Surgical Management of Polycystic Ovarian Syndrome
Sumathi Kotikela, MD, Michelle Tham, Alan B. Copperman, Lin Lin, MD, Michal Sellin
Polycystic ovarian syndrome (PCOS) is the most common manifestation of hormonal dysfunction in reproductive-age women today. PCOS is a heterogeneous condition, both clinically and biochemically. The prevalence of PCOS is reported to be anywhere from 4% to 12%, with mild racial variations.1 It is a complex disorder affecting multiple organ systems whose historical roots lie in Stein and Leventhal’s 1935 case reports2 describing seven women with a constellation of clinical symptoms, including amenorrhea, infertility, obesity, hirsutism and polycystic-appearing ovaries. What initially may have been considered primarily a reproductive disorder has since evolved into a disease entity that we now understand profoundly affects the cardiovascular, metabolic and endocrine systems.
CLINICAL FEATURES
The typical clinical manifestations of PCOS occurring at puberty and adolescent age include irregular menses, particularly oligomenorrhea, increased LH levels, signs of androgen excess, such as hirsutism or acne.
However, they also exhibit hyperinsulinemia, obesity, hypertension, dyslipidemia, and an increased prothrombotic state. They have an increased risk of type 2 diabetes and impaired glucose tolerance, infertility and sleep apnea.
Information in a young or adult woman suspected to have PCOS should be obtained: age of menarche, presence of symptoms of ovulation or of premenstrual symptoms (ovulatory pain, premenstrual discomfort, and breast tenderness), previous pregnancies or abortion and particularly oral contraceptive (OC) use. Most young women, in fact, have a history of long-term OC use, which may have masked or delayed the recognition of menstrual dysfunction or hyperandrogenic symptoms. In women presenting while taking OC, blood testing or pelvic ultrasounds should not be performed until they have discontinued OC use for at least 3 months.
The menstrual irregularity of PCOS typically manifests in the peripubertal period, although some women may apparently have regular cycles at first and subsequently develop menstrual irregularity in association with weight gain. Menstrual irregularities include mild or severe oligomenorrhea or amenorrhea. Anovulation is very common in the presence of mild oligomenorrhea, but also when normal cycles are present. Some cycles may be associated with dysfunctional bleeding. Endometrial atrophy may be present in some women with PCOS who have prolonged amenorrhea, which may be related to androgen excess.
Chronic anovulation is one of the most important criteria in the diagnosis of PCOS. However, occasional ovulation may occur, particularly in women with less severe oligomenorrhea. Ovulation can be easily detected by measuring progesterone levels in the luteal phase, at approximately days 20 to 22 after cycle onset. Appropriate hormone levels suggesting an adequate luteal phase are 6ng/mL to 8ng/mL.
Hyperandrogenic signs and symptoms are the hallmark characteristic of PCOS. Most women with PCOS have clinical evidence of hyperandrogenism, which includes hirsutism, acne, oily skin, and sometimes, male pattern balding or alopecia. Rarely, virilizing symptoms may be present, such as increased muscle mass, deepening of the voice, or clitoromegaly. In general, hirsutism is the most representative sign of clinical hyperandrogenism. It is defined as excess terminal (thick, pigmented) body hair in a male distribution, which usually starts during pubertal development or right after. Typical areas of androgen-dependent terminal hair are the face (particularly upper lip and chin); around the nipples and the breast area; and the abdomen, along the linea alba. Acne is typically the first manifestation of hyperandrogenism after menarche. The typical acne lesions vary in increasing order of severity, which are highly dependent on previous topical, systemic, and cosmetic treatments. Terminal hair growth is age dependent, and it may not be apparent until the early twenties after several years of exposure to excess androgens.
Hyperinsulinemia directly stimulates both ovarian and adrenal androgen secretion and suppresses liver sex hormone-binding globulin (SHBG) synthesis, resulting in an increase in free, biologically active androgens. This excess in local ovarian androgen production, augmented by hyperinsulinemia, causes premature follicular atresia and anovulation along with the other clinical manifestations of hyperandrogenism, such as hirsutism and acne.3,4
The prevalence of risk factors for CVD (obesity, diabetes, IR, hypertension, dyslipidemia, increased serum plasminogen activator inhibitor levels, and increased carotid artery intima-media thickness on ultrasound) is increased in PCOS.
