Complications of Laparoscopic and Robotic Nephron Sparing Surgery
Craig Rogers, Matthew Sand, Firas Petros, Michael Stifelman
INTRODUCTION
Laparoscopic and robotic nephron sparing surgery (NSS) for the treatment of renal cell carcinoma is an attractive option among urologists who are experienced in laparoscopic and robotic techniques. Partial nephrectomy (PN) is considered a standard of care for the treatment of small renal tumors. Laparoscopic partial nephrectomy (LPN) has demonstrated comparable oncologic outcomes to open PN with decreased patient morbidity and shorter recovery times.1,2,3 Robotic-assisted laparoscopic partial nephrectomy (RALPN) is an emerging technique that offers minimally invasive advantages of LPN with technical advantages specific to robotic systems (e.g. improved visualization and dexterity during reconstruction).4,5
As the experience with RALPN is early, the incidence and risk of complications are not well documented. However, complications in early reports seem to parallel those of LPN. This chapter reviews common complications related to laparoscopic and robotic nephron sparing surgery and describes ways for prevention and management.
LPN AND RPN PREPARATION AND TECHNIQUE
LPN and RPN should be performed only by surgeons with experience in laparoscopic and robotic surgery as the procedure is technically challenging and should be completed within 30 minutes of warm ischemia time. The surgeon should start with smaller more exophytic tumors and progress to larger more endophytic tumors with experience.
Ideally, surgeons should practice minimally invasive partial npehrectomy in an animal lab before attempting their first procedure to ensure that all necessary steps can be performed within 30 minutes of warm ischemia time. Proper patient selection and preoperative imaging is important in avoiding complications. Preoperative evaluation with a high quality computerized tomography (CT) scan with precontrast, early and delayed intravenous contrast enhanced images can help delineate anatomy and help identify the presence of aberrant vessels.
Preoperatively, patients should be consented for the possibility of radical nephrectomy or open surgery. A bowel prep is given the day prior to surgery, which may include a bottle of magnesium citrate and a clear liquid diet. Go-lytely is preferred to magnesium citrate in patients with renal insufficiency. Sequential compression devices are applied to the lower extremities and the patient is given prophylactic intravenous antibiotics. The patient is placed in a flank position with all pressure points carefully padded. A transperitoneal or retroperitoneal approach for nephron sparing surgery may be selected based on considerations such as tumor location, amount for surrounding fat, and prior abdominal surgeries, etc. The transperitoneal approach is preferred in most cases because it offers a larger working space with familiar anatomical references for performance of surgical tasks.
The abdomen is insufflated and ports are placed. For patients with prior abdominal surgery and suspected abdominal adhesions, placement of the initial trocar may be performed using an open Hasson technique or by a direct vision technique using an optical trocar. A detailed description of ports, instruments, and technique of RPN is beyond the scope of this chapter, but has been described previously.4 Various laparoscopic and robotic instruments may be used per surgeon preference. If the nondominant hand instrument is attached to cautery, care must be taken not to inadvertently hit the wrong pedal during dissection, which could result in injury to structures as the bowel, ureter, and vessels.
Wide exposure is important to provide adequate exposure of the renal hilum for vascular control. For right-sided tumors, the right colon is reflected medially, the liver is retracted superiorly and the duodenum is kocherized. On the left side, lateral attachments to the spleen are divided and the spleen, pancreas and colon are reflected medially. If an opening in the bowel mesentery is made during mobilization, this should be closed to decrease the risk of internal hernia formation. The ureter is isolated, and the lower pole of the kidney is retracted anteriorly. All posterior attachments to the kidney are dissected.
The kidney is placed on traction using the assistant or fourth arm to place the renal hilum on stretch while the console surgeon has two hands free to perform the dissection. A skilled side surgeon is helpful in appropriately manipulating tissues and minimizing risk of iatrogenic injury during RPN. The renal artery and vein are dissected sufficiently for complete enblock or individual control of vessels. All vessels should be accessible for clamping as incomplete clamping can lead to severe hemorrhage. A laparoscopic Doppler probe (VTI, Nashua, NH) may be used to improve recognition of hilar vessels during dissection, particularly if abberent vessels are suspected. Perirenal fat is removed to obtain wide exposure of the real capsule surrounding the tumor to enable complete resection and reconstruction. A laparoscopic ultrasound probe is used to locate the tumor and determine margins in three dimensions to guide tumor excision. The anticipated excision line is scored on the capsule with cautery prior to clamping of the renal artery.
