Laparoscopic Pelvic Reconstructive Surgery
Bulent Berker, MD, Babac Shahmohamady, MD, Naghmeh Saberi, MD, Camran Nezhat, MD, FACOG, FACS
Pelvic organ prolapse is a common concern for women and their physicians. Up to 50% of parous women may experience this type of prolapse, which is associated with a variety of urinary, bowel, and sexual symptoms. The estimated lifetime risk of undergoing surgical intervention for prolapse is 11% for women who reach 80 years of age, and this figure is likely to increase with the current aging of the population. Pelvic organ prolapse can occur when pelvic support structures are subjected to increased intraabdominal pressure. An intrinsic defect of the pelvic floor is commonly present. Several theories exist regarding the cause of pelvic organ prolapse; none fully explains the origin and natural history of the process. Proposed causes include denervation of the pelvic floor musculature, direct injury to the pelvic floor musculature, abnormal synthesis or degradation of collagen, and defects in endopelvic fascia.1 Although support for the pelvic viscera, the vagina, and neighboring structures involves a complex interplay between muscles, fascia, nerve supply, and appropriate anatomic orientation, the endopelvic fascia and pelvic floor muscles provide most of the support function in the female pelvis.2
Loss of support of the pelvic organs can involve one or all of the 3 following areas: anterior, posterior, and apical compartments. Defects in the anterior vaginal compartment result in cystourethrocele formation and sometimes stress urinary incontinence. Posterior compartment defects result in rectocele and enterocele. Apical defects result in uterovaginal prolapse and vaginal vault prolapse.3 Usually pelvic floor defects occur in several places requiring multiple procedures in the same patient. The existence of numerous surgical techniques for treating genitourinary prolapse and incontinence demonstrates that no single method is completely satisfactory.
The anatomy, pathophysiology, and treatment of pelvic organ prolapse has significantly evolved over the last decade with increasing understanding of anatomy and development of minimally invasive surgical procedures.2 The introduction of videolaparoscopy by Nezhat et al4,5 has revolutionized modern day gynecologic and general surgery. Although operative laparoscopy has been used for decades, only recently has it gained widespread popularity for major operative procedures. Advances in minimally invasive surgery have led to an increasing adoption of laparoscopic techniques in pelvic reconstructive surgery and treatment of urinary incontinence. Interest in the use of laparoscopic procedures to correct pelvic organ prolapse is growing. The laparoscopic approach has been successfully adopted for many procedures that previously relied on an abdominal or transvaginal route. The principles of laparoscopic surgery are based on those used in the corresponding open procedures. The laparoscopic approach offers several important advantages, including excellent intraoperative visualization of the pelvic anatomy, reduction in adhesion formation, decreased postoperative pain and quicker postoperative recovery. However, a longer time is required to train surgeons to be highly skilled in both prolapse surgery and advanced laparoscopic techniques. In particular, a high level of laparoscopic suturing skill is necessary, if the efficacies of their laparoscopic prolapse procedures are to be comparable to those of their open abdominal counterparts.6,7 Laparoscopic reconstructive pelvic surgery requires a thorough knowledge of pelvic floor anatomy and its supportive components before repair of defective anatomy is attempted.
RETROPUBIC URETHRAL SUSPENSION
The normal continence mechanism in the female consists of an intrinsic coapting ability of the proximal urethra, a critical functional urethral length, the ability of the pelvic floor to increase urethral pressure at the time of stress, and the proper anatomic location of this sphincteric unit.8 Leakage resulting from the loss of the normal supporting backboard effect of the levator muscles and the urethral pelvic ligaments on the bladder neck and the proximal urethra is termed anatomic stress urinary incontinence. Urinary incontinence is becoming more prevalent as the population ages9,10 with 20% to 40% of women having this complaint.9-12 The psychological status of these patients improves significantly after a successful surgical cure.13 Despite more than 160 different corrective operations, an optimal approach has not been demonstrated.14 However, retropubic bladder neck suspensions have become recognized as the most efficacious procedures for long-term management of stress urinary incontinence.
The principles of surgical correction of anatomic stress urinary incontinence include securing the anterior vaginal wall with its overlying endopelvic fascia to a strong ligamentous or bony structure anterosuperiorly in such a manner that the bladder neck and proximal urethra are elevated and secured in a retropubic position. Retropubic urethropexy has produced excellent results with relatively few complications.15-17 Although needle urethropexy18-22 is a shorter operation and is less invasive, it is not as effective and is associated with more complications.23-26 Tanagho’s modification of the Burch procedure has succeeded in curing patients with pure genuine urinary stress incontinence (GUSI) and an intact urethral sphincter.27-31 Laparoscopic bladder neck suspension has been carried out with good results.32-34
Laparoscopic retropubic urethral suspension is an outpatient procedure that potentially provides a long-term solution for this condition. The advantages include excellent exposure and access to the retropubic space because of videolaparoscopic magnification, enhancing the surgeon’s ability to place the sutures precisely. The improved exposure allows proper support and avoids urethral obstruction and compression. Laparoscopic retropubic colposuspension (Burch method) has produced good results with relatively few complications.
Preoperative Evaluation
The workup should include a history and physical examination and gynecologic and neurologic examinations. Attempts should be made to evaluate and correct factors that contribute to urinary incontinence. The onset, duration, and severity of incontinence and prior surgeries for associated conditions should be documented. Any symptoms of urgency and urge incontinence mandate a urodynamic evaluation to determine detrusor instability or other overt neurologic causes of incontinence. A Valsalva leak point pressure of <90cm of water is considered suggestive of intrinsic sphincter dysfunction, and patients with this finding are advised to undergo sling urethropexy rather than a bladder neck suspension.35 Office tests include stress test (lithotomy and standing), the Q- tip test, urinalysis, urine culture and sensitivity, and blood chemistry analysis.36 Multichannel urodynamic evaluation of patients while in the lithotomy and standing positions is done with an emphasis on voiding time, voiding volume, and postvoid residual urine volume.37-40 Additional tests include a water fill cystometrogram with continuous urethral pressure monitoring and subtracted rectal and abdominal pressures.
GUSI is diagnosed by a positive stress test in the absence of simultaneous detrusor contractions or pressure equalization on the stress urethral closure pressure profile. Patients are encouraged to keep 24-hour to 48-hour symptom diaries.
Operative Technique
After induction of general endotracheal anesthesia, the patient is placed in Allen stirrups, which permit the assistant to do a vaginal examination. A Foley catheter is placed. A 10- mm operative video laparoscope is introduced infraumbilically, and three 5-mm accessory cannulas are inserted (Figure 1).5
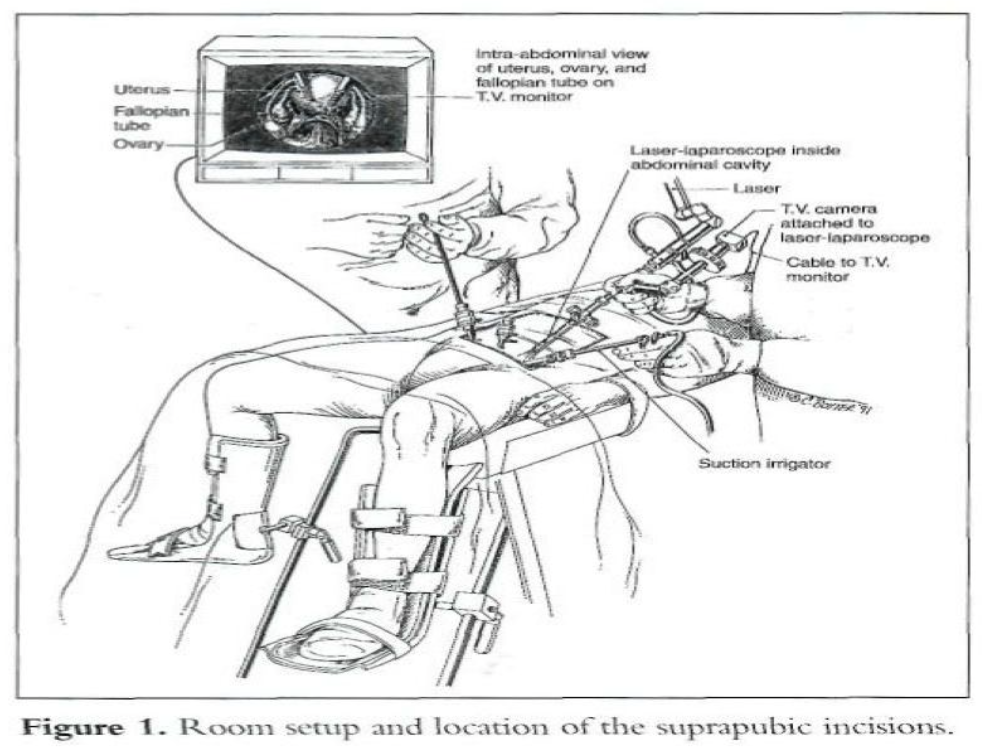
The middle cannula is 5cm to 6cm above the pubic symphysis, and the other 2 cannulas are 7cm to 8cm above the pubic symphysis lateral to the umbilical ligaments, avoiding injury to the inferior epigastric vessels. The carbon dioxide (CO2) laser may be placed through the operative channel of the 10-mm laparoscope and used as a long knife.
However, any cutting modality is acceptable. A suction-irrigator, a grasping forceps, a needle holder, and a bipolar electrocoagulator are introduced through the accessory cannulas.
The intraperitoneal cavity is inspected to detect any pelvic abnormalities that require surgical treatment. Initially, indicated gynecologic procedures are done. After the ureters are identified, a culdoplasty is carried out if indicated. The enterocele sac is excised, and a laparoscopic purse-string suture (1-0 polybutilate-coated polyester) is placed that incorporates the posterior wall of the vagina, the peritoneum laterally at the left and right pararectal areas, and shallow bites of serosa over the anterior rectosigmoid colon. In patients who have undergone previous hysterectomy, remnants of uterosacral ligaments are included in the suture. Closure of the cul-de-sac should leave no defect that could result in bowel entrapment.
