Laparoscopic Pediatric Surgery
Gustavo Stringel, MD, MBA
INTRODUCTION
History of Pediatric Laparoscopy
The first case of laparoscopy in pediatric surgery was reported by Stephen Gans in 1971, in his landmark publication, “Advances in Endoscopy of Infants and Children,” as a peritoneoscopy. The term peritoneoscopy was soon replaced by Pediatric Laparoscopy.1 The availability of smaller instruments expanded the role and applications of laparoscopy and thoracoscopy in very small infants and newborns. Initially, application of laparoscopy in children was for diagnostic purposes.
Gans’ first case was the successful verification of a hernia on the opposite side by an endoscope introduced through the contralateral known hernia sac. In his series, he also included a 2 kg one day old baby with ascites. This was the first report of a laparoscopic procedure in a newborn. Gans also reported a laparoscopic therapeutic procedure to remove a broken ventriculo-peritoneal shunt in a child.1,2
Thoracoscopy developed in a similar fashion. The first report of pediatric thoracoscopy appeared in 1971 when Klimkovich et al reported a variety of successful thoracoscopic procedures in children.3 Rodgers performed pediatric thoracoscopic procedures in children as young as 17 months of age.4
The weight of a pediatric surgical patient ranges from a few pounds in a newborn to over 200 pounds in an adolescent. Pediatric surgeons operate on a variety of anatomical areas, including the abdomen, the chest, the head and neck, and the extremities. Pediatric surgery is probably the last truly general surgical specialty. It is a unique specialty because of the variability in patients’ size and anatomical and physiological characteristics. Laparoscopic pediatric surgeons perform procedures in newborn babies to correct congenital anomalies such as diaphragmatic hernia, esophageal atresia, duodenal atresia and many others. At the other extreme, bariatric procedures are performed in morbidly obese adolescents. Pediatric surgeons are well acquainted with state of the art technology like the use of robotic surgery in newborns and infants, the use of single incision surgery, single port laparoscopic surgery, and minilaparoscopy.
“Children are not small adults.” This statement is true not only because of anatomical and physiological characteristics, but also because of psychological and emotional differences compared to the adult population.
An increasingly sophisticated and informed patient population often requests laparoscopy over open traditional procedures. Parents frequently select surgeons based on their laparoscopic skills. The older generation of pediatric surgeons has been forced to learn this new technique in contrast with the new trainees who are versed in laparoscopy and thoracoscopy. When dealing with laparoscopic complications, the older surgeons who usually have more experience with traditional open procedures may not hesitate to convert to an open procedure, while the new generation with less exposure to open procedures may be more reluctant to do it or may fail to recognize a potentially dangerous situation. An example of this is laparoscopic pyloromyotomy, a technique adopted by many teaching hospitals; the residents may learn this approach readily and never perform the open procedure. The lack of experience by the resident with the open operation may be a downside when faced with a complication of laparoscopic pyloromyotomy. In our institution open appendectomies, cholecystectomies, antireflux procedures and others are seldom performed. Consequently, we are concerned about the ability of our trainees to perform well when compelled to convert to an open procedure. Most of the complications occur during the learning curve stage. Even an expert surgeon performing a new procedure should be considered an inexperienced surgeon.5
The incidence of complications in pediatric laparoscopy is reported to be around 4-5%.5-8 In most cases the complication can be managed laparoscopically. A conversion rate of 5- 10% has been reported as acceptable.6 There are complications specifically related to the minimally invasive approach and complications of the actual procedure. Problems secondary to the laparoscopic/thoracoscopic approach may be physiologic disturbances due to insufflation, access, or equipment. Complications that are procedure specific may occur with an open technique as well as a minimally invasive approach.5-9
ANATOMICAL AND PHYSIOLOGICAL ISSUES
The reason for the establishment of the subspecialty of pediatric surgery is the recognition of significant issues regarding the care of the pediatric surgical patients that warrant special attention. This is certainly applicable to minimally invasive surgery for pediatric patients. Anatomical and physiological differences in children may require a modification of the techniques used in adults.5-11
Anatomical considerations in the pediatric patient are related to the variation in size and the stage of development. The smaller chest and abdomen in infants make access and exposure more problematic. The relatively large size of the liver and spleen in young children can compromise the working space. This has been a limitation for abdominal robotic procedures performed in children weighing less than 3 kg and in children below 4 kg in thoracoscopic operations. The failure to carefully consider trocar placement can make a procedure more difficult or limit the ability to complete the procedure. The umbilical vein may remain patent in the infant and care should be taken when using the umbilicus as the portal of entry to avoid causing gas embolism. The bladder is an intra- abdominal organ in the younger pediatric population; if not emptied at the start of the procedure, it may limit exposure and be at risk of injury during trocar placement. The closer proximity of organs and associated structures such as blood vessels and nerves requires careful identification and dissection to avoid injury.
Physiological changes secondary to insufflation of the abdomen or the chest are generally well tolerated in the adult patient but can present a challenge to the anesthesiologist in the pediatric patient.6,10,12 The greater compliance of the thoracic cavity in the child and the dependence on diaphragmatic excursion for ventilation can be compromised with the establishment of pneumoperitoneum. Elevations in carbon dioxide and decreased oxygenation can occur with the higher intra abdominal pressures normally used in adult laparoscopy. The lowest pressure possible should be used to elevate the abdominal wall or create a pneumothorax. An intra-abdominal pressure of less than 14 mmHg appears to be well tolerated even in infants smaller than 5 kg.12 Intra-thoracic pressures of 5 mmHg by carbon dioxide insufflation have minimal hemodynamic effects; higher intra-thoracic pressures (10-15 mmHg) cause marked hemodynamic changes, including myocardial ischemia.13 In patients with severe congenital cardiac or pulmonary disease even low pressures may not be tolerated and conversion to an open technique may be the safest action. Hypercarbia is improved by increasing the minute ventilation and an increase in the fraction of inspired oxygen will increase oxygenation.12 There is no general consensus regarding an acceptable degree or duration of hypercapnea and whether the associated acidosis results in discernable negative consequences. A report of hypercarbia, hypercapnea and associated acidosis during congenital diaphragmatic hernia repair in 31 consecutive neonates recorded intraoperative mean highest end-tidal CO2 of 64 ±13 torr and highest arterial PCO2 of a mean of 78±29 torr. The authors found a poor correlation between simultaneous measures of ETCO2 and arterial PCO2 and that the administration of buffering agents was not necessary to correct the associated respiratory acidosis.14 The development of new techniques such as mass spectrometry to measure absorption of carbon dioxide during laparoscopy may help to correlate the effects of CO2 and pathophysiological changes occurring during laparoscopy.15The debate continues regarding safe levels of hypercarbia and hypercapnea during pediatric laparoscopic and thoracoscopic surgery; hopefully future research projects will shed more light on this subject.
Transient oliguria and even anuria have been reported during laparoscopic surgery in infants and children. Pneumoperitoneum causes anuria in most children younger than one year of age and significant oliguria in about one third of older children; this is usually a reversible event. Some authors cautioned that urine output during laparoscopic surgery might not be an adequate parameter for calculating intravenous fluid administration.16
Cardiac performance can be compromised as a result of decreased cardiac filling and preload with insufflation. Patients may be hypovolemic after preoperative fasting and this contributes to the decreased preload, especially in children who have greater fluid requirements due to an elevated basal metabolic rate. Intraoperative hydration and judicious fluid boluses should improve this problem. Even patients with severe congenital heart disease such as hypoplastic heart left syndrome can tolerate laparoscopic procedures; however, a multidisciplinary approach with a skilled and experienced cardiac anesthesia team is essential to optimize outcomes in these critically ill children.17
Thermoregulation is a primary concern in the pediatric patient. Introduction of cool gas into a body cavity can result in a rapid decrease in body temperature at higher flow rates. The use of gas warming insufflators and low flow rates can ameliorate this problem.7,12 Overall, these disturbances are minor and correctable with interventions by the anesthesiologist. Hypercapnia is the main concern of the anesthesiologist and usually resolves rapidly with decompression of the pneumoperitoneum or pneumothorax. This elevated carbon dioxide is generally well tolerated in the otherwise healthy pediatric patient. Muscle relaxation during the procedure is important to avoid the unnecessary use of high pressures. Close communication between anesthesiologist and surgeon is critical for a successful outcome.
Open surgical procedures cause tissue trauma that results in a complex systemic inflammatory response causing stress to the organism. One of the advantages of laparoscopy is to produce less systemic inflammatory response than open procedures. However a recent study questions this assumption in cases of laparoscopic appendectomy.18 More studies are necessary to evaluate this phenomenon because appendicitis produces a variable inflammatory response in different patients.
PAIN CONTROL
Laparoscopy and thoracoscopy cause less tissue trauma and, theoretically, less postoperative pain than open surgery. However, up to 80% of adult patients require postoperative opiates.12 It is difficult to evaluate postoperative pain in the pediatric population, especially in small children, infants, and newborns. It is extremely important to use a pain scale card that is easily interpreted by children in order to provide appropriate pain relief. Pain can be secondary to gas insufflation, over distention of the diaphragm and the phrenic nerve, or residual carbon dioxide gas in the abdomen. Every effort should be made to remove all gas from the abdomen at the end of the procedure and avoid using unnecessarily high intra-abdominal pressures.10,12
An important source of pain is related to the incisions for port placement. The use of pre- emptive analgesia by infiltration of local anesthesia before the incision at the trocar site is very helpful. We prefer the use of a long-acting local anesthetic; bupivacaine at a ¼% concentration works well. When thoracoscopy is performed, we often use an intercostal nerve block under direct visualization as well as intrapleural instillation of bupivacaine.
The use of epidural analgesia can help to decrease pain and the use of opiates. The preoperative administration of rectal acetaminophen and ketorolac is beneficial.12
Postoperative pain is always a concern and we do not hesitate to prescribe opiates as well as patient-controlled analgesia (PCA pump) in older children.
COMPLICATIONS OF ACCESS
A potential source of complications in laparoscopic pediatric surgery is the insertion and removal of trocars.5-7,19,20 The placement of trocars into the abdomen or chest can be accomplished using an open or closed technique. The closed technique, using a Veress needle and non-visualized trocar insertion, has the disadvantage of inadvertent perforation of organs and vascular structures during placement of the Veress needle or the trocar.5-7,20,21 The smaller size and closer proximity of organs in the child make this a particular concern in this patient population.
These complications in pediatric patients can be minor, such as an enterotomy, or major, such as an injury to the iliac vessels or aorta.20,21 There are also reports in the adult literature of death from gas embolism due to insufflation with the Veress needle in a major blood vessel.7 Abdominal entry with a Veress needle inserted into the umbilical vein caused carbon dioxide embolism in a recently reported case during a laparoscopic pyloromyotomy in a 3 week old infant; the infant sustained sudden cardiovascular collapse and cyanosis, requiring cardiovascular resuscitation.22 In the pediatric patient, the relatively small size of the body cavity and close proximity of the structure can make closed access difficult. The use of an open technique is the favored method of access in the pediatric patient. Although it does not eliminate the risk of visceral/blood vessel injury, it allows for better control and visualization when inserting trocars.
After insertion of the first trocar and insufflation, the abdomen or chest should be inspected for sign of injury to the viscera or vasculature. The presence of excessive blood or sulcus entericus mandates a careful search for an injury. Conversion to an open procedure may be necessary to treat any iatrogenic injuries that may have occurred. After the insufflation and inspection, all other trocars should be placed under direct vision. This will not eliminate the possibility of trocar-related injuries but it will allow for better control and the avoidance of most injuries. Abdominal wall lifting or a radial dilating trocar system may help overcome the relative laxity of the body wall. Good visualization with gentle controlled trocar insertion will help to minimize complications related to trocar placement.
We have abandoned the use of the Veress needle. The preferred approach of the author is to make a small infraumbilical incision, expose the root of the umbilicus, grasp it with a Kocher clamp, pull the abdominal wall upward, make a small midline incision in the rectus fascia, and proceed to dissect with a mosquito clamp until entering the peritoneum; subsequently, a blunt trocar is inserted. Since this technique was adopted several years ago, there have been no visceral or vascular injuries in hundreds of cases. In addition, this technique is very useful in obese patients. In infants and small children, the distance between the umbilicus and pubis is shorter than the distance between the umbilicus and the xiphoid. For this reason, the initial umbilical trocar should be directed towards the upper abdomen to avoid injuries.
A potential problem area is the lower abdomen. The inferior epigastric vessels should be identified in order to avoid trocar injury which can result in bleeding or abdominal wall hematoma. A large falciform ligament or insufflation of the falciform ligament can interfere with the port placement and exposure of the upper abdomen. At the completion of the procedure, problems can occur with removal of the trocar from the insertion site. Bleeding and evisceration may occur at the time of removal (Figures 1 and 2).
Subcutaneous or body wall blood vessels that were divided and subsequently tamponaded with trocar placement may hemorrhage upon removal of the trocar. If unrecognized, this may lead to significant blood loss. Careful inspection of trocar site at the time of removal can identify bleeding which can be controlled at that time. A useful maneuver when profuse port bleeding is encountered is to place a Foley or similar catheter through the port, inflate the balloon, and apply traction to tamponade the bleeding. This can facilitate exposure and suturing of the bleeding area.
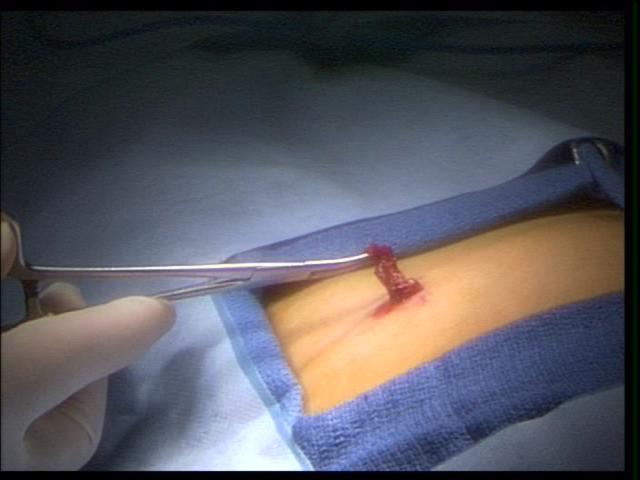 Figure 1. External view of eviscerated omentum through a 5 mm port, in a small child.
Figure 1. External view of eviscerated omentum through a 5 mm port, in a small child.
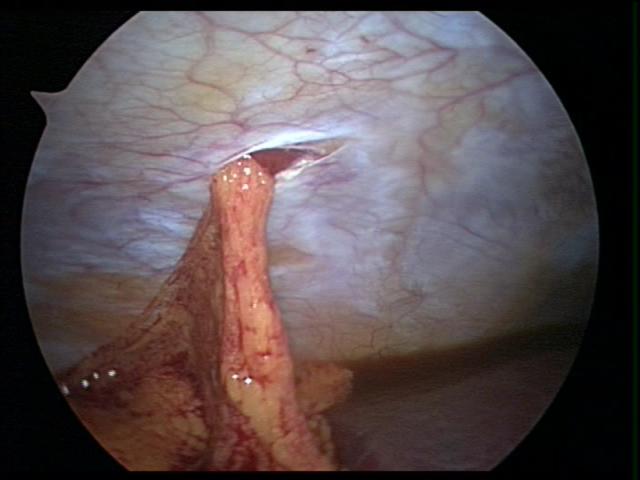 Figure 2. Internal view of eviscerated omentum through a 5 mm port, in a small child.
Figure 2. Internal view of eviscerated omentum through a 5 mm port, in a small child.
Evisceration is usually obvious and can be easily fixed. The omentum in infants may be flimsy and sutured into the trocar closure (Figures 3 and 4). Omental evisceration can be a problem when even small trocar incisions are not properly closed. Careful inspection and closure helps to avoid problems with entrapped viscera or tissue. When closing the port areas in small and thin patients, care should be taken to avoid suturing the intestine or omentum to the port site (Figure 5). This maneuver can cause postoperative bowel obstruction (Figure 6). A more delayed complication related to trocar removal is the development of hernias at the trocar sites.
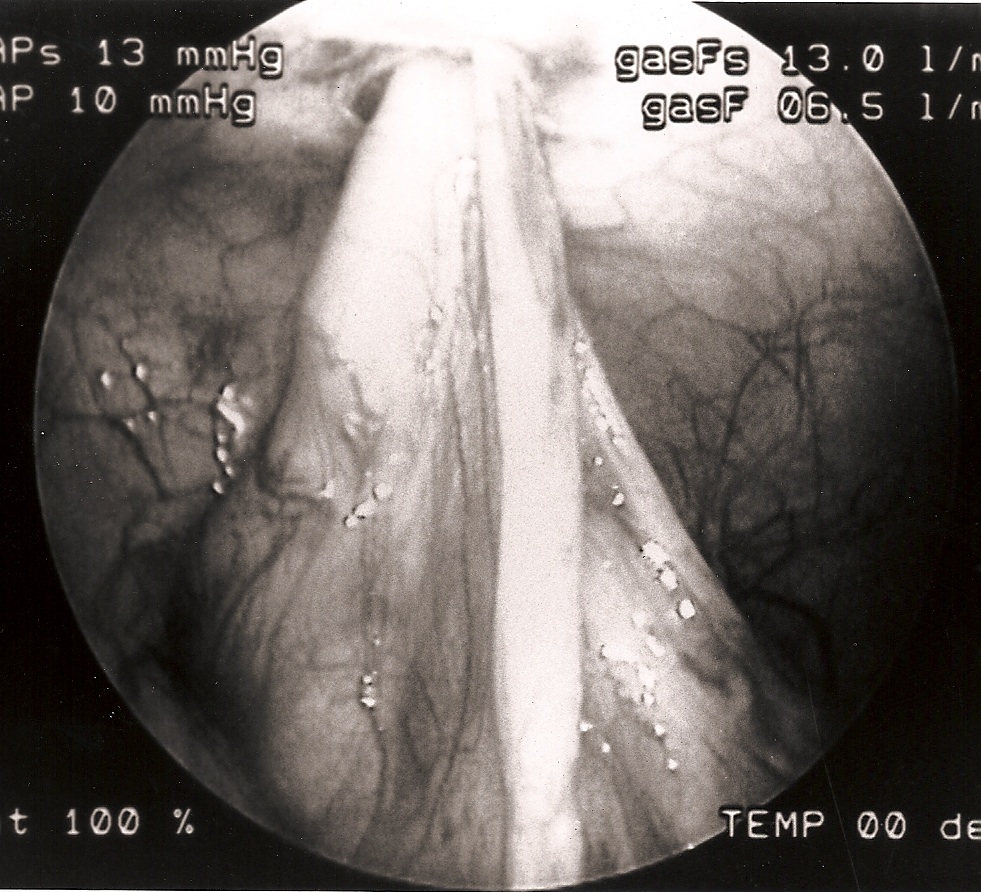 Figure 3. Small intestine sutured to the umbilical port site.
Figure 3. Small intestine sutured to the umbilical port site.
 Figure 4. Loops of small intestine sutured to two port sites.
Figure 4. Loops of small intestine sutured to two port sites.
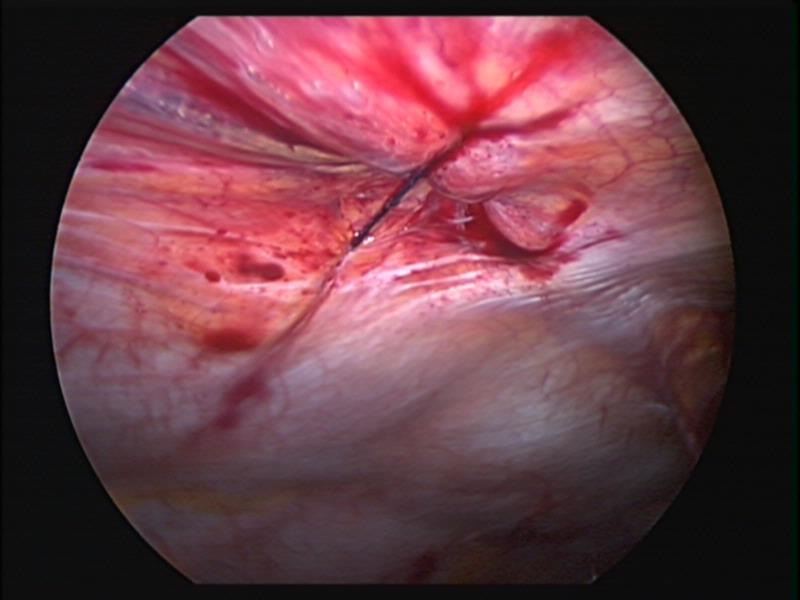 Figure 5. Secure closure of a 5mm umbilical port in a small child.
Figure 5. Secure closure of a 5mm umbilical port in a small child.
 Figure 6. Loop of small intestine sutured to a port site, causing localized volvulus with intestinal obstruction.
Figure 6. Loop of small intestine sutured to a port site, causing localized volvulus with intestinal obstruction.
