Laparoscopic Treatment of Chronic Pelvic Pain: Presacral Neurectomy
Odysseas Ath. Savvouras, Arathi Veeraswamy, James E. Carter.
Presacral neurectomy is the interruption of the superior hypogastric plexus, defined by Dr. M.G. Cotte in 1938. Tjaden used the surgical technique first described in 1899 by Jaboulay and Ruggi.3,4 Which was described as complete removal of the nerve tissue within the interiliac triangle, now known as the triangle of Cotte. Laparoscopic techniques for presacral neurectomy have been described by Perez,6 Biggerstaff,7 Carter,8 Chen,9 and the first series of laparoscopic presacral neurectomy described from Dr.
Nezhat et al in 1992.10 Kwok reviewed laparoscopic pre-sacral neurectomy and concluded that patients for whom this operation is recommended should be carefully selected.11 They should have midline dysmenorrhea as the main symptom and should have failed or not tolerated medical therapy. Presacral neurectomy is useful in the treatment of severe, disabling dysmenorrhea secondary to endometriosis and pelvic pain associated with pelvic inflammatory disease.1 The efficacy of presacral neurectomy for the relief of midline dysmenorrhea was confirmed by a randomized study performed at the Johns Hopkins University School of Medicine.2 Black estimated a 75-80% success in 9937 cases of pre-sacral neurectomy.5 Presacral neurectomy has been shown to have long-run effectiveness for the treatment of severe dysmenorrhea due to endometriosis.12 As has been pointed out by Stones and Jacobson, a percentage of women with chronic pelvic pain and/or dysmenorrhea do not respond or respond poorly to medical treatment.13,14 (25% of dysmenorrheic patients reporting no improvement with nonsteroidal anti-inflammatory drugs.)3 Surgery may represent the final therapeutic option for these patients. In a prospective double-blind randomized, controlled study, Zullo et al. demonstrated the effectiveness of presacral neurectomy for women with severe dysmenorrhea due to endometriosis who had been treated with conservative laparoscopic surgical intervention.15 The authors continued to follow their patients for an additional year and published on the 2-year success of this procedure. They found a significant reduction in the frequency and severity of dysmenorrhea, dyspareunia, and chronic pelvic pain observed 24 months after surgery. The addition of presacral neurectomy was also associated with significant improvement in quality of life. In the conclusion to the study, Zullo et al. stated, “We demonstrate that presacral neurectomy is a safe and useful surgical procedure to improve the cure rate and the quality of life in patients with severe dysmenorrhea treated with laparoscopic conservative surgery based on a long-term followup of two years, but chronic constipation and/or urinary urgency may be consequences of this therapy.”12
ANATOMY
The autonomic plexuses comprise named parts: aortic, superior mesenteric, inferior mesenteric, superior hypogastric, and inferior hypogastric (hypogastric nerves).31
The presacral nerve is a plexus of nerves known as the superior hypogastric plexus. Kwok11 elegantly summarized the anatomy of the pelvic autonomic nerves as originally described by Curtis16:
The lumbar and lower thoracic sympathetic ganglia, and the superior, middle, and inferior hypogastric plexus provide the afferent pathways for the pelvic viscera.
However, an exception to this is the pain afferent fibers from the ovaries and distal fallopian tubes, which travel to the ovarian plexus, and then via the infundibulopelvic ligaments to the aortic and renal plexuses. The sigmoid colon sends visceral afferents to the inferior mesenteric plexus. Interruption of the presacral plexus will affect a decrease in central pain perception and perhaps also a change in the function of the sigmoid colon. Pain afferents from the uterus and cervix and proximal part of the fallopian tubes travel with the sympathetic nerves and travel via the uterosacral and cardinal ligaments to join with the pelvic plexus. (Frankenhauser’s ganglion, uterovaginal ganglion).
The fibers from the pelvic plexus course proximally to become the inferior, the middle hypogastric plexus over the sacral promontory, and then the superior hypogastric plexus. The ‘presacral nerve’, the common name for the superior hypogastric plexus, is a misnomer because it is actually pre-lumbar in position and lies in front of the fifth lumbar vertebrae. In addition, it is usually not a nerve but rather a nerve plexus. It is a single trunk in only approximately 20% of the anatomical sections.11
The presacral nerve is a direct extension of the aortic plexus below the aortic bifurcation. This plexus spreads out behind the peritoneum in the loose areolar tissue lying over the fourth and fifth lumbar vertebrae. Between the vertebrae and the presacral nerve lies the middle sacral artery, which may be traumatized during surgical dissection. In a series of 30 cadaveric dissections, Curtis et al. reported 75% of the time the superior and middle hypogastric plexus lie on the left, 25% in the midline, and none on the right.16 On the right of the presacral nerve, lie the right ureter and common iliac vein and artery. On the left lie the sigmoid colon, inferior mesenteric vessels, and the left ureter. The left ureter is seen less commonly in surgical dissections because it is obscured by the sigmoid colon.11
Pain impulses from the cervix, the body of the uterus, and the proximal fallopian tube are transmitted through afferent fibers that accompany sympathetic nerves into the spinal cord at the thoracic and lumbar levels. The sympathetic nerves that emerge from the uterus pass through the uterosacral ligament along the cardinal ligament and join the pelvic plexus. Parasympathetic fibers from S1 through S4 travel with the phrenic nerve through the pelvic plexuses (Frankenh¨auser ganglia) lateral to the cervix to reach the bladder, rectum, and uterus.