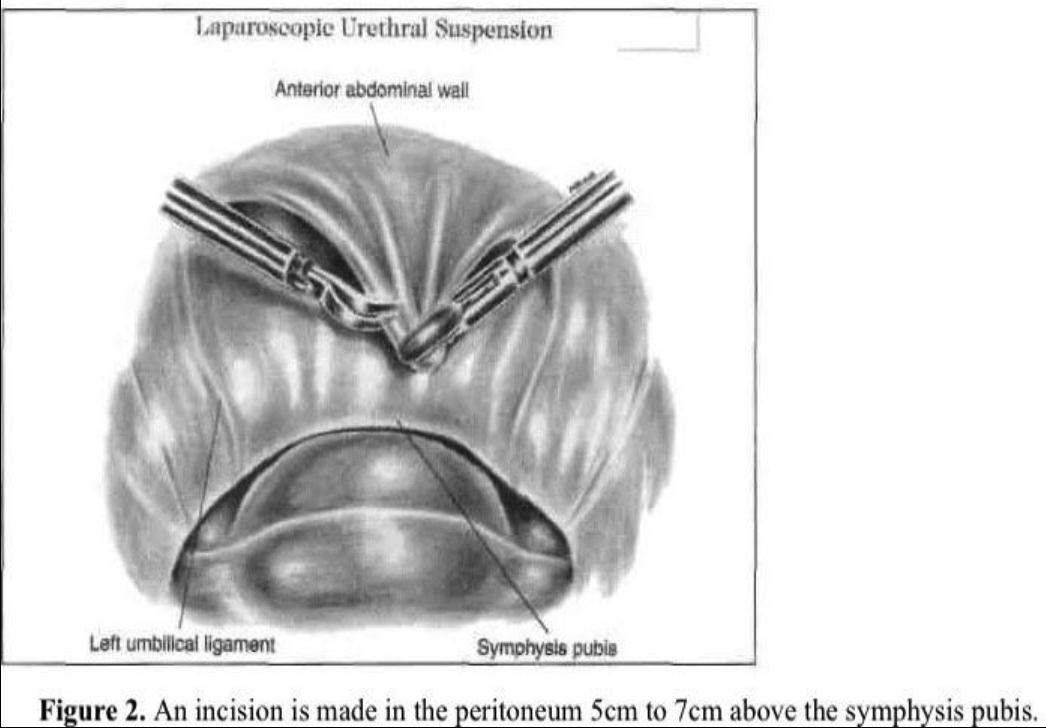
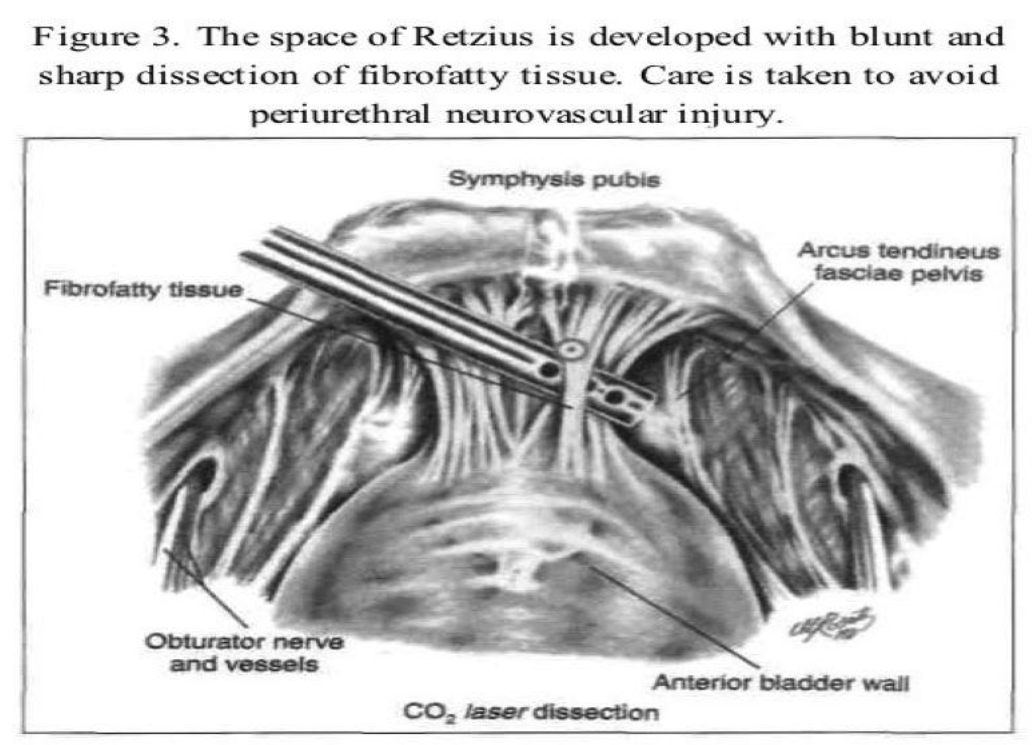
The transperitoneal technique is one of the procedures that can be used to enter the space of Retzius. The anterior abdominal wall peritoneum 5cm to 7cm above the pubic symphysis is pulled down with grasping forceps placed through a lateral accessory cannula (Figure 2). A transverse incision is made with the CO2 laser, scissors, or Harmonic scalpel caudal to the midsuprapubic cannula above the pubic symphysis on the peritoneum between the 2 umbilical ligaments. The midline cannula entry and anatomic landmarks, including the round ligament from the internal ring, are used to avoid injury to the bladder. The retropubic space is dissected bluntly with hydrodissection or any of the above-mentioned instruments (Figure 3). The incision should remain close to the back of the pubic bone to drop the anterior bladder wall, vaginal wall, and urethra downward. Dissection is limited over the urethra in the midline to approximately 2cm lateral to the urethra to protect its delicate neuromusculature. An assistant does a vaginal examination with one finger on each side of the catheterized urethra, elevating the lateral vaginal fornix. The overlying fibrofatty tissue is cleared from the anterior vaginal wall under videolaparoscopic magnification. Beginning laterally, the bladder is dissected medially from the paravaginal fascia. The thin-walled venous plexus is identified and protected from trauma. Pneumoperitoneal pressure and the coagulating effect of the cutting instrument, such as a radiofrequency operated knife or a Harmonic scalpel, help control bleeding from small vessels.
Balloon Dissector
The balloon dissector (Tyco-Connetic, USA) consists of a cannula, a guide rod, and a balloon system. The dissector is inserted through a 1-cm infraumbilical incision. It is advanced between the rectus muscle and the anterior surface of the posterior rectus sheath to the pubic symphysis. The external sheath of the dissector is removed, and the balloon is inflated with approximately 750mL of saline solution. During inflation, the balloon unrolls sideways and exerts a perpendicular force that separates tissue layers. Blunt dissection of the connective tissues is propagated as the balloon expands. When the maximal volume is reached, the balloon is deflated and removed through the incision.
The dissected space is insufflated with CO2 at a pressure of 8mm Hg to 10mm Hg. The predefined shape of the balloon, its nonelastomeric material, and the incompressible character of the saline ensure a large, relatively bloodless working space of predictable size and shape. The space is adequate for identifying pertinent landmarks and for unencumbered manipulation of endoscopic surgical instruments.
Preperitoneal Approach
The preperitoneal technique for access to the space of Retzius uses the patient position and trocar placement described above. To begin dissection of the space, the midsuprapubic can-nula is withdrawn into the preperitoneal area and a 16-gauge laparoscopic needle is inserted through it. Approximately 30mL to 50mL of dilute vasopressin (20U in 60mL to 100mL of lactated Ringer’s solution) is injected subperitoneally into the lower anterior abdominal wall above the bladder to decrease oozing. The needle is replaced with a suction-irrigator probe by injecting 300mL to 500mL of lactated Ringer’s solution or normal saline at a pressure of 300mm Hg to form a subperitoneal space in the anterior abdominal wall. The laparoscope is retracted from the abdominal cavity and directed toward the newly created subperitoneal space in the anterior abdominal wall. A long hydrodissection probe is inserted through the space under direct observation, and the space is expanded further, using hydrodissection. The laparoscope is advanced into this space, which is insufflated with CO2. The lateral suprapubic trocars are retracted to the pneumosubperitoneal space. Blunt or sharp dissection may be required to lyse adhesions that remain after hydrodissection. The pubic symphysis and Cooper’s ligaments can be seen. The patient is placed in a deep Trendelenburg position and rotated to the left to facilitate left-handed suture placement.
Experience with this technique in 10 patients resulted in good outcomes.41 The technique has several advantages over transperitoneal and balloon dissection.42 Extension of the peritoneal incision is avoided, eliminating repair and minimizing the risks of bowel herniation and adhesions. The danger of trauma to the thin-walled venous plexus in this area during blunt or sharp dissection is minimized. The balloon dissector is an effective alternative to manual dissection of the space of Retzius42 but is more costly to use. The balloon should be inserted between the rectus muscle and the anterior surface of the posterior rectus sheath. It may be advanced inadvertently into an incorrect plane. The predefined shape of the balloon and its nonelastomeric material can force dissection that is less dependent on anatomic planes.
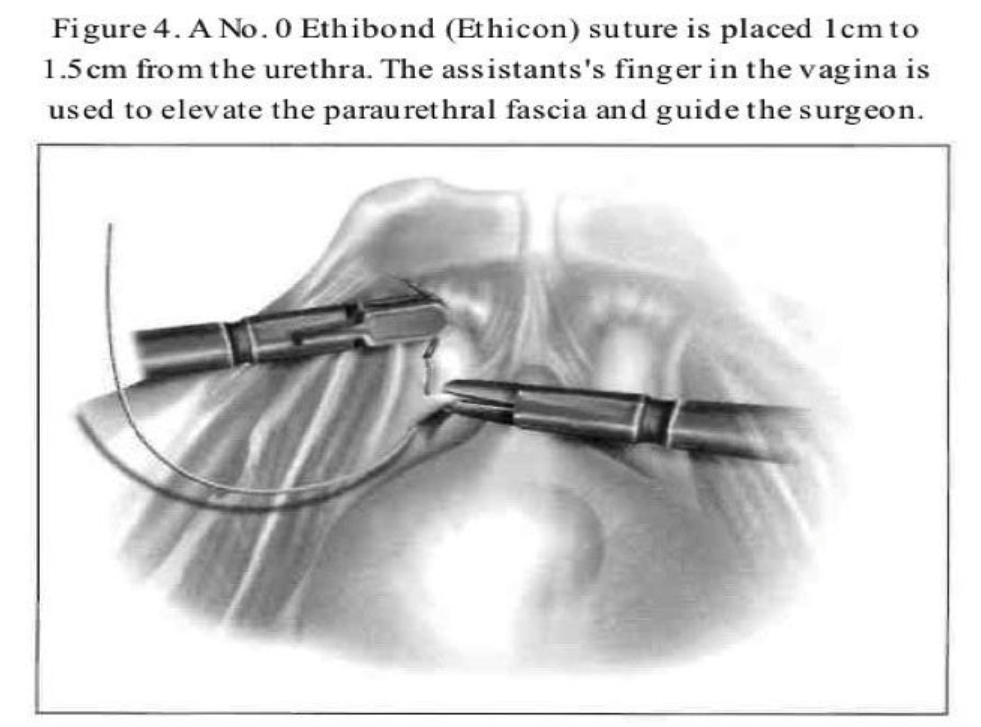
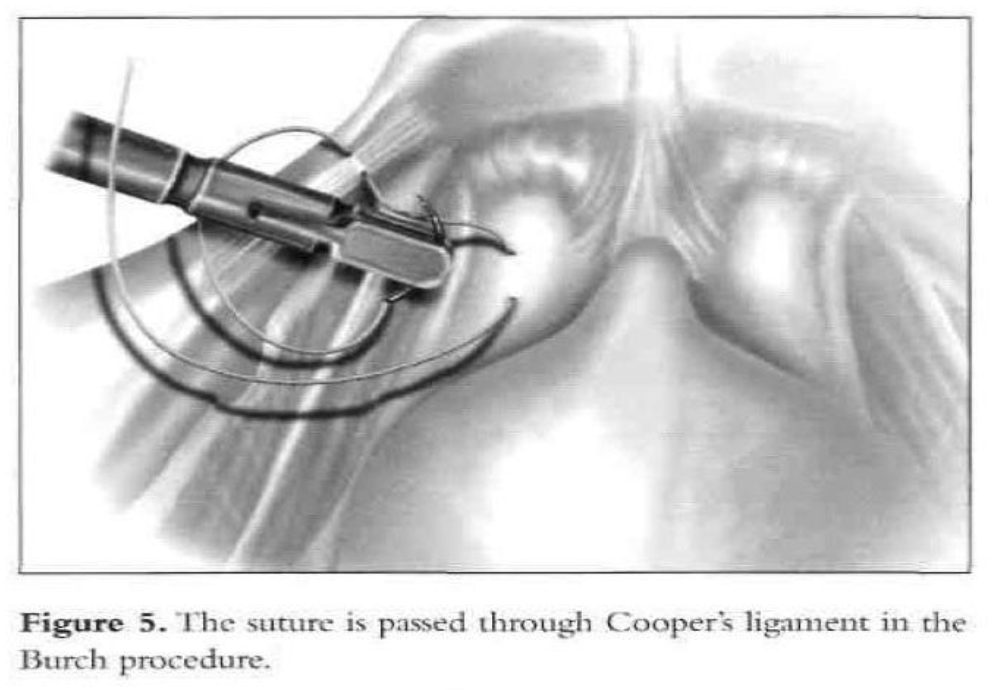
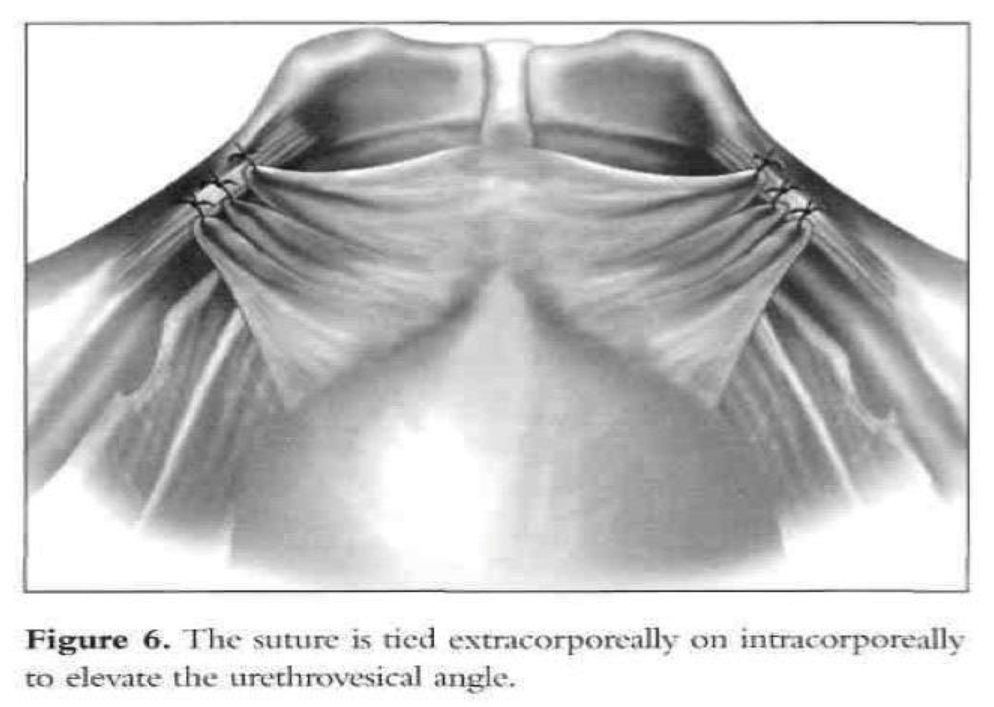
After dissection of the space of Retzius by any of the routes mentioned above is complete and the paravaginal fascia is identified, using an atraumatic grasping forceps, the paravaginal fascia is elevated and a 1-0 polybutilate-covered polyester endoscopic (Ethibond) suture is placed at the level of the urethrovesical junction, approximately 1cm to 1.5cm from the urethra (Figure 4). The assistant’s finger is used as a guide, and traction on the bulb of the Foley catheter facilitates the placement of the suture. The suture is inserted perpendicular to the vaginal axis to include approximately 1cm to 2cm of tissue (the complete vaginal fascia) but not the vaginal mucosa and is fixed to Cooper’s ligament (Figures 5, 6, and 7). The sutures are tied intracorporeally or extracorporeally with help from an assistant, who lifts the vagina upward and forward. Direct observation allows the surgeon to gauge the tension in the vaginal wall while tying the sutures. The urethra is observed to ensure that it is not compressed against the pubic bone. Suturing is repeated on the opposite side to create a platform on which the bladder neck rests while cinching is avoided. If the suspension is judged inadequate by visual inspection, manual elevation, or cystoscopy, a second set of sutures is placed cephalad along the base of the bladder. Alternative techniques include staple and mesh placement on each side of the bladder neck. Ou et al40 reported on 40 patients treated with placement of nylon mesh strips between the vagina and Cooper’s ligament. They used an Endostapler to secure the material. The proposed advantage was to simplify the suspension by eliminating suturing and tying.
