Laparoscopic Surgery During Pregnancy
James F. Carter, MD
Initially, laparoscopic surgery in pregnancy was felt to be more dangerous and, in fact, was contraindicated. The reasons were many, including injuring the pregnant uterus, elevating the CO2 level in the fetus, and an increase in spontaneous abortion, to name just a few considerations. These concerns have proven to be false as surgical skills have increased, more experience has been gained, and the envelope has continued to be pushed with ever more elegant instrumentation. In fact, the laparoscope has made it possible to make a difficult diagnosis in pregnancy earlier, if one is comfortable with the use of this formidable tool.
PHYSIOLOGIC CHANGES IN PREGANCY
The minute ventilation in pregnant women is 50% higher than that in nonpregnant women. This change results in a marked decrease in carbon dioxide in the arterial concentration, and a resulting mild respiratory alkalosis.1 The fetus has a mild respiratory acidosis in the normal state that may facilitate the delivery of oxygen. Many other changes in pregnancy occur including a normal mild anemia, increased cardiac output, increased heart rate, and an increased oxygen consumption that allows the mother and fetus to be adequately oxygenated. These changes are most remarkable, but must be considered when planning general anesthesia.
Often overlooked are the hematologic “abnormalities” in the pregnant patient. These changes include an increase in fibrinogen, factor VII, and factor XII, but a decrease in antithrombin III. All of these changes result in an increased risk of venous thromboembolism.2,3
In considering the acute abdomen in pregnancy, making the correct diagnosis is even more difficult. Nausea and vomiting are common. Leukocytosis is the norm. Low-grade fever, mild hypotension, and anorexia are common. In addition, the gravid uterus is well known to push the abdominal contents cephalad, altering landmarks by displacing organs and possibly inhibiting the migration of the omentum.1,4 Clearly, these changes can alter the clinical picture. The gravid uterus often causes a decrease in gastric motility and an increase in the risk of gastroesophageal reflux disease, including aspiration (Mendelson’s syndrome),5 a life-threatening issue feared by anesthesiologists and obstetricians alike, remaining to this day the number one cause of maternal death.
ABDOMINAL SURGERY IN PREGNANCY
The incidence of pelvic pain requiring surgery ranges from approximately 1:440 to 1:1300.6 The incidence of surgery during pregnancies is approximately 0.75%.6 One in 600 pregnancies are complicated by the presence of adnexal masses.7 There are multiple concerns in abdominal operations in pregnancy, because the life of the mother and fetus must be considered. Earlier studies have shown that abdominal surgery in the first trimester is associated with a higher (12%) rate of spontaneous abortions, which is reduced to zero in the third trimester. Abdominal pregnancy increases the rate of preterm labor: in the second trimester, it is 8% but it increases to 30% to 40% during the third trimester.3,8
Mazze and Kallen6 reported a large study of adverse outcomes of nonobstetrical operations during pregnancy, obtaining data from 3 Swedish Health Care registries in which 5405 operative cases were evaluated, 16.1% of which were laparoscopic. This sentinel report noted that no increase occurred in stillbirths or congenital anomalies in the pregnant patient who underwent an operation, even when the operation was performed in the first trimester. They showed an increase in low birth weight infants from intrauterine growth restriction and premature delivery and infants who died within the first 7 days of delivery compared with women who had not undergone an operation. The crux of their article was that there was no increase in adverse outcomes between the laparoscopic group compared with the open surgical procedure group.
Appendicitis is the most common general surgical condition during pregnancy.9 Approximately 0.05% to 0.1% of pregnant women will have acute appendicitis evenly distributed in all trimesters.2,4 Compared with nonpregnant patients, there is a high negative appendicitis rate. There is a 35% to 55% negative appendicitis rate compared with a 10% negative appendicitis rate in the nonpregnant patient.10,11
Clearly, the presence of leukocytosis, nausea, vomiting, and abdominal pain make the diagnosis of acute appendicitis, biliary disease, and the adnexal mass more difficult in pregnancy.8 The rate of fetal loss in uncomplicated appendicitis is only 1.5% compared with fetal loss as high as 35% with perforated appendicitis. More importantly, a delay in diagnosis of appendicitis leads to a 10% to 15% perforation rate, which leads to increases in fetal mortality as high as 35% and a premature delivery rate of 40% in all trimesters.
