Leiomyomas: Minimally Invasive Approaches to Myomectomy
Mojgan Mohammadi, Mark H. Glasser
As more articles debating the prevalence of hysterectomies appear in the lay press and on the Internet, increasing numbers of women are demanding alternative procedures. A recent New York Times1 article decrying the prevalence of hysterectomies states that by the age of 60, 1 woman in 3 in the United States will have had her uterus removed. By comparison, in Italy the figure is 1 in 6, whereas in France, it is 1 in 18 women. Of the 600,000 hysterectomies performed annually in the United States, one-third are done for leiomyomas.2 This number rises dramatically for women over the age of 40 and for those in certain ethnic groups. For African American women, 61.3% of hysterectomies are done for leiomyomas, and for women in the 45 to 54 age group,
53% of all hysterectomies, regardless of race, are done for this indication. It is estimated that more than 25% of women over the age of 36 have one or more leiomyomas, with 50% of these being symptomatic.3
Uterine leiomyomas (myomas) are benign smooth muscle tumors arising from the myometrium. Despite the fact that myomas are quite common, very little is known about their cause. They are monoclonal, arising from a single myometrial cell. Different karyotypes of multiple myomas have been found in the same patient. What causes abnormal myometrial cells to transform into clinically detectable myomas is also unknown. There are, however, more estrogen receptors in myomas than in the surrounding myometrium and less estradiol conversion, so hormonal factors certainly play a role. There has also been speculation that chromosome rearrangement may contribute to tumor initiation and growth as well as stimulation by growth hormone and other insulin-like growth factors. Myomas are heterogeneous; some women have solitary myomas, whereas others have multiple myomas. Myomas may be subserous, intramural, pedunculated, or submucosal. There are even different classifications of submucosal myomas, according to the percentage of the myoma that is within the endometrial cavity versus the percentage within the myometrium. Treatment options depend greatly on careful classification of the myoma or myomas present in an individual patient and on what symptoms are most bothersome to her and require treatment.
Most myomas do not cause any symptoms, and the first lesson physicians must learn is that if the patient is asymptomatic, no treatment is necessary. The presence of an abdominal mass is not an indication for a hysterectomy or myomectomy unless it is of significant concern to the patient. Also, the old teaching that a hysterectomy is necessary in uteri larger than 12 gestational weeks because the adnexa cannot be adequately examined is no longer true in the age of modern imaging technology, such as ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI). Patients with myomas may present with a wide variety of symptoms, including menometrorrhagia, dyspareunia, pelvic pressure or discomfort, urinary frequency, bowel or back discomfort, infertility or repeated pregnancy loss, and the presence of a large pelvic mass.4 Symptoms very often are related to the size, type, and location of the myoma as well as the lifestyle of the individual patient. The criteria for intervention as outlined by the American College of Obstetrics and Gynecology in their published Quality Assurance criteria sets are as follows:
- Clinically apparent myomas that are a significant concern to the patient, even if otherwise asymptomatic;
- Myomas causing excessive bleeding and/or anemia;
- Myomas causing acute or chronic pain;
- Myomas causing significant urinary problems not due to other abnormalities
Various modalities besides the bimanual pelvic and rectal examination have been used to diagnose and classify myomas, the mainstay being transabdominal and transvaginal ultrasound. MRI has been used for more accurate myoma “mapping” as well as differentiation of pedunculated myomas from adnexal masses. To evaluate whether an intracavitary component is present, saline infusion sonography as well as hysteroscopy are valuable tools. Hysterosalpingography is useful in the evaluation of infertile patients with myomas, because tubal patency and the size and shape of the uterine cavity can be assessed.
The treatment of symptomatic leiomyomas for women who have completed childbearing has been, in the vast majority of cases, hysterectomy. Perimenopausal women are often given no choice other than hysterectomy, with myomectomy offered in only a small number of cases. Recent advances in the nonsurgical management of leiomyomas, including medical management with gonadotropin-releasing hormone (GnRH) analogues or antagonists, mifepristone, raloxifene, and progesterone receptor modifiers as well as uterine artery embolization, have been promising for these patients but may be inappropriate for those who want to preserve childbearing, because none has been shown to enhance fertility.5 Certainly, for women in the reproductive age group wanting to maintain fertility, a myomectomy remains the “gold standard.” Abdominal myomectomy, however, is associated with significant morbidity, including excessive blood loss, a high rate of blood transfusion, infection, and postoperative adhesions.6 Recurrence rates have been reported to be between 5% and 30%, with 20% to 25% of patients requiring a subsequent hysterectomy.7
LAPAROSCOPIC MYOMECTOMY
Laparoscopic myomectomy is an alternative to the abdominal approach, with fewer complications, shortened hospital stay, and less disability,8,9 but it is a difficult and tedious operation. Widespread acceptance of this procedure has been limited because of the advanced skills required, but the advent of better insufflators, light sources, and cameras as well as the electronic morcellator has increased the use of this procedure. It is felt by some authors that women with large intramural myomas who want to bear children should not be managed laparoscopically because meticulous repair of the uterus is difficult. Parker10 has established selection criteria for patients with symptomatic myomas for laparoscopic myomectomy, which include uterine size equal to or less than 14 weeks after a 3-month course of GnRH agonist therapy, no individual myoma larger than 7 cm, no myoma near the uterine artery or near the tubal ostia if fertility is desired, and at least 50% of the myoma subserosal, to be accessible and to allow adequate repair through the laparoscope.11 Certainly there are several highly skilled laparoscopists in the world who are comfortable managing larger myomas, but the vast majority of practicing gynecologists would resort to standard laparotomy in these cases. Sinha et al.,11 in a study published in 2003, removed 78 myomas in 51 patients. Three patients had two myomas between 5 cm and 9 cm (in addition to one ≥9 cm), and one had three myomas between 5 cm and 9 cm (in addition to one ≥9 cm). Mean number of myomas removed per patient was 1.53 ± 1.17 (range, 1 to 6); 12 women (23.5%) had multiple myomectomy. The largest myoma removed was 21 cm. Mean myoma weight was 698.47 ± 569.13 g (range, 210 to 3400 g). Mean operating time was 136.67 ± 38.28 minutes (range, 80 to 270 minutes). Mean blood loss was 322.16 ± 328.2 mL (range, 100 to 2000 mL). One patient developed a broad ligament hematoma, two developed postoperative fever, and one underwent open subtotal hysterectomy 9 hours after surgery for dilutional coagulopathy. Twenty women (39.2%) were given blood transfusions postoperatively; 10 received a single unit, six were given 2 U, three were given 3 U, and one was given 4 U.12 This study has a far higher incidence of blood transfusion than any study in the literature and may be explained by different criteria for transfusion in India. Nezhat and associates9 reported on myomectomy in 137 women, from whom 196 leiomyomas were removed.
The fibroids ranged in size from 2 to 14 cm. The operations lasted from 50 to 160 minutes (mean, 116 minutes). Estimated blood loss was between 10 mL and 600 mL, and two women received transfusions because of intraoperative blood loss. The hospital stay ranged from 7 to 48 hours, with a mean of 19.6 hours. In a retrospective multicenter study comparing myomectomy by laparoscopy and laparotomy, Marret et al.12 found that compared with women undergoing laparoscopic myomectomy, women undergoing open myomectomy had more myomas that were larger and that were generally interstitial and anterior. More of them received GnRH analogues. Excised myomas weighed four times more, the decrease in hemoglobin was greater (1 g/dL), fever was more frequent, and nine patients needed transfusions (compared to none for laparoscopic myomectomy).
There were 37 conversions to laparotomy (29%) after laparoscopic myomectomy. The conversion rate was high for inexperienced surgeons. Length of hospital stay was reduced by half for laparoscopic myomectomy (without conversion). Recurrence rate at 2 years was 2.5% for laparoscopic myomectomy versus 3.6% for open myomectomy (P = 0.506). The authors concluded that preoperative evaluation by ultrasound was essential to establish myoma number, size, type, and location to choose the most appropriate surgical procedure. The ideal candidate for laparoscopic myomectomy is a patient with fewer than three myomas, none larger than 8 to 9 cm. Those with pedunculated myomas are ideal for a minimally invasive approach regardless of the size. Patients with multiple myomas whose imaging studies report “fibroids too numerous to count” are not candidates for myomectomy because the myometrial damage created by excising them would be like doing a virtual hysterectomy. Certainly these patients should be counseled to accept hysterectomy if future childbearing is not desired. If the patient strongly desires keeping her uterus, she should be referred for uterine fibroid embolization.
Surgical Technique
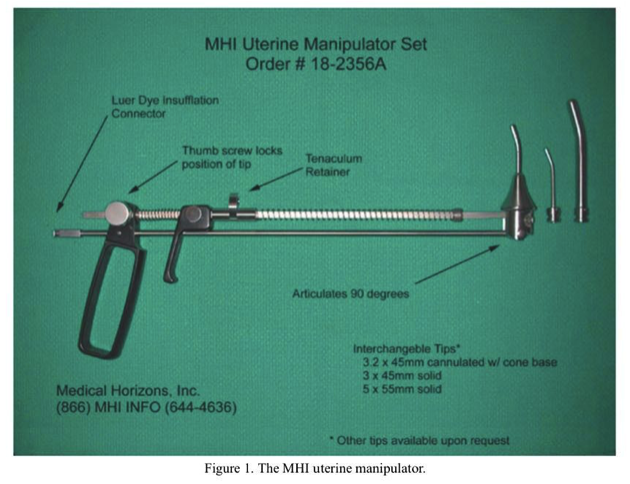
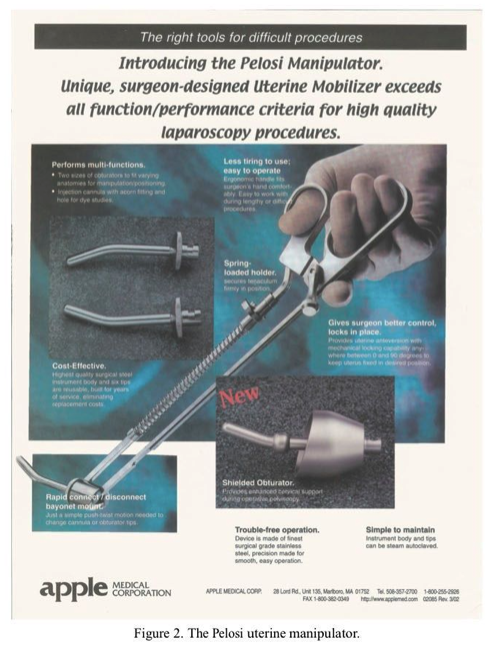
The patient is placed in the low lithotomy position using Allen stirrups (Allen Medical Systems, Cleveland, OH). Ten milliliters of a dilute vasopressin solution (2 U in 60 mL NaCl) is then injected intracervically about 1 to 2 cm deep at both the 8 and 4 o’clock positions. Laparoscopic myomectomy is facilitated by the use of a uterine manipulator. Although manipulators that use a balloon for stabilization of the device within the uterus work well for most laparoscopic procedures, these may be counterproductive in myomectomy. If the uterine cavity is entered, as occasionally occurs inadvertently or intentionally during the course of myoma enucleation, the balloon may be ruptured, causing the manipulator to fall out. We prefer the MHI uterine manipulator (Medical Horizons, Fair Oaks, CA; Figure 1) or the Pelosi manipulator (Apple Medical, Marlborough MA; Figure 2). The procedure is facilitated by the use of four ports, the sizes of which are dependent on which suturing technique is employed or whether an electronic morcellator is used. Generally, one can perform most laparoscopic myomectomies with an 11-mm umbilical port, a high lateral port on each side placed 3 cm below the umbilicus and 6 to 8 cm lateral to the midline. The lateral ports may be placed above the umbilicus for larger uteri. Finally, a suprapubic 11- to 12-mm port is placed, through which the morcellator can be inserted after dilating the port site to 15 mm. Alternatively, this port may be placed in the left lower quadrant. Before proceeding with the insertion of multiple accessory ports, the size and position of the myomas are carefully assessed. If there is any doubt about the safe performance of the myomectomy via laparoscopy, such as the presence of large inaccessible posterior or broad ligament myomas, a conventional laparotomy should be performed.
A dilute vasopressin solution (2 U in 60 mL of normal saline) is then injected into the serosa and myometrium overlying the myoma until the tissue blanches. This is easily accomplished using a control-top syringe and a 4-inch 22-gauge spinal needle, which is inserted percutaneously over the uterus. The tip of the needle is then guided into the proper sites along the myoma with a 5-mm grasper from a lateral port. The use of vasopressin for gynecologic surgery has been controversial but is widely accepted in the United States. In two prospective randomized studies, dilute vasopressin solution was found to decrease blood loss at time of myomectomy by laparotomy compared with placebo or a tourniquet.13 An alternative to the use of vasopressin is to inject 0.25% bupivacaine with epinephrine into the serosa and myometrium in the same fashion. This also has a vasoconstrictive effect and has been found in one randomized study to decrease the need for postoperative pain medication in women undergoing laparoscopic myomectomy as well as to decrease blood loss.14
Pedunculated myomas are the least difficult to manage laparoscopically. After the dilute vasopressin solution is injected into the stalk, the myoma is removed by cutting and coagulating the stalk. Care must be taken to stay close to the myoma and avoid thermal damage to the normal myometrium from which the stalk arises. An alternative technique is to place one or two ties around the pedicle (Endoloops [Ethicon] may be used) and to excise the myoma by electrosurgically cutting through the serosa around its base about 1 to 2 cm above the insertion of the pedicle. The pedicle can then be oversewn to assure hemostasis. This technique minimizes the risk of later uterine rupture, which has been reported during pregnancy following laparoscopic removal of a pedunculated myoma.15
The removal of subserous myomas is less challenging than the removal of deep intramural myomas. Dilute vasopressin is injected in multiple sites between the myometrium and the fibroid capsule. An incision is made on the serosa overlying the leiomyoma, using the CO2 laser (superpulse or ultrapulse mode), a monopolar electrode, a fiber laser, or harmonic scalpel. The incision is extended until it reaches the capsule.
The myometrium retracts as the incision is made and the myoma bulges outward. Two grasping, toothed forceps hold the edges of the myometrium, and the suction–irrigator can be used as a blunt probe to shell the leiomyoma from its capsule. A myoma screw is inserted into the tumor to apply traction while the suction–irrigator is used as a blunt dissector. An alternative is to insert a finger through the 12-mm suprapubic port site incision and manually dissect the myoma free from the myometrium. Once this is accomplished, the cannula is reinserted. Vessels are electrocoagulated before being cut. After complete removal of the myoma, the uterine defect is irrigated. Bleeding points are identified and controlled with bipolar or monopolar electrocoagulation. Point coagulation of identifiable vessels can be accomplished by using short bursts of cutting current while the vessel is grasped with a Maryland dissecting forceps, much as one would do in an open case with a hemostat. The edges of the uterine defect are approximated by superficial suturing.