Cystoscopy is accomplished to ensure that no suture material is in the bladder and to assess the urethrovesical junction angle and urethral patency. Also, at the end of the procedure, generally intravenous indigo carmine is given to document ureteral patency.
Pneumosubperitoneal pressure is decreased, and the retropubic space is observed for bleeding. Hemostasis is achieved with the bipolar electro-coagulator. The laparoscope is withdrawn from the space of Retzius into the abdomen. No drain is used, and the peritoneal defect is closed with an absorbable suture if needed. The laparoscope is removed from the abdomen, and the procedure is complete. The transurethral Foley catheter is left in place for 2 days or 3 days, or the patient is instructed on self- catheterization. Patients should receive perioperative intravenous antibiotic prophylaxis followed by a course of oral antibiotics for 5 days or until self-catheterization is discontinued when postvoiding residuals are less than 100mL. Postoperative osteitis pubis after this procedure is a concern,41 but that complication has not occurred in the authors’ series.42
In 62 operations, one bladder perforation occurred during bladder dissection and entry into the space of Retzius. It was repaired laparoscopically in one layer by using 0 polyglactin interrupted sutures. One patient, who also had an oophorectomy, developed an incisional hernia at the site of the midline suprapubic cannula, although the fascia had been repaired with one interrupted absorbable suture. Another patient who was unable to void postoperatively required self-catheterization for 10 days. Nine women had dysuria without infection that resolved with phenazopyridine hydrochloride (Pyridium) treatment. During the 8-month to 30-month follow-up, all patients reported relief of stress incontinence. Subjective success was ascertained by a questionnaire on urinary leakage and the absence of a need to wear pads (none were required). Objective success was assessed by comparison of pre- and postoperative symptom diaries, postvoid residual volume, urethrovesical junction angle as detected by catheter and Q-tip placement, bladder support, and a negative standing stress test.42
Since Vancaillie and Schuessler33 reported the first laparoscopic colposuspension case series in 1991, many other investigators have reported their experience. Published cure rates range from 69% to 100%, with the majority of the studies43,44 reporting cure rates greater than 80%. A recent Cochrane review on these 2 approaches included 9 trials.45 All of these favored the laparoscopic approach, because it was associated with decreases in blood loss, postoperative pain, hospital stay, and duration of catheterization. Laparoscopy did, however, increase operative times by a mean of 13.7 minutes. Perioperative complications were greater overall in the open group, but there were significantly more bladder injuries in the laparoscopic group. No statistically significant differences existed in objective cure, de-novo detrusor overactivity, and voiding dysfunction between the laparoscopy and laparotomy groups on follow-up at 18 months to 5 years after surgery.
Subjective cure rates were in the ranges of 58% to 96% in the laparotomy group and 62% to 100% in the laparoscopy group. In one study, patients were randomized to placement of 1 suture or 2 sutures on each side of the urethra. At 1 year, the results revealed a higher objective cure rate with 2 sutures compared with a single suture bilaterally (83% vs. 53%) and higher subjective cure rates (89% vs. 69%).46
This further supports the assertion that laparoscopy is nothing more than a mode of surgical access; it should not mandate modification of surgical technique, and subsequent cure rates should be comparable to those of open surgical techniques. During laparoscopic retropubic urethral suspension, although the principles of an abdominal approach are followed, the disadvantages of a laparotomy incision are avoided. Elevation of the bladder neck depends on the scarring of the perivesical tissue to the pubic symphysis or pelvic sidewall, not on the 2 periuretheral sutures as in a needle procedure. GUSI can be corrected laparoscopically with comparable results. Such an approach appears to be less traumatic and invasive, with decreased operative and postoperative recovery times and less morbidity.
SACRAL COLPOPEXY
Vaginal vault prolapse occurs whenever the apex of the vagina descends below the introitus, turning the vagina inside out. It is uncommon in the United States, affecting between 900 and 1200 women annually.47 Vaginal vault prolapse occurs as a result of damage to the supporting structures of the vaginal apex, the cardinal or the uterosacral ligaments. The most common cause of this condition is hysterectomy with failure to adequately reattach the cardinal-uterosacral complex to the pubocervical fascia and rectovaginal fascia at the vaginal cuff. Other predisposing factors include enterocele, damage to the endopelvic fascia or pelvic floor ligaments during labor and delivery, and postmenopausal atrophy. Vaginal vault prolapse is usually associated with cystocele, rectocele, enterocele, or a combination of these defects.1-6
The approaches to the treatment of vaginal vault prolapse include the use of a pessary, vaginal reconstruction, and vaginal closure. For the obliterative approach, patient selection criteria should include the patient’s physiologic age, sexual desires, general health status, and symptoms. Total colpocleisis is not an option for sexually active women.
The goal of vaginal vault suspension is to correct all anatomic defects, maintain or restore normal bowel and bladder function, and restore a functioning vagina. Transvaginal sacrospinous vault suspension and needle urethropexy can result in a satisfactory outcome in most operations.48 The vaginal route can be used in women whose preference or medical disorders contraindicate the abdominal approach. However, studies have shown a 33% rate of recurrent prolapse associated with sacrospinous fixation and transvaginal needle suspension. The probability of an optimal surgical outcome is twice as great with a transabdominal operation.
Among the proposed surgical techniques to prevent and correct this condition is abdominal sacral colpopexy using the interposition of a synthetic suspensory hammock between the prolapsed vaginal vault and the anterior surface of the sacrum.49-51 However, this technique usually requires a midline abdominal incision, abdominal packing, and extensive bowel manipulation. It has a potential for infection, wound separation or dehiscence, and ileus or bowel obstruction. To minimize these drawbacks, sacral colpopexy can be carried out laparoscopically. Laparoscopic sacrocolpopexy was first described by Nezhat et al52 in 1994. The clinical outcomes and the morbidity reported in the clinical studies appear to be comparable to those for the abdominal approach.
Potential advantages over open surgery include the enhanced view deep in the pelvis, facilitating safe dissection and accurate suture placement. It allows a good view of the anterior and posterior compartments, so a global approach for the prolapse is possible by the same surgical route. If the surgeon uses laparoscopy as a means of surgical access and performs the sacral colpopexy in the same manner as in the open abdominal approach, the operative cure rate should theoretically be equivalent.
Patient evaluation and preparation for the endoscopic procedures should focus on the degree of prolapse and associated rectocele, cystourethrocele, and incontinence.
Preoperative mechanical and antibiotic bowel preparation is essential.
Technique
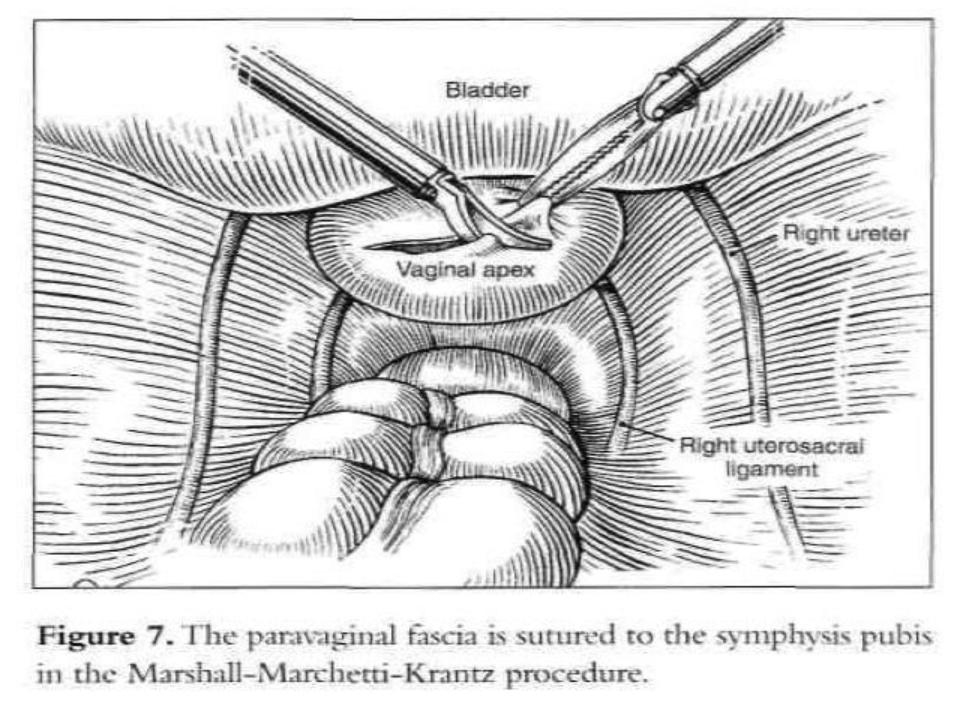
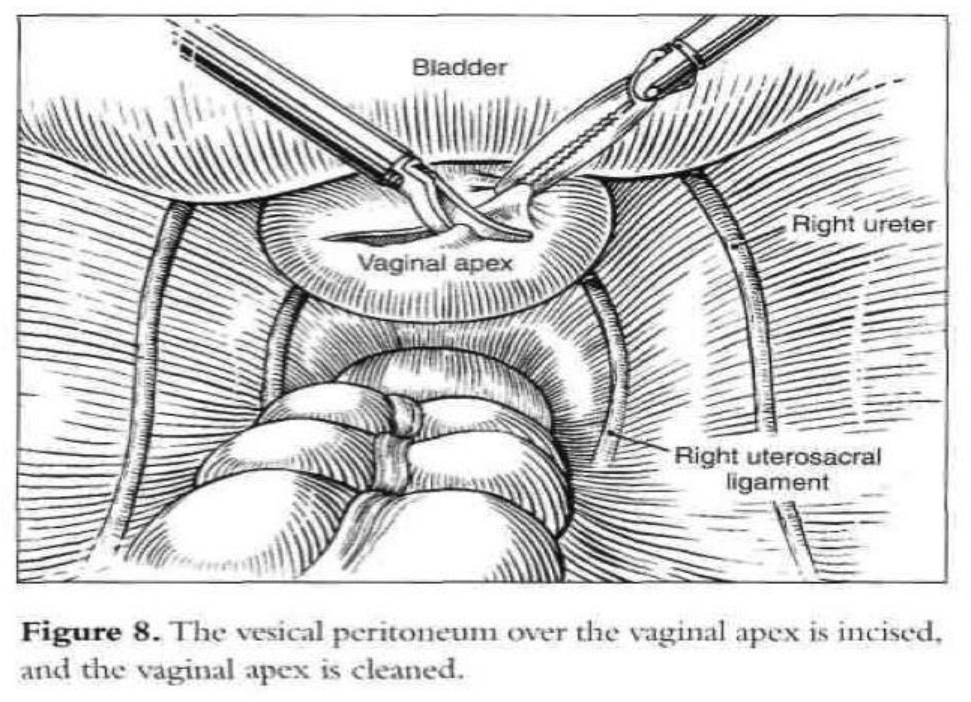
After the introduction of the operative laparoscope and ancillary 5-mm suprapubic trocars, the patient is placed in a Trendelenburg position and tilted to the left. After evaluation of the peritoneal cavity and completion of other indicated procedures, a sponge on a ring forceps is used to elevate the vaginal vault.53 The vaginal apex is prepared by removing the peritoneum and connective tissue until the vaginal fascia and scar are seen (Figure 8). The bladder is dissected from the anterior vaginal wall, and the rectum is dissected from the posterior vaginal wall so that approximately 4cm of the vaginal vault is exposed. If the vagina is opened because of hysterectomy or partial vaginectomy, pneumoperitoneum is maintained by placing an inflated surgical glove in the vagina.