When performing presacral neurectomy, the surgeon will encounter variable anatomic findings. For this reason, the nerve-bearing tissue, especially on the left, should be thoroughly exposed when performing the procedure.17 In 8% to 15% of dissections, the mesocolon was over the area of the presacral nerve, making neurectomy difficult or impossible.17 Labate found a single nerve in 8% to 13% of dissections.18 In 75 dissections, he found a plexus in 84% of the cases, parallel nerve trunks in 8%, and single nerves in 8%.
Within the interiliac trigone, the common iliac artery and ureter are on the right and the common iliac vein is on the left. The inferior mesenteric, superior hemorrhoidal, and midsacral arteries are in the center of the prelumbar space. This trigone is defined caudally by the sacral promontory and laterally by the common iliac arteries. The superior edge of the triangle is delineated by the aortic bifurcation. Centrally and to the left, multiple nerve fibers, sometimes in bundles, run caudally from the aortic plexus above and through the interiliac trigone to form the superior hypogastric plexus. These fibers, representing the presacral nerve, are buried in loose areolar tissue. They display no particular patterns and vary among individuals. Both ureters, which lie to the right and left of the trigone, are identified before transection of the nerve bundle continues. The left ureter is more difficult to see because it lies underneath the rectosigmoid and mesocolon.
INDICATIONS
Dysmenorrhea can be a severe and debilitating symptom in many women. Although most women may find adequate relief of symptoms from pharmacological approaches, there remain a few with resistant pain.11 The presacral neurectomy is indicated for patients who have disabling midline dysmenorrhea and pelvic pain and have not responded to appropriate and adequate medication. Presacral neurectomy, although technically challenging, may be offered after other approaches are unsuccessful. The operation is likely to relieve pain in 50% to 75% of patients. The operation is now performed increasingly by laparoscopic and recently by robotic approach. When associated with complete resection of endometriosis, cure rates are improved.
Presacral neurectomy does not alleviate adnexal pain because ovarian innervation originates from the ovarian plexus, a meshwork of nerve fibers that arise from the aortic and renal plexuses and accompany the ovarian artery throughout its course.
