Cystoscopy
Shabnam Dadgar, Ceana Nezhat
The reproductive and urinary tracts in women are closely related anatomically and because of this proximity, pathogenesis or diagnosis of gynecologic conditions may need evaluation of the urinary tract. Cystoscopy is an endoscopic technique for examining the internal aspect of the bladder. It is the principle way to diagnose and surveil bladder conditions.
ANATOMY OF FEMALE URINARY TRACT
The female urethra is a tubular structure 2.5 to 4 cm in length. The upper one-third is lined by transitional epithelium and the lower two-thirds are lined by squamous epithelium. The periurethral glands are located medially and posterolaterally (Figure 1).
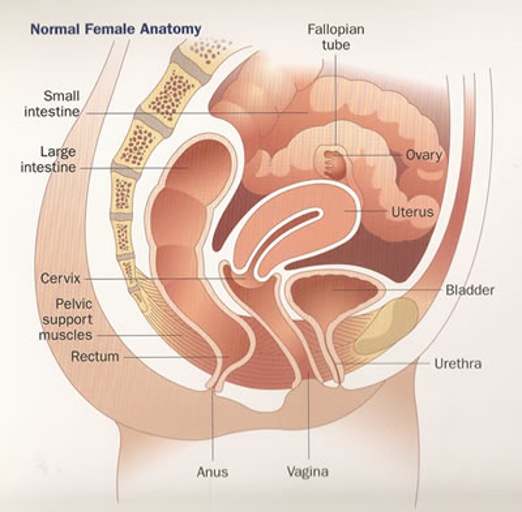 Figure 1.
Figure 1.
INDICATIONS TO PERFROM CYSTOSOCPY
Cystoscopy can be used for diagnostic and operative indications. It is being used in gynecologic and non-gynecologic conditions.4,5
Indications to perform cystoscopy in gynecology to evaluate the following:
- Identifying intraoperative injuries (e.g., ureteral injury, intravesical placement of mesh or suture)
- Gynecologic malignancies involving urinary tract
- Genitourinary fistulas
- Traumatic involvement of the urinary tract
- Irritative voiding symptoms in the absence of urinary tract infection (urinary urgency or frequency or urge incontinence)
- Urethral diverticulum
- Verification of suprapubic catheter placement
Indications to perform cystoscopy for non-gynecologic conditions:
- Recurrent urinary tract infections
- Hematuria
- Urinary incontinence or overactive bladder
- Abnormal urine cytology
- Chronic pelvic pain, interstitial cystitis and painful urination
- Urinary blockage caused by stricture, or narrowing of the urinary tract
- Stone, unusual growth, polyp, tumor, or cancer in the urinary tract
URETHRAL DIVERTICULUM
A diverticulum is a sac pouching out from a hollow organ. It is usually located posteriorly somewhere along the urethra. In females it is located in the space between the periurethral fascia and anterior vaginal wall (Figure 3). The prevalence of female urethral diverticula ranges from 1-5%.7,8 Diverticula occur most commonly in people aged 30-60 years. The mean age at diagnosis is 45 years. The exact etiology is not well understood. It can be congenital or acquired. In women it is commonly acquired.
Potential causes are vaginal birth trauma, urethroscopy, trauma from prior vaginal or urethral surgery, urethrotomy and repetitive transurethral surgical procedures. It is also reported to be a rare complication of tension free vaginal tape sling.9,10
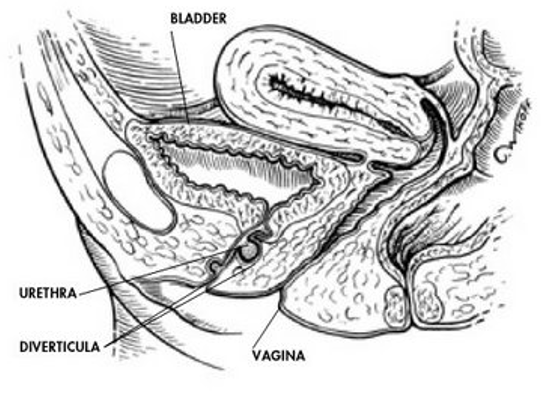 Figure 3.
Figure 3.
Congenital urethral diverticula are rare and most commonly present themselves in children. They may arise from a remnant of Gartner duct, faulty union of priordial folds, wall cyst mullerian origins, or congenital dilation of periurethral cysts.
Clinical symptoms of urethral diverticulum range from being asymptomatic to recurrent urinary tract infection, incontinence, dysuria, hematuria, finding a vaginal mass (Figure 2), frequency and urgency, urinary retention, and pain. The presenting symptoms depend on the size of the diverticulum. The small sizes are associated with pain and recurrent urinary tract infections and larger sizes more commonly cause incontinence.