Deep intramural or broad ligament intramural myomas are the most difficult to properly manage laparoscopically and should be done only by surgeons skilled in laparoscopic suturing. This is especially true if the patient plans future childbearing. The gold standard of myometrial closure after open myomectomy is a three-layered closure beginning at the base of the defect to obliterate the dead space with figure-of-eight or horizontal mattress sutures. A second layer of continuous suture is then placed to further approximate the myometrium and finally ending with a continuous imbricating “baseball” stitch on the serosa. Synthetic absorbable polyglactin sutures (Vicryl, Ethicon, Somerville, NJ; Polysorb, USSC, Norwalk, CT) are recommended because they produce less inflammatory reaction than catgut.
Intraligamentous and broad ligament myomas require careful observation of the course of the ureters and large blood vessels. Depending on the location of the myoma, an incision is made on the anterior or posterior leaf of the broad ligament. The myoma is removed with the techniques described above for subserosal and intramural tumors. Throughout the procedure, the location of the ureters is noted. Hemostasis is obtained with the sutures, clips, or bipolar forceps. None of the available lasers, despite the power setting or focus of the beam, can adequately coagulate bleeding myometrial vessels. A bipolar forceps, monopolar fine dissecting forceps (Maryland), or argon beam coagulator is excellent for this purpose. The broad ligament and peritoneum are not closed but allowed to heal spontaneously. Drains are used infrequently.
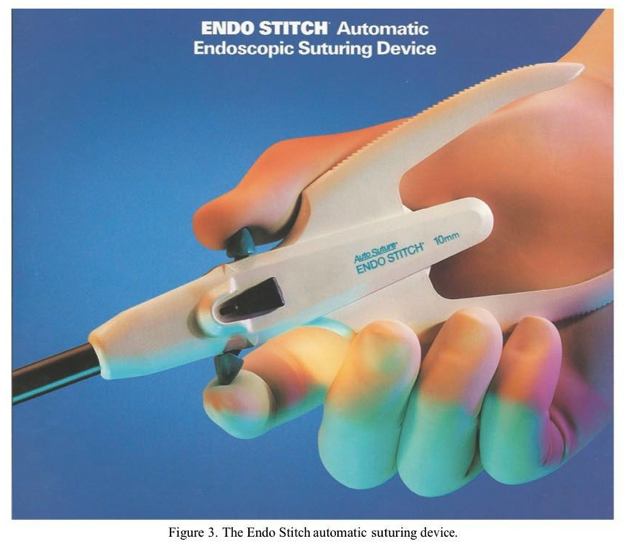
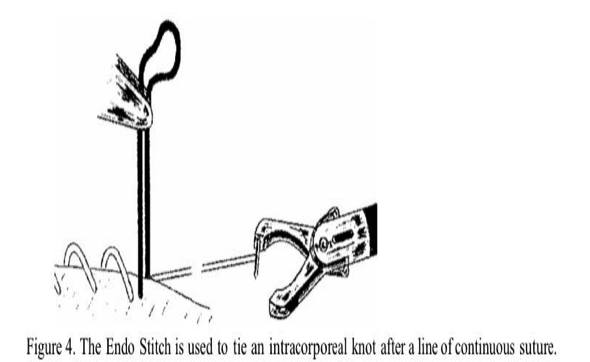
Stringer and associates16 described a simplified way of closing deep myometrial defects using the Endo Stitch automatic suturing device (United States Surgical, Norwalk CT).16 Multiple layers of continuous interlocking sutures are placed using this device, which captures the 9-mm needle in the opposite jaw when the handles are squeezed (Figure 3). The suture is held taut by the assistant, and finally an intracorporeal knot is tied with the device (Figure 4). The Endo Stitch must be used through a 10-mm port. Its limitations are a semi-straight needle that is relatively short. Once adept at using the device, one can often stitch faster than by using conventional open suturing techniques.
Recently, the da Vinci surgical robot (Intuitive Surgical) has been advocated as a suturing aid in performing laparoscopic myomectomy. The initial published report done in a university hospital setting showed a conversion rate of 8.6% to laparotomy and an average operating time of 230.8 ± 83 minutes. The average length of stay was 1 day.17Although this technology is certainly “space age,” it is very expensive in capital outlay, per case cost, and annual service contract cost. This device performs suturing precisely and because of its high cost, will be available only in a very few centers. The average operating time of close to 4 hours as reported above is unacceptable and far longer than the average operating time for laparoscopic myomectomy in the published literature. The degree of precision the system affords is hardly necessary or appropriate for myomectomy, which can be accomplished safely by many of the techniques discussed in this chapter. Until good data are presented clearly demonstrating the superiority of this instrument, gynecologists should approach it with a critical eye.
Specimen Removal Techniques
Other than laparoscopic suturing, the greatest challenge and often the most frustrating step in laparoscopic myomectomy is specimen removal. For multiple small myomas, the enucleated specimens can be placed in a specimen retrieval bag, brought up to the largest port site, and morcellated in the bag at the skin line using a #11 scalpel blade. Another technique for intra-abdominal morcellation of larger myomas is to grasp opposite sides of the myoma with strong-toothed graspers from each lateral port and to suspend it in the lower abdominal airspace near the anterior peritoneum. A narrow scalpel with a #11 blade is then passed through the suprapubic 12-mm port, and the myoma is cut into small segments, placed in a specimen retrieval bag, and removed through the umbilical or suprapubic port site after the cannula is removed. The port site incision can be made larger to facilitate this. If the cannula needs to be reinserted, a disposable cone- shaped adaptor or a Hasson cannula can be used.
The laparoscopic morcellator has been a significant advance for specimen removal during laparoscopic myomectomy and has saved many patients from major laparotomies. The most widely used device in the United States is made by Gynecare (Johnson & Johnson, Somerville, NJ). Other excellent electronic morcellators are made by Karl Storz, WISAP, and Richard Wolf. Because the morcellator blade is very sharp and turns at a high rate of speed, it is most important that the cutting edge of the blade be kept in view at all times. The morcellator is best inserted through the lateral or suprapubic port and held parallel to the abdominal wall. A full pneumoperitoneum must be maintained to maximize the airspace and minimize the risk of a loop of bowel or other structure being injured by coming into contact with the active blade. The myoma should be grasped with a heavy claw forceps or laparoscopic tenaculum and drawn into the morcellator blade without changing position of the morcellator. Slow, gentle pressure will withdraw the morcellated myoma in a single strip from the end of the morcellator. Excess traction should be avoided because it will sever the strip and necessitate regrasping. The surgeon should work from the periphery of the myoma and around it, almost like peeling a fruit. It is not advisable to drill through the center of the myoma because this technique takes much longer and increases the risk of injury to organs hidden from view by the myoma. It is also important to not lose sight of any fragments of myoma that have been morcellated and dropped into the abdomen or any small myomas that have been enucleated.
Transfixing these small myomas with a single suture and suspending them from the anterior abdominal wall is a good way to prevent their loss in the abdomen. One study reported the loss of a 6-cm myoma during a laparoscopic supracervical hysterectomy. The specimen became entangled and fixed to the mesentery of the small bowel in the upper abdomen and caused a bowel obstruction, resulting in the need for an exploratory laparotomy.18 Seeding of port sites with malignant cells or endometriosis is a well-known sequela of laparoscopic procedures. This may also occur with morcellated myoma tissue, as was shown in a case report by Ostrzenski.19
In a randomized trial, Sinha and associates20 evaluated two groups of patients undergoing laparoscopic myomectomy with at least one myoma 7 cm in diameter to assess the feasibility of enucleation of myomas by morcellation while the myoma is still attached to the uterus compared with standard enucleation and morcellation as described above. The mean weight of the myomas removed in each group was about 600 g. Blood loss, length of stay, and complication rates were similar in both groups, but operating time was significantly decreased in the group employing the technique of morcellation of the myomas while still attached to the uterus. The authors speculate that this technique may allow larger myomas to be managed laparoscopically. In situ morcellation of large myomas is the technique employed in minilap myomectomy, which is discussed at length later in this chapter.
Another variation of laparoscopic myomectomy used as an alternative to the morcellator is specimen removal through a colpotomy incision. The colpotomy incision can be done vaginally below the cervix between the uterosacral ligaments. This is facilitated by transfixing the myoma with a tumor screw and pushing it into the cul-de-sac laparoscopically. This allows the vaginal surgeon to make the colpotomy incision directly over the myoma without fear of injury to intra-abdominal structures. Once the peritoneum is opened, the myoma is grasped vaginally with a tenaculum or Leahy clamp and removed intact or progressively morcellated using a coring technique. Alternatively, the vagina can be identified laparoscopically by the uterine manipulator, vaginal probe, or a sponge stick placed in the posterior fornix vaginally. An incision is then made laparoscopically using an electrosurgical needle or scissors, harmonic scalpel, or CO2 laser. The disadvantage of this approach is the pneumoperitoneum is rapidly lost, making it difficult to bring the myoma into the cul-de-sac. A wet lap pad may be placed in the vagina to facilitate restoring the pneumoperitoneum to view the pelvis. Multiple small myomas can be removed with a specimen retrieval bag placed through the colpotomy incision. The colpotomy incision can be sutured laparoscopically, but it is far quicker and easier to close the colpotomy vaginally.
In a retrospective cohort study, Ou and associates21 compared two groups of patients undergoing laparoscopic myomectomy with specimen removal by colpotomy versus morcellation. They found that multiple myomas can be removed more quickly via posterior colpotomy than by morcellation. The incisions were closed vaginally and then inspected laparoscopically to ensure hemostasis. Certainly, avoiding the use of electronic morcellators and the high cost of disposable morcellator blades is an economical way of performing laparoscopic myomectomy.
VAGINAL MYOMECTOMY AND LAPAROSCOPICALLY ASSISTED VAGINAL MYOMECTOMY
Several authors have reported on vaginal myomectomy and laparoscopically assisted vaginal myomectomy (LAVM), a version of laparoscopic myomectomy in which the dominant myoma is incised and partially enucleated by the techniques described above and the enucleation is completed through a transverse colpotomy incision. Smaller myomas are then incised and enucleated vaginally. The fundus is then delivered through the colpotomy and the defects repaired.22,23 This technique is possible only if there is adequate room vaginally and the cul-de-sac can be reached easily. For large dominant posterior myomas, the use of the laparoscope may not be necessary. In a small pilot study, Birsan and associates24 compared two similar groups of women undergoing laparoscopic myomectomy versus vaginal myomectomy for large posterior myomas.
There was no difference in parity or myoma size between the two groups. Vaginal myomectomy was found to be feasible and safe and was associated with a shorter operating time and lower morphine consumption than laparoscopic myomectomy.24 LAVM through an anterior approach was reported by Chin and associates.25 Seven women with symptomatic fundal and anterior wall myomas were treated by laparoscopically placing a suture through the myoma and bringing it down through the anterior cul-de-sac into the vagina via an anterior colpotomy. Resection and suturing were performed transvaginally. There were no complications, although four patients developed transient hematuria.
LAPAROSCOPICALLY ASSISTED MYOMECTOMY/MINILAP MYOMECTOMY
First reported by Nezhat et al.26 in 1994, laparoscopically assisted myomectomy (LAM) is a safe alternative to laparoscopic myomectomy. It is less difficult and requires less time to complete than other modes of myomectomy. These considerations are summarized in Table 1. The decision to do LAM usually is made in the operating room after the diagnostic laparoscopy and treatment of other pelvic abnormalities are completed. The criteria for LAM are a myoma greater than 8 cm, many myomas requiring extensive morcellation, and a deep, large, intramural myoma that requires uterine repair in multiple layers.
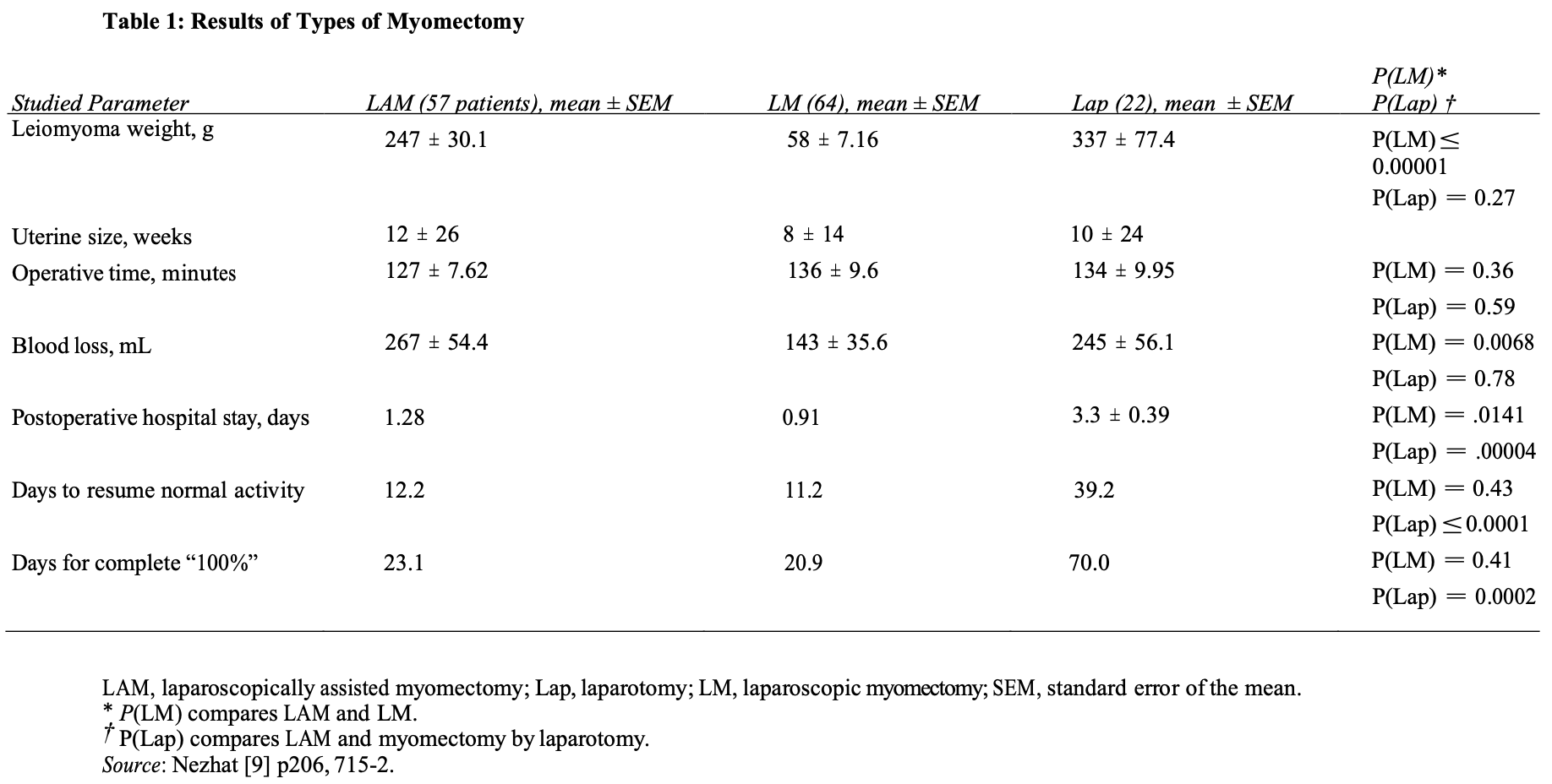
A combination of laparoscopy with a 2- to 4-cm abdominal incision may enable more gynecologists to apply this technique. The conventional uterine suturing in two or three layers reduces the potential for uterine dehiscence, fistulas, and adhesions. Better pelvic exposure during the laparoscopy allows the gynecologist to diagnose and treat associated endometriosis or adhesions.