Prior to clamping, it is important to ensure that all necessary stitches and instruments are available to prevent unnecessary prolongation of warm ischemia time. Bolsters and needles may be preplaced in the abdomen off to the side to be readily available for renal reconstruction after tumor removal in order to minimize warm ischemia time. Intravenous mannitol may be administered prior to arterial clamping for osmotic diuresis. Hilar clamping is done by the assistant through the 12 mm port using either laparoscopic bulldog clamps or en-block clamping using a Statinsky clamp. The renal artery is clamped first, followed by the renal vein. For small or exophytic tumors, the renal artery alone may be clamped. For hilar or endophytic tumors, both the renal artery and renal vein may be clamped to improve visualization to help achieve a negative surgical margin. Small, exophytic tumors may be resected without hilar clamping; However, the renal hilum should be exposed in case clamping is required. Gentle application and removal of clamps is important in avoiding vascular injury. Bulldog clamps obviate the risk of inadvertent tugging or external collision with the Satinsky clamp while locked on vessels and free up the port for the surgeon to use. However, the Satinski clamp offers the advantage of quick enblock control of hilar vessels with less dissection and improved ability to do early unclamping techniques.5
The tumor may be excised using various energy sources (ie. “hot” scissors, Harmonic shears, etc), but “cold” excision allows precise visualization of the excised tissue to help avoid positive margins. Exposed vessels and/or collecting system injury at the base of the resection are oversewn using running or figure of 8 absorbable suture. Precise closure of the resection bed with the avoidance of deep passes with large needles may decrease the likelihood of AV fistula formation.6 The renal defect is then closed with larger absorbable sutures placed through the renal capsule across the defect. The sutures may be anchored with hemolock clips that can be slid down to adjust the tension and tightened using the sliding hemolock clip technique for addition compressive hemostasis.7 Bolsters and hemostatic agents may also be placed in the renal defect, but may not be necessary if the renal defect can be closed without them.
Following reconstruction of the renal defect, the hilar clamps are removed. If bulldog clamps are used, the venous clamp is removed first, followed by flashing of the arterial clamp to confirm hemostasis. The arterial clamp is removed typically after the renal capsule is closed, but may be removed before closure of the renal capsule to ensure specific vessels are oversewn and to reduce warm ischemia time.5
Confirmation of adequate hemostasis with the insufflation pressure decreased to 5mm Hg and the mean arterial pressure increased to >90mm Hg should be performed prior to completing the procedure to help prevent postoperative bleeding. If the kidney has been mobilized, it is important to place the kidney back into normal anatomic position and perform a nephropexy with clips or suture to prevent renal torsion with ureteral or vascular obstruction. A single JP drain is placed near the resection bed in a dependent position and left to straight drainage. All the ports are removed under direct vision.
LITERATURE REVIEW: ROBOTIC AND LAPAROSCOPIC PARTIAL NEPHRECTOMY
Robotic assistance may offer technical advantages for minimally invasive partal nephrectomy offers significant benefits over conventional laparoscopy, such as increased precision and visualization for tumor resection and renorrhaphy. Improved magnification may enable improved recognition of the tumor’s gross margin. Even for skilled laparoscopists, tumors that are difficult to access (e.g. posterior) or more complex (e.g. near the hilum) may be facilitated with the precision and improved range of motion of robotic tools. However, robotic assistance may also have some potential disadvantages for partial nephrectomy. There is a learning curve that must be overcome as the surgeon adapts to a new surgical environment, although potentially shorter than with learning LPN. There is an increased reliance on the side surgeon for tasks such as providing traction, exposure, suction, irrigation, hilar clamping and passing of instruments, needles and adjunct hemostatic agents.
Multiple case series of RALPN have been published and are presented in Table 1.6,8-16
Complications in these published series of RALPN are summarized in Table 2. Intra-operative complications in these series include two cases of intra-operative hemorrhage requiring conversion to open surgery.9 One hemorrhage occurred after unclamping of the hilum and the other occurred due to venous back-bleeding from an unclamped renal vein.9 One robotic camera malfunction occurred requiring conversion to traditional laparoscopy.10 One series reported losing a free needle in the patient as it was being removed without direct vision through a port requiring open conversion to locate and remove the needle.11 Another series reported open conversion in two patients for failure to progress.17 One enterotomy occurred during lysis of adhesions that was repaired robotically.18
The 46 post-operative complications reported in these series of 495 cases (9.3%) included 15 hemorrhagic complications (3%), 11 urine leaks (2.2%), 6 (1.2%) gastrointestinal complications (ie. ileus, C. difficile colitis, etc), and 12 (2.4%) medical complications (pulmonary embolism, congestive heart failure, and myocardial infarction). One post- operative abscess was reported adjacent to the tumor resection bed which failed intravenous antibiotic therapy and eventually required nephrectomy.11 One patient with platelet dysfunction required re-exploration for delayed rupture of a hepatic subcapsular hematoma.18
Postoperative complications appear similar in RALPN and LPN series. One LPN series focusing specifically on complications reported a rate of urinoma and post-operative hemorrhage after LPN of 4.5% and 6%, respectively.19 Another LPN series of 123 patients reported 26 overall complications (21%); specifically three hemorrhagic complications (2.4%), 13 urine leaks (10.6%), and 10 medical complications (8%).20 In a study of 217 LPN patients, Link et al. reported four hemorrhagic complications (1.8%), three urine leaks (1.4%), three wound infections (1.4%), two cases of post-operative renal failure (0.9%) and 15 medical complications (6.9%).21
Early literature on RALPN has demonstrated potential advantages to LPN, including reduction in warm ischemia time (WIT) and blood loss.6,8 However, there have been no reported differences between RALPN and LPN in terms of intra- or post-operative complications in any series.6,8-16 Definitive comparisons of RALPN with LPN in terms of outcomes and complications should be drawn from prospective and, ideally, randomized studies.