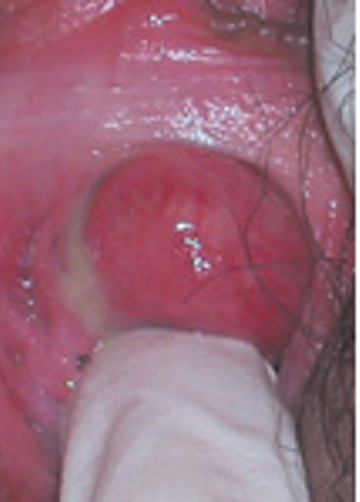 Figure 2.Urethral diverticulum in distal urethra
Figure 2.Urethral diverticulum in distal urethra
During vaginal examination, a cyst may be visible under the urethra, and pus may be expressed by compression and movement of the examining fingers along the urethra. Urine should be sent for analysis and culture.
There are different imaging studies available to aid diagnose diverlicula. MRI is the most sensitive imaging option for diagnosing urethral diverticula.11-13 Ultrasonography (US) can also be used to evaluate urethral diverticula and to measure the overall size and circumference of the urethral mass.14,15 Urethroscopy permits visual examination of the urethral epithelium and urethrovesical junction.
Surgical correction of urethral diverticula is indicated in patients with significant symptoms, including recurrent urinary tract infections, severe pain, dyspareunia, frequency, urgency, and postvoid dribbling.
PAINFUL BLADDER SYNDROME/INTERSTITIAL CYSTITIS
Interstitial cystitis (IC) is more common in females than males. It occurs between 40 and 60 years of age. In this disorder there is chronic inflammation of the bladder wall with unknown etiology, although an autoimmune etiology is generally accepted.
Interstitial cystitis is characterized by bladder pain of variable severity (ranging from mild burning to severe and debilitating). The symptoms vary from patient to patient, but there are many common clinical characteristics.16,17 A common complaint of a patient with IC is pain in association with bladder filling and/or emptying. The pain is subrapubic or urethral in origin and some will describe unilateral lower abdominal or low back pain.18,19
Diagnosis of IC is based upon the presence of characteristic symptoms, with no other identifiable cause to explain their symptoms and characteristic cystoscopic findings. The use of pelvic pain and urgency/frequency symptom scale will promote early diagnosis.
A thorough history and physical examination is essential to exclude all the possible causes such as genitourinary cancers, urinary tract stones, urinary infection, urinary retention, or pelvic masses. Laboratory tests such as urinalysis with microscopy, urine culture, and post-void residual urine volume, should be done in all patients.
Cystoscopy, hydrodistension, bladder biopsy, and potassium sensitivity testing are not necessary for diagnosis of PBS/IC, however cystoscopy should be performed if there is high suspicion for other pathologies. During cystoscopy the bladder epithelium should be examined for characteristic findings of IC, which include glomerulations (petechial red areas) and reddened patches (Hunner’s patches). Biopsies are taken from any suspicious areas (Figure 4).
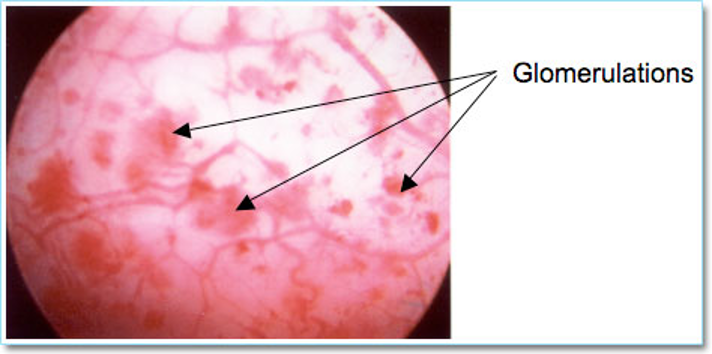 Figure 4. Interstitial cystitis → Glomerulation
Figure 4. Interstitial cystitis → Glomerulation
IDENTIFYING BLADDER INJURY
The bladder must be inspected for suture or mesh, adhesions, erythema, edema, lesions, perforation or masses.6Bladder injury is often grossly apparent to the surgeon, but cystoscopy can aid diagnosis in more subtle injuries (Figure 5). Although cystoscopy can detect most of the bladder injuries, devascularization, denervation, or thermal injuries are not identifiable by cystoscopy. The rate of missed diagnoses of bladder injury in women, who underwent benign gynecologic surgery with routine intraoperative use of cystoscopy, was estimated by systemic review of 17 retrospective studies, to be 0.8 per 1000 procedures.1 Bladder injuries indentified should be repaired by identification of the ureters, by mobilization, vascularization and closure followed by bladder rest. (Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11).