Three major objectives of LAM are reduction of blood loss, prevention of postoperative adhesions, and maintenance of myometrial integrity. LAM with morcellation and conventional suturing reduces the duration of the operation and the need for more extensive laparoscopic experience.
The use of minilaparotomy in surgery for benign gynecologic disease has been well established.27 In a randomized controlled trial, Benassi and associates28 evaluated the efficacy and applicability of the minilaparotomy technique in abdominal myomectomies and compared it with traditional laparotomy. They found duration of surgery and days of postoperative hospital stay were significantly lower in the minilaparotomy group, as well as higher treatment satisfaction reported by the patients (P ≤ 0.05). Moreover, each minilaparotomy operation ended up saving 620 Euros.
Based on a review of 139 cases, Glasser29 found that myomectomy performed through a 3- to 6-cm minilaparotomy incision affords the advantage of same-day discharge as well as the ability to palpate the uterus and close the defect using a standard three-layered suturing technique. Of the original 139 patients, 66 had LAM, during which the laparoscope was used to identify and mark the incision site or to perform adhesiolysis.
The vast majority of those procedures were done during our early experience. For the last 4 years, virtually all myomectomies were done without the use of the laparoscope. All patients with leiomyomas complaining of “bulk” symptoms and desiring intervention were offered minilap myomectomy as one of their treatment options. Those who had completed childbearing were also offered vaginal or minilap supracervical hysterectomy, uterine artery embolization, or medical therapy if they were perimenopausal.
Laparoscopic myolysis was also offered as a treatment option. Laparoscopic myolysis was our first choice for the management of large symptomatic myomas in the mid-1990s. We did perform 102 myolysis procedures from 1994 to 2001 but have not done any in the past 4 years. The procedure is technically much easier to perform than myomectomy, blood loss is less, and operating time is shorter. Over time, however, both patients and physicians found this procedure to be distasteful. Patients, in particular, were unhappy with leaving “dead fibroids” in their uterus. We did see some regrowth, and subsequent hysterectomies done on myolysis patients were difficult secondary to adhesions. As our skill level with minilap myomectomy improved, more of our physicians encouraged this procedure as a first option – even when fertility was not an issue. Certainly, myomectomy is always the first option in patients desiring future childbearing.
All patients underwent pelvic ultrasonography to assess uterine size, individual myoma size, and number and location of myomas. Those with equivocal sonography and those with multiple myomas desiring future fertility underwent MRI studies to “map” the uterus more accurately. Those with abnormal uterine bleeding underwent diagnostic office hysteroscopy and hysteroscopic resection at the time of myomectomy if submucous myomas were present. Myomas larger than 2 cm were removed transmyometrially. Patients with more than five measurable myomas on imaging studies and not desiring future fertility were strongly urged to undergo hysterectomy or uterine artery embolization. The “ideal” patient for this procedure is thin, with a single large anterior fundal myoma. We have, however, performed this procedure on patients weighing as much as 280 lbs and on those with multiple or posterior myomas.
Pretreatment with GnRH analogues was used in all patients in the early part of the study, but this practice has largely been abandoned. GnRH analogues are given only to reduce massive bulk in uteri greater than 20 weeks’ gestational size or to patients who are anemic (Hgb<10 GMS.) to create amenorrhea and increase hemoglobin levels. An 8- week course is generally sufficient to achieve these results. The results of this series are summarized in Table 2. The average age of the patients was 38.9 years, with a range of 23 to 56 years. There were also a few perimenopausal patients who were poor candidates for either vaginal hysterectomy (nulliparous, no uterine decensus and huge fibroids) or laparoscopic supracervical hysterectomy (LSH) and wanted a minimally invasive approach rather than a conventional abdominal hysterectomy. Those patients are now offered minilap supracervical hysterectomy as an alternative.
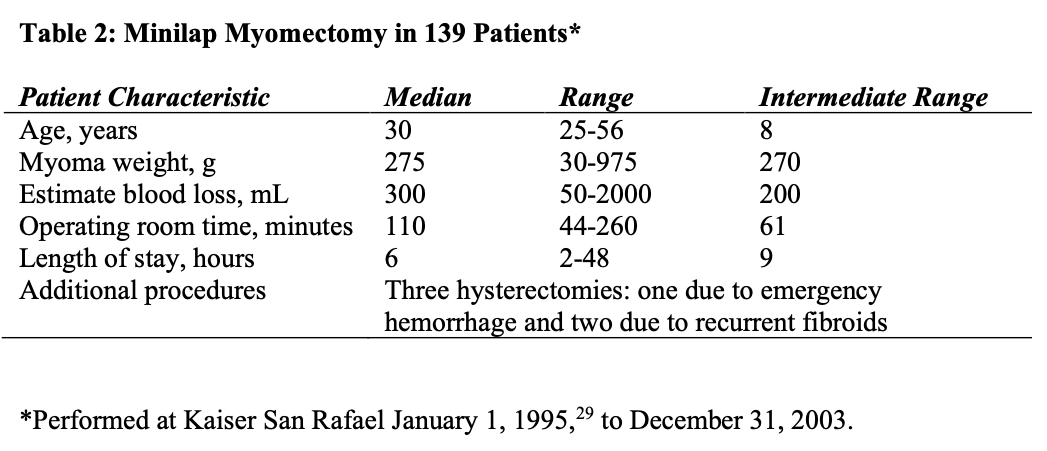
The average weight of the myomas removed was 285.6 g, with a range of 30 to 925 g. The patient with a 30-g myoma was an infertility patient with a 5-cm type 2 myoma penetrating the entire myometrium. Two patients had 15 separate myomas removed that were not evident on preoperative imaging studies. Seventy of 139 patients had pretreatment with GnRH analogues.
The average length of stay was 13.6 hours, with a range of 4 to 48 hours. One of the two patients who spent 48 hours in the hospital developed a fever, probably secondary to atelectasis, and was kept for observation. The other patient had nausea and vomiting, which resolved with antiemetics. Twenty-four of 139 patients were discharged within 4 hours of surgery and 61 within 8 hours. Of the 51 patients discharged between 8 and 23 hours post surgery, 27 lived more than 50 miles from the hospital and chose to spend the night rather than drive home.
Average operating time for this procedure was 110 minutes, with a range of 55 to 260 minutes. The operating time was, to some degree, related to the skill and experience of the surgeon. There was a direct relationship between operating time and number of myomas, rather than size, although individual myomas larger than 10 cm took longer to morcellate.
The average blood loss was 330 mL, with a range of 50 to 2000 mL. The patient with the 2000-mL blood loss had a large broad ligament myoma that was avulsed by too vigorous upward traction before being completely morcellated. She had uncontrolled bleeding at the myoma bed and underwent emergency hysterectomy after efforts at uterine artery ligation failed. She was the only patient to undergo a blood transfusion in our series. This complication occurred early in our series in a procedure performed by a relatively inexperienced surgeon.
The two other hysterectomies performed in this group were for recurrent myomas. Both these patients had multiple myomas at the time of initial myomectomy. One had a hematometra following surgery and gradual uterine growth over a 6-month period. Her bulk symptoms recurred, and she elected to have an abdominal hysterectomy at that time. The second patient had multiple myomas removed at age 38 and had recurrence of symptoms 5 years later. She underwent a supracervical hysterectomy for a uterus that weighed 900 g and contained multiple myomas.
Surgical Technique
If LAM is being performed, the following technique is used. In patients with multiple myomas, the most prominent myoma is injected at its base with 3 to 7 mL of diluted vasopressin. A vertical incision is made over the uterine serosa onto the surface of the tumor and extended until the capsule of the leiomyoma is reached. A corkscrew manipulator is inserted into the leiomyoma and used to elevate the uterus toward the midline suprapubic puncture. With the trocar and manipulator attached to the myoma, this midline 5-mm puncture is enlarged to a 4-cm transverse skin incision. After the incision of the fascia transversely, the rectus muscle is divided using a monopolar electrode. If the inferior epigastric vessels are found, they are coagulated. This approach provides excellent access to the abdominal cavity.
The peritoneum is entered transversely, and the leiomyoma is observed. It is brought to the laparotomy incision by using the corkscrew manipulator to raise the uterus. A corkscrew manipulator is replaced with two Lahey tenacula. The tumor is shelled and morcellated sequentially, and after its complete removal, the uterine wall defect shows through the incision. If uterine size allows, the uterus is exteriorized to complete the repair. When multiple leiomyomas are found, as many as possible are removed through one uterine incision if it can be accomplished without excessive tunneling. When other myomas are located that cannot be removed through the initial uterine incision, the 4-cm abdominal opening is approximated temporarily with two or three Allis clamps or an inflated latex glove. The laparoscope is reintroduced, and the remaining myomas are identified and brought to the level of the abdominal incision. They are removed under laparoscopic control. The uterus is exteriorized through the 4-cm abdominal incision. The myometrium is closed in layers with 2-0 and 0 polydioxanone sutures. The serosa is closed microsurgically with 5-0 sutures. The uterus is palpated to ensure that no small intramural leiomyomas remain. It is returned to the peritoneal cavity. The fascia is closed with a 1-0 polyglactin suture, and the skin is closed in a subcuticular manner. The laparoscope is used to evaluate hemostasis. The pelvis is observed to detect and treat endometriosis and adhesions that may have been obscured previously by myomas.
Copious irrigation is used, blood clots are removed, and Interceed (Gynecare, Somerville, NJ) is applied over the uterus to help prevent adhesions.
Intraoperatively, injections of dilute vasopressin into the myoma help reduce blood loss. Vertical uterine incisions bleed less than do transverse incisions,4 and pneumoperitoneum seems to decrease intraoperative bleeding.
A recent innovation in LAM is the use of the LAP DISK abdominal wall sealing device (Ethicon Endosurgery, Somerville, NJ), which allows the surgeon to place a hand in the abdomen during laparoscopic surgery, facilitating exposure and dissection. In a retrospective study of 43 patients who underwent LAM using the LAP DISK, Tanaguchi and associates30 in Japan removed myomas that ranged in weight from 40 to 700 g (mean, 208 g) and diameter from 2 to 10 cm (mean, 5.4 cm). Mean blood loss was 42.3 mL. Half of the 18 patients who had been diagnosed with primary infertility for 2 years or longer became pregnant with- out postoperative assisted reproductive techniques. The authors concluded that the LAP DISK abdominal wall-sealing device was useful for LAM, allowing surgeons to remove myomas safely and repair uterine defects effectively while minimizing blood loss and trauma.
In minilap myomectomy without the use of laparoscopy, the patient is placed in the low lithotomy position using Allen stirrups (Allen Medical Systems, Cleveland, OH). Ten milliliters of a dilute vasopressin solution (6 U in 60 mL NaCl) is then injected intracervically about 1 to 2 cm deep at both the 8 and 4 o’clock positions according to the technique described by Phillips et al.,31 and we have noticed a marked blanching of the entire uterus when this is done before making the abdominal incision. A firm uterine manipulator with a 5-mm obturator (Medical Horizons, Fair Oaks, CA) is then placed in the cervix (Figure 1). A Pelosi (Figure 2) or Valtchev manipulator also works well. It is important not to use a uterine manipulator with an inflatable balloon because it may be mistaken for a myoma on palpation. The midline is identified on the abdomen and a 3- to 5-cm horizontal line is drawn on the skin about two to three finger breaths above the top of the symphysis. The incision should be made slightly higher if the myoma is posterior. Also, the incision should be made slightly longer for patients with central obesity. For patients who are massively obese with a large pannus, this can be raised and taped to the top of the table with 6-inch cloth tape secured with tincture of benzoin. The incision then is made at the skin fold above the pubic bone, which is often the thinnest part of the abdomen.
The operation is performed using a cruciate incision. The cruciate incision, as described by Pelosi32 for minilaparotomy, affords excellent exposure. By dissecting the fascia vertically rather than horizontally, the abdominal opening is round rather than ovoid, giving an increased working area. Before making the skin incision, 10 mL of 0.25% bupivacaine (Marcaine, AstraZeneca) with epinephrine solution is injected superficially, extending laterally beyond the limits of the incision. The skin and subcutaneous tissue are opened horizontally to the level of the fascia, and a finger is used to bluntly tunnel under the subcutaneous fat close to the fascia in the midline both cephalad toward the umbilicus and caudad to the pubic bone. The fascia is then opened in the midline for a total length of about 6 cm. The rectus muscle is then separated in the midline and the peritoneum grasped, nicked, and entered longitudinally. Care must be taken not to injure the bladder when extending the peritoneal incision downward. Once the peritoneum is opened, the surgeon’s finger is swept circumferentially, to make sure there are no adhesions to the anterior abdominal wall. A Mobius elastic retractor (Apple Medical, Marlborough, MA) is then inserted (Figure 5). The bottom blue ring of the retractor is first inserted under the peritoneum, and the polyethylene membrane is then rolled up on the top yellow ring. This is facilitated by the surgeon grasping the top ring at the 10 and 2 o’clock positions and twisting it down toward the abdominal wall. The assistant then completes the twist while the surgeon holds the top ring firmly. Usually two or three twists are sufficient to create a nice round opening in the abdominal wall with a diameter equal to the length of the skin incision. The Mobius elastic self-retaining retractor33 enhances this exposure, and the incision can be moved to the areas of dissection using small Deaver or Lorenz vein retractors. A 4-cm incision results in a working area of 12.5 cm2, whereas a 6-cm incision will increase this working area to 28 cm2. The retractor gently compresses the layers of the abdominal wall and allows the specimen to be elevated to the skin line. Also, the retractor allows the skin to stretch so that much larger specimens can be removed. This is not possible using a fixed metal self-retaining retractor. There is also minimal trauma to the rectus muscles. The vast majority of our patients requires only oral analgesics in the immediate postoperative period and manages well on nonsteroidal anti-inflammatory drugs or acetaminophen at home. The retractor is soft and very atraumatic to the tissues of the abdominal wall while it keeps subcutaneous fat, muscle, and peritoneum out of the operative field. We have found that a 4- to 5-cm incision gives us adequate room to remove myomas by morcellation and repair the uterine defect by conventional suturing techniques.