Chronic anovulation and resultant lack of menses may lead to endometrial hyperplasia and the increase the risk for endometrial cancer through unopposed estrogen exposure. Studies have shown that PCOS women may have an increased risk of obstructive sleep apnea syndrome (OSAS), diagnosed either by questionnaire or by overnight polysomnography. This sleeping disorder is much more common in the presence of obesity. Thus, women with PCOS should be questioned about signs and symptoms of OSAS. Such symptoms include habitual snoring, nocturnal restlessness, and daytime sleepiness.5
PCOS is a common cause of counseling in infertility clinics. The primary cause is chronically irregular ovulation, leading to a reduced number of ovulations and unpredictable timing. An increased rate of early pregnancy loss in PCOS may be an additional cause of infertility. A reduced rate of conception relative to the rate of ovulation after therapy with clomiphene citrate and exogenous gonadotropins is also well known.
Evidence of decreased insulin sensitivity is seen in both lean (30%) and obese women (75%) with PCOS; but IR accompanied by compensatory hyperinsulinemia is most marked when an interaction occurs between obesity and the syndrome. Obesity and particularly abdominal obesity as indicated by an increased waist to hip ratio is correlated with reduced fecundity, menstrual disorders, and hyperinsulinemia. Obesity correlates with an increased rate of menstrual cycle disturbance and infertility.
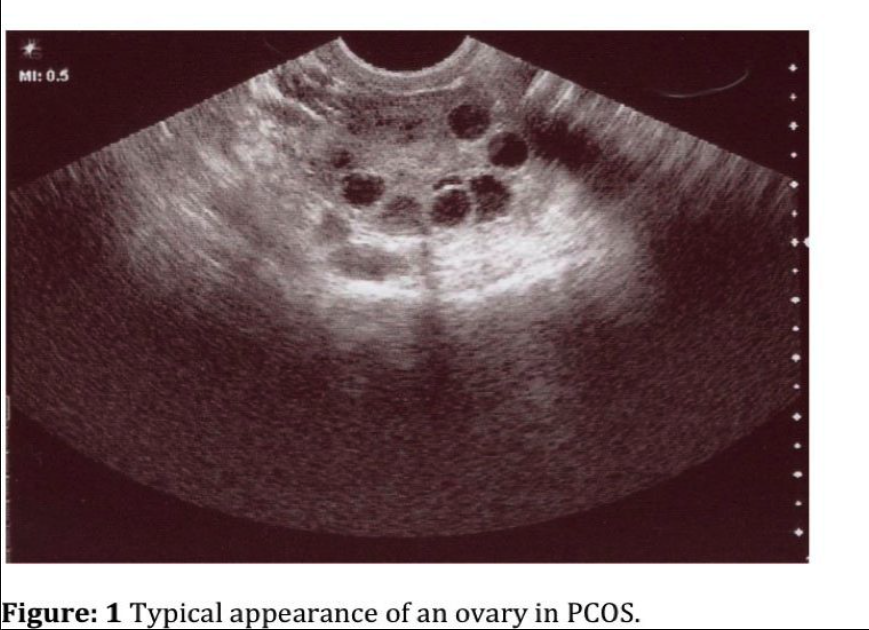
PCOS patients generally have enlarged ovaries with increased central stroma and an abundance of peripheral cystic follicles (Figure 1). It is unclear whether there are simply a baseline elevated number of follicles or if the rate of their programmed cell death is retarded.1 The hyperplastic ovarian stroma occupies approximately 25% of the medullary portion of the ovary. Ultrasound criteria have been defined and include 12 or more follicles 2mm to 9mm in diameter in each ovary and/or increased ovarian volume (³10mL).6
DIAGNOSIS
In 1990, the National Institutes of Health sponsored a conference to systematically describe polycystic ovarian syndrome, laying the foundation for a more dynamic definition by the 2003 Rotterdam European Society of Human Reproduction/American Society for Reproductive Medicine (ESHRE/ASRM) PCOS consensus workshop. The Rotterdam Consensus Group 2003, recommended that all clinicians and investigators now use a new internationally agreed upon definition of PCOS (Rotterdam ESHRE 2004).
