Adhesion Prevention and Management
Ceana H. Nezhat, MD, Vadim V. Morozov, MD, Daniel S. Seidman, MD
INTRODUCTION
Peritoneal adhesions following pelvic and abdominal surgery are a frequent cause of intestinal obstruction, reduced fertility, and pelvic pain. In gynecology, adhesion formation at the vaginal cuff and pelvic sidewall frequently involves bowel, omentum, and adnexa. This may result in pelvic pain, dyspareunia, small bowel obstruction, and residual ovary syndrome when salpingo-oophorectomy is not performed. Adhesions were identified as the primary cause of chronic pelvic pain in 13-26% of females. In many surveys of postoperative bowel obstruction, abdominal surgery was found to be the leading cause of adhesion formation.
Intra-abdominal adhesions between the previous abdominal scar and the underlying viscera are a common consequence of laparotomy. Patients undergoing laparoscopy after a previous laparotomy should be considered at risk for the presence of adhesions between the old scar and the underlying viscous, such as bowel or omentum. It has been reported that after laparotomy, 68-82% of patients will have intra-abdominal adhesions. Patients with midline abdominal incisions had more adhesions (59%) than did those with Pfannenstiel incisions (27%). Patients with midline incisions done for gynecologic indications had more adhesions (42%) than did patients with any abdominal incisions performed for obstetric indications (22%). The type of incision did not affect the presence of adhesions in patients with previous obstetric operations. Adhesions to the bowel were more common after midline incisions above the umbilicus. Twenty-one women had direct injury to adherent omentum and bowel during the laparoscopicprocedure.
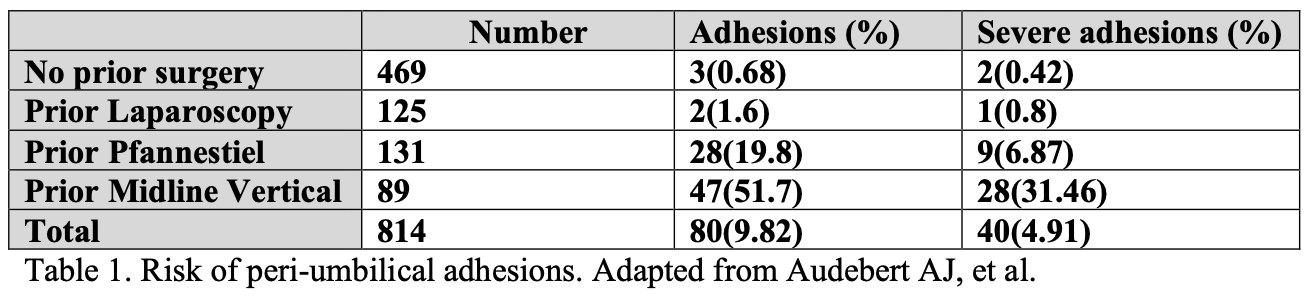
Even more important for laparoscopic surgery is the presence of peri-umbilical adhesions. In most cases, the umbilicus remains the primary site of entry for a variety of laparoscopic procedures. Table 1 demonstrates the incidence of peri-umbilical adhesions following various surgical interventions.
Peritoneal adhesions are thought to cause pelvic pain by indirectly inhibiting the motion of involved organs, thereby stretching the smooth muscle of adjacent viscera or the abdominal wall. Sulaiman et al., examined adhesions found in women undergoing laparotomy, most of whom had chronic pelvic pain. Analysis revealed that all adhesions studied had sensory nerve fibers although the location of the adhesion, its size, and its estimated age did not influence the type of nerve fibers found.
A recent prospective blinded randomized multicenter trial compared laparoscopic adhesiolysis to diagnostic laparoscopy for the relief of chronic pelvic pain attributed to adhesions. Both groups exhibited significant improvement in their pain and quality of life but there were no differences found between the two groups. This finding in the diagnostic laparoscopy group could be attributed to a possible placebo effect or to the fact that many women were reassured that they had no serious pelvic pathology. In spite of these results, adhesiolysis for pelvic pain should still be considered in cases where dense adhesions between organ systems exist or for situations such as ovarian remnant syndrome.
The effect of adhesions on fertility is unquestioned. Caspi and associates reported an inverse relation between the severity of pelvic adhesions and pregnancy rates. After adhesiolysis, pregnancy rates varied according to the extent of adnexal damage and, to a lesser degree, the severity of the adhesions. It has been repeatedly shown that both extent and severity of adhesions are significantly reduced by laparoscopic adhesiolysis.
However, the initial extent and severity of adhesions do not predict recurrence. The involved organ, adnexa being most frequent, was shown to be the only predictive factor.
Since the development of postoperative adhesions is a major factor in deciding the outcome of fertility-promoting operative procedures, gynecologists should understand the mechanism of their formation, use optimal techniques for adhesiolysis, and apply agents or devices to reduce their development.
ADHESION FORMATION
Adhesions form when there is damage to the visceral or parietal peritoneum, and the basement membrane of the mesothelial layer is exposed to the surroundings. Causes of peritoneal injury include irritation and damage from infection, ischemia, mechanical trauma, or exposure to various chemicals. This injury induces an inflammatory reaction whereby mast cell breakdown occurs, vascular permeability increases, and a fibrinous exudate is produced. This exudate is then transformed into a fibrin matrix covered with mesenchymal cells, fibroblasts, and macrophages, and enables tissue repair to occur.
Although this fibrin matrix is essential for the healing process of normal peritoneum, its dissolution is also necessary so that adhesion formation does not occur. This dissolution is mediated by the fibrinolytic pathway where plasminogen is converted to its active form, plasmin, which then degrades fibrin. Normal fibrinolytic activity usually prevents fibrinous attachments for 72 to 96 hours after surgery. Mesothelial repair occurs within 5 days of trauma and this process of peritoneal healing differs from that of skin as observed by Hertzler. When a peritoneal defect is made, repair is not initiated at the borders of the defect, but rather, the entire surface becomes epithelialized spontaneously. This phenomenon allows small defects to heal as quickly as larger ones. Figure 1.
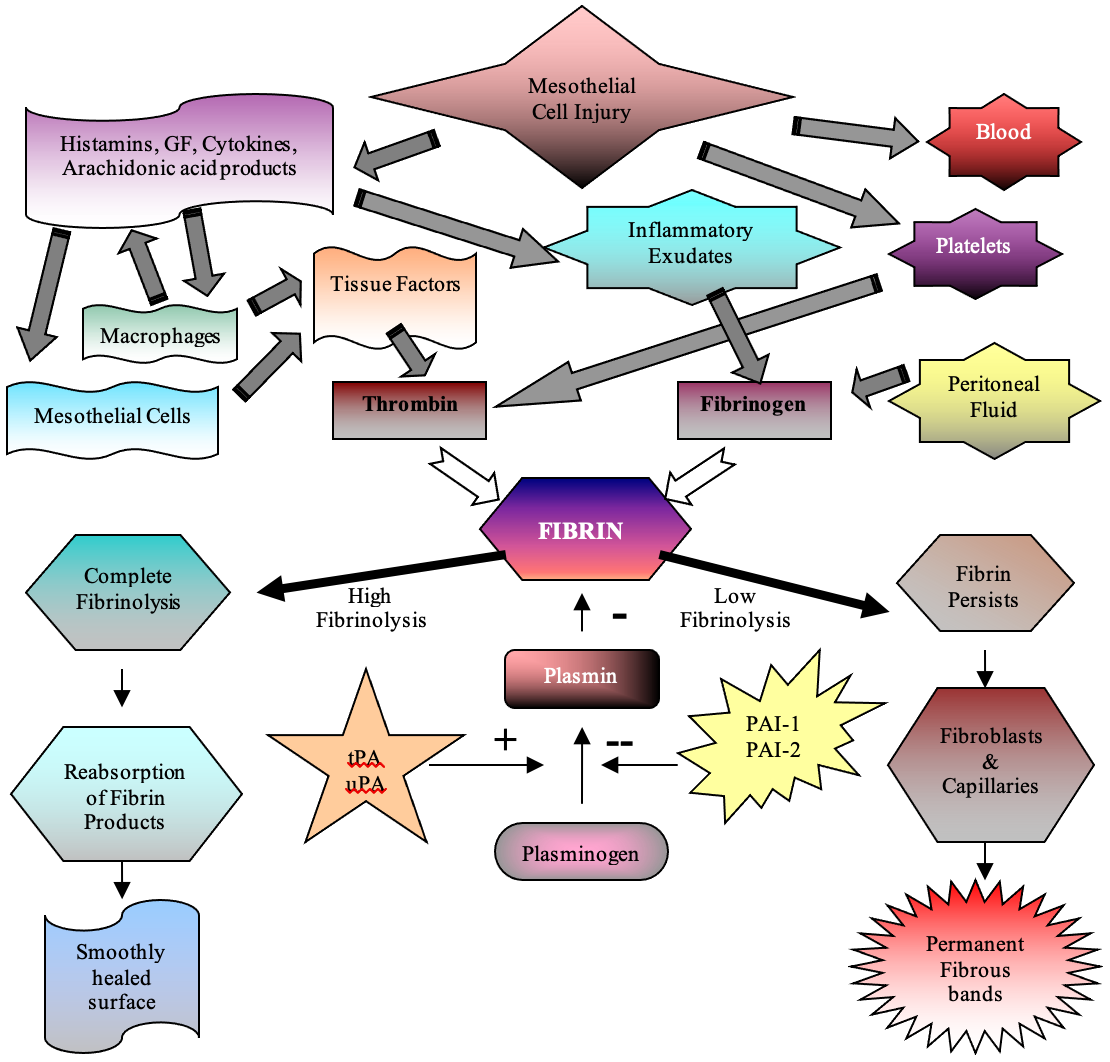 Fig.1. Schematic representation of adhesion formation and peritoneal surface healing following tissue insult. (reproduced from Hatipoglu A, Turkyilmaz Z, Mert S. The effects of melatonin on postoperative intraabdominal adhesion formation. Yonsei Med J. 2007 Aug 31; 48(4): 659-664.)