Before proceeding with the myomectomy, the size and position of the myomas are carefully assessed. If there is any doubt about the safe performance of the procedure via minilap, such as the presence of large inaccessible posterior or broad ligament myomas, a conventional laparotomy should be performed. This can easily be accomplished by removing the Mobius retractor, extending the skin and fascial incisions, and reinserting the Mobius to its full 6-inch diameter. Conversion to laparotomy when the myoma is partially dissected results in increased blood loss secondary to the delay, so this decision should be made early. Assessment as to where the uterine incision should be placed is the next step. We prefer anterior midline fundal incisions for large solitary intramural myomas. If multiple myomas are present, a strategic incision site through which most of the myomas can be removed is attempted, but it is sometimes preferable to make separate incisions for each myoma. Posterior myomas can usually be approached through a transverse incision well below the tubal insertions. For large subserosal myomas, it is important to make the initial incision high on the myoma, usually 2 to 3 cm distal to the junction between the myoma and the uterus. This allows for easier closure of the defect.
Also, pedunculated myomas should be incised above where the pedicle inserts on the myoma to avoid cautery damage to the fundal myometrium. Attempts should be made to avoid the cornual region, thus minimizing trauma to the tubal ostia.
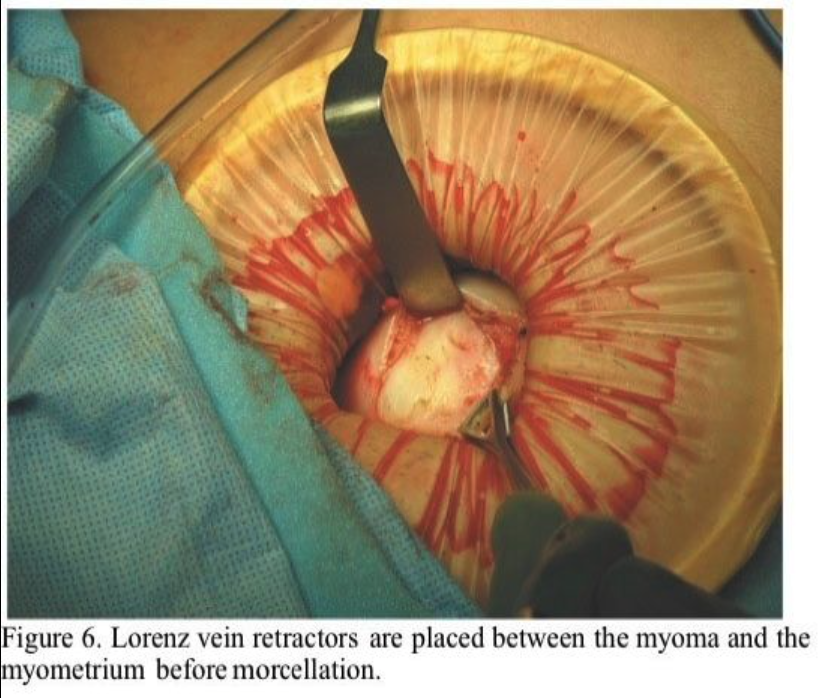
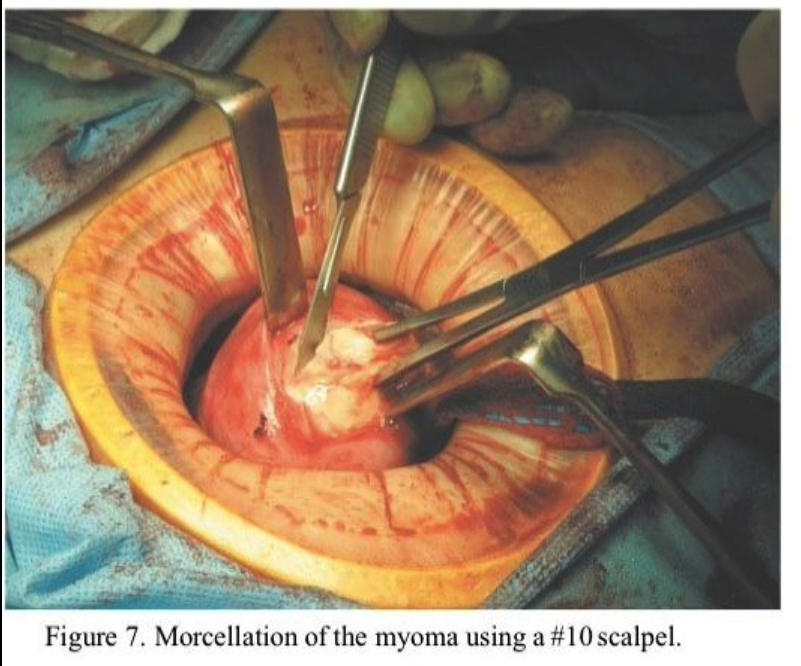
Using the uterine manipulator, the uterus is elevated to the abdominal incision. The serosa and myometrium along the course of the proposed incision is then injected with the same dilute vasopressin solution used intracervically to a depth of 1 to 2 cm. This is easily accomplished with the use of a standard 22-gauge 1.5-inch needle. Additional vasopressin is injected deeply into the myoma. Subserous myomas should be injected at the base, directing the needle both into the myoma and into the uterus. A distinct blanching of the myoma becomes readily apparent. Usually, 10 to 15 mL of vasopressin is sufficient to achieve hemostasis. A 4- to 6-cm incision is then made through the serosa and myometrium and carried down through the pseudo capsule of the myoma. The myoma usually bulges out at this point. Small “army–navy,” Jarit “S,” or Lorenz vein retractors are then inserted between the myoma and the myometrium inside the pseudocapsule (Figure 6). The myoma is grasped with Lahey thyroid clamps and strong upward traction applied. This tamponades the uterine incision against the upper abdominal wall and markedly reduces blood loss. The myoma is then progressively morcellated, much as one would do in a vaginal hysterectomy using a #10 scalpel blade on a long handle (Figure 7). This is more ergonomic and thus less tiring than using a short-handled scalpel. It is most important to regrasp a remaining edge of the myoma before the morcellated core of tissue is removed so that upward traction and tamponade are maintained (Figure 8). Attempts to enucleate the large myoma should be avoided because this tears blood vessels both in the periphery and at the base of the myoma.
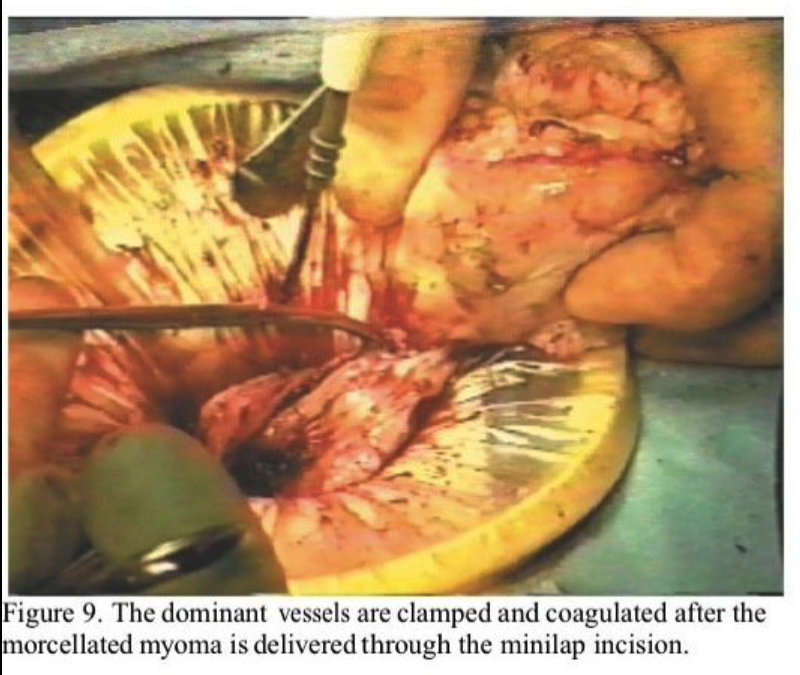
Careful dissection around the capsule of the myoma with coagulation of blood vessels as they are encountered significantly reduces blood loss. The large blood vessels at the base are easily identified and desiccated when the remains of the myoma are brought through the incision (Figure 9). Careful palpation at this point allows additional myomas to be identified and, if possible, removed through this incision by a combination of morcellation and enucleation. Also, palpation of the obturator of the manipulator identifies the uterine cavity. If additional myomas are identified in other areas, it is more prudent to first close the original uterine incision and approach the others through separate incisions using the same technique described above.
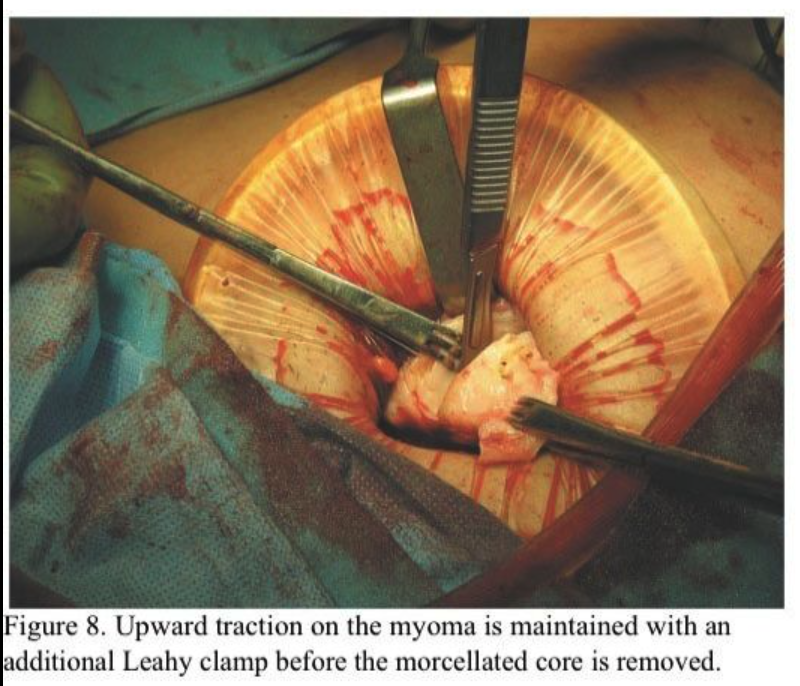
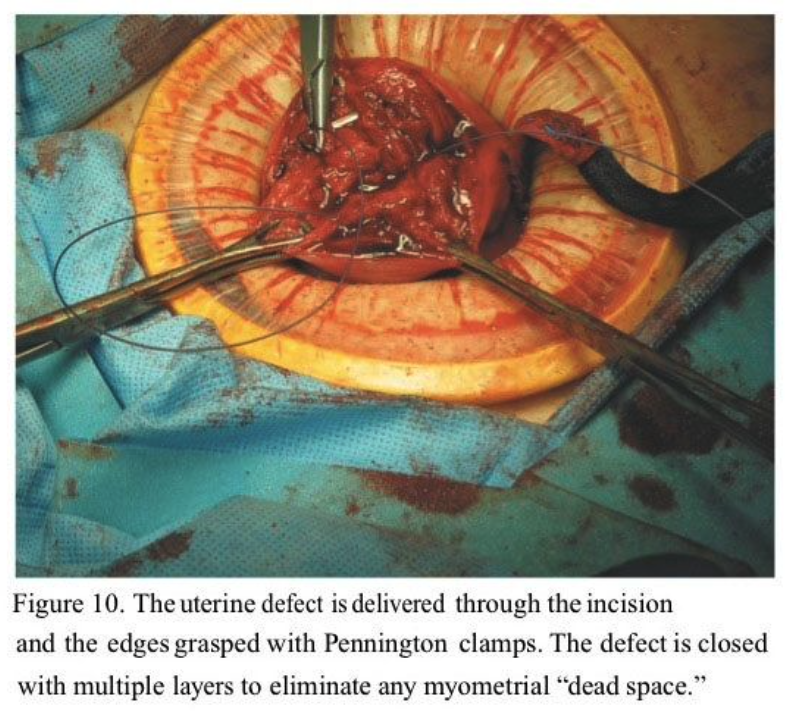
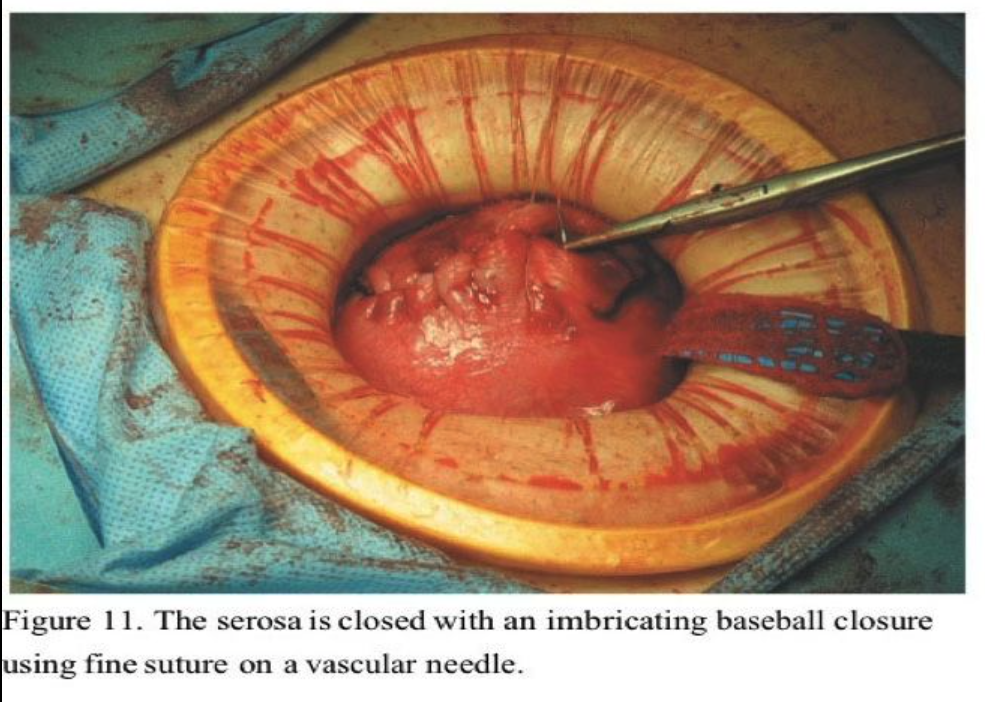
The uterine elevator is helpful in bringing the uterus up to the anterior abdominal wall, where the edges of the uterine incision are then grasped with Pennington clamps. The Pennington clamps are placed as deep as possible into the myometrial defect and an attempt is made to evert the edges. The entire uterine defect can often be exteriorized completely for easy repair on the abdomen (Figure 10). This allows placement of deep figure-of-eight or horizontal mattress sutures. We use #1 Polysorb on an HGS 21 needle (USSC, Norwalk, CT) and take deep myometrial bites to close off the base of the incision and approximate the myometrium. A second layer of continuous 0 Polysorb or interrupted horizontal mattress sutures are placed to further approximate the myometrium. The serosa is then closed with a continuous “baseball” imbricating layer using 3-0 Polysorb on a V20 (vascular) needle for minimal trauma and adhesion prevention (Figure 11). All anterior and pedunculated myomas should be removed first so that the uterus can be sharply anteflexed by the uterine manipulator to approach posterior lower-segment myomas. We have been surprised that even posterior cervical myomas can be removed by this technique.