In the adult laparoscopic practice, only trocar sites equal to or greater than 10-mm require closure of the fascial layer. The small size of the abdominal contents in infants and small children allows for herniation through a 5-mm trocar site.23,24 We recommend closing 5- mm trocars sites in infants, small children, or thin patients, at the fascial level to avoid herniation. Teenagers and obese patients do not need a 5-mm trocar closure. We close the skin only in ports that are 3mm or smaller.
Endo close™ or similar devices can injure adjacent organs when not properly used during the closure of port sites. In one of our cases a blunt needle-type closing device injured the mesentery causing profuse bleeding and required placing a suture to stop the hemorrhage (Figures 7, 8, and 9).
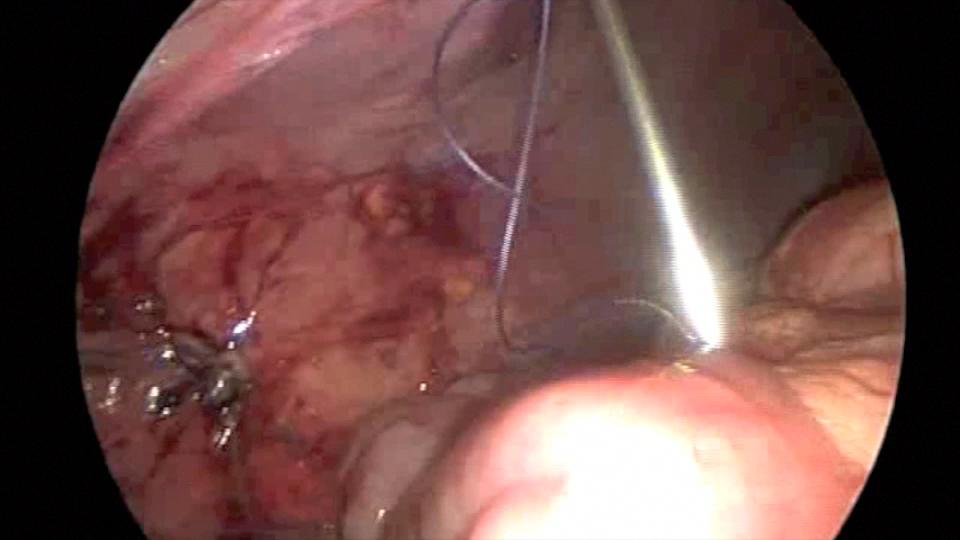 Figure 7. Endo close™ needle causing injury to the mesentery.
Figure 7. Endo close™ needle causing injury to the mesentery.
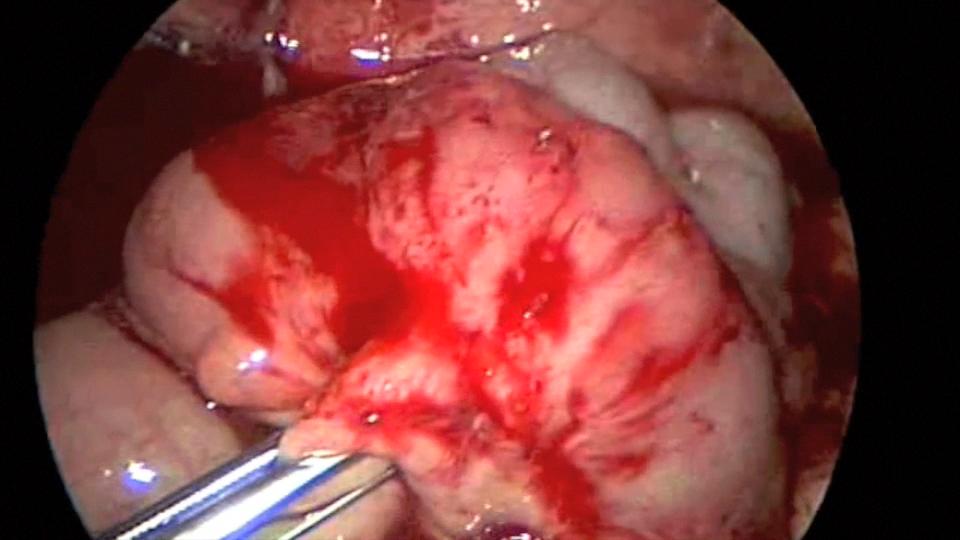 Figure 8. Profuse bleeding caused by endo close™ needle injury to mesentery.
Figure 8. Profuse bleeding caused by endo close™ needle injury to mesentery.
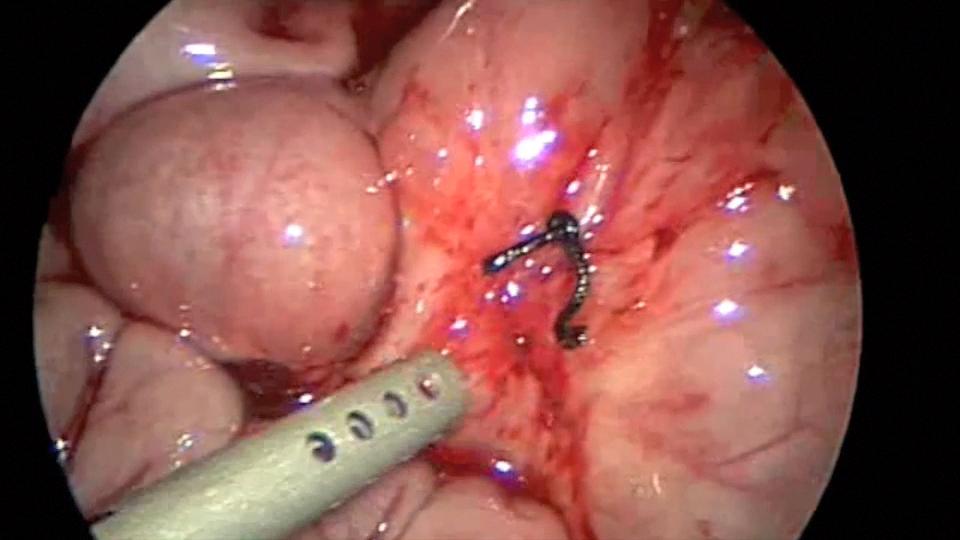 Figure 9. Mesenteric bleeding controlled with one figure of 8 suture.
Figure 9. Mesenteric bleeding controlled with one figure of 8 suture.
The type of trocar used is another factor in minimizing trocar-related complications. There are essentially two types of trocars: bladed cutting and noncutting or blunt dilating. The cutting trocar gains access by sharply dividing tissues to allow insertion. The noncutting type spreads and dissects tissues without disrupting their integrity.7 The theoretical advantages of blunt dilation are that the tissues will return to their normal configuration following removal of the trocar and that the nerves and blood vessels are pushed aside rather than divided. The thinner abdominal wall in pediatric patients may suffer more extensive disruption or injury with a cutting-type trocar and thus be more prone to hernias.
Postoperative subcutaneous emphysema may be worrisome but it usually has minimal clinical consequence and resolves within a few days. Carbon dioxide in the scrotum may alert the surgeon to the presence of an inguinal hernia.
Ports are easily dislodged in small or thin children. Accidental removal of the port causes loss of the pneumoperitoneum and the port’s replacement wastes operative time. It can be a potential source of visceral injury by intra-abdominal instruments. Using serrated ports can minimize this problem. Suturing the ports to the abdominal wall is often necessary to prevent this complication.
Port placement is extremely critical in small children. Sufficient working distance must be available between the ports. Using shorter ports is important to minimize this problem.
COMPLICATIONS RELATED TO INSTRUMENTATION AND EQUIPMENT
The development of improved optics and video-technology capabilities has contributed to the evolution of laparoscopic surgery. The increased application of laparoscopic and thoracoscopic procedures has led to the need for more sophisticated instrumentation, such as stapling and suturing devices, clip appliers, and retrieval bags. The majority of laparoscopic instruments has been designed with the adult patient in mind and as a result can present technical difficulties when used in the pediatric patient. These devices facilitate the performance of more complex procedures but they also increase the risk of serious problems when they fail or are improperly utilized. The use of high-energy cutting devices such as electrocautery or the harmonic scalpel can cause significant harm if improperly applied in laparoscopic pediatric procedures where the tissue is thinner and the structures are in closer proximity, thus allowing for extension of the cautery effect.7,25 The author favors the use of bipolar cautery and harmonic scalpel. It is important to remember that the harmonic scalpel can get very hot after repeated use and cause thermal injury by direct contact.
Stapling technology is well established as safe and effective in both adult and pediatric patients in open surgery. This technology has been adapted to the laparoscopic minimally invasive world with equal success. Effective stapling requires knowledge of and familiarity with the device as well as a properly functioning instrument. As with any mechanical device, there is a risk of failure regardless of the high quality standards that are required in their construction. Multi-fire stapling devices require the use of reload cartridges, which, if not properly replaced, can lead to problems. Staples can fail to engage or deploy the cutting device without staple application.6,7,9,10 Staple lines may also bleed, even if the stapler is correctly applied and such bleeding may be difficult to control without converting to an open technique. These problems can be, at best inconvenient, and, at worst potentially lethal. It is important to select the appropriate staple size to avoid complications. When dealing with vascular structures such as splenic vessels, we prefer the use of 2-mm stapling devices; intestine and stomach need a larger staple size.
Clip-applying devices can create problems if the clip does not close correctly or is not positioned well. There is also the danger of inadvertently clipping adjacent structures which may occur when using clip appliers intended for adults in children, such as when a 10-mm clip is used on a small child’s cystic duct during laparoscopic cholecystectomy. The advent of 5-mm clipping devices has facilitated the control of small vessels and other structures without the use of 10-mm ports.
The removal of specimens or organs may require the use of retrieval devices, such as endobags. The placement of the specimen in the bag and extraction can be difficult in children because the bag or device may be too large for the pediatric body cavity. Failure of the bag to deploy, premature separation, or perforation and spillage of its contents may occur while trying to maneuver in a small space. Attempts to place large organs, such as the spleen, into extraction bags for manual or mechanical morcellation can disrupt the bag. A gentle extraction technique, together with knowledge of the limitations of the bag’s size and strength, will avoid these problems (Figure 10). It is important to observe the bag with the laparoscope at all times during morcellation and specimen retrieval (Figure 11).7,26
 Figure 10. Very large spleen fitting with difficulty inside a large endo bag.
Figure 10. Very large spleen fitting with difficulty inside a large endo bag.
 Figure 11. Monitoring of extraction of large spleen through morcelization.
Figure 11. Monitoring of extraction of large spleen through morcelization.
During instrumentation, devices are subjected to mechanical stress, especially when they are being aggressively manipulated to fit into a confined space. This equipment is composed of small and larger parts that can break off or disengage intracorporeally (Figures 12 and 13). These fragments, large or small, can usually be retrieved with judicious laparoscopic manipulation. In the event that a piece of an instrument cannot be safely extracted laparoscopically, conversion to an open procedure should be performed. A device removed forcefully in frustration can do significant damage to the thin pediatric body wall upon removal. Careful inspection of the instrumentation prior to its use can minimize this problem.
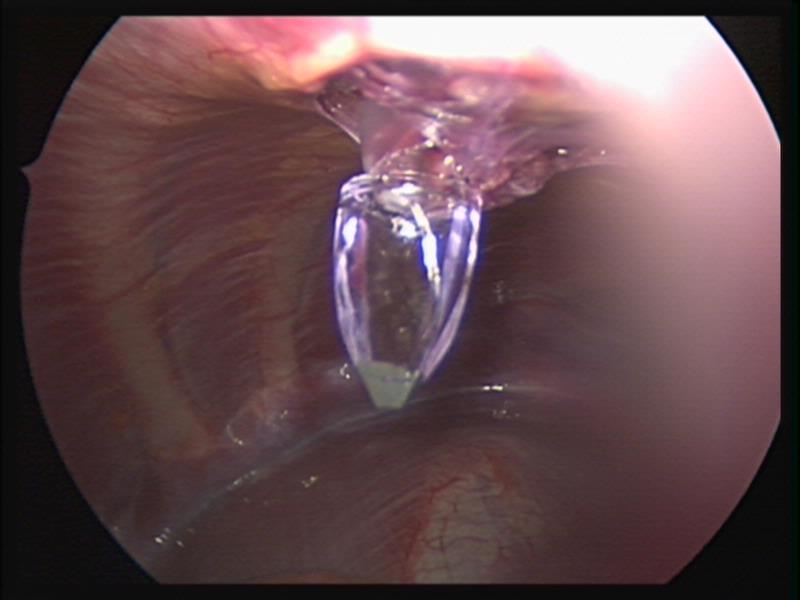 Figure 12. Lost trocar tip.
Figure 12. Lost trocar tip.
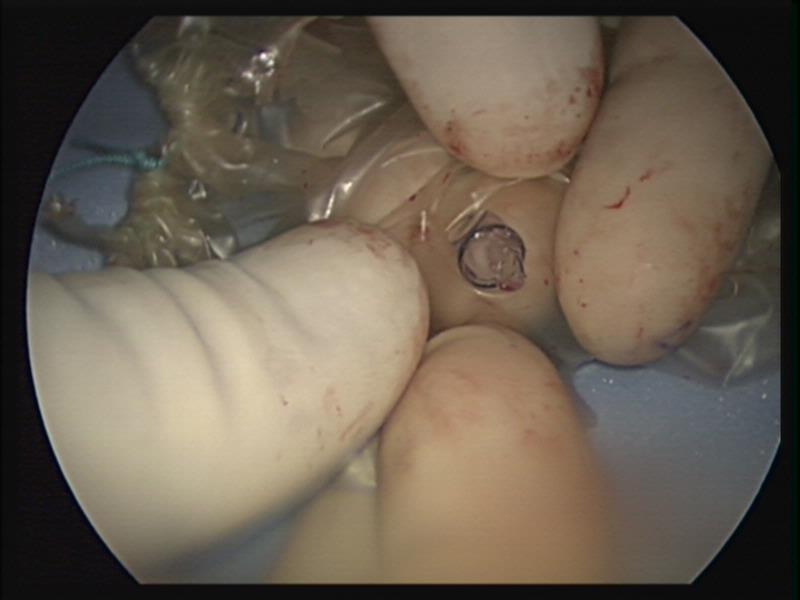 Figure 13. Lost trocar tip after retrieval.
Figure 13. Lost trocar tip after retrieval.
In two of our patients, the cartridge of the automatic endo-stapler became separated from the handle after firing the instrument (Figures 14, 15, 16, and 17). In one of those cases, the stapler was fired across the splenic vessels (Figure 18). We immediately contacted the manufacturer’s hotline and they provided invaluable advice to manipulate and remove the device without harm to the patient and without conversion to an open procedure.
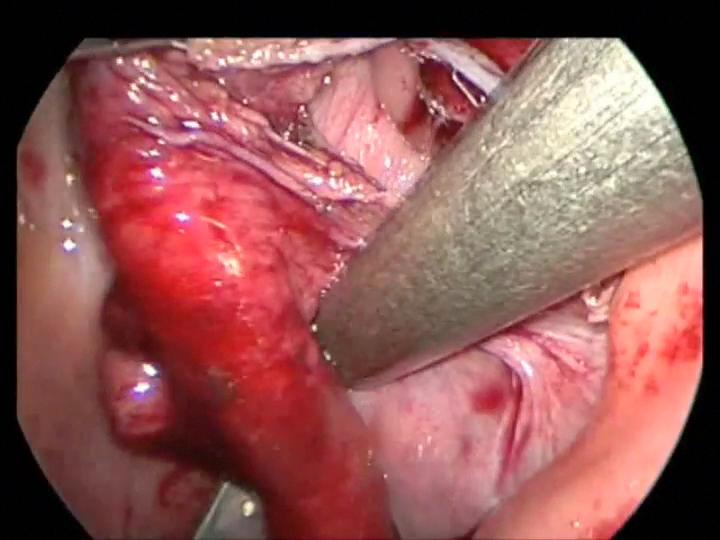 Figure 14. Stapler being fired across the mesentery of the appendix. Notice lost stapler cartridge in the left lower corner.
Figure 14. Stapler being fired across the mesentery of the appendix. Notice lost stapler cartridge in the left lower corner.
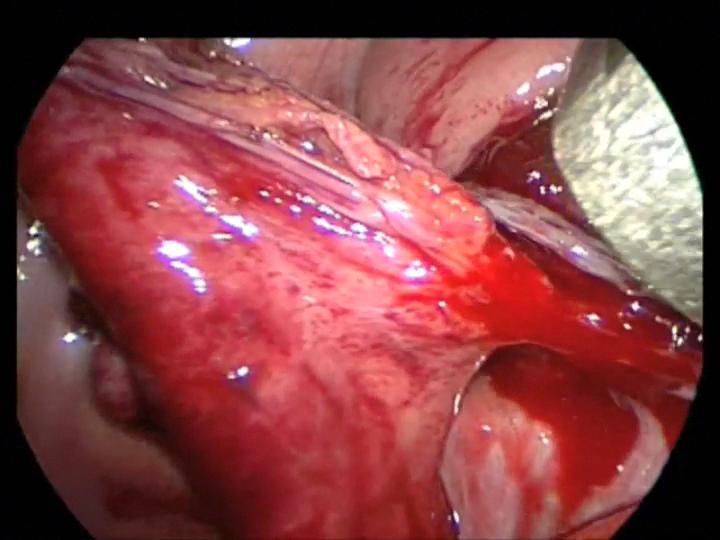 Figure 15. Appendiceal mesenteric bleeding caused by stapler failure.
Figure 15. Appendiceal mesenteric bleeding caused by stapler failure.
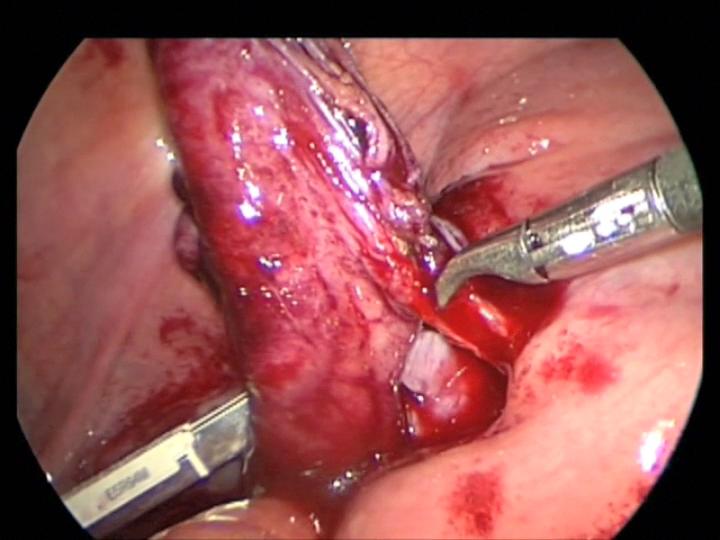 Figure 16. Appendiceal mesenteric bleeding controlled with a clamp. Notice the lost stapler cartridge in the left lower corner.
Figure 16. Appendiceal mesenteric bleeding controlled with a clamp. Notice the lost stapler cartridge in the left lower corner.
![]() Figure 17. Lost stapler cartridge being retrieved after failure to fire.
Figure 17. Lost stapler cartridge being retrieved after failure to fire.