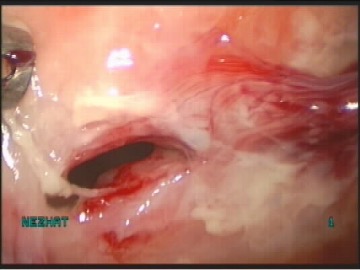 Figure 5. Bladder injury
Figure 5. Bladder injury
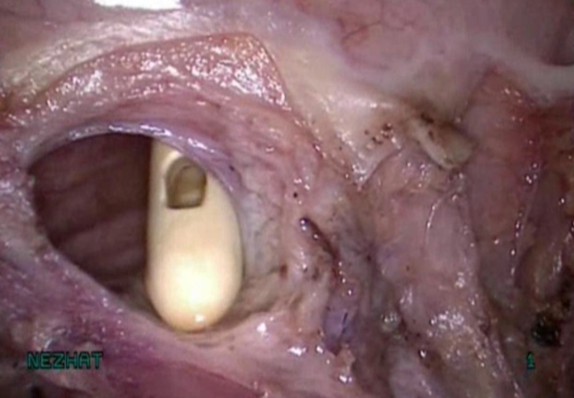 Figure 6. Cystotomy
Figure 6. Cystotomy
 Figure 7. Identification of ureters
Figure 7. Identification of ureters
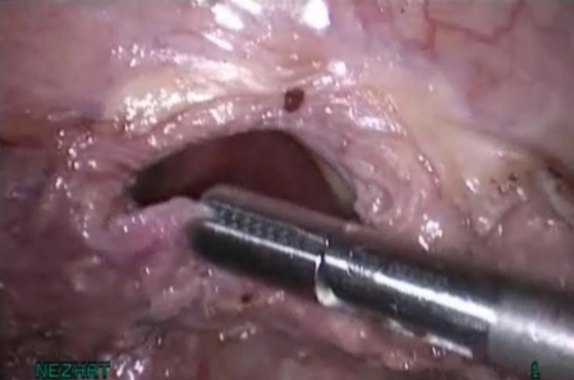 Figure 8. Adequate mobilization
Figure 8. Adequate mobilization
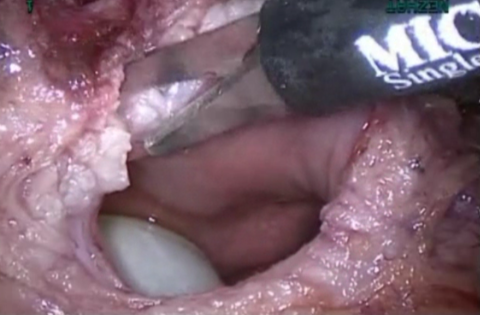 Figure 9. Vasularization to obtain healthy margins.
Figure 9. Vasularization to obtain healthy margins.
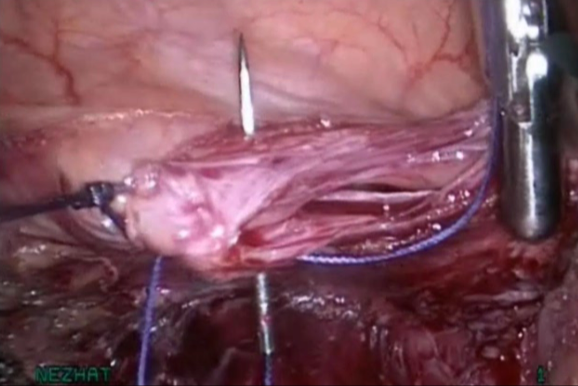 Figure 10. Closure without tension
Figure 10. Closure without tension
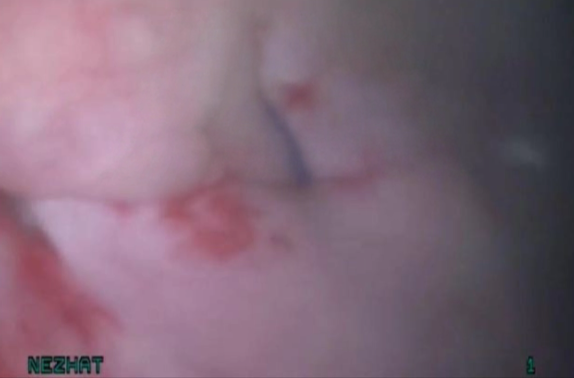 Figure 11. Bladder rest
Figure 11. Bladder rest
EVALUATING GENITOURINARY FISTULA
The etiology of urogenital tract fistulas varies geographically. In the United States, these fistulas are mostly related to gynecologic surgery, radiation therapy, severe pelvic pathology or injuries incurred in the healing process.
Ischemia is attributable to external pressure (crush or clamp injury), kinking of urinary tract tissue (proximity to a ligated pedicle), or severe inflammation with tissue fibrosis (Figure 12). Once tissue becomes ischemic, it will lead to tissue necrosis.
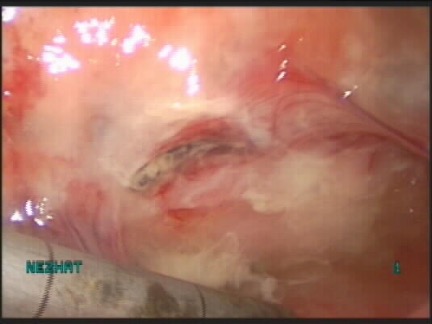 Figure 12.