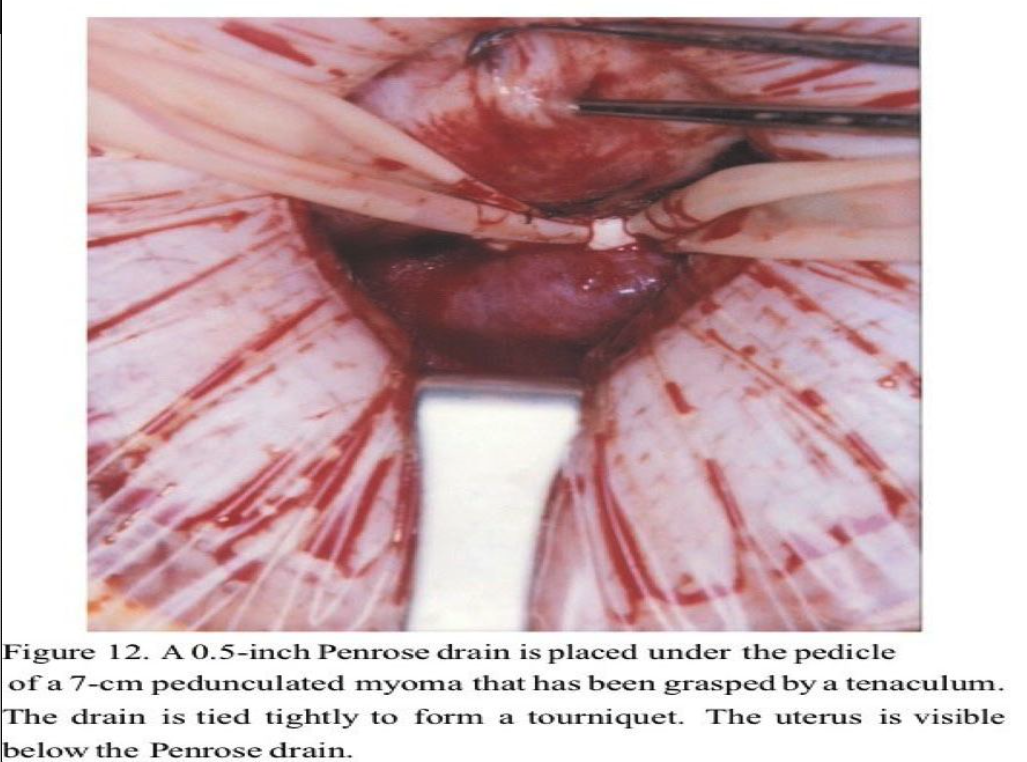
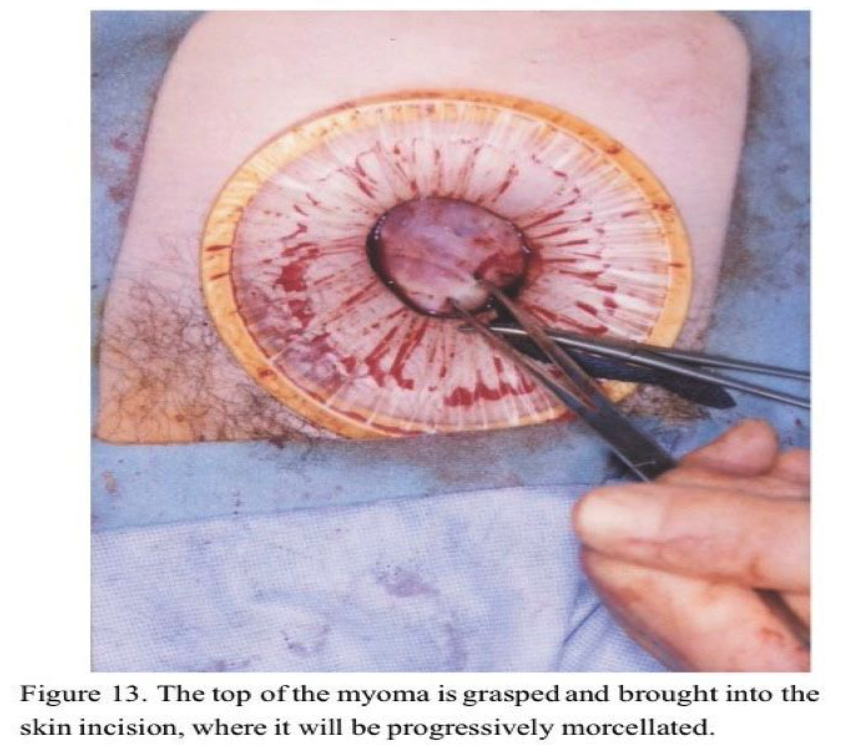
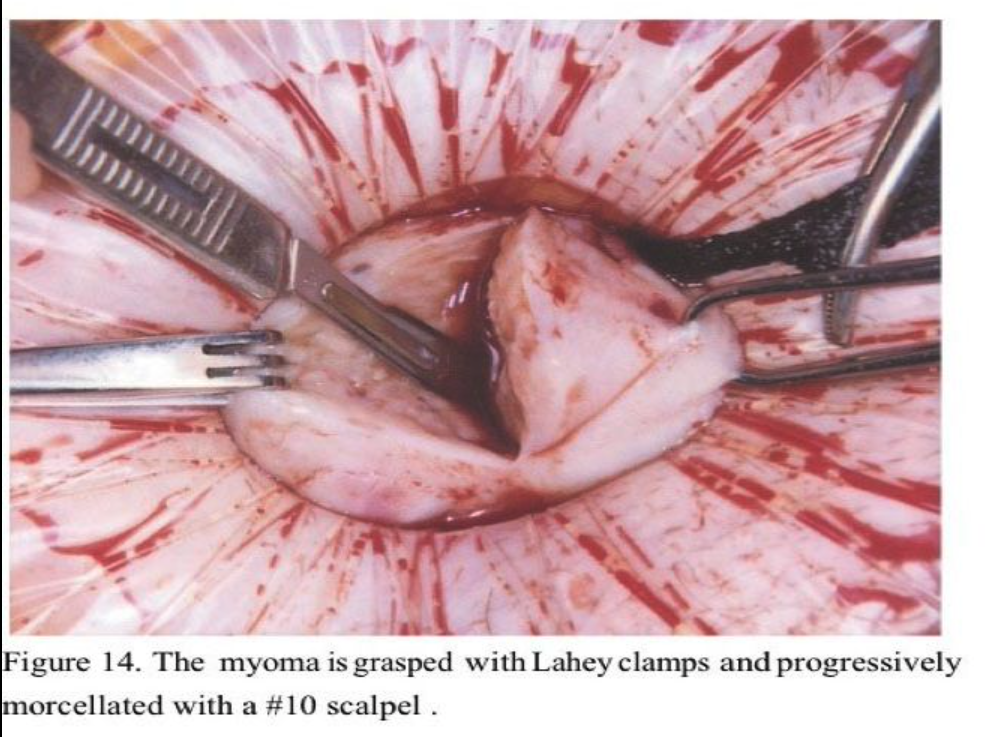
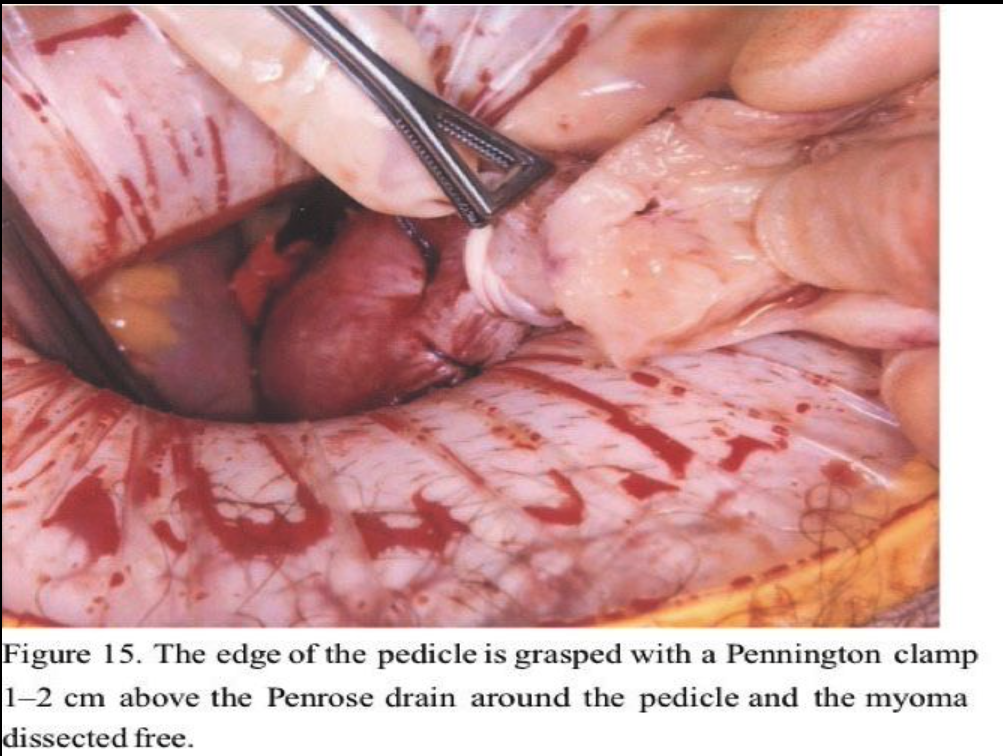
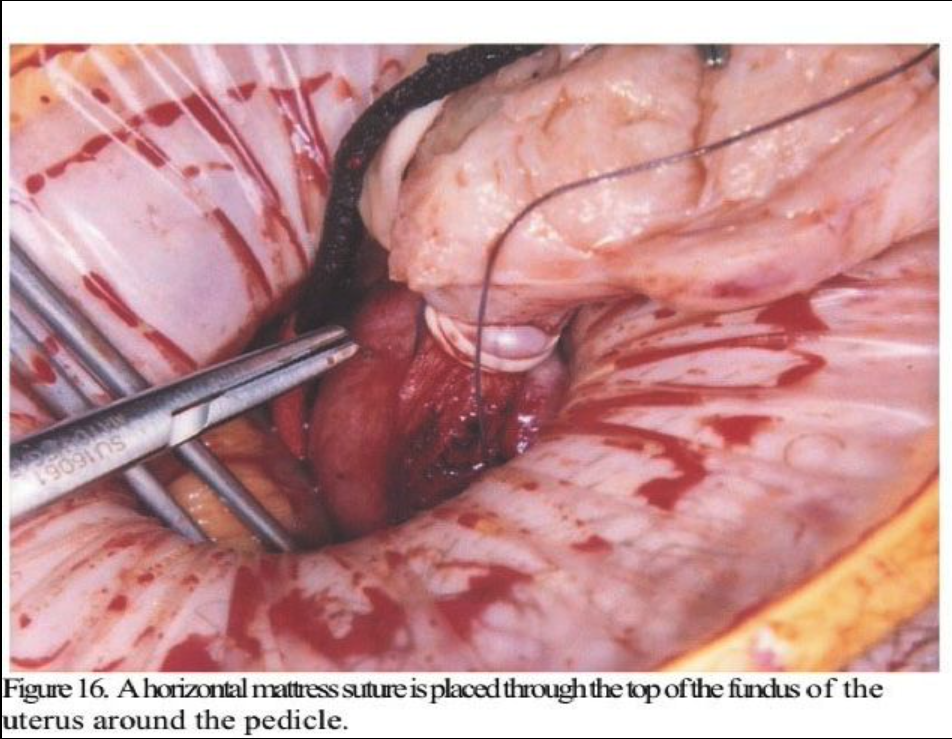
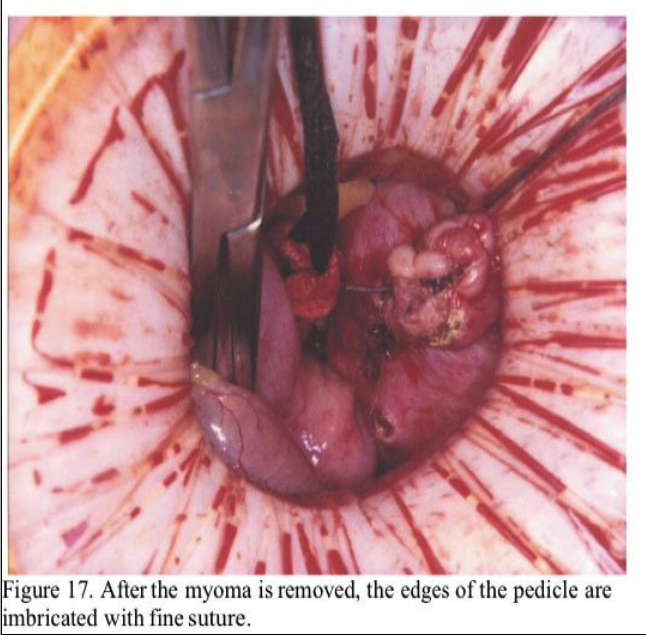
Large pedunculated fundal myomas are most easily managed by minilap because extensive deep suturing usually is not necessary. Usually, a 3-cm incision suffices. Once the myoma pedicle is identified, it is injected with the dilute vasopressin solution as described above. A large, blunt right-angled vascular clamp (Grover or Satinsky) is placed under the myoma pedicle and a 0.5-inch Penrose drain is drawn under and tightly tied around the myoma pedicle. (Figure 12). Using the electrosurgical pencil, a circumferential mark is made at the myoma base about 1 to 2 cm above the tourniquet. The top of the myoma is then brought to the incision (Figure 13) and progressively morcellated with a scalpel until the mark at the base is reached (Figure 14). It is then carefully dissected free from the pedicle (Figure 15). A horizontal mattress suture is then placed in the fundus of the uterus incorporating the base of the pedicle and tied tightly (Figure 16). The tourniquet is then removed and the edges of the pedicle sutured and imbricated (Figure 17). This technique assures that the myometrium at the fundus of the uterus is not subject to electrosurgical or thermal injury. After hemostasis is assured in the uterine incision, the uterus is dropped back into the pelvis and the pelvis is irrigated with copious amounts of lactated Ringer’s solution or normal saline. Interceed adhesion prevention barrier is placed over the uterine incisions. The minilaparotomy incision is repaired in layers, with an attempt to eliminate the dead space in the subcutaneous tissue to prevent seroma formation. A subcuticular closure for the skin is used after Marcaine 0.25% with epinephrine is injected in the fascia and the skin edges. Steri-Strips (3M) and a large Band- Aid are placed on the incision, and a vertical pressure dressing is applied until the patient is discharged.
