Patient Preparation for Laparoscopic Surgery
Bradford W. Fenton, MD, PhD, FACOG, Shruti Malik, MD
INTRODUCTION
“Sweat saves blood.” Although this is a military quotation, its essence is critical to the safe conduct of laparoendoscopic surgery, and indeed all of medicine. With proper preparation, morbidity and mortality can be minimized, and patient outcomes optimized. The topics of this chapter, perhaps more than any other, have been extensively researched in the hope of improving outcomes for the greatest number of patients. Many of these topics are so important that they have been taken out of the hands of practicing surgeons (for better or worse) and are now codified by federal regulatory agencies into institutional mandates. The purpose of this is clear: standardized, checklist driven preparatory protocols have been clearly demonstrated to decrease errors of omission in a number of venues, and the federal government has taken the position that this type of approach is necessary to ensure patient safety across all hospitals and systems.
The Surgical Care Improvement Project (SCIP) represents a series of guidelines for perioperative care that originated with payors and quality assurance reviewers in an effort to decrease the cost of surgical care by decreasing the incidence of certain expensive complications in the immediate (30-day) postoperative time frame. Over time, it was discovered that decreasing these costs actually translated into better patient outcomes, and thus the program was expanded with the new goal of improving patient safety.
Because it has its roots in quality assurance, many of the guidelines have built-in performance measures, which are checklist items designed to allow a nurse or lay reviewer to read through a chart and determine whether an item was or was not done. If a checklist item is not done (for good reason or not), then that incident is flagged and the institution’s failure to comply with the guidelines is noted. It can be safely assumed that institutions (hospitals) are unlikely to permit surgeons to operate on their premises if that surgeon’s charts continually lead to guideline violation flags, because hospitals with too many flags will lose their accreditation and insurance companies will refuse to pay for services rendered there.
Although most surgeons are independent thinkers, it should be realized that the SCIP guidelines are culled from current medical society guidelines, and that their ultimate goal is to improve the outcomes of patients on a national scale. The SCIP guidelines cover the prevention of perioperative infection (which includes preoperative antibiotics, appropriate skin preparation, proper hair removal, and maintenance of normothermia), the prevention of venous thromboembolism, the prevention of perioperative cardiac events, and the prevention of perioperative pulmonary events.
The Joint Commission for the Accreditation of Healthcare Organizations (JCAHO) has also taken an interest in assuring that patient safety is made a documentable priority in perioperative care.1 To this end, JCAHO has developed the 2010 National Patient Safety Goals that provide (mandate) guidelines for the care of patients in a wide variety of settings, particularly for procedure-based care, and these have been codified into a document called the Universal Protocol.2 JCAHO has implemented guidelines on other topics, including forbidden abbreviations, proper electrolyte solution use, and infections control, which are less pertinent to the conduct of surgery but may be encountered in other venues. The Universal Protocol addresses the prevention of wrong-person, wrong- site, and wrong-procedure surgeries, in addition to improving communication among care-team members, including the patient. The stated objective is to put into place a standardized method, used in all hospitals, surgery centers, and offices where procedures are performed across the nation, which prevents these types of “never” events.
The preparation for surgery starts before the patient even arrives in the preoperative area, and includes a discussion of informed consent and home bowel preparation. Many patients with comorbid medical conditions undergo a review of their treatment strategy with their primary care physicians. Although commonly termed “medical clearance,” this process really provides an opportunity to optimize the patient’s medical management prior to scheduled surgery. The implementation of a method to prevent venous thromboembolism begins based on the procedure and the patient’s risk factors.
Preoperative antibiotic prophylaxis typically commences before arrival in the surgical theater, with the goal of having therapeutic levels of the medication in the skin prior to incision. Proper skin preparation and sterile draping of the patient is also necessary to decrease infectious mortality, as has been done for almost a century. The national standard has been set that requires a “time out” in the operating room, including proper identification of the patient, the surgery, and the surgical site. All of these measures are clearly demonstrated to improve patient outcomes and are important components of a modern approach to surgical intervention.
Many of the current recommendations arise from the Agency for Healthcare Research and Quality report3generated in 2001 in response to the Institute of Medicine report To Err is Human: Building a Safer Health System.4
UNIVERSAL PROTOCOL: PATIENT IDENTIFICATION, PROCEDURE VERIFICATION, CONSENT VERIFICATION
History
The Universal Protocol5 is concerned with assuring patient safety through the elimination of certain “never” events, which are events that should never occur in surgery. This includes surgery on the wrong patient and the wrong surgery being performed or the wrong site operated on. Currently, these concepts are most obviously wrapped up in the “time out” called at the beginning of a procedure. The objective of the Universal Protocol is to have a system of multiple, complimentary strategies in place to prevent wrong person, wrong procedure, or wrong site surgery.
Anatomy
The anatomic consideration pertinent to patient safety is the proper marking of unilateral procedures, also known as the “sign your site” approach. Hospitals have some flexibility in setting regulations for site marking based on their own unique circumstances.
Procedures on midline or unpaired organs are unlikely to benefit from site marking.
Unilateral procedures on paired organs (such as right ovarian cystectomy or left adrenalectomy) will likely benefit from site marking. Although in laparoscopic surgery the marks on the skin of the abdomen are only visible at the time of trocar insertion, marking the abdomen on one side or another to indicate the site of surgery (not the unoperated side) reminds the team of the appropriate laterality for the procedure.
Physiology
Marking should be done with as permanent an ink marker as possible. Most pen marks will be at least partially removed in skin preparation, which may be a reason to have the performing surgeon be the one who marks the site. The Universal Protocol specifically recommends that the attending surgeon mark the site, although it is not mandated, which allows preoperative nursing staff to do so. As the one ultimately responsible for patient safety, it seems prudent for the attending surgeon to abide by these recommendations.
Pathophysiology
A wide range of disorders affect paired organs in the abdomen and pelvis, which may require laparoscopic treatment. One of the most insidious disorders that affects proper adherence to the Universal Protocol is surgeon hubris. “The Universal Protocol is implemented most successfully in hospitals with a culture that promotes teamwork and where all individuals feel empowered to protect patient safety.”5 Surgeons who are overbearing, defiant, arrogant, and unable to comprehend that the objective of these guidelines is to improve their patients’ outcomes have the capacity to circumvent and sabotage most of this protocol.
Wrong-site surgery is a “never” event and is reportable not just to the local hospital quality assurance program, but can be used to deny accreditation to that institution. Such ramifications go beyond the basic courtesy of assuring patient safety and conducting the correct procedure, and go well beyond the physician patient interaction.
Treatment
Compliance with the Universal Protocol is designed to be straightforward, is clearly meant to improve patient safety and outcomes, and is to be implemented in essentially all healthcare institutions. Using multiple complimentary strategies generally means that the preoperative nursing staff, attending physician at the immediate preoperative evaluation, and the operating room staff all cover the same basic facts in some manner. Preoperative nursing staff will often have a checklist, which is also known as the preprocedure verification. This is supposed to be matched to the patient’s expectations; thus, it cannot be performed after induction of anesthesia. Additionally, there is the stated expectation that supporting documentation must be accounted for by using a checklist, including the history and physical examination, pertinent laboratory studies, and special instruments or implants.
The surgeon may verbally review the patient, procedure, and site, or have a structured note to fill out. Portions of this are part of the informed consent process, and this is the final step that assures both the patient and surgeon that the correct site and procedure are performed on the correct patient. The OR staff will call a “time out,” which at a minimum will identify the patient, surgery, and site.
Institutions must have in place a policy for determining which procedures require preoperative marking and which do not. Regardless of institutional requirements, surgeons are urged to mark any site when there is the possibility for more than one location for the procedure or when there would be negative consequences to performing the procedure in a different location.
The responsibility for marking the site is currently unregulated, and could be relegated to a junior resident, nurse practitioner, or nurse. The only recommendation is that the individual who knows the most about the patient should mark the site, which would imply that patients themselves should do the marking. As the individual with ultimate responsibility for the outcome of the patient, the attending surgeon would be hard pressed to argue that he or she does not know the patient best if he or she is the one performing the surgery.
Complications
Failure to abide by JCAHO regulations results in loss of accreditation for the institution, which leads to license revocation and loss of essentially all payors. Surgeons who fail to abide by institutional regulations will be identified by the (mandatory reporting) peer review process, which typically results in the loss of hospital privileges if repetitive.
The creation of multiple complimentary strategies is designed to eliminate the occurrence of all wrong patient, wrong site, and wrong procedure surgeries. It is the expectation of JACHO that with proper implementation of this system these complications will never occur.
Surgeon hubris is a difficult complication to overcome, because rescue strategies generally require extreme punitive measures. Identification of a “difficult physician” is typically done through a series of nursing and support staff complaints, generally centered around a failure to promote an empowered team. Local administration is generally averse to addressing this problem, which may fester for an extended period if no objective investigation is started. Many professional organizations (ACOG) have a service that provides an external “quality review” that includes an evaluation of team building and collegiality of an individual physician. Truly disruptive physicians in smaller institutions may not get the benefit of a professional society review, and may instead undergo review by an expert witness. In general, an expert witness is both selected by and beholden to the administration that contracts with them, typically assuring that they support the position of their employer. Such reviews are unlikely to be complimentary to the surgeon who incurred such an expense upon the hospital.
Rescue Strategies
Compliance with the Universal Protocol will help avoid ever having one of the targeted errors, which minimizes the need for rescue strategies. Confounding the system by avoiding the proscribed steps that are designed to be difficult due to the requirement that they be multiple and complimentary does occur. As proven by American surgeons at the turn of the last century, despite clear and convincing evidence, including the death of a President, surgeon hubris can be exceedingly difficult to overcome.6 Although sometimes-difficult physicians can be identified prospectively and avoided,7 the best method is likely to be a solid foundation of communications skills learned in medical school.8
INFORMED CONSENT
History
The guiding ethical principle behind the doctrine of informed consent (autonomy) requires that a patient understand that any course of treatment has risk to it, and that to receive any potential benefit from a treatment, these risks must be undertaken with the understanding that alternatives have been considered, even though it is not clear how much technical information a patient is likely to retain.9 The word “doctor” means “teacher” in Latin (derived from docere: to teach), and this role is most prominent in a discussion of informed consent.