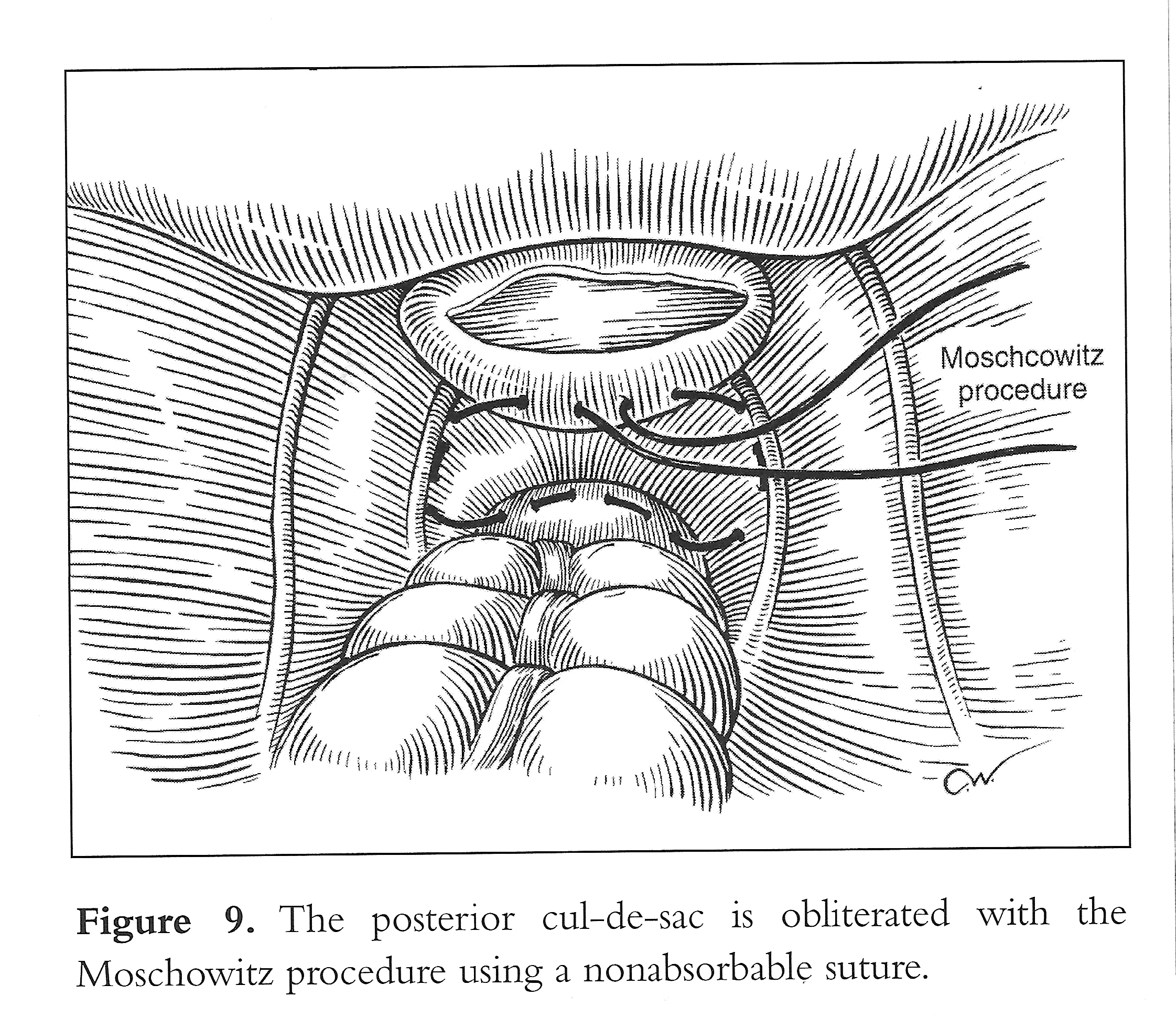
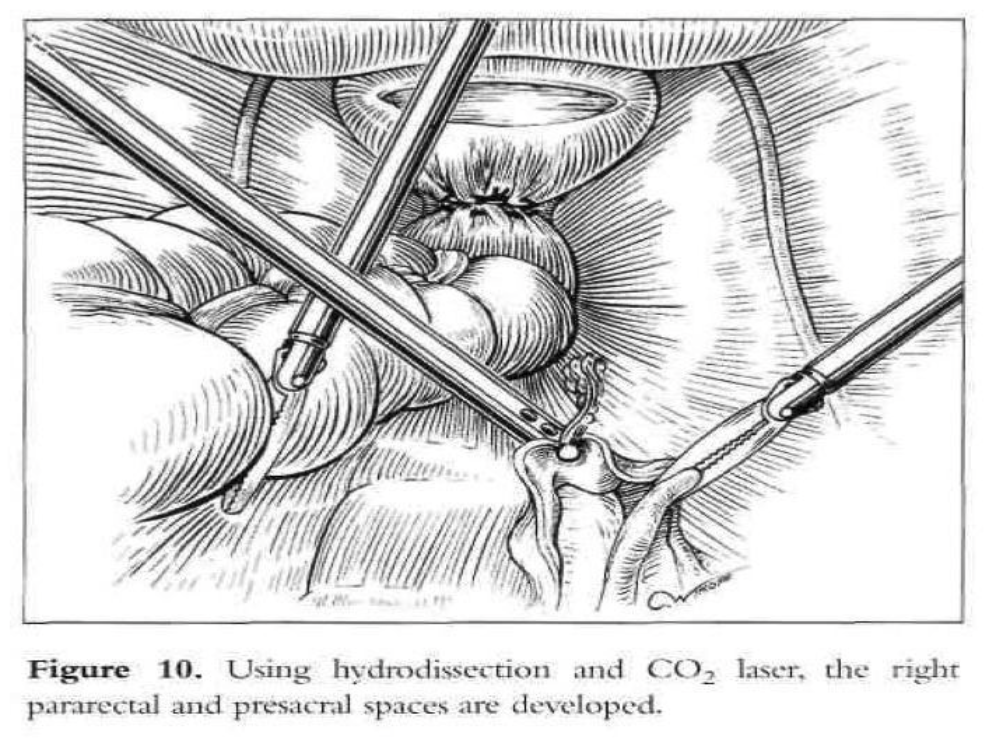
Vaginal vault prolapse is usually seen together with enterocele. At the time of vaginal hysterectomy or in the vaginal correction of vault prolapse, it is imperative that the surgeon identifies any existing enterocele. This can readily be accomplished preoperatively as well as intraoperatively by paying particular attention to anatomy. An intraoperative rectal examination should be performed by inserting the finger as far into the rectum as possible to ensure that the upper posterior wall defect is indeed an enterocele and not a high anterior rectocele. An enterocele is defined as a pelvic hernia where the parietal peritoneum comes into direct contact with vaginal epithelium with no intervening fascia. The development of an enterocele is likely to be directly related to a disruption of the fusion of the proximal margins of the anterior pubocervical fascia and posterior rectovaginal fascia or failure to surgically reattach these 2 fascial margins at the time of vaginal cuff closure.2 Repair of an enterocele is achieved laparoscopically by excising the sac and using a modified Moschcowitz procedure (Figure 9). The rectosigmoid colon is pushed to the left side to expose the sacral area. The posterior parietal peritoneum at or below the sacral promontory is lifted with grasping forceps and incised to the level of the third and fourth sacral vertebrae (S3-S4), and the anterior sacral fascia is exposed. The peritoneal incision is extended from the right pararectal area downward toward the vagina through the presacral space (Figure 10). The right ureter, internal iliac artery and vein, descending colon, and presacral vessels are identified.
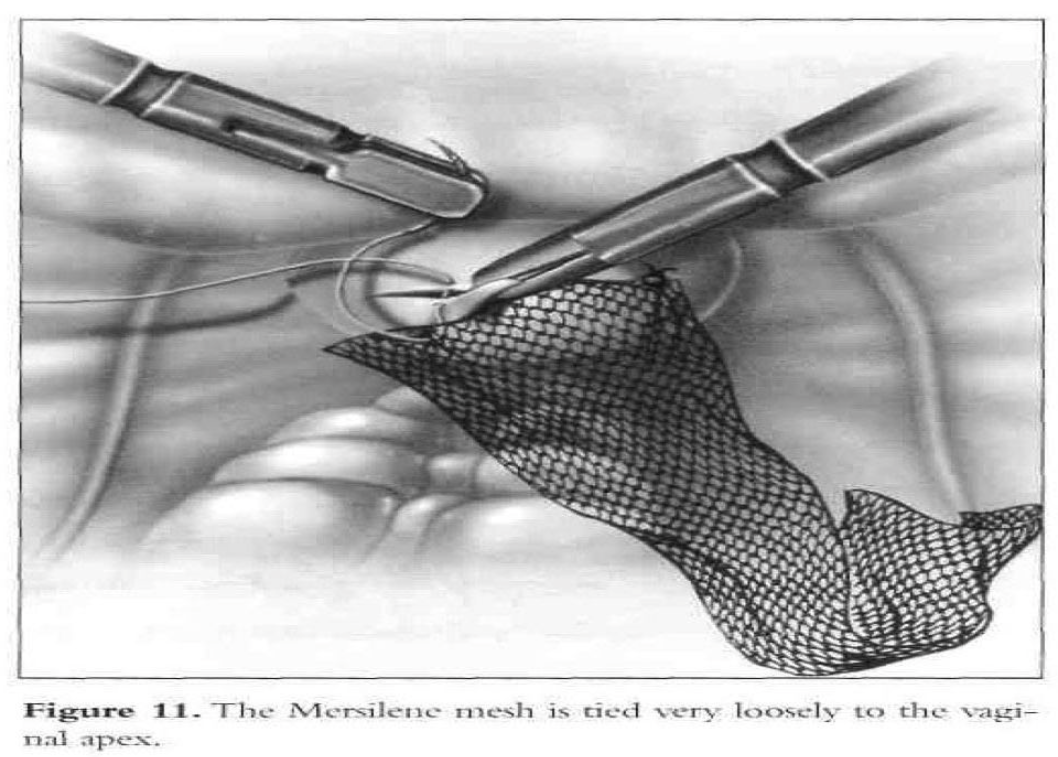
A 2.5-mm by 10-mm piece of Mersilene or Gore-Tex (WX. Gore and Associates, Inc., Phoenix, AZ) mesh is rolled and introduced into the abdomen through the 10-mm suprapubic port. Three to five 0 Ethibond sutures (Ethicon, Inc., Somerville, NJ) are placed in a single row in the posterior vaginal wall apex (excluding the vaginal mucosa) from one lateral fornix to the other. Each suture is placed through one end of the mesh and tied loosely (Figure 11).