These effects of delay have remained the same since being initially reported in 1905.12 It is recommended that a pregnant patient with acute appendicitis be treated as a nonpregnant patient with rapid resuscitation with intravenous fluids, antibiotics, and prompt surgical treatment.
Gallstones are also common in pregnancy and are found in 12% of all pregnancies. It is estimated that 4.5% to 12% of pregnant women will have asymptomatic cholelithiasis, whereas 0.05% of pregnant women will experience symptomatic cholelithiasis. This is 30% to 40% higher than that in the general population.13 Forty percent of those who have symptoms will require surgical intervention, with 3 to 8 out of 10,000 pregnant women requiring a cholecystectomy.2,11,14 Clearly, pregnancy predisposes women to biliary disease with an increase in risk of gallstone disease, because of a secretion of cholesterol compared with bile acids, and increased secretions of phospholipids15 as well as an increase in gallbladder size during fasting, higher volume emptying, and an increased saturation of bile with cholesterol. All of these changes plus a decrease in the circulating pool of bile salt also contribute to the large number of pregnant women with cholelithiasis.3
Delay in treatment of biliary disease can be life threatening to both mother and fetus. The rate of spontaneous abortion is 5% in uncomplicated cholecystectomies and as high as 60% in gallstone pancreatitis.4 There is approximately a 12% risk of spontaneous abortion with nonoperative management of symptomatic cholelithiasis during the first trimester.14,15 However, nonoperative management of symptomatic cholelithiasis increases the risk of gallstone pancreatitis to 13%,11 and gallstone pancreatitis increases maternal mortality to 15% and fetal mortality to 60%.2,3
The initial management of symptomatic cholelithiasis is conservative: (1) restrict oral intake (GI rest), (2) rehydrate with intravenous fluids, (3) manage pain, and (4) initiate antibiotic therapy if needed.3,14,15 Often (70%) patients treated conservatively will relapse, and 90% will require hospitalization. The risk of relapse is greatest in the first trimester at 92%, 64% in the second trimester, and 44% in the third trimester.3 Muench et al13 found that of those patients diagnosed with biliary disease who were treated nonoperatively, 12% had spontaneous abortions and 30% failed medical management, culminating in a cholecystectomy. Thus, indications for surgery in the pregnant patient are failed medical management, acute cholecystitis, obstructive jaundice, gallstone pancreatitis, and peritonitis.14,15 The same indication for intraoperative cholangiography exists for the pregnant patient and includes a total bilirubin ≥1.5ng/dL, a dilated common bile duct ≥8mm, and the presence of gallstone pancreatitis.4
There is a growing body of evidence that laparoscopy for gynecologic indications can be performed safely during pregnancy.3,16-19 We have recently compared the management of pregnant patients with symptomatic abdominal pain managed with laparoscopic surgery with those managed with laparotomy.20 More and more gynecologic surgery is being accomplished via laparoscopic techniques, requiring similar basic cautions and techniques as well as surgeons skilled in advanced laparoscopic surgery and techniques modified for the pregnant patient.
TECHNIQUES
The landmark survey by Reedy et al17,18 of 413 laparoscopic procedures performed during pregnancy for general surgical and gynecological indications appeared to result in no higher fetal or maternal complications than those in a population undergoing laparotomy, or the complications were associated with the laparoscopic procedure itself. Nongynecologic laparoscopic procedures have been reported far more than gynecologic procedures have, with cholecystectomy being the most commonly reported. A fetal loss of 10% to 25% and a preterm delivery rate of approximately 20% have been reported,6,14,17 but these data are associated with laparotomy.