Figure 12.
Fistulas between the urinary tract and vagina are painless and result in leakage of urine from the vagina.26 Intermittent leakage, particularly when positional, can be a sign of ureterovesical fistula, whereas continuous urine loss is characteristic of vesicovaginal fistulas. Other associated problem can also occur such as vulvar irritation and recurrent infections. On vaginal examination the fistula will be a small, red area of granulation tissue with no visible opening. To identify the exact location other tests may be performed such as retrograde filling the bladder with methylene blue or sterile milk.
Even though Cystoscopy is not usually helpful in the initial diagnosis of ureterovaginal or vesicovaginal fistulas, it is important to document the size and position of the defect before attempting repair. Prevention is the key for development of fistulas.
TYPES OF CYSTOSCOPY
There are two main types of cystoscopy – flexible and rigid – differing in the flexibility of the cystoscope. Flexible cystoscopy is carried out without the use of local anaesthesia on both sexes. Typically, xylocaine gel is used as an anesthetic, instilled in the urethra. Rigid cystoscopy can be performed under local anesthesia, but is generally carried out under general anesthesia (Figure 13). In flexible cystoscopy, the diameter is usually 15 to 18 French. The fiberoptic telescope and irrigation channel are combine in a single unit.1
 Figure 13. Cystoscopy lens
Figure 13. Cystoscopy lens
DIFFERENCES BETWEEN RIGID AND FLEXIBLE CYSTOSCOPE
Use of flexible fiberoptic cystoscopes is associated with less pain and postoperative morbidity than rigid cystoscopes.3 However, the flow rate of irrigation fluid is less than with rigid cystoscope and visualization is not as clear. Use of a flexible cystoscope requires more extensive training than a rigid scope. We suggest use of flexible cystoscopes when no anesthesia, topical anesthetic or conscious sedation is used and rigid cystoscopes when regional or general anesthesia is used.
INSTRUMENTATION
Cystoscope — A rigid cystoscope consists of a telescope, bridge connector, sheath, and obturator (fits inside the sheath).1 A 30 or 70 degree telescope is usually used for cystoscopy, whereas 0 degree lens is preferred for urethroscopy (Figure 14, Figure 15). The 0 and 12 degrees lenses focuses largely straight ahead and are excellent to evaluate the urethra, but often times are inadequate for visualization of the entire bladder.20,21
 Figure 14. 70 degree lens, obturator and the bridge connector
Figure 14. 70 degree lens, obturator and the bridge connector
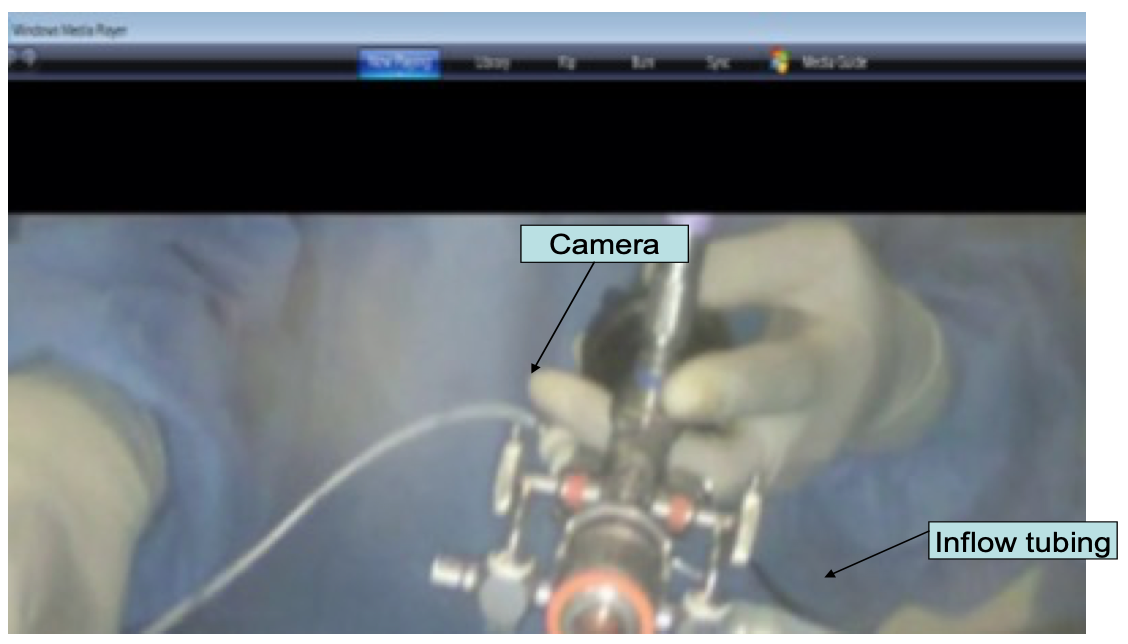 Figure 15. Assembled cystosccope
Figure 15. Assembled cystosccope
The 30-degree lens allows optimal visualization of the posterior wall and the base of the bladder. The 70-degree lens allows visualization of the dome, anterolateral walls, and into an elevated urethrovesical junction, such as after colposuspension procedures.
