogra PN, Gupta NP. Laparoscopic radical cystectomy and extracorporeal urinary diversion: a single center experience of 48 cases with three years of follow-up. Urology. 2008;71:41-46.
- Haber GP, Campbell SC, Colombo JR, Jr., et al. Perioperative outcomes with laparoscopic radical cystectomy: “pure laparoscopic” and “open-assisted laparoscopic” approaches. Urology. 2007;70:910-915.
- Haber GP, Gill IS. Laparoscopic radical cystectomy for cancer: oncological outcomes at up to 5 years. BJU Int. 2007;100:137-142.
- Moinzadeh A, Gill IS, Desai M, Finelli A, Falcone T, Kaouk J. Laparoscopic radical cystectomy in the female. J Urol. 2005;173:1912-1917.
- Gill IS, Fergany A, Klein EA, et al. Laparoscopic radical cystoprostatectomy with ileal conduit performed completely intracorporeally: the initial 2 cases. Urology. 2000;56:26-29.
- Menon M, Hemal AK, Tewari A, et al. Nerve-sparing robot-assisted radical cystoprostatectomy and urinary diversion. BJU Int. 2003;92:232-236.
- Chang SS. Cookson MS, Baumgartner RG, et al. Analysis of early complications after radical cystectomy: results of a collaborative care pathway. J Urol. 2002;167:2012- 2016.
- Hollenbeck BK, Miller DC, Taub D, et al. Identifying risk factors for potentially avoidable complications following radical cystectomy. J Urol. 2005;174:1231-1237.
- Konety BR, Allareddy V, Herr H. Complications after radical cystectomy: analysis of population-based data. Urology. 2006;68:58-64.
- Quek M.L, Stein J.P, Daneshmand S, et al. A critical analysis of perioperative mortality from radical cystectomy. J Urol. 2006;175:886-889.
- Studer UE, Burkhard FC, Schumacher M, et al. Twenty years experience with an ileal orthotopic low pressure bladder substitute—lessons to be learned. J Urol. 2006;176:161-166.
- Novotny V, Hakenberg OW, Wiessner D, et al. Perioperative complications of radical cystectomy in a contemporary series. Eur Urol. 2007;51:397-401.
- Fairey A, Chetner M, Metcalfe J, et al. Associations among age, comorbidity and clinical outcomes after radical cystectomy: results from the Alberta Urology Institute radical cystectomy database. J Urol. 2008;180:128-134.
- Pycha A, Comploj E, Martini T, et al. Comparison of complications in three incontinent urinary diversions. Eur Urol. 2008;54:825-832.
- Boström PJ, Kössi J, Laato M, et al. Risk factors for mortality and morbidity related to radical cystectomy. BJU Int. 2009;103:191-196.
- Ng CK, Kauffman EC, Lee MM, et al. A Comparison of Postoperative Complications in Open versus Robotic Cystectomy. Eur Urol. 2010;57:274-281.
- Haber GP, Crouzet S, Gill IS. Laparoscopic and robotic assisted radical cystectomy: a critical analysis. Eur Urol. 2008;54:54-62.
- Novara G, Marco VD, Aragona M, et al. Complications and Mortality After Radical Cystectomy for Bladder Transitional Cell Cancer. J Urol. 2009;182:914-921.
- Hemal AK. Role of robot-assisted surgery for bladder cancer. Curr Opin Urol. 2009;19:69-75.
- Nunez R, Andrews PE, Martin AD, et al. Comparison of Open and Robot Assisted Radical Cystectomy. J Urol. 2009;181:A1008.
- Hemal Ak, Kolla AB, Wadhwa P. First case series of robotic radical cystoprostatectomy, bilateral pelvic lymphadenectomy, and urinary diversion with the da Vinci S system. J Robotic Surg. 2008;2:35-40.
- Sala LG, Matsunaga GS, Corica FA, Ornstein DK. Robot-assisted laparoscopic radical cystoprostatectomy and totally intracorporeal ileal neobladder. J Endourol. 2006;20:233-235.
- Rhee JJ, Lebeau S, Smolkin M et al. Radical cystectomy with ileal conduit diversion: early prospective evaluation of the impact of robotic assistance. BJU Int. 2006;98:1059-1063.
- Menon M, Hemal AK, Tewari A, et al. Robot-assisted radical cystectomy and urinary diversion in female patients: technique with preservation of the uterus and vagina. J Am Coll Surg. 2004;98:386-393.
- Hemal AK, Abol-Enein H, Tewari A, et al. Robotic radical cystectomy and urinary diversion in the management of bladder cancer. Urol Clin North Am. 2004;31:719-729.
- Pruthi RS, Smith A, Wallen EM. Evaluating the learning curve for robot-assisted laparoscopic radical cystectomy. J Endourol. 2008;22:2469-2474.
- Wang GJ, Barocas DA, Raman JD, et al. Robotic vs open radical cystectomy: prospective comparison of perioperative outcomes and pathological measures of early oncological efficacy. BJU Int. 2008;101;89-93.