Adherence to several technical aspects is important when laparoscopy is performed during pregnancy. Clearly, safe laparoscopic access is paramount, and we feel that the open (Hasson) technique is appropriate. Modification of trocar sites must be individualized but must be anticipated once access is initially obtained to ensure an effective procedure with minimal, if any, uterine manipulation; the location of these trocars in relation to the enlarged uterus is a key to success. Smaller trocars, diameters down to 2-mm, are used. In addition, 3-mm laparoscopes superior to the umbilicus were used in our study (on uteri that were at or above the umbilicus). Minimizing CO2 insufflation to maximize both cardiac output, maternal hepatic flow, and to minimize fetal acidosis is best accomplished by keeping intraperitoneal pressures <12mm Hg.19,21 Communication with the anesthesiologist for patient’s O2 and CO2 status should be routine. To minimize risk of venous stasis, one should use sequential compression devices below the knees and keep the patient out of the pure supine position with use of the operating room table. Foley catheters have been routinely used after general anesthesia has been obtained to decompress the bladder to prevent compromise of the operative field.20,22,23 The same parameters should always be present in the surgeon’s planning, whether one is performing emergency surgery or the elective laparoscopic abdominal cerclage in pregnancy. It must be emphasized that in some of these laparoscopic procedures laparoscopic suturing techniques may be needed, and advanced laparoscopic surgeons whether master surgeons or residents, must constantly practice their suturing skills in the “dry laboratory.” The inability to be able to instantly move to intra- or extracorporeal knot tying is a must if one is to attain these advanced skills and push the envelope in minimally invasive surgery.
LAPAROSCOPIC SURGERY IN PREGNANCY
As noted earlier, the explosion of laparoscopic surgery in the recent past has failed to impact pregnant patients. There are many reasons for this fact, including the mechanical difficulties of the pregnant uterus and concern over fetal injury from instrumentation, the pneumoperitoneum, or both of these.24 Weber et al25 published the first data on successful laparoscopic cholecystectomy during pregnancy. Many reports have followed.26,27,28,29,30,31 There have been many reports of successful laparoscopic appendectomies in pregnancies with the largest study being performed by Rollins et al.11 The rate of preterm delivery was 21.4%, which is statistically higher than the rate of preterm deliveries for those not undergoing surgery. There were no fetal losses reported. In his report, both the Hasson and Veress needle techniques of abdominal cannulation were used in all 3 trimesters without any increase in maternal or fetal mortality. Lyass et al26 observed no abnormal fetal organogenesis in the laparoscopic appendectomies performed in the first trimester, which was previously thought to be unsafe. They also noted a shorter length of stay when comparing the laparoscopic with the open group.
Affleck et al27 published the largest case series of laparoscopic cholecystectomies and found no fetal losses, no birth defects, and no uterine injuries. Amos et al28 published the frequently cited report in which 4 out of 7 laparoscopic cases resulted in adverse outcomes (2 intrauterine fetal deaths and 2 incomplete abortions). However, 3 of the 4 patients in the report who experienced adverse outcomes suffered from gallstone pancreatitis or perforated appendicitis, which as we discussed earlier has a higher fetal mortality rate. In addition, rarely discussed or known in this study is that the operating time for these procedures was significantly longer than those in similar reports. We think, and it has been argued that, the underlying maternal disease contributed to the fetal demise and not necessarily the procedure.
Some authors8,20,21 have compared laparoscopic procedures with open procedures in the pregnant patient, comparing various outcomes, such as hospital length of stay, use of tocolytics, need for postoperative analgesia, and the time of return to a regular diet and mobilization. Curet et al8 compared 16 pregnant patients undergoing laparoscopic procedures with 18 pregnant patients undergoing open procedures (both groups were comparable in age, trimester, intraoperative oxygenation, and end-tidal CO2) and found that gestational age at delivery, birth weight, and Apgar scores were not statistically different in the 2 groups. In addition, the laparoscopic group had a shorter hospital stay, fewer days of postoperative narcotic use, and resumed a regular diet sooner postoperatively than the open surgery group did. These differences were statistically significant.
Reedy et al17 published a significant survey in which surgeons from the Society of Laparoendoscopic Surgeons (SLS) were queried about their unpublished experiences with pregnant patients. They reported data for 199 laparoscopic cholecystectomies and 67 laparoscopic appendectomies, demonstrating an overall trend toward an increased usage of laparoscopic surgery in pregnant patients.