Other supportive measures in the lower vagina, such as anterior and posterior colporrhaphy, may be necessary for the lower and middle third of the vagina. If required, partial vaginectomy is accomplished. The mesh is sutured to the posterior vaginal wall with a nonabsorbable suture and placed intraperitoneally before the vaginal cuff is closed with delayed absorbable sutures.
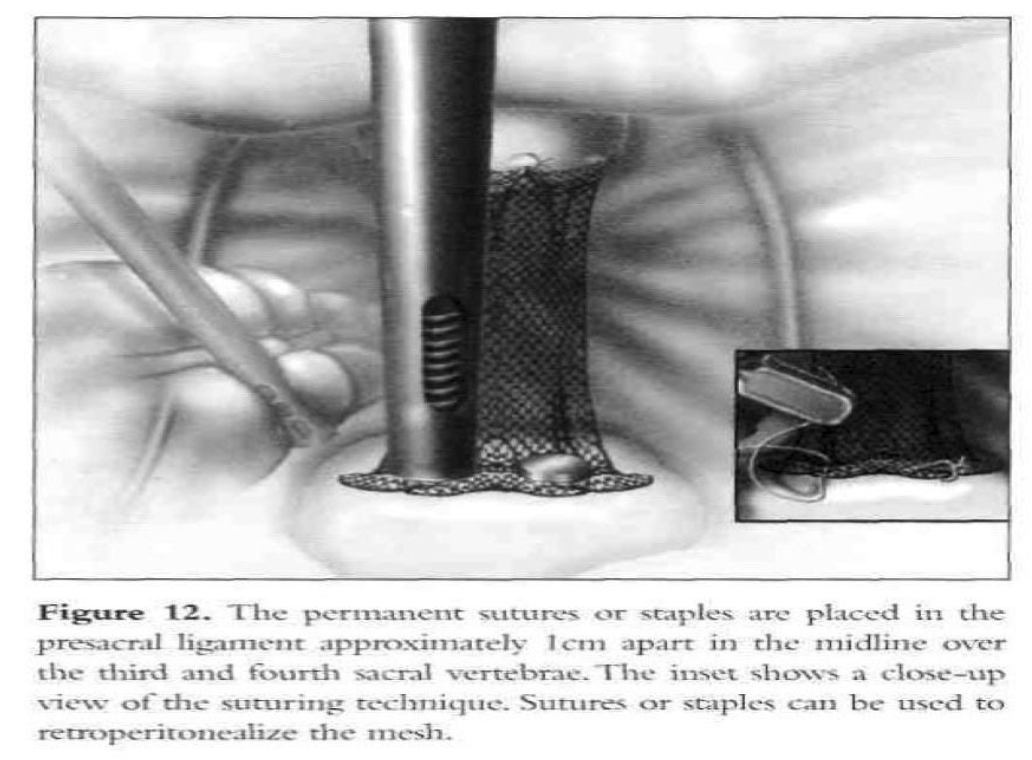
The mesh is adjusted to hold the vaginal apex in the correct anatomic position without being tight. Two permanent sutures or staples are placed in the longitudinal ligaments of the anterior surface of the sacrum, approximately 1cm apart in the midline over S3-S4 (Figure 12). The peritoneum is sutured over the graft from the sacrum toward thevagina. Usually, only a small segment of the graft cannot be covered. The serosa of the tail of the bladder (posterior peritoneal fold) and the superficial serosa of the sigmoid colon are used to cover the exposed mesh completely. If indicated, laparoscopic urethropexy is carried out.
Postoperatively, the patient is instructed to limit her activity, avoid intercourse for 6 weeks, and avoid strenuous exercise and heavy lifting for 2 months. Diet is advanced as tolerated, and a mild laxative is prescribed to prevent constipation.
Although data are somewhat limited, objective cure rates reported for laparoscopic sacral colpopexy range from 75% to 100%, with published subjective cure rates ranging from 89% to 98%. In a prospective cohort study, Sarlos et al54 followed 101 laparoscopic sacrocolpopexy patients to a median of 12 months. The objective cure rate, as defined by no prolapse in any compartment on physical examination, was 92%. Six (5.9%) had recurrence in the anterior wall, each with ≤stage 2 prolapse. One underwent further surgery. There were 2 (2%) posterior wall recurrences with ≤stage 2 prolapse. Only 2 (2%) patients with recurrent prolapse were symptomatic, yielding a subjective cure rate of 98%.
Laparoscopic sacrocolpopexy is a relatively safe procedure. In small comparative studies, complication rates seem similar between the laparoscopic and open approaches. Among 101 women followed prospectively for a median of 12 months after laparoscopic sacrocolpopexy, Sarlos et al54 encountered 4 (4%), bladder injuries, 3 (3%) rectal injuries, and 1 (1%) postoperative ileus. The cystotomies were repaired intraoperatively using laparoscopy. One of these patients developed mesh erosion into the bladder 6 months after the initial cystotomy repair. Two of the bowel injuries were noted intraoperatively, and one postoperatively. Twenty-four patients (23.8%) also presented with postoperative stress urinary incontinence, despite uniform administration of preoperative urodynamics and suburethral sling placement in patients with positive testing. Additional complications include urinary tract infection (16.8%), mesh erosion (1%), accidental colpotomy (1%), epigastric vessel injury (1%), and wound infection (1%). Thirty-nine of 47 (83%) previously sexually active patients reported their sexual function was stable or improved postoperatively, whereas 1 (1%) developed de novo dyspareunia. The authors postulate that the cohort’s previous prolapse surgery rate of >20% contributed to their relatively high complication rates.
The laparoscopic approach offers reduced morbidity, shorter hospitalization, and decreased postoperative pain. The disadvantages of the laparoscopic approach include longer operating time and the need for advanced laparoscopic surgical skills including suturing. Robotic-assisted laparoscopic technology allows the performance of complex laparoscopic maneuvers with less difficulty, thereby simplifying the procedure and possibly making it available to more patients with pelvic organ prolapse. The da Vinci robotic surgical system (Intuitive Surgical Inc., Sunnyvale, CA, USA) may decrease the difficulty of the procedure. This surgical system was introduced in 1999 and was approved by the Food and Drug Administration (FDA) for performing gynecologic procedures in April 2005. The robotic system was designed to overcome the current limitations of conventional laparoscopy by providing the surgeon with improved dexterity, precision, and 3-dimensional imaging. There were 2 main studies in the literature on RASCP. Both studies included a limited number of patients. Elliot et al55 reported on 30 patients who had RASCP with a mean operative time of 186 minutes.
However, the authors described a technique in which the robot was only utilized to facilitate suturing, whereas all of the dissection was otherwise performed with conventional laparoscopy. Daneshgari et al56 reported on 15 patients with RASCP. The authors reported a mean operative time of 317 minutes. Recently, Akl and et al57 reported the largest case series of RASCP reported in the literature to date. They noted that their mean operative time generally decreased as more procedures were done. After completion of the first 10 cases, their mean operative time decreased significantly by 25.4%. The mean operative time of the last 30 cases was 167.3 minutes. Although the robot decreases the complexity of the procedure and may shorten its learning curve, the procedure still requires some degree of advanced laparoscopic skills.
REPAIR OF CYSTOURETHROCELE
Prolonged, bothersome vaginal protrusions and pelvic pressure that worsens with ambulation and daily activity are common symptoms in women who have vaginal prolapse. Other symptoms include difficulty walking, voiding, or defecating; urinary incontinence; recurrent mucosal irritation; ulceration; and coital difficulty. Improvement in the quality of life is achievable in certain patients with behavioral modification and nonsurgical vaginal devices.
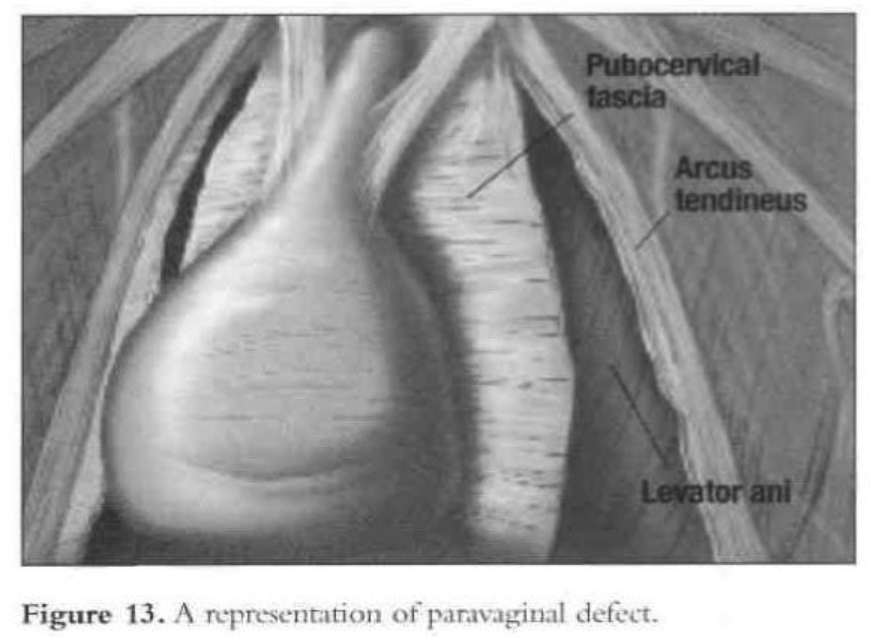
The arcus tendinous fascia pelvis is a band of dense regular connective tissue stretched between the pubic bone and the ischial spine. The pubocervical fascia forms a trapezoidal layer spanning the area between the 2 arcus tendineae.1 Paravaginal repair is required when cystourethrocele results from a separation of the pubocervical fascia from its lateral attachment to the pelvic sidewall. If this defect is accompanied by GUSI, the paravaginal repair almost always will correct the problem.
Dissection during anterior colporrhaphy splits the vaginal muscularis, and vaginal repair involves plication of the muscularis and adventitia in the midline and can pull the lateral attachments farther from the pelvic sidewall. Paravaginal repair restores the lateral attachments to the pelvic sidewall at the linea alba. Reported failure rates range from 0% to 20% for anterior colporrhaphy and from 3% to 14% for paravaginal repair.58
In determining the correct surgical approach, preoperative clinical assessment of the patient is very important. Successful surgical correction of the cystocele depends on the type of defect found in the pubocervical fascia. On examination of the anterior vagina, anterolateral support should be confirmed. If one or both anterolateral sulci are absent and vaginal rogation is present, then a detachment of the pubocervical fascia from the fascial white line—a paravaginal defect—should be suspected.2 Four different pubocervical fascial defects can cause cystocele. Distinguishing these defects is important, as each type requires a different operative procedure.
- The paravaginal defect results from detachment of the pubocervical fascia from its lateral attachment to the fascia of the obturator internal muscle at the level of the arcus tendineus fascia of the pelvis.55,59,60 This is the most common cause of The repair consists of reestablishing the lateral pelvic side-wall attachments of the pubocervical fascia and restoring the stability of this “hammock” by correcting the fundamental anatomic defect.
- The transverse defect is caused by transverse separation of the pubocervical fascia from the peri-cervical ring into which the cardinal and uterosacral ligaments The base of the bladder herniates into the anterior vaginal fornix and forms a cystocele without displacing the urethra or urethrovesical junction.
- The midline or central defect results from a break in the central portion of the hammock between its lateral, dorsal, or ventral attachments.
- With the distal defect, the distal urethra becomes avulsed or separated from its attachment to the urogenital diaphragm as it passes under the pubic symphysis.