Finally, the 120-degree retro-lens allows the visualization of the bladder and the urethra, and optimizes visualization of the bladder neck.20,21
The sheath contains channels for instilling irrigation fluid and inserting operative instruments. The diameter of the sheath that is used commonly is 17 to 24 French. It is advised to use the smallest cystoscope that allows good visualization and to minimize pain and trauma to urethra. In selected cases, such as urethral compression by a tumor, using a pediatric cystoscope (8 French) may be helpful.2 At times, even with the smallest scope, Urethra dilatation is necessary. Gradual dilatation using Hegar dilators may be done.20,21
Telescope cables, which are either fibroptic or fluid-filled, serve as the illuminating system,20,21 even though the fluid-filled cables tend to last longer, the fibroptic cables are being used more commonly because they are less expensive.
DISTENDING MEDIUM
Irrigating fluid is instilled to distend the bladder and improve visualization. There are different distending media conductive fluids (laclated ringer’s, normal saline), nonconductive or non-electrolyte (sterile water, 5% glycine, 3% sorbitol, 5% mannitol), and gas. The usual distending medium for most surgeons for diagnostic procedures is isotonic saline or sterile water, because of better visualization. Use of any distending medium requires monitoring of fluid absorption to avoid volume overload. Use of nonconductive fluids (eg, glycine) may result in hyponatremia, and therefore, these fluids are reserved for operative procedures.
EXAMINATION OF THE LOWER URINARY TRACT USING CYSTOSCOPE
Urethra —The urethral mucosa must be examined for pallor, exudates, polyps, erythema, condylomata, or diverticulae.1
Bladder —The dome of the bladder can often be identified by the presence of an air bubble. Slight suprapubic pressure can facilitate visualization of the dome.
A bubble-like appearance to the trigone, or bullous edema, may indicate tumor encroaching upon the bladder, but does not document bladder invasion for staging purposes. Frond-like papillary growths or shaggy, necrotic tissue may be a tumor (either primary or extending into the bladder) and should be biopsied. A biopsy is performed with a cystoscopic biopsy instrument. After the biopsy, the biopsy site usually has no bleeding or minimal bleeding that does not require cautery.
Ureteral orifices — the trigone is directly in front of the urethra and is identified by locating the interureteric ridge. The cystoscope may need to be rotated to visualize the ureteral orifices. Several minutes of observation may be needed to identify the orifices, which are small, slit-like openings that only become apparent when a burst of urine is released (Figure 16, Figure 17).