Although assurance of informed consent is based on the ethical principle of autonomy, as a standard component of care, it began around the end of the 1800s in America,10 and formally arose out of a medical malpractice case in 1914.11 Now thoroughly etched into the health care system by payors (Centers for Medicare & Medicaid Services, CMS) and accrediting organizations (The Joint Commission on Accreditation of Healthcare Organizations, JCAHO) informed consent requires that a patient understand what they are consenting to. A layman about to undergo a technically sophisticated procedure does not need to describe it in depth, but needs to be capable of describing an understanding of the overall condition being treated, a rough outline of the procedure, the location of the surgical site, the expected benefits, the major risks, and the alternatives (including no treatment). These items are part of a minimum list that is becoming a requirement for preoperative personnel to use to question patients before anyprocedure.12
Anatomy
The origin (if not insertion) of the doctrine of informed consent is attributed to Hippocrates:13 Life is short, the Art long, Opportunity fleeting, Experiment treacherous, Judgment difficult. The physician must be ready, not only to do his duty himself, but also to secure the cooperation of the patient, of the attendants, and of externals.
Further refinement and documentation of informed consent for surgical intervention is documented in the treatment of Justin II, Emperor of Byzantium. Facing possible execution for failure to save the life of the Emperor, his surgeons insisted that he himself hand them the scalpel to be used, assuring them that he used his own free will for surgical intervention.14
Physiology
The application of the doctrine of informed consent essentially shifts the responsibility for complications arising from a surgical procedure from the surgeon onto the patient. Although codified into law in America, the concept that surgical procedures have risk to them and that these risks must be weighted against the risks of no treatment was well known to the ancients. Some of the first records of informed consent are between Alexander the Great and his battlefield surgeons. Faced with several life-threatening wounds, Alexander assured his surgeon of his immunity and asked: “Do you perhaps fear that you may be blamed because I have received an incurable wound?”15 Further review of the development of the doctrine of informed consent is available from more modern sources.16,17
Pathophysiology
Certain patients are unable to give informed consent, either due to an inability to comprehend the issues at hand (mentally disabled), a legal barrier to giving informed consent (minors, prisoners), or an acute lack of capacity in an emergency (trauma victims).18 The nature of emerging surgical technologies, such as laparoscopy, means that some patients will be undergoing a procedure by a surgeon with less experience with that technique. Of course, one patient must be the first to undergo any procedure, but even so an open discussion with the patient regarding the risks of a novel approach compared with the benefits of a minimally invasive surgery should be done.19
Treatment
In most cases where informed consent is limited, a legal guardian typically is the one who provides informed consent. If at all possible, informed assent, which takes the place of consent in a patient not legally capable of granting it, should be sought. For patients who are acutely incapacitated, a surgeon acts on the principle of beneficence to provide reasonable and prudent care for a patient. The items required for assurance of informed consent (procedure to be performed, location or organ system, an understanding of the consent form used, and marking of the site) have been affirmed by the American College of Surgeons.20
Complications
The actual complications that need to be addressed in the discussion of surgical risk will vary significantly depending on the procedure. Different hospitals in different jurisdictions may have particular issues that are required to be emphasized based on local case law. The complications that arise from failure to obtain informed consent generally are visited upon the surgeon rather than the patient, in the form of legal proceedings. As noted in the original informed consent case, performing surgery without a patient’s consent constitutes assault.
Rescue Strategies
Currently, several computer-based prompting systems (search for “automated informed consent”) are available for use in the preoperative area, which go through essentially a quiz on the primary principles of informed consent: an understanding of the overall condition being treated, a rough outline of the procedure, the location of the surgical site, the expected benefits, the major risks, and the alternatives (including no treatment).
These automated informed consent tools not only assure that patients understand their treatment regimen, but also provide documentation of this for the chart.
COMMUNICATION
History
The problem of communication among the caregivers of a medical team is one that is a global problem among essentially all industrialized countries that use multiple individuals with specialized tasks and skills to provide medical care.21 One of the standardized National Patient Safety Goals is to improve the effectiveness of communication among caregivers.22
Anatomy
Physiology
Implementation of standardized techniques for caregiver communication provides an opportunity to improve patient care and lower costs, because failure to adequately communicate pertinent information in a complex or critical care setting leads to poor patient outcomes, with the attendant cost and complexity of managing the induced medical issues and litigation costs.23
Pathophysiology
The World Health Organization statement on communication identifies the underlying problems in caregiver communication as a lack of provider education in team training and communication, a lack of good role models, and a health care system that promotes autonomy and individual performance.
Treatment
Management of patient information exchange at change of shift or patient transfer includes use of the Situation, Background, Assessment, and Recommendation (SBAR) technique, allocation of sufficient time for communicating important information, and for staff to ask and respond to questions without interruptions wherever possible (repeat-back and read-back steps should be included in the hand-over process) with provision of information regarding the patient’s status, medications, treatment plans, advance directives, and any significant status changes.21
Complications
The WHO monograph on patient hand-overs lists several potential obstacles to caregiver communication. These can be divided into individual and system-wide failures.
Individual failures center on a resistance to change communication behavior with the goal of improving patient care. Part of this may be cultural, and part may be due to a lack of education or training in improved communication skills. System-wide failures focus on an inability to implement and enforce proper caregiver communication techniques, either through lack of resources, leadership, or knowledge.21
Rescue Strategies
Detecting a breakdown in caregiver communication is usually done through the identification of untoward consequences, such as sentinel event monitoring or the peer review process. Smaller individualized problems that do not lead to complications are unlikely to be remedied. Once a complication has occurred and communication breakdown has been identified as the problem, then remedial measures can be taken. This may include individual or group counseling, or education in the use of the SBAR technique. Interestingly, although SBAR and other patient handoff mnemonics have been embraced by regulatory agencies, which assumes a certain due diligence on their part to assure that the recommendations are based on fact, the actual literature demonstrating a measurable benefit from these techniques is relatively thin.24
PREOPERATIVE MEDICAL CLEARANCE
History
The first step to preventing both intraoperative and postoperative complications lies in obtaining a thorough medical assessment and physical examination before entering the operating room. Although many surgeons are accustomed to problem-focused evaluations and physicals, it is critically important to gain a comprehensive history and physical examination. This alone can help decrease the overall incidence of preventable complications. Preoperative assessment can also be used to help modify a patient’s risk factors, such as smoking cessation, diabetes, or hypertension.
Medical History
A patient’s medical history is highly beneficial in guiding a surgeon’s preoperative laboratory work and imaging. Special care must be given to cardiac, pulmonary, and renal systems, because laparoscopic procedures may subject a patient to longer operating times and increased strain due to pneumoperitoneum and patient positioning compared with open surgery.
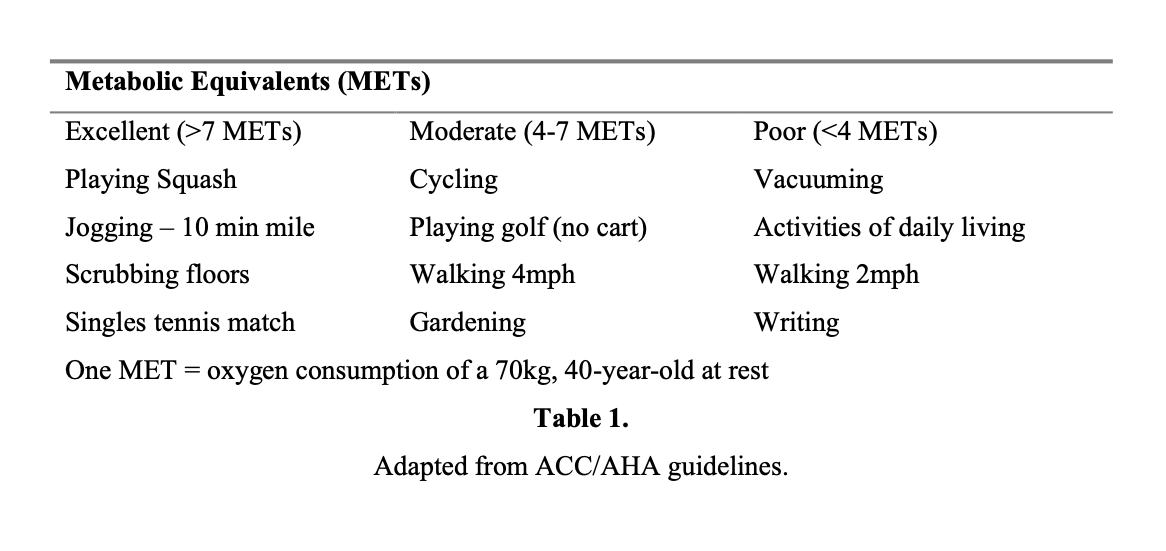
A significant cardiac history, such as that of congestive heart failure, hypertension, cardiac arrhythmias, or coronary artery disease, warrants a baseline ECG. Patients with noncardiac diseases, such as asthma with COPD, emphysema, renal failure, or diabetes, as well as men over 45 years old and women over 55 years old should be screened with preoperative ECGs. Patients over 75 years old, those with a history of cardiopulmonary disease, or abnormalities on physical examination should also receive a chest radiograph.25 A history of myocardial infarction (MI) carries a 36% risk of reinfarction within 3 months of the episode and 5% to 6% overall in the perioperative period. General recommendations state that elective surgery should be postponed for at least 6 months after an MI.26,27 Patients with hypertension should also strive for optimal blood pressure control before surgery: current guidelines state a goal of 140/90.26 Another important consultation point for patients is an assessment of cardiac function. One way to evaluate this is by measurement of left ventricular ejection fraction. Patients with an ejection fraction <35% are more susceptible to perioperative complications.27 Another appraisal of cardiac function uses metabolic equivalents of tasks (METs). A patient is considered high risk if he is unable to increase oxygen consumption to more than 4 METs.26 Stress testing is recommended with low functional capacity25 (Table 1).