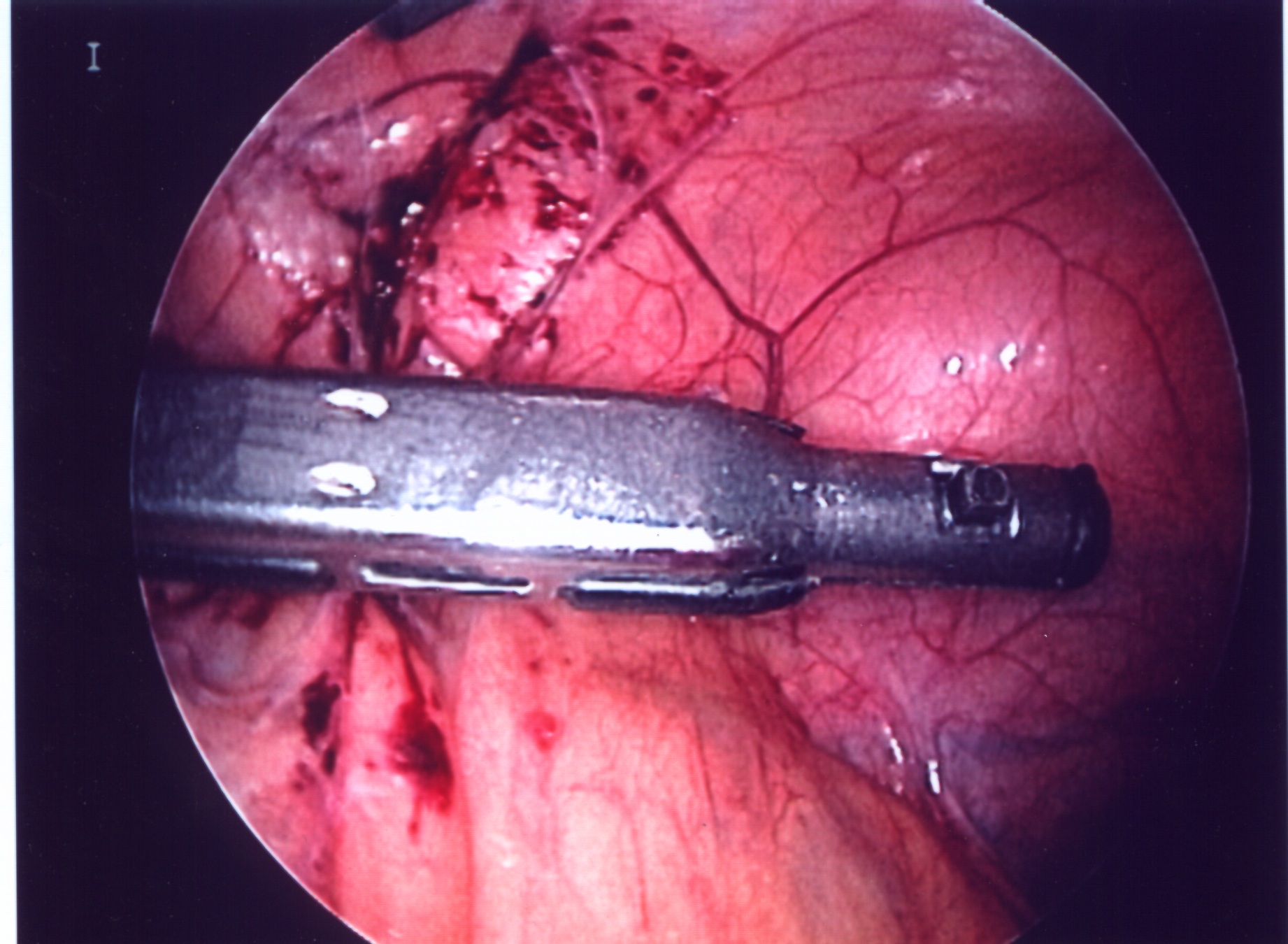 Figure 18. Photograph demonstrating an endostapler cartridge fired across the splenic hilum. The stapler separated from the instrument handle. It was safely removed without converting to an open procedure.
Figure 18. Photograph demonstrating an endostapler cartridge fired across the splenic hilum. The stapler separated from the instrument handle. It was safely removed without converting to an open procedure.
Extreme care must be taken to avoid damage to the staple line. Bleeding from the staple line can be managed with bipolar electrocautery and the harmonic scalpel. A carefully and properly placed clip can control the bleeding.
The loss of a suturing needle can be a significant problem. A methodical search needs to be done and a radiograph may need to be taken to find the needle. Every effort should be made to remove the needle. In most cases, the needle is easily found but in some cases, the use of a combination of laparoscopy and intraoperative fluoroscopy may be required in order to safely and successfully retrieve the lost needle.
Grasping forceps can damage the intestine and stomach, especially in small children. The smaller the forceps, the higher the pressure applied to the tissue. These instruments can cause perforation and necrosis (Figure 19).7,25 A laparoscopic hook dissector can damage abdominal organs inadvertently (Figure 20). Sharp instruments should be kept inside the laparoscopic visual field to prevent injuries. A careful laparoscopic inspection of the peritoneal cavity should be done and any injury should be repaired before closure.
Compromised areas or areas of necrosis should be repaired prophylactically even if there is no frank perforation.25 Intestinal hematomas may be difficult to differentiate from necrotic bowel (Figures 21 and 22). The surgeon should always be alert during the postoperative period to the possibility of unrecognized intestinal perforations. Symptoms usually appear between 24 and 48 hours after the procedure, but can be delayed until 4-10 days after thermal injury.25 The signs and symptoms of intestinal perforation may be persistent abdominal pain, fever, general discomfort, nausea and vomiting, and localized or diffuse peritonitis. Although imaging studies can be helpful, laparoscopic exploration generally gives the best results.
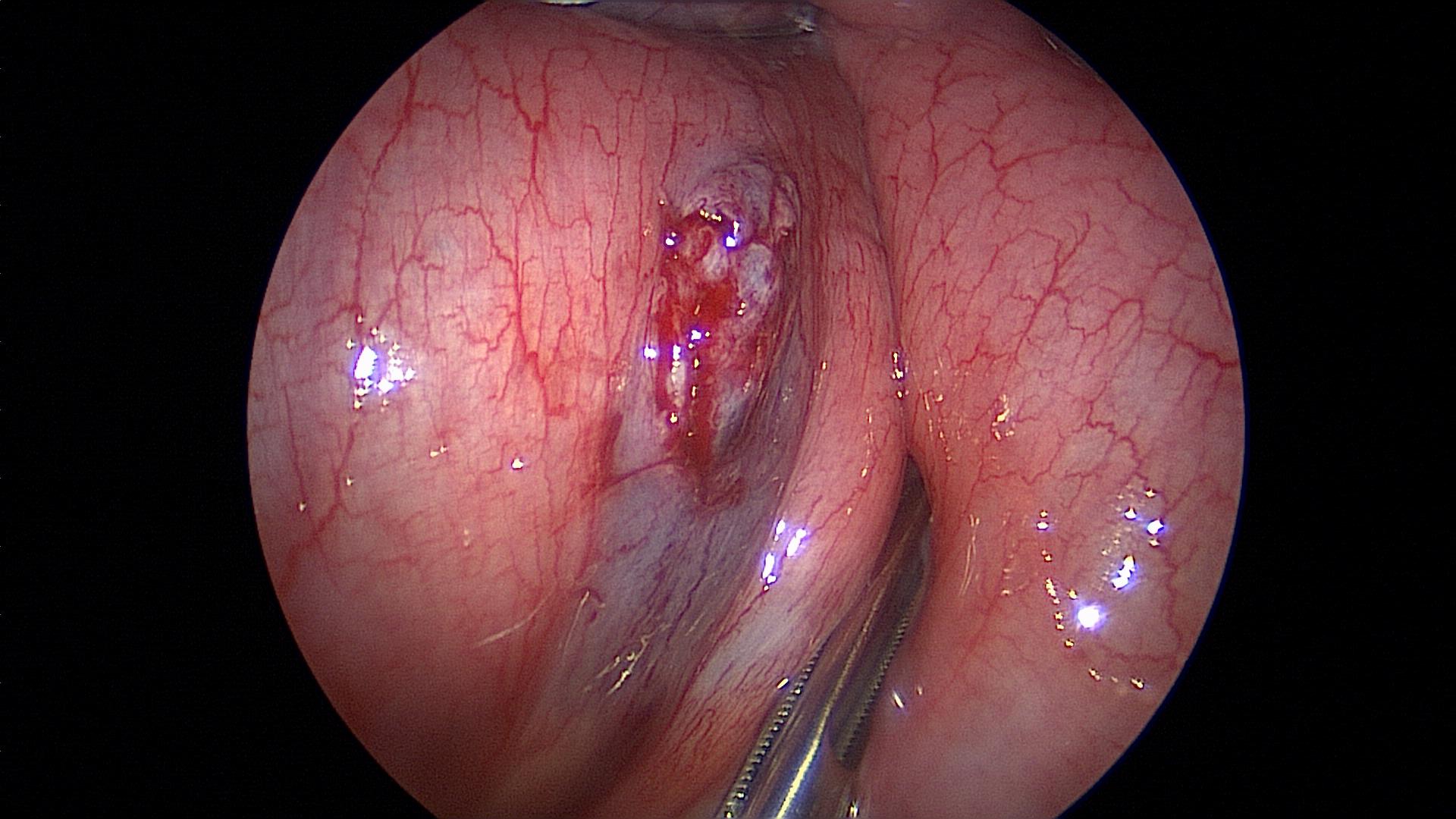 Figure 19. Serosal tear caused by rough manipulation of the intestine.
Figure 19. Serosal tear caused by rough manipulation of the intestine.
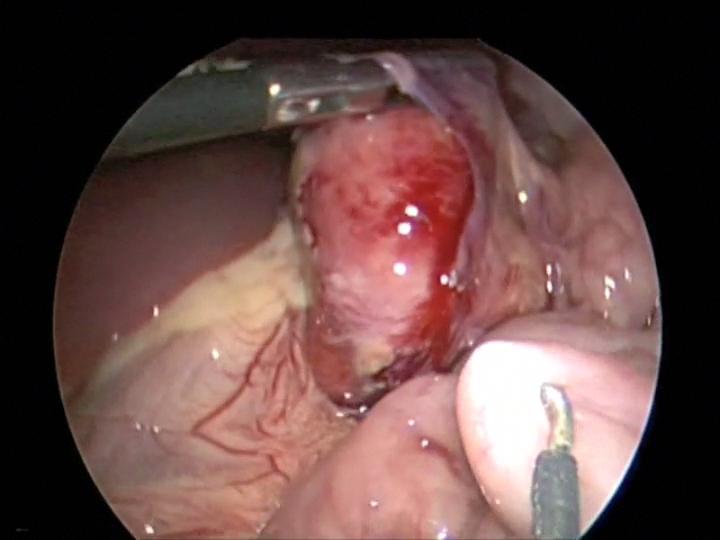 Figure 20. Inadvertent hook injury to the small bowel during mesenteric lymph node dissection. Notice hook in the right lower quadrant.
Figure 20. Inadvertent hook injury to the small bowel during mesenteric lymph node dissection. Notice hook in the right lower quadrant.
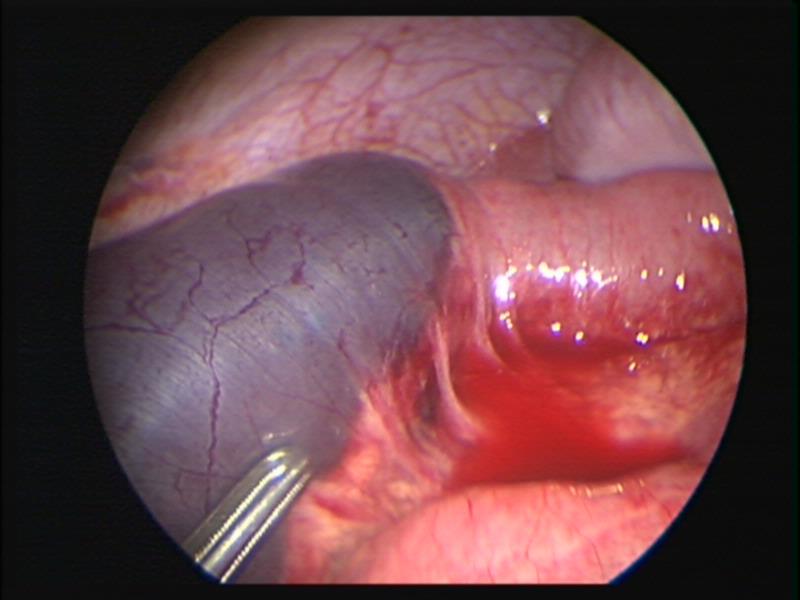 Figure 21. Intestinal hematoma caused by rough manipulation of intestine with a laparoscopic clamp.
Figure 21. Intestinal hematoma caused by rough manipulation of intestine with a laparoscopic clamp.
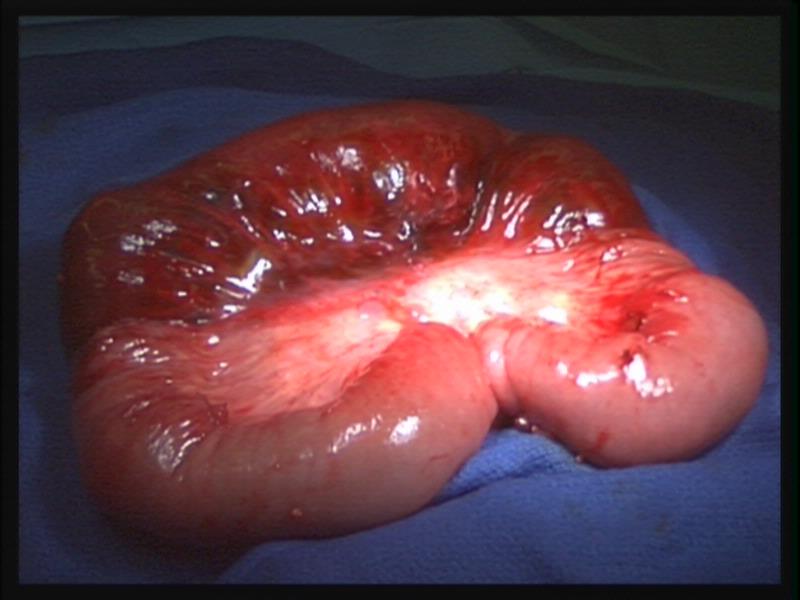 Figure 22. Necrotic bowel with normal mesentery may have a similar appearance to that of hematoma of the intestine as seen in figure 21.
Figure 22. Necrotic bowel with normal mesentery may have a similar appearance to that of hematoma of the intestine as seen in figure 21.
COMPLICATIONS RELATED TO SPECIFIC PROCEDURES
While it is beyond the scope of this chapter to detail all the possible complications in each laparoscopic or thoracoscopic pediatric procedure, there are some common types of complications that can be discussed in the context of particular procedures. The major categories of complications may be related to learning a new procedure or technique, anatomic disorientation, loss of control of vascular pedicles, clipping or division of misidentified structures, and perforation or laceration from dissection or myotomy.5-9 These problems can occur in open procedures as well as in minimally invasive surgery, and may not be purely a function of the laparoscopic approach.
The “learning curve” issue is one that is frequently implicated in complications related to a new surgical approach. This is particularly true with minimally invasive surgery. Brief instruction with limited operative supervision by those proficient in laparoscopic techniques sets the scenario for problems. This same standard needs to be applied to the development of minimally invasive surgical skills. To avoid problems related to the “learning curve,” mentoring from a surgeon experienced in laparoscopic minimally invasive surgery is required.
The anatomic approach in laparoscopic and thoracoscopic surgery is different from that taught in gross anatomy and in open surgical procedures. While the anatomy is unchanged, the perspective from which it is viewed is different. This can lead to disorientation and misidentification of structures in the infant and the small child.
The most frightening situation in laparoscopic or thoracoscopic procedures is uncontrolled bleeding which can occur as a result of inadvertent division of vascular structure, or failure of stapling or clipping devices designed for the adult patient. The initial response is panic, but at this point two options are available: immediate conversion to an open procedure or an attempt to obtain control via the laparoscope. Blind clipping or electrocautery should be avoided. First, identify the site of bleeding. If the field is obscured by blood and cannot be visualized, conversion to open surgery should be done. Disruption of the short gastric vessel may be encountered during mobilization for fundoplication (Figure 23). This can usually be controlled without conversion to an open procedure. Bleeding from the appendiceal mesentery can look impressive but can usually be controlled laparoscopically with cautery or clips. Bleeding from the splenic vessels or renal pedicle may be difficult to control using a closed technique and significant blood loss may occur. The rate of blood loss is the major factor when considering whether to convert to an open procedure versus continuing the laparoscopic procedure. A calm demeanor, rapid assessment of the rate of bleeding, and consideration of all available options, including conversion to an open procedure, will help resolve the problem of uncontrolled hemorrhage.
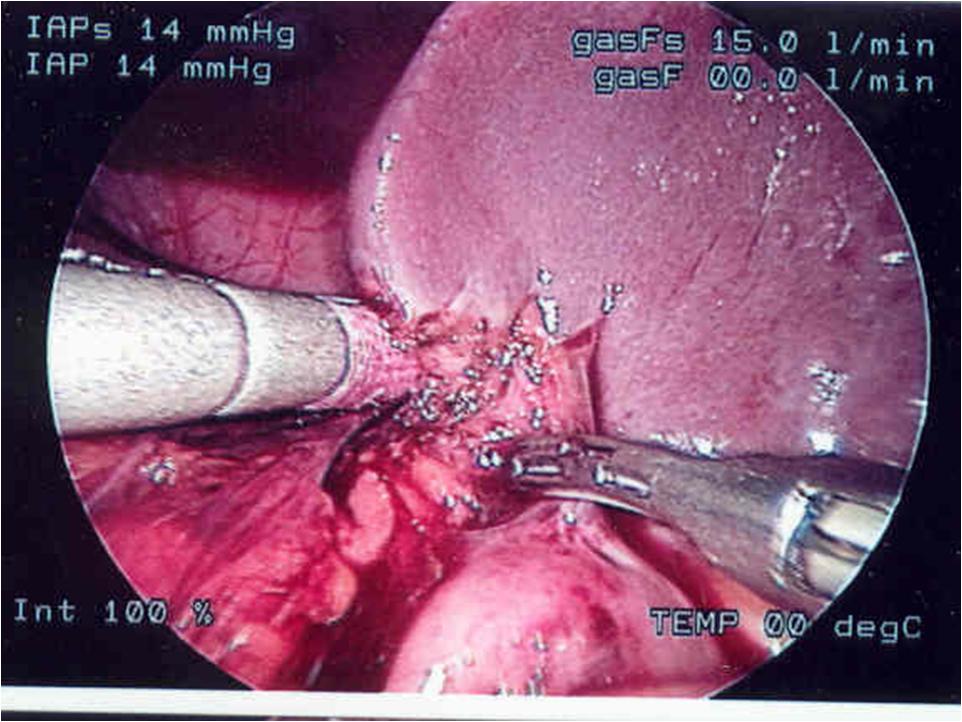 Figure 23. Division of short gastric vessels with harmonic scalpel during Nissen fundoplication in a small child.
Figure 23. Division of short gastric vessels with harmonic scalpel during Nissen fundoplication in a small child.
Clipping or division of unintended structures is the most common cause of long-term problems and malpractice claims. Failure to properly identify structures or the misapplication of clips or staples is the cause of this problem. The clipping of the common bile duct with or without division is a concern of anyone who does laparoscopic cholecystectomy (Figure 24).5,27 Failure to visualize structures to be clipped or the entire stapling/clipping instrument can lead to injury of adjacent tissue.
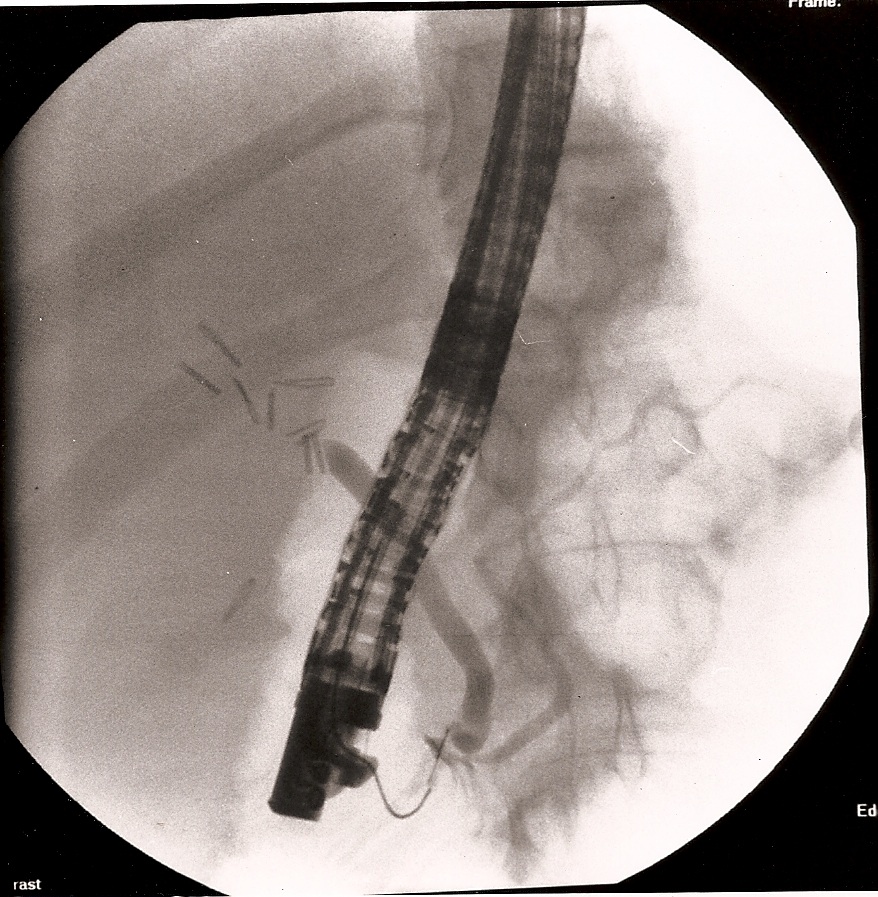 Figure 24. A 12-year-old female patient was transferred from another institution with a diagnosis of retained common bile duct stone with obstructive jaundice and peritonitis, 7 days after laparoscopic cholecystectomy. The photograph shows an ERCP demonstrating a clipped and transected common bile duct. Notice the large number of clips above the transected common bile duct.
Figure 24. A 12-year-old female patient was transferred from another institution with a diagnosis of retained common bile duct stone with obstructive jaundice and peritonitis, 7 days after laparoscopic cholecystectomy. The photograph shows an ERCP demonstrating a clipped and transected common bile duct. Notice the large number of clips above the transected common bile duct.