COMPLICATIONS: PREVENTION, DIAGNOSIS AND MANAGEMENT
Maintaining a high index of suspicion and a consistent diagnostic protocol are important to enable early detection of complications of minimally invasive partial nephrectomy.
This section will focus on strategies to diagnose and manage the most common complications of LPN and RPN.
Urine Leak
Urine leak is one of the most common complications of RALPN and LPN. Excision into the collecting system can be performed with increasing experience, although there is a higher risk of bleeding and urinary leakage.22 Preoperatively, careful review of imaging may suggest tumor proximity to the collecting system which might necessitate entry into a calyx to obtain a negative deep margin. Intra-operatively, various techniques have been described to facilitate recognition of collecting system entry. Close inspection of the resection bed may reveal gross evidence of collecting system entry. Previous retrograde placement of a ureteral catheter connected to methylene blue solution for intraoperative irrigation may be used to identify the collecting system and test the integrity of collecting system repair.23 Indigo carmine can also be given intravenously to help identify collecting system defects. We do not routinely perform these maneuvers as gross inspection is typically sufficient to identify collecting system entry, particularly with the increased 3-D magnification provided with robotic assistance. No data has been published to show that these identification techniques result in lower incidence of urine leaks, however they may be useful tools. Intra-operative repair of collecting system defects is important to help prevent urine leak after partial nephrectomy. Robotic systems make suture reconstruction technically easier than conventional laparoscopy. Multiple synthetic compounds are available to augment creation of a water-tight seal in collecting system closure.
The urinary tract is drained to help prevent urine leakage. A foley catheter is left and a Jackson-Pratt drain is placed near the resection bed in a dependent position and placed to bulb suction. After the foley catheter is removed, the JP drain is observed for increased output and the JP fluid is sent for creatinine level at least 4-6 hours later. If it is consistent with serum creatinine, the JP is removed. If there is evidence of leakage (i.e. continued high output, elevated JP creatinine level), the drain is left in place and output is monitored. If a leak persists, a CT urogram may be performed (Figure 1) to determine if there is urinoma that needs to be drained, the position of the drain in relationship to the area of leak, and to rule out ureteral obstruction. If there is a urinoma, the position of the drain within the collection should be confirmed. If there is an undrained collection, percutanous drainage should be performed. If the drain is too close to the site of the leak in the resection bed, it may prevent sealing of the leak and, therefore, should be repositioned away from the site and/or taken off suction. Serial imaging should be performed to confirm improvement and resolution of the urinoma. If there is persistent leak without urinoma, the drain may be converted to straight drainage. If leakage does not resolve with straight drainage, the drain should be gradually pulled back over several weeks to allow for fistulization and gradual sealing of the collecting system. If there is persistent high output drainage or evidence of distal obstruction on CT urogram (ie. secondary to blood clot), retrograde placement of a ureteral stent should be considered.
Foley catheter drainage should be used in patients with known or suspected high pressure voiding, or if a ureteral stent is placed to prevent reflux. Most urine leaks will resolve with maximal drainage of the urinary tract and patience. Re-exploration and repair of the collecting system versus nephrectomy are feasible, but rarely indicated.23
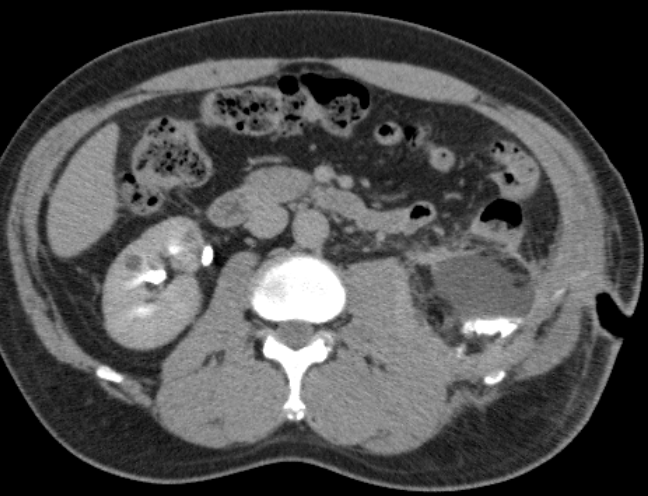 Figure 1. CT urogram demonstrating urinoma inferior to kidney after lower pole RALP. Contrast-enhancement within urinoma occurred during delayed phase of CT indicating communication between collecting system and fluid collection.