This consensus group recommended that diagnosis of PCOS requires that at least 2 of the following 3 criteria be met: The Rotterdam criteria for the diagnosis of PCOS state that 2 of the 3 features need to be present to make the diagnosis and with the exclusion of other causes (congenital adrenal hyperplasia, androgen-secreting tumors, Cushing’s syndrome).
These features include:
(1) oligo- or anovulation,
(2) clinical and/or biochemical signs of hyperandrogenism, and (3) polycystic ovaries (either 12 or more follicles measuring 2mm to 9mm in diameter, or an ovarian volume of >10cm7).
Recently, it has become clear that PCOS is linked to several metabolic disturbances, including type 2 (non-insulin dependent) diabetes mellitus and possibly cardiovascular disease.
PCOS is associated with insulin resistance, and measurements of fasting glucose levels and insulin may also be performed, although the specificity of these tests tends to be poor. The gold standard of the hyperinsulinemic euglycemic clamp is not commonly used in clinical practice. Classically, PCOS has also been associated with an elevated luteinizing hormone (LH) to follicle-stimulating hormone (FSH) ratio; however, the actual levels of these pituitary peptides do not correlate with the severity of the disease, and it is not mandatory to measure them to establish a clinical diagnosis.
The diagnosis is also contingent on ruling out other endocrinologic disorders, such as hyperprolactinemia, ovarian hyperthecosis, congenital adrenal hyperplasia (CAH), Cushing’s syndrome, and androgen-secreting neoplasm, or acromegaly.7 Initial laboratory assessment of the patient with suspected PCOS should serve to narrow the differential diagnosis and includes serum total testosterone, 17a-hydroxyprogesterone (to rule out CAH from 21-hydroxylase deficiency), and dehydroepiandrosterone (DHEA) levels. A 24-hour urine collection to measure free cortisol will assist in diagnosing Cushing’s syndrome.
How and why PCOS develops remains unclear, but both genetic and environmental factors are involved. The diagnosis is made primarily on clinical criteria.
PATHOGENESIS
At its core, PCOS is an ovarian dysfunction. The precise pathogenesis of this entity, however, involves far more than ovarian structure. The hyperandrogenic state seems to be the product of increased LH secretion in combination with enhanced ovarian theca cell responsiveness. The cause of the increased LH levels has been purported to be secondary to increased pulse frequency of gonadotropin-releasing hormone (GnRH). This increased pulse frequency may be the result of an inherent defect in the GnRH pulse generator or of low circulating levels of progesterone from few ovulations leading to decreased negative feedback.8
The exact pathophysiological mechanism leading to the characteristic PCOS phenotype remains unknown. Some investigators explain it as primarily an intrinsic ovarian problem (excess ovarian production of androgens), others as adrenal (excess adrenal gland production of androgens), and again others as hypothalamic-pituitary dysfunction (exaggerated gonadotropin releasing hormone pulsatility that results in hypersecretion of luteinizing hormone). In the last 15 years, a large body of evidence has indicated that increased insulin resistance and compensatory hyperinsulinemia play a key role in the pathogenesis of PCOS.9
In combination with elevated ovarian production of androgen, the amount of circulating sex hormone-binding globulin (SHBG) also plays a role in pathogenesis. There is an inverse relationship between insulin and SHBG. Insulin inhibits hepatic production of SHBG. Intuitively, in the hyperinsulinemic state of PCOS, SHBG is decreased and there are resultant higher levels of free testosterone.
Hyperinsulinemia directly stimulates both ovarian and adrenal androgen secretion and suppresses liver sex hormone-binding globulin synthesis, resulting in an increase in free, biologically active androgens. This excess in local ovarian androgen production, augmented by hyperinsulinemia, causes premature follicular atresia and anovulation along with the other clinical manifestations of hyperandrogenism.