Perforation or laceration of organs both solid and hollow may occur during dissection or as a result of retraction. During the dissection of the posterior esophagus, the relatively thinner wall in the child can be perforated if the correct plan of dissection is not identified. Esophageal or pyloric myotomy is an opportunity for inadvertent mucosal perforation. The limited tactile sense increases the risk for perforation. Proper equipment and experience can decrease the risks (Figure 25).
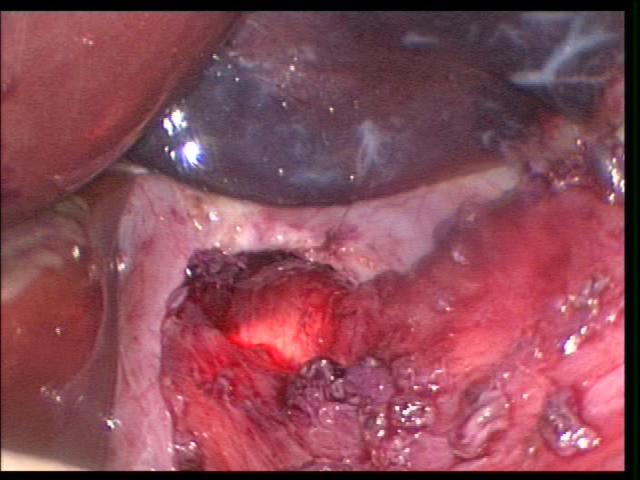 Figure 25. Transillumination with a flexible GI scope helps to identify possible mucosal perforations.
Figure 25. Transillumination with a flexible GI scope helps to identify possible mucosal perforations.
Colonic perforation can occur during biopsy for ganglion cells to assess for Hirschsprung’s disease. Care is required to insure mucosal integrity, and if there is any question, a suture should be used to close the biopsy site. Laceration of organs can occur both with dissection and retraction. Over-aggressive retraction of the liver or spleen can lead to injury and bleeding. This is seen in infants where the capsule is thinner and more delicate. Clamp injuries to the viscera can also occur and lead to delayed perforation if the clamp is tightly applied for an extended period of time, as with retraction of the stomach during fundoplication. Adult-type clamps and forceps may be too rough for the more delicate pediatric tissues. Careful handling of the tissues with the laparoscopic instruments is as important as in open surgery.
Removal of tissue from the chest or abdomen can be problematic with the small incisions used in laparoscopic surgery. Large specimens, such as spleen or tumors, can be particularly vexing, and the need to contain infectious tissue, such as appendix, bowel, or gallbladder, can also be problematic. This requires the introduction, deployment, and retrieval of endobags. Vigorous manipulation during extraction or disruption of a specimen for removal can lead to rupture of the bag and spillage of contents or injury to the abdominal or chest wall. Potential seeding of splenic tissue or tumor cells can be a serious problem.7,26 Spillage of infectious material can also complicate the recovery or prolong hospitalization. Knowledge of the limitations of the endobags and familiarity with their operation should minimize problems related to tissue extraction in pediatric patients.
Overzealous dissection of the mesentery during mobilization or mesenteric lymph node biopsy can result in devascularization of the intestine with gangrene requiring bowel resection (Figures 26, 27, and 28).
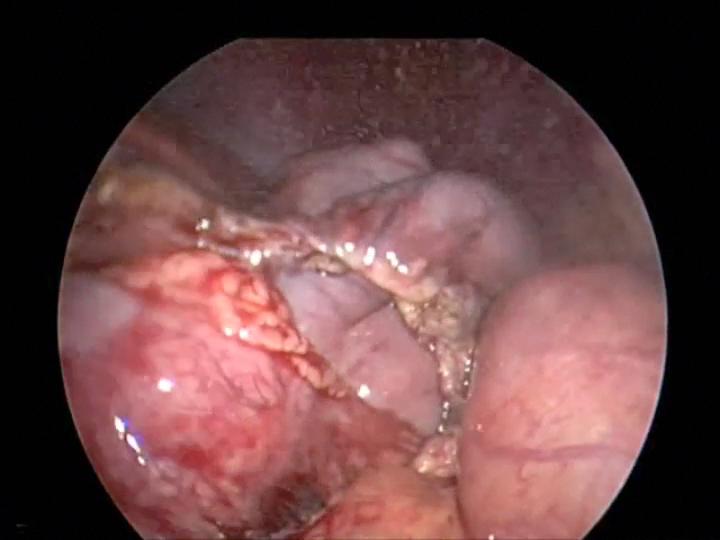 Figure 26. Mesenteric injury with harmonic scalpel causing devascularization of small bowel which required small bowel resection.
Figure 26. Mesenteric injury with harmonic scalpel causing devascularization of small bowel which required small bowel resection.
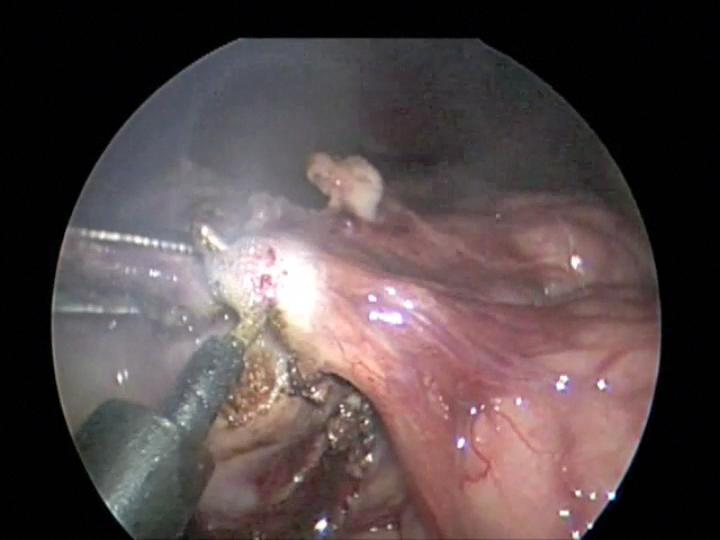 Figure 27. Monopolar hook injury to mesentery with subsequent intestinal ischemia.
Figure 27. Monopolar hook injury to mesentery with subsequent intestinal ischemia.
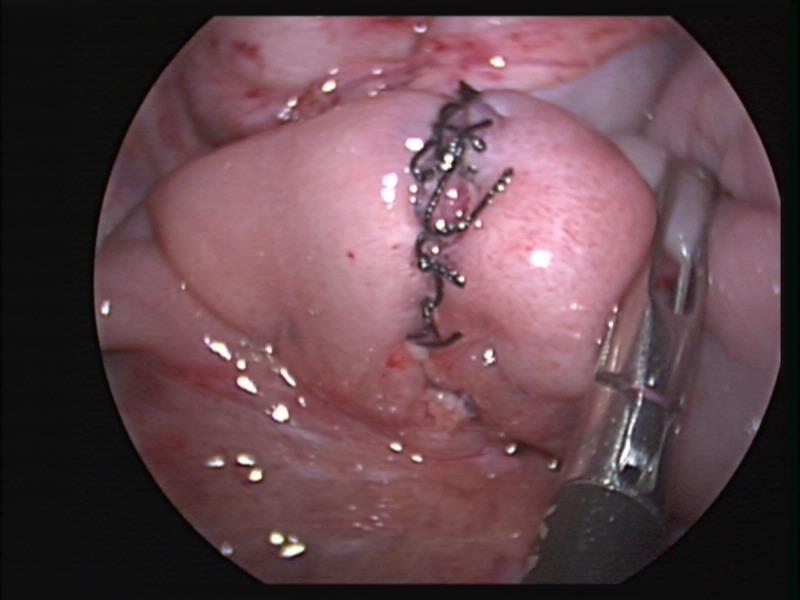 Figure 28. Small bowel anastomosis following bowel resection after intestinal necrosis caused by mesenteric monopolar hook injury seen in figure 27.
Figure 28. Small bowel anastomosis following bowel resection after intestinal necrosis caused by mesenteric monopolar hook injury seen in figure 27.
COMMON PEDIATRIC PROCEDURES
Cholecystectomy
Gallbladder disease is not as common in the pediatric population as it is in adults.27,28 There has been an increase of gall bladder disease in children in the past 15 years with a reported increase from 1.5 to 2.5 cholecystectomies/100,000 population.
Cholecystectomy is performed through three or four ports, generally 5-mm ports, but two can be smaller ports. Intraoperative cholangiogram is not routinely done but we do not hesitate to do a cholangiogram if the anatomy is confusing. When choledocholithiasis is suspected, we rely heavily on preoperative endoscopic retrograde cholangiopancreatography (ERCP). The author now performs cholecystectomies almost exclusively with the harmonic scalpel, trying to avoid the use of monopolar electrocautery and its inherent complications. The triangle of Calot and Hartmann’s pouch can be dissected with the harmonic scalpel and the cystic artery can be safely divided with it. The gallbladder can easily be removed from the liver bed without bleeding. The presence of even a small amount of blood can obscure the anatomy; it is important to identify the bleeder and achieve hemostasis avoiding the indiscriminate use of endoclips or electrocautery, which can cause severe harm to the patient. An adolescent was referred to our institution with jaundice, sepsis and severe abdominal pain a few days after an “uneventful” cholecystectomy. ERCP demonstrated complete transection of the common bile duct requiring a Roux-en-Y choledochojejunostomy (Figure 24).
When the anatomy is unclear, we usually remove the gallbladder in a retrograde fashion. We have been very satisfied with this technique and have had no complications in a significant number of cases. This technique is also useful in cases of a large cystic duct which can be confused with the hepatic ducts or the common bile duct. The author has used the endoscopic stapler to divide large cystic ducts after the anatomy has been clearly delineated. The retrograde technique is also useful in cases of a short cystic duct and cases of acute cholecystitis (Figures 29, 30, and 31).
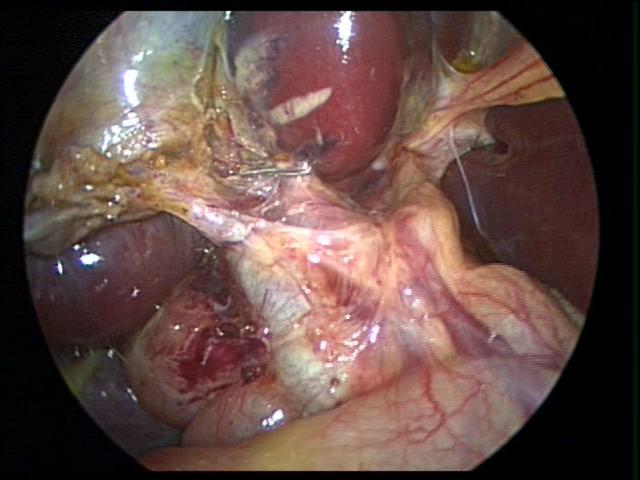 Figure 29. Confusing anatomy during cholecystectomy for acute cholecystitis managed by retrograde dissection of the gallbladder (dome down).
Figure 29. Confusing anatomy during cholecystectomy for acute cholecystitis managed by retrograde dissection of the gallbladder (dome down).
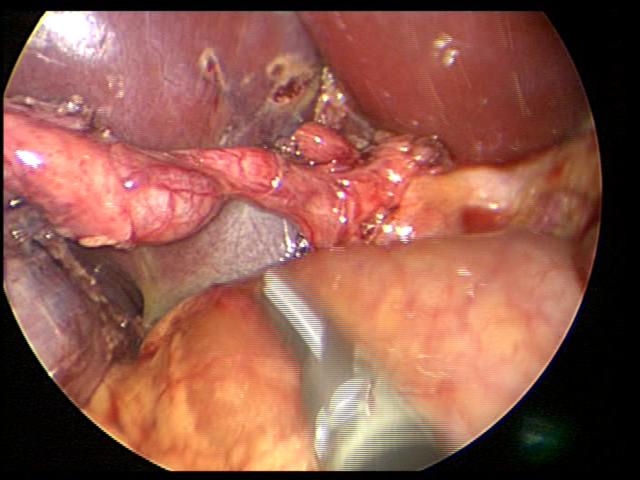 Figure 30. Junction of cystic duct and common bile duct clearly identified after gallbladder has been detached from gallbladder bed by retrograde resection (dome down – figure 29).
Figure 30. Junction of cystic duct and common bile duct clearly identified after gallbladder has been detached from gallbladder bed by retrograde resection (dome down – figure 29).
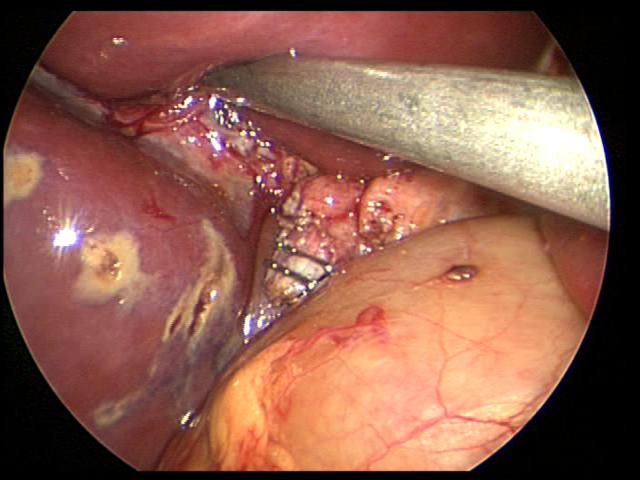 Figure 31. Cystic duct divided with two endoclips at its junction with the common bile duct. Notice liver burns caused by harmonic scalpel.
Figure 31. Cystic duct divided with two endoclips at its junction with the common bile duct. Notice liver burns caused by harmonic scalpel.
We had three cases of postoperative retained common bile stones. The problem was identified and the stones removed by ERCP (Figure 32). Exploration of the common bile duct in small children is difficult and can have a significant rate of complications.
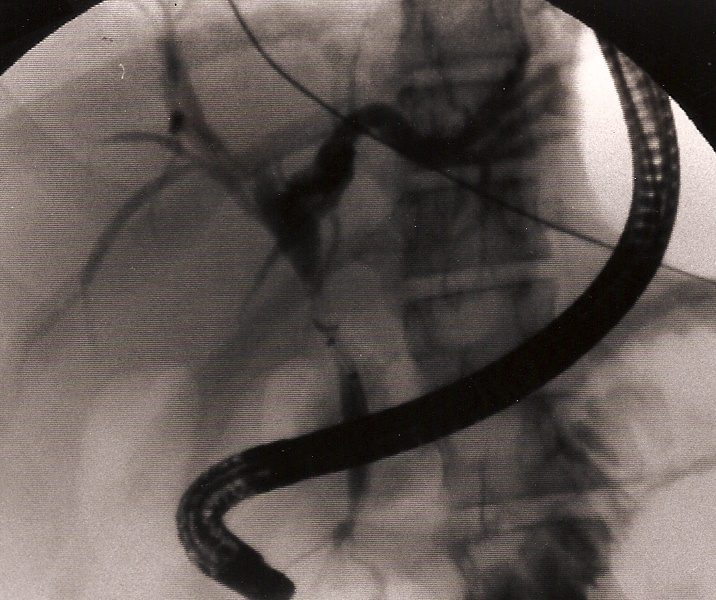 Figure 32. Retained common bile duct stone retrieved by ERCP.
Figure 32. Retained common bile duct stone retrieved by ERCP.
The gallbladder is removed through the umbilical port with an endobag. The bag can be introduced directly into the abdomen after removing the 5 mm trocar; this maneuver eliminates the need for a 10-mm port.
Appendectomy
Appendicitis is the most common condition requiring abdominal emergency surgical intervention in children. It occurs in 1%-8% of children presenting to the emergency room with acute abdominal pain. The lifetime risk of appendicitis is 8.6% in male patients and 6.7% in female patients.29,30
The reported accuracy of diagnosing appendicitis by clinical examination varied from 70- 80% in the general population, 54-70% in children and 50-70% in women of childbearing age.29,30 The widespread use of preoperative imaging has improved the diagnostic accuracy of acute appendicitis. Initially, ultrasound was more frequently used in the initial evaluation; recently Computed Tomography (CT scan) has become the gold standard. The reported sensitivity and specificity of ultrasound was 77% and 86%, while CT scan has a sensitivity and specificity of 95%. The combination of both diagnostic modalities has been reported to yield a high diagnostic accuracy without adverse events from delay in treatment.29-32 The benefits of this approach need to be weighed against the increasing costs of healthcare and the risk of radiation from CT scan.
We have been performing laparoscopic appendectomies since 1993.33 Almost all cases of appendicitis can be managed laparoscopically. The patient with a large appendiceal abscess is initially treated with intravenous antibiotics, with or without radiological- guided percutaneous drainage followed by interval appendectomy 8-12 weeks later (Figure 33). The patient needs to be followed up closely; prompt laparoscopic surgical intervention is indicated if there is no significant improvement after initiation of treatment. We perform repeated physical examinations; closely follow the clinical course of the patient with special attention to resolution of the adynamic ileus or bowel obstruction, fever, white cell blood count, and perform imaging as needed. If the patient shows clinical improvement a percutaneous inserted central catheter (PICC line) is placed for home antibiotics; some patients are treated with oral antibiotics at home.
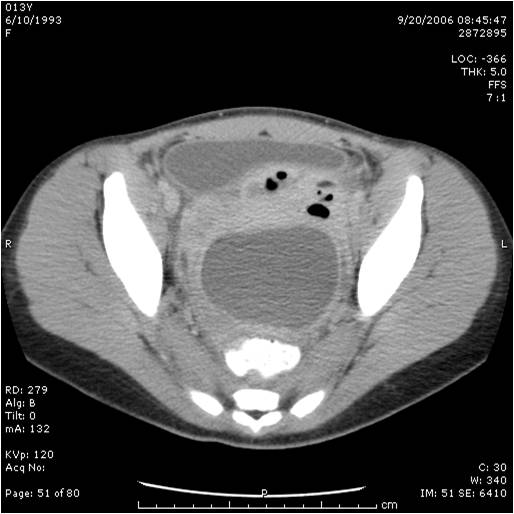 Figure 33. Large appendiceal abscess managed with intravenous antibiotics and interval appendectomy eight weeks later.
Figure 33. Large appendiceal abscess managed with intravenous antibiotics and interval appendectomy eight weeks later.
We generally use three ports for appendectomy. Our port placement has been modified over the past 10 years. We place a 5-mm port in the umbilicus, a 5-mm port in the left lower quadrant, and a 5-mm port in the left suprapubic area; the umbilical port can be converted to a 12 mm port if the endoscopic stapler is being used. The surgeon and the assistant work from the left side of the abdomen, allowing the surgeon to work comfortably with both hands. If a difficult dissection is anticipated, the harmonic scalpel is used; otherwise, the procedure can be done with the bipolar cautery. The appendiceal stump is not inverted. It can be ligated with coated Vicryl endoloops™ or the endoscopic stapler (Figures 34, 35, and 36). If the base of the appendix is not necrotic or perforated, the use the endoloops™ is preferred because they are operator friendly and less expensive than the stapling device. When necrosis or perforation compromises the base of the appendix, we prefer the stapler and occasionally staple across the lower part of the cecum (Figures 37, 38, and 39). Perioperative antibiotics are always used. We have had a very low incidence of complications with this technique.33,34
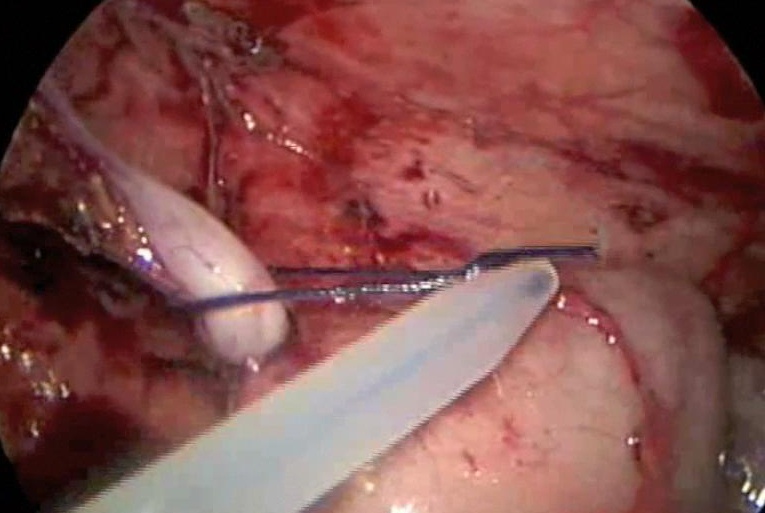 Figure 34. Appendectomy managed with use of Endoloop™.
Figure 34. Appendectomy managed with use of Endoloop™.