Figure 1. CT urogram demonstrating urinoma inferior to kidney after lower pole RALP. Contrast-enhancement within urinoma occurred during delayed phase of CT indicating communication between collecting system and fluid collection.
Hemorrhage
Hemorrhage can occur at different points during the case, such as hilar dissection, tumor resection, clamp removal, and the immediate or delayed postoperative period.
Intraoperative hemorrhage can be prevented with meticulous dissection and avoiding using monopolar cautery near large vessels. Intraoperative venous bleeding can usually be addressed with a combination of direct pressure, increased insufflation pressure, hemostatic agents, and patience. If a small bleeding vessel is identified, a grasping instrument can be used to occlude the vessel until cautery or clips control the bleeding. Injury to larger vessels (e.g. inferior vena cava, renal vein) may require sutured repair with prolene suture. Arterial bleeding will not typically resolve with pressure and/or hemostatic agents and requires control or repair of vessels. Bleeding while on clamp can occur due to a number of reasons. (1) The main renal artery has a branch which was missed during clamping or (2) an accessory artery is still perfusing the kidney. For persistent bleeding, a long bulldog clamp can be placed across the renal hilum to encompass all branches of the artery, and another bulldog clamp placed in the fat between the kidney and the adrenal gland to occlude any accessory arteries. In addition, if the renal vein is clamped and unoccluded arterial inflow is suspected, the venous clamp may be removed to help alleviate renal congestion and improve vision. (3) Old bulldog clamps may have decreased clamping force allowing flow to enter the kidney. In this case a second bulldog is placed. Pneumoperitoneum can temporally be increased to reduce bleeding as needed.
Bleeding may occur intraoperatively after unclamping the renal hilum from inadequate closure of the renal defect. This may allow for identification of specific vessels that require oversewing. If significant bleeding occurs, the insufflation pressure should be temporarily increased and local pressure applied while additional large compressing sutures are placed. If significant bleeding is noted and does not resolve with pressure, additional sutures or hemostatic agents, it may be necessary to take down the reconstruction and address a specific area of bleeding. Reclamping of the renal hilum is discouraged unless absolutely necessary because the additional warm ischemia time will be detrimental to the kidney. Intermittent renal hilar clamping results in greater ischemic damage than continuous clamping for the same amount of time.24 If bleeding remains uncontrolled, conversion to a hand-assist or open procedure should be considered to allow for manual parenchymal compression until hemostasis is achieved. A low threshold for open conversion and good judgement are important to safely control bleeding and prevent a manageable problem from escalating into a life-threatening problem.
Post-operative hemorrhage is usually readily identified clinically by hemodynamic instability and a decreasing hematocrit. A baseline hematocrit should be obtained postoperatively and compared to serial follow up hematocrit values. Immediate post- operative hemorrhage can usually be managed conservatively initially with close monitoring of vital signs, blood count, and transfusions as indicated. Delayed postoperative hemorrhage is usually due to formation of a pseudoaneurysm, arteriovenous (AV) fistula, or arteriocalyceal fistula. The typical pathogenesis involves injury to the wall of a segmental branch of the renal artery. An incomplete disruption of the arterial wall can result in pseudoaneurysm or AV fistula formation as the wall attempts to heal. Communication of a pseudoaneurysm or AV fistula with the collecting system can result in significant hematuria. Hematuria is the most common presentation of delayed hemorrhage after RALPN and any degree of hematuria should be carefully evaluated. It is important to have a low threshold to evaluate post-operative hematuria as rapid diagnosis and treatment of vascular complications is important to avoid more serious morbidity.
If there is mild gross hematuria, a CT or MR angiogram is the initial study of choice and will identify most vascular complications.25 Renal ultrasonography with Doppler flow study may also be useful.25 If bleeding is substantial (ie. hematuria requiring clot evacuation, decreasing hematocrit despite transfusions), patients should be taken directly for renal angiography with potential embolization by interventional radiology (Figure 2). Proceeding directly to angiography with a therapeutic option will decrease the overall intravenous contrast load, decrease radiation exposure for a patient and avoid any potential delay in treatment. If angio-embolization fails to control the bleeding or hemodynamic instability precludes interventional radiology management, operative re- exploration is indicated. If bleeding still cannot be controlled, nephrectomy may be necessary.