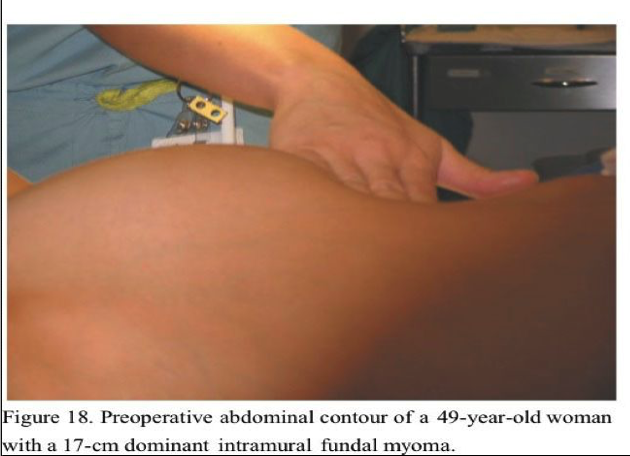
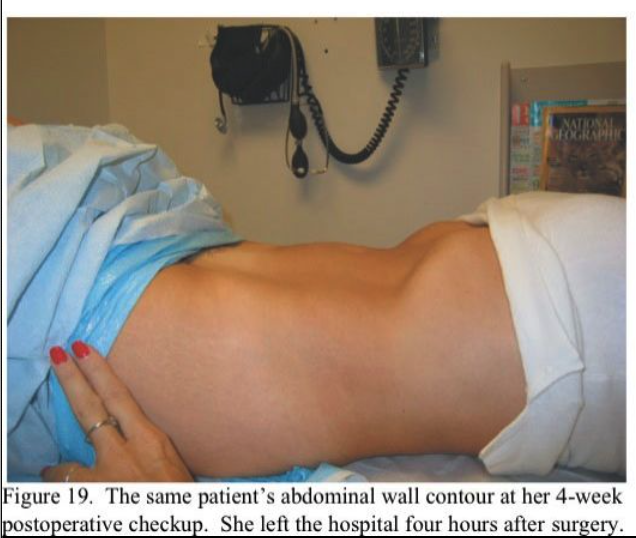
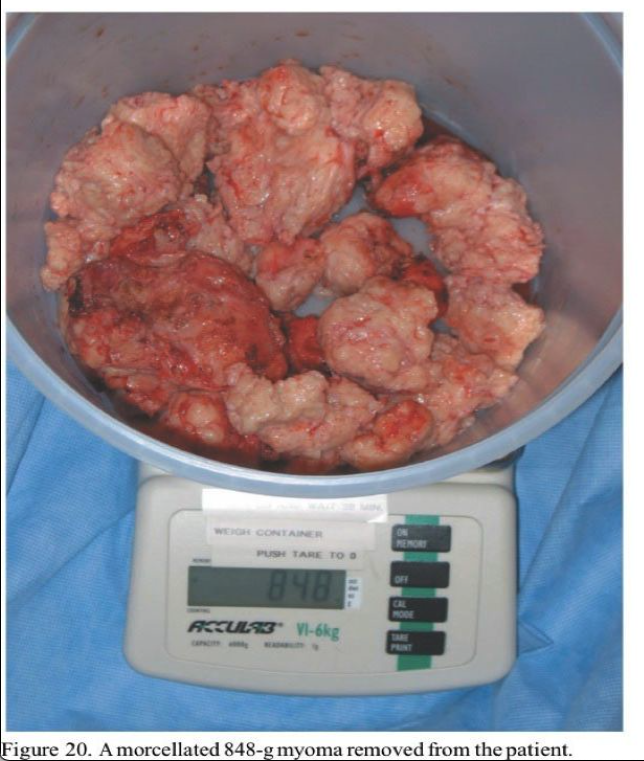
Most patients receive ketorolac 60 mg intramuscularly (Toradol, Roche Pharmaceuticals, Nutley, NJ) and dexamethasone 8 mg intravenously (IV) (Decadron, Merck & Co., West Point, PA) intraoperatively and are discharged after approximately 4 hours in our ambulatory surgery recovery area. Patients who have late-afternoon surgery or are insufficiently recovered are discharged the following morning. All patients without cephalosporin allergies are given cefazolin 1 g IV pre- operatively. Figure 18, Figure 19 and Figure 20 show the pre- and postoperative abdominal wall contours of a 49-year-old patient who had an 848-g myoma excised through a 5-cm minilap incision. The patient left the hospital 4 hours postoperatively and went shopping for shoes 4 days postoperatively.
Use of GnRH Agonists
Pretreatment with GnRH agonists before myomectomy remains controversial. In a systematic review of randomized controlled trials of the use of GnRH analogues before hysterectomy and myomectomy, it was found that pre- and postoperative blood counts were improved, uterine and myoma volume decreased, and operative time and blood loss reduced by GnRH agonist therapy.34 Reduction in leiomyoma size makes the procedure less time consuming because a smaller uterine incision can be made and less morcellation is required. The individual myomas, however, are softer, which often results in the Lahey clamps tearing through the tissue, resulting in the loss of upward traction, which is counterproductive and may result in increased bleeding. For anemic patients, preoperative GnRH treatment may enable restoration of a normal hematocrit, decrease the size of the myoma, and reduce the need for transfusion.35 Although GnRH agonist use may soften the myoma (facilitating morcellation) and shorten operative times, it also may increase the duration of laparoscopic or minilap myomectomy.36 However, not all studies have shown the benefits described above. Increased difficulty in identifying and dissecting the cleavage planes and shrinking small myomas to the point at which they cannot be seen or palpated may increase recurrence.37 We do not use preoperative GnRH agonists unless the patient is anemic or the myoma is above the umbilicus.
Adhesion Prevention
Although myomectomy is done to preserve fertility, postoperative adhesions may jeopardize this goal. Single, vertical, anterior, and midline uterine incisions cause fewer adhesions.4 Although sutures predispose patients to adhesions,38 they are always necessary to close the uterine defect. Although several adhesion barriers are either available or under development, none is completely effective.39 A prospective, randomized, blinded multicenter study evaluated 127 women undergoing uterine myomectomy with at least one posterior uterine incision 1 cm or greater in length.40 Patients were randomized to Seprafilm (HAL-F) bioresorbable membrane (Genzyme Corporation, Cambridge, MA) or to no treatment. At second-look laparoscopy, the incidence, measured as the mean number of sites adherent to the uterine surface, was significantly lower in the treated group as were the mean uterine adhesion severity scores and mean area of adhesions. In another study, 50 pre-menopausal, nonpregnant women who underwent laparoscopic myomectomy were randomized to the control group (25 patients) with surgery alone or to the treatment group (25 patients) using Interceed, an oxidized regenerated cellulose.41 At second-look laparoscopy 12 to 14 weeks later, 12% (three) of the women in the control group were adhesion-free, compared with 60% (15) of those treated with Interceed (P ≤ 0.05).
In a randomized, prospective, controlled, multicenter clinical trial of a sprayable, site- specific adhesion barrier system (SprayGel, Confluent Surgical, Waltham, MA) in 66 women with a mean age at surgery of 34.9 years (range, 23 to 52 years) undergoing laparoscopic or open uterine myomectomy, Mettler and associates42 found when compared with initial surgery, the mean adhesion tenacity score of adhesions seen at second-look laparoscopy was significantly reduced in treatment patients com- pared with control patients (0.6 vs. 1.7, a 64.7% reduction). Mean adhesion extent score at second- look laparoscopy compared with initial surgery was 4.5 cm2 versus 7.2 cm2, and the mean adhesion incidence score was 0.64 versus 1.22. Of 64 patients, 40 (62.5%) returned for second-look laparoscopy. The Mettler group’s conclusions were that this adhesion barrier was safe and well-tolerated and demonstrated efficacy in a population of patients known to be at risk for adhesion formation. There were no adverse effects attributable to the product and no patients in whom it could not be applied.
Regardless of the adhesion prevention material used, most are ineffective in the absence of hemostasis. This was shown very early in a study on animal models by Linsky and associates.43 Using an imbricating baseball-type closure on the uterine serosa is both hemostatic and minimizes the presence of raw edges, which also predispose to adhesion formation.
LAPAROSCOPIC MYOMECTOMY AND LAPAROSCOPICALLY ASSISTED (MINILAP) MYOMECTOMY: COMPARISON OF RESULTS
Nezhat and associates<9 evaluated 143 charts from their practice, including those of patients who had myomectomy by laparotomy (15.3%), laparoscopic myomectomy (44.7%), or LAM (39.8%) (Table 1). The 22 myomectomies by laparotomy were done before the development of LAM. Because LAM replaced myomectomy by laparotomy and patient selection criteria were comparable, the myoma weights of the two groups were similar.
Mean operating times were the same for laparoscopic myomectomy and LAM despite larger myomas, difficult locations of intramural myomas, and adjunctive laparoscopy in the latter group. The leiomyoma weights were greater in the LAM group than in the laparoscopic myomectomy group (P ≤ 0.05).
The mean estimated blood loss of the LAM and laparotomy groups was not different. In contrast, blood loss among the laparoscopic myomectomy patients was significantly lower, and this may be attributed to the smaller leiomyomas. In comparing the hospitalization time of the LAM and laparoscopic myomectomy patients, that of the LAM group appeared to be longer (P(LM) = 0.014). This may be explained by the initial reluctance of some physicians to discharge LAM patients on the day of the operation or on postoperative day 1. After the initial 10 to 15 operations, all the women underwent LAM on an outpatient basis. In fact, when the initial 15 cases are removed from the LAM group, the mean hospital stay drops to 1.06 days. This period is not statistically different from that for the laparoscopic myomectomy group.
The comparison of postoperative recovery times shows important distinctions between the LAM and laparoscopic myomectomy groups. Here, despite the differences in size and location of myomas, the recovery time can be compared because of the diverse incisions. The time elapsed before patients resumed work or regular activity was similar (P ≤ 0.05). Introducing a 4-cm incision in the LAM group did appear to prolong (P ≤0.05) the subjectively perceived time for the women to achieve100% recovery.
Previous studies44,45 underscored the need to decrease the operative time of laparoscopic myomectomy. Whereas myomas less than 8 cm are managed laparoscopically, larger tumors and intramural lesions require prolonged morcellation and laparoscopic suturing of the uterine defect. The largest reported myomas removed by laparoscopy were 15 to 16 cm,9,44 and one group wrote that 10 cm was its limit.46 Both laparoscopic morcellation and myometrial suturing are difficult and prolong operations. Hospitalization was much longer for the patients who underwent myomectomy by laparotomy (P ≥ 0.05) compared with both the LAM and the laparoscopic myomectomy groups.
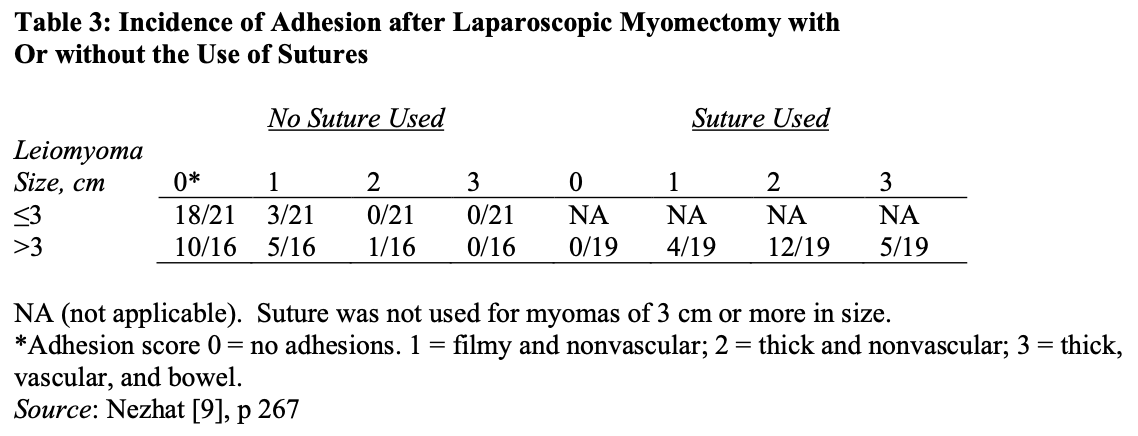
Second-look laparoscopies done on postmyomectomy patients who had pedunculated and superficial subserosal myomas without sutures showed complete uterine healing. In contrast, intramural myomas were associated with granulation tissue and indentation of the uterus proportional to the size of the leiomyoma excised unless sutures were used.
The use of sutures is associated with more adhesion (Table 3).9
Most patients are observed in an outpatient unit and discharged the morning after the operation, although some can leave the hospital on the afternoon or evening of the procedure.
PREGNANCY FOLLOWING MYOMECTOMY
Pregnancy rates after myomectomy, both open and laparoscopic, have been variable. One study comparing reproductive outcomes after laparoscopic or abdominal myomectomy showed no significant difference between the two approaches.53 There are many factors that determine the reproductive outcome following myomectomy, including the age of the patient and the number, size, and location of the myomas. In a retrospective study of 67 women who had undergone myomectomy, the majority of which were done by laparotomy, Sudik and associates54 reported higher pregnancy rates in women with fewer than six myomas removed. Although myoma location did not influence the results in this study, women with a larger volume (≥100 mL) of myomas removed had higher pregnancy rates. Age of the patient was not a factor in this study because there was no significant difference in the age of women who did not become pregnant (33.8 ± 5.1 years) and those who did (31.4 ± 4.5 years). In a retrospective review of 103 women who had undergone laparoscopic myomectomy in France, Desolle and associates55 found a significantly higher pregnancy rate in women with unexplained infertility, those less than 35 years of age, and those with less than 3 years of infertility. Neither the number of myomas removed nor their location or size had any significant influence on pregnancy rates. The authors concluded other factors involved in the patients’ infertility were probably more important than the characteristics of the myomas. In a 5-year review of the anticipated benefits of myomectomy, Olufowobi and associates56 in the United Kingdom found that the symptomatic benefit was less (36%) in the “infertility group.” Following an observation period of 12 to 36 months, 17 patients in the infertility group were lost to follow-up. Two of the 14 patients (14%) who attempted in vitro fertilization (IVF) were successful. In the non-IVF group, 13 of the 28 (46%) achieved natural conception. These results suggested that symptomatic improvement and fertility enhancement may be possible in some patients with fibroids. It was also the feeling of the authors that in view of the risks and potential failure of treatment associated with myomectomy, their results, yet again, support the fact that patients should be properly counseled before embarking on myomectomy. They strongly advocate local data to form the basis of the advice given during the consultation rather than what is published in the literature.