Well-known hemodynamic changes occur during laparoscopic surgery.22,29 First, CO2 pneumoperitoneum causes a decrease in cardiac index, an increase in mean arterial pressure, and an increase in systemic vascular resistance. The increased intraabdominal pressure decreases venous return and cardiac output, which may lead to decreased uterine blood flow, increased intrauterine pressure, and possibly decreased fetal perfusion.
Significantly, the decreased venous return seen during laparoscopic surgery due to increased intraabdominal pressure is volume dependent, and in adequately resuscitated pregnant women, increased intraabdominal pressure should not lead to decreased CO2 and fetal blood flow.26 The low pressure of 8mm Hg to 12mm Hg should be used, if possible, to minimize any adverse effect on fetal perfusion. This pressure was elicited by Hunter et al16 when they studied pregnant ewes. It may lead to maternal hypercapnia, tachycardia, and hypertension, but it was only a transient effect. Though Hunter felt that CO2 was the detrimental agent in pregnant ewes, no abnormal organogenesis has been observed in laparoscopic appendectomies performed in humans in the first trimester with CO2 as the insufflation agent.26
A risk of hypercapnia certainly does exist with a CO2 pneumoperitoneum. We recommend monitoring the end-tidal CO2, with a goal of <35mm Hg. It is also recommended to hyperventilate the mother to keep end-tidal CO2 concentration in expired air less to ≤35mm Hg.28 We prefer continuous O2 saturation monitoring, while some authors prefer the more controversial serial arterial blood gases to monitor arterial CO2 partial pressure in women predisposed to hypercarbia.
DISCUSSION
The key to operative laparoscopy in pregnancy is that all patients (even in the first trimester) undergoing laparoscopy are positioned in the dorsal supine position with anesthesia in control of a leftward tilt. Positional changes are modified based on close communication with the anesthesia personnel. General endotracheal anesthesia is used in all patients. Sequential compression devices are placed below the knees.20,21 The individual placement of the laparoscope and operating trocars should be modified, depending on uterine size and gestational age. It is always preferable to go higher on the abdominal wall even above the umbilicus if the uterine size warrants it. There must NOT be preconceived trocar positions or numbers. We never hesitate to place two 5-mm trocar sites on the same side of the enlarged uterus always under direct visualization (Figure 1). The trocar sizes are also modified according to gestational age. Our rule is to use the open technique (Hasson) for all initial trocar placements. If the uterus is 18 weeks to 20 weeks or greater, the initial trocar is placed above the umbilicus by using a 5-mm trocar via the open technique. The remainder of trocar sites are placed under direct visualization, varying from 5mm to 12mm on the affected side.
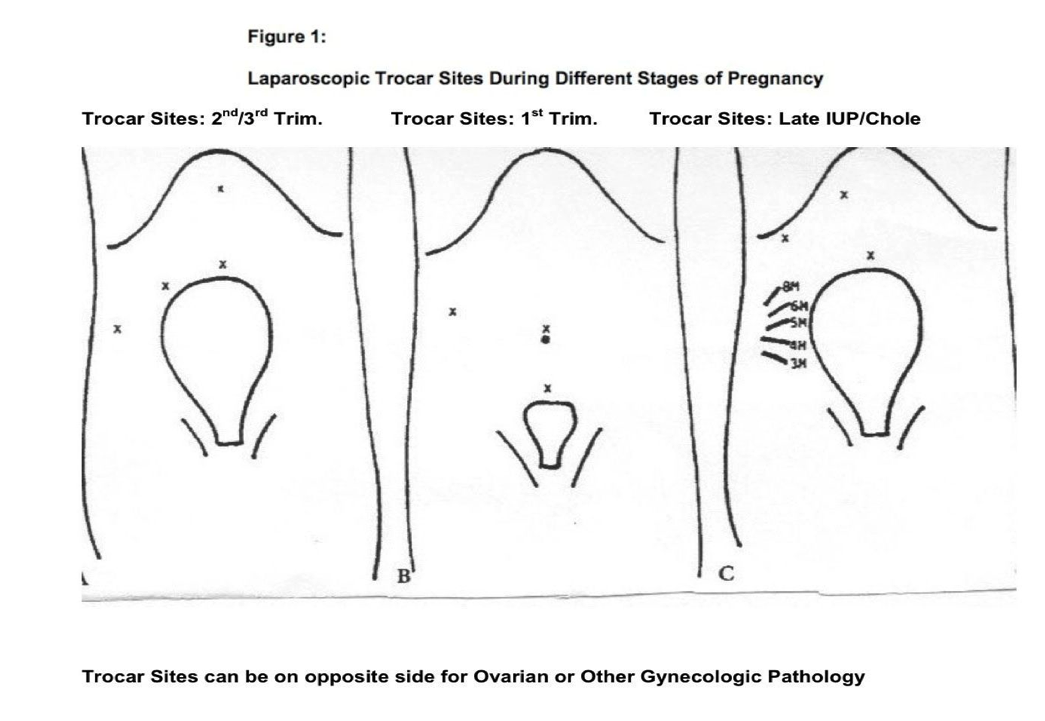