Clear evidence now exists that women with PCOS have a 5-fold to 10-fold increased risk of developing T2DM compared with age- and weight-matched women. PCOS is also associated with an increased risk of impaired glucose tolerance (IGT). There is a 31% to 35% prevalence of IGT and 7.5% to 10% prevalence of T2DM in women with PCOS. Preliminary data indicate that 10% and 30% of PCOS women with a normal or impaired oral glucose tolerance test, respectively, will develop T2DM over 2 years to 3 years of follow-up.10
TREATMENT
Treatment of PCOS should be targeted toward the patient’s primary complaint. The therapy should be individual, and includes normalizing biochemical and clinical hyperandrogenism, restoring reproductive function, improving reproductive outcomes, and managing metabolic (diabetic and cardiovascular) morbidity and mortality. When the clinical features of PCOS are worsened by IR or obesity, preventative and therapeutic interventions and use of insulin sensitizing agents, including the glitazones and metformin, have increasingly been adopted as preferable pharmacological strategies both in isolation or in combination with other pharmacological options to improve treatment response. One recent randomized, double-blinded placebo trial, however, failed to show effects of metformin on weight loss or menstrual frequency in obese PCOS patients, and suggested that weight loss alone for that subset was the only successful factor.11 Further efforts are being made to identify prognostic indicators to determine which modality is most likely to succeed in an individual patient.
Treatment of PCOS includes normalizing biochemical and clinical hyperandrogenism, restoring reproductive function, improving reproductive outcomes, and managing metabolic morbidity and mortality.
Treatment of PCOS includes combination oral contraceptives to suppress luteinizing hormone and enhance SHBG production, antiandrogens (spironolactone and flutamide) to inhibit androgen binding to peripheral androgen receptors,5-alpha-reductase inhibitors (finasteride) for treatment of hirsutism, glucocorticoids to reduce adrenal androgen levels, ovulation induction agents (clomiphene citrate) and gonadotrophins for the treatment of anovulatory infertility and laparoscopic ovarian surgery.13
However, lifestyle (dietary, exercise, or both) interventions to reduce the features of obesity, IR, or hyperinsulinemia are preferable and cost-effective initial treatment strategies compared with surgical and pharmacological options.
Weight loss improves the endocrine profile, cyclic menstruations, likelihood of ovulation, and a healthy pregnancy. In addition, insulin resistant PCOS women also had a lower ovulation rate and are more likely to develop clomiphene resistance compared with non- insulin resistant PCOS patients. Women with PCOS who developed menstrual disturbance had lower insulin sensitivity than controls had, while those with regular cycles had normal insulin sensitivity, similar to that in controls.
Oral contraceptive pills (OCP) have been the traditional therapy for the long-term treatment of PCOS, to provide endometrial protection, regularize and lighten menses, and to improve hirsutism and acne by reducing ovarian androgen production. A recent review, based on limited and contradictory evidence, raised the concern that OCPs may reduce insulin sensitivity and glucose tolerance in women with PCOS. The most common side effects in women taking OCP included headache, mood changes, gastrointestinal disturbances, and breast pain.
Problems in inducing ovulation and anovulation are well recognized in women with polycystic ovary syndrome. Medical ovulation inducted with clomiphene and gonadotrophins and ovulation induction with clomiphene citrate are not always successful, with approximately 20% of women described as “clomiphene-resistant.” Women who are clomiphene resistant can be treated with gonadotrophins but often have an overproduction of follicles and are exposed to the risks of ovarian hyperstimulation syndrome (OHSS) and multiple pregnancies.
Treatment of polycystic ovaries should be targeted toward the patient’s primary complaint, be it infertility, clinical signs of hyperandrogenism, or prolonged amenorrhea. In a large percentage of cases, treatment is ultimately focused on balancing the elevated circulating androgens and restoring the normal endocrine axis either via weight reduction of pharmacologic assistance.
In the patient not desiring fertility, oral contraceptive agents assist in decreasing ovarian steroidogenesis and increasing SHBG. Clinically, oral contraceptive pills may diminish hirsutism and protect the endometrium by inducing regular shedding.