In a retrospective analysis of 72 women with intramural and subserosal myomas, Marchionni and associates57 in Italy found the conception rate was 28% before myomectomy and 70% after surgery. The corresponding figures were 69% and 25% for pregnancy loss and 30% and 75% for live birth rate, respectively. Age 30 years or younger and number of fibroids removed were the only significant and independent predictors of obstetric outcome by multivariate analysis. Their results suggested that abdominal myomectomy might improve reproductive outcome in patients with intramural and subserosal fibroids. The reproductive performance was particularly good when the patients were younger than 30 years and had a single myoma to remove. In a prospective randomized study of 131 women comparing myomectomy by laparoscopy versus laparotomy, Seracchioli and associates58 found pregnancy rates were no different (55.9% in the laparotomy group compared with 53.6% in the laparoscopy group).
In a retrospective study comparing reproductive outcomes after uterine artery embolization for fibroids versus laparoscopic myomectomy, Goldberg and associates59 found that pregnancies after uterine artery embolization had higher rates of preterm delivery (odds ratio, 6.2; 95%CI, 1.4-27.7) and malpresentation (odds ratio, 4.3; 95%, 1.0-20.5) than did pregnancies after laparoscopic myomectomy. The risks of postpartum hemorrhage (odds ratio, 6.3; 95% CI, 0.6-71.8) and spontaneous abortion (odds ratio, 1.7; 95%CI, 0.8-3.9) after uterine artery embolization were similarly higher than the risks after laparoscopic myomectomy; however, these differences were not statistically significant. They concluded that pregnancies in women with fibroids who were treated by uterine artery embolization, compared with pregnancies after laparoscopic myomectomy, were at increased risk for preterm delivery and malpresentation.
Surrey and associates60 evaluated the impact of myomectomy on in vitro fertilization- embryo transfer (IVF-ET) and oocyte donation cycle outcome. The study was carried out within one center and involved treatment of myomas that distorted the uterine cavity only, whether submucosal or intramural. A total of 101 patients underwent surgical treatment for leiomyomas: 46 submucosal with hysteroscopic resection and 55 intramural treated with open laparotomy. Patients who underwent surgical myomectomy for what was felt to be clinically significant leiomyomas had assisted reproductive technology cycle outcomes similar to those of a control population with regard to implantation, ongoing pregnancy, and early pregnancy loss. Neither the size nor the surgical approach altered the outcome. Pregnancy rates in the donor oocyte group were higher in patients who underwent surgical myomectomy as opposed to the control group, with no increase in biochemical pregnancies. Size was not the criterion for a surgical approach in this study; uterine cavity distortion was the criterion used. The study also mandated a diagnostic hysteroscopy in all patients to rule out any cavity distortions or endometrial lesions, including postoperatively. The authors stress the lack of strong correlation between either hysterosalpingography or traditional standard transvaginal sonography and hysteroscopic findings. As would be expected, the mean number and size of leiomyomas were significantly larger in patients who underwent abdominal myomectomy. However, neither ongoing pregnancy nor implantation rates were significantly different in comparison with controls among either oocyte donor recipients (group A – hysteroscopic resection: 86.7%, 57.8%; group B – myomectomy: 84.6%, 55.2%; group C – no surgery: 77%, 49.1%). The findings were similar for those undergoing IVF-ET in comparison with controls (group 1: 61%, 24%; group 2: 52%, 26%; group 3: 53%, 23%).
Oliveira and associates61 in Brazil did a study to further evaluate the effects of intramural and subserosal uterine fibroids on the outcome of IVF-ET when there is no compression of the endometrial cavity. In a retrospective, matched-control study from January 2000 to October 2001 done in a private IVF center, 245 women with subserosal and/or intramural fibroids that did not compress the uterine cavity (fibroid group) and 245 women with no evidence of fibroids anywhere in the uterus (control group) were studied. The type of fibroid (intramural, subserosal) and the number, size (centimeters), and location of intramural leiomyomas (fundal, corpus) were recorded and outcomes of IVF– intracytoplasmic sperm injection (ICSI) cycles were compared between the two groups. There was no correlation between location and number of uterine fibroids and the outcomes of IVF-ICSI. Patients with subserosal or intramural fibroids 4 cm or smaller had IVF-ICSI outcomes (pregnancy, implantation, and abortion rates) similar to those of controls. Patients with intramural fibroids 4 cm or greater had lower pregnancy rates than did patients with intramural fibroids 4 cm or less. There were no statistical differences related to delivery rates (31.5% vs. 32%, respectively) between all patients with fibroids and controls. The authors concluded that patients having subserosal or intramural leiomyomas 4 cm or smaller not encroaching on the uterine cavity have IVF-ICSI outcomes comparable to those of patients without such leiomyomas. Therefore, these patients might not require myomectomy before being scheduled for assisted reproduction cycles. However, the investigators recommend caution for patients with fibroids 4 cm or larger and that such patients be submitted to treatment before they are enrolled in IVF- ICSI cycles.
In summary, there is no consensus as to superior pregnancy rates associated with a laparoscopic route versus myomectomy by laparotomy versus no intervention at all. Preemptive myomectomy in the asymptomatic patient who wants fertility remains one of the most controversial subjects in gynecology. One must remember that myomectomy carries the risk of emergency hysterectomy in about 1% of cases.4 Also, the chance of infection and adhesion formation might in itself cause infertility. In a review of fibroids in pregnancy, Cooper and Okolo stated that “the true incidence of fibroids during pregnancy is, however, unknown, but reported rates vary from as low as 0.1% of all pregnancies to higher rates of 12.5%. It seems that pregnancy has little or no effect on the overall size of fibroids despite the occurrence of red degeneration in early pregnancy.
Fibroids, however, affect pregnancy and delivery in several ways, with abdominal pain, miscarriage, malpresentation, and difficult delivery being the most frequent complications. The size, location, and number of fibroids and their relation to the placenta are critical factors.”62
It stands to reason that a 4-cm intracavitary myoma will certainly interfere with the ability to get pregnant or maintain a pregnancy. It is our practice to perform preemptive myomectomies on all intramural myomas 6 cm or greater in diameter in asymptomatic patients desiring pregnancy. If patients are symptomatic from their myomas, then they should be treated for the symptoms that bother them. It is most important that gynecologists employ only the minimally invasive techniques they are skilled in performing and not be afraid to convert to standard laparotomy if they encounter difficulty.
COMPLICATIONS
UTERINE RUPTURE FOLLOWING MYOMECTOMY
Women of childbearing age who plan a future pregnancy and require a myomectomy for an intramural tumor should probably undergo a minilaparotomy or standard laparotomy to ensure proper closure of the myometrial incision unless the surgeon is very skilled in laparoscopic suturing. A cesarean delivery is safest for such patients. The laparoscopic approach is appropriate for pedunculated or subserosal tumors because smaller intramural tumors may be missed by the laparoscopic approach. Also, the necessity for complete closure of the defect cannot be emphasized enough. It is inadequate to place two or three large through-and-through interrupted sutures in a deep myometrial defect if the patient plans future childbearing. Recent reports of uterine rupture following laparoscopic myomectomy47–49 emphasize the importance of adequate closure of the myometrial defect. A dramatic photograph of a uterine rupture at 27 weeks’ gestation submitted by Riccardo Marana’s group in Rome appeared on the cover of the July/August issue of the Journal of Minimally Invasive Gynecology (Figure 21).50 A recent study reported the appearance of myomectomy scars in 15 women undergoing elective cesarean section because of previous myomectomy.51 The scars in the five women who had the myomectomies performed laparoscopically were all described as “strained with thinned tissue and ill-defined edges” despite being sutured. In the 10 women who had their myomectomy performed by laparotomy, the majority of scars were symmetric, with the tissue being of uniform thickness compared with the surrounding myometrium. Certainly the liberal use of electrocautery for hemostasis might be the etiologic factor in uterine rupture.
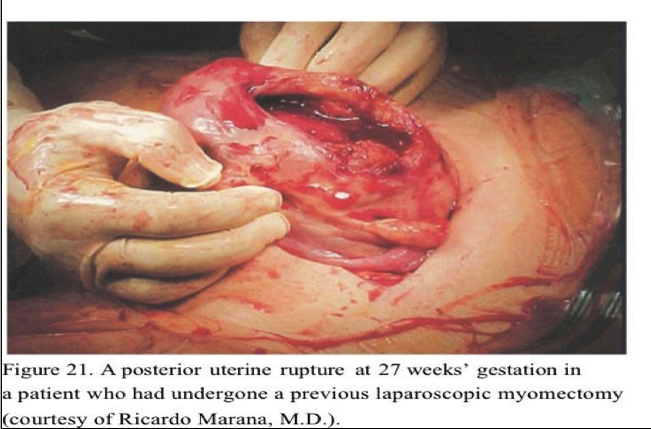
Spontaneous rupture of the uterine scar after laparoscopically assisted minilaparotomy has also been reported.52 Chromic catgut was used to close the uterine defect in that case, but whether or not this played a role is a matter of conjecture. In summary, patients must be thoroughly counseled about the possibility of uterine rupture at any time during the pregnancy and be made aware of the possibility of losing the baby and also losing the uterus if hemorrhage cannot be controlled. They must be followed very carefully and all complaints of abdominal pain taken seriously.
PARASITIC MYOMAS
The conventional thinking is that parasitic myomas are a rare variant of pedunculated subserosal myomas. It has been suggested that if a pedunculated subserosal myoma develops a long stalk and becomes what is termed a “wandering or migrating leiomyoma,” such a tumor can then go on to adhere to surrounding structures such as the omentum or broad ligament and develop an auxiliary blood supply. In this way, a “parasitic myoma” is formed when a “wandering myoma” loses its uterine blood supply and becomes attached and fed from a nonuterine source.
The literature has been limited to case reports and small series of such findings since the early 1900s; more recently a few case studies have identified parasitic myomas after laparoscopic morcellation procedures. Diagnosis of parasitic myomas is often incidental at the time of abdominal surgery for treatment of symptoms due to uterine myomas; however, there are case reports in which parasitic myomas have been found to cause symptoms themselves.
Kho and Nezhat63 reported a series of 12 cases of parasitic myomas in different locations. Eighty-three percent of patients had prior abdominal surgery, Sixty-seven percent had a prior myomectomy, and each of the patients with multiple parasitic myomas had a prior laparoscopic myomectomy with morcellation.
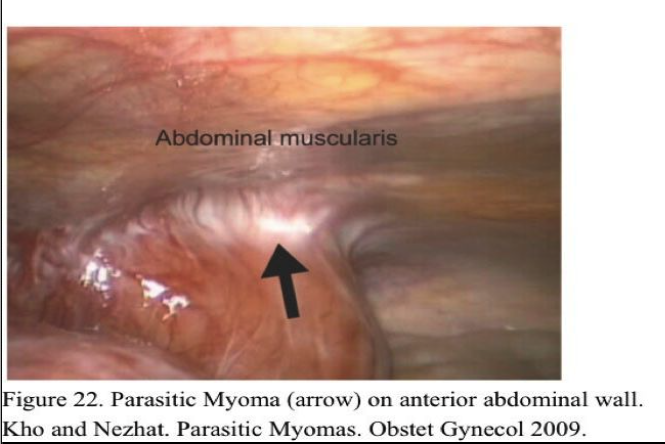
This case series reveals several interesting trends suggesting possible risk factors and causes for parasitic myomas. The greatest risk factor for development of parasitic myomas is the presence of uterine leimyoma. The next most frequent risk factor is a history of prior morcellation of the uterus or myomas. However, given the high rate of association and the biologic plausibility of this relationship, it is important that gynecologic surgeons consider the increased risk of parasitic myoma formation associated with morcellation of myomas at the time of myomectomy and similarly, with the morcellation of the uterus during hysterectomy. As suggested by case reports in the literature, the most likely locations of parasitic myomas are in areas “local” to a myomentous uterus. For example, one patient who had no prior history of abdominal surgery went on to have a large anterior abdominal wall myoma penetrated her rectus abdominus (Figure 22).
The most common indication for surgery was pain, followed by uterine bleeding – nonspecific symptoms which were attributed to concomitant uterine myomas. Because these symptoms are more commonly associated with uterine myomas, the diagnosis of parasitic myomas was almost always incidental at the time of laparoscopy for management of what were perceived to be symptoms of uterine myomas. The upper abdomen and retroperitoneal spaces (Figure 23) are often obscured by other organs and can be difficult to access; an exploration of these areas may be limited at the time of laparotomy, especially if performed through a mini laparotomy or Pfannestiel incision.