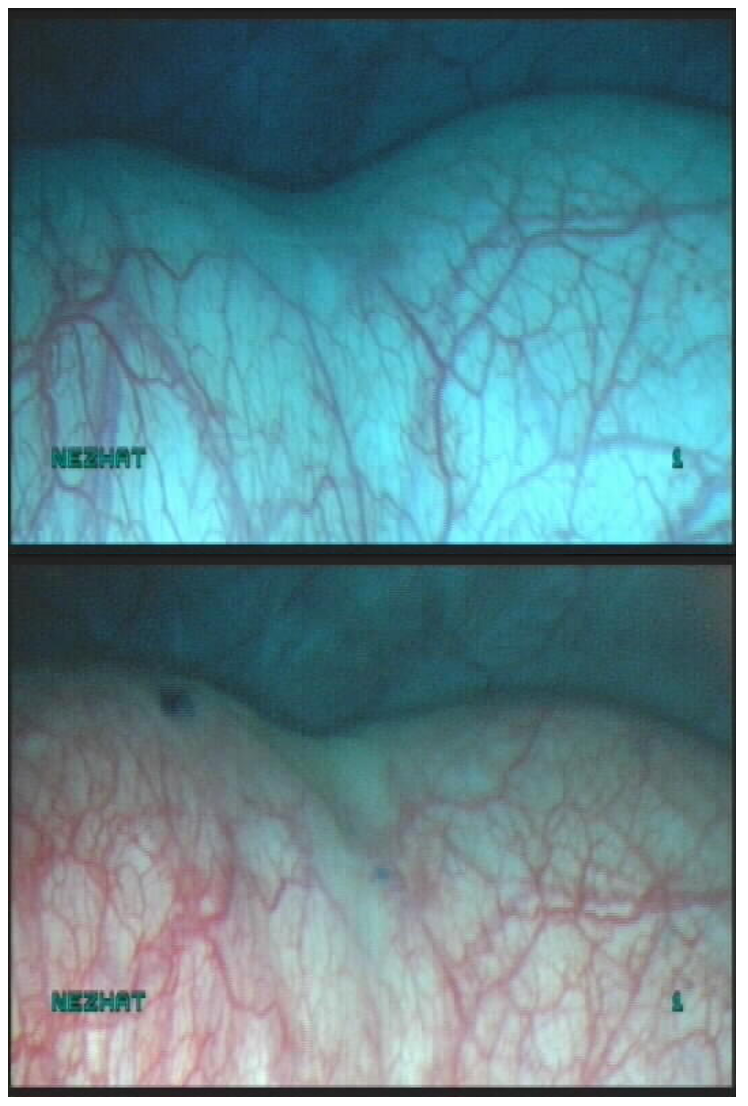 Figure 16. Ureteral orifice
Figure 16. Ureteral orifice
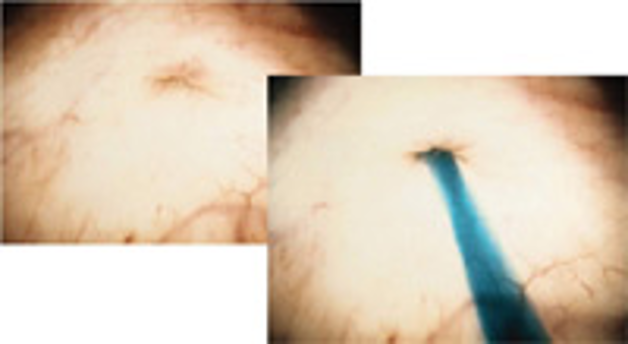 Figure 17. Ureteral orrifice
Figure 17. Ureteral orrifice
In women in whom the bladder is distorted by tumor or cystocele, a finger placed in the vagina and pressed upward under the bladder base may facilitate visualization of the ureteral openings. Large cervical tumors can cause distortion of the bladder such that the trigone sits in a valley behind the ridge made by the tumor.
Use of any distending medium requires monitoring of fluid absorption to avoid volume overload. It is important to mention that hyponatremia can happen following use of nonconductive irrigation solution.
DOUBLE URETER
The most common congenital anomaly of the urinary tract is the duplication of the renal collecting system. The duplication can be complete or partial. In complete duplication, the kidney has two separate pelvicaliceal and two ureters (Figure 18). The ureter from the lower system enters the bladder in the trigone, whereas the ureter from the upper collecting system can insert into the trigone or be inserted in the bladder or ectopically somewhere else.24 In partial duplication, the kidney has two separate pelvicaliceal systems with one ureter or two ureters that unite before inserting to the bladder.
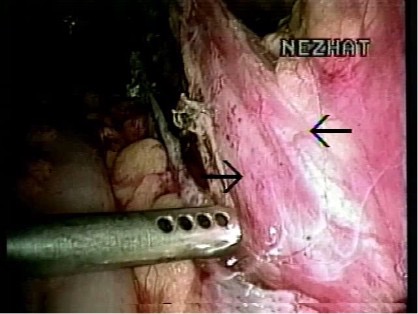 Figure 18. Double ureter
Figure 18. Double ureter
The incidence of congenital anomalies and urethral endometriosis is very low, and the presence of both together is very rare.25 Figure 19.
 A
A
 B
B
 C
C
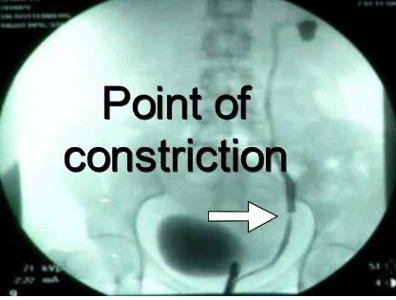
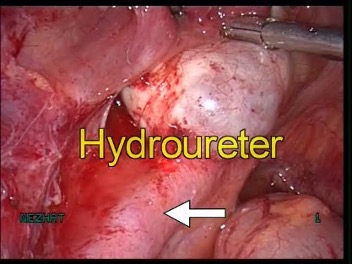
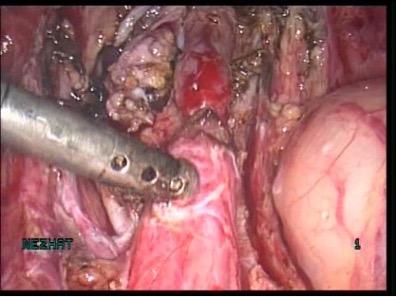
Figure 19 A-C. Ureteral stricture by endometriosis
PROCEDURE
The principle of performing cystoscopy remains the same, weather it is performed in the operating room or it’s being done in the office setting (Figure 20). The patient informed consent is obtained, then the patient is placed in the dorsal lithotomy position, the buttocks should be positioned at the edge of the table and the legs should be supported with well-padded knee crutches or stirrups.20,21 The vulva, vagina, and periurethral area should be sterilely prepared. If sterile urine needs to be collected, it should be done at the beginning of the procedure. Topical anesthetic gel can be used, if necessary, and it should be sterile and applied after sterile preparation.20,21
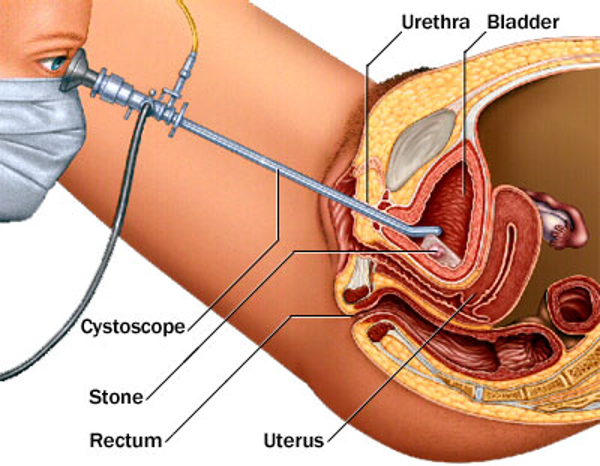 Figure 20. Cross- sectional view of cystoscope in the bladder.