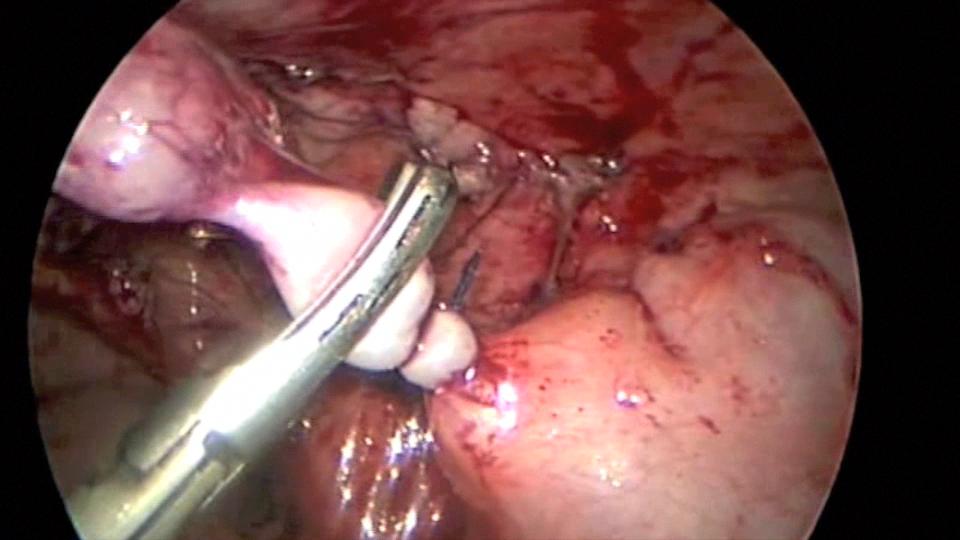 Figure 35. Appendix being divided with the harmonic scalpel after application of Endoloop™.
Figure 35. Appendix being divided with the harmonic scalpel after application of Endoloop™.
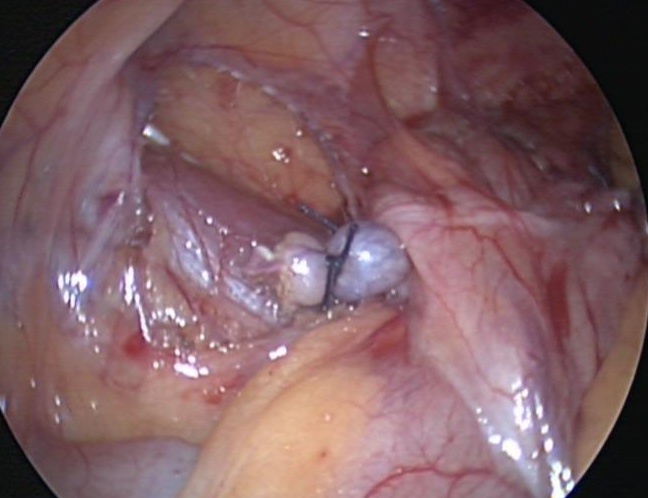 Figure 36. Appendiceal stump with two Endoloops™.
Figure 36. Appendiceal stump with two Endoloops™.
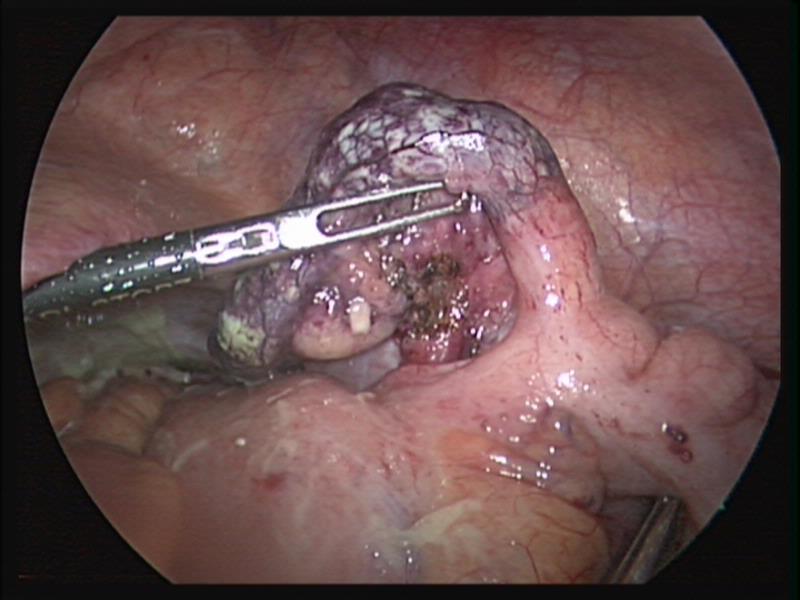 Figure 37. Gangrenous appendix with necrotic base.
Figure 37. Gangrenous appendix with necrotic base.
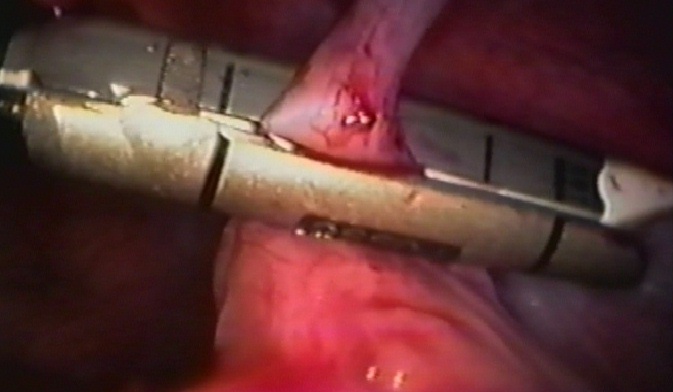 Figure 38. Endostapler being fired across appendiceal stump close to the cecum.
Figure 38. Endostapler being fired across appendiceal stump close to the cecum.
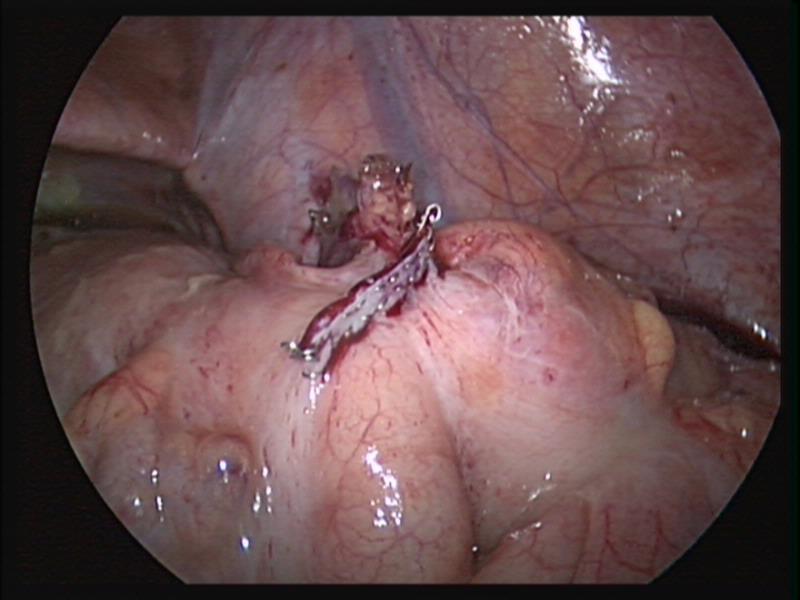 Figure 39. Stapler line across the cecum after appendectomy.
Figure 39. Stapler line across the cecum after appendectomy.
We use single access laparoscopic appendectomy in cases of uncomplicated appendicitis. After placing the umbilical port, a quick assessment of the status of the appendix will guide the decision to proceed with standard laparoscopic appendectomy or single access. If we find complicated appendicitis, we proceed with a standard laparoscopic approach. Complicated appendicitis which comprises approximately 20% to 30% of all cases of appendicitis has been defined as perforation with purulent peritoneal collection, abscess formation and generalized peritonitis.35,36 It is important to identify the fallopian tube because this structure can sometimes be confused with the appendix (Figures 40 and 41). When using single access laparoscopy we prefer to use two 5 mm ports through the umbilicus, generally a long and short port; this can be ordinary 5 mm ports or anchor ports™. The use of two ports of different lengths avoids the two heads of the ports hitting each other and restricting laparoscopic motion. We also exclusively use 30 or 45 degree laparoscopic lenses. The 10 mm working laparoscope may be useful in some occasions.
Manipulation of the appendix can be done with an endoloop™ suture placed through the 5 mm umbilical port around the tip or body of the appendix and exteriorized through the lower abdomen using an endoclose™ needle suture device (Autosuture, CT). In more difficult cases a mini alligator grasper forceps (2 mm) used for bronchoscopy can be introduced directly through the suprapubic area to manipulate the appendix. The appendix can be removed with an endo catch™ bag introduced directly through one of the 5 mm umbilical incisions after one of the ports is removed.
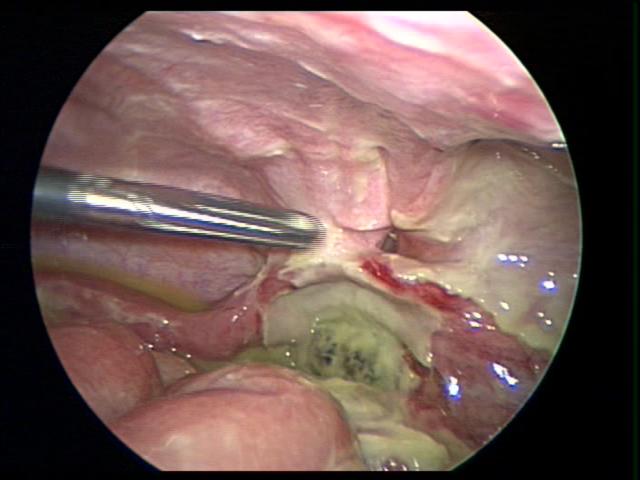 Figure 40. Fallopian tube can be easily confused a necrotic appendix.
Figure 40. Fallopian tube can be easily confused a necrotic appendix.
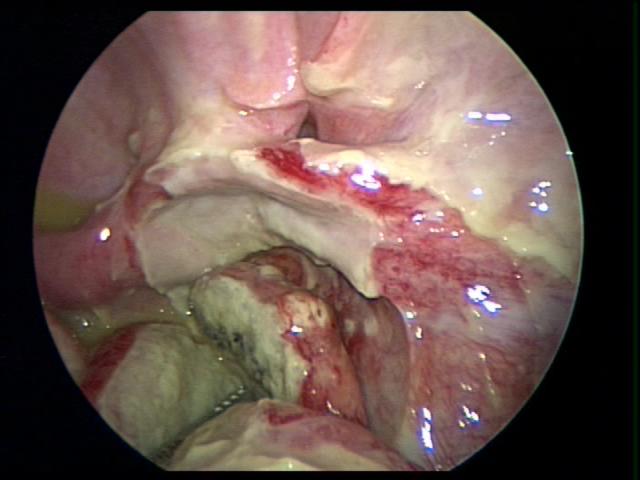 Figure 41. Fallopian tube identified after dissection of a necrotic perforated appendix.
Figure 41. Fallopian tube identified after dissection of a necrotic perforated appendix.
We currently use a delay appendectomy approach in stable children presenting with appendicitis after 8 PM. After admission we resuscitate the patient with intravenous fluids and administer broad-spectrum intravenous antibiotics. Appendectomy is performed the next day, within 24 hours of admission. We confirm the experience of other authors who found that delayed management did not significantly affect operating time, perforation rate or complications and allowed for better efficiency and effective use of physician and hospital resources especially with the 80 hour/week work limitations for residents.37,38
Splenectomy
Laparoscopic splenectomy is currently the standard procedure for the treatment of many hematological disorders in children.26,39 The procedure can be performed either with the patient supine, left side elevated at a 45-degree angle, or with the right lateral decubitus position which allows for the spleen to be suspended in the left upper quadrant by the splenic ligaments. At least three ports are used for this procedure. A fourth port, which can be a 3- or 5-mm port in the left flank posteriorly, is extremely useful to elevate and manipulate the spleen. The other three ports are a 5-mm in the epigastrium, a 12-mm in the left lower quadrant, and a 5-mm in between these two ports.
We use the harmonic scalpel or bipolar cutting forceps for the dissection. The hilum is divided with the endoscopic stapler. We prefer using 2-mm staples, although we have used 2.5-mm staples without significant bleeding. First, the gastroepiploic splenic branch is divided carefully in order to avoid bleeding. The short gastric vessels can bleed significantly, especially when there is little space between the stomach and the spleen.
Care is required to avoid damage to the greater gastric curvature. When placing the stapler across the splenic vessels, special attention should be taken to avoid injury to the tail of the pancreas.
A careful search for accessory spleens should be done. If an accessory spleen remains, it may result in failure of the splenectomy to improve the hematological condition (Figure 42).
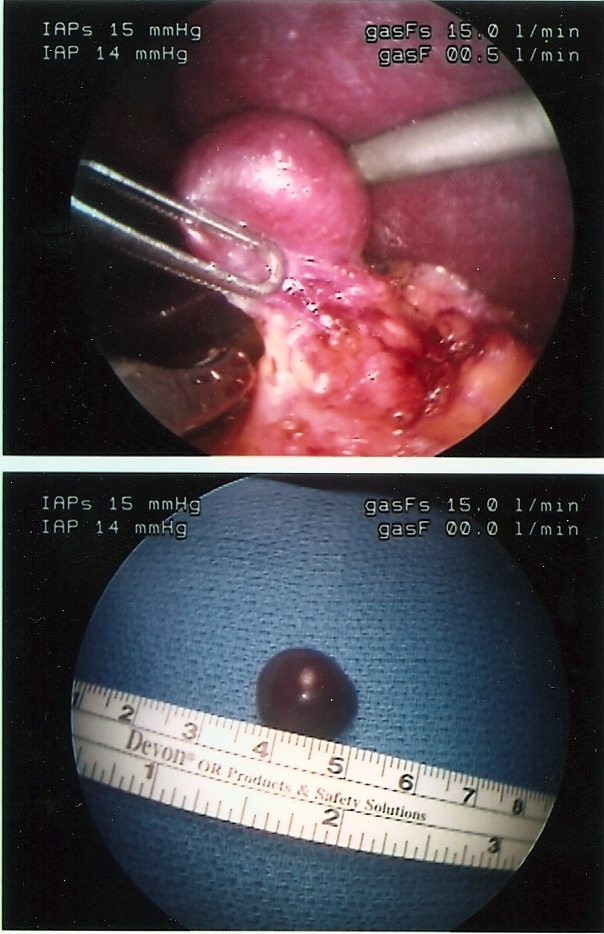 Figure 42. Accessory spleen.
Figure 42. Accessory spleen.
The spleen is placed over the liver in the subphrenic space to facilitate the bagging. Rupture of the spleen during splenectomy or spillage during removal can result in splenosis. Laparoscopic partial splenectomy has been reported with variable success in the management of hereditary spherocytosis. More experience is needed to assess the effectiveness of laparoscopic partial splenectomy in the management of some hematological pediatric disorders.40
When cholelithiasis is present, cholecystectomy can be performed concurrently. Special care is needed to correct coagulation problems. The platelet count should be raised above 50,000 in patients with idiopathic thrombocytopenic purpura. Patients with sickle cell anemia should be transfused to obtain a hemoglobin value above 10 g/dL.26 All children undergoing splenectomy should receive preoperative pneumococcal and meningococcal vaccines and postoperative prophylactic oral penicillin twice a day.
Gastrostomy and Nissen Fundoplication
We have been performing laparoscopic gastrostomy since 199241,42 and laparoscopic Nissen fundoplication since 1995.43 These two procedures are common in pediatric surgery because of the large number of neurologically impaired children with swallowing incoordination and gastroesophageal reflux.44 The placement of gastrostomy alone can cause or aggravate gastroesophageal reflux.45 We recommend that all neurologically impaired children requiring gastrostomy should be evaluated for reflux preoperatively because reflux following gastrostomy, can be devastating to these children and their parents. Laparoscopic placement of gastrostomy can be done alone or assisted by gastrointestinal endoscopy. The use of laparoscopy avoids the complications of a blind percutaneous procedure or an open operation and facilitates the placement of the gastrostomy close to the lesser curvature to prevent the development of reflux.41,45
Nissen fundoplication is an ideal operation for laparoscopy. It can be performed even in small infants and newborns. We do this operation through five ports, which can be 5 mm or smaller. Port placement is extremely important in small children. One must allow for enough separation of the ports to avoid constant interference (sword fighting) when dissecting and suturing. The liver can be elevated with endo-peanuts or a small fan retractor. The liver of small children is extremely friable and can bleed profusely. Our operative technique has changed over the years. We now routinely divide the short gastric vessels to avoid wrap tension, and we perform a floppy two or three-stitch 360-degree wrap. Dissection of the esophagus is done with extreme care to avoid perforation. The vagus nerves and its hepatic branches should be preserved. We routinely close the crura. Tension on the wrap can cause dysphagia and postoperative retching.
We perform both intra- and extra-corporeal knot tying. Extracorporeal tying is very useful in small infants in whom there is limited working space and it has the advantage of requiring only one port for the tying.
We used a reconstructive tissue matrix mesh (Strattice™) in a case of a recurrent large hiatal hernia, with excellent results.
Pyloric Stenosis
Hypertrophic pyloric stenosis is a pediatric surgical condition with an incidence of 2 to 4 in 1000 infants.46,47 Early diagnosis has been facilitated by the use of ultrasound.
Recently, most infants with pyloric stenosis present early in good clinical condition and generally with normal serum electrolytes. The open pyloromyotomy first described by Ramstedt in 1911 has been the gold standard and produced excellent results.46-48 The main problem with this operation is increased postoperative pain and poor cosmetic results since the operation is generally performed through a right upper quadrant muscle cutting transverse incision. There is also a significant associated risk of infection, incisional hernias and possible evisceration. In 1991, Alain described the laparoscopic approach adopted by many pediatric surgeons because it is technically easy, reproducible and effective.46 The alternative to laparoscopic pyloromyotomy is the umbilical and supraumbilical approach.47,48 Few pediatric surgeons currently perform the classical Ramstedt operation.49,50 There are proponents for both the laparoscopic and the umbilical approach. Both techniques offer comparable results. The final approach generally depends on the personal preference of the surgeon and the environmental circumstances such as availability of equipment.
A recent survey of British pediatric surgeons reported that umbilical pyloromyotomy is the most commonly used technique by British surgeons with only a minority still performing the classic approach.51 In our institution we perform both the laparoscopic as well as the supraumbilical approach with equally good results.
The laparoscopic technique is generally performed trough 3 ports: a 5 mm umbilical port, a 3mm right upper quadrant port and a left epigastric working port. It is important to stabilize the duodenum. The myotomy is done with an arthrotomy knife and finalized with a Benson spreader (Figure 43).
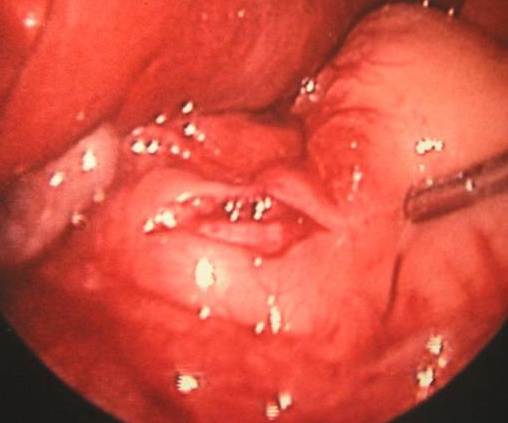 Figure 43. Laparoscopic pyloromyotomy.
Figure 43. Laparoscopic pyloromyotomy.
The umbilical approach consists of a supraumbilical semi-circumferential incision. A subcutaneous flap is developed towards the epigastrium and a one-inch long midline fascial incision is performed. The stomach and pyloric olive are easily delivered through this incision. If there is difficulty delivering the pylorus, the skin incision can be slightly extended laterally on both sides (wings). Our learning curve for this technique was approximately 10 cases. Careful closure of the midline fascia with interrupted suture is necessary to prevent herniation or evisceration (Figure 44).
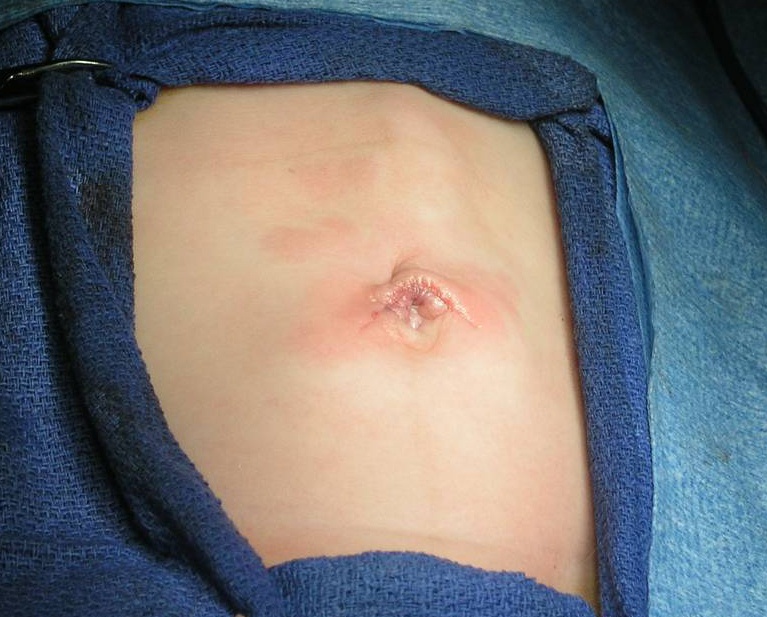 Figure 44. Incision following supraumbilical pyloromyotomy.