Technique
Many operations have been described to correct loss of pelvic support. The abnormalities are identified, and the operation is planned with the intention of correcting each defect to achieve the optimal outcome.49 The patient should be able to tolerate general anesthesia, increased intraabdominal pressure, and the Trendelenburg position.
The principles of the transabdominal approach used in laparotomy are used during laparoscopy. This technique has evolved as an alternative in reconstructive pelvic operations.50,51 Laparoscopy involves a smaller incision, eliminates the need for abdominal packing, causes less manipulation of the viscera, affords a better view of the pelvis, and allows precise hemostasis.
The patient is given intravenous antibiotics prophylactically. After induction of general endotracheal anesthesia and placement, a 10-mm to 11-mm umbilical trocar is inserted. Three lower abdominal 5-mm ancillary trocars also are placed; 2 are lateral to the epigastric vessels at the level of the iliac crest, and one is in the midline 5cm above the pubic symphysis. The patient is put in the Trendelenburg position and tilted to the left to shift the bowel away from the operating field. After evaluation of the peritoneal cavity and completion of other indicated procedures, the pelvic reconstruction can proceed. The retropubic space is entered and dissected. The pubic symphysis, obturator foramen, and obturator neurovascular bundle are identified. The paravaginal defect (the lateral vaginal sulci) can be seen detached from the arcus tendineus fascia (Figures 13 and 14).
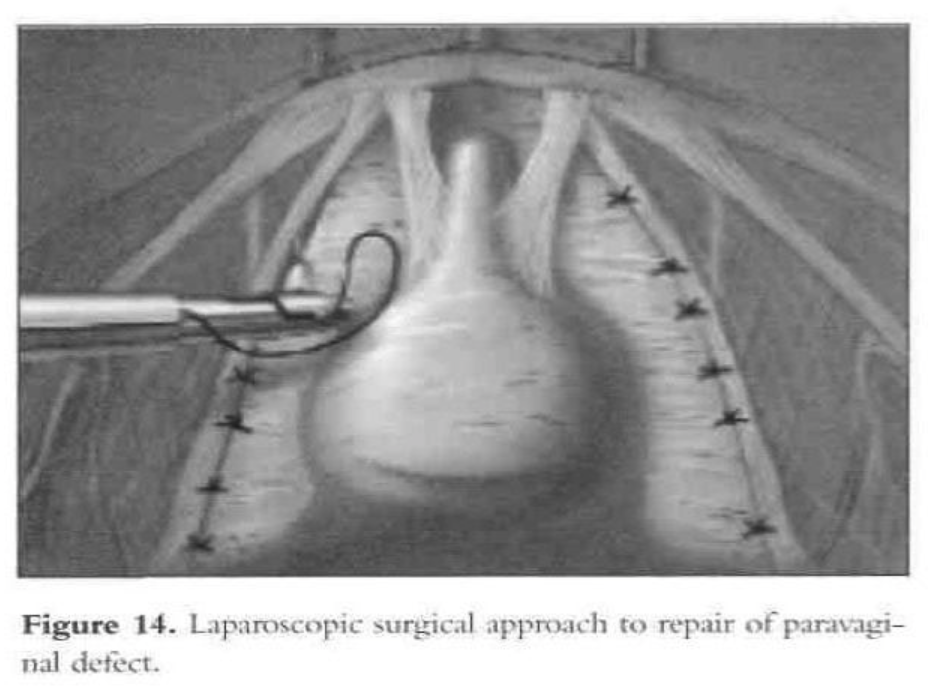
The bladder is mobilized medially, and the pubocervical fascia is exposed. The ischial spine can be located digitally by the operator’s fingers placed inside the vagina while he or she is viewing through the laparoscope. During mobilization of the bladder, the lateral superior sulcus of the vagina is lifted by the assistant’s fingers in the vagina to facilitate dissection.
Separation of the lateral sulcus from the pelvic sidewall can be seen laparoscopically. Permanent sutures (2-0 Prolene) are used to attach the superior lateral sulcus of the vagina to the arcus tendineus fascia (white line). The superior lateral sulcus of the vagina is elevated with the assistant’s fingers in the vagina. Beneath the prominent paraurethral vascular plexus, the vagina is sutured to the linea alba of the pelvic sidewall.
The paraurethral vascular plexus runs longitudinally along the axis of the vagina and is electrodesiccated before the placement of sutures. Otherwise, bleeding can occur if the plexus is penetrated by the needle. Such bleeding invariably stops when the suspension sutures are tied. To avoid bleeding, the first paravaginal suspension stitch should be placed close to the ischial spine. Figure-eight sutures are used for the suspension stitches to obtain good hemostasis and suspension. After placement of the first stitch, additional sutures are placed through the vaginal sulcus with its overlying fascia and the arcus tendineus fascia ventrally toward the pubic symphysis. The last stitch should be as close as possible to the pubic ramus.
Before this first suture is placed, the gynecologist should identify the ischial spine by vaginal palpation and by viewing through the laparoscope to avoid injuring the pudendal vessels and nerve. The initial stitch is placed through the linea alba approximately 1cm to 1.5cm ventral to the ischial spine.53 Frequent vaginal examinations are done while suturing to assist the proper placement of the stitches, assess the adequacy of suspension, and establish anterior support. The procedure is completed by bladder neck suspension.
VESICOVAGINAL FISTULA REPAIR
The primary cause of vesicovaginal fistula in developed countries is surgical trauma associated with gynecologic procedures. Most fistulas occur after hysterectomy for benign conditions, because these procedures are far more common than surgery for cancer. However, the risk of fistula formation is higher after radical surgery because of the scope of surgery, the presence of tumor, and, in some cases, radiation-induced changes. In contrast, urogenital fistulas in developing countries are usually associated with childbirth. Despite the best efforts of the surgeon, injuries to the urinary tract may still occur as part of the healing process in pelvic surgery. Tissue necrosis follows tissue ischemia, attributable to external pressure (crush or clamp injury), kinking of urinary tract tissue (proximity to a ligated pedicle), or marked inflammation with tissue fibrosis. Direct injury to the urinary tract by laceration or puncture usually results in immediate urine leakage, while delayed injury from retroperitoneal fibrosis, tissue pressure, or partial obstruction may not result in fistula formation and urine leakage for days or weeks.
Postoperative patients with a vesicovaginal fistula usually are easily diagnosed with urine leaking through the vagina. Classically, fistulas occur between the seventh and the twelfth day after obstetric or gynecologic surgery. The diagnosis can be confirmed by filling the bladder with a dilute solution of methyl blue. The vaginal vault is then directly inspected to visualize the fistula. If no defect is clearly seen, then cystoscopy can be a valuable diagnostic help. In a patient who is experiencing urinary incontinence the tampon test, where a tampon is inserted into the vagina after filling the bladder with a dilute solution of methyl blue and the patient is ambulated, can help confirm the diagnosis. In addition to the cystoscopy and the cystourography, an intravenous pyeloureterogram is recommended to rule out concomitant ureteral fistulas before proceeding with the surgical repair.61
Debate exists in the literature regarding the most appropriate period of time to wait before proceeding to surgery should the defect not heal, with approximately equal numbers advocating early (1 to 3 months) intervention as those advocating later (more than 3 months) intervention. The purpose of waiting is to allow recovery from inflammation, infection, and tissue necrosis. Although this might be true for complicated fistula and postpartum fistula, extensive infection and tissue necrosis are uncommon with gynecologic surgery related fistula. Thus, waiting is less relevant for fistula following hysterectomy.
Vesicovaginal fistulas are treated with different surgical techniques, depending on their cause and location.62 Small vesicovaginal fistulas that are not responsive to nonsurgical management are usually easily repaired.63 The edges of the fistula are removed, and the defect is closed. Latzko’s technique64 is commonly used for fistulas that are surrounded by severe fibrosis and close to the bladder neck or urethral meatus. Lee et al65 recommended an abdominal approach for fistulas in the upper part of a narrow vagina, cases of multiple fistulas, those associated with other pelvic abnormalities, and those close to the ureter. A combined abdominal and vaginal approach is used in some instances.66 Nezhat et al67 first reported the laparoscopic approach in 1994. Laparoscopic repair of vesicovaginal fistula may offer the patient less morbidity and a quicker recovery. The laparoscopic approach significantly reduces the access trauma of traditional laparotomy with additional advantages of magnified vision of pelvic organs and less traumatic tissue handling.
Technique
The basic principles for repair include adequate exposure, excision of fibrous tissue from the edges of the fistula, approximation of the edges without tension, the use of suitable suture material, and efficient postoperative bladder drainage.65,68
A 10-mm infraumbilical incision is made for the insertion of the operative laparoscope. Three 5-mm trocars are inserted in the lower abdomen for the suction-irrigator probe, grasping forceps, and bipolar forceps.32 A simultaneous cystoscopy is done, and both ureters are catheterized to aid in their identification and protection during excision and closure of the fistula. A ureteral catheter is pulled through the fistula into the vagina to facilitate identification during excision.
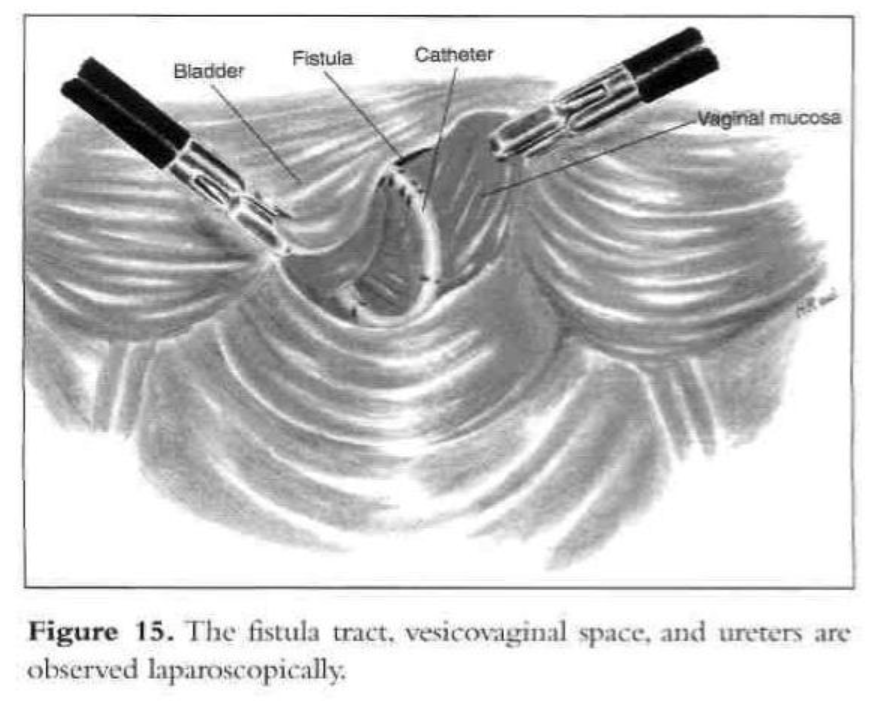
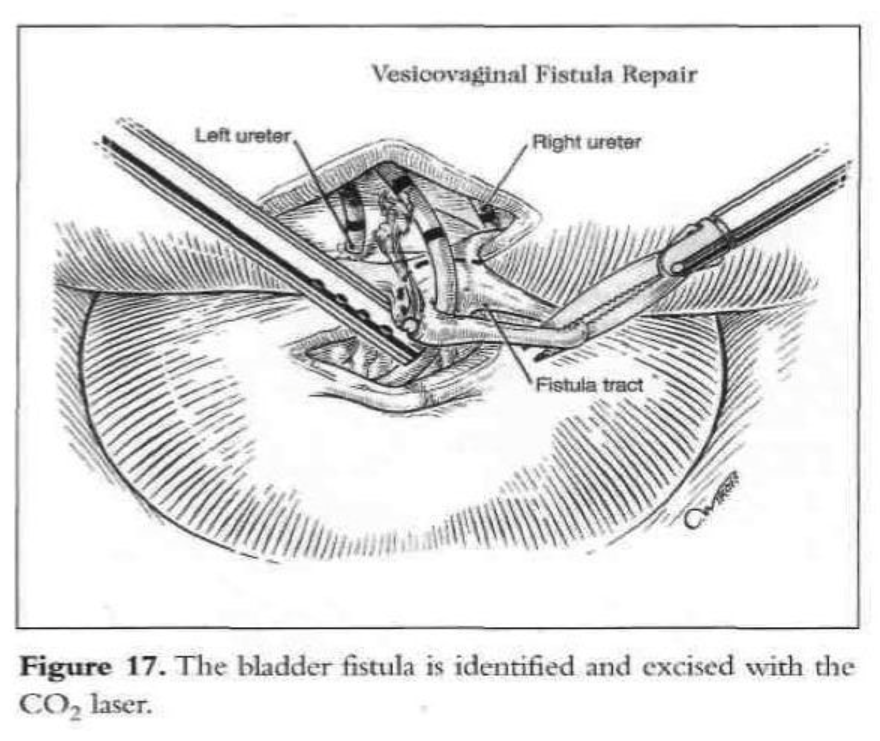
A digital rectovaginal examination is carried out to exclude rectal involvement. An opening is made in the vagina, avoiding the bladder and rectum, and an inflated glove in the vagina helps maintain pneumoperitoneum. The anterior vaginal wall is elevated with a grasping forceps, and the fistula is identified with the previously inserted catheter, which also delineates the posterior bladder wall. The bladder is filled with water, and a cystotomy is made above the fistula. The water is evacuated as the bladder is distended by the pneumoperitoneum from the cystotomy. The fistula tract, vesicovaginal space, and ureters are observed laparoscopically (Figures 15 and 16). The vesicovaginal space is developed laparoscopically with the CO2 laser and hydrodissection or any other cutting modality. The bladder is freed posteriorly from the vaginal wall. The fistula is identified, held with a grasping forceps, and excised (Figure 17). Adequate bladder dissection and mobilization are essential to eliminate tension upon suturing.