Figure 20. Cross- sectional view of cystoscope in the bladder.
OPERATIVE TECHNIQUE
The cystoscope should be placed into urethral meatus, under direct visualization.20,21 During rigid cystourethroscopy, some surgeons insert the obturator into the sheath to assist entry and some will insert the sheath and scope directly into the urethra without using the obturator, depending on the the experience of the surgeon. It is important to note that obturator may cause urethral injury and performing cystoscopy without using obturator may expose the urethral tissues to the sharp edges of the instrument and may cause small amounts of bleeding.
As discussed earlier in this chapter, if there is a need to dilate, then Hegar dilators should be used with care to avoid overdilation. Before introducing the cystoscope, lubricant must be applied to the scope. The cystoscope should be flushed to minimize air entry to the bladder. Expose the periurethral area with the fingers of one hand and gently introduce under the cystoscope direct visualization. It is essential to make sure to hold the scope nearly vertically to the urethral meatus and the tip of the sheath of a rigid scope, which has a blunt bevel, should always be pointing posteriorly. Once the cystoscope is introduced in to the bladder, then systemic evaluation should take place. The urethra should be evaluated for diverticula, stenosis, or other lesions such as microabscess.
One of the essentials in performing cystoscopy is to maintain orientation at all times. The light cord is 180 degrees opposite the direction the lens is illuminating. For instance, the light cord will be at 1 o’clock if the surgeon is visualizing the bladder at 7 o’clock. To evaluate the bladder, the surgeon must use an imaginary clock, starting at 12 o’clock and moving clockwise. The bladder and the urethral orifices must be examined, as mentioned earlier, as well as demonstration of bilateral jets. Administration of intravenous indigo carmine dye facilitates the visualization of jets. Visualization of bladder may be difficult in patients with big cystocele, in such a cases, placing one finger in the vagina will help bladder visualization improve.
PLACEMENT OF URETERAL STENTS
Ureteral stents are thin hollow tubes with multiple side-holes. Stent are inserted into the ureter with cystoscopic guidance in an operating room setting or in the office (Figure 21). The distal and the proximal ends are usually curled to prevent migration.
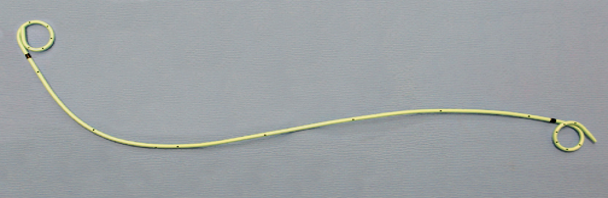 Figure 21. Double pigtail ureteral stent
Figure 21. Double pigtail ureteral stent
It is used to prevent or treat obstruction of the urine flow from the kidney. Indications for stent placement are:
- Ureteral obstruction caused by tumor, fibrosis, endometriosis and so on.
- Prophylactic stent prior to surgery for identification of location of ureter during pelvic surgery.
- Urethral anastomosis to promote healing process and ensure continued urine flow.
Various material has been used such as polyethylene and polyurethane, silicone-based, Biodegradable and metal stents.22-23 Stents are usually coated with materials such as, heparin, silver nitrate, ofloxacin and hydrogel to improve function, decrease complications. Stent length depends on the patient’s height. A 24 or 26 cm stent is typically used in the average adult patient and its diameter ranges from 4 to 7 French. The most commonly used diameter in 6 French.
Steps necessary to place stent:
- Urinalysis prior to placement or removal
- Administration of prophylactic antibiotic
- Patient should be placed in low dorsal lithotomy
- Perform cystoscopy to identify the ureteral orifices
- Placement of the guide wire up to the ureter into the renal pelvic
- Placement of access catheter over the guide wire
- Once the placement of the guide wire is confirmed, the access catheter should be removed
- Stent should then be placed over the guide wire (Figure 22).