Respiratory complications are the most prevalent cause of morbidity postoperatively and occur more often in older patients. Laparoscopic surgery, concurrent pneumoperitoneum, and the Trendelenburg position can place pressure on the diaphragm and a strain on lung expansion, especially in those with suboptimal pulmonary status. For this reason, it is essential to assess pulmonary status preoperatively. Patients with a history of COPD, shortness of breath, or orthopnea may need pulmonary function tests to evaluate baseline function. Patients with significant pulmonary disease, malignancy, or possible respiratory infections will also need chest X-rays.25
A thorough renal assessment is also pertinent, especially in patients with suspected insufficiency. Laparoscopy and operative time both affect renal function.
Pneumoperitoneum has been noted to cause a decrease in renal blood flow and renal function. This is especially important in patients with a compromised renal status and is more likely to occur at pressures >12mm Hg to 15mm Hg, suboptimal fluid hydration, or in the reverse Trendelenburg position.28 Preoperative evaluation of electrolytes, BUN, and creatinine is recommended in patients with diabetes, hypertension, liver or kidney disease, diuretic use, lupus, or procedures involving radiographic dye. Hepatic disease should be evaluated with a complete blood count, coagulation studies, and a complete metabolic panel, because many anesthetic agents such as propofol are metabolized in the liver.25
Endocrine conditions like diabetes are increasingly prevalent, and optimal glucose control before surgery can lead to decreased complications and improved postoperative healing. It is important to understand the physiology of hyperglycemia and the effect of the stress response due to surgery. Improved outcomes are associated with a preoperative glucose goal of <200mg/dL.29 In addition to blood glucose checks, the surgeon should also check serum electrolytes, BUN, creatinine, and order an ECG.25
A history of thrombophilias and bleeding disorders is necessary to amend any current anticoagulation regimens. Patients with anemia should be evaluated with a complete blood count and type and screen. Low preoperative hematocrit is associated with increased postoperative pneumonia and hospital stay.30
Family History
Closely examining a patient’s family history can be an insightful predictor for potential complications or underlying medical conditions that have yet to surface. A history of diabetes, hypertension, thyroid disease, cardiomyopathy, or inheritable thrombophilias can lend to a more detailed preoperative evaluation, because these conditions may initially present under perioperative conditions.
Social History
Eliciting a patient’s social history is often overlooked. In patients who smoke, it is beneficial to encourage complete cessation. Patients who quit smoking 6 to 8 weeks before surgery demonstrate some return of ciliary function and improved perioperative oxygen consumption.26 A thorough assessment of alcohol intake and illicit drug use can aid in monitoring for withdrawal symptoms or delirium tremens. Additionally, any woman of childbearing age should be evaluated for pregnancy, because pregnancy may influence anesthesia and medication choices, or the need to postpone or cancel an elective surgery.
Medications and Allergies
Obtaining a detailed list of current medications is essential in a preoperative assessment. While a patient may recall prescription medications, it is equally important to include any supplements or herbal medications, because these may affect coagulation and wound healing. Although it is advisable to stop aspirin prior to surgery, a daily low-dose pill may be considered safe to continue into the perioperative period for patients with a strong cardiovascular history; the risks and benefits of anticoagulation should be balanced with the potential for increased blood loss. Warfarin should be withheld 5 days to 7 days preoperatively and bridged with low molecular weight or unfractionated heparin.
Medications that affect blood clotting, such as ibuprofen, ginko biloba, clopidogrel, and others, should be discontinued 7 days to 10 days before surgery. Angiotensin-converting enzyme inhibitors and angiotensin II antagonists should be discontinued at least 10 hours before general anesthesia is administered to reduce intraoperative hypotension.31 Patients receiving steroid therapy, such as prednisone, for a period of >2 weeks to 3 weeks should be advised to continue their usual doses or be given stress doses of steroids perioperatively to avoid adrenal insufficiency. Drugs like monoamine oxidase inhibitors and tricyclic antidepressants have been reported to react with anesthestic agents and should be discontinued approximately 2 weeks to 3 weeks before surgery. However, it is advisable that serotonin specific reuptake inhibitors (SSRIs) are continued, because withdrawal can precipitate anxiety, dizziness, lethargy, palpitations, and gastrointestinal complaints.32
Lastly, a history of medication and contact allergies should not be overlooked. Allergic reactions can range from rashes, pruritis, and gastrointestinal complaints to bronchospasms and anaphylaxis. Although only 10% of these adverse reactions are true allergies, identifying them allows the physician to choose alternative medications that are more suitable for the patient. Any allergies to latex or adhesive tapes should also be noted in advance to plan for latex-free supplies and more appropriate materials in the operating room.
Anatomy
Any history of surgical complications, postoperative wound infections, or problems with anesthesia are helpful in guiding the patient’s next surgery. While laparoscopy has a significantly decreased risk of infection in comparison with laparotomy, it is important to optimize any conditions like obesity or diabetes that might predispose a patient to complications.33
It may also be useful to gain an overview of any previous abdominal surgeries and examine the abdomen for surgical scars. A review of any available operative notes may convey knowledge of extensive adhesions by a prior surgeon. A history of cesarean deliveries or laparotomies may raise suspicion for pelvic adhesions. It is also important to assess whether hernia repairs were completed with or without mesh. In a patient with known dense adhesions or periumbilical mesh, it is advisable to consider initial trocar entry above the umbilicus or in the left upper quadrant.
Physiology
As a result of patient positioning in a Trendelenburg or reverse Trendelenburg and potentially prolonged operating times, it is necessary to understand the increased stresses placed on the body. An extended amount of time in a steep Trendelenburg position, such as in robotic surgery, can lead to increased intraocular pressure and orbital swelling due to the dependent edema.34 Similarly, the reverse Trendelenburg position can lead to venous pooling and stasis in the lower extremities and predispose patients to venous thrombosis.
A laparoscopist also needs to consider certain physiologic aspects of pneumoperitoneum that place the patient at different risks than that of laparotomy. Abdominal insufflation and pneumoperitoneum may cause organ ischemia and subsequent reperfusion injury after desufflation. This leads to an increase in reactive oxygen species and oxidative stress.35 The effect of pneumoperitoneum on the diaphragm can additionally diminish vital capacity and needs to be balanced with positive pressure ventilation by anesthesia. Functional residual capacity is decreased 6% to 21% in the Trendelenburg position.36 In morbidly obese patients, intraabdominal pressure is noted to be 2 to 3 times that of nonobese patients. This increased pressure can lead to venous stasis, decreased portal venous blood flow, decreased intraoperative urinary output, and impaired cardiopulmonary function.37
As mentioned earlier, renal function and blood flow can also be adversely affected by pneumoperitoneum and the reverse Trendelenburg position. This is usually transient in healthy patients and can be avoided with adequate hydration in surgery.
Pathophysiology
Laparoscopic surgeons should pay special attention to particular medical issues that may affect the patient in the operating room. Careful evaluation of these issues may prevent complications and save time on the day of surgery. As more and more patients with chronic hypertension are presenting to the office, special consideration should be taken to closely monitor fluctuations in blood pressure in the operating room. Long periods of hypotension intraoperatively lead to decreased renal perfusion and renal ischemia. This may predispose the patient towards acute tubular necrosis and oliguria after surgery.
During some medical or invasive procedures, it is believed that bacteremia is incited and endocardial surfaces are exposed to various bacterial or fungal microemboli that circulate in the bloodstream. Bacteria such as streptococcus have virulence factors that cause adherence of these organisms to endovascular surfaces, while coagulase-negative staphylococci are known for their adherence to prosthetic surfaces. Infective endocarditis occurs when nonbacterial thrombi form due to turbulent valvular blood flow and are seeded during this bacteremia. Over time, these organisms can proliferate within the thrombus and create vegetations that may interfere with valvular function or pass into the systemic circulation and initiate secondary infections. This transient bacteremia can occur when mucosal surfaces, such as the oropharynx, GI tract, urethra or vagina, are disrupted and endogenous microfloras are released.
With the increasing prevalence of diabetes, it is important to understand the physiology of hyperglycemia and the stress response due to surgery. The additional stress placed on the body perioperatively activates the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system, causing a hyperglycemic response.38 This surplus glucose load can cause concern during surgery or carry over to the recovery period. While this is rarely of consequence in healthy individuals, it is especially important in poorly controlled diabetic patients.
Treatment
Certain medical conditions may necessitate treatment in the perioperative period. For this reason, it is essential to gain a thorough medical history during the initial preoperative visit. Persons with asthma may be treated with bronchodilators or aerosol breathing treatments to treat any reversible bronchoconstriction.26 A course of systemic corticosteroids in those with a peak flow or forced expiratory volume in one second (FEV1) <80% of predicted function may also be helpful in optimizing the patient’s condition. These patients should be observed closely to maintain adequate oxygen saturation and may be administered aerosol treatments to facilitate bronchodilation intraoperatively.
Similarly, as intubation and steep Trendelenburg positioning can predispose patients with an upper respiratory tract infection towards complications, a preoperative course of antibiotics may be recommended. Obese patients, those with COPD, and patients in the Trendelenburg position will already have a decreased functional capacity and may need increased pressure support or hyperventilation to correct respiratory acidosis.25 As stated previously, patients with renal compromise will be more affected by decreased renal blood flood and diminished renal function. These individuals or those with underlying hypertension may need additional fluid support intraoperatively to maintain adequate perfusion. In anemic patients, iron supplementation and preoperative blood transfusion may be considered in patients with a hemoglobin <8.0g/dL, because these patients are more than 16 times more likely to die than patients with higher hemoglobin levels.25 Persons with diabetes may need special attention. Although it is preferable for patients to run slightly above than slightly below goal glucose levels in surgery, tight control is key. This may require adjustment of intravenous fluids or even administration with short- acting glucose. Although much of this management will fall on the anesthetist while the patient is still in the operating room, it is important for the surgeon to be aware of these interventions so he is able to better guide postoperative recovery.