TECHNIQUE
Trendelenburg position is used and the patient is tilted slightly to the left. The aortic bifurcation, common iliac arteries and veins, ureters, and sacral promontory are identified. The peritoneum overlying the promontory is elevated with grasping forceps, and a small opening is made with the CO2 laser, scissors, or other cutting modality (Figure 1).
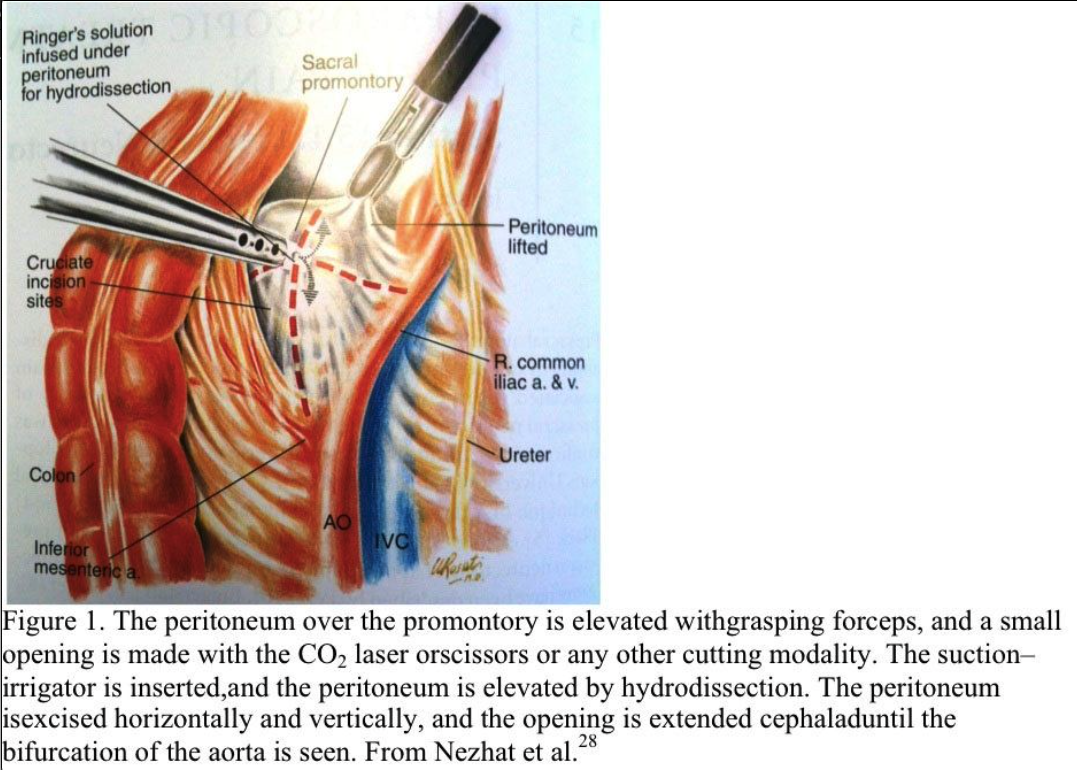
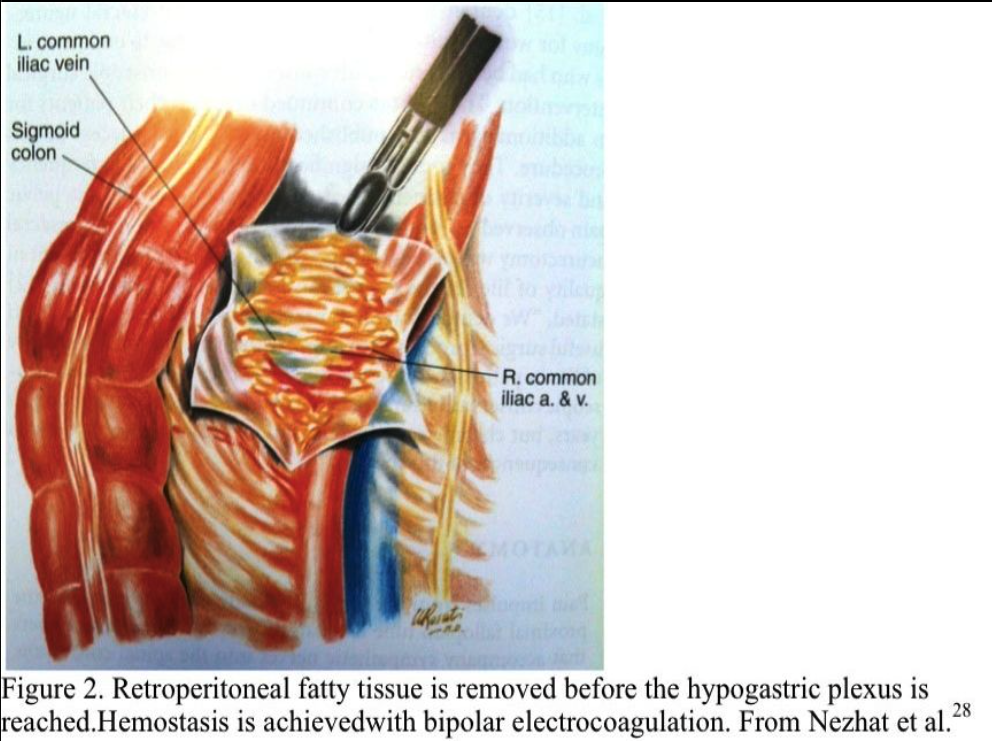
The suction–irrigator is inserted through this opening, and the peritoneum is elevated by hydrodissection. The peritoneum is incised horizontally and vertically, and the opening is extended cephalad to the aortic bifurcation (Figure 2). Bleeding from the peritoneal vessels is controlled with the bipolar electrocoagulator. Retroperitoneal fatty tissue is removed before the hypogastric plexus is reached. The mesocolon does not cover the sacral promontory in most patients. If the mesocolon covers the sacral promontory, the procedure is more difficult, and the surgeon must avoid injuring the inferior mesenteric artery and its branches. Hemostasis is obtained with bipolar electrocoagulation.
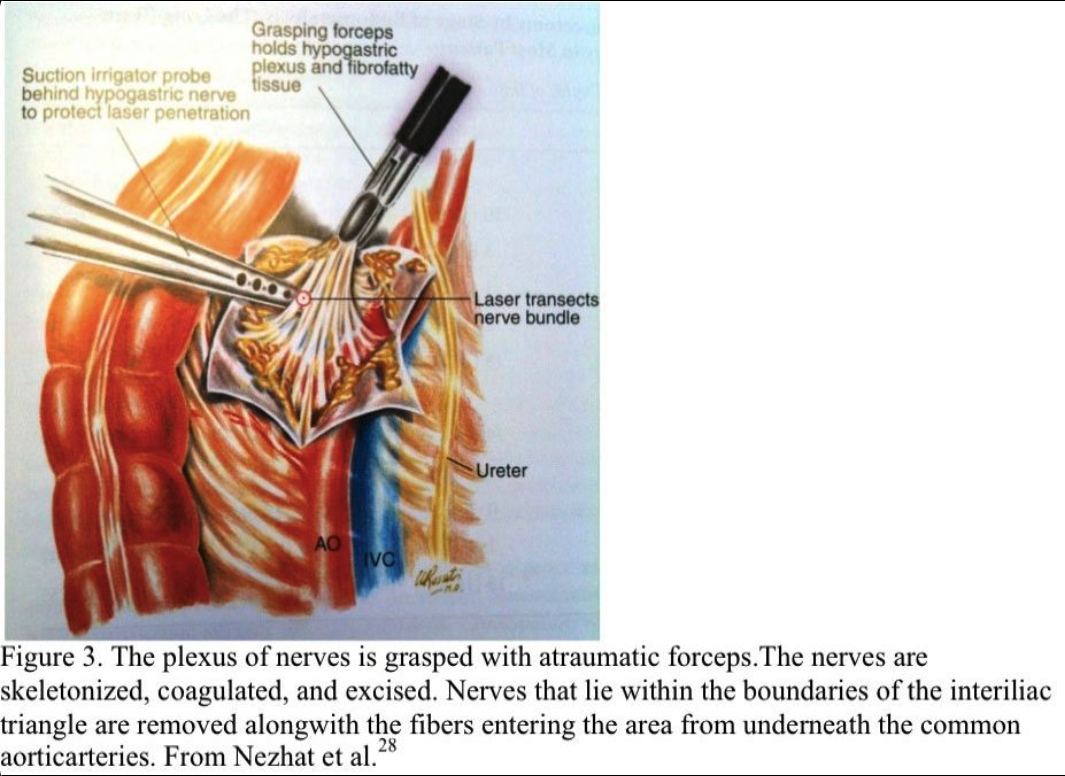
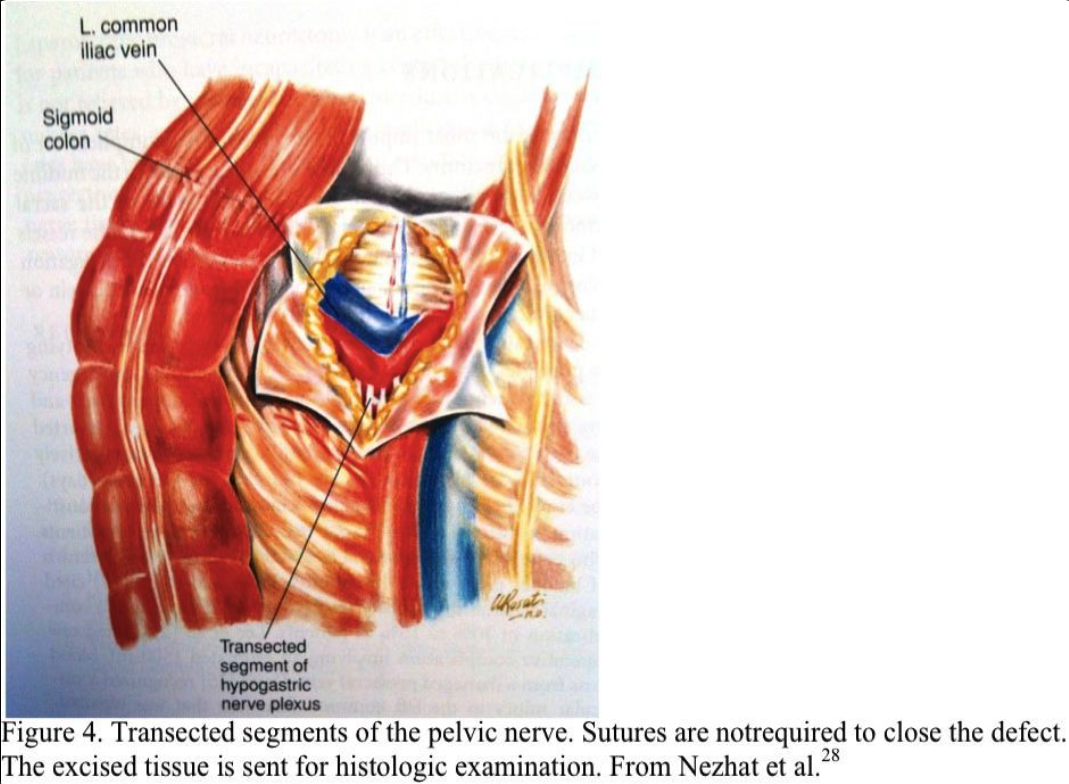
The nerve plexus is grasped with an atraumatic forceps. Using blunt and sharp dissection, the nerve fibers are skeletonized, coagulated, and excised (Figure 3). All the nerves that lie within the boundaries of the interiliac triangle are removed, including any fibers entering the area from under the common iliac arteries (Figure 4). The retroperitoneal space is irrigated, and bleeding points are coagulated. Sutures are not required. The excised tissue is sent for histologic confirmation of nerve removal. At second-look laparoscopy, the presacral area should appear healed. Usually, no small bowel is attached to this area. If a mesocolon detachment is required at the initial procedure, the mesocolon usually reattaches itself to the presacral area.
The da Vinci Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA) is used for the robotic portion of the procedure. Availability of the robot is the sole determining factor for the procedure chosen.30
RESULTS