Fig.1. Schematic representation of adhesion formation and peritoneal surface healing following tissue insult. (reproduced from Hatipoglu A, Turkyilmaz Z, Mert S. The effects of melatonin on postoperative intraabdominal adhesion formation. Yonsei Med J. 2007 Aug 31; 48(4): 659-664.)
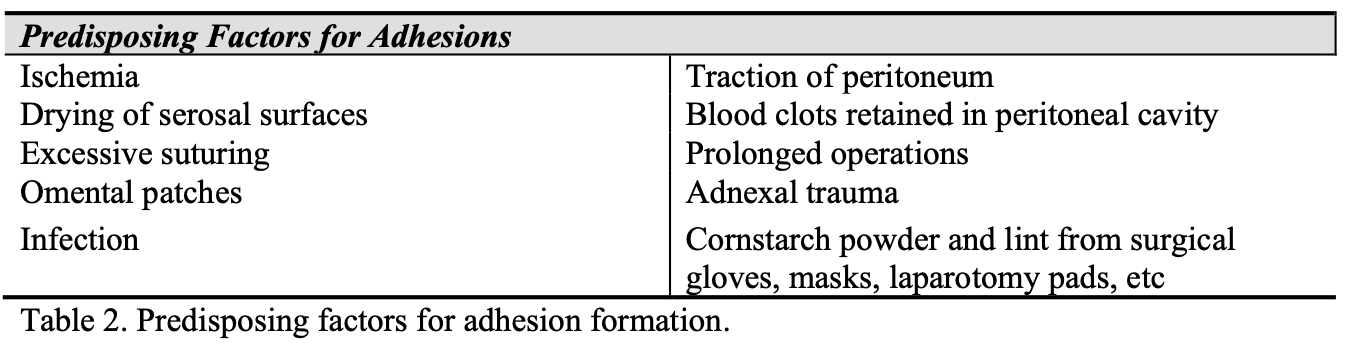
When the fibrinolytic activity of the peritoneum is suppressed, fibrinous adhesions are formed and persist, as collagen deposition and neovascularization occur. The factors that suppress fibrinolytic activity and promote formation of postoperative adhesions are listed in Table 2.
Prevention of fibrinolytic suppression can be achieved to a certain extent with microsurgical principles and techniques. The use of magnification, proper handling of tissues, constant irrigation, meticulous hemostasis, and the use of microsurgical instruments and fine nonreactive sutures are of utmost importance. Peritoneal reapproximation, which was previously advocated, is now no longer recommended. After peritoneal tissue is resected, natural healing is associated with less adhesion formation than occurs after reapproximation with staples or sutures, as demonstrated by McDonald in a rabbit model.
In the past, fertility-promoting operations done by laparotomy were often followed by reformation of adhesions and the development of new adhesions even when proper microsurgical techniques were employed. Reformation of adhesions is found at 37 to 72% of operative sites, and 51% of patients develop new adhesions after reproductive procedures by laparotomy.
Several animal and clinical studies compared the formation of postoperative adhesions after fertility-promoting operations by laparoscopy and laparotomy. With few exceptions, operative laparoscopy resulted in the development of fewer reformed and de novo adhesions. These results are consistent with the observations made a century ago by Von Dembrowski and later confirmed by Ellis. They reported that uncomplicated peritoneal injuries, such as those likely to occur at operative laparoscopy, heal without the development of adhesions.
Decreased adhesion formation after laparoscopic procedures has been attributed to the reduced presence of foreign bodies within the peritoneum that tend to stimulate more numerous and dense adhesions. In addition, laparoscopic operations may lead to fewer adhesions because tissue trauma distant from the site of adhesions increases their formation. Laparoscopy, although associated with less adhesion formation than laparotomy, is still associated with a substantial risk of adhesion formation. This has been estimated in one extensive evaluation of second-look procedures to be 67% after fimbrioplasty and 80% following ovarian cystectomy. Adhesions developed in 40-60% of patients after laparoscopic removal of an ectopic pregnancy. The type of injury to the peritoneum can control the formation of intra-abdominal adhesions. The potential to form adhesions is significantly higher in visceral than in parietal peritoneal lesions.
Transperitoneal laparoscopy did not increase adhesion formation compared with extraperitoneal laparotomy in an animal model. The transperitoneal laparoscopy approach also induced fewer adhesions than did transperitoneal laparotomy. A presumed advantage of the extraperitoneal approach is the avoidance of adhesions because the peritoneum is not entered and direct contact with intraabdominal structures is avoided. However, when the peritoneum is dissected from the abdominal wall, it is partially devascularized, leading to scars and potential adhesions. Dissection of the peritoneum from the overlying abdominal wall in a murine model was shown to cause intra-abdominal adhesions.
Even though laparoscopic procedures result in fewer adhesions than do laparotomy procedures, adhesions can develop even after laparoscopy. To minimize the formation of adhesions, good surgical technique involves the basic principles of microsurgery as mentioned previously. Alternatively, an early second-look laparoscopy can be useful for assessing the degree of postoperative adhesions, allow technically easy adhesiolysis, and result in lower adhesion scores, as shown by third-look procedures.
ANTI-ADHESION ADJUVANTS
The pathogenesis of adhesion formation that has been discussed should direct surgeons toward the best adhesion-preventing techniques. While meticulous microsurgical technique should always be maintained, there are new modalities that can aid in adhesion prevention. Multiple agents have been developed to decrease the formation of adhesions with varying degrees of success. They achieve this result by one of two means. They either disrupt the inflammatory cascade or fibrin-forming process leading to adhesion formation, or they provide a mechanical barrier between affected tissues preventing their apposition. Numerous studies exist for a wide variety of products and these will be discussed individually below.
Mechanical Barriers: intraperitoneal solutions
Mechanical barriers are available in two forms: free-floating abdominal instillates or membrane barriers; both prevent adhesion formation by preventing tissue apposition during the time period of peritoneal repair and adhesion development. High viscosity, absence of side effects, biocompatibility, and low rate of peritoneal reabsorbtion are important characteristic of fluid barriers. There are several abdominal instillates previously utilized in adhesion prevention, which are currently no longer in use: crystalloids, dextran 70, and Intergel.
Crystalloid solutions such as lactated ringers were previously instilled at the end of a surgical procedure to prevent tissue apposition. A volume of 200 to 500cc was most commonly used, but multiple studies have shown no decrease in adhesion formation, with some studies actually showing an increase. Crystalloids have been shown to be reabsorbed from the peritoneal cavity at approximately 35-60cc/hr so that a 500cc volume would be evacuated in approximately 12 hours. As discussed previously, adhesion formation of 5-7 days extends far beyond this time period and renders this technique ineffective.
A 32% solution of dextran 70, Hyskon (Medisan Pharmaceuticals Inc., Parsipany, NJ) is also no longer utilized for adhesion prevention. Hyskon did prevent adhesion formation in prospective studies, although it tended to do so in the dependent areas of the pelvis where the solution pooled. However, because of its high osmotic gradient, Hyskon was found to cause multiple side effects such as fluid overload in the form of ascites, vulvar and leg edema, and pleural effusions. There were also reports of resultant coagulopathies, disseminated intravascular coagulation, hypotension, and anaphylactic shock. These complications, although rare, far outweighed Hyskon’s anti-adhesionbenefits.
More recently, a new hyaluronic based solution called Intergel adhesion prevention solution (Gynecare – Johnson & Johnson Inc., Somerville, NJ) was widely utilized. Hyaluronic acid is a naturally occurring component of peritoneal fluid that aids in tissue lubrication and structural integrity. However, its low viscosity and high rate of peritoneal reabsorbtion make hyaluronic acid unsuitable for adhesion prevention. Intergel combines hyaluronic acid with ferric ion, increasing its viscosity and time spent in the peritoneal cavity. In a multicenter American and European prospective randomized trial by Johns et al., Intergel was found to decrease the number, severity, and extent of postoperative adhesions after laparotomy. Adhesion prevention occurred not only at the sites of application but throughout the peritoneal cavity. However, what is called “possible Intergel Reaction Syndrome” (pIRS) including prolonged ileus, peritonitis, and anastomotic dehiscence was described after using Intergel. In March of 2003, Gynecare conducted a voluntary withdrawal of Intergel due to “post market reports of late-onset, post-operative pain, repeat surgeries following the onset of pain, non-infectious foreign body reactions, and tissue adherence.” The etiology of these complications is uncertain and currently under investigation.
ACP gel (Baxter, Italy) is a new crosslinked derivative of hyaluronic acid that exhibits a higher viscosity in addition to hyaluronic acid’s other beneficial properties. In a randomized controlled prospective study utilizing the rabbit cecal abrasion model, ACP gel decreased both the incidence and grade of adhesions compared to controls. In a follow up study, ACP gel was found to decrease the incidence and surface area of adhesions in the same rabbit model in the setting of mild and severe intra-abdominal bleeding. A similar auto-crosslinked hyaluronic gel was used in a prospective randomized controlled human study following laparoscopic myomectomy. This gel was found to decrease adhesion formation from 77.8% in the control group to 27.8% in the study group. This product is available in Europe.
SprayGel (Confluent Surgical, Waltham, MA) is another gel-based adhesion barrier. It is a hydrophilic polyethylene glycol based barrier formed by simultaneously spraying two liquid precursors onto specified sites. The liquids then polymerize to form an adherent gel that coats tissues. After 5 days, the hydrogel layer is reabsorbed and undergoes renal clearance. SprayGel was found to decrease the incidence, extent, and severity of adhesions in a rat cecal abrasion and rabbit uterine horn models, although inconclusive results were obtained in the porcine uterine horn model. In a recent prospective human trial, SprayGel was utilized after operative hysteroscopy and was found to be easily applied with no adverse side effects. There was no intrauterine adhesion development in the 20 patients studied and SprayGel remained in the uterine cavity for 14 to 21 days.