Complications
Because many complications of laparoendoscopic surgery may be prevented with a strong preoperative assessment, failure to do so can be disadvantageous. In this section, we will focus on some complications encountered and touch on treatment modalities.
Cardiac complications including congestive heart failure (CHF) and postoperative cardiac arrthythmias can occur after laparoscopic surgery. Fluctuation in mean arterial pressure and inadequate volume support can predispose patients to CHF in the postoperative period. Careful monitoring of volume status and appropriate use of diuretics can be used to treat these acute exacerbations. Fluid and electrolyte imbalances as well as increased adrenergic tone due to surgery or inotropic agents can make patients more vulnerable to these arrhythmias.39 Careful assessment of electrolytes and volume status as well as telemetry monitoring may be considered in these conditions.
Pulmonary complications can occur after many different types of surgery but are of special consideration in laparoscopy. Given the additional strain placed on the respiratory system by pneumoperitoneum, positioning, and longer operating times, it is important to monitor the return of respiratory function. Atelectasis and hypoxemia are commonly encountered after laparoscopic surgery as a result of reduced tidal volume and increased bronchial secretions. This may result in low-grade temperatures in the first 48 hours.25,36 Early ambulation and frequent use of incentive spirometry can be useful in these situations. Nosocomial and aspiration pneumonia may also present in the postoperative period. While preoperative antibiotics have not been shown to prevent pneumonia, therapy should be tailored to the clinical situation at the time of diagnosis and then targeted to any specific organism discovered in blood or sputum cultures.
One of the other important organ systems affected during laparoscopic surgery is the renal system. Two commonly encountered causes of acute renal failure are dehydration and acute tubular necrosis (ATN). This may be more likely after long laparoscopic surgeries. Although maintaining volume status intraoperatively is key, rapid volume resuscitation is necessary in acute dehydration. A fractional excretion of sodium (FE-Na) <1% correlates with dehydration and other prerenal causes of acute decompensation. Meanwhile FE-Na >2% to 3% is consistent with ATN or a postrenal cause.25 ATN is considered a diagnosis of exclusion after other intrinsic or obstructive causes have been ruled out. Careful fluid resuscitation, correction of serum electrolytes, and discontinuation of nephrotoxic agents is key.40 Cautious use of diuretics may be warranted in patients with oliguria. In more severe cases, dialysis may be necessary; although there is no consensus on when treatment should be initiated.
Rescue Strategies
As discussed earlier in this chapter, many common complications of laparoscopic surgery can be prevented with judicious assessment of risk factors before surgery and careful intraoperative management. Close observation of fluid status and electrolyte abnormalities can lead to early diagnosis and treatment of cardiopulmonary conditions and impending acute renal compromise. Early ambulation and incentive spirometry should be encouraged. Sedentary patients are predisposed towards venous thrombosis due to prolonged stasis and endothelial injury due to surgery, and prophylaxis with sequential compression devices or heparin should be considered. In patient presentations with a high suspicion for pneumonia or postoperative infection, antibiotics should be initiated early and prudently. As always, a detailed patient assessment and examination can guide a surgeon in both the preoperative evaluation and postoperative care.
BOWEL PREPARATION
History
Before the 1970s, bowel surgery was considerably more dangerous, because the risk for infection was a large cause of postoperative morbidity and mortality. As the notion of cleansing the bowel before surgery came about, complication rates began to decline. The purpose of bowel preparation is to decrease the bulk of feces in the colon and to aid manipulation of the bowel, visualization in the surgical field, and decrease the risk of infection and anastamotic leakage should bowel opening occur. With the increasing frequency of multiple abdominal surgeries, the prevalence of postoperative adhesions is also on the rise. In the event of incidental bowel perforation during dissection or lysis of adhesions, an adequate bowel preparation has been considered helpful in preventing the spill of intestinal contents into the abdomen and possible infection.
Although it is a necessity for patients to complete a full bowel preparation before a colonoscopy, it is common to consider this preparation prior to laparoscopic surgery. Recently, surgeons have debated the utility of mechanical and antibiotic bowel preparations. While increasing data are being presented to support the abandonment of mechanical bowel preparations, it is still a controversial topic regarding a long- established practice.
Anatomy
The gastrointestinal (GI) tract is composed of the oropharynx, esophagus, stomach, small intestine, large intestine, rectum, and anal canal. The small intestine, which is further broken down into the duodenum, jejunum and ileum, along with the large intestine are the most important components to consider in bowel preparation. The small intestine is approximately 6.5m (20ft) in length if laid out straight. The main function of the small intestine is digestion and absorption as the contents move to the large intestine. The colon is 1.5m in length, and like the rest of the GI tract is composed of 4 layers: the adventitia, muscularis, submucosa, and mucosa. Each of these has a separate function. The muscularis of the colon contains 2 layers of smooth muscle: the outer longitudinal layer and the inner circular fibers. These 2 layers work together in a peristaltic motion to propel food through the colon.41
Physiology
It is important to understand the physiology of absorption to understand the mechanism of bowel preparation. Osmotic laxatives, such as sodium phosphate (NaP), magnesium citrate, and mannitol, function by drawing extracellular fluid into the lumen of the colon. This increased colonic volume stimulates peristaltic activity and evacuation. However, due to this process, these laxatives are associated with extracellular volume depletion and electrolyte abnormalities. Polyethylene glycol (PEG) is a high molecular weight nonabsorbable macrogol polymer that is combined with a dilute electrolyte solution. The polymer helps retain the electrolyte solution in the colon through an osmotic effect and aids in cleansing the bowel. Since there is minimal fluid exchange across the intestinal mucosa, there is less risk of electrolyte imbalances.42 Stimulant laxatives, such as senna or bisacodyl, function by stimulating the nerve endings in the colonic mucosa and augmenting the smooth muscle activity in the bowel wall. They have a small degree of osmotic activity as well.
Pathophysiology
Special considerations and adjustment must be made in patients with particular comorbidities. Sodium phosphate is associated with more notable fluid losses and electrolyte changes. Due to its adverse effects, it is contraindicated in patients under 5 years old, patients with serum electrolyte imbalances, considerable hepatic or renal compromise, recent myocardial infarction, congestive heart failure, or malabsorption.43 In these situations, PEG, bisacodyl, or a combination of the 2 may be a more suitable alternative. Older patients and those with renal insufficiency or uncontrolled diabetes may need modified regimens due to the heavy load placed on renal function. Patients with impaired swallowing or altered consciousness may not tolerate oral preparations and can be stimulated withsuppositories.
Treatment
The use of mechanical bowel preparation (MBP) before a colonoscopy is necessary to fully cleanse the colon and achieve optimal visualization of lesions or polyps. Although surgeons do not debate this, the use of these preparations prior to elective colorectal surgery has come into question.
There are limited data dealing directly with laparoscopic colorectal surgery, and most of the recent literature has been in regards to elective open colorectal surgery. One recent study looking at MBP in laparoscopic colectomies determined no significant difference in anastomotic leaks or postoperative wound infections in patients without a preoperative bowel preparation. It also found that the slightly increased rate of conversion to laparotomy in patients without MBP was not statistically significant.44
In general, 2 components of bowel preparation are taken into consideration before diagnostic laparoscopy or planned colorectal surgery: mechanical and antibiotic preparation.
In laparoscopy where there is no planned entry into the bowel, studies show that the incidence of incidental enterotomy is so low that MBP does not decrease the incidence of leakage or wound infection but does increase patient discomfort and may be unnecessary.45 This is especially true for benign cases. Special consideration may be taken for patients with severe endometriosis, previous abdominal surgery, or abdominal malignancy, because the risk of perforation increases in these complex cases.
In elective colorectal surgery, there is a strong academic divide over the utility of preoperative mechanical bowel preparation. Historically, mechanical bowel preparation had been given without question. As evidence-based medicine becomes more and more prominent today, this tradition may not prove to be as beneficial as previously assumed. It was believed that reducing the amount of fecal matter would decrease wound infections, intraabdominal abscesses, and anastomotic leaks after bowel surgery.