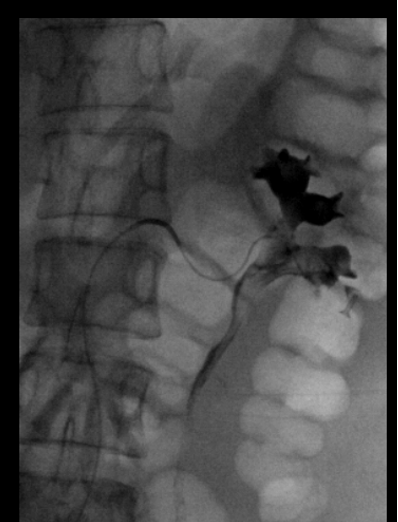 Figure 2a. Arterio-caliceal fistula: Renal angiogram demonstrating 3rd order inferior branch of renal artery with abnormal fistulous connection with adjacent lower pole calyx. Note the filling of renal collecting system with contrast during angiographic phase.
Figure 2a. Arterio-caliceal fistula: Renal angiogram demonstrating 3rd order inferior branch of renal artery with abnormal fistulous connection with adjacent lower pole calyx. Note the filling of renal collecting system with contrast during angiographic phase.
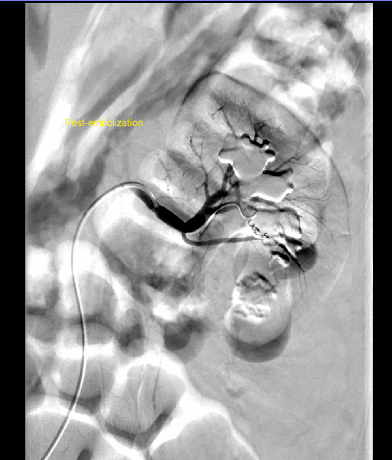 Figure 2b. Renal angiogram of same kidney after super-selective arterial embolization. Note coils in position preventing contrast from perfusing affected segment of kidney.
Figure 2b. Renal angiogram of same kidney after super-selective arterial embolization. Note coils in position preventing contrast from perfusing affected segment of kidney.
Bowel Injury
Bowel injury during RALPN and LPN can be a serious complication, particularly if unrecognized. Incidence of laparoscopic bowel injury during urologic surgery was reported in a large meta-analysis to be 0.13% (266/205,969 cases), of which, 69% were unrecognized at the time of surgery.26 Bowel injury can occur during laparoscopic port placement or removal/closure or may occur as a result of thermal injury during the procedure. The degree of bowel injury varies from superficial abrasions to small enterotomies to frank perforation. If a serosal injury (e.g. abrasion) is noted, this should be oversewed. If a small laceration is noted, this can be repaired in two layers, but larger injuries may require bowel resection. There should be a low threshold to consult general surgery for intraoperative consultation for both practical and legal purposes. For extensive injuries or contamination, bowel diversion may be necessary.
Early recognition of bowel injury is critical, as a delay in treatment may result in progression to sepsis and cardiovascular collapse. Early signs of unrecognized bowel injury include trocar site pain, abdominal distention, nausea/vomiting, fever and leukopenia or leukocytosis, and enteric output from the drain.26 Immediate surgical exploration and repair is indicated if suspicion of bowel injury is high. A CT scan with oral and IV contrast may be performed to identify the injury or associated abscess if the patient is stable and the clinical picture is less clear. A small subset of these patients may be able to be managed conservatively with TPN.26
Rhabdomyolysis
Rhabdomyolysis may occur due to compression at pressure points related to prolonged positioning in the lateral decubitus position. Risk factors for developing rhabdomyolysis during laparoscopic procedures include male sex, high body mass index, prolonged operative times, and the lateral decubitus position.27 Diagnosis of rhabdomyolysis is suspected when a patient reports discrete musculoskeletal pain immediately upon recovering from anesthesia. Serum creatinine phosphokinase and creatinine should be trended to follow the clinical course. There is no absolute cut-off with regards to the level of creatinine phosphokinase (CK) that is used to define clinical rhabdomyolysis. Many clinicians use 5x the upper limit of normal (1000 IU/L) but typically much higher levels of CK are necessary to cause clinical concern. Imaging studies are not typically necessary but, if performed, may show a hematoma within the affected muscle. If compartment syndrome occurs, fasciotomy may be necessary.27 Rhabdomyolysis can be complicated by acute renal failure (occurring in 4%-33% of patients), compartment syndrome, cardiac dysrhythmias via electrolyte abnormalities, and disseminated intravascular coagulopathy.28 If patients exhibit clinical findings consistent with rhabdomyolysis, careful screening for these associated complications is imperative. Management includes intravenous fluid hydration with the initiation of sodium bicarbonate therapy for urine alkalinization in order to prevent myoglobin deposition in the glomeruli and progressive nephropathy.28
Renal Insufficiency
Renal vascular occlusion is often necessary during RALPN or LPN to minimize hemorrhage during resection and to allow for satisfactory visualization for precise excision and repair. Renal hilar clamping with warm ischemia may result in transient renal insufficiency postoperatively. Renal ischemia can result in acute tubular necrosis (ATN) demonstrated by an elevated serum creatinine over several days.