A relatively new agent, Adept (Baxter, Deerfield, IL), has been approved by the FDA to be used in laparoscopy and holds promising results as an adhesion preventing agent. It is a 4% Icodextrin solution that in the recent ARIEL (Adept Registry for Clinical Evaluation) study demonstrated to be an effective treatment for the reduction of adhesion formation following surgery. Adept was reportedly easy to use in a wide range of general surgical procedures and does not adversely affect viewing of the surgical field or the handling of tissues. In the study, Icodextrin 4% solution was well tolerated by patients undergoing laparotomy or laparoscopy. It may play an important role as part of an adhesion reduction strategy. Nevertheless, multiple concerns have been raised regarding Adept, including septic/infective events (4.2% and 3.4% in the laparotomy and laparoscopy groups) and anastomotic wound-healing problems (7.6%). Adept can also cause severe labial swelling, and cases of severe DIC have been reported.
Multitude of experimental agents has been studied for the prevention of adhesions. Intraperitoneal phospholipid solutions were studied for their effect on adhesion development. In a rabbit uterine horn model, a phospholipid solution was found to inhibit adhesion formation when compared to Ringer’s lactate. In another study utilizing the rabbit model, phospholipids were once again found to decrease adhesion incidence while not impeding would healing, incisional tensile strength, or anastamotic bursting pressures.
Various other combinations of cellulose derivatives and hyaluronic acid, in addition to other unrelated compounds, are currently being tested in animal models with scattered reports available in the medical literature. Examples of such compounds are LM 200, Lipiodol, and chondroitin sulfate solution.
Mechanical barriers: membrane form
There are multiple surgical membranes that are currently utilized for adhesion prevention. Unlike their liquid counterparts, membrane forms prevent adhesions only in their area of application. The membranes most commonly used are Interceed (Johnson & Johnson Medical, Summevile, NJ), Seprafilm (Genzym Corporation, Cambridge, MA), and Gor- tex Surgical Membrane (W.L. Gore & Associates, Inc., Flagstaff, AZ).
Interceed is an absorbable adhesion barrier made of oxidized regenerated cellulose that has been shown to be safe and effective in reducing adhesions after various laparoscopic procedures. It has been postulated to compete for the macrophage scavenger receptor because of its polyanionic nature resulting in decreased secretion of matrix components, inflammatory mediators, and cellular growth factors. It has been shown to be both nonreactive and bacteriostatic. It usually forms a gelatinous mass within hours of placement, metabolized within 4 days and absorbed within 28 days. In a Cochrane Database review, thirteen trials assessed the effect of Interceed versus no treatment on adhesion formation following a variety of gynecologic procedures by both laparoscopy and laparotomy. The use of Interceed in women was associated with a reduced incidence of pelvic adhesion formation, both new formation and reformation following both laparoscopy and laparotomy.
Interceed is easily applied via laparotomy or laparoscopy to affected tissues. However the traumatized area must be covered completely while sparing healthy tissue as much as possible. Effective application and optimal results are impeded by the presence of blood or excess peritoneal fluid. It is also contraindicated for use in the presence of ongoing infection in the pelvic or abdominal cavities.
Gore-Tex is an expanded polytetrafluoroethylene membrane, which is both nonabsorbable and non-reactive. It has been used to repair both the pericardium and peritoneum. A Cochrane Database review compared the use of Gore-Tex versus both Interceed and controls in three studies on women following various gynecologic procedures via laparoscopy and laparotomy. Gore-Tex was found to be more effective than Interceed or no barrier in preventing adhesion formation. This review confirmed prior animal studies where Gore-Tex was found to be better than Interceed with respect to the size of the adhesion area, tenacity, and vascularity, with a significant improvement in the total adhesion score. The Gore-Tex surgical membrane can easily be positioned over a peritoneal defect by laparoscopy, as its handling properties do not worsen when wet.
Gore-Tex’s utility, however, is greatly diminished by its nonabsorbable nature and need for subsequent removal. It must be secured into place with sutures or laparoscopic staples. As a result of this, and the development of newer absorbable adhesion barriers, the use of Gore-Tex has fallen out of favor in recent years. Nevertheless, studies have shown that removal of Gore-Tex at early second look laparoscopy (11 days after myomectomy) was not associated with adhesions.
Seprafilm is another widely used surgical membrane. It is a bioresorbable translucent membrane composed of chemically modified hyaluronic acid and carboxymethylcellulose. It has been widely studied in the general setting of intraperitoneal adhesion formation and specifically when polypropylene mesh is utilized for abdominal hernia repair. It is however difficult to use during laparoscopy because of handling limitations as a continuous sheath of surface-covering membrane. However, there are off-label reports of rolling Seprafilm in a wrap or making a slurry to make it applicable in laparoscopy.
In a unique study by Tsapanos et al., the intrauterine application of Seprafilm was found to significantly decrease the formation of adhesions after D&C in a randomized controlled trial. Postoperative hysterosalpingogram was utilized to detect adhesions, which were found to be lower in women with either one or multiple prior D&C’s in the Seprafilm group.
Seprafilm forms a hydrophilic gel within 24 hours of its application and covers serosal surfaces for 7 days. It is excreted from the body in about 28 days. Conflicting studies exist regarding its effectiveness. A recent study by Bulbuller et al. utilizing a rat uterine horn model, compared Seprafilm to a no treatment control and adhesion scores in the Seprafilm group (.3 +/- 0.1;P=.000, MWU test) were significantly lower than in the control group (3.6 +/- 0.1). Buckenmaier et al. studied Seprafilm in a rat colonic transection and reanastamosis model and found no difference in adhesion formation versus controls. Vrijland et al. found that Seprafilm decreased the severity but not the incidence of adhesions in a prospective study of patients undergoing a Hartmann procedure for diverticulitis or obstructed rectosigmoid.
While controlled clinical studies have shown that Seprafilm is effective in reducing postoperative adhesion formation, a Cochrane Database review revealed that there was limited evidence that Seprafilm was effective in preventing adhesion formation in women following myomectomy.
Seprafilm’s ability to prevent adhesion to polypropylene mesh has also been widely studied. Polypropylene mesh is utilized in the repair of abdominal wall defects but has a tendency to cause dense adhesions. Dinsmore et al. found an 80-90% decrease in adhesion formation to the mesh when Seprafilm was employed in a rabbit model.
Altuntas et al. achieved similar results in a rat model.
Oxiplex (FzioMed, Inc., San Luis Obispo, CA) is another promising membrane barrier. Viscoelastic gel, consisting of a high molecular weight polyethylene oxide and carboxymethylcellulose, has been shown to be effective in adhesion prevention. It is a biocompatible, resorbable film with good handling properties and tissue adherence and is easily applied laparoscopically with FilmSert forceps through a 5-mm trocar. It transforms into a gel-like material and is cleared by phagocytosis within 96 hours.
Rodgers et al. studied different formulations of the Oxiplex membrane in the rabbit, rat, and pig model. The optimal Oxiplex membrane prevented adhesion formation in 100% of rabbits in the cecal abrasion model and adhesion reformation in 91% of cases. The presence of blood at the membrane-tissue interface, although not studied directly did not seem to affect the results. Additionally, its effect on intraperitoneal infection was also studied. In a prospective, controlled study utilizing a rat model, fecal-filled gelatin capsules were placed intraabdominally, and Oxiplex was found to have no negative effect on survival or abscess formation. The study by diZerega et al. found that in humans, the treated adnexa with application of Oxiplex was less likely to develop adhesions compared with the control.
SurgiWrap (MAST Biosurgery, San Diego, CA) Bioresorbable Adhesion Barrier Film is a transparent polymer film that is designed to separate opposing tissue during the critical period of peritoneal healing. Made of polylactide (PLA), it comes in 8 different sizes. A copolymer of 70:30 Poly(L-lactide-co-D,L-lactide), it is composed of lactic acid similar to that which occurs naturally in the human body, the material maintains its strength during the healing process, and is slowly hydrolyzed into lactic acid. The molecules are then metabolized into carbon dioxide and water and are released from the body through the lungs. SurgiWrap received FDA clearance to be used in urological, gynecological, and gastroenterological procedures, either by laparotomy or laparoscopy.
There are multiple other barrier membranes that are currently under review.
Preclude (W.L. Gore Co., Flagstaff, AZ), an expanded polytetrafluoroethylene membrane similar to Gore-Tex, is approved for use as a pericardial substitute. Noninflammatory and inert, it is applied over the areas at risk just prior to closure. Yet it is also a nonabsorbable material, requiring suturing and subsequent removal. It has been shown to decrease adhesions to peritoneal defects when compared to the contralateral nontreated side in a rat model.
SupraSeal, another solid adhesion barrier consisting of D,L-polylactid (PDLA- Copolymer), was studied and showed efficacy similar to Adeptas reported by Rajab et al. in rat model.
Human amniotic membrane and a chitosan/poloxamer gel have also been found to decrease adhesions in animal models, but require further testing.
Fibrinolytic agents
The theory that normal peritoneum has fibrinolytic activity which could prevent adhesion formation was first described by Hartwell in 1955, with subsequent evidence of this theory provided by Von Benzer in 1963. Recombinant human tissue plasminogen activator (rt-PA), which converts plasminogen to plasmin, causing fibrin degradation, has been widely studied in animal models with great success. A significant reduction in adhesion formation was achieved when compared to controls. Side effects such as changes in hematologic indices, postoperative bleeding, and altered wound healing were not noted in the vast majority of studies performed. Although these results are promising, there is little known about the pharmacokinetics of intraperitoneal rt-PA administration and the proper dosage and length or treatment necessary for optimal results.
An increase in the main inhibitor of fibrinolysis, plasminogen activator inhibitor type 1 (PAI-1), is also a major factor in the loss of fibrinolytic activity and subsequent adhesion formation. Falk et al. studied the effects of inhibition of PAI-1 on adhesion formation.
Fragments for antigen binding of polyclonal rabbit antibody against PAI-1 were injected intraperitoneally in a murine model, and were found to decrease adhesion formation compared to controls with no episodes of postoperative bleeding noted.