Figure 44. Incision following supraumbilical pyloromyotomy.
The main complications of pyloromyotomy are bleeding, mucosal perforation and incomplete myotomy with recurrence of the vomiting. Bleeding is generally self-limited; monopolar cautery is not used close to the mucosa to avoid perforation. Mucosal perforations are best managed by conversion to an open approach with direct repair of the mucosa with fine absorbable sutures and an omental patch. An experienced laparoscopic surgeon may elect to repair the perforation laparoscopically. It is very important to recognize mucosal perforations during the procedure as it is a potentially lethal complication. We have had infants referred to our institution with unrecognized mucosal perforations following pyloromyotomy.
Some infants continue to vomit postoperatively. This is generally attributed to gastritis and it resolves spontaneously. It must be differentiated from incomplete pyloromyotomy which is rare. It is important to notice that ultrasound of the pylorus following a successful pyloromyotomy is not a good indicator to evaluate the adequacy of the myotomy because it often looks almost identical to the preoperative study.
Proponents of the umbilical approach argue that the laparoscopic approach has the potential for undetected mucosal perforation which can produce significant morbidity and even mortality. At the present time both techniques offer comparable outcomes.
Inguinal Hernia
The gold standard of inguinal hernia repair in children has traditionally been a high ligation of the inguinal hernia sac through an inguinal open approach. This procedure is very effective and applies to most children since the overwhelming majority of pediatric hernias are of the indirect type. Recently, the laparoscopic approach to repair inguinal hernias in children has been gaining popularity.52-55 Laparoscopy through the open hernia sac has been used effectively for many years to diagnose an open processus vaginalis in the contralateral side.
There are many techniques described for repair of inguinal hernias in children, from simple suturing of the internal ring with a purse string to complicated approaches such as the flip-flap technique and the hook method.55,56
Laparoscopic repair of inguinal hernias in children was initially reserved for female patients because of the fear of damage to the vas deferens and the spermatic blood vessels. There are currently large series of laparoscopic repairs, with few complications and a recurrence rate of approximately 4 %.52-56
We currently prefer the inguinal open repair with high ligation of the sac. We perform laparoscopy through the hernial sac to exclude a hernia in the opposite side. Laparoscopic repair is done with a simple purse string or a figure of eight suture with non-absorbable suture material when the hernia is found incidentally during another laparoscopic procedure.
With the advent of new and smaller laparoscopic instruments the future of laparoscopic inguinal hernia repair is very promising. Refinement of the currently used techniques is necessary to decrease the incidence of complication and recurrence. It is critical to avoid damage to the vas deferens and to protect the blood supply to the testicle; in addition it is important to lower the incidence of recurrence.
The best approach to laparoscopic repair of inguinal hernias in adolescents remains controversial. We offer both the open and laparoscopic technique to our adolescent patients. When the laparoscopic approach is chosen by the patient or their family, the repair is done with mesh placement, similar to the repair done in adults.
Some authors recommend that pediatric laparoscopic inguinal hernia repair should be limited to children under the age of 6 years to decrease the incidence of recurrence.54
There are recent reports of successful laparoscopic repair of femoral hernias in children but the experience is limited at this time.57
Intussusception
Intussusception is the most common cause of small bowel obstruction in pediatric patients.58 The initial management consists of hydrostatic or pneumatic reduction (air enema) under radiographic surveillance. Ultrasound guided saline enema has been reported with comparable results. The main advantage of this technique is the avoidance of radiation exposure; however this method has not gained popularity in the United States.59 In our institution, we use almost exclusively pneumatic reduction.
The success of radiographic reduction has been reported from 70 to 85%. Failed pneumatic reduction is generally defined as 3 attempts using adequate pressure failing to elicit reflux of air into the small bowel.58-61
The traditional method of treatment of irreducible intussusception has been open laparotomy with bowel resection when indicated. Laparoscopy has gained popularity as an effective technique to manage irreducible intussusception with excellent results comparable to the open procedures and adding the benefits of laparoscopy.58-62
One of the initial concerns about the laparoscopic approach was the inability to palpate the intestine to exclude the presence of a leading point; this is especially risky in older patients generally over 3 years of age. Another concern was that the laparoscopic approach deviated from the traditional open method, which consisted on gently pushing the intussuceptum avoiding pulling (Figures 45, 46, and 47). This maneuver is difficult to achieve with laparoscopy. Some authors have reported similar approaches by pushing gently on the intussuscipiens with atraumatic bowel clamps just distal to the tip of the intussuceptum. A “Chinese Fan Spread” distraction technique has been also reported with success.62
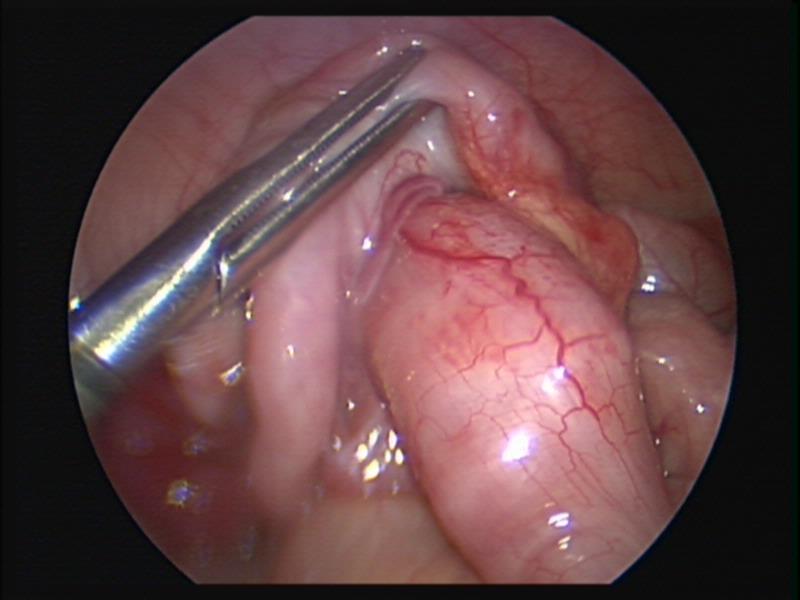 Figure 45. Ileocolic intussusception before laparoscopic reduction.
Figure 45. Ileocolic intussusception before laparoscopic reduction.
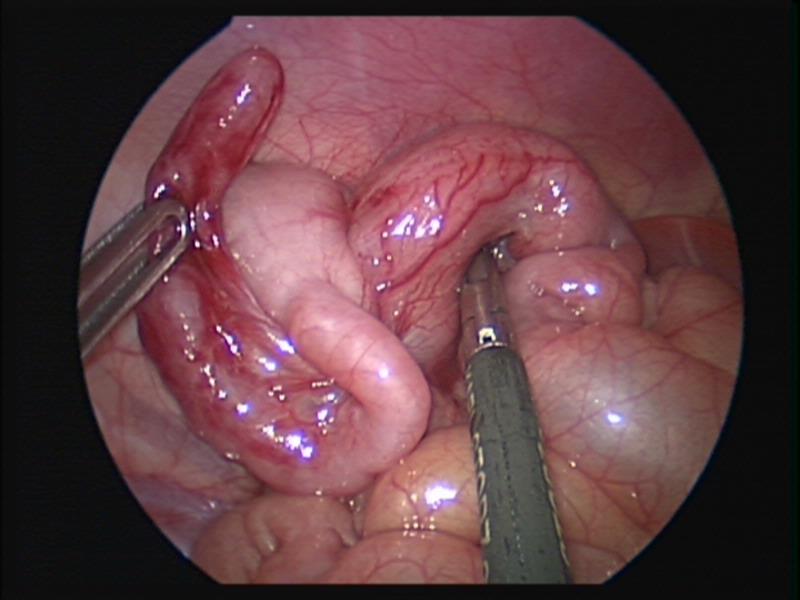 Figure 46. Ileocolic intussusception after laparoscopic reduction.
Figure 46. Ileocolic intussusception after laparoscopic reduction.
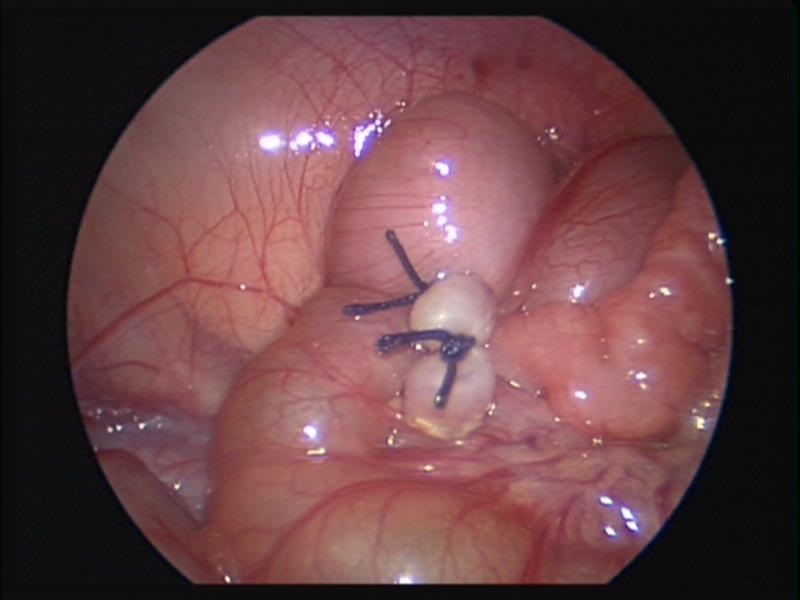 Figure 47. Appendectomy after laparoscopic reduction of ileocolic intussusception.
Figure 47. Appendectomy after laparoscopic reduction of ileocolic intussusception.
The laparoscopic procedure can generally be done through 3 ports. Port placement is similar to the one used for appendectomy. We found that it is preferable for the surgeon to work from the left side of the patient. The ports can be 5 mm or smaller but one may be converted to a 12 mm port if the endostapler is used.
Laparoscopy is very useful in the management of intussusception. Frequently the intussuception is found in the cecum, almost reduced by the therapeutic enema, allowing for easy laparoscopic reduction. Laparoscopy allows for planning of the incision to achieve optimal exposure without doing a large laparotomy in cases, when a leading point is suspected, the intussusception is irreducible, or perforation or necrotic bowel is encountered. The intestine can be mobilized laparoscopically and delivered through an extended umbilical incision or a muscle sparing a McBurney approach. Laparoscopic bowel resection may be performed by experienced laparoscopic surgeons.
Pediatric tumors
Laparoscopy and thoracoscopy have been used successfully for biopsy and resection of abdominal and thoracic tumors.63-65 We have biopsied and removed adrenal and retroperitoneal neuroblastomas, pheochromocytomas, teratomas and many other tumors. The retroperitoneal space is suitable to the laparoscopic approach. Biopsies can be achieved with biopsy needles, by wedge excision, or using laryngeal biopsy forceps. We have successfully used the laryngeal biopsy forceps which can be introduced through a small port to obtain adequate specimens in a variety of tumors. When bleeding is a problem, conversion to open laparotomy may be necessary. It is not advisable to use cautery or the harmonic scalpel before removal of the biopsied tissue because of the potential damage to the specimen. Once an adequate biopsy has been taken, the electrocautery or other hemostatic devices can be used. Even though adequate mobilization of the tumor may be done laparoscopically, it may be necessary to make a small incision in order to remove the specimen. We have not attempted resection of Wilms tumor laparoscopically because of the possibility of spillage which may upgrade the staging although we have removed metastatic Wilms tumors in the chest through thoracoscopy.
More reports of the successful application of minimally invasive surgery in the management of benign and malignant tumors in children continue to appear in the literature (Figures 48 and 49).
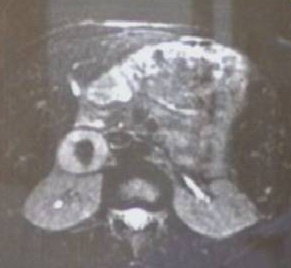 Figure 48. CT scan of right adrenal pheochromocytoma.
Figure 48. CT scan of right adrenal pheochromocytoma.
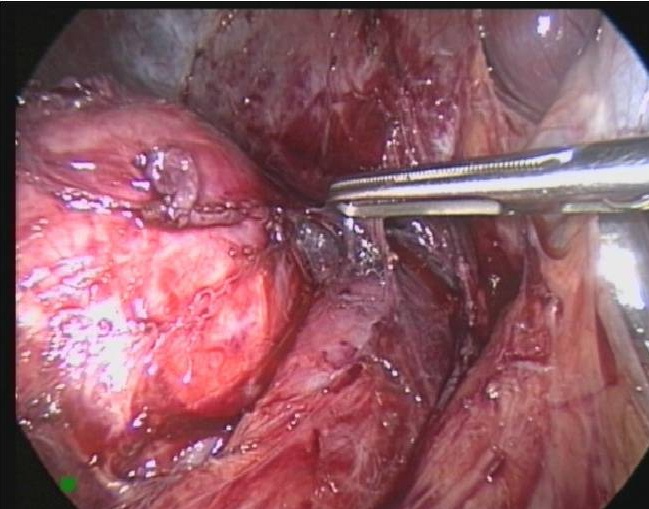 Figure 49. Laparoscopic removal of right adrenal pheochromocytoma seen in figure 48.
Figure 49. Laparoscopic removal of right adrenal pheochromocytoma seen in figure 48.
Trauma
The role of laparoscopy and thoracoscopy is well established in the treatment of penetrating and blunt trauma in the stable pediatric patient.66-68 Laparoscopy and thoracoscopy can be used as a diagnostic tool and very often provide definite treatment of the injury. In many occasions the use of laparoscopy avoids the morbidity associated with open laparotomy (Figures 50, 51, and 52). The unstable patient needs to be treated with conventional laparotomy.
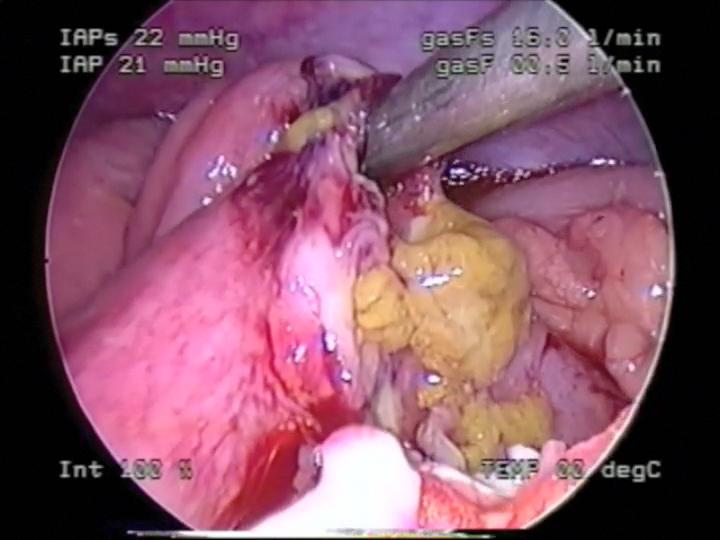 Figure 50. Small bowel perforation secondary to seatbelt injury.
Figure 50. Small bowel perforation secondary to seatbelt injury.
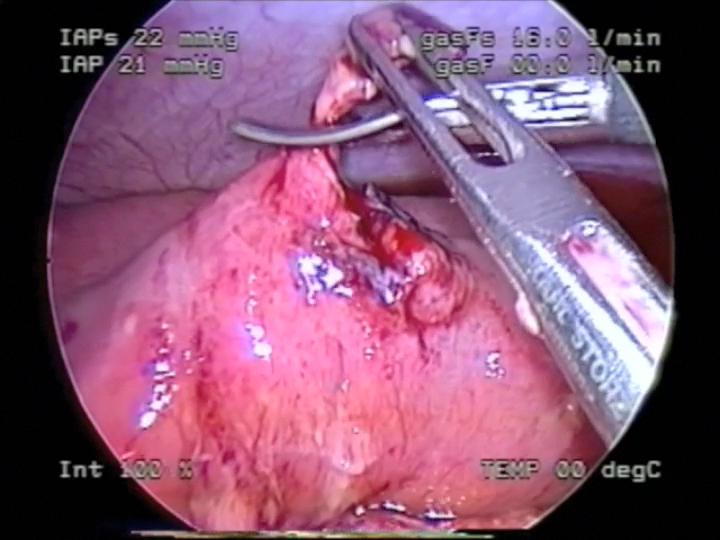 Figure 51. Debridement of necrotic edges of intestinal perforation seen in figure 50.
Figure 51. Debridement of necrotic edges of intestinal perforation seen in figure 50.
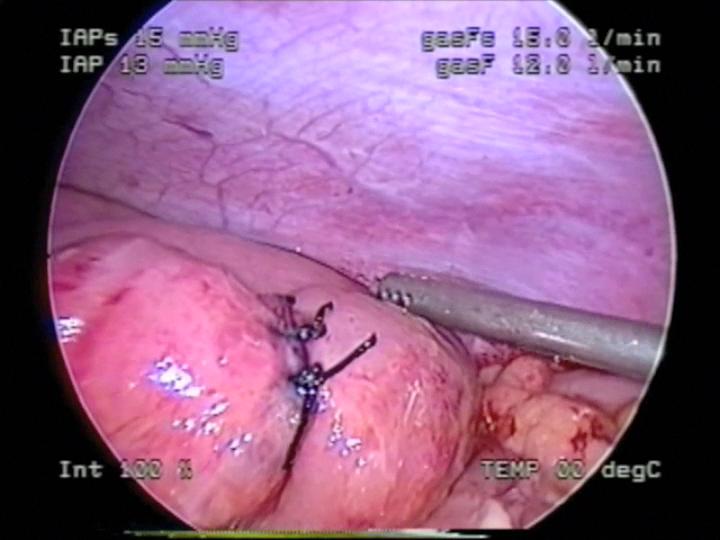 Figure 52. Laparoscopic repair of intestinal perforation seen in figures 50 and 51.
Figure 52. Laparoscopic repair of intestinal perforation seen in figures 50 and 51.
We have been using laparoscopy and thoracoscopy for over ten years in cases of intestinal and duodenal perforations, mesenteric injuries, diaphragmatic injuries, bleeding from lung lacerations, splenic and hepatic injuries and other injuries. When the injury cannot be corrected with laparoscopy, this technique allows for better planning of the incision for a more effective open approach.
A systematic examination of the whole peritoneal cavity should be done when an injury is suspected. The whole intestine should be carefully inspected from the ligament of Treitz to the rectum. The use of two monitors is strongly recommended, one at the head and one at the foot, in order to expedite the procedure.
Laparoscopy for Bowel Obstruction
We routinely use laparoscopy for the management of small bowel obstruction. The obstruction is frequently caused by a band or adhesions that can be released laparoscopically (Figures 53, 54, and 55). The use of the harmonic scalpel™ is preferred for lysis of adhesions but the monopolar hook can also be useful. One of the problems can be the lack of domain to work inside the abdomen. A slightly higher intra- abdominal pressure, usually 16 or 18 mmHg, may help to gain more working space. A careful examination of the whole intestine is often necessary. We find the distended proximal intestinal loop and follow it down to the area of blockage, trying to find the transitional area. An alternative way is to find the collapsed distal intestine and follow it proximally to locate the area of obstruction. Diagnostic laparoscopy can identify unsuspected problems causing the obstruction, such as the presence of congenital bands or Meckel’s diverticulum (Figures 56, 57, and 58). Laparoscopic evaluation can guide the placement of the abdominal incision in cases that need to be converted to laparotomy.
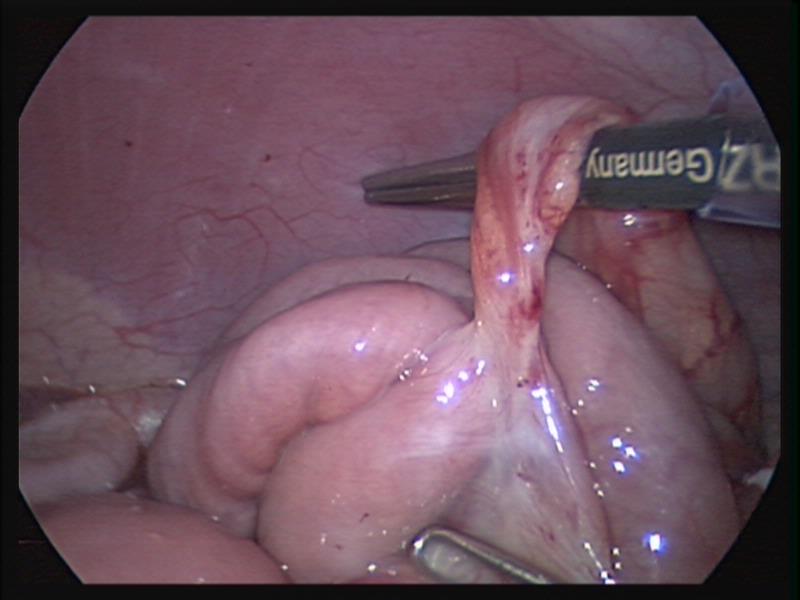 Figure 53. Fibrotic band causing small bowel obstruction.