However, as more data have emerged, it appears that withholding mechanical bowel preparations has no significantly increased risk of wound infection or anastomotic leaks.46,47 In some analyses, it has been opined that omission of mechanical preparation has a decreased risk due to the fluidity of the fecal matter. Some evidence suggests that mechanical bowel preparation can increase the rate of structural alterations and inflammation in the wall of the colon. There is concern that this inflammation leads to increased leakage of the anastomosis as well.48
Laparoscopic surgeons may still favor some degree of mechanical preparation to aid in manipulation and visualization of the surgical field despite proven significance.45
So while mounting evidence suggests that mechanical bowel preparation should be reconsidered, the long-established tradition is not one that will easily be abandoned. There is also debate over the use of antibiotic bowel preparation, but to a lesser degree. These regimens should target both aerobic and anaerobic pathogens. Common oral regimens include metronidazole, erythromycin, or neomycin due to their bioavailability and absorption into the systemic circulation through the gastrointestinal mucosal surfaces. However, surgeons must consider the inherent risks of oral agents attacking normal bowel flora, such as diarrhea, Clostridium difficile infection, or pseudomembranous colitis. The concomitant use of probiotics is being considered to decrease the incidence of such complications, but limited data are available at the current time.49
Parenteral antibiotics are widely used before colorectal operations and are typically administered within one hour of surgery. They have been shown to significantly decrease the risk of surgical-site infections.50 Typical agents include second- or third-generation cephalosporins. Overall, the use of oral and parenteral antibiotics before colorectal surgery has been shown to lower the concentration of colonic bacteria and decrease the rate of infection.51,52
Complications
Electrolyte abnormalities have been associated with osmotic laxatives. Sodium phosphate has been associated with hyperphosphatemia, hypokalemia, elevated blood urea nitrogen levels, hypocalcemia, and hyponatremia. Although elevated phosphate levels are generally asymptomatic, they can occur in up to 40% of patients and be significant in patients with renal failure.43 In 2008, manufacturers recalled the oral preparation of Fleets Phospho-soda after the FDA issued a safety alert warning consumers of the possible risks of electrolyte abnormalities and renal failure. The over-the-counter use of the product was found to be associated with acute phosphate nephropathy, a severe form of kidney disease that could lead to failure, dialysis, or death. This oral preparation is now available by prescription only. Mannitol is also not used often due to studies demonstrating an increased concentration of hydrogen gas when fermented by E. coli that can lead to a potentially explosive gas when electrocautery is used.53
Rescue Strategies
In high-risk patients, such as the elderly, children, or those with renal compromise, inpatient bowel preparation should be considered to ensure proper hydration. A large number of patients complain of nausea and abdominal discomfort during the bowel preparation process and have difficulty with maintaining adequate oral hydration. In select patients, close monitoring, antiemetics, and intravenous hydration may help prevent electrolyte abnormalities and large fluctuations in fluid status.54
DVT PROPHYLAXIS
History
Prophylaxis against the development of an intraoperative thromboembolism is an important consideration in all surgical procedures, with significant health-care wide implications for morbidity, mortality, and health care costs. Because of this, the federal government has compiled the recommendations of several expert groups to produce a guideline based on the Americal College of Chest Physicians (ACCP) and the Institute for Clinical Systems Improvement’s consensus statements, all of which are highly congruent.55,56 These guidelines have been expanded and modified over the years, and currently explicity address laparoscopic procedures. These expand on older SAGES guidelines.57 The current guidelines are part of the SCIP program to limit surgical complications in several areas through standardization of perioperative care.
Anatomy
The understanding of the genesis of perioperative DVT has been well described and reviewed.58 The key anatomic abnormalities that lead to thrombus formation are based on venous stasis occurring during immobilization, which may be prolonged in advanced laparoscopic surgery. The vessels of particular interest include the deep calf, pelvic, and abdominal veins, where thrombi originate.
Physiology
The classic Virchow’s triad includes abnormalities of blood flow and blood clotting components. In combination with vessel wall injury due to surgery, these risk factors form the basis for the genesis of DVTs. The current understanding of the development of thrombi is focused on the generation of inflammatory intermediate generation and has been recently reviewed.59
Pathophysiology
Vessel wall injury, venous stasis, and the generation of cytokines during surgery all contribute to the formation of thrombi. Other risk factors commonly encountered in laparoscopy significantly impact the risk of DVT, including obesity, major medical comorbidity, cancer, and age >40. Acquired and inherited hypercoagulable states (such as lupus, protein C or S deficiency) also increase the risk of DVT. Management of perioperative DVT prophylaxis is based on the presence of the risk factors55 listed below.
Risk Factors for determining DVT prevention methods
Hospital based factors
Extended immobility or estimated length of stay of 4 or more days
Admission to the intensive care unit
Acute medical conditions
Active cancer
Acute infection
Acute respiratory failure
Uncompensated congestive heart failure
Chronic medical conditions
Inflammatory bowel disease
Nephrotic syndrome
Rheumatoid/collagen vascular disorder
Individual risk factors
Prior history of deep vein thrombosis/pulmonary embolism (the most important predictor of thromboembolism development)
Age >60
Thrombophilia – congenital or acquired
Obesity (body mass index >30)
Treatment
Current guidelines recommend that all patients be screened for DVT prophylaxis upon admission, and surgery is really a special case of this general category. Aspirin alone is generally not recommended for anyone anymore. All patients should undergo early ambulation as part of their postoperative care plan. Patients who cannot ambulate should at least have a method of mechanical DVT prophylaxis in place, such as intermittent pneumatic compression or graded compression stockings. Patients at moderate to high risk should have pharmacologic prophylaxis unless contraindicated, in which case mechanical methods are acceptable. The guidelines of the ACCP that pertain to laparoscopy include the following recommendations:56
For patients undergoing entirely laparoscopic procedures who do not have additional thromboembolic risk factors, they recommend against the routine use of thromboprophylaxis, other than early and frequent ambulation (Grade 1B).
For patients undergoing laparoscopic procedures in whom additional VTE risk factors are present, or major surgery, they recommend the use of thromboprophylaxis with one or more of low molecular weight heparin or its analogs, intermittent pneumatic compression, or graded compression stockings (all Grade 1C). These include dalteparin (2500 U sq 1-2 hours preoperative, then q 24 hours until discharge), enoxaparin (40 mg sq 2 hours preoperative, then q 24 hours until discharge), or unfractionated heparin (5000 U sq q 12 hours postoperative until discharge).
For patients with a history of a previous thromboembolism, concurrent malignancy, or more than one risk factor, longer term (4 weeks total duration), and higher dose pharmacologic prophylaxis is recommended: dalteparin (5000 U sq 1 to 2 hours preoperative, then q 24 hours), enoxaparin (40 mg sq 2 hours preoperative, then q 24 hours), or unfractionated heparin (5000 U sq q 8 hours postoperative).
For patients undergoing bariatric surgery, the duration of treatment is until discharge or up to 10 days postoperatively with higher doses of enoxaparin (40 mg sq 12 hours) and unfractionated heparin (5000 U sq q 8 hours postoperative or a continuous infusion to target antiXa levels of 0.15 to 2.0).
Complications
The complications of thromboembolism can be divided into 2 categories: superficial vein thrombosis or DVT confined to the calf, and deep venous thrombosis with pulmonary embolism. The former are managed much more conservatively than the latter, for which standard treatment guidelines are available. The development of DVT leads progressively to short-term consequences (thrombus extension, pulmonary embolism, and death) and long-term sequellae (postphlebetic syndrome).
One of the thrombotic complications that seems to be most specifically associated with laparoscopic surgery is the development of mesenteric venous thrombosis.60 Since this is a relatively newly identified condition, the actual incidence of it is likely to be underreported in the literature, but given the substantial volume of laparoscopic surgeries being performed, it is most likely quite rare although frequently catastrophic.
Cases of mesenteric venous thrombosis that occur following laparoscopy present primarily with extreme abdominal pain following surgery. In this condition, thrombosis occurs throughout the mesentery of the small or large intestine, leading to venous stasis and necrosis of the bowel. This can include thrombosis of the portal venous system as well. If not identified promptly, bowel infarction leads to fulminant sepsis and death.
Patients may present immediately or up to 2 months after surgery, but most will occur in the first 2 weeks. Risk factors for thromboembolism have been identified in about half of patients. Surgical exploration, anticoagulation, and thrombolytic therapy are potential treatment options.
Rescue Strategies
Management will be determined by the location and extent of thromboembolism present, along with other risk factors. As with prophylaxis, the American College of Chest Physicians published guidelines for thromboembolism treatment in 2008, which have been reviewed and summarized recently.61 The current recommendations are scaled according to a grading system, where numbers (1-3) represent risk (cost)/benefit analysis (grade 1 treatments have costs and risks that are clearly outweighed by the benefits) and letters (A-C) that correspond to the strength of the available research evidence. Although current recommendations focus on medical management, the possibility of thrombolysis62 or thrombectomy63 may be considered depending on institutional capabilities.
The goal of medical therapy for thromboembolism is to begin heparin therapy immediately (even before diagnostic tests are completed if a high clinical suspicion is present) along with warfarin until the patients international normalized ratio (INR) is >2 for at least 24 hours. Because of paradoxical hypercoagulability, heparin therapy should be continued for at least 5 days after warfarin has been started. Currently, unfractionated heparin is not preferred for initial therapy in most patients, which means that outpatient treatment is appropriate for the majority of cases. These agents do not require monitoring of laboratory values. Since they are metabolized by the kidney, in patients with renal failure or who are pregnant, dosing may need to be adjusted, and this is done based on measurement of anti-Xa activity. For patients with a high risk of bleeding (such as in the postoperative period), a shorter acting agent (unfractionated heparin) may be preferable to longer acting agents because it can be discontinued rapidly in the case of hemorrhage.
Thromboembolism treatment guidelines
Enoxaparin* 1 mg/kg SC every 12 h or 1.5 mg/kg SC daily
Tinzaparin* 175 IU/kg SC daily
Dalteparin* 100 IU/kg SC every 12 h or 150 IU/kg SC daily
Fondaparinux* (based on body weight (BW)) 5 mg (BW<50 kg), 7.5 mg (BW: 50-100 kg), or 10 mg (BW>100 kg)
Unfractionated heparin IV infusion, adjust rate based on plasma levels or 333 units/kg SC once, followed by fixed dose 250 units/kg SC twice daily
SURGICAL-SITE INFECTION PREVENTION
History
In a manner similar to the creation of the Universal Protocol by JCAHO, several healthcare-related organizations have come together to create the Surgical Care Improvement Project (SCIP).64 This program originally was started by the Centers for Disease Control in an effort to decrease postoperative surgical-site infections.65 Since then, the Center for Medicaid Services, ACS, and ASA have joined in along with the IHI to create a series of perioperative care guidelines designed to decrease postoperative complications from several causes. Just as with the Universal Protocol, the federal government and accreditation bodies have become involved, and all guidelines now have specific performance measures. These are checklist driven review notes that allow a chart abstractor or reviewer to evaluate whether certain protocols were followed. In this manner, the SCIP program is really designed to develop a process of care across the country, rather than direct the management of individual patients.