Fibrin glue and Coseal
Fibrin glue has also been found to decrease adhesion formation in multiple animal models. It is composed of human fibrinogen obtained either from cryoprecipitate or fresh frozen plasma, thrombin, calcium chloride, factor XIII, and aprotinin solution. It has been used as a hemostatic agent and biological sealant in cardiothoracic surgery and in cases of hepatic and splenic trauma. It is counterintuitive to think that the addition of a large amount of exogenous fibrin would decrease adhesion formation, but this has been found to be true in multiple animal studies.
Fibrin glue is applied easily during laparotomy and laparoscopy and remains in a liquid form for a few minutes following application, at which time it exerts its hemostatic effect. It then forms a gel, and is thought to act as a mechanical barrier that covers wound surfaces and prevents adherence of other organs. This fibrin gel may also inhibit diffusion of inflammatory substances throughout the peritoneum and suppress endogenous production of fibrin. These various clinical applications make fibrin glue a versatile product and one that deserves further studies as an anti-adhesion agent in humans.
Coseal Surgical Sealant (Baxter, Deerfield, IL), originally introduced as a sealant for vascular reconstruction is composed of two synthetic polyethylene glycols (PEGs), which when mixed together form a hydrogel that adheres to tissue, synthetic graft materials and covalently bonds to itself. Although introduced as a tissue sealant, Coseal has been found to prevent adhesion formation at much higher rates as compared to fibrin glue.
Anti-inflammatory agents
Nonsteroidal anti-inflammatory drugs (NSAIDs) and steroids have been extensively studied for their possible anti-adhesion benefits. They both decrease the inflammatory response associated with adhesion formation. Systemic administration of these medications has met with mixed results in addition to being associated with infection and delayed wound healing. This ineffectiveness in the prevention of adhesions may be due to the inability of a systemically administered drug to reach devascularized sites where adhesions are likely to form.
Recent studies utilizing local drug delivery systems such as miniosmotic pumps or incorporation of these drugs into a cream or gel-form have yielded more promising results and warrant further studies.
Newer experimental agents with anti-inflammatory properties are being studied and tested. Camptothecin-loaded films in animal studies have shown to inhibit inflammation and angiogenesis. Camptothecin is an anti-inflammatory, antiproliferative, and antiangiogenic agent. The use of crosslinked hyaluronic acid film containing camptothecin was evaluated in rats by Cashman et al. and has shown the decrease in adhesion formation. No toxicity was observed. Whether this substance is safe for human use is still unknown.
Sodium chromoglycate is another experimental agent for adhesion prevention. It stabilizes the membranes of mast cells and in a rabbit cecal abrasion model has shown to decreases adhesion formation. Adding of aprotinin, a proteolytic enzyme that inhibits kallikrein and plasmin, and dexamethasone increases sodium chromoglycate efficiency. Dexamethazone alone is clinically ineffective and aprotinin’s efficacy when used alone is inconclusive.
LAPAROSCOPIC ADHESIOLYSIS
Preoperative detection of adhesions is difficult and the overall sensitivity of imaging with standard ultrasound technique is low. However, the patient’s history of previous surgery, endometriosis, or pelvic inflammatory disease can help predict the likelihood of encountering adhesions during laparoscopy.
Recently, Nezhat et al. demonstrated the novel technique of detecting obliterating subumbilical adhesions in patients at risk for viscous injury during original trocar insertion. Called PUGSI (Peri-umbilical Ultrasound Guided Saline Injection), it relies on ultrasound detection of the visceral slide and fluid dispersion in a paralyzed, intubated patient.
Visceral slide testing, displacement of viscous with deep inspiration in a paralyzed patient observed with ultrasound peri-operatively, has 50% sensitivity and 98% specificity. Combined with PUGSI, where 10cc of the sterile solution injected intraperitoneally under direct ultrasound guidance and observation of fluid dispersion is documented, the test was able to detect 100% of obliterating subumbilical adhesions before placement of the primary umbilical trocar. Figures 2, 3, 4, 5.
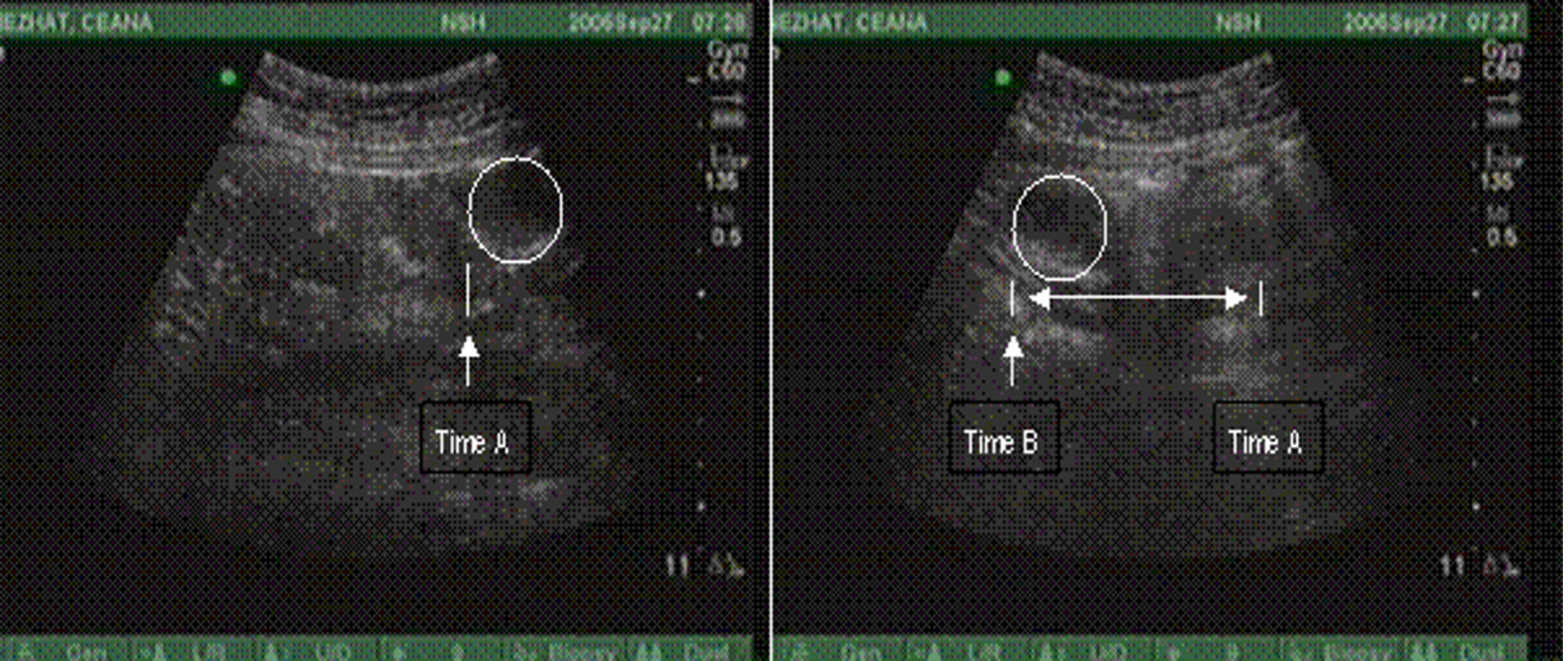 Figure 2. Normal Visceral slide. Displacement of the viscous of more than 1 cm in paralyzed patient under deep forced inspiration.
Figure 2. Normal Visceral slide. Displacement of the viscous of more than 1 cm in paralyzed patient under deep forced inspiration.
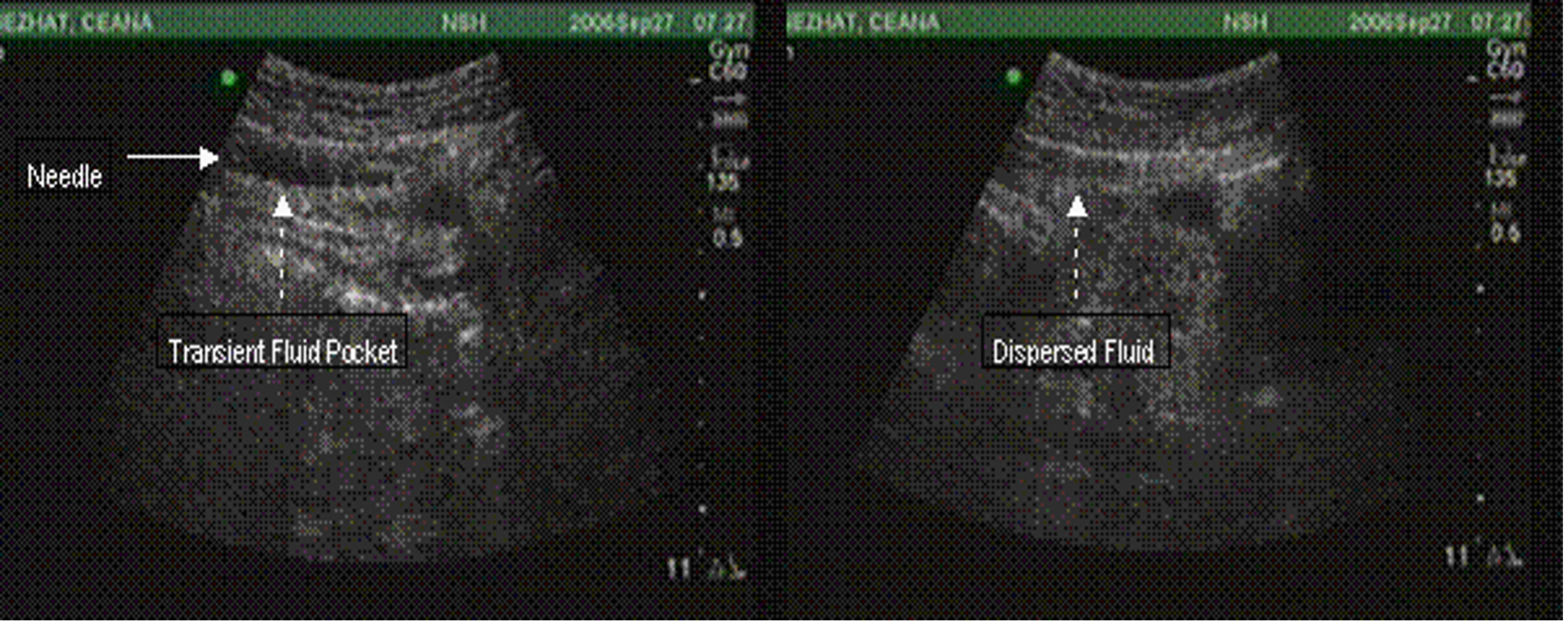 Figure 3. Normal PUGSI with observed dispersion of intaperitoneal “bubble” after injection of 10cc of sterile saline with 21 gauge needle.