The majority of women (70%) with PCOS who exhibit infertility as a result of chronic anovulation will respond to clomiphene citrate. Approximately 50% of these women will eventually then go on to conceive.12 Gonadotropin therapy has traditionally been the next step in therapy for those who fail clomiphene; however, the ability to achieve monofollicular ovulation is challenging, and there is considerable risk for ovarian hyperstimulation syndrome. In fact, recent data reported from our center comparing controlled ovarian hyperstimulation with IVF for patients with PCOS shows 3 times the pregnancy rate and less than one-third the higher-order multiple gestation rate with IVF.13 We concluded that though traditionally considered a more “aggressive” treatment of infertility, in patients with PCOS, IVF might actually be expensive and invasive but more “conservative.”
Because of that increased risk, there have been some advocates for mechanical destruction of excess ovarian tissue. Decreasing functional ovarian mass may diminish intraovarian androgen production and possibly encourage increased FSH levels. This theory dates back to Stein and Leventhal’s original work involving wedge resection of the ovary (though surgical management of this disease began to fall out of favor in the early 1980s). The technique, however, had been shown to be effective in as many as 90% of reported cases.14 Unfortunately, although improving the hormonal milieu and often restoring ovulatory function, the technique often caused severe pelvic adhesions and subsequently resulted in mechanical infertility due to tubal disease.
Over the past 2 decades, laparotomy has largely been abandoned in these patients, and laparoscopy has taken its place. In fact, laparoscopic-assisted ovarian diathermy (most often via monopolar electrocautery) has been reported in more than 1000 patients, with variable success rates. Though too few to establish a consensus on the technique, the randomized trials that do exist comparing laparoscopic diathermy and gonadotropin therapy show similar rates of conception.12 Laparoscopic ovarian drilling also offers the benefit of altering the hormonal composite of PCOS patients after surgery. There is often a reported decrease in serum LH, androgen concentration, and DHEA levels. This reduction in the intraovarian androgen levels allows for the development of functional follicles.15 Inhibin levels also fall more permanently, but the overall improvement in hormonal status does not seem to affect the peripheral sensitivity to insulin. Although electrocautery is the most well studied, laser drilling and multiply biopsy technique have also been used. In the electrocautery technique, the ovary is first isolated with laparoscopic grasping forceps. An insulated 8-mm monopolar needle is introduced at a 90° angle to the ovarian cortex, and a series of puncture sites are created. A cutting current of 100W is used to initially enter the cortex and is followed by 2 seconds of coagulation current at 40 W. The whole length of the needle may be placed into the ovary. Based on ovarian site, anywhere from 10 to 15 puncture sites may be made to adequately destroy ovarian tissue. The surface of the ovary is then irrigated with crystalloid before the trocars are removed. Of note, the use of the laser has diminished secondary to anecdotal increases in adhesion formation and increased surface damage to the ovarian cortex. Laparoscopic ovarian drilling was first described by Gjonnaess in 1984. Both laparoscopic ovarian cautery and laser vaporization using carbon dioxide (CO2), argon or Nd: YAG (neodymium-doped yttrium aluminum garnet; Nd:Y3Al5O12) crystal lasers have been used to create multiple perforations (approximately 10 holes per ovary) in the ovarian surface and stroma (inner area of the ovary). LOD has lesser trauma and fewer postoperative adhesions than with ovarian wedge resection.
The mechanism of action of LOD is that it may destroy ovarian androgen-producing tissue and reduce the peripheral conversion of androgens to estrogens. A fall in the serum levels of androgens and LH and an increase in FSH levels have been demonstrated after ovarian drilling. The endocrine changes following the surgery are thought to convert the adverse androgen-dominant intrafollicular environment to an estrogenic one and to restore the hormonal environment to normal by correcting disturbances of the ovarian- pituitary feedback mechanism. Thus, both local and systemic effects are thought to promote follicular recruitment, maturation, and subsequent ovulation.