VersaStep trocars (US Surgical, Covidien, Norwalk, CT) are used in some cases per surgeon’s discretion. In other cases, nonbladed trocars are used also under direct visualization. One should not confine oneself any longer to a maximum or minimum number of trocar sites used, including the laparoscope. If the uterus is <18 weeks, the initial trocar placement is in the umbilicus, not subumbilical. CO2 pneumoperitoneum is obtained with open placement of the laparoscopic trocar that ranges from a 10-mm to 3- mm diagnostic laparoscope. As our technology has advanced, in the last few years we almost exclusively have been using only the 5-mm laparoscope, even if we place a 10- mm Hasson trocar in the navel. This gives one the flexibility to move the laparoscope to virtually any other port if the pathology warrants superior visualization from another trocar site (one could use the 10-mm laparoscope at the Hasson port and then change to the 5-mm laparoscope for the smaller trocar sites). In pregnancies associated with a uterine size ≥18 weeks, the initial trocar is placed above the navel with the lateral ports being placed under direct visualization. Intraabdominal pressure is monitored in all patients, and care is taken not to exceed 12mm Hg pressure to ensure adequate venous return and to minimize pressure on the inferior vena cava and decrease CO2 absorption to the fetus.4,19,22,23 By using multiple graspers and manipulators, the abdominal contents were manipulated based on the location, symptoms, and characteristics of the pelvic pathology. The upgraded 5-mm bowel retractor fan has become indispensable in the larger uterus.
Ovarian cystectomy, oophorectomy/salpingectomy, appendectomy, cholecystectomy, or lysis of adhesions has been performed in virtually all uterine sizes. In all cases, uterine manipulation was kept to a minimum. Copious irrigation is used. Postoperatively, the patients are observed closely in the Labor and Delivery room for increased uterine activity. We use a tocolytic (indomethacin) in a one-time dose of 50mg per rectum in >12-week’s gestation if our Maternal Fetal Medicine colleagues feel it is indicated. No further tocolysis is used. General endotracheal anesthesia has been used in all cases.
Preoperatively, the fetus was monitored. Postoperatively, patients were observed closely in Labor and Delivery for increased uterine activity and fetal heart tones. One may, in the high-risk patient, consider continuous transvaginal monitoring of the fetus. We feel that transabdominal monitoring, though possible, is cumbersome and could contaminate the operative site. One should monitor the fetus, but the type of monitoring is not the purpose of this chapter. In one of our studies, there was no difference between infants’ outcomes, and it was not statistically significant. The infants born preterm also did well.21 The blood loss for the laparoscopies was noted to be minimal. The blood loss for the laparotomies ranged from a low of 50cc to a high of 300cc in the same study. More recent data show no increased morbidity associated with laparoscopy performed during pregnancy. In the landmark survey by Reedy et al, 413 laparoscopic procedures performed during pregnancy for general surgical and gynecological indications appeared to have no higher fetal or maternal complications compared with a population undergoing laparotomy or complications were associated with the laparoscopic procedure itself.17,18
Nongynecologic laparoscopic procedures have been reported far more than gynecologic procedures have been, with cholecystectomy being the most commonly reported.