Figure 3. Normal PUGSI with observed dispersion of intaperitoneal “bubble” after injection of 10cc of sterile saline with 21 gauge needle.
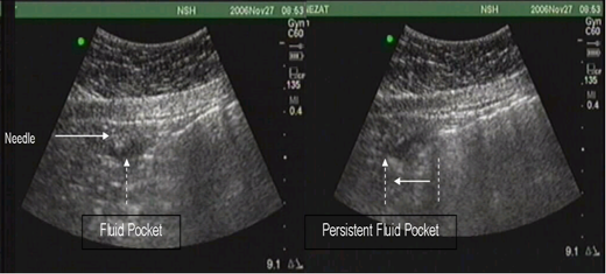 Figure 4. Abnormal PUGSI. Intraperitoneal fluid pocket persists with deep inspiration and slides in unison with limited motion of the viscous.
Figure 4. Abnormal PUGSI. Intraperitoneal fluid pocket persists with deep inspiration and slides in unison with limited motion of the viscous.
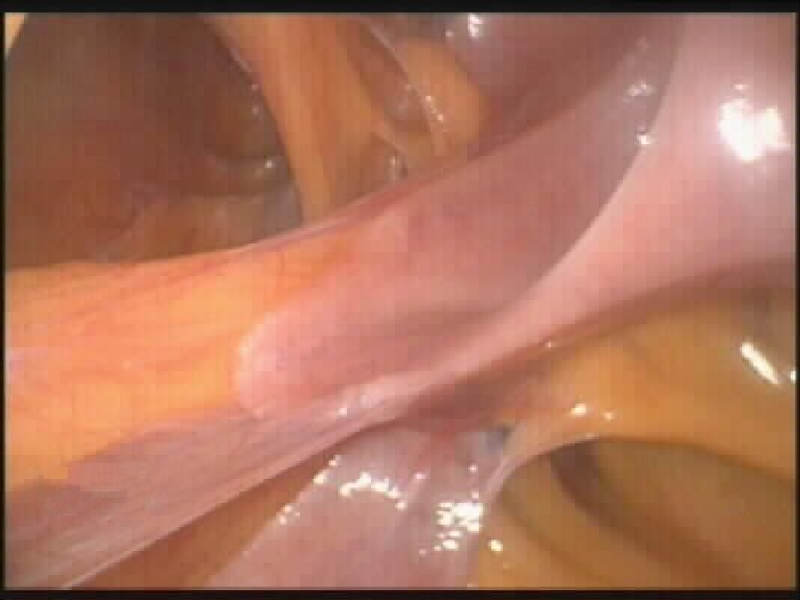 Figure 5. Actual laparoscopic picture of obliterating subumbilical adhesions.
Figure 5. Actual laparoscopic picture of obliterating subumbilical adhesions.
The possibility of injury during abdominal entry with a Veress needle or trocar exists in patients with or without a previous history of laparotomy (Figures 6, 7a, 7b). In order to perform laparoscopic adhesiolysis adequately, three or four abdominal punctures are required: the intraumbilical incision for the operative laparoscope and two to three lower, lateral suprapubic punctures about 2-4cm below the level of the iliac crests. Successful insertion depends on adequate skin incisions; the trocar’s working condition, proper orientation, and control over the instrument’s force and depth of insertion. Small diameter (<3 mm in diameter) laparoscopy as an alternative to open laparoscopy can be utilized for initial abdominal entry in patients at risk for adhesions. Additionally, in patients with extensive adhesions, a mapping technique can be used to lessen the chance of complications. After insertion of the laparoscope, the abdominal wall with adherent bowel or omentum is explored. If the adhesions are severe and no clear space for accessory ports insertion is seen, the abdominal wall is observed through the laparoscope and gentle external compression applied, marking areas that seem free of adhesions.
Before inserting the cannula, the surgeon should simulate its path with a 21-gauge spinal needle. If a clear path is identified, the cannula is introduced next to the needle, or the needle is removed and the port is introduced. An advantage of first inserting the 21-gauge needle is its small diameter because the injury incurred does not require repair.
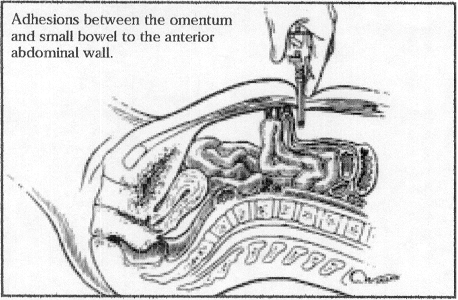 Figure 6. Trocar insertion in a patient with bowel adhesions from a previous laparotomy
Figure 6. Trocar insertion in a patient with bowel adhesions from a previous laparotomy
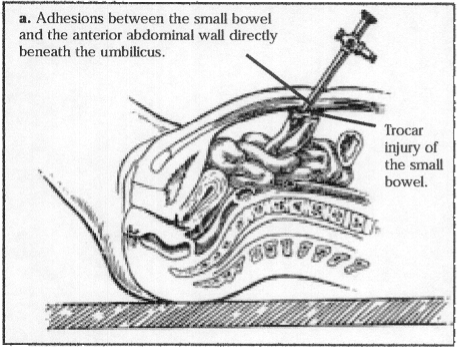 Figure 7a. Attachment of the bowel to the anterior abdominal wall. The bowel is attached directly under the umbilicus.
Figure 7a. Attachment of the bowel to the anterior abdominal wall. The bowel is attached directly under the umbilicus.
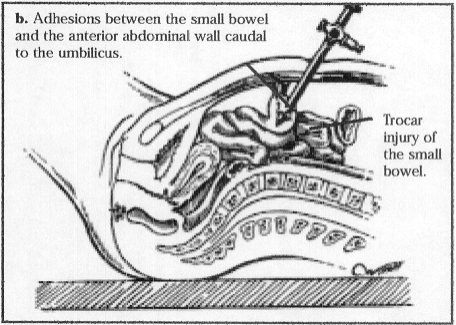 Figure 7b. The attachment is below and distal to the umbilicus.
Figure 7b. The attachment is below and distal to the umbilicus.
Once the trocars are in place, an atraumatic grasping forceps is inserted through the lateral trocar on the side of the assistant to hold the adhesion or involved organ, stretch it, and identify its boundaries and avascular planes. The opposite trocar, on the side of the primary surgeon, is used for microscissors or the suction-irrigator probe. This probe can serve as a manipulator or backstop when the CO2 laser is used (Figure 8).
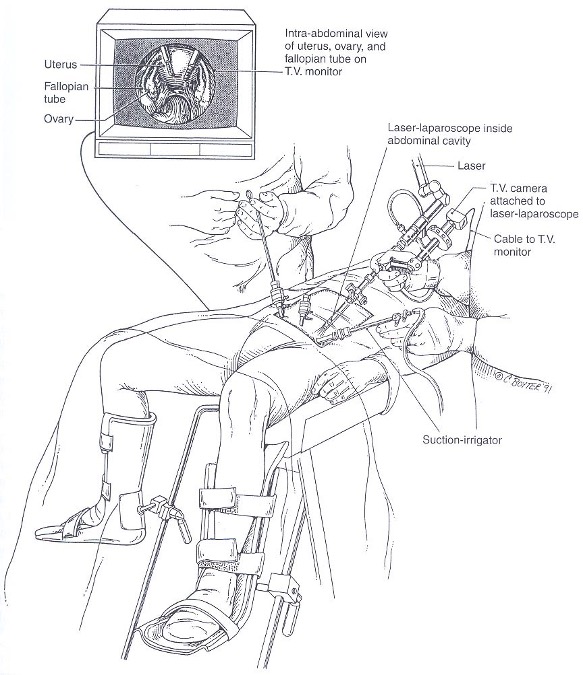 Figure 8. Example of patient positioning with trocar and instrument placement. Reproduced with permission from Nezhat et al. from Operative Gynecologic Laparoscopy: Principles and Techniques.
Figure 8. Example of patient positioning with trocar and instrument placement. Reproduced with permission from Nezhat et al. from Operative Gynecologic Laparoscopy: Principles and Techniques.
Adhesions are cut close to the affected organ at both ends and, if possible, removed from the abdomen. Vascular adhesions are coagulated with lasers or electrosurgery. When scissors are used, filmy and avascular adhesions are stretched and then cut. Thick, vascular adhesions must be desiccated before being cut.
Intestinal adhesions are severed first, followed by periovarian adhesions and peritubal adhesions (Figure 9). This approach allows progressive exposure of the pelvic structures. Once the intestines are freed from adjacent structures, they are pushed gently cephalad.
Adherent ovaries are freed from the pelvic sidewall, broad ligament, tubes, and uterus (Figure 10). Grasping forceps are essential for applying traction to the ovary, tube, intestines, or abdominal wall so that a plane of dissection can be identified.