Although laparoscopic ovarian drilling is efficacious and carries with it the benefit of multiple ovulatory cycles, relatively short operative time, a decrease in spontaneous abortions, and a lowered risk of multiple gestations, there may be disadvantages as well. A recent article16 suggests that bilateral ovarian drilling may result in diminished ovarian reserve. The authors suggest that unilateral drilling might have comparable results, without deleterious long-term effects. Even with laparoscopy, however, significant postoperative tubo-ovarian adhesions, and thus compromised fertility, may result. With these potential iatrogenic effects on the patient, selection for this technique should be carefully assessed before proceeding.
Perhaps Stein and Leventhal’s initial paper was our best clue for a cure. Wedge resection via laparotomy restored fertility in their case studies, corroborating their theory of mechanical crowding of the diseased ovary with follicles lacking the signal of programmed cell death. The true pathogenesis of PCOS, however, remains elusive.
Whether a selection or a combination of oral antihyperglycemics, selective estrogen receptor modulators, aromatase inhibitors, oral contraceptives, dietary modification, mechanical ablation of excess ovarian tissue, or a combination of these, is the best treatment is still uncertain and must be individualized. What is clear is that a multisystem approach to this complex disease state must be used for cardioprotection, optimal fertility, and long-term diminished risk for neoplastic processes.
References
- Chang RJ. Polycystic ovarian syndrome and hyperandrogenic states. In: Strauss J, Barbieri R, eds. Yen and Jaffe’s Reproductive Endocrinology. 5th ed. Philadelphia: Saunders; 2004:597-632
- Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181-191.
- Setji TL, Holland ND, Sanders LL, Pereira KC, Diehl AM Brown AJ. Nonalcoholic steatohepatitis and nonalcoholic fatty liver disease in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:1741-1747.
- Salley KE, Wickham EP, Cheang KI, Essah PA, Karjane NW, Nestler JE. Glucose intolerance in polycystic ovary syndrome–a position statement of the Androgen Excess Society. J Clin Endocrinol Metab. 2007; 92:4546-4556.
- Diamanti-Kandarakis E. Polycystic ovarian syndrome: pathophysiology, molecular aspects and clinical implications. Expert Rev Mol Med. 2008; 10:e3.
- Balen AH, Laven JS, Tan SL, Dewailly D. Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update. 2003; 9:505-514.
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovarian syndrome. Hum Reprod. 2004; 19;41-47.
- Ehrmann D. Polycystic ovarian syndrome. N Engl J Med. 2005; 352; 1223-1236.
- Diamanti-Kandarakis E, Papavassiliou AG. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol Med. 2006; 12:324-32.
- Balen AH, Anderson RA. Policy & Practice Committee of the BFS. Impact of obesity on female reproductive health: British Fertility Society, Policy and Practice Guidelines. Hum Fertil (Camb). 2007;10:195-206.
- Tang T, Glanville J, Hayden CJ, White D, Barth JH, Balen AH. Combined lifestyle modification and metformin in obese patients with PCOS. A randomized, placebo- controlled, double-blind multicentre study. Hum Reprod. 2006; 21:80-89.
- Farquhar CM, Williamson K, Gudex G, Johnson NP, Garland J, Sadler L. A randomized controlled trial of laparoscopic ovarian diathermy versus gonadotropin therapy for women with clomiphene citrate-resistant polycystic ovarian syndrome. Fertil Steril. 2002; 78:404-411.
- Grunfeld L, Mukherjee T, Sandler B, Scott RT, Copperman AB. IVF, with a maximum of two embryo replacement is the treatment of choice for high responding patients. Fertil Steril. 80:101.
- Adashi EY, Rock JA, Guzick D, Wentz AC, Jones GS, Jones HW Jr. Fertility following bilateral ovarian wedge resection. Fertil Steril. 1981; 36:320-325.
- Felemban A, Tan SL, Tulandi T. Laparoscopic treatment of polycystic ovaries with insulated needle cautery: a reappraisal. Fertil Steril. 2000; 73:266-269.
- Kandil M, Selim M. Hormonal and sonographic assessment of ovarian reserve before and after laparoscopic ovarian drilling in PCOS. BJOG Int Obstet Gynecol. 2005; 112:1427-1430.