Jedrzejczak P et. Al. in 2009 reported dysmenorrhoea decreased at 3 months by 75% in those without endometriosis and by 78% in those with endometriosis. At 12-months, dysmenorrhea increased in women with endometriosis, but not in those without endometriosis. Pelvic pain not related to menses decreased by 67% and by 87% , respectively, in women with and without endometriosis. Dyspareunia, declined dramatically at 3 and 12 months to a median score of 0.32 Cotte reported favorable results with presacral neurectomy in 1500 selected patients with only a 2% failure rate.19 Meigs reported an 85% relief rate.20 In a review of 2516 patients, Black noted 70% of the patients experienced reliefs, 19% were improved, and 11% were unimproved.5 Polan and DeCherney reported in 1980 that 14 of 20 patients (70%) were relieved of pain after pre- sacral neurectomy.1 In the control group, 14 of 54 (26%) showed significant pain relief.
Lee and colleagues reported a 74% success rate, with 14% experiencing a partial cure.21 There was a 12% failure rate. Perez studied 25 patients and concluded that 96% experienced pain relief.6 The mean preoperative score for patients in the study was 8.5 (on a scale of 0 to 10, with 0 being no pain and 10 being the worst pain), whereas the postoperative mean score was 2.2. In a randomized prospective study on the efficacy of presacral neurectomy initiated by Tjaden and colleagues, 17 of the 26 patients had a presacral neurectomy.2 Fifteen of the 17 (88%) noted relief, whereas two (12%) had no improvement. Pain persisted in all nine of the patients whodid not undergo presacral neurectomy. In 1992, Nezhat and Nezhat described a simplified method of presacral neurectomy in one of the earliest reports on the laparoscopic approach.22 The authors performed laparoscopic presacral neurectomy in 52 patients with dysmenorrhea unresponsive to medical treatment. The severity of endometriosis varied among the patients (31 had minimal, 13 had mild, five had moderate, and three had severe endometriosis). Forty-eight of the 52 patients (92.3%) reported relief of dysmenorrhea, including 27 (51.2%) who reported complete pain relief. Of the 27 patients reporting complete pain relief, 16 (59%) had minimal, six (22%) had mild, three (11%) had moderate, and two (8%) had severe endometriosis. Carter reported on presacral neurectomy in 20 patients with follow-up of up to 18 months.8 The pain level in these women decreased from an average of 9.4 to 2.0 (on a scale of 0 to 10, with 0 being no pain and 10 the worst).