- Abraham JB, Young JL, Box GN, et al. Comparative analysis of laparoscopic and robot-assisted radical cystectomy with ileal conduit urinary diversion. J Endourol. 2007;21:1473-1480.
- Pruthi RS, Wallen EM. Robotic-assisted laparoscopic radical cystoprostatectomy. Eur Urol. 2008;53:310-322.
- Guru KA, Kim HL, Piacente PM, et al. Robot-assisted radical cystectomy and pelvic lymph node dissection: initial experience at Roswell Park Cancer Institute. Urology. 2007;69:469-474.
- Beecken WD, Wolfram M, Engl T, et al. Robotic-assisted laparoscopic radical cystectomy and intra-abdominal formation of an orthotopic ileal neobladder. Eur Urol. 2003;44:337-339.
- Murphy DG, Challacombe BJ, Elhage O, et al. Robotic-assisted laparoscopic radical cystectomy with extracorporeal urinary diversion: initial experience. Eur Urol. 2008;54:570-580.
- Galich A, Sterrett S, Nazemi T, et al. Comparative analysis of early perioperative outcomes following radical cystectomy by either the robotic or open method. JSLS. 2006;10:145-150.
- Balaji KC, Yohannes P, McBride CL, et al. Feasibility of robot-assisted totally intracorporeal laparoscopic ileal conduit urinary diversion: initial results of a single institutional pilot study. Urology. 2004;63:51-55.
- Menon M, Hemal AK, Tewari A, et al. Nerve-sparing robot-assisted radical cystoprostatectomy and urinary diversion. BJU Int. 2003;92:232-236.
- Guillotreau J, Game X, Mouzin M, et al. Radical Cystectomy for bladder cancer: morbidity of laparoscopic versus open surgery. J urol. 2009;181:554-559.
- Hemal AK, Kolla SB, Wadhwa P, Dogra PN, Gupta NP. Laparoscopic radical cystectomy and extracorporeal urinary diversion: a single center experience of 48 cases with three years follow-up. Urology. 2008;71:41-46.
- Cathelineau X, Arroyo C, Rozet F, Barret E, Vallancien G. Laparoscopic assisted radical cystectomy: the montsouris experience after 84 cases. Eur Urol. 2005;47:780-784.
- Gerullis H, Kuemmel C, Popken G. Laparoscopic cystectomy with extracorporeal- assisted urinary diversion: experience with 34 patients. Eur Urol. 2007;51:193-198.
- Lavery HJ, Thaly R, Albala D, et al. Robotic equipment malfunction during robotic prostatectomy: a multi-institutional study. J Endourol. 2008;22:2165-2168.
- Winfree CJ, Kline DG. Intraoperative positioning nerve injuries. Surg Neurol. 2005;63:5-18.
- Kretschmer T, Heinen CW, Antoniadis G, et al. Iatrogenic nerve injuries. Neurosurg Clin N Am. 2009;20:73-90.
- Bocca G, van Moorselaar JA, Feitz WF, et al. Compartment syndrome, rhabdomyolysis and risk of acute renal failure as complications of the lithotomy position. J Nephrol. 2002;15:183-185.
- Choi SJ, Gwak MS, Ko JS, et al. The effects of the exaggerated lithotomy position for radical perineal prostatectomy on respiratory mechanics. Anaesthesia. 2006;61:439- 443.
- Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;36:62-72.
- Gerges FJ, Kanazi GE, Jabbour-Khoury SI. Anesthesia for laparoscopy: a review. J Clin Anesth. 2006;18:67-78.
- Rauh R, Hemmerling TM, Rist M, et al. Influence of pneumoperitoneum and patient positioning on respiratory system compliance. J Clin Anesth. 2001;13:361-365.
- Wu MP, Ou CS, Chen SL, et al. Complications and recommended practices for electrosurgery in laparoscopy. Am J Surg. 2000;179:67-73.
- Nduka CC, Super PA, Monson JR, et al. Cause and prevention of electrosurgical injuries in laparoscopy. J Am Coll Surg. 1994;179:161–170.
- van der Voort M, Heijnsdijk EA, Gouma DJ. Bowel injury as a complication of laparoscopy. Br J Surg. 2004;91:1253-1258.
- Chang SS, Baumgartner RG, Wells N, et al. Causes of increased hospital stay after radical cystectomy in a clinical pathway setting. J Urol. 2002;167:208-211.
- Maffezzini M, Campodonico F, Canepa G, et al. Current perioperative management of radical cystectomy with intestinal urinary reconstruction for muscle-invasive bladder cancer and reduction of the incidence of postoperative ileus. Surg Oncol. 2008;17:41-48.
- Naselli A, Andreatta R, Introini C, Fontana V, Puppo P. Predictors of Symptomatic Lymphocele After Lymph Node Excision and Radical Prostatectomy. Urology. 2010;75:630-635.
- Goldhaber SZ. Prevention of recurrent idiopathic venous thromboembolism. Circulation. 2004;14:20-24.
- Hautmann RE, Abol-Enein H, Hafez K, et al. Urinary diversion. World Health Organization (WHO) Consensus Conference on Bladder Cancer. Urology. 2007;69:17- 49.