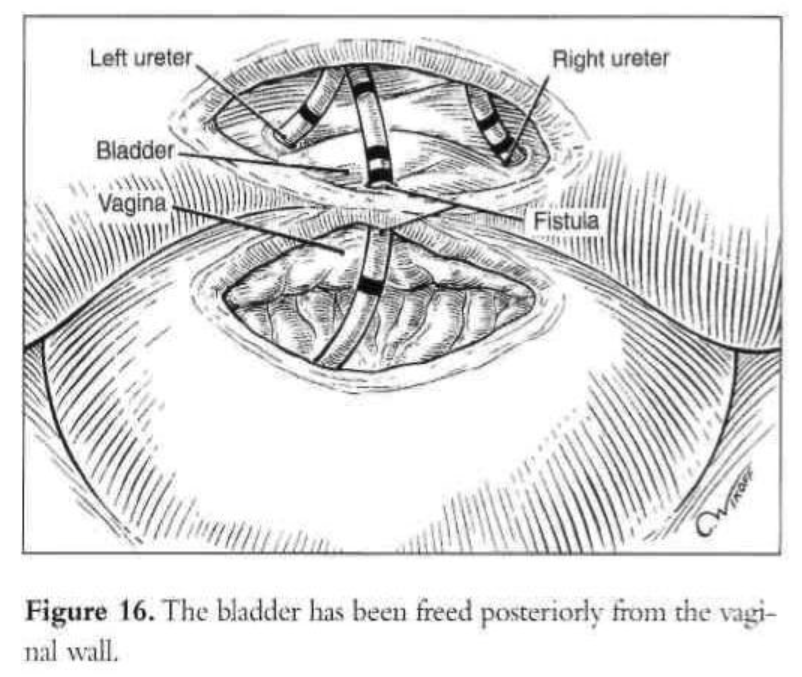
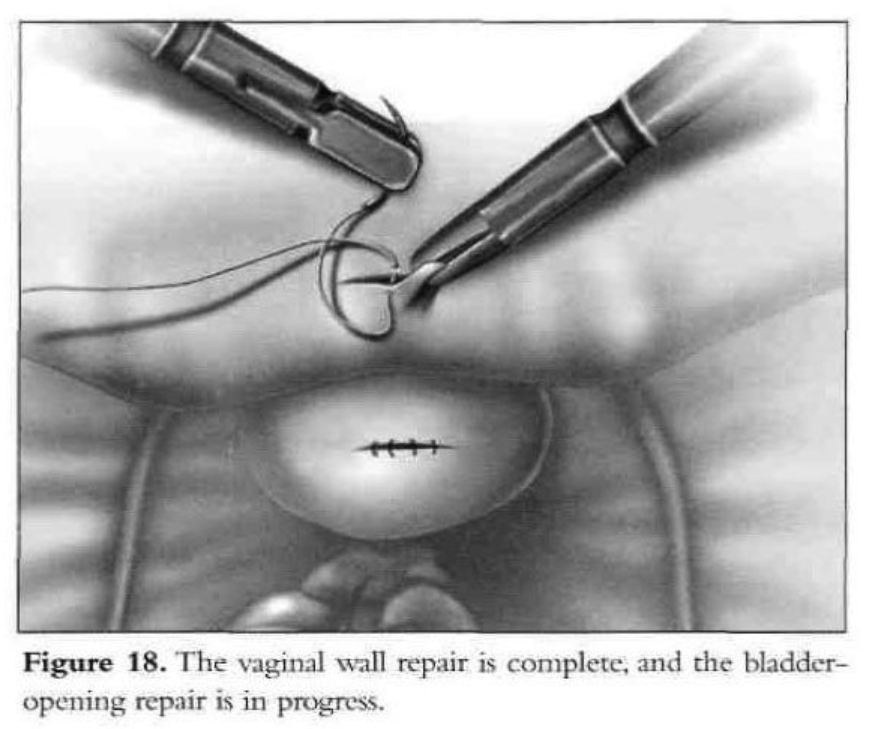
Initially, the vaginal wall opening of approximately 1.5cm is closed with one layer of interrupted polyglactin suture (Figure 18). Then the vesical defect is repaired in one layer with interrupted 1-0 Endoknot polyglactin sutures (Ethicon), using extracorporeal knotting. Defects in the vagina and bladder are closed separately. Hemostasis in the vesicovaginal space and fistula area is essential. A peritoneal flap is obtained superior and lateral to the bladder dome, close to the round ligament and diverted toward the bladder base. The flap is used to separate the vesicovaginal space. It is secured with 2 interrupted polyglactin sutures. The dissected peritoneal area heals secondarily. No intraperitoneal drainage is used. After the procedure, a suprapubic or transurethral catheter is inserted and ureteral catheters are removed.
Results
Laparoscopic repair of a vesicovaginal fistula in 2 patients has been reported.67 After a laparoscopic excision of an ovarian remnant, a 45-year-old patient developed a posterior vesicovaginal fistula in the upper third of the vagina that failed to resolve with prolonged bladder drainage. A vaginal approach was not appropriate because of the complexity of the location and condition of the defect. The morning after the repair of the fistula, the patient was discharged. Prophylactic antibiotics and estrogen were prescribed, and on postoperative day 10, the Foley catheter was removed. A cystogram was done, and no evidence of the fistula was seen. The patient was cured. A 48-year-old woman had a history of multiple previous abdominal operations. After a total abdominal hysterectomy with bilateral salpingo-oophorectomy and an inadvertent cystotomy, she developed a vesicovaginal fistula. After the repair, she was cured.
LAPAROSCOPIC UTEROSACRAL LIGAMENT VAGINAL VAULT SUSPENSION
Uterosacral ligament (USL) suspension allows reattachment of the vaginal vault high within the pelvis. Through the decades, both abdominal and vaginal renditions have been explored, and, still today, pelvic surgeons vary in their approaches. Although the uterosacral ligament (USL) has long been identified as a strong suspensory ligament, only more recently have surgical modifications allowed for a more durable and effective option. The exact attachment of the USL from the ischial spine has been the subject of controversy as some believe it connects to the sacrum, whereas others postulate there are attachments to the sacrospinous ligament and coccygeus muscle. With identification of the USL high or above the ischial spines, strong supportive tissue can be identified and used for vault suspension. Even in advanced cases of uterovaginal prolapse, a stronger segment of the USL can be palpated closer to the sacrum. Although USLs are strong attachment points, they lie close to anatomic structures that can be injured during surgery. Specifically, the ureters may be kinked, tied, or injured during suspension.69
The purpose of the USL vault suspension is to attach a strong segment of the USL to the rectovaginal and anterior pubocervical fascia. Yazdany et al69 used the same basic principles of the vaginal repair during the laparoscopic approach. A 10-mm umbilical port and two 5-mm lateral ports are placed on each side of the patient. The ureters are identified throughout their course below the pelvic brim and a relaxing incision is placed below the level of the ureter within the peritoneum. A rectal probe is placed within the vagina and the ischial spines identified by placing tension on the cuff in the contralateral direction. Using gortex or other permanent suture material, the USLs are attached to the posterior surface of the vaginal vault. Cystoscopy is then performed to confirm ureteral flow.
There is a paucity of literature regarding the laparoscopic uterosacral ligament suspension, which mostly comprises case reports and small cohort studies. Similar to the other laparoscopic procedures in urogynecology and reconstructive pelvic surgery, patients are rarely followed beyond 2 years. Over the past 10 years, few studies have examined this technique. In the largest cohort to date, Lin et al70 performed a retrospective analysis of the laparoscopic vaginal vault suspension using uterosacral ligaments in 133 patients with advanced symptomatic vaginal vault prolapse to evaluate the efficacy and durability of this laparoscopic technique. Efficacy and anatomicoutcome were assessed by the Baden-Walker halfway scoring system before and after the surgical procedure. Preoperatively, all patients showed evidence of grade 2 or greater prolapse (descent to the level of the hymen). Fifty-one patients (38.4%) had uterovaginal prolapse, and 82 patients (61.6%) had vaginal vault prolapse. The patients were reevaluated at 1, 6, and 12 months postoperatively and yearly thereafter. The postoperative follow-up ranged from 2.0 to 7.3 years. Postoperatively, 116 patients (87.2%) had no recurrence of prolapse, and 17 patients (12.8%) had recurrence of prolapse. The major complication rate was 2.25%.
In the study published by Schwartz et al,71 which is one of the few recent studies that reports on objective outcome data after uterosacral ligament repair, they state that laparoscopic uterosacral ligament repair improves symptoms and Pelvic Organ Prolapse- Quantification (POP-Q) scores over the long term in patients with uterine or vaginal vault prolapse. Schwartz et al followed 72 patients with stage II or greater uterine prolapse (n=42) and vaginal vault prolapse (n=30) who underwent laparoscopic uterosacral ligament repair. There were no major complications. After 12 months, there was a statistically significant improvement in POP-Q scores by 14.4 in the uterine prolapse group and 9.28 in the vaginal vault prolapse group. Patient self-reported symptom scores also improved significantly.
We conclude that laparoscopic uterosacral ligament vaginal vault suspension is a safe, efficacious, and durable alternative for the management of vaginal vault prolapse. While performing USL suspensions, surgeons must have a high level of suspicion for ureteral injuries. Performing an intraoperative cystoscopy is an absolute necessity. Laparoscopic instrumentation kits facilitate procedure performance for the surgeon with expedited surgery times. Laparoscopic uterosacral ligament vaginal vault suspension is an effective procedure and enables us to combine the advantages of laparotomy with the low morbidity of the vaginal route. Additionally, uterosacral ligament suspension is the most natural anatomic repair of defects, and hence, the least likely to be predisposed to future defects in the anterior or posterior vaginal wall or to compromise vaginal function.
References
- Deval B, Haab F. What’s new in prolapse surgery? Curr Opin Urol. 2003;13(4):315- 323.
- Miklos JR, Moore RD, Kohli N. Laparoscopic surgery for pelvic support defects. Curr Opin Obstet Gynecol. 2002;14(4):387-395.
- Birnbaum SJ. Rational therapy for the prolapsed vagina. Am J Obstet Gynecol. 1973;115(3):411-419.
- Nezhat C. Videolaparoscopy and videolaseroscopy: a new modality for the treatment of endometriosis and other diseases of reproductive organs. Colposc Gynecol Laser Surg. 1986;2:221-224.