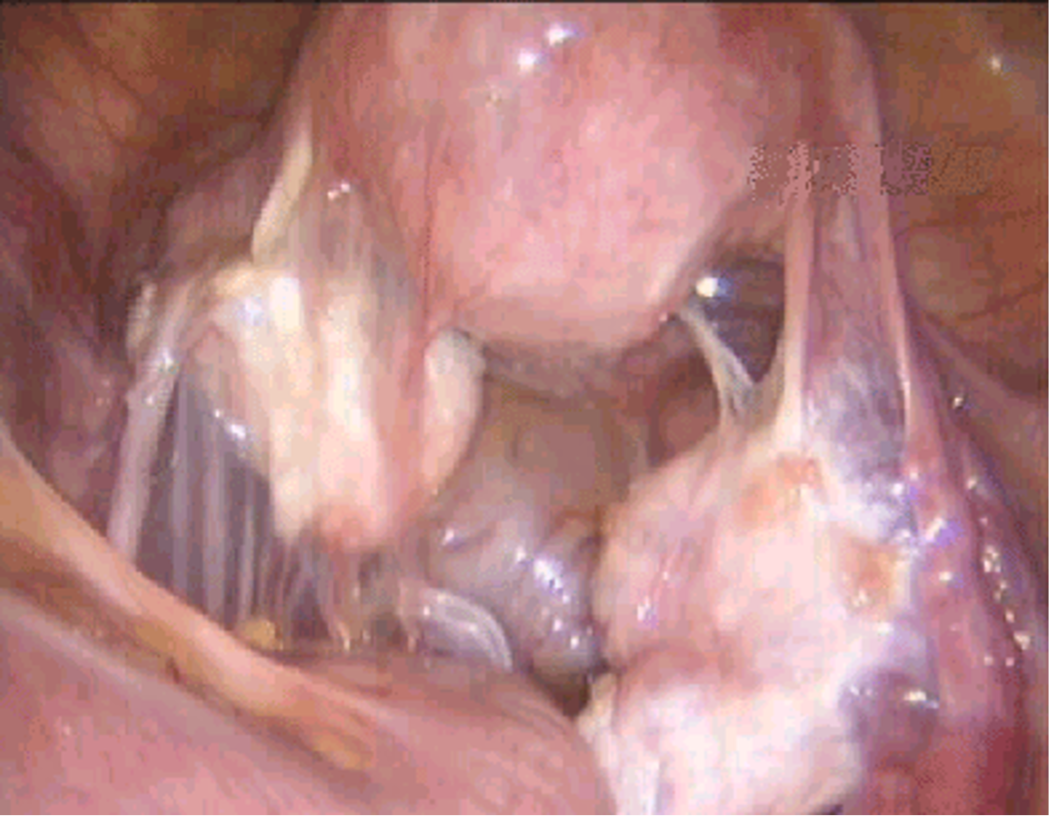 Figure 9. Adhesions between the bowel, adnexa, and uterus.
Figure 9. Adhesions between the bowel, adnexa, and uterus.
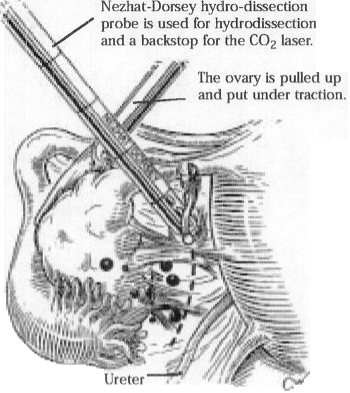 Figure 10. Ovary with endometriosis and adhesions.
Figure 10. Ovary with endometriosis and adhesions.
Bleeding areas are coagulated with the laser or a bipolar electrocoagulator. Whenever possible, either the adhesions or the ovarian ligaments are grasped instead of the ovarian cortex to reduce trauma. Once the ovaries are lifted from the cul-de-sac and mobilized, the peritubal adhesions are removed.
Adhesions can be coagulated effectively and incised with a CO2 laser, superpulse (40 W) laser, ultrapulse (20 to 80 W and 25 to 200 mJ) laser, or fiber laser (15 to 20 W, cutting mode). When there are dense adhesions among different organs (bowel, uterus, ovaries, pelvic sidewall, and anterior abdominal wall), hydrodissection with the suction-irrigator probe is useful to create tissue planes before dissection (Figure 10).
Since an intestinal injury can occur during enterolysis, patients with a history of previous laparotomies or severe endometriosis should undergo a preoperative bowel preparation. Inadvertent enterorrhaphy can be repaired with the use of a one-layer closure of 0 polyglactin (Vicryl, Ethicon) or an endoloop. Once the pelvic structures are freed and hemostasis is achieved, the cul-de-sac is filled with lactated Ringer’s solution and the adnexa are allowed to float in the clear fluid. Filmy adhesions that are difficult to identify on the surface of the ovary become visible as they float from the ovarian cortex. These adhesions are grasped with the forceps, cut, and removed from their attachments, using laparoscopic microscissors (Figure 11). They are filmy and avascular, and so coagulation is not required.
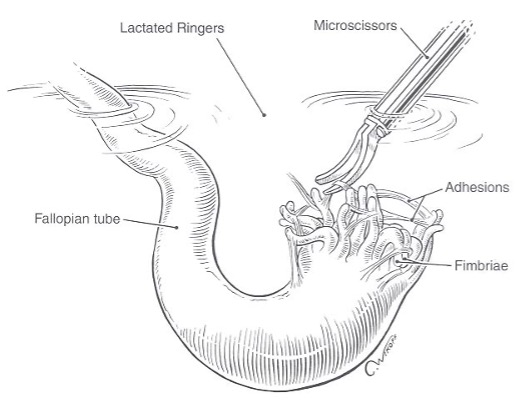 Figure 11. Hydroflotation of the tube and fimbria is used to detect and remove filmy adhesions. Reproduced with permission from Nezhat et al. from Operative Gynecologic Laparoscopy: Principles and Techniques.
Figure 11. Hydroflotation of the tube and fimbria is used to detect and remove filmy adhesions. Reproduced with permission from Nezhat et al. from Operative Gynecologic Laparoscopy: Principles and Techniques.
Agglutinated fimbrial folds are caused by fine avascular adhesions. As the fimbrial folds float and disperse in the fluid, the adhesions become visible; they are grasped, stretched, and sharply cut with fine scissors or an ultrapulse laser. The laser beam (other than ultrapulse) delivered through the laparoscope is at least 1 mm in diameter and is too wide for these narrow bands of adhesions. Thermal damage can occur with electrosurgery and the fiber laser (neodymium-yttrium aluminum garnet, potassium titanyl phosphate, or argon). When performing delicate microscopic procedures such as fimbriolysis and salpingo-ovariolysis, the microscissors or ultrapulse laser are preferable.
COMPLICATIONS
Complications due to adhesiolysis are not infrequent, occurring in approximately 6% of cases, and the organ most frequently involved is the bowel. Injuries can occur following thermal, blunt, or sharp trauma. The possibility of injury during abdominal entry with a Veress needle or trocar exists in patients with or without a previous history of laparotomy.
Factors which contribute to an increased risk of gastrointestinal injuries include 1) failure to establish an adequate pneumoperitoneum, 2) the use of dull trocars which require excessive force, 3) uncontrolled, sudden entry of sharp instruments, and 4) gastric distension. Poorly controlled or sudden trocar entry can result in rectosigmoid laceration. Gastric distension can displace the transverse colon toward the pelvis, where it can be punctured by the Veress needle or lacerated with the trocar. This complication can be eliminated by using an orogastric tube intra-operatively.
The rectosigmoid can be injured if the depth of penetration by endometriosis is underestimated or the cul-de-sac is obliterated. When the rectum is adherent to the posterior aspect of the cervix or uterosacral ligaments, blunt dissection may lacerate the rectum. Sharp dissection with scissors or the CO2 laser is recommended. The combination of high-power superpulse or ultrapulse CO2 laser and hydrodissection is relatively safe for working around the bowel.
When the cul-de-sac is dissected, identification of the vagina and rectum is facilitated by placing a probe or an assistant’s finger in both the vagina and rectum. Dissection should begin lateral to the uterosacral ligaments, where anatomy is less distorted, and proceed toward the obliterated cul-de-sac. Similarly, when posterior culdotomy is performed for tissue removal or during laparoscopic hysterectomy, correct identification of vagina and rectum is important.
A high degree of awareness is necessary for prompt diagnosis of intraoperative complications due to adhesions. Electrical injuries to the bowel are not always apparent intraoperatively, but when detected, conservative management should be employed. If the area of blanching on the intestinal serosa exceeds 5 mm, the extent of thermal damage will probably exceed the apparent damage, and therapy should be instituted immediately. The actual area of injury can extend up to 5 cm from the apparent injury. Electrical injury to the right colon is managed by resection of the injured segment and primary anastomosis. Diverting ileostomy facilitates healing and reduces morbidity and mortality. Injury to the descending colon, sigmoid, or rectum in an unprepared bowel is usually not managed by primary closure or resection with primary anastomosis. Diverting colostomy with resection of the injured portion is traditionally performed, although this treatment may be excessive.
The small bowel is the portion of bowel most frequently injured during laparoscopy. The severity of the injury is related to the extent of damage and the time lapsed before the injury is discovered. If the small bowel has been lacerated by a trocar, the surgeon may initially view a mucosal surface or notice a foul smell when the laparoscope is inserted.
Small bowel contents may be observed leaking from a laceration, or a hematoma may be present on the small bowel serosa.
Sharp injuries to the bowel can be limited to the serosa or deep, involving the entire wall. Small punctures or superficial lacerations seal readily and require no further treatment, assuming that careful inspection of the affected bowel reveals no leakage of bowel contents or bleeding. Small (< 5 mm) superficial lacerations need to be inspected to assure that only the serosa is involved. In these cases, the patient may be treated conservatively and discharged the day of surgery with dietary instructions and orders to report any untoward reaction.