Chen and coworkers reported on presacral neurectomy in 67 patients with primary dysmenorrhea who had a poor response to medical treatment.23 The patients were divided into two groups, with 33 undergoing laparoscopic presacral neurectomy and 34 undergoing LUNA. The efficacy of the two procedures was identical after 3 months, but after 12 months, laparoscopic presacral neurectomy was significantly more effective than LUNA.
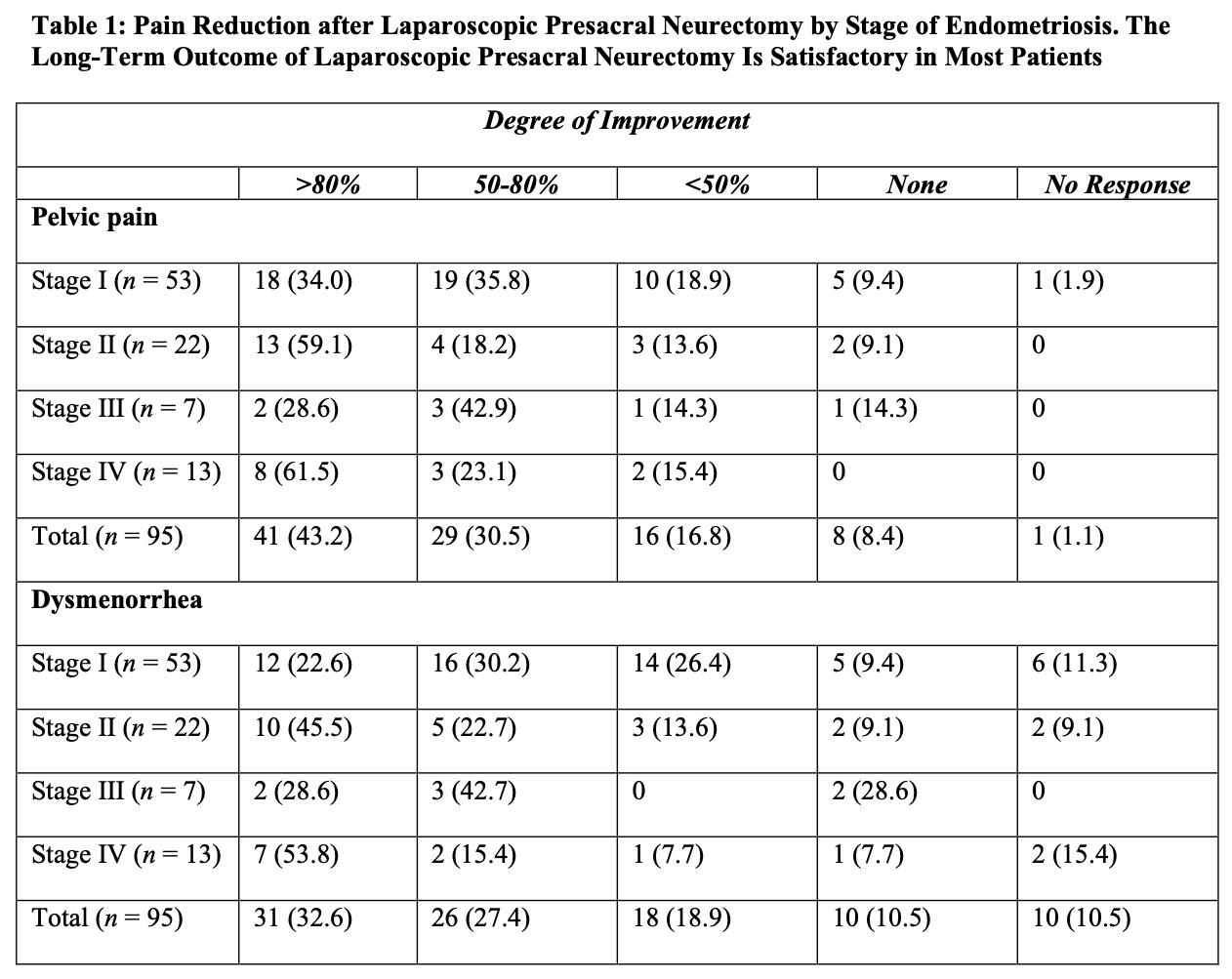
The authors concluded that presacral neurectomy was preferable to uterine nerve ablation for long-term relief of primary dysmenorrhea. In a retrospective review of 655 patients who had laparoscopic conservative surgery and laparoscopic presacral neurectomy, Chen and Soong found that 527 (80%) reported significant alleviation of pain.9 Cure was achieved in 22 (52%) of the 42 patients with adenomyosis, 75 (73%) of the 103 patients with moderate to severe endometriosis with dysmenorrhea, 123 (75%) of the 164 patients with minimal to mild endometriosis with dysmenorrhea, 64 (77%) of the 83 patients with primary dysmenorrhea, and 84 (62%) of the 135 patients with chronic pelvic pain. Nezhat et al. evaluated long-term outcomes of laparoscopic presacral neurectomy in 176 women who underwent presacral neurectomy and treatment of endometriosis.10 More than 50% alleviation of pain was reported in 69.8% of the women with stage I endometriosis (using the revised classification of the American Fertility Society), 77.3% of those with stage II, 71.4% with stage III, and 84.6% with stage IV (Table 1). The authors concluded that long-term outcome of laparoscopic presacral neurectomy is satisfactory in most patients, and the stage of endometriosis is not related directly to the degree of pain improvement achieved.