Figure 53. Fibrotic band causing small bowel obstruction.
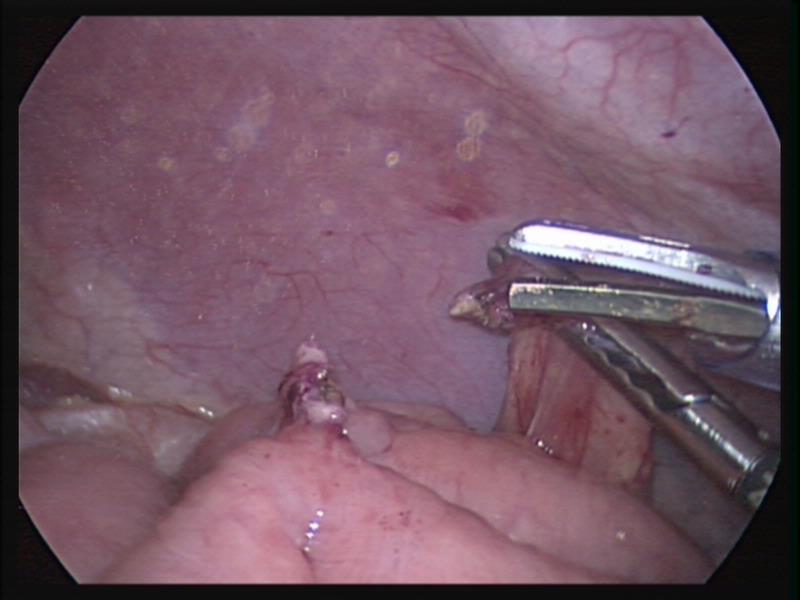 Figure 54. Fibrotic band seen in figure 53 divided with the harmonic scalpel.
Figure 54. Fibrotic band seen in figure 53 divided with the harmonic scalpel.
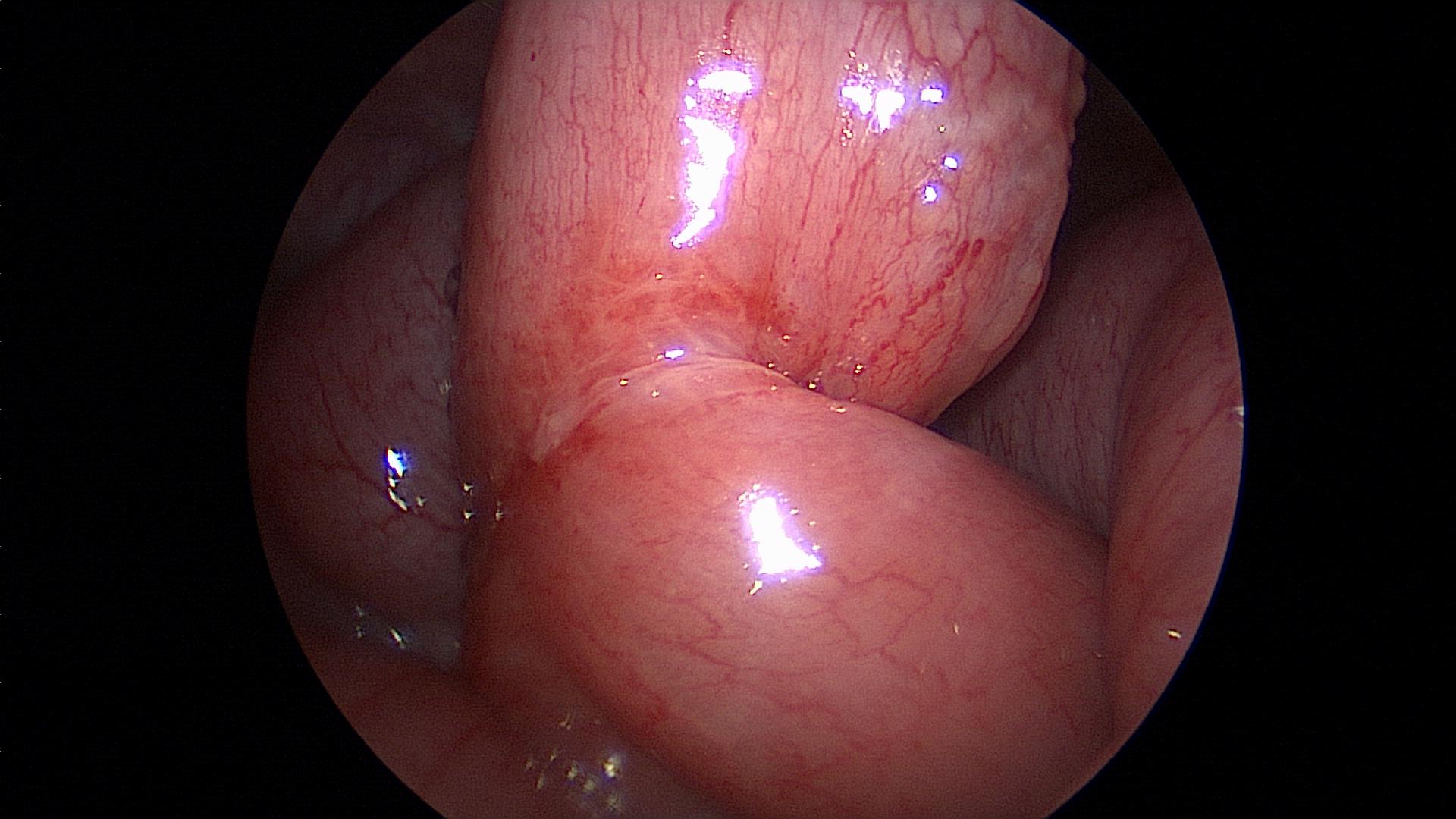 Figure 55. Transition point of obstructed small bowel seen in figures 53 and 54 after division of fibrotic band.
Figure 55. Transition point of obstructed small bowel seen in figures 53 and 54 after division of fibrotic band.
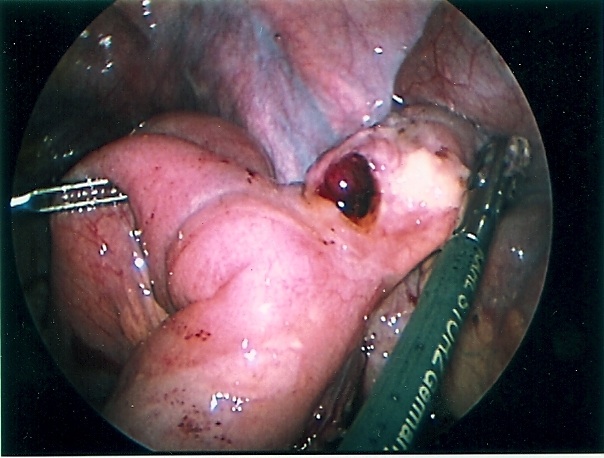 Figure 56. Perforated Meckel’s diverticulum causing small bowel obstruction.
Figure 56. Perforated Meckel’s diverticulum causing small bowel obstruction.
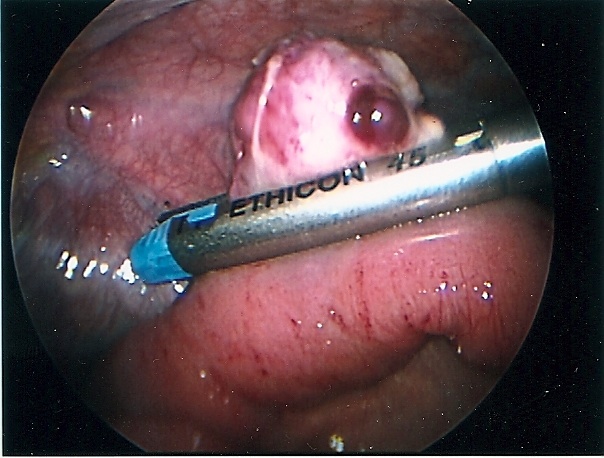 Figure 57. Perforated Meckel’s diverticulum seen in figure 56 being divided with the endostapler.
Figure 57. Perforated Meckel’s diverticulum seen in figure 56 being divided with the endostapler.
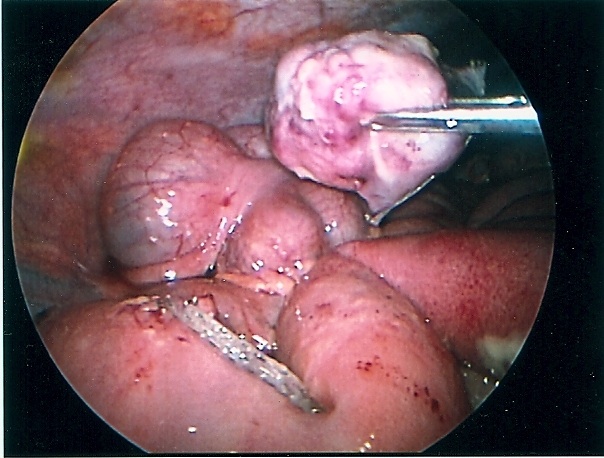 Figure 58. Staple line in the small bowel after removal of Meckel’s diverticulum seen in figures 56 and 57.
Figure 58. Staple line in the small bowel after removal of Meckel’s diverticulum seen in figures 56 and 57.
Robotic Surgery
Robotic surgery is an advanced technology that enables the surgeon to perform a wide variety of minimally invasive procedures. There are theoretical advantages of robotic surgery over standard laparoscopic surgery: articulating instruments that permit increased degrees of freedom, high quality of optical resolution and magnification, elimination of tremor, improved ergonomics for the surgeon, precise instrument manipulation, and the ability to perform precise and expeditious intracorporeal suturing.69-72
The only FDA approved robot is the DaVinci™ (Intuitive Surgical, Sunnyvale,CA). Originally designed for use in adult patients, it weighs 567 kg and stands about 6 feet tall.71 Despite the size and cost of this technology, the robot has been successfully applied to pediatric surgery, even in neonates weighting less than 3 kg. Abdominal procedures include Nissen fundoplication, removal of tumors, repair of congenital defects such as duodenal atresia, gastric duplications and diaphragmatic hernia. Thoracic procedures include lobectomy and segmentectomy, repair of esophageal atresia with tracheoesophageal fistula and other.69-72
Robotic surgery in children is currently reserved for advanced minimally invasive centers. It is equivalent to standard laparoscopic techniques but may have an advantage when fine dissection and suturing is needed. Robotic surgery is difficult to perform in children weighing less than 4 kg because of lack of domain. The learning curve in setting the robot and equipment has been cited as a disadvantage of this technique. The cost of this technology has to be evaluated in comparison to standard laparoscopy. At the present time further experience with robotic surgery is needed to objectively evaluate its impact on clinical outcomes. In the future, smaller and less expensive robotic equipment may be more amenable to use in pediatric surgery.71
Bariatric Surgery
Obesity in America affects approximately 50% of adults and 18% of children. The guidelines of the American Academy of Pediatrics state that a BMI (basal metabolic index) between 85th and 95th percentile based on normals from 1973 for age and gender identifies children are overweight or at risk for obesity. A BMI over 95th percentile for age and gender identifies children who are obese.73
Bariatric procedures are commonly performed in adults. Although bariatric surgery has increased in adolescents, there are still many problems that need resolution. The clinical comorbidities of obesity in adolescents are similar to those of the adult population and can have significant long term consequences. In addition, there are psychological, social and ethical issues that are unique to adolescents.74
There is no general consensus about the best bariatric procedure for adolescents. The Roux en Y gastric bypass, one of the most common procedures in adults, is generally considered irreversible and its long term consequences over several decades are unknown. There are some restrictive procedures that may be more appropriate for the adolescent. In addition, newer reversible procedures such as intragastric ballon, vagal stimulator, and others may be more appropriate for adolescents.73,74
Current medical intervention programs such as behavioral therapy, diets and increase in physical activity are only effective in 50% of adolescent patients.74 A multidisciplinary approach is preferred for the treatment of morbid obesity in children and adolescents. Informed consent for a bariatric pediatric patient should involve the patient’s family and include a medical weight reduction program and a behavioral modification treatment.
Neonatal laparoscopic surgery
Neonatal laparoscopic surgery has advanced significantly over the last few years. Laparoscopy and thoracoscopy are safe techniques with equivalent or even better results than open surgery in some situations. Laparoscopic procedures have been successfully performed in babies weighing as little as 1 kg.75-78
Laparoscopy has been used to correct congenital anomalies such as duodenal atresia, duodenal stenosis, intestinal malrotation, imperforate anus with recto urethral and recto vesical fistula, congenital diaphragmatic hernia and many others.75-83
Congenital diaphragmatic hernia of Bochdalek can be repaired by laparoscopy or thoracoscopy. Most pediatric surgeons prefer the thoracoscopic approach which allows for easy reduction of the viscera back into the peritoneal cavity by applying low pressure pneumothorax with carbon dioxide, generally a pressure of 4 to 6 mmHg. The pneumothorax facilitates the reduction of the viscera lodged into the chest cavity. The repair can be accomplished with or without prosthesis. The potential disadvantage of this approach is the inability to evaluate the intestine for malrotation, midgut volvulus, and associated atresia. The procedure is generally performed through 3 ports; these can be 5mm or even 3 mm ports. When the defect is too large, a prosthetic patch can be applied. The patch can be secured to the ribs or chest wall by applying the suture through the chest wall, tying them extracorporeally, and burying the knot under the skin. The laparoscopic approach provides less exposure but allows for a better development of the lower edge of the diaphragm. The laparoscopic repair can be done through 3 or 4 ports. The intestine must be manipulated carefully because undue traction can cause serosal damage or hematomas. The survival of neonates with diaphragmatic hernia depends largely on the degree of pulmonary hypoplasia and pulmonary hypertension as well as the presence of associated anomalies. The thoracoscopic and laparoscopic repair should be limited to stable patients (Figures 59, 60, 61, 62, and 63).
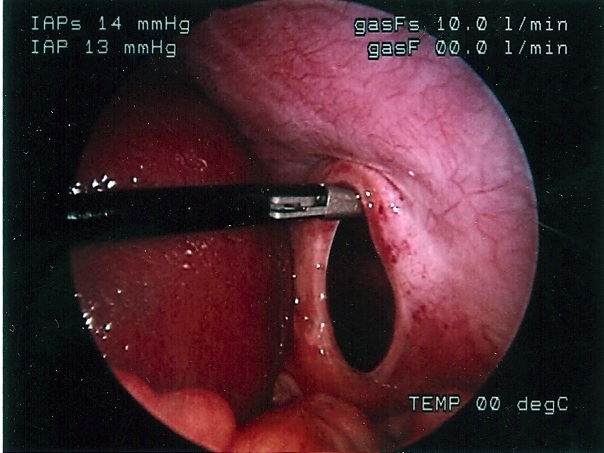 Figure 59. Laparoscopic repair of diaphragmatic hernia of Bochdalek.
Figure 59. Laparoscopic repair of diaphragmatic hernia of Bochdalek.
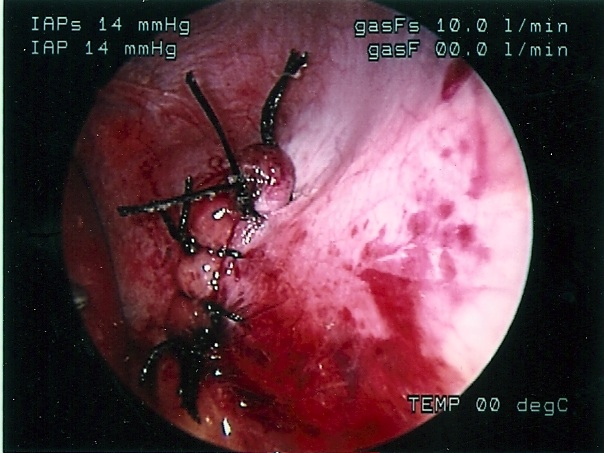 Figure 60. Completed laparoscopic repair of diaphragmatic hernia of Bochdalek seen in figure 59.
Figure 60. Completed laparoscopic repair of diaphragmatic hernia of Bochdalek seen in figure 59.
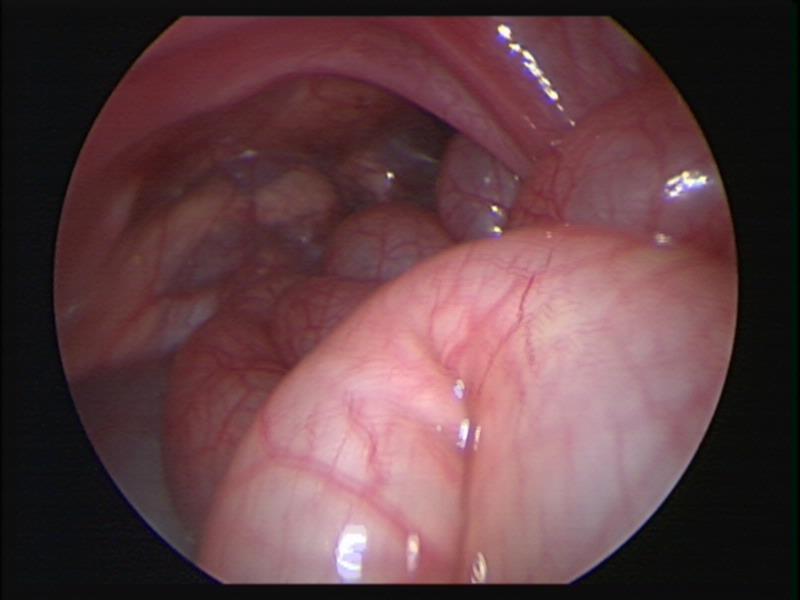 Figure 61. Thoracoscopic view of left sided diaphragmatic hernia of Bochdalek.
Figure 61. Thoracoscopic view of left sided diaphragmatic hernia of Bochdalek.
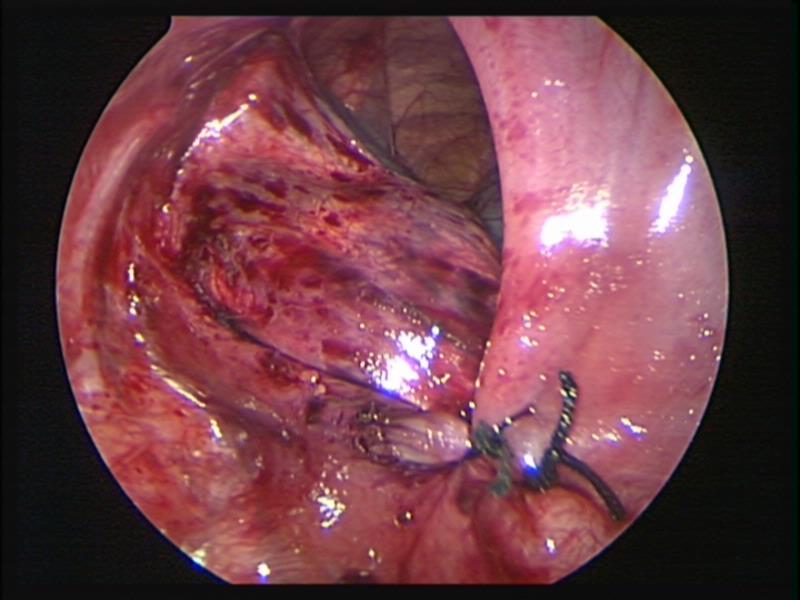 Figure 62. Thoracoscopic repair of left sided diaphragmatic hernia of Bochdalek.
Figure 62. Thoracoscopic repair of left sided diaphragmatic hernia of Bochdalek.
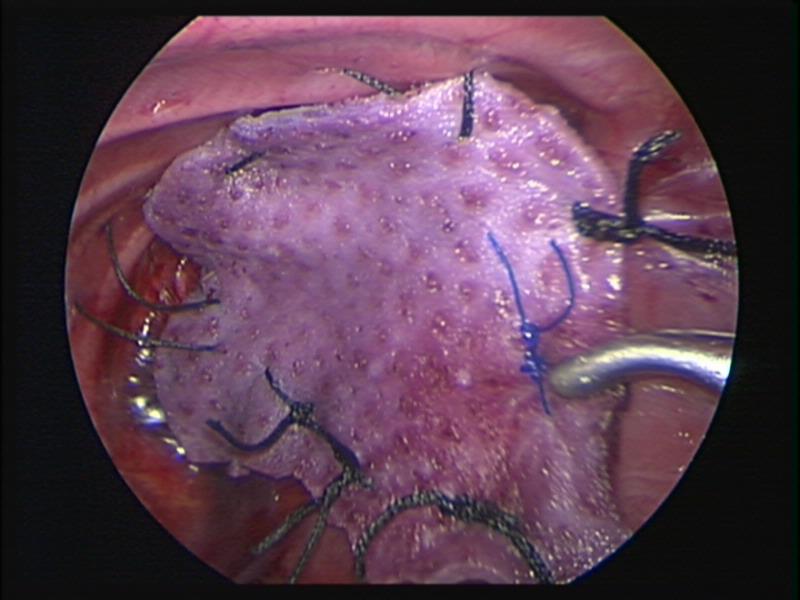 Figure 63. Thoracoscopic repair of left sided diaphragmatic hernia of Bochdalek with Strattice™ patch.