Anatomy
The SCIP program covers the prevention of perioperative infection, thromboembolism prevention, cardiac complication prevention, and respiratory complication prevention.66 The most in-depth guidelines are for infection prevention and cover preoperative antibiotic dose and (dis)continuation, proper skin preparation, proper hair removal, and the maintenance of normothermia. A review of hospitals where these processes have been implemented has demonstrated an improvement in surgical outcomes.67
Hair Removal
For many procedures, hair removal is required for a clear operative field; however, there are many different methods to accomplish this task: removal the day of or the day before surgery, and the use of a razor, clippers, or depilatory cream. Since all methods will cause some degree of skin irritation, it is possible that hair removal could lead to an increase in surgical-site infections. Implementation of a preoperative patient management pathway including the SCIP protocol measures has been shown to decrease surgical-site infections.68
Skin Preparation
As part of a team effort to reduce perioperative surgical-site infections, topical reduction in skin flora using some method of site preparation is a mainstay of modern surgical practice. However, a wide range of traditional methods are available, as are more innovative approaches, such as skin sealants that immobilize bacterial migration into the wound.69
Pathophysiology
The occurrence of surgical-site infections is due to breaks in the protective dermis of the skin that occurs due to the inherently invasive nature of surgical procedures. The risk factors for the development of surgical-site infection (SSI) are well known and include the contamination of the wound site, advanced age, poor nutritional status, smoking, chronic steroid use, immune deficiency, external catheters or prostheses, prolonged hospitalization, coexistent infection, body mass index, operative approach, length of procedure, significant blood loss, and medical comorbidities.70,71 Using the laparoscopic approach generally decreases SSI by at least half in digestive tract surgery72 and gynecologic surgery.73
Administering prophylactic antibiotics to all patients undergoing any type of surgery has several drawbacks, which are the impetus for the development of the various guidelines. The widespread use of any particular antibiotic will rapidly induce resistance among bacteria, thus limiting the effectiveness of preoperative administration. Antibiotic administration has individual risks as well, typically based on an individual patient’s allergic reaction, which is rarely severe, but a risk that can be avoided by limiting administration to select individuals. Cost effectiveness is often difficult to measure, and for many procedures antibiotics are both inexpensive and effective; however, limiting their use to indicated cases can provide savings when applied across the entire healthcare system.
Prophylactic antibiotic administration for nondental surgeries solely for the purpose of preventing infective endocarditis is no longer recommended.74
Treatment
Currently, a variety of specialty society guidelines are available that provide recommendations on the use of prophylactic antibiotics to prevent surgical infection. In gynecology, according to the American College of Gynecology guidelines,75. laparoscopy, either diagnostic or operative, does not require the use of prophylactic antibiotics. One reason is that these preocedures are classified as clean, rather than clean/contaminated, which would include all urogynecology procedures and hysterectomy. Laparoscopic supracervical hysterectomy could be considered a more extensive operative laparoscopic procedure and thus not necessarily require the use of preoperative antibiotics; however, current guidelines do not provide such a fine distinction, and it is included with all other hysterectomies.
Guidelines for the use of prophylactic antibiotic use in general surgery have been summarized in the SCIP reccomendations.66 These guidelines do recommend the use of prophylactic antibiotics in cardiovascular, orthopedic, and colorectal surgeries. One of the drawbacks of these consensus statements is that the use of antibiotics in many particular types of surgery, especially laparoscopy, is not addressed. This provides flexibility for the surgeon; however, one of the impetuses for the development of guidelines is to decrease inappropriate antibiotic administration, with the goal of reducing anitbiotic resistance among common pathogens.
Preoperative antibiotic prophlyaxis for urologic procedures has been promulgated by the American Urological Association.76 The administration guidelines agree with those of other specialty societies: antibiotics should begin within 60 minutes of the incision (120 minutes if quinolone or vancomycin is used), active prophylaxis should be provided during the course of the surgery, and should be discontinued within 24 hours. Procedure- specific recommendations are abstracted based on the surgical site: antibiotic prophlyaxis is recommended if entry into the genitourinary tract or intestines is anticipated. For cases where the skin only is incised, antibiotics are indicated only if risk factors for infection are present.
Hair Removal
A systematic Cochrane database review77 found no evidence for or against hair removal on the day of surgery compared with the day before. Similarly, there was no difference between clipping or the use of a depilatory cream for hair removal. The only positive finding was that using a razor resulted in more surgical-site infections.78
Skin Preparation
Randomized clinical trials have compared the 3 common skin preparation techniques: ChloraPrep (2% chlorhexidine gluconate and 70% isopropyl alcohol), DuraPrep (0.7% iodophor and 74% isopropyl alcohol), and povidone-iodine scrub and paint (0.75% iodine scrub and 1.0% iodine paint). However, even the most recent studies have failed to consistently demonstrate the superiority of one approach over another.79,80 For this reason, the current SCIP guidelines do not endorse any one method as superior to another, and simply state that some method of skin preparation must be accomplished prior to incision.
Normothermia
Human body temperature is tightly regulated under physiologic conditions, to within a tenth of a degree. However, during surgery, the combination of sedation, paralysis, and breaks in the insulating layer (surgical incisions) all combine to disrupt normal homeostatic thermoregulation and lead to hypothermia if not treated. Although many regulatory systems are disrupted by hypothermia, the SCIP guidelines target maintenance of temperature as part of the surgical-site infection prevention program. Although including all of the protocol items may appear burdensome, in actual practice they can be implemented and this does decrease infection and complications.81,82 The molecular mechanisms underlying the pathological changes encountered in hypothermia are under investigation and lend further support to this approach.83
Complications
Complications of surgical-site infection fall into 2 categories: the development of infection in the patient, and failure to follow SCIP guidelines for the surgeon. Generally, most hospitals will have mandatory protocols in place that limit the opportunity for the surgeon to skip any of the required elements, because removing razors from the surgical suites and the determination of skin preparation typically is not specified by the individual surgeon. Improving compliance with antibiotic reccomendations, including termination of prophylactic antibiotics, can be more challenging from an institutional perspective. Failure to abide by hospital guidelines will generally result in an opportunity to discuss the matter with the surgical quality assurance committee. The consequences of violating hospital policies vary depending on medical staff bylaws.
Surgical-site infection occurs at a variable rate that depends heavily on all of the listed risk factors.84 Aggressive infections with nosocomial bacteria are among the most serious, and their management has been reviewed.85 In general, use of the laparoscopic approach leads to a 50% decrease or more in surgical-site infections compared with open procedures.86,87 Obviously, the best method for management of surgical-site infections is to prevent them by using the techniques described.88
Rescue Strategies
Management of surgical-site infection follows an algorithm ranging from conservative (antibiotics, debridement, packing) to aggresseive (hyperbaric oxygen, vacuum-assisted wound healing). Use of any particular approach will depend on the location of the wound, likely organisms (although wound infections are frequently polymicrobial), response to previous treatments, and medical comorbidities. Management protocols for complex wound infections are available.89 These guidelines have been recently reviewed and expanded for difficult surgical infections.90,91
PATIENT POSITIONING
History
Since the introduction of laparoscopy over a century ago, more and more patient positions are being utilized to improve visualization and maneuverability. The Trendelenburg and reverse Trendelenburg positions are 2 of the most commonly used variations of the supine position and have become immensely helpful in laparoscopic surgery. It is essential that a surgeon understand these different positions so he can take advantage of the most beneficial ones and take into account any possible consequences.
Anatomy
Traditionally, the supine position has been the most commonly utilized for surgical procedures. Most surgeons find the position to be safe, because it resembles the natural sleeping position. In this position, patients are lying flat on their backs. While in this position, patients can be placed in the Trendelenburg, reverse Trendelenburg, or a lateral tilt. Arms should be placed on padded arm boards at no more than a 90-degree angle and supinated according to the ASA guidelines.92
When the surgeon is focusing on the pelvis, such as in laparoscopically assisted hysterectomies or tubal sterilization, placing a patient in the Trendelenburg position a can help move the bowel out of the surgical field. In a similar fashion, the reverse Trendelenburg position can shift the abdominal contents into the pelvis for better visualization during laparoscopic cholecystectomies and fundoplication. In cases with steep Trendelenburg, placing nonsliding mattresses and gel pads underneath the patient may be useful. The use of shoulder supports is discouraged, because recent studies have shown an increased correlation with brachial nerve injury.
A lateral tilt may be accomplished by tucking a roll under one side of the patient and may be useful when gaining access to the gallbladder or retroperitoneal structures, such as the kidney. In laparoscopic splenectomy, a 45-degree tilt is often beneficial. Additionally, women who may be pregnant at the time of surgery should be placed in a left lateral decubitus position to take pressure off of the inferior vena cava and improve blood flow to the uterus and placenta.
The lithotomy position is also very familiar to surgeons and is often used for access in gynecologic and genitourinary procedures. In this position, the patient is supine and has his legs elevated and abducted with flexion at the hips and knees. The lower extremities are suspended either with stirrups or foot straps. It is important that the heel of the foot and leg are supported evenly and are in-line with the hip and contralateral shoulder.
The lateral decubitus position places the patient on one side and is helpful in accessing the retroperitoneal structures, such as in laparoscopic nephrectomies. Proper padding and support are necessary. Placing a roll under the chest may also help in decreasing the risk of upper extremity neuropathy.
The lateral jackknife position is a variation of this position used in laparoscopic adrenalectomy and thoracoscopy. The operative table is flexed downwards to provide flexion and lateral extension of the sidewall and provide optimal exposure.
Physiology
The human cardiovascular system plays an important role in patient positioning. As a patient moves from upright to supine or Trendelenburg, the heart and peripheral vasculature alter the cardiac output and vascular resistance to maintain a relatively constant blood pressure and ensure perfusion of the central nervous system and vital organs.
The basic effect of positioning on pulmonary function is also important to understand. With spontaneous ventilation, the diaphragm, rib cage, and abdomen all function as a unit to expand the chest wall and assist in normal respiratory function. In the upright position, the diaphragm is pulled downwards and helps maintain functional residual capacity. In the supine and Trendelenburg positions, abdominal expansion is more difficult and the contents of the abdomen begin to exert upward pressure on the diaphragm.93
Pathophysiology
While the Trendelenburg and reverse Trendelenburg positions are very helpful to the surgeon in gaining excellent visualization of the surgical field, they are not without their risks. When a patient is head down during laparoscopic surgery, the combined pressure of the abdominal contents and pneumoperitoneum exert pressure on the diaphragm and decrease functional residual capacity. Dependent edema can also contribute to swelling of the eye, face, or throat in the Trendelenburg position. Conversely, this can also lead to venous stasis in the lower extremities in a reverseTrendelenburg.