Adherence to several technical aspects is important when laparoscopy is performed during pregnancy. Clearly, safe laparoscopic access is paramount, and we feel that modification must be anticipated once access is initially obtained to ensure an effective procedure with minimal, if any, uterine manipulation; the location of these trocars in relation to the enlarged uterus is a key to success. Smaller trocars, diameters down to 2- mm, are used. In addition, 3-mm laparoscopes superior to the umbilicus were used in a study by Carter and Soper20,21 on uteri that were at or above the umbilicus. Minimizing CO2 insufflation to maximize both cardiac output, maternal hepatic flow, and minimizing fetal acidosis is best accomplished by keeping intraperitoneal pressures <12-mm Hg.19,22 Communication with the anesthesia department must be routine. To minimize risk of venous stasis, we used sequential compression devices below the knees and kept the patient out of the pure supine position with use of the operating room table. Foley catheters were routinely used after general anesthesia was obtained to decompress the bladder to prevent compromise of the operative field.22,23 These same parameters should always be present in planning, whether performing emergency surgery or the elective laparoscopic abdominal cerclage in pregnancy. It must be emphasized that in some of these laparoscopic procedures laparoscopic suturing techniques may be needed, and advanced laparoscopic surgeons whether master surgeons or residents must constantly practice their suturing skills in the “dry laboratory.” The ability to be able to instantly move to intra- or extracorporeal knot tying is a must if one is to attain these advanced skills and push the envelope in minimally invasive surgery.
CONCLUSION
Both the Society of Gastrointestinal and Endoscopic Surgeons (SAGES) and the Society of Laparoendoscopic Surgeons (SLS) recommend obtaining access to the abdomen through an open technique, using left side-down positioning, and minimizing the intrauterine pneumoperitoneum pressure to 8-mm Hg to 12-mm Hg.21,30 Laparoscopic surgery associated with pregnancy is feasible and carries a low morbidity. Although the operative times are longer, pregnant women undergoing operative laparoscopy with the appropriately skilled laparoscopist benefit from the minimally invasive procedures with brief hospital stays, rapid postoperative recoveries, earlier return to normal gastrointestinal function and ambulation compared with pregnant women undergoing laparotomies.2,3,12 We feel that less manipulation of the uterus and a decrease in delay of the diagnosis can decrease maternal and fetal/infant mortality with subsequent successful pregnancy outcomes with fewer complications. It must be emphasized that these procedures should not be undertaken without the surgeon having the proper skills, constant communication with anesthesia personnel monitoring CO2 and arterial blood gases, as well as our maternal fetal medicine colleagues and an operating room team that is skilled, knowledgeable, and comfortable with advanced minimally invasive surgery. The combination of multi-specialty communication, skilled and caring surgeons, trained nursing personnel, and attention to detail will surely give our patients (both mother and fetus) the best possible outcome.
References
- Barone JE, Bears S, Chen S, Tsai J, Russell JC. Outcome study of cholecystectomy during pregnancy. Am J Surg. 1999;177:232-236.
- Curet MJ. Special problems in laparoscopic surgery. Previous abdominal surgery, obesity and pregnancy. Surg Clin North Am. 2000;80:1093-1110.
- Graham G, Baxi L, Tharakan T. Laparoscopic cholecystectomy during pregnancy: a case series and review of the literature. Obstet Gynecol Surv. 1998;l9:556-574.
- Gurbuz AT, Peetz ME. The acute abdomen in the pregnant patient. Is there a role for laparoscopy? Surg Endosc. 1997;11:98-102.
- Mendelson CL. Aspiration of stomach contents into the lungs after obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205.
- Mazze RI, Kallen B. Reproductive outcome after anesthesia and operation during pregnancy: a registry study of 5,405 cases. Am J Obstet Gynecol. 1989;161:1178-1185.
- Kohler MF. The adnexal mass in pregnancy. Postgrad Obstet Gynecol. 1994;14:1-5.
- Curet MJ, Allen D, Josloff RK, et al. Laparoscopy during pregnancy. Arch Surg. 1996;131:546-55o; discussion 550-551.