The small bowel should be repaired in 1 or 2 layers by placing an initial row of interrupted sutures of 2-0 Vicryl to approximate the mucosa and muscularis. A reinforcing layer of 3-0 silk Lembert sutures is used to approximate the muscularis and serosal edges. Injuries caused by trocars or the CO2 laser can be repaired in one layer by using 3-0 silk or 4-0 polydioxanone without complication or laparotomy. Injuries less than 2 cm (small or large bowel) may be repaired transversely or longitudinally. All lacerations greater than 2 cm should be closed transversely to minimize the occurrence of stricture and stenosis of the bowel lumen. Repair of the laceration could be accomplished with laparoscopic suturing. This closure is appropriate only if the laceration is less than one-half the diameter of the bowel. If the laceration exceeds this amount, segmental resection and anastamosis should be performed. If the mesenteric blood supply is interrupted by the puncture, a resection must be performed regardless of the size or length of the laceration. For those surgeons who are not familiar with bowel repair and its postoperative care, consultation with a general or colorectal surgeon is appropriate whenever significant bowel trauma occurs.
Perforation of the large bowel with the Veress needle can sometimes be recognized by the saline aspiration test where recovery of brownish fluid is pathognomonic. Fecal odor may also be detected. If large bowel entry is suspected based on these two tests, the needle should be promptly withdrawn and another sterile Veress needle reinserted. Once the laparoscope is inserted, the entry site should be sought and examined. Because of the high bacterial concentration, minor leaks of fecal material into the peritoneal cavity can be the source of serious infection.
Colonic lacerations in prepared bowel can be repaired laparoscopically after excising endometriosis nodules and identifying the extent of the laceration. A single layered repair using 2-0 silk, 4-0 polydioxanone, or 0 polyglactin sutures is used.
Patients with obvious peritoneal soiling require special attention. Laparoscopic repair should only be performed by an experienced laparoscopist. The bowel should be inspected on both sides to detect through-and-through injuries, especially if produced by a trocar. If only one entry is found and repaired, peritonitis may develop postoperatively. If the laparoscope has been inserted through the bowel laceration and laparotomy is performed, leaving the trocar in place allows the defect to be readily identified and closed by a purse-string suture as the laparoscope is withdrawn (so as to minimize peritoneal contamination).
Following repair of any bowel laceration, the entire abdomen is irrigated. In order to evaluate the repair, the abdomen and posterior cul-de-sac are filled with lactated Ringer’s solution. A sigmoidoscope is used to insufflate the bowel and the presence of air bubbles indicates inadequate repair. The sigmoidoscope can also be used to directly visualize the integrity of the repair if it was performed in the area of the rectosigmoid. The use of a nasogastric tube postoperatively is usually not necessary.
Injuries involving the small and large bowel may not be recognized at the time of surgery and symptoms may not become apparent until the third or fourth postoperative day.
Patients generally present with lower abdominal pain, fever, nausea, and anorexia. By the fifth or sixth postoperative day, symptoms include fever, severe abdominal pain, nausea, vomiting, constipation, elevated white blood cell count, and peritonitis. Radiographs reveal multiple air/fluid levels or air under the diaphragm.
When possible bowel laceration becomes apparent after the patient has been discharged, conservative management is successful in patients who have not developed peritonitis. In-hospital management consists of hydration, bowel rest, and close observation with white blood count and physical exam. Wheeless reported that over one-half of patients treated conservatively required no surgical intervention. Those whose condition deteriorated during observation underwent laparotomy and had no complications attributable to delayed surgery.
Immediate surgical intervention by laparoscopy or possible laparotomy may be indicated in patients who present with fever, severe abdominal pain, nausea, vomiting, obstipation or peritonitis, or in those whose clinical condition worsens. Surgical consideration for managing bowel injuries discovered postoperatively differ somewhat from those discovered and managed intra-operatively. The damaged bowel must be repaired or resected. In addition, resection of all necrotic tissue in the pelvis is mandatory and at times this may require a hysterectomy and bilateral salpingo-oophorectomy. If burned or necrotic tissue that has been bathed in intestinal contents and blood or serum is not excised, it could lead to a pelvic abscess.
Wheeless presented a 7-point plan to manage patients with peritonitis secondary to bowel perforation:
- Preoperative stabilization with fluids, electrolytes and nasogastric suction
- Eploratory laparotomy with repair or resection of the injured bowel
- Resection of all necrotic tissue
- Cpious and repeated saline lavage of the abdomen
- Pelvic drainage using a closed drainage system
- Aggressive antibiotic therapy
- Embolus prophylaxis with mini-does heparin (5000 units TID)
In conclusion, intraperitoneal adhesions remain a primary concern, not only as a risk factor for laparoscopy (especially abdominal entry) but also as a result of surgery. Many modalities are being studied to reduce this risk, however none can eliminate the risk of adhesion formation. Knowledge of the risk factors, good surgical technique to minimize tissue trauma and utilization of proper instruments and ancillary technology has shown to decrease complications and the severity of adhesion formation.
Suggested Reading
Altuntas I, Tarhan O, Delibas N. Seprafilm reduces adhesions to polypropylene mesh and increases peritoneal hydroxyproline. Amer Surg. 2002;68(9):759-761.
Belluco C, Meggiolaro F, Pressato D, Pavesio A, Bigon E, Dona M, Forlin M, Nitti D, Lise M. Prevention of postsurgical adhesions with an autocrosslinked hyaluronan derivative gel. J Surg Research. 2001;10092):217-221.
Berker B, Hsu S, Nezhat CH, Nezhat F, Nezhat C. Laparoscopic adhesiolysis and adhesion prevention. In: Nezhat C, Nezhat F, Nezhat CH, eds. Nezhat’s Operative Gynecologic Laparoscopy and Hysteroscopy. 3rd edition. New York: Cambridge University Press, 2008: 304-315.
Bertacca S, Bassanino C, Dossena P, Piloni S, Schiro G. Use of “Interceed” in adhesion prevention after laparoscopic myomectomy. In: World Meeting on Minimally Invasive Surgery in Gynecology: Proceedings and Abstract Book, 2nd meeting. Rome: 2003:206.
Brill AI, Nezhat F, Nezhat CH, Nezhat C. The incidence of adhesions after prior laparotomy: a laparoscopic appraisal. Obstet Gynecol. 1995;85(2):269-272.
Buckenmaier CC 3rd, Summers MA, Hetz SP. Effect of the antiadhesive treatments, carboxymethylcellulose combined with recombinant tissue plasminogen activator and Seprafilm, on bowel anastamosis in the rat. Amer Surg. 2000;66(11):1041-1045.
Chan CLK, Wood C. Pelvic adhesiolysis: the assesment of symptom relief by 100 patients. Aust NZ Obstet Gynaecol. 1985;25:295-298.
De Iaco PA, Muzzapapa G, Bigon E, Pressato D, Dona M, Pavesio A, Bovicelli L. Efficacy of a hyaluronan derivative gel in postsurgical adhesion prevention in the presence of inadequate hemostasis. Surg. 2001;130(1):60-64.
Dinsmore RC, Calton WC Jr., Harvey SB, Blaney MW. Prevention of adhesions to polypropylene mesh in a traumatized bowel model. J Amer College Surg. 2000;191(2):131-136.
diZerega GS. Contemporary adhesion prevention. Fertil Steril. 1994;61(2):219-235.
diZerega GS, senior ed. Peritoneal Surgery. New York: Springer-Verlag; 2000.
diZerega GS, Verco SJ, Young P, Kettel M, Kobak W, Martin D, Sanfillippo J, Peers EM, Scrimgeour A, Brown CB. A randomized controlled pilot study of the safety and efficacy of 4% icodextrin in the reduction of adhesions following laparoscopic gynecological surgery. Human Reprod. 2002;17(4):1031-1038.
Dunn R, Lyman MD, Edelman PG, Campbell PK. Evaluation of the SprayGel adhesion barrier in the rat cecum abrasion and rabbit uterine horn adhesion models. Fertil Steril. 2001;75(2): 411-416.
Duran HE, Kuscu E, Zeyneloglu HB, Saygili E, Batioglu S. Lipiodol versus methylene blue for prevention of postsurgical adhesion formation n a rat model. Eur J Obstet Gynecol & Reprod Biology. 2002;102(1):80-82.
Ellis H. The clinical significance of adhesions: focus on intestinal obstruction. Eur J Surg. 1997;577(suppl):5-9.
Falk K, Bjorquist P, Stromqvist M, Holmdahl L. Reduction of experimental adhesion formation by inhibition of plasminogen activator inhibitor type 1. Brit J Surg. 2001;88(2):286-289.
Falk K, Lindman B, Bengmark S, Larsson K, Holmdahl L. Sodium polyacrylate potentiates the anti-adhesion effect of a cellulose-derived polymer. Biomaterials. 2001;22(16):2185-2190.
Farquhar C, Vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Database of Systematic Reviews. 2000;(2):CD000475.
Fayez JA, Clark RR. Operative laparoscopy for the treatment of localized chronic pelvic- abdominal pain caused by postoperative adhesions. J Gynecol Surg. 1994:10:79-83.
Ferland R, Mulani D, Campbell PK. Evaluation of a sprayable polyethylene glycol adhesion barrier in a porcine efficacy model. Human Reprod. 2001;16(12):2718-2723.
Francois Y, Mouret P, Tomagluk, Vignal J. Postoperative adhesive peritoneal disease. Surg Endosc. 1994;8:781-783.
Freys SM, Fuchs KH, Heimbucher J, Thiede A. Laparoscopic adhesiolysis. Surg Endosc. 1994;8:1202-1207.
Goldstein DP, deCholnoky C, Emans SJ, Leventhal JM. Laparoscopy in the diagnosis and management of pelvic pain in adolescents. J Reprod Med. 1980;2(6):251-256.
Gutman JN, Diamond MP. Principles of laparoscopic microsurgery and adhesion prevention. In: Practical Manual of Operative Laparoscopy and Hysteroscopy, 2nd ed. New York: Springer-Verlag; 1997:94-107.
Guvenal T, Cetin A, Ozdemir H, Yanar O, Kaya T. Prevention of postoperative adhesion formation in rat uterine horn model by nimesulid: a selective COX-2 inhibitor. Hum Reprod. 2001;16(8):1732-1735.
Hellebrekers BWJ, Trimbos-Kemper TCM, Trimbos JB, Emeis JJ, Kooistra T. Use of fibrinolytic agents in the prevention of postoperative adhesion formation. Fertil Steril. 2000;74(2):203-211.