Minimizing ischemia time is important for maximizing postoperative renal function, although significant debate is seen in the literature regarding acceptable WIT to minimize renal functional impairment. One study reported minimizing WIT to under 40 minutes as ideal to minimize risk of ATN and to potentially avoid more permanent renal insufficiency.29 The incidence of renal functional impairment was found to be more than two-fold higher in cases with a WIT greater than 40 minutes in patient with a pre- operative GFR>60 ml/min per 1.73m2. Other earlier studies demonstrated stable post- operative renal function with WIT up to 50 mins.30-33 An additional study reports that nadir glomerular filtration rate (GFR) is most affected by WIT greater than 20 minutes with each additional minute over 20 resulting in significantly reduced nadir GFR.34 Patient with preexisting renal insufficiency, risk factors for nephropathy or solitary kidney will have less renal reserve34 and consideration should be given to open partial nephrectomy with cold ischemia.
A large multi-instutional series comparing robotic to conventional laparoscopic PN showed significantly shorter WIT in the robotic cohort (19.7 min vs. 28.4 min; p<0.001).8 The techniques of early unclamping of the renal hilum after running an initial suture along the resection bed, with complete hemostasis and closure being obtained off-clamp, has also been shown to decrease ischemia times.35 Clamping of the renal artery alone may provide sufficient control of bleeding during resection while allowing for some venous backflow perfusion to limit total renal ischemia during resection.34 Further investigation is needed to determine if shorter warm ischemia times result in superior long term post-operative renal functional outcomes.
Adequate renal perfusion pre- and post-clamping also minimize the degree of ischemia- reperfusion injury to the kidney. As patients are NPO pre-operatively, sufficient fluid resuscitation should be given at the beginning of the case to return patients to a normovolemic fluid status. Intraoperative administration of Mannitol before and after arterial clamping may decrease the risk of ATN associated with renal ischemia. The proposed mechanisms of action of mannitol include acting as a free radical scavenger, decreasing intracellular edema, decreasing intra-renal vascular resistance, increasing blood flow and GFR of superficial nephrons, and promoting osmotic diuresis.36 Administration of furosemide after unclamping of the renal hilum may also reduce the risk of ischemic nephropathy.36
Postoperative monitoring of urine output and serum creatinine are performed to look for renal insufficiency after RALPN. If renal function further deteriorates or persists postoperatively, there should be a low threshold for obtaining nephrology consultation. A renal ultrasound may be performed to ensure there is no postrenal etiology including clot obstruction. A urine leak may cause an increase in serum creatinine secondary to peritoneal absorption.
LAPAROSCOPIC CRYOABLATION
The risk of urologic complications of ablative therapies is less that that of LPN or RPN (4.9% vs. 6.3-9%), but there is a slightly lower local recurrence free survival rate (88- 91% vs. 95-98%).37 Postoperative hemorrhage is a potential complication of laparoscopic renal ablation.38,39 The cryoprobe should be inserted gently, avoiding lateral movements that could cause parenchymal fracture and bleeding. After the second thaw cycle has been completed, the surgeon should wait until the probe is completely free before attempting removal. Testing the ability to rotate the needle can help confirm it is free prior to pulling it back. Application of hemostatic agents and/or sealants into the cryoprobe cavity after probe removal with light local compression may help prevent bleeding. Careful hemostatic confirmation of hemostasis at a reduced insufflation pressure of < 5 mm Hg is important. If diffuse parenchymal oozing is observed, additional hemostatic measures such as argon beam coagulation (ConMed, Utica, NJ), hemostatic agents, and compression may be used to resolve the bleeding. Suturing of treated tissue is discouraged because the ablated tissue is extremely friable and may lead to increased bleeding.
Calyceal cryoinjury is another potential complication. In pig models, intentional freezing of the collecting system showed microscopic necrosis extending up to the mucosa but no signs of perforation or leakage with up to 3 months of follow-up.40,41 Nevertheless, cautious selection of patients with peripherally localized tumors and the use of real-time ultrasound while performing the procedure may help to avoid urinary fistula formation.
Injury to contiguous organs when cryoablation is being performed is another complication to avoid. Complete small bowel obstruction and the development of severe strictures of the ureteropelvic junction have been reported in animal models.42 Adequate mobilization of the kidney, away from the bowel, and visual control of surrounding structures, such as the renal vessels and the ureter, are imperative to prevent these potential complications. Furthermore, intraoperative ultrasound monitoring of the iceball formation may further increase the safety of the procedure. A retroperitoneal approach may offer a decreased risk of bowel injury, particularly for lateral and posterior lesions.
LAPAROSCOPIC RADIOFREQUENCY ABLATION (RFA)
The same safety precautions relevant to cryoablation should be followed for RFA. Small laparoscopic series describe the combined the use of radiofrequency as a preceding step to LPN. Although no complications occurred, these results are not interpretable in terms of complications specific to RFA.43,44 Jacomides et al44 reported on a series of 17 renal tumors treated with RFA. In 11 of these tumors, the mass was left in situ after treatment, with no perioperative complications. This treatment modality may hold promise as a minimally invasive treatment for organ-confined renal tumors.