- Once stent is securely placed the guide wire can be removed.
 Figure 22. Stent placement
Figure 22. Stent placement
References
- Cundiff, GW, Bent, AE. Endoscopic evaluation of the lower urinary tract. In: Urogynecology and Reconstructive Pelvic Surgery, 3rd ed, Walters, MD, Karram, MM (Eds), Mosby Elsevier, Philadelphia. 2007; 114.
- Cervigni, M, Scotto, V, Panei, M, Sbiroli, C. Acute urethral obstruction due to condylomata acuminata. Obstet Gynecol. 1991; 78:970.
- Denholm, SW, Conn, IG, Newsam, JE, Chisholm, GD. Morbidity following cystoscopy: comparison of flexible and rigid techniques. Br J Urol. 1990; 66:152.
- ACOG Committee Opinion. Number 372. July 2007. The Role of cystourethroscopy in the generalist obstetrician-gynecologist practice. Obstet Gynecol. 2007; 110:221.
- Gilmour, DT, Das, S, Flowerdew GJ. Rates of urinary tract injury from gynecologic surgery and the role of intraoperative cystoscopy. Obstet Gynecol 2006. 107;1366.
- Burrows, LJ, Howden, NL, Meyn, L, Weber, AM. Surgical procedures for urethral diverticula in women in the United States, 1979-1997. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:158.
- Aldridge, CW Jr, Beaton, JH, Nanzig, RP. A review of office urethroscopy and cystometry. Am J Obstet Gynecol. 1978;131:432.
- Martensson, O, Duchek, M. Translabial ultrasonography with pulsed colour-Doppler in the diagnosis of female urethral diverticula. Scand J Urol Nephrol. 1994;28:101.
- Athanasopoulos, A, McGuire, EJ. Urethral diverticulum: a new complication associated with tension-free vaginal tape. Urol Int. 2008; 81:480.
- Clemens,JQ, Bushman, W. Urethral diverticulum following transurethral collagen injection. J Urol. 2001;166:626.
- Neitlich, JD, Foster, HE Jr, Glickman, MG, Smith, RC. Detection of urethral diverticula in women: comparison of a high resolution fast spin echo technique with double balloon urethrography. J Urol. 1998;159:408.
- Blander, DS, Rovner, ES, Schnall, MD, et al. Endoluminal magnetic resonance imaging in the evaluation of urethral diverticula in women. Urology. 2001; 57:660.
- Foster, RT, Amundsen, CL, Webster, GD. The utility of magnetic resonance imaging for diagnosis and surgical planning before transvaginal periurethral diverticulectomy in women. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:315.
- Lee, JW, Fynes, MM. Female urethral diverticula. Best Pract Res Clin Obstet Gynaecol. 2005;19:875.
- Siegel, CL, Middleton, WD, Teefey, SA, et al. Sonography of the female AJR Am J Roentgenol. 1998;170:1269.
- Bogart, LM, Berry, SH, Clemens, JQ. Symptoms of interstitial cystitis, painful bladder syndrome and similar diseases in women: a systematic review. J Urol. 2007;177:450.
- Teichman, JM, Parsons, CL. Contemporary Clinical Presentation of Interstitial Cystitis. Urology. 2007;69:41.
- FitzGerald, MP, Brensinger, C, Brubaker, L, Propert, K. What is the pain of interstitial cystitis like?. Int Urogynecol J Pelvic Floor Dysfunct. 2006;
- Koziol, JA. Epidemiology of interstitial cystitis. Urol Clin North Am. 1994; 21:7.
- Nezhat C, Siegler A ,Nezhat F, Nezhat C, Seidman D, Lociano A. Operative gynecology: principles and techniques. Second ed. New York, NY: McGraw- Hill Company.
- Nezhat C, Nezhat F, Nezhat C. Nezhat’s Operative Gynecologic Laparoscopy and Hysteroscopy. 3rd ed. New York, NY: Cambridge University Press; 2008.
- Zimskind, PD, Fetter, TR, Wilkerson, JL. Clinical use of long-term indwelling silicone rubber ureteral splints inserted cystoscopically. J Urol. 1967;97:840.
- Marx, M, Bettmann, MA, Bridge, S, et al. The effects of various indwelling ureteral catheter materials on the normal canine ureter. J Urol. 1988;139:180.
- Williams H.Renal revision: from lobulation to duplication–what is normal?. Arch Dis Child Educ Pract Ed. 2007;92(5):ep152.
- Nezhat C, Rottenberg H .Laparoscopic ureteroneocystostomy and vesicopsoas hitch with double ureter for infiltrative endometriosis: a case report. J Reprod Med. 2009;Jun;54(6):407-10.
- Nezhat CH, Nezhat F, Nezhat C, Rottenberg H. Laparoscopic repair of a vesicovaginal fistula: a case report. Obstetrics and Gynecology. 1994;83(5):899-901.