Nerve injury is another factor to consider when positioning a patient before surgery. Predominant mechanisms of injury are excess stretching, compression, metabolic imbalance, generalized ischemia, or by direct surgical transection. Even a 10% to 15% extension of a peripheral nerve can affect sensation or function.94
The 3 risk factors found to be strongly associated with development of a postoperative neuropathy in the lithotomy position were prolonged surgery (over 3 hours), very thin body habitus, and recent cigarette smoking. Nerve compression sustained over a long period can adversely affect myelination, leading to transient neuropathies. Smoking is also known to cause vasoconstriction and predispose patients to ischemia along the distribution of certain nerves. Patients with poorly controlled diabetes or preexisting neuropathies or peripheral vascular disease may also be at an increased risk for neurologic and vascular complications.
Treatment
Most neuropathies encountered are transient and due to demyelination of the peripheral nerve fibers. These patients generally have a return to normal function and sensation in 4 to 6 weeks.93 More severe injury or complete disruption of the nerve takes considerably longer and can require surgery in some cases.
Complications
Neuropathies are one of the most notable complications associated with patient positioning in surgery. Most injuries are due to some degree of hyperextension, flexion, compression, or surrounding ischemia of the nerve. The ulnar nerve can often be compressed when the arms are being tucked or by surgeons leaning against the extremity. The dorsal lithotomy position can lend to various neuropathies due to flexion or extension at the joints or direct contact with the stirrups. The obturator nerve can be injured in the course of pelvic node dissection. A case of obturator neuropathy was even noted with laparoscopic tubal sterilization.95 Keeping in the mind the common causes of these neuropathies can help guide the surgeon in their prevention (Table 2). Other complications, such as lower extremity compartment syndrome, have been reported due to increased pressure and decreased tissue perfusion in the lithotomy position.96

Rescue Strategies
While each surgery varies from patient to patient, positioning can also need to be modified accordingly. However, it is always important to place a patient in an anatomical position that places the least amount of undue stress on his extremities. Proper positioning and adequate cushioning with foam padding, pillows, or specially molded gel pads can go a long way in preventing injury. Taking care to properly pad the elbows before tucking the arms may aid in preventing compression of the ulnar or radial nerves. While postoperative ulnar neuropathy has been shown to occur despite proper positioning and padding, it can decrease the incidence overall. Stirrups should be adjusted to distribute pressure equally over the leg while keeping the ankle and knee in line with the contralateral shoulder. Taping the eyes early after the induction of anesthesia can decrease the incidence of corneal abrasions and injury to the eyes duringsurgery.93
In addition, a surgeon should be cognizant of special issues such as finger safety, especially in the lithotomy position where the foot of the bed is lowered. This can result in serious injury if the hand or fingers are caught in the gap of the bed when the foot of the bed is returned to a vertical position. Another subject that is becoming increasingly prevalent is robotic surgery. Whether a patient is supine or in the lithotomy position, the surgeon must be vigilant in placement of the robotic arms and cords. A thorough inspection of robotic arm placement should take place after docking to ensure that neither the robot nor the attached cables are resting on the patient.
In elderly patients, it is important to assess range of motion and any possible limitations before the induction of anesthesia. Some of these patients may have limited mobility and diminished peripheral vasculature. It is important to avoid hyperextension or excessive abduction of the extremities. Previous hip or other joint surgeries should be taken into account as well. Obese patients may have special requirements; special stirrups, or nonslip mattresses, and gel pads should be considered prior to surgery.
References
- Joint Commission for the Accreditation of Healthcare Organizations. Surgical Care Improvement Project Core Measure Set. Oakbrook Terrace, IL: The Joint Commission; 2006.
- Joint Commission for the Accreditation of Healthcare Organizations. Accreditation program: Hospital. National Patient Safety Goals. JCAHO;2010:20-24.
- Shojania KG, Duncan BW, MacDonald KM, Wachter RM, Markowitz AJ. Making health care safer: a critical analysis of patient safety practices. Evid Rep Technol Assess (Summ). 2001;43:i-x, 1-668.
- To Err is Human: Building a Safer Health System. Washington, DC: The Institute of Medicine; 1999.
- Accreditation program: Hospital. National Patient Safety Goals. JCAHO;2010:20-24.
- Herr HW. Ignorance is bliss: the Listerian revolution and education of American surgeons. J Urol. 2007;177(2):457-460.
- Peters JA. The devil in the doctor. How to cope with problem physicians. MGMA Connex. 2003;3(2):50-53, 1.
- Towle A, Hoffman J. An advanced communication skills course for fourth-year, post- clerkship students. Acad Med. 2002;77(11):1165-1166.
- Falagas ME, Korbila IP, Giannopoulou KP, Kondilis BK, Peppas G. Informed consent: how much and what do patients understand? Am J Surg. 2009;198(3):420-435.
- Powderly KE. Patient consent and negotiation in the Brooklyn gynecological practice of Alexander J.C. Skene: 1863-1900. J Med Philos. 2000;25(1):12-27.
- Schloendorff v Society of New York Hospital. 211 N.Y. 127, 105 N.E. 92. 1914.
- Forum NQ. Safe Practices for Better Healthcare – 2009 Update: A Consensus Report. Washington, DC: NQF; 2009.
- Jones W. Vol II. London: Heinemann. 1923;6-7:192-193.
- Ephesos IV. Historia Ecclesiastica. Louvain: EW Brooks; 1964;3:91-96.
- Curtius Q. History of Alexander. London: Heinemann;1971;(2):99-103.
- Dalla-Vorgia P, Lascaratos J, Skiadas P, Garanis-Papadatos T. Is consent in medicine a concept only of modern times? J Med Ethics. 2001;27(1):59-61.
- Moskop JC, Marco CA, Larkin GL, Geiderman JM, Derse AR. From Hippocrates to HIPAA: privacy and confidentiality in emergency medicine–Part I: conceptual, moral, and legal foundations. Ann Emerg Med. 2005;45(1):53-59.
- Moskop JC, Marco CA, Larkin GL, Geiderman JM, Derse AR. From Hippocrates to HIPAA: privacy and confidentiality in emergency medicine–Part II: Challenges in the emergency department. Ann Emerg Med.2005;45(1):60-67.
- Gates EA. New surgical procedures: can our patients benefit while we learn? Am J Obstet Gynecol. 1997;176(6):1293-1298; discussion 1298-1299.
- American College of Surgeons. Statement on ensuring correct patient, correct site, and correct procedure surgery. Bull Am Coll Surg. 2002;87(12):26.
- WHO Collaborating Centre for Patient Safety Solutions. Communication During Patient Hand-Overs. Patient Safety Solutions. 2007;1(3):1-4.
- Organizations, HCAHO. Accreditation program: Hospital. National Patient Safety Goals. JCAHO. 2010:20-24.
- O’Byrne WT, 3rd, Weavind L, Selby J. The science and economics of improving clinical communication. Anesthesiol Clin. 2008;26(4):729-744, vii.
- Riesenberg LA, Leitzsch J, Little BW. Systematic review of handoff mnemonics literature. Am J Med Qual. 2009;24(3):196-204. Epub 2009 Mar 5.
- Miller RD, Miller’s Anesthesia. Vol. 1. 6th ed. Miller RC, ed. Philadelphia: Elsevier Churchill Livingstone; 2005.
- Yates D, Caldicott L. Preoperative assessment of vascular patients. Anaesthesia and Intensive Care Medicine.2007;8(6):239-243.
- Bill K. Anaesthesia for patients with cardiac disease undergoing non-cardiac surgery. Anaesthesia and Intensive Care Medicine. 2006;7(9):331-334.
- Demyttenaere S, Feldman LS, Fried GM. Effect of pneumoperitoneum on renal perfusion and function: a systematic review. Surg Endosc. 2007;21(2):152-60. Epub 2006 Dec 9.
- Power M, Ostrow CL. Preoperative diabetes management protocol for adult outpatients. J Perianesth Nurs. 2008;23(6):371-378.
- Dunne JR, Malone D, Tracy JK, Napolitano LM. Perioperative anemia: an independent risk factor for infection, mortality, and resource utilization in surgery. J Surg Res. 2002;102(2): 237-244.
- Jones DR, Lee HT. Surgery in the patient with renal dysfunction. Med Clin North Am. 2009;93(5):1083-1093.
- Huyse FJ, Touw DJ, van Schijndel RS, De Lange JJ, Slaets JP. Psychotropic drugs and the perioperative period: a proposal for a guideline in elective surgery. 2006;47(1):8-22.
- Boni L, Benevento A, Rovera F, et al. Infective complications in laparoscopic surgery. Surg Infect (Larchmt). 2006;7(Suppl 2):S109-S111.
- Awad H, Santilli S, Ohr M, et al. The effects of steep Trendelenburg positioning on intraocular pressure during robotic radical prostatectomy. Anesth Analg. 2009;109(2):473-478.
- Sammour T, Mittal A, Loveday BP, et al. Systematic review of oxidative stress associated with pneumoperitoneum. Br J Surg. 2009;96(8):836-850.
- Regli A, Habre W, Saudan S, et al. Impact of Trendelenburg positioning on functional residual capacity and ventilation homogeneity in anaesthetised Anaesthesia. 2007;62(5):451-455.
- Nguyen NT, Wolfe BM. The physiologic effects of pneumoperitoneum in the morbidly obese. Ann Surg.2005;241(2):219-226.
- Hager P, Permert J, Wikström AC, Herrington MK, Ostenson CG, Strömmer Preoperative glucocorticoid administration attenuates the systemic stress response and hyperglycemia after surgical trauma in the rat. Metabolism. 2009;58(4):449-455.