CONCLUSION
LPN, RPN, and renal ablation are safe and effective approaches to the treatment of small renal tumors with similar profiles of potential complications. Key principles to minimize complications include maximal drainage of the urinary tract for urine leakage, prompt diagnosis and treatment of vascular complications, minimizing warm ischemia time to prevent renal dysfunction, and having a low threshold to diagnose and treat suspected complications.
References
- Porpiglia F, Volpe A, Billia M, Scarpa RM. Laparoscopic versus open partial nephrectomy: analysis of the current literature. Eur Urol. Apr 2008;53(4):732-742; discussion 742-733.
- Romero FR, Rais-Bahrami S, Muntener M, Brito FA, Jarrett TW, Kavoussi LR. Laparoscopic partial nephrectomy in obese and non-obese patients: comparison with open surgery. Urology. May 2008;71(5):806-809.
- Gong EM, Orvieto MA, Zorn KC, Lucioni A, Steinberg GD, Shalhav AL. Comparison of laparoscopic and open partial nephrectomy in clinical T1a renal tumors. J Endourol. May 2008;22(5):953-957.
- Patel MN, Bhandari M, Menon M, Rogers CG. Robotic-assisted partial nephrectomy. BJU Int. 2009;103(9):1296-1311.
- Nguyen MM, Gill IS. Halving ischemia time during laparoscopic partial nephrectomy. J Urol. Feb 2008;179(2):627-632; discussion 632.
- Wang AJ, Bhayani SB. Robotic partial nephrectomy versus laparoscopic partial nephrectomy for renal cell carcinoma: single-surgeon analysis of >100 consecutive procedures. Urology. Feb 2009;73(2):306-310.
- Benway BM, Wang AJ, Cabello JM, Bhayani SB. Robotic partial nephrectomy with sliding-clip renorrhaphy: technique and outcomes. Eur Urol. 2009;55(3):592-599.
- Benway BM, Bhayani SB, Rogers CG, et al. Robot assisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: a multi-institutional analysis of perioperative outcomes. J Urol. Sep 2009;182(3):866-872.
- Caruso RP, Phillips CK, Kau E, Taneja SS, Stifelman MD. Robot assisted laparoscopic partial nephrectomy: initial experience. J Urol. 2006 Jul 2006;176(1):36-39.
- Aron M, Koenig P, Kaouk JH, Nguyen MM, Desai MM, Gill IS. Robotic and laparoscopic partial nephrectomy: a matched-pair comparison from a high-volume BJU Int. 2008;102(1):86-92.
- Michli EE, Parra RO. Robotic-assisted laparoscopic partial nephrectomy: initial clinical experience. Urology. Feb 2009;73(2):302-305.
- Deane LA, Lee HJ, Box GN, et al. Robotic versus Standard Laparoscopic Partial/Wedge Nephrectomy: A Comparison of Intraoperative and Perioperative Results from a Single Institution. J Endourol. Apr 8 2008.
- Gettman MT, Blute ML, Chow GK, Neururer R, Bartsch G, Peschel R. Robotic- assisted laparoscopic partial nephrectomy: technique and initial clinical experience with DaVinci robotic system. Urology. Nov 2004;64(5):914-918.
- Kaul S, Laungani R, Sarle R, et al. Da vinci-assisted robotic partial nephrectomy: technique and results at a mean of 15 months of follow-up. Eur Urol. 2007 Jan 2007;51(1):186-192.
- Ho H, Schwentner C, Neururer R, Steiner H, Bartsch G, Peschel R. Robotic-assisted laparoscopic partial nephrectomy: surgical technique and clinical outcomes at 1 year. BJU Int. Mar 2009;103(5):663-668.
- Rogers CG, Metwalli A, Blatt AM, et al. Robotic partial nephrectomy for renal hilar tumors: a multi-institutional analysis. J Urol. Dec 2008;180(6):2353-2356; discussion 2356.
- Rogers CG, Menon, M., Weise, E. S., Gettman, M. T., Frank, I., Shephard, D. L., Abrahams, H. M., Green, J. M., Savatta, D. J., Bhayani, S. B. Robotic partial nephrectomy: a multi-institutional analysis. J Robotic Surg. 2008;2(3):141-143.
- Patel MN, Krane LS, Bhandari A, et al. Robotic partial nephrectomy for renal tumors larger than 4 cm. Eur Urol. Feb 2010;57(2):310-316.
- Ramani AP, Desai MM, Steinberg AP, et al. Complications of laparoscopic partial nephrectomy in 200 cases. J Urol. Jan 2005;173(1):42-47.
- Venkatesh R, Weld K, Ames CD, et al. Laparoscopic partial nephrectomy for renal masses: effect of tumor location. Urology. Jun 2006;67(6):1169-1174; discussion 1174.