- Nezhat C, Siegler A, Nezhat F, Nezhat C, Seidman D, Luciano A, eds. Appendectomy. In: Operative Gynecologic Laparoscopy, Principles and Techniques. 2nd ed. New York, NY: McGraw-Hill; 2000:339-353.
- Price N, Jackson SR. Advances in laparoscopic techniques in pelvic reconstructive surgery for prolapse and incontinence. Maturitas. 2009;62(3):276-80.
- Margossian H, Walters MD, Falcone T. Laparoscopic management of pelvic organ prolapse. Eur Obstet Gynecol Reprod Biol. 1999;85(l):57-62.
- Staskin DR, Zimmern PE, Hadley HR, Raz S. Pathophysiology of stress incontinence. Clin Obstet Gynaecol. 1985;12(2):357-368.
- Thomas TM, Plymat KR, Blannin J, Meade TW Prevalence of urinary incontinence. Br Med J. 1980;281(6250):1243-1245.
- Diokno AC, Brock BM, Brown MD, Herzog AR. Prevalence of urinary incontinence and other urological symptoms in the noninstitutionalized elderly. J Urol. 1986;136:1022-1025.
- Yarnell JW, St. Leger AS. The prevalence, severity and factors associated with urinary incontinence in a random sample of the elderly. Age Ageing. 1979;8:81-85.
- Hoist K, Wilson PD. The prevalence of female urinary incontinence and reasons for not seeking treatment. N Z Med J. 1988;101:756.
- Rosenzweig BA, Hischke MD, Thomas S, Bhatia NN. Stress incontinence in women: Psychological status before and after treatment. J Reprod Med. 1991;36:835-838.
- Horbach NS. Genuine SUI: best surgical approach. Contemp Obstet Gynecol. 1992;37:53.
- Marshall VF, Marchetti AA, Krantz KE. The correction of stress incontinence by simple vesicourethral suspension. J Urol. 2002;168(4pt 1):1326-1331.
- Burch JC. Cooper’s ligament urethrovesical suspension for stress incontinence. Nine years’ experience—results, complication, techniques. 1968. Am J Obstet Gynecol. 2002;187:512.
- Stanton SL. Colposuspension. In: Stanton SL, Tanagho E, eds. Surgery of Female Incontinence. 2nd ed. New York, NY: Springer-Verlag; 1986.
- McGuire EJ, Lytton B. Pubovaginal sling procedure for stress incontinence. J Urol. 1978;119:82-84.
- Pereyra AJ, Lebherz TB. Combined urethrovesical suspension and vaginourethroplasty for correction of urinary stress incontinence. Obstet Gynecol. 1967;30:537-536.
- Raz S. Modified bladder neck suspension for female stress incontinence. Urology. 1981;17:82-85.
- Hohnfellner R, Petrie E. Sling procedures in surgery. In: Stanton SL, Tanagho E, eds. Surgery of Female Incontinence. 2nd ed. New York, NY: Springer-Verlag; 1986.
- Gittes RF, Loughlin KR. No incision pubovaginal suspension for stress incontinence. J Urol. 1987;138:568-570.
- Bhatia NN, Bergman A. A modified Burch versus Pereyra retropubic urethropexy for stress urinary incontinence. Obstet Gynecol. 1985;66:255-261.
- Mundy AR. A trial comparing the Stamey bladder neck suspension procedure with colposuspension for the treatment of stress incontinence. Br J Urol. 1983;33:687-690.
- Green DF, McGuire EJ, Lytton B. A comparison of endoscopic suspension of the vesical neck versus anterior urethropexy for the treatment of stress urinary incontinence. J Urol. 1986;136:1205-1207.
- Karram MM, Bhatia NN. Transvaginal needle bladder neck suspension procedures for stress urinary incontinence: a comprehensive review. Obstet Gynecol. 1989;73(5 pt 2):906-914.
- Tanagho EA. Colpocystourethropexy: the way we do it. J Urol. 1976;116:751-753.
- Ballard C, Bergman A, Koonings PP. Primary stress urinary incontinence and pelvic relaxation: Prospective randomized comparison of three different operations. Am J Obstet Gynecol. 1989;161:97-101.
- Bergman A, Ballard C, Koonings PP. Comparison of three different surgical procedures for genuine stress incontinence: Prospective randomized study. Am J Obstet Gynecol. 1989; 160:1102-1106.
- Penttinen J, Kindholm EL, Kaar K, Kauppila A. Successful colposuspension in stress urinary incontinence reduces bladder neck mobility and increases pressure transmission to the urethra. Arch Gynecol Obstet. 1989;224:233-238.
- Van Geelen JM, Theeuwes AGM, Eskes TK, Martin CB Jr. The clinical and urodynamic effects of anterior vaginal repair and Burch colposuspension. Am J Obstet Gynecol. 1989;159:137-144.
- Nezhat CH, Nezhat F, Nezhat C. Operative laparoscopy (minimally invasive surgery): state of the art. J Gynecol Surg. 1992;8:111-141.
- Vancaillie TG, Schuessler W Laparoscopic bladder neck suspension. J Laparoendosc Surg. 1991;1:169-173.
- Nezhat CH, Nezhat F, Nezhat CR, Rottenberg H. Laparoscopic retropubic cystourethropexy. J Am Assoc Gynecol Laparosc. 1994;l(4):339-349.
- McDougall EM. Laparoscopic management of female urinary incontinence. Urol Clin North Am. 2001;28(1):145-149.
- Walters M, Shields L. The diagnostic value of history, physical examination and the Q-tip cotton swab test in women with urinary incontinence. Am J Obstet Gynecol. 1988;159:145-149.
- Hilton P, Stanton SL. A clinical and urodynamic assessment of the Burch colposuspension for genuine stress incontinence. Br J Obstet Gynaecol. 1983;90:934- 939.
- Bergman A, Bhatia NN. Uroflowmetry for predicting postoperative voiding difficulties in women with SUI. Br J Obstet Gynaecol. 1985;92:835-838.
- Bhatia NN, Bergman A. Use of preoperative uroflowmetry and simultaneous urethra cystometry for predicting risk of prolonged postoperative bladder drainage. Urology. 1986;28:440-445.
- Ou CS, Presthus J, Beadle E. Laparoscopic bladder neck suspension using hernia mesh and surgical staples. J Laparoendosc Surg. 1993;3(6):563-566.
- Nezhat CH, Nezhat F, Seidman DS, Nasserbakht F, Nezhat C, Roemisch M. A new method for laparoscopic access to the space of Retzius during retropubic cystourethropexy. J Urol. 1996; 155:1916-1918.
- Nezhat CH, Nezhat F, Nezhat CR, Rottenberg H. Laparoscopic retropubic cystourethropexy. J Am Assoc Gynecol Laparosc. 1994;1:339-349.
- Kitchener HC, Dunn G, Lawton V, Reid F, Nelson L, Smith AR; COLPO Study Group. Laparoscopic versus open colposuspension: results of a prospective randomised controlled trial. BJOG. 2006;113(9):1007-13.
- Miklos JR, Kohli N, Moore RD. Laparoscopic management of urinary incontinence, ureteric and bladder injuries. Curr Opin Obstet Gynecol. 2001;13(4):411-47.
- Dean MN, Ellis G, Wilson PD, Herbison GP. Laparoscopic colposuspension for urinary incontinence in women, Cochrane Database Syst Rev (2006), CD002239.
- Dunton CJ, Mikuta J. Post-hysterectomy vaginal vault prolapse. Postgrad Obstet Gynecol. 1988;8:1.
- Sze EH, Miklos JR, Partoll L, Roat TW, Karram MM. Sacrospinous ligament fixation with transvaginal needle suspension for advanced pelvic organ prolapse and stress incontinence. Obstet Gynecol. 1997;89:94-96.
- Arthur HG, Savage D. Uterine prolapse and prolapse of vaginal vault treated by sacral hysteropexy. J Obstet Gynaecol Br Emp. 1957;64:355-360.
- Randall CL, Nichols DH. Surgical treatment of vaginal inversion. Obstet Gynecol. 1971;38:327-332.
- Symmonds RE, Williams TJ, Lee RA, Webb MJ. Posthysterectomy enterocele and vaginal vault prolapse. Am J Obstet Gynecol. 1981;140:852-859.
- Nezhat C, Nezhat F, Gordon S, Wilkins E. Laparoscopic versus abdominal hysterectomy. J Reprod Med. 1992;37:247-250.
- Nezhat CH, Nezhat F, Nezhat C. Laparoscopic sacral colpopexy for vaginal vault prolapse. Obstet Gynecol. 1994;84 (5):885-888.
- Richardson AC, Lyon JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126:568-573.
- Sarlos D, Brandner S, Kots Laparoscopic sacrocolpopexy for uterine and post- hysterectomy prolapse: anatomical results, quality of life and perioperative outcome- a prospective study with 101 cases. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1415- 1422.
- Elliott DS, Krambeck AE, Chow GK. Long-term results of robotic assisted laparoscopic sacrocolpopexy for the treatment of high grade vaginal vault prolapse. J Urol. 2006;176:655-659.
- Daneshgari F, Kefer JC, Moore C, Kaouk J. Robotic abdominal sacrocolpopexy/sacrouteropexy repair of advanced female pelvic organ prolapse (POP): utilizing POP-quantification-based staging and outcomes. Br J Urol. 2007;100:875-879.
- Akl MN, Long JB, Giles DL, Cornella JL, Pettit PD, Chen AH, Magtibay Robotic-assisted sacrocolpopexy: technique and learning curve. Surg Endosc. 2009;23(10):2390-4.
- Weber AM, Walters MD. Anterior vaginal prolapse: Review of anatomy and techniques of surgical repair. Obstet Gynecol. 1997;89:311-318.
- Liu CY. Laparoscopic cystocele repair: paravaginal suspension. In: Laparoscopic Hysterectomy and Pelvic Floor Reconstruction. Cambridge, MA: Blackwell; 1996.
- Delancey JO. Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol. 2002;187(1):93- 8.
- Angioli R, Penalver M, Muzii L, et al. Guidelines of how to manage vesicovaginal fistula. Crit Rev Oncol Hematol. 2003;48(3):295-304.
- Drutz HP. Urinary fistulas. Obstet Gynecol Clin North Am. 1989;16(4):911-921.
- Falk HC, Orkin LA. Nonsurgical closure of vesicovaginal fistulas. Obstet Gynecol. 1957;9:538-541.
- Latzko W. Behandlund Hochsitzender Blasen und Mastdarmscheidenfisteln nach Uteruseztipation mit hohom Schedienverschluss. Zentralbl Gynakol. 1914;38:904.
- Lee RA, Symmonds RE, William TJ. Current status of genitourinary fistula. Obstet Gynecol. 1988;72:313-319.
- Taylor JS, Hewson AD, Rachow P. Synchronous combined transvaginal repair of vesicovaginal fistulas. Aust N Z J Surg. 1980;50:23-25.
- Nezhat CH, Nezhat F, Nezhat LC, Rottenberg H. Laparoscopic repair of a vesicovaginal fistula: a case report. Obstet Gynecol. 1994;83:899-901.
- Moir JC. Principles and methods of treatment of vesicovaginal fistulae. In: The Vesicovaginal Fistula. London, UK: Bailliere, Tindall, and Cassell; 1967: 52.
- Yazdany T, Bhatia N. Uterosacral ligament vaginal vault suspension: anatomy, outcome and surgical considerations. Curr Opin Obstet Gynecol. 2008;20(5):484-488.
- Lin LL, Phelps JY, Liu CY. Laparoscopic vaginal vault suspension using uterosacral ligaments: a review of 133 cases. J Minim Invasive Gynecol. 2005;12:216-220.
- Schwartz M, Abbott KR, Glazerman L, et al. Positive symptom improvement with laparoscopic uterosacral ligament repair for uterine or vaginal vault prolapse: interim results from an active multicenter trial. J Minim Invasive Gynecol. 2007; 14:570-576.
Illustrations for figures 1-3, 7-10, and 15-17 reproduced with permission from Nezhat CR, et al. Operative Gynecologic Laparoscopy: Principles and Techniques. 2nd ed. New York, NY: McGraw Hill; 2000. Illustrations for figures 4-6, 11, 12, and 18 by Medical Illustration Services, Inc., Sarita Hleap, CEO, Senior Illustrator; Jessica Pastula, Resident Illustrator.