- Rizzo AG. Laparoscopic surgery in pregnancy: long-term follow up. J Laparoendosc Adv Surg Tech A. 2003;13:11-15.
- Lemaire BM, van Erp WR. Laparoscopic surgery during pregnancy. Surg Endosc. 1997;11:15-18.
- Rollins MD, Chan KJ, Price RR. Laparoscopy for appendicitis and cholelithiasis during pregnancy. A new standard of care. Surg Endosc. 2004;8:237-241.
- S. Department of Health, Education, and Welfare, Department of Health, National Vital Statistics Division, Vital Statistics of the U.S.
- Muench J, Albrink M, Serafini F, et al. Delay in treatment of biliary disease during pregnancy increases morbidity and can be avoided with safe laparoscopic cholecystectomy. Am Surg. 2001;67:539-542; discussion 542-543.
- Sungler P, Heinerman PM, Steiner H, et al. Laparoscopic cholecystectomy and interventional endoscopy for gallstone complications during pregnancy. Surg Endosc. 2000;14:267-271.
- Sen G, Nagabhushan JS, Joypaul V. Laparoscopic cholecystectomy in 3rd trimester of pregnancy. J Obstet Gynaecol. 2002;22:556-557.
- Hunter JG, Swanstrom L, Thornberg K. Carbon dioxide pneumoperitoneum induces fetal acidosis in a pregnant ewe model. Surg Endosc. 1995;9:272- 279.
- Reedy MB, Galan HL, Richards WE, Preece CK, Wetter PA, Kuehl TJ. Laparoscopy During Pregnancy, A survey of Laparoendoscopic Surgeons. J Reprod Med. 1997;42:33- 38.
- Reedy MB, Kallen B, Kuehl TJ. Laparoscopy during pregnancy: a study of five fetal outcome parameters with use of the Swedish Health Registry. Am J Obstet Gynecol. 1997;177:673- 680.
- Duncan PG, Pope WDB, Cohen MM, et al. Fetal risk of anesthesia and surgery during pregnancy. Anesthesiology. 1986;64:790-794.
- Carter JF, Soper DE. Laparoscopy vs. laparotomy in pregnancy. Laparoscopy Today. March 2005.
- Carter J, Chang E, Haynes G, Scardo J. Hemodynamic effects of pneumoperitoneum during gynecologic laparoscopic surgery. J Gynecol Surg. 1997;13:169-173.
- Carter JF, Soper DE. Laparoscopy in pregnancy. JSLS. 2004;8:57-60.
- Iafrati MD, Yarnell R, Schwaitzberg S. Gaseless laparoscopic cholecystectomy in pregnancy. J Laparoendosc Surg. 1995;5:127-130.
- Abuabar SF, Gross FWW, Sirinek KR. Laparoscopic cholecystectomy during pregnancy is safe for both mother and fetus. J Gastrointest Surg. 1997;1:48-52.
- Weber AM, Bloom GP, Allan TR, Curry SL. Laparoscopic cholecystectomy during pregnancy. Obstet Gynecol. 1991;78:958-959.
- Lanzafame RJ. Laparoscopic cholecystectomy during pregnancy. Surgery. 1995;118: 627-631.
- Lyass S, Pikarsky A, Eisenberg H, et al. Is laparoscopic appendectomy safe in pregnant women? Surg Endosc. 2001;15:377-379.
- Affleck DG, Handrahan DL, Egger MJ, Price RR. The laparoscopic management of appendicitis and cholelithiasis during pregnancy. Am J Surg. 1999;178:523- 528.
- Amos JD, Schorr SJ, Norman PF, et al. Laparoscopic surgery in pregnancy. Am J Surg. 1996;171:435-437.
- Steinbrook RA, Bhavani-Shankar K. Hemodynamics during laparoscopy in pregnancy. Anesth Analg. 2001;93:1570-1571.
- The Society of Gastrointestinal and Endoscopic Surgeons. SAGES Guidelines for Laparoscopic Surgery during Pregnancy. Los Angeles: SAGES; 2000.