Hellebrekers BW, Trimbos-Kemper GC, van Blitterswijk CA, Bakkum EA, Trimbos JB. Effects of five different barrier materials on postsurgical adhesion formation in the rat. Human Reprod. 2000;15(6):1358-1363.
Hendrikx M, Mees U, Hill AC, Egbert B, Coker GT, Estridge TD. Evaluation of a novel synthetic sealant for inhibition of cardiac adhesions and clinical experience in cardiac surgery procedures Heart Surgery Forum 2001;4:204-210.
Jahoda AE, Albala DM, Dries DJ, Kovacs EJ. Fibrin sealant inhibits connective tissue deposition in a murine model of peritoneal adhesion formation. Surg. 1999;125(1):53-59.
Johns A. Evidence-based prevention of post-operative adhesions. Hum Reprod Update. 2001;7(6):577-579.
Johns DB, Keyport GM, Hoehler F, diZerega GS, Intergel Adhesion Prevention Study Group. Reduction of postsurgical adhesions with Intergel adhesion prevention solution: a multicenter study of safety and efficacy after conservative gynecologic surgery. Fertil Steril. 2001;76(3):595-604.
Liakakos T, Thomakos N, Fine PM, Dervenis C, Young RL. Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Digest Surg. 2001;18(4):260-273.
Lunberg WI, Wall JE, Mathers JE. Laparoscopy in evaluation of pelvic pain. Obstet Gynecol. 1973;42(6):872-876.
Lundorff P, Hahlin M, Kallfelt B, Thorburn J, Lindblom B. Adhesion formation after laparoscopic surgery of tubal pregnancy: a randomized trial versus laparotomy. Fertil Steril. 1991;55(5):911-915.
Lundorff P, Thornburn J, Lindblom B. Second-look laparoscopy after ectopic pregnancy. Fertil Steril. 1990;53(4):604-609.
Lundorff P, van Geldorp H, Tronstad SE, Lalos O, Larsson B, Johns DB, diZerega GS. Reduction of post-surgical adhesions with ferric hyaluronate gel: a European study. Hum Reprod. 2001;16(9):1982-1988.
Malinik LR. Operative management of pelvic pain. Clin Obstet Gynecol. 1980;23(1):191- 200.
Mettler L, Audebert A, Lehmann-Willenbrock E, Jacobs VR, Schive K. New adhesion prevention concept in gynecological surgery. JSLS. 2003;7(3):207-209.
Miller EM, Winfield JM. Acute intestinal obstruction secondary to postoperative adhesion. Arch Surg. 1959;8(6):952-957.
Mueller MD, Tschudi J, Herrmann U, Klaiber C. An evaluation of laparoscopic adhesiolysis in patients with chronic abdominal pain. Surg Endosc. 1995;9:802-804.
Muller SA, Treutner KH, Tietze L, Anurov M, Titkova S, Polivoda M, Oettinger AP, Schumpelick V. Influence of intraperitoneal phospholipid dosage on adhesion formation and wound healing at different intervals after surgery. Langenbecks Arch Surg. 2001;386(4):278-284.
Muller SA, Treutner KH, Jorn H, Anurov M, Oettinger AP, Schumpelick V. Phospholipids reduce adhesion formation in the rabbit uterine horm model. Fertil Steril. 2002;77(6):1269-1273.
Nemir P Jr. Intestinal obstruction. Ann Surg. 1952;135:67-375.
Nezhat C, Cho J, Morozov V, Yeung P. Preoperative periumbilical ultrasound-guided saline infusion (PUGSI) as a tool in predicting obliterating subumbilical adhesions in laparoscopy. Fertil Steril 2009;91(6):2714-2719.
Nezhat C, Nezhat F, Ambroze W, et al. Laparoscopic repair of small bowel, colon, and rectal endometriosis: a report of twenty-six cases. Surg Endosc. 1993;7:88-89.
Nezhat CR, Nezhat FR, Metzger DA, Luciano AA. Adhesion reformation after reproductive surgery by videolaparoscopy. Fertil Steril. 1990;53:1008-1011.
Nezhat F, Brill AI, Nezhat CH, et al. Adhesion formation after endoscopic posterior colpotomy. J Reprod Med. 1993;38:534-536.
Nezhat FR, Crystal RA, Nezhat CH, Nezhat CR. Laparoscopic adhesiolysis and relief of chronic pelvic pain. J Society Laparoendoscopic Surg. 2000:4(4):281-285.
Ozgun H, Cevikel MH, Kozaci LD, Sakarya S. Lexipafant inhibits postsurgical adhesion formation. J Surg Research. 2002;103(2):141-145.
Pellicano M, Bramante S, Cirillo D, Palomba S, Bifulco G, Zullo F, Nappi C. Effectiveness of autocrosslinked hyaluronic acid gel after laparoscopic myomectomy in infertile patients: a prospective, randomized, controlled study. Fertil Steril. 2003;80(2):441-444.
Operative Laparoscopy Study Group. Postoperative adhesion development after operative laparoscopy: evaluation at early second-look procedures. Fertil Steril. 1991;55(4):700- 704.
Raf LE. Causes of abdominal adhesions in cases of intestinal obstruction. Acta Chir Scand. 1969;135:73-76.
Rapkin AJ. Adhesion and pelic pain: a retrospective study. Obstet Gynecol. 1986;68(1):13-15.
Rodgers KE, Schwartz HE, Roda N, Thornton M, Kobak W, diZerega GS. Effect of oxiplex films (PEO/CMC) on adhesion formation and reformation in rabbit models and on peritoneal infection in a rat model. Fertil Steril. 2000;73(4):831-838.
Saravelos HG, Li TC, Cooke JD. An analysis of the outcome of microsurgical and laparoscopic adhesiolysis for infertility. Hum Reprod. 1995;10:2888-2894.
Sawada T, Nishizawa H, Nishio E, Kadowaki M. Postoperative adhesion prevention with an oxidized regenerated cellulose adhesion barrier in infertile women. J Reprod Med. 2000;45(5):387-389.
Shepard BB, De Virgilio C, Bleiweis M, Milliken JC, Robertson JM. Inhibition of intra- abdominal adhesions: fibrin glue in a long term model. The Amer Surg. 1993;59(12):786- 790.
Stenchever M, ed. Commentary: Adhesiolysis for chronic abdominal pain is controversial. Acog Clinical Review. 2003;8(6):10-11.
Sulaiman H, Gabella G, Davis MSc C, Mutsaers SE, Boulos P, Laurent GJ, Herrick SE. Presence and distribution of sensory nerve fibers in human peritoneal adhesions. Annals Surg. 2001;234(2):256-261.
Swank DJ, Swank-Bordewijk SC, Hop WC, van Erp WF, Janssen IM, Bonjer HJ, et al. Laparoscopic adhesiolysis in patients with chronic abdominal pain: A blinded randomised controlled multi-center trial. Lancet. 2003;361(9365):1247-1251.
Szabo A, Haj M, Waxsman I, Eitan A. Evaluation of seprafilm and amniotic membrane as adhesion prophylaxis in mesh repair of abdominal wall hernia in rats. Eur Surg Research. 2000;32(2):125-128.
Takeuchi H, Toyonari Y, Mitsuhashi N, Kuwabara Y. Effects of fibrin glue on postsurgical adhesions after uterine or ovarian surgery in rabbits. J Obstet Gynaecol. 1997;23(5):479-484.
Tsapanos VS, Stathopoulou LP, Papathanassopoulou VS, Tzingounis VA. The role of Seprafilm bioresorbable membrane in the prevention and therapy of endometrial synechiae. J Biomedical Materials Research. 2002;63(1):10-14.
Tran HS, Chrzanowski FA Jr., Puc MM, Patel NG, Geldziler B, Malli D, Ramsamooj R, Hewitt CW, DelRossi AJ. An in vivo evaluation of a chondroitin sulfate solution to prevent postoperative intraperitoneal adhesion formation. J Surg Research. 2000;88(2):78-87.
Uberoi R, D-Costa H, Brown C, Dubbins P. Visceral slide for intraperitoneal adhesions: a prospective study in 48 patients with surgical correlation. J Clin Ultrasound. 1995;23:363-366.
Verco SJ, Peers EM, Brown CB, Rodgers WE, Roda N, diZerega G. Development of a novel glucose polymer solution (icodextrin) for adhesion prevention: pre-clinical studies. Human Reprod. 2000;15(8):1764-1772.
Vrijland WW, Tseng LN, Eijkman HJ, Hop WC, Jakimowicz JJ, Leguit P, Stassen LP, Swank DJ, Haverlag R, Bonjer HJ, Jeekel H. Fewer intraperitoneal adhesions with use of hyaluronic acid-carboxymethylcellulose membrane: a randomized clinical trial. Annals Surg. 2002;235(2):193-199.
Vlahos A, Yu P, Lucas CE, Ledgerwood AM. Effect of a composite membrane of chitosan and poloxamer gel on postoperative adhesive interactions. Amer Surg. 2001;67(1):15-21.
Watson A, Vandekerckhove P, Lilford R. Liquid and fluid agents for preventing adhesions after surgery for subfertility. Update in Cochrane Database Syst Rev. 2000;(3):CD001298.
Wheeless CR. Gastrointestinal injuries associated with laparoscopy. In Phillips JM, ed. Endoscopy in Gynecology. California: AAGL; 1978:317.
Zhang YD, Yao W, Wu CX, Chi QM, Zhang JY, Li M. Topical application of halcinonide cream reduces the severity and incidence of intraperitoneal adhesions in a rat model. Amer J Surg. 2002;184(1):74-77.
Zupi E, Piredda A, Marconi D, Exacoustos C, Sorrenti G, Zumpano A, Szabolcs B, Valli E. Initial feasibility study of a hydrogel adhesion barrier system in patients treated by operative hysteroscopy for intrauterine benign pathologies. In: World Meeting on Minimally Invasive Surgery in Gynecology: Proceedings and Abstract Book, 2nd meeting. Rome: 2003:202-203.