Zullo reported on a 2-year study of presacral neurectomy for the treatment of severe dysmenorrhea due to endometriosis.12 The frequency and severity of dysmenorrhea, dyspareunia, and chronic pelvic pain, and quality of life were evaluated at entry and 24 months postoperatively. At follow-up visit, the 83.3% cure rate (P. 0.05) was significantly higher in the group with laparoscopic surgery and presacral neurectomy than the 53.3% cure rate in the group with only conservative laparoscopic surgical intervention. The frequency and severity of dysmenorrhea, dyspareunia, and chronic pelvic pain were significantly lower in both groups compared with baseline values (P. 0.05), and only severity was significantly lower in the group with presacral neurectomy and endometriosis surgery (P. 0.05). A significant improvement in quality of life was observed after surgery in both groups (P. 0.05) and was significantly better in the presacral neurectomy group (P. 0.05) compared with the conservative surgery–only group. Zullo concluded that presacral neurectomy improves long-term cure rates and quality of life in women treated with conservative laparoscopic surgery for severe dysmenorrhea due to endometriosis.
COMPLICATIONS
Bleeding is the most important intraoperative complication of presacral neurectomy. The middle sacral vessels are in the midline between the presacral nerve and the periosteum of the sacral promontory. Usually, the nerve is dissected anterior to the vessels and ligation is not necessary. Hemostasis is obtained by ligation or coagulation. However, an injury to the common iliac vein or vena cava may require an immediate laparotomy.
Ureteral injury, urinary urgency, and poor bladder emptying are potential complications. Meigs noted urinary urgency in some patients that persisted for 7 years postoperatively and persistent constipation in 32% of the patients.20 Black reported the need for catheterization in 13 of 26 patients postoperatively (four for 1 day, six for 2 days, and one each for 3, 5, and 6 days).5 Lee et al. noted bladder problems and urgency and constipation problems in 4% of 50 patients.21 Eight of 45 patients (18%) who benefited from presacral neurectomy initially had a return of bladder pain within 19 months. Jones and Rock cited vaginal dryness that usually resolved within 6 months as a complication in 10% to 15% of patients.24 Lee et al. noted one operative complication involving an estimated 1500-mL blood loss from a damaged presacral vein.21 Davis recognized a vascular injury to the left common iliac vein that was repaired.25 Cotte reported one incidence of damage to the left ureter among 1500 operations and noted postoperative bleeding in four other patients.19 Two required a second operation and repair of the posterior peritoneum. The other two cases, which involved sub-peritoneal blood infiltrating the posterior rectal areas, resolved spontaneously. Chen and coworkers reported four cases of chylous ascites after laparoscopic presacral neurectomy.26 This rare complication is caused by intraoperative injury to the retroperitoneal lymphatic plexus.
Of the four injuries, two were treated successfully with bipolar cauterization. One was managed by compression with Gelfoam (Pharmacia & Upjohn Inc., Peapack, NJ), and closure of the peritoneum was achieved by laparoscopic suturing. The fourth patient had persistent chylous leakage from the drainage tube. This complication was resolved by conservative management, removal of the drainage tube, and a low-fat diet. Yen reported postlaparoscopic vulvar edema in two cases after laparoscopic presacral neurectomy.27 This was associated with chyloperitoneum. Both cases were managed expectantly. Yen now closes the presacral neurectomy wound with bipolar coagulation to seal the cutting edge and the cannula wound with precise and layer-by-layer repair, and no further cases of chyloperitoneum and vulvar edema have occurred in 2 years since that modification was introduced.
Zullo reported constipation and urinary urgency as a complication of presacral neurectomy performed concomitantly with laparoscopic surgery for endometriosis.15 Constipation was reported in 21 (3.3%) and nine patients (14.3%) at 6-and 12-month follow-up, respectively. In 15 of 21 cases (71.4%), constipation was treated successfully with medical therapies. At the 6and 12-month follow-up visits, urinary urgency was observed in three patients (4.8%).
Novel techniques
The Robotic surgery has the potential to enable a laparoscopic approach for procedures performed by laparotomy due to the technical difficulties intrinsic to laparoscopy.