- Lan YT, Lee JC, Wetzel G. Postoperative arrhythmia. Curr Opin Cardiol. 2003;18(2):73-78.
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, the ADQI workgroup. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204-12. Epub 2004 May 24.
- Brunicardi F. Schwartz’s Principles of Surgery. 8th ed. New York, NY: McGraw- Hill; 2005.
- Schanz S, Kruis W, Mickisch O, et al. Bowel preparation for colonoscopy with sodium phosphate solution versus polyethylene glycol-based lavage: a multicenter Diagn Ther Endosc. 2008;713521.
- Belsey J, Epstein O, Heresbach D. Systematic review: oral bowel preparation for colonoscopy. Aliment Pharmacol Ther. 2007;25(4):373-384.
- Zmora O, Lebedyev A, Hoffman A, et al. Laparoscopic colectomy without mechanical bowel preparation. Int J Colorectal Dis. 2006;21(7):683-687. Epub 2005 Oct 18.
- Muzii L, Bellati F, Zullo MA, Manci N, Angioli R, Panici PB. Mechanical bowel preparation before gynecologic laparoscopy: a randomized, single-blind, controlled trial. Fertil Steril. 2006;85(3):689-693.
- Slim K, Viczaut E, Launay-Savary MV, Contant C, Chipponi J. Updated systematic review and meta-analysis of randomized clinical trials on the role of mechanical bowel preparation before colorectal surgery. Ann Surg. 2009;249(2): 203-209.
- Pineda CE, Whelton ML. Mechanical bowel preparation in intestinal surgery: a meta-analysis and review of the literature. J Gastrointest Surg. 2008;12(11):2037-2044. Epub 2008 Jul 12.
- Bucher P, Gervaz P, Egger JF, Soravia C, Morel P. Morphologic alterations associated with mechanical bowel preparation before elective colorectal surgery: a randomized trial. Dis Colon Rectum. 2006;49(1):109-112.
- Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. 2007;335(7610):80. Epub 2007 Jun 29.
- Howard DD, White CQ, Harden TR, Ellis CN. Incidence of surgical site infections postcolorectal resections without preoperative mechanical or antibiotic bowel preparation. Am Surg. 2009;75(8):659-663; discussion 663-664.
- Hayashi MS, Wilson SE. Is there a current role for preoperative non-absorbable oral antimicrobial agents for prophylaxis of infection after colorectal surgery? Surg Infect (Larchmt). 2009;10(3):285-288.
- Nichols RL, Choe EU, Weldon CB. Mechanical and antibacterial bowel preparation in colon and rectal surgery. 2005;51(Suppl 1):115-121.
- Ladas SD, Karamanolis G, Ben-Soussan E. Colonic gas explosion during therapeutic colonoscopy with electrocautery. World J Gastroenterol. 2007;13(40):5295-5298.
- Lichtenstein GR, Cohen LB, Uribarri J. Review article: Bowel preparation for colonoscopy–the importance of adequate hydration. Aliment Pharmacol Ther. 2007;26(5):633-641.
- Venous thromboembolism prophylaxis. Bloomington, MN: Institute for Clinical Systems Improvement (ICSI). 2008
- Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. 8th ed. 2008;133(6 Suppl):381S-453S.
- Guidelines for deep venous thrombosis prophylaxis during laparoscopic surgery. Surg Endosc. 2007;21(6):1007-1009. Epub 2007 Apr 5.
- Turpie AG, Chin BS, Lip GY. Venous thromboembolism: pathophysiology, clinical features, and prevention. 2002;325(7369):887-890.
- Henke PK, Wakefield T. Thrombus resolution and vein wall injury: dependence on chemokines and leukocytes. Thromb Res. 2009;123(Suppl 4):S72-S78.
- James AW, Robl C, Westphalen AC, Fogarty PF, Posselt AM, Campos GM. Portomesenteric venous thrombosis after laparoscopic surgery: a systematic literature review. Arch Surg. 2009;144(6):520-526.
- Houman Fekrazad M, Lopes RD, Stashenko GJ, Alexander JH, Garcia D. Treatment of venous thromboembolism: guidelines translated for the clinician. J Thromb Thrombolysis. 2009;28(3):270-275.
- Bick RL. Proficient and cost-effective approaches for the prevention and treatment of venous thrombosis and thromboembolism. 2000;60(3):575-595.
- Rao AS, Konig G, Leers SA, et al. Pharmacomechanical thrombectomy for iliofemoral deep vein thrombosis: an alternative in patients with contraindications to thrombolysis. J Vasc Surg. 2009;50(5):1092-1098. Epub 2009 Sep 26.
- Surgical Care Improvement Project Core Measure Set. Oakbrook Terrace, IL: The Joint Commission; 2006.
- Bratzler DW. The surgical infection prevention and surgical care improvement projects: promises and pitfalls. Am Surg. 2006;72(11):1010-1016; discussion 1021-1030, 1133-1148.
- Bratzler DW, Houck PM. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189(4):395-404.
- Dellinger EP, Hausmann SM, Bratzler DW, et al. Hospitals collaborate to decrease surgical site infections. Am J Surg. 2005;190(1):9-15.
- Graf K, Sohr D, Haverich A, Kühn C, Gastmeier P, Chaberny IF. Decrease of deep sternal surgical site infection rates after cardiac surgery by a comprehensive infection control program. Interact Cardiovasc Thorac Surg. 2009;9(2):282-286. Epub 2009 May 5.
- Owens CD, Stoessel K. Surgical site infections: epidemiology, microbiology and prevention. J Hosp Infect. 2008;70(Suppl 2):3-10.
- Poon JT, Law WL, Wong IW, et al. Impact of laparoscopic colorectal resection on surgical site infection. Ann Surg. 2009;249(1):77-81.
- Schaeffer A, Schaeffer E. Infections of the urinary tract. In: Campbell-Walsh Urology. Wein A, et al, eds. Philadelphia: Saunders-Elsevier; 2007:223-303.
- Imai E, Ueda M, Kanao K, et al. Surgical site infection risk factors identified by multivariate analysis for patient undergoing laparoscopic, open colon, and gastric surgery. Am J Infect Control. 2008;36(10):727-731. Epub 2008 Oct 3.
- Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009(3):CD003677.
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. J Am Dent Assoc. 2008;139(Suppl):3S-24S.
- ACOG practice bulletin No. 104: antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2009;113(5):1180-1189.
- Wolf JS, Jr, Bennett CJ, Dmochowski RR, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol. 2008;179(4):1379-1390. Epub 2008 Feb 20.
- Tanner J, Woodings D, Moncaster K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev. 2006;3:CD004122.
- Ko W, Lazenby WD, Zelano JA, Isom OW, Krieger KH. Effects of shaving methods and intraoperative irrigation on suppurative mediastinitis after bypass operations. Ann Thorac Surg. 1992;53(2):301-305.
- Saltzman MD, Nuber GW, Gryzlo SM, Marecek GS, Koh JL. Efficacy of surgical preparation solutions in shoulder surgery. J Bone Joint Surg Am. 2009;91(8):1949-1953.
- Swenson BR, Hedrick TL, Metzger R, Bonatti H, Pruett TL, Sawyer RG. Effects of preoperative skin preparation on postoperative wound infection rates: a prospective study of 3 skin preparation protocols. Infect Control Hosp Epidemiol. 2009;30(10):964-971.
- Dellinger EP, Hausmann SM, Bratzler DW, et al. Hospitals collaborate to decrease surgical site infections. Am J Surg. 2005;190(1):9-15.
- Forbes SS, Stephen WJ, Harper WL, et al. Implementation of evidence-based practices for surgical site infection prophylaxis: results of a pre- and postintervention study. J Am Coll Surg. 2008;207(3):336-341. Epub 2008 May 19.
- Qadan M, Gardner SA, Vitale DS, Lominadze D, Joshua IG, Polk HC Jr. Hypothermia and surgery: immunologic mechanisms for current practice. Ann Surg. 2009;250(1):134-140.
- Young MH, Washer L, Malani PN. Surgical site infections in older adults: epidemiology and management strategies. Drugs Aging. 2008;25(5):399-414.
- Anderson DJ, Kay KS. Staphylococcal surgical site infections. Infect Dis Clin North Am. 2009;23(1):53-72.
- Szomstein S, Lo Menzo E, Simpfendorfer C, Zundel N, Rosenthal RJ.. Laparoscopic lysis of adhesions. World J Surg. 2006;30(4):535-540.
- Boni L, et al. Infective complications in laparoscopic surgery. Surg Infect (Larchmt). 2006;7(Suppl 2):S109-S111.
- Casey AL, Elliott TS. Progress in the prevention of surgical site infection. Curr Opin Infect Dis. 2009;22(4):370-375.
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41(10):1373-406. Epub 2005 Oct 14.
- May AK, Stafford RE, Bulger EM, et al. Treatment of complicated skin and soft tissue infections. Surg Infect (Larchmt). 2009;10(5):467-499.
- Cainzos M. Review of the guidelines for complicated skin and soft tissue infections and intra-abdominal infections–are they applicable today? Clin Microbiol Infect. 2008;14(Suppl 6):9-18.
- Neuropathies, American Society of Anesthesiologists. Practice advisory for the prevention of perioperative peripheral neuropathies. 2000;92(4):1168- 1182.
- Miller RD. Miller’s Anesthesia. Vol 1. 6th ed. Miller RD, ed. Philadelphia: Elsevier Churchill Livingstone; 2005.
- Coppieters MW, Van de Velde M, Stappaerts KH. Positioning in anesthesiology: toward a better understanding of stretch-induced perioperative neuropathies. 2002;97(1):75-81.
- Jirsch JD, Chalk CH. Obturator neuropathy complicating elective laparoscopic tubal occlusion. Muscle Nerve.2007;36(1):104-106.
- Brunicardi F. Schwartz’s Principles of Surgery. 8th ed. New York, NY: McGraw- Hill; 2005.