- Link RB, SB, Allaf, ME, et al. Exploring the learning curve, pathological outcomes and perioperative morbidity of laparoscopic partial nephrectomy for renal mass. J Urol. 2005;173:1690-1694.
- Desai MM, Gill IS, Kaouk JH, Matin SF, Novick AC. Laparoscopic partial nephrectomy with suture repair of the pelvicaliceal system. Urology. Jan 2003;61(1):99- 104.
- Meeks JJ, Zhao LC, Navai N, Perry KT, Jr., Nadler RB, Smith ND. Risk factors and management of urine leaks after partial nephrectomy. J Urol. Dec 2008;180(6):2375- 2378.
- Neely WA, Turner MD. The effect of arterial, venous, and arteriovenous occlusion on renal blood flow. Surg Gynecol Obstet. Jun 1959;108(6):669-672.
- Cohenpour M, Strauss S, Gottlieb P, et al. Pseudoaneurysm of the renal artery following partial nephrectomy: imaging findings and coil embolization. Clin Radiol. Nov 2007;62(11):1104-1109.
- Bishoff JT, Allaf ME, Kirkels W, Moore RG, Kavoussi LR, Schroder Laparoscopic bowel injury: incidence and clinical presentation. J Urol. Mar 1999;161(3):887-890.
- Glassman DT, Merriam WG, Trabulsi EJ, Byrne D, Gomella L. Rhabdomyolysis after laparoscopic nephrectomy. Jsls. Oct-Dec 2007;11(4):432-437.
- Reisiger KE, Landman J, Kibel A, Clayman RV. Laparoscopic renal surgery and the risk of rhabdomyolysis: diagnosis and treatment. Urology. Nov 2005;66(5 Suppl):29-35.
- Mufarrij PW, Shah OD, Berger AD, Stifelman MD. Robotic reconstruction of the upper urinary tract. J Urol. Nov 2007;178(5):2002-2005.
- Bhayani SB, Rha KH, Pinto PA, et al. Laparoscopic partial nephrectomy: effect of warm ischemia on serum creatinine. J Urol. Oct 2004;172(4 Pt 1):1264-1266.
- Desai MM, Gill IS, Ramani AP, Spaliviero M, Rybicki L, Kaouk JH. The impact of warm ischaemia on renal function after laparoscopic partial nephrectomy. BJU Int. Feb 2005;95(3):377-383.
- Lane BR, Gill IS. 5-Year outcomes of laparoscopic partial nephrectomy. J Urol. Jan 2007;177(1):70-74; discussion 74.
- Saranchuk JW, Touijer AK, Hakimian P, Snyder ME, Russo P. Partial nephrectomy for patients with a solitary kidney: the Memorial Sloan-Kettering experience. BJU Int. Dec 2004;94(9):1323-1328.
- Lane BR, Babineau DC, Poggio ED, et al. Factors predicting renal functional outcome after partial nephrectomy. J Urol. Dec 2008;180(6):2363-2368; discussion 2368-2369.
- Baumert H, Ballaro A, Shah N, et al. Reducing warm ischaemia time during laparoscopic partial nephrectomy: a prospective comparison of two renal closure techniques. Eur Urol. Oct 2007;52(4):1164-1169.
- Nosowsky EE, Kaufman JJ. The protective action of mannitol in renal artery occlusion. J Urol. Mar1963;89:295-299.
- Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. Oct 2009;182(4):1271-1279.
- Carvahal E NA, Gill IS. Renal Cryoablation application in nephron-sparing treatment. Brazilian J Urol. 2000;26(6):558-570.
- Rodriguez R, Chan DY, Bishoff JT, et al. Renal ablative cryosurgery in selected patients with peripheral renal masses. Urology. Jan 2000;55(1):25-30.
- Shingleton WB, Farabaugh P, Hughson M, Sewell P. Effects of cryoablation on short-term development of urinary fistulas in the porcine kidney. J Endourol. Feb 2003;17(1):37-40.
- Sung GT, Gill IS, Hsu TH, et al. Effect of intentional cryo-injury to the renal collecting system. J Urol. Aug 2003;170(2 Pt 1):619-622.
- Campbell SC, Krishnamurthi V, Chow G, Hale J, Myles J, Novick AC. Renal cryosurgery: experimental evaluation of treatment parameters. Urology. Jul 1998;52(1):29-33; discussion 33-24.
- Gettman MT, Bishoff JT, Su LM, et al. Hemostatic laparoscopic partial nephrectomy: initial experience with the radiofrequency coagulation-assisted Urology. Jul 2001;58(1):8-11.
- Jacomides L, Ogan K, Watumull L, Cadeddu JA. Laparoscopic application of radio frequency energy enables in situ renal tumor ablation and partial nephrectomy. J Urol. Jan 2003;169(1):49-53; discussion 53.