Advocates of robotic-assisted gynecologic surgery revere the system’s wristed instrumentation, ergonomic positioning, and three-dimensional high-definition vision system as significant improvements over laparoscopic equipment’s four degrees of freedom (DOF) and two-dimensional laparoscope that demand, the surgeon stand throughout a procedure. With improvements in operative times, decreased blood loss, and decrease in length of stay after surgery, the computer-enhanced technology (robot) has enabled surgeons to perform procedures laparoscopically formerly performed by laparotomy, while providing patients the benefits of minimally invasive surgery. The cost, lack of haptic feedback, and the bulky size of the equipment make robotics less attractive to others.33,34
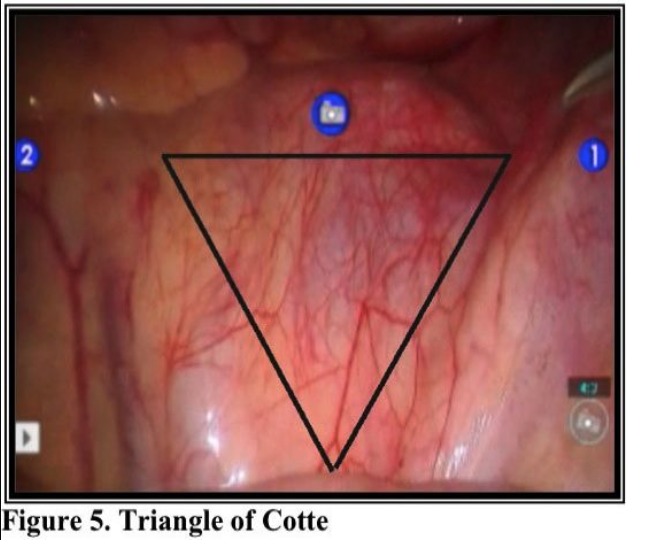
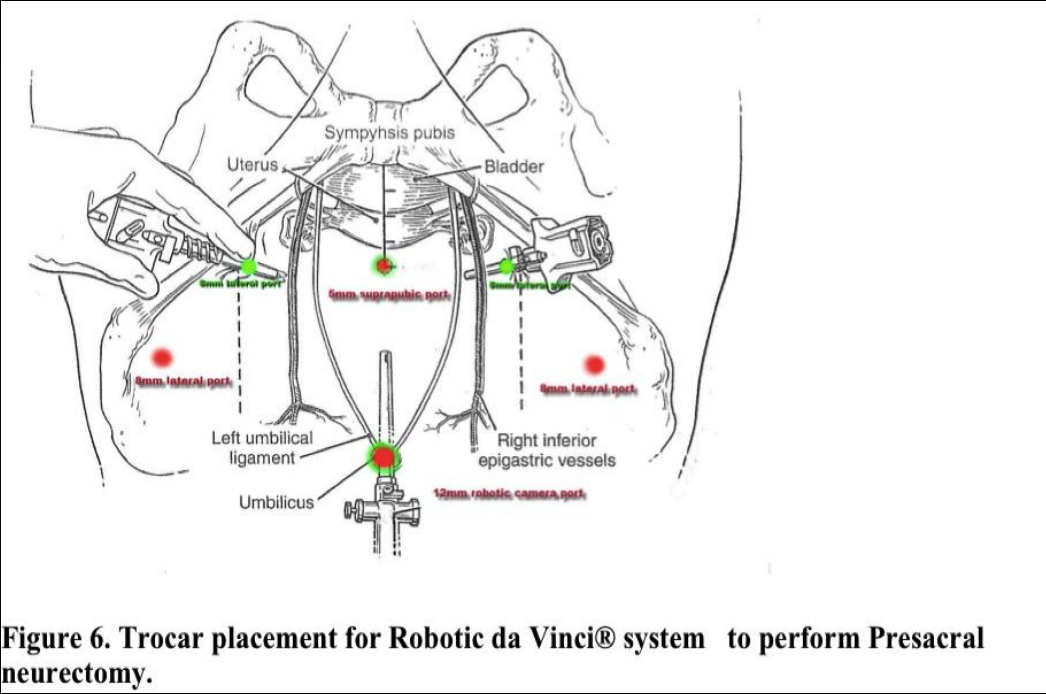
Laparoscopic presacral neurectomy (LPSN), despite its ability to provide magnification and excellent exposure of surgical field, has yielded variable results. Attention to details of anatomy, combined with the proper technique, are essential to successful outcomes. Due to the dangerous location of the superior hypogastric nerves, multiple modifications of the techniques have contributed to inconsistent surgical outcomes.35 Mechanics of the robotic arms require higher port placement and larger diameter cannula. One 12mm cannula was introduced intra-umbilically and two 8mm cannulas were placed laterally, 2- 3 cm above the level of anterior superior iliac crest (Figure 6). Precise™ or Maryland bipolar grasper on the left and monopolar Hot Shears™ on the right.5mm suprapubic port for introduction of suction-irrigator and tissue grasper. Multifunctional devices as PreciseTM forceps, may offset the high cost when used as both forceps and bipolar instrument. The inter-iliac hypogastric nervous plexus are completely excised by stripping the interiliac triangle from all the lymphatic, aereolar, and nervous tissue (Figure 5).
CONCLUSION
Laparoscopic presacral neurectomy is an effective, safe operation for patients who have incapacitating central dysmenorrhea that is not relieved by medication. The procedure is empiric because success rates are not predictable. Complications and mortality rates have been minimal. Dr. Nezhat C. noted in an article in 2010 that robot-assisted laparoscopic presacral neurectomy is feasible and safe, without added risk of short- or long-term complications. It compares favorably to the conventional laparoscopic approach of presacral neurectomy. The surgical robot provides a better angle and 3-dimensional visualization of the operating field, similar to laparotomy, and supplemented with magnification. This combined with elimination of hand tremor enables better surgeon control.30
Poor patient selection and incomplete neurectomy due to neurologic variability or failure to remove all nerve tissue within the interiliac trigone are the most common reasons for poor results.
References
- Polan M, DeCherney A. Presacral neurectomy for pelvic pain in infertility. Fertil Steril. 1980; 34:557–560.
- Tjaden B, Schlaff WD, Kimball A, Rock JA. The efficacy of presacral neurectomy for the relief of midline dysmenorrhea. Obstet Gynecol. 1990;76:89–91.
- Jaboulay M. Le traitment de la neuralgie pelvienne par la paralysie du sympathetique sacre. Lyon Med.1899;90:102.
- Ruggi T. Della sympathectamia al collo ed ale avome. Policlinico. 1899;1:193.
- Black WT. Use of presacral sympathectomy in the treatment of dysmenorrhea. Am J Obstet Gynecol. 1964;89:16–22.