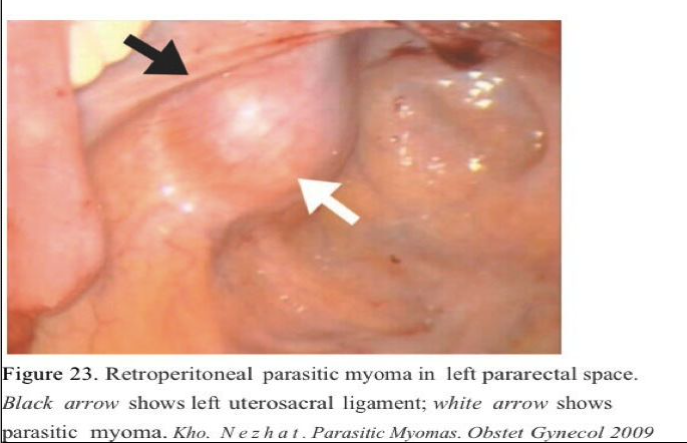
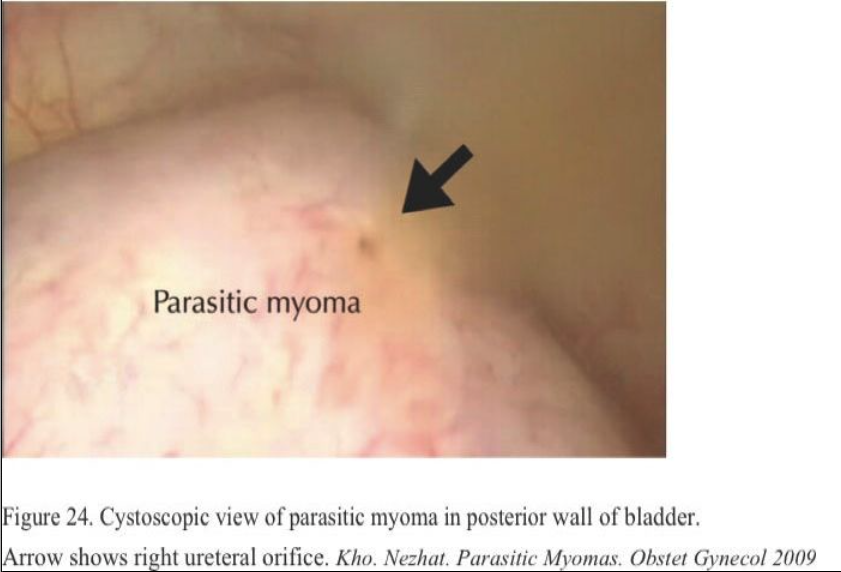
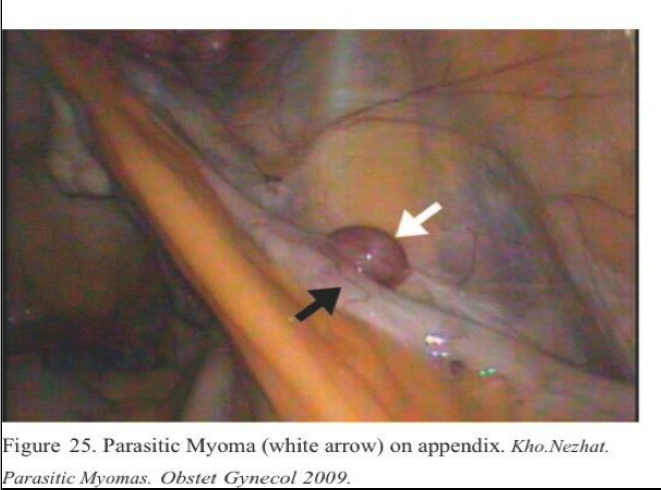
A parasitic myoma could account for secondary complaints such as constipation and dyschezia in the patient with sigmoid myomas, urinary frequency in the case of the patient with a posterior bladder myoma and right lower quadrant pain in the patient with an appendiceal myoma (Figures 24 and 25).
The use of ultrasonography and even magnetic resonance imagining or computed tomography scans may be beneficial in these cases in identifying an unanticipated mass. Given the association between morcellation and parasitic myomas, these observations hold especially true for patients who have had a prior uterine surgery, namely myomectomy or even hysterectomy.
Perhaps most importantly it has been our practice to thoroughly irrigate the entire abdominal cavity with approximately 3 L. of lactated Ringer’s solution while alternating from Trendelenburg to reverse Trendelenburg positioning after all our procedures, thereby floating and dislodging any reminant tissue. Whether access is by laparotomy or by laparoscopy, meticulous attention should be paid to basic surgical principles, including attention to complete removal of small fragments of fibroid that may be buried under bowel or bladder or stuck in cannulas and wedged in the abdominal wall.64
Although minimally invasive approaches have provided multiple benefits to patients and surgeons, they must continue to be vigilant about the potential complications and side effects of this approach, and we must be certain that we are not creating future problems while dealing with those at present.
References
- Angler NA. Different battle over a woman’s womb. New York Times. February 17, 1997.
- Farquahar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99:229–234.
- Pokras R. Hysterectomy: Past, Present and Future. Statistical Bulletin of Metropolitan Life Insurance Company. New York: Metropolitan Life Insurance Company; 1989.
- Buttram VC, Reiter RC. Uterine leiomyomata: etiology, symptomatology and management. Fertil Steril. 1981;36:433–445.
- Olive DL, Lindheim SR, Pritts EA. Nonsurgical management of leiomyomata: impact on fertility. Curr Opin Obstet Gynecol. 2004;16(3):239–243.
- Berkeley AS, DeCherney AH, Polon ML. Abdominal myomectomy and subsequent infertility. Surg Gynecol Obstet. 1983;156:319–322.
- Candiani GB, Fedele L, Parazzini F, Villa Risk of reoccurrence after myomectomy. Br J Obstet Gynaecol. 1991;98:385–389.
- Daniell JF, Gurley LD. Laparoscopic treatment of clinically significant symptomatic uterine fibroids. J Gynecol Surg. 1991;7:37.
- Nezhat C, Nezhat F, Silfen SL, et al. Laparoscopic myomectomy. IntJ Fertil. 1991;36:275–280.
- Parker WH, Rodie IA. Patient selection for laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 1994;2:23–26.
- Sinha R, Hegde A, Warty N, Patil N. Laparoscopic excision of very large myomas. J Am Assoc Gynecol Laparosc. 2003;10(4):461–468.
- Marret H, Chevillot M, Giraudeau B. A retrospective multicentre study comparing myomectomy by laparoscopy and laparotomy in current surgical practice. What are the best patient selection criteria? Eur J Obstet Gynecol Reprod Biol. 2004;117:82–86.
- Fletcher H, Frederick J, Hardie M, et al. A randomized comparison of vasopressin and tourniquet as hemostatic agents during myomectomy. Obstet Gynecol. 1996;87:1014–1018.
- Zullo F, Palomba S, Corea D, et al. Bupivacaine plus epinephrine for laparoscopic myomectomy (a randomized placebo-controlled trial). Obstet Gynecol. 2004;104:243– 249.
- Lieng M, Istre O. Uterine rupture after laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 2004;11(1):92–93.
- Stringer NH, McMillan NA, Jones RI, Nezhat A, Park E. Uterine closure with the endo stitch 10-mm laparoscopic suturing device – a review of 50 laparoscopic myomectomies. Int J Fertil Womens Med. 1997;42(5):288–296.
- Advincula AP, Song A, Burke W. Preliminary experience with robot assisted laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 2004;11(4):511–518.
- Hutchins FL, Reinoehl EM. Retained myoma after laparoscopic supracervical hysterectomy with morcellation. J Am Assoc Gynecol Laparosc. 1998;5:293–295.
- Ostrzenski A. Uterine leiomyoma particle growing in an abdominal-wall incision after laparoscopic retrieval. Obstet Gynecol. 1997;89:853–854.
- Sinha R, Hegde A, Warty N. Laparoscopic myomectomy: enucleation of the myoma by morcellation while it is still attached to the uterus. J Minim Invasive Gynecol. 2005;12(3):284–289.
- Ou CS, Harper A, Liu YH, et al. Laparoscopic myomectomy technique (use of colpotomy and the harmonic scalpel). J Reprod Med. 2002;47:849–853.
- Pelosi MA 3d, Pelosi MA. Laparoscopic-assisted transvaginal myomectomy. J Am Assoc Gynecol Laparosc. 1997;4:241–246.
- Goldfarb HA, Fanarjian MA. Laparoscopc-assisted transvaginal myomectomy: a case report and literature review. J Soc Laparosc Surg. 2001;5:81–85.
- Birsan A, Deval B, Detchev R. Vaginal and laparoscopic myomectomy for large posterior myomas: results of a pilot study. Eur J Obstet Gynecol Reprod Biol. 2003;10(1):89–93.
- Chin HY, Lee CL, Yen CF, et al. Laparoscopic-assisted vaginal myomectomy through an anterior approach. J Laparoendosc Adv Surg Tech A. 2004;14(3):135–138.
- Nezhat C, Nezhat F, Bess O. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil Menopausal Stud. 1994;39:39–44.
- Benedetti-Panici P, Maneschi F, Cutillo G. Surgery by minilaparotomy in benign gynecologic disease. Obstet Gynecol. 1996;87:456–459.
- Benassi L, Marconi L, Benassi G, et al. Minilaparotomy vs. laparotomy for uterine myomectomies: a randomized controlled trial. Minerva Ginecol. 2005;57(2):159–163.
- Glasser MH. Minilaparotomy myomectomy: a minimally invasive alternative for the large fibroid uterus. J Minim Invasive Gynecol. 2005;12(3):275–283.
- Tanaguchi F, Harada T, Iwabe T, et al. Use of the LAP DISK (abdominal wall sealing device) in laparoscopically assisted myomectomy. Fertil Steril. 2004;81(4):1120– 1124.
- Phillips DR, Nathanson HG, Millim SJ. The effect of dilute vasopressin on blood loss during hysteroscopy: a randomized con- trolled trial. Obstet Gynecol. 1996;88:761– 766.
- Pelosi MA 3d, Pelosi MA. The suprapubic cruciate incision for laparoscopic-assisted microceliotomy. JSLS. 1997;1(3):269–272.
- Pelosi MA 2d, Pelosi MA 3d. Self-retaining abdominal retractor for minilaparotomy. Obstet Gynecol. 2000;96:775–778.
- Lethaby A, Vollenhoven B, Sowter M. Efficacy of pre-operative gonadotrophin releasing hormone analogues for women with uterine fibroids undergoing hysterectomy or myomectomy: a systematic review. Br J Obstet Gynaecol. 2002;109(10):1097–1108.
- Freidman AJ, Rein NS, Harrison-Atlas D, et al. A randomized, placebo-controlled, double blind study evaluating leuprolide acetate depot treatment before myomectomy. Fertil Steril. 1989;52:728.
- Campo S, Garcea N. Laparoscopic myomectomy in pre- menopausal women with and without preoperative treatment using gonadotrophin-releasing hormone analogues. Human Reprod. 1999;14:44–48.
- Vercellini P, Trespidi L, Zaina B, Vicentini S, Stellato G, Crosig- nani PG. Gonadotropin-releasing hormone agonist treatment before abdominal myomectomy: a controlled trial. Fertil Steril. 2003;79(6):1390–1395.
- Operative Laparoscopy Study Group. Postoperative adhesion development after operative laparoscopy: evaluation at early second-look procedures. Fertil Steril. 1991;55:700.
- Bayers SP, Jansen D. Gore-Tex surgical membrane. In: DeCherney A, Diamond MP, eds. Treatment of Post-Surgical Adhesions. New York: Wiley-Liss; 1990.
- Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrance (HAL-F): a blinded, prospective, randomized, multicenter clinical Seprafilm Adhesion Study Group. Fertl Steril. 1996;66:904.
- Melis GB, Ajossa S, Piras B, et al. A randomized trial to evaluate the prevention of de novo adhesion formation after laparoscopic myomectomy using oxidized regenerated cellulose (Interceed) barrier. J Am Assoc Gynecol Laparosc. 1995;2:S31.
- Mettler L, Audebert A, Lehmann-Willenbrock E, et al. A randomized, prospective, controlled, multicenter clinical trial of a sprayable, site-specific adhesion barrier system in patients under- going myomectomy. Fertil Steril. 2004;82(2):398–404.
- Linsky CB, Diamond MP, DiZerega GS. Effect of blood on the efficacy of barrier adhesion reduction in the rabbit uterine horn model. Infertility. 1988;11:273.
- Fedele L, Vercellmi P, Bianchi S, et al. Treatment with GnRH agonists before myomectomy and the risk of short-term myoma recurrence. Br J Obstet Gynaecol. 1990;97:393.
- Hasson HM, Rotman C, Rana N, et al. Laparoscopic myomectomy. Obstet Gynecol. 1992;80:884.
- Dubuisson JB, Lecuru F, Herve F, et al. Myomectomy by laparoscopy. Fertil Steril. 1991;56:827.
- Dubuisson JB, Chavet X, Chapron C, et al. Uterine rupture during pregnancy after laparoscopic myomectomy. Hum Reprod. 1995;10(6)1475–1477.
- Friedman W, Maier RE, Luttkus A, et al. Uterine rupture after laparoscopic myomectomy. Acta Obstet Gynecol Scand. 1996; 75(7):683–684.
- Malberti S, Ferrari L, Milani R. Spontaneous uterine rupture in the third trimester of gestation after laparoscopic myomectomy. A case report. Minerva Ginecol. 2004;56(5):479–480.
- Grande N, Catalano GF, Ferrari S, Marana R. Spontaneous uterine rupture at 27 weeks of pregnancy after laparoscopic myomectomy. J Minim Invasive Gynecol. 2005;12(4):301.
- Cobellis L, Pecori E, Cobellis G, et al. Comparison of intramural myomectomy scar after laparotomy or laparoscopy. Int J Gynaecol Obstet. 2004;84:87–88.
- Hockstein S. Spontaneous uterine rupture in the early third trimester after laparoscopically assisted myomectomy (a case report). J Reprod Med. 2000;45:139–141.
- Campo S, Campo V, Gambadauro P. Reproductive outcome before and after laparoscopic or abdominal myomectomy for sub- serous or intramural myomas. Eur J Obstet Gynecol Reprod Biol. 2003;110(2):215–219.
- Sudik R, Husch K, Steller J, et al. Fertility and pregnancy out- come after myomectomy in sterility patients. Eur J Obstet Gynaecol Reprod Biol. 1996;65:209–214.
- Dessolle L, Soriano D, Poncelet C, et al. Determinants of pregnancy rate and obstetric outcome after laparoscopic myomectomy for infertility. Fertil Steril. 2001;76:370–374.
- Olufowobi O, Sharif K, Papainnou S, et al. Are the anticipated benefits of myomectomy achieved in women of reproductive age? A 5-year review of the results at a UK tertiary hospital. J Obstet Gynaecol. 2004;24(4):434–440.
- Marchionni M, Fambrini M, Zambelli V, et al. Reproductive performance before and after abdominal myomectomy (a retrospective analysis). Fertil Steril. 2004;82:154–159.
- Seracchioli R, Rossi S, Govoni F, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata (a randomized comparison with abdominal myomectomy). Hum Reprod. 2000;15:2663–2668.
- Goldberg J, Pereira L, Berghella V, et al. Pregnancy outcomes after treatment for fibromyomata: uterine artery embolization versus laparoscopic myomectomy. Am J Obstet Gynecol. 2004;191(1):18–21.
- Surrey ES, Minjarez DA, Stevens JM, Schoolcraft WB. Effect of myomectomy on the outcome of assisted reproductive technologies. Fertil Steril. 2005;83(5):1473–1479.
- Oliveira FG, Abdelmassih VG, Diamond MP, et al. Impact of subserosal and intramural uterine fibroids that do not distort the endometrial cavity on the outcome of in vitro fertilization– intracytoplasmic sperm injection. Fertil Steril. 2004;81(3):582–587.
- Cooper NP, Okolo S. Fibroids in pregnancy–common but poorly understood. Obstet Gynecol Surv 2005;60(2):132–138.
- Kho, K.A., Nezhat, C. Parasiic Myomas. Obstet. Gynecol. 2009 Sep 114(3):611-5.
- Nezhat, C., Kho, K. Iatrogenic Myomas: New Class of Myomas? J Minim. Invasive Gynecol. 2010 Jun 25.