- Perez JJ. Laparoscopic presacral neurectomy. Results of the first 25 cases. JReprodMed. 1990;35:625–630.
- Biggerstaff ED 3rd, Foster SN. Laparoscopic presacral neurectomy for treatment of midline pelvic pain. J Am Assoc Gynecol Laparosc. 1994;2:31–35.
- Carter JE. Laparoscopic presacral neurectomy utilizing contact-tip Nd: YAG laser. KeioJMed. 1996;45:332–335.
- Chen FP, Soong YK. The efficacy and complications of laparoscopic presacral neurectomy in pelvic pain. Obstet Gynecol. 1998; 91:701–704.
- Nezhat CR, Nezhat FR, Luciano AA, et al. Uterine Surgery in Operative Gynecologic Laparoscopy: Principles and Techniques.New York: McGraw-Hill; 1995.
- Kwok A, Lam A, Ford R. Laparoscopic presacral neurectomy: a review. Obstet Gynecol Surv. 2001;56:99–104.
- Zullo F, Palomba S, Zupi E, et al. Long-term effectiveness of presacral neurectomy for the treatment of severe dysmenorrhea due to endometriosis. J Am Assoc Gynecol Laparosc. 2004, 11:23–28.
- Stones RW, Mountfield J. Interventions for treating chronic pelvic pain in women. Cochrane Database Syst Rev. 2000:CD000387.
- Jacobson TZ, Barlow DH, Garry R, et al. Laparoscopic surgery for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2001:CD001300.
- Zullo F, Palomba S, Zupi E, et al. Effectiveness of presacral neurectomy in women with severe dysmenorrhea caused by endometriosis who were treated with laparoscopic conservative surgery: a 1-year prospective randomized double-blind study. Am J Obstet Gynecol. 2003, 189:5–10.
- Curtis AH, Anson BJ, Ashley FL, Jones T. The anatomy of the pelvic autonomic nerves in relation to gynecology. Surg Gynecol Obstet. 1942;75:743.
- Rosenshein NB, Rock JA. Surgery in the Retroperitoneal Space. Philadelphia: JB Lippincott; 1988:31–41.
- Labate JS. The surgical anatomy of the superior hypogastric plexus“presacral nerve.” Surg Gynecol Obstet. 1938;67:199.
- Cotte MG. Technique of presacral neurectomy. Am J Surg. 1949;78:50.
- Meigs JV. Excision of the superior hypogastric plexus (presacral nerve) for primary dysmenorrhea. Surg Gynecol Obstet. 1939;68:723.
- Lee RB, Stone K, Magelssen D, et al. Presacral neurectomy for chronic pelvic pain. Obstet Gynecol. 1986;68:517.
- Nezhat C, Nezhat F. A simplified method of laparoscopic pre-sacral neurectomy for the treatment of central pelvic pain due to endometriosis. Br J Obstet Gynecol. 1992;99:659.
- Chen FP, Chang SD, Chu KK, et al. Comparison of laparoscopic presacral neurectomy and laparoscopic uterine nerve ablation for primary JReprodMed. 1996;41:463.
- Jones HW, Rock JA. Reparative and Constructive Surgery of the Female Generative Tract. Baltimore: Williams & Wilkins; 1983.
- Davis AA. The technique of resection of the presacral nerve (Cotte’s operation). Br J Surg. 1933;20:516.
- Chen FP, Lo TS, Soong YK. Management of chylous ascites following laparoscopic presacral neurectomy. Hum Reprod. 1998;13: 880.
- Yen CF, Wang CJ, Lin SL, Lee CL, Soong YK. Post-laparoscopic vulvar edema, a rare complication. J Am Assoc Gynecol Laparosc. 2003;10:123–126.
- Nezhat C, Siegler A, Nezhat F, Nezhat C, Seidman D, Luciano A. Operative Gynecologic Laparoscopy. Principles and Techniques. 2nd Edition. New York: McGraw- Hill; 2000.
- Jedrzejczak P, Sokalska A, Spaczyński RZ, Duleba AJ, Pawelczyk L. Effects of presacral neurectomy on pelvic pain in women with and without endometriosis. Ginekol Pol. 2009 Mar;80(3):172-8.
- Nezhat C, Morozov V. Robot-Assisted Laparoscopic Presacral Neurectomy: Feasibility, Techniques, and Operative Outcomes. J Minim Invasive Gynecol. 2010 Jul;17(4):508-512.
- Mirilas P, Skandalakis JE. Surgical anatomy of the retroperitoneal spaces, Part IV: retroperitoneal nerves. Am Surg. 2010 Mar;76(3):253-62.
- Jedrzejczak P, Sokalska A, Spaczyński RZ, Duleba AJ, Pawelczyk L. Effects of presacral neurectomy on pelvic pain in women with and without endometriosis. Ginekol Pol. 2009 Mar;80(3):172-8.
- Nezhat C, Lavie O, Lemyre M, Unal E, Nezhat CH, Nezhat F. Robot-assisted laparoscopic surgery in gynecology: scientific dream or reality? Fertil Steril. 2009 Jun;91(6):2620-2.
- Nezhat C, Saberi NS, Shahmohamady B, Nezhat F. Robotic-assisted laparoscopy in gynecological surgery. JSLS. 2006 Jul-Sep;10(3):317-20.
- Chang Y, Tsai E, Long C, Lin W. A modified method of laparoscopic presacral neurectomy for the treatment of midline dysmenorrheal. J Min Invas Gynecol. 2006;13:211-215.