Figure 63. Thoracoscopic repair of left sided diaphragmatic hernia of Bochdalek with Strattice™ patch.
Thoracoscopic repair of esophageal atresia and associated tracheoesophageal fistula is being reported with increasing frequency. This approach should be reserved for pediatric surgeons with advanced laparoscopic skills.84,85
The repair of imperforate anus is controversial; there are no long-term results available at this time.86,87
The laparoscopic Kasai portoenterostomy procedure has had poor results compared to the traditional open approach and is not recommended at this time.88
Single Port and Single Incision Laparoscopic Surgery. Natural Orifice Translumenal Endoscopic Surgery (NOTES) and Mini-laparoscopy.
There is increasing enthusiasm about decreasing the number of ports in laparoscopic and thoracoscopic surgery. The main techniques are single port and single access, NOTES and the use of smaller instruments such as 3mm and 2 mm graspers and scopes.
There are successful reports of cholecystectomy, appendectomy, splenectomy, reduction of intussusception, gastrostomy tube placement, thoracoscopic lung biopsy, decortications and other procedures with single port, single incision or a modified technique.89-92 The main advantage of this technique is improved cosmetic results. The experience is limited at the present time. The most common techniques are the placement of 2 or 3 ports, usually 3 or 5 mm through the umbilicus or the placement of modified single ports which measure 15 to 20 mm and allow the introduction of the scope and two instruments.
The concern with very large ports through the abdomen in smaller children is the potential for umbilical port hernias, and the increased pain caused by such large ports. We prefer to place two ports, usually 5 mm or smaller, directly through the umbilicus. The two ports need to be of different lengths to avoid the heads of the ports interfering with each other and limiting the surgeon’s motion. We generally use a regular 5 mm port and a short anchorport™ (Surgiquest, CT). We use an endoloop™ suture exteriorized through the abdominal wall or a mini grasper (2 mm) introduced directly through the abdominal wall to mobilize the appendix. A 30 or 45 degree lens is necessary for this approach. Sometimes we use the 10 mm working scope; this scope is a zero degree scope and has a working port. It has been traditionally used by adult gynecologists for many years. It requires experience and provides limited exposure. The two 5 mm ports need to be joined into one at the end of the procedure in order to remove larger specimens.
Experience with NOTES in children is limited at this time, with only isolated reports.91 There are significant reservations about the value of this technique in children at the present time. On the other hand the use of 2 an 3 mm (minilaparoscopy) seems to be gaining popularity in children.93,94
Thoracoscopy
Thoracoscopy can be performed even in small infants and newborns.94-96 The indications for thoracoscopy continue to increase. Common thoracoscopic pediatric procedures include lung biopsy, drainage of empyema and decortication, mediastinal tumor biopsy, creation of a pericardial window, ligation of patent ductus arteriosum, repair of diaphragmatic hernias and diaphragmatic plication, repair of esophageal atresia and tracheoesophageal fistula, removal of pulmonary nodules and tumors, removal of blebs and pleurodesis, ligation of the thoracic duct, repair of pectus excavatum, and other more complex procedures.94-99 Thoracoscopy for trauma care in the pediatric population is also indicated.
Anesthetic considerations are important.12,13 Thoracoscopy can be performed with one- lung ventilation in older children by applying a double-lumen tube or a bronchial blocker. In small children, this technique is not possible, and the surgeon needs to resort to creating a pneumothorax with carbon dioxide. We recommend that the pressure should be kept at 5-7 mmHg or below to avoid complications.13,94,98,99 If desaturation is noticed, the pneumothorax should be immediately released. Port placement is important, and triangulation is necessary to provide sufficient working space. The first port should be applied under direct vision with blunt technique between the fifth and sixth intercostal spaces along the mid axillary line. Lower ports can cause diaphragmatic and liver injuries on the right side and splenic injuries on the left. The use of cutting or sharp trocars should be avoided to prevent injury to the intercostal vessels that can produce profuse bleeding and damage to internal organs. After placement of the first port, subsequent ports need to be placed under direct vision guided by the endoscope. The placement of postoperative chest tubes is indicated if bleeding or air leak is anticipated. Otherwise, the gas can be suctioned through the last port with a small catheter, as the anesthesiologist keeps the lung expanded until closure is completed to seal the area.
CONCLUSIONS
Laparoscopic surgery is becoming the standard of care for many pediatric conditions. There is an increased demand from referring physicians as well as the public for laparoscopic minimally invasive surgery. It is the responsibility of surgeons to insure that these procedures are as safe as possible for their patients. Careful preparation and training are the cornerstones of successful outcomes. The willingness to convert to an open procedure when faced with significant difficulty should not be regarded as failure on the part of the surgeon, but rather as an exercise in good surgical judgment. The possibility of converting to an open procedure should always be explained to the parents and patient and informed consent should be obtained with specific reference to the possibility of conversion. There are many new and advanced laparoscopic procedures in pediatric surgery, some still controversial. As new procedures are implemented, we will need to learn more about their potential complications and how to avoid them. Rapid recognition of complications and the appropriate treatment of these problems can minimize their impact.
References
- Gans AL. Historical development of pediatric endoscopic surgery. In: Holcomb, GW, editor. Pediatric Endoscopic Surgery. East Norwalk, CO: Appleton & Lange, 1994:1-7.
- Gans S, Berci G: Advances in endoscopy of infants and children. J Pediatr Surg, 1971; 6:199-234.
- Klimkovich IG, Geldt VG, Okulov, AB et al: Thoracoscopy in children. Khirurgiia (Mosk). 1971; 47:19-24.
- Rodgers BM, Ryckman FC, Moazam F, et al: Thoracoscopy for intrathoracic tumors. Ann Thorac Surg. 1981; 31: 414-420.
- Esposito C, Montupet P. Complications of laparoscopic minimally invasive surgery. Pediatr Endosurg Innov Tech. 2003;7:13-8.
- Chen MK, Schropp KP, Lobe TE. Complications of minimal-access surgery in children. J Pediatr Surg. 1996;31:1161-5.
- Bax NM, van der Zee DC. Complications in laparoscopic surgery in children. In: Bax NM, Georgeson KE, Najmaldin A, Valla JS, editors. Endoscopic surgery in children. Berlin: Springer-Verlag, 1999; 357-68.
- Esposito C, Montupet P, Amici G, Desruelle P. Complications of laparoscopic antireflux surgery in childhood. Surg Endosc. 2000;14:622-4.
- Esposito C, Mattioli G, Monguzzi GL, Montinaro L, Riccipetiotoni G, Aceti R, et Complications and conversions of pediatric videosurgery: the Italian multicentric experience on 1689 procedures. Surg Endosc. 2002;16:795-8.
- Warner PM, Andrus CH. Hemodynamic and pulmonary implications and complications in laparoscopic surgery. Semin Laparosc Surg. 1997;4:135-8.
- McMahon MJ. Complications of laparoscopic access. Semin Laparosc Surg. 1997;4:139-46.
- Siedman Anesthesia for neonatal minimal access surgery. Pediatr Endosurg Innov Tech. 2002;6:153-9.
- Sato M, Muraji T, Asai T, Hamada Y, Hioki K. Hemodynamic effects of carbon dioxide insufflation of the thoracic cavity during thoracoscopic surgery. Pediatr Endosurg Innov Tech. 2002;6:185-9.
- Bliss D, Matar M, Krishnaswami S. Should intraoperative hypercapnea or hypercarbia raise concern in neonates undergoing thoracoscopic repair of diaphragmatic hernia of Bochdalek? J Laparoendosc Adv Surg Tech. 2009;19(S1):S55-S58.
- Pacilli M, Pierro A, Kingsley C, et al. Absorption of carbon dioxide during laparoscopy in children measured using a novel mass spectrometric technique. Br J Anaesth 2006; 97:215-219.
- Gomez Dammeier BH, Karanik E, Gluer S, et al. Anuria during Pneumoperitoneum in infants and children: a prospective study. J Pediatr Surg. 1995;40:1454-8.
- Slater B, Rangel S, Ramamoorthy C et al. Outcomes after laparoscopic surgery in neonates with hypoplastic heart left heart syndrome. J Pediatr Surg. 2007;42:1118-21.
- Simon P, Burkhardt U, Sack U, et al. Inflammatory response is no different in children randomized to laparoscopic or open appendectomy. J Laparoendosc Adv Surg Tech. 2009;19 (S1): S71-S76.
- Esposito C, Ascione G, Garipoli V, De Bernardo G, Esposito G. Complications of pediatric laparoscopic surgery. Surg Endosc. 1997;11:655-7.
- Esposito C, Porreca A, Esposito G. Vascular complications during laparoscopy. An analysis of a personal case. Minerva Chir. 1999;54:163-5.
- Montero M, Tellado MG, Rios J, Mendez R, Somoza I, Pais E, et al. Aortic injury during diagnostic pediatric laparoscopy. Surg Endosc. 2001;15:519.
- Kudsi Oy, Jones SA, Brenn BR. Carbon dioxide embolism in a 3 week old neonate during laparoscopic pyloromyotomy: a case report. J Pediatr Surg. 2009;44:842-5
- Nakajima K, Wasa M, Kawahara H, Hasegawa T, Soh H, Taniguchi E, et al. Revision laparoscopy for incarcerated hernia at a 5-mm trocar site following pediatric laparoscopic surgery. Surg Laparosc Endosc Percutan Tech. 1999;9:294-5.
- Waldhaussen JH. Incisional hernia in a 5-mm trocar site following pediatric laparoscopy. J Laparoendosc Surg. 1996;6(1 Suppl):89S-90S.
- Valla JS, Trevino E, Demay A, Steyaert H. Laparoscopic bowel injuries in children. Pediatr Endosurg Innov Tech. 2001;5:253-7.
- Sandoval C, Stringel G, Ozkaynak MF, Tugal O, Jayabose S. Laparoscopic splenectomy in pediatric patients with hematologic diseases. JSLS. 2000;4:117-20.
- Esposito C, Gonzalez Sabin MA, Corcione F, Sacco R, Esposito G, Settimi A. Results and complications of laparoscopic cholecystectomy in childhood. Surg Endosc. 2001;15:890-2.
- Della Corte C, Falchetti D, Nebbia G, et al. Management of cholelithiasis in Italian children: A national multicenter study. World J Gastroenterol. 2008;14:1383-1388.
- Callahan MJ, Rodriguez DP, Taylor GA. CT of appendicitis in children. Radiology. 2002; 224:325-332.
- York D, Smith A, Phillips JD, et al. The influence of advanced radiographic imaging on the treatment of pediatric appendicitis. J Pediatr Surg. 2005;40:1908-1911.
- Terasawa T, Blackmore CC, Bent S et al. Systematic review: computed tomography and ultrasonography to detect acute appendicitis in adults and adolescents. Ann Inter med. 2004; 14 (7):537-46.
- Poortman P, Oostvogel HJ, Bosma E, et al. Improving diagnosis of acute appendicitis: results of a diagnostic pathway with standard use of ultrasonography followed by selective use of CT. J Am Coll Surg. 2009;208: 434-41.
- Stringel G, Zitsman J, Shehadi I, Kithir S. Laparoscopic appendectomy in children. JSLS. 1997;1:37-9.
- Paya K, Fakhari M, Rauhofer U, Felberbauer FX, Rebhandl W, Horcher E. Open versus laparoscopic appendectomy in children: A comparison of complications. JSLS 2000;4:121-4.
- Kiriakopoulos A, Tsakayannis D, Linos D. Laparoscopic management of complicated appendicitis. JSLS. 2006; 10:453-456.
- Moraitis D, Kini SU, Annamaneni RK, et al. Laparoscopy in complicated appendicitis. JSLS. 2004;8:310-313.
- Taylor M, Emil S, Nguyen N, et al. Emergent vs urgent appendectomy in children: a study of outcomes. J Pediatr Surg. 2005;40:1912-15.
- Yardeni D, Hirschl R, Drongowski RA. Et al. Delayed versus immediate surgery in acute appendicitis: Do we need to operate durging the night? J Pediatr Surg. 2004;39:464-69.
- Qureshi FG, Ergun O, Sandulache VC, et al. Laparoscopy splenectomy in children. JSLS. 2005; 9:389-392.
- Morinis J, Dutta S, Blanchette V, et al. Laparoscopic partial vs splenectomy in children with hereditary spherocytosis. J Pediatr Surg. 2008;43:1649-52.
- Stringel G, Robinson E, Maisel S. Laparoscopic gastrostomy. Pediatr Surg Int. 1993;8:382-4.
- Stringel G, Geller ER, Lowenheim MS. Laparoscopic-assisted percutaneous endoscopic gastrostomy. J Pediatr Surg. 1995;30:1209-10.
- Hopkins MA, Stringel G. Laparoscopic Nissen fundoplication in children: a single surgeon’s experience. JSLS. 1999;3:261-6.
- Stringel G, Delgado M, Guertin L, Cook JD, Maravilla A, Worthen H. Gastrostomy and Nissen fundoplication in neurologically impaired children. J Pediatr Surg. 1989;24:1044-8.
- Stringel G. Gastrostomy with antireflux properties. J Pediatr Surg. 1990;25:1019-21.
- Hendrickson RJ, Yu S, Bruny JL. Et al. Early experience with laparoscopic pyloromyotomy in a teaching institution. JSLS. 2005;9:386-388.
- Lazar D, Naik B, Fitch ME, et al. Transumbilical pyloromyotomy with umbilicoplasty provides ease of access and excellent cosmetic results. J Pediatr Surg. 2008;43:1408-10.
- Cozzi DA, Ceccanti S, Mele E, et al. Circumumbilical pyloromyotomy in the era of minimally invasive surgery. J Pediatr Surg. 2008;43:1802-06.
- Rey LR, Fernandez M. Open vs. laparoscopic pyloromyotomy—a retrospective analysis. Minim Invasive Ther Allied Technol. 2008;17: 313-7
- Sola JE, Neville Laparoscopic vs open pyloromyotomy: a systematic review and meta-analysis. J Pediatr Surg. 2009;44:1631-37.
- Mullassery D, Perry D, Goyal A, et al. Surgical practice for infantile hypertrophic pyloric stenosis in the United Kingdom and Ireland—a survey of members of the British Association of Pediatric Surgeons. J Pediatr Surg. 2008;43:1227-9.
- Chinnaswamy P, Malladi V, Jani KV, et al. Laparoscopic inguinal hernia repair in children. JSLS. 2005; 9:393-398.
- Saranga Bharathi R, Arora M, Baskaran V. Pediatric inguinal hernia: laparoscopic versus open surgery. JSLS. 2008; 12:277-281.
- Sneider EB, Jones S, Danielson PD. Refinements in selection criteria for pediatric laparoscopic inguinal hernia repair. J Laparoendosc Adv Surg Tech. 2009,19: 237-40
- Him Tam Y, Hung Lee K, Yin Sihoe JD, et al. Laparoscopic hernia repair in children by the hook method: a single center series of 433 consecutive cases. J Pediatr Surg. 2009;44:1502-05.
- Hassan ME, Mustafawi AR. Laparoscopic flip-flap technique versus conventional inguinal hernia repair in children. JSLS. 2007; 11: 90-93.
- Matthyssens LEM, Philippe P. A new minimally invasive technique for the repair of femoral hernia in children. About laparoscopic repairs in 10 children. J Pediatr Surg. 2009;44:967-71.
- Fraser JD, Aguayo P, Ho B, et al. Laparoscopic management of intussusception in pediatric patients. J Laparoendosc Adv Surg Tech. 2009,19: 563-65.
- Gonzalez Spinola J, Del Pozo G, Tejedor D, et al. Intussusception: The accuracy of ultrasound-guided saline enema and the usefulness of a delayed attempt reduction. J Pediatr Surg. 1999;34:1016-20.
- Bonnard A, Demarche M, Dimitriu C, et al. Indications for laparoscopy in the management of intussusception. A multicenter retrospective study conducted by the French study group for pediatric laparoscopy (GECI). J Pediatr Surg. 2008;43:1249-53.
- Burjonrappa S. Laparoscopic reduction of intussusception: an evolving therapeutic option. JSLS. 2007; 11:235-37.
- Hon Chui C, Yin Ong L, Yling Chua JH, et al. “Chinese Fan Spread” distraction technique of laparoscopic reduction of intussusception. JSLS. 2007; 11: 238-41.
- Sandoval C, Strom K, Stringel G. Laparoscopy in the management of pediatric intraabdominal tumors. JSLS. 2004; 8:115-118.
- Metzelder MI, Kuebler JF, Shimotakahara A, et al. Role of diagnostic and ablative minimally invasive surgery for pediatric malignancies. Cancer. 2007; 109:2343-2348.
- Laje P, Mattei PA. Laparoscopic adrenalectomy for adrenal tumors in children: A case series. J Laparoendosc Adv Surg Tech. 2009,19: s27-s29.
- Gandhi R, Stringel G. Laparoscopy in pediatric abdominal trauma. JSLS. 1997; 1:349-351.
- Shultz FA, McKenna B, Gaines BA. Diagnostic and therapeutic laparoscopy in pediatric abdominal trauma. J Pediatr Surg. 2006;41:72-77.
- Nikfarjam M, Rosen M, Ponsky T. Early management of traumatic pancreatic transection by spleen-preserving laparoscopic distal pancreatectomy. J Pediatr Surg. 2009;44:455-58.
- Meehan JJ. Sandler A. Pediatric robotic surgery: A single institutional review of the first 100 consecutive cases. Surg Endosc. 2008; 22:177-82.
- Copeland DR, Boneti C, Kokoska ER, et al. Evaluation of initial experience and comparison of the DaVinci surgical system with established laparoscopic and open pediatric Nissen fundoplication surgery. JSLS. 2008;12:238-240.
- Meehan JJ. Robotic surgery in small children: is there room for this? J Laparoendosc Adv Surg Tech. 2009,19:707-712.
- Slater BJ, Meehan JJ. Robotic repair of congenital diaphragmatic hernia. J Laparoendosc Adv Surg Tech. 2009,19: s123-s127.
- Browne AF, Inge T. How young for bariatric surgery in children? Semin Pediatr Surg. 2009; 18: 176-185.
- Caniano DA. Ethical issues in pediatric bariatric surgery. Semin Pediatr Surg. 2009; 18: 186-192.
- Iwanaka T, Uchida H, Kawashima H, et al. Complications of laparoscopic surgery in neonates and small infants. J Pediatr Surg. 2004;39:1838-41.
- Clark C, Mackinlay GA. Laparoscopy as an adjunct to peritoneal drainage in perforated necrotizing enterocolitis. J Laparoendosc Adv Surg Tech. 2006,16: 411-13.
- Tan HL, Tantoco JG, Ee MZ. The role of diagnostic laparoscopy in micropremmies With suspected necrotizing enterocolitis. Surg Endosc. 2007; 21: 485-487.
- Pierro A, Hall N, Ade-Ajayi A et al. Laparoscopy assists surgical decision making in infants with necrotizing enterocolitis. J Pediatr Surg. 2004; 39:902-906.
- Martinez Ferro M, Bignon H, Figueroa M. Ladd laparoscopic procedure in the neonate. Cir Pediatr. 2006; 19: 182-4.
- Spilde TL, St Peter SD, Keckler SJ, et al. Open vs laparoscopic repair of congenital duodenal obstruction: a concurrent series. J Pediatr Surg. 2008; 43:1002-5.
- Kay S, Yoder S, Rothenberg S. Laparoscopic duodenoduodenostomy in the neonate. J Pediatr Surg. 2009; 44:906-908.
- Kim AC, Bryner BS, Akay B, et al. Thoracoscopic repair of congenital diaphragmatic hernia in neonates: lessons learned. J Laparoendosc Adv Surg Tech. 2009,19: 575-80.
- Kalfa N, Allal H, Raux O, et al. Tolerance of laparoscopy and thoracoscopy in neonates. Pediatrics.2005;116:e785-91.
- Lugo B, Malhotra A, Guner Y, et al. Thoracoscopic versus open repair of tracheoesophageal fistula and esophageal atresia. J Laparoendosc Adv Surg Tech. 2008,18: 753-55.
- Patkowski D, Rysiakiewicz K, Jaworski W, et al. Thoracoscopic repair of tracheoesophageal fistula and esophageal atresia. J Laparoendosc Adv Surg Tech. 2009,19: s19-s22.
- Hay SA. Transperineal rectovesical fistula ligation in laparoscopic-assisted abdominoperineal pull through for high imperforate anus. J Laparoendosc Adv Surg Tech. 2009,19: s77-s79.
- Lima M, Tursini S, Ruggeri G, et al. Laparoscopically assisted anorectal pull-through for high imperforate anus: three years experience. J Laparoendosc Adv Surg Tech. 2006,16: 63-6.
- Wong KK, Chung PH, Chan KL, et al. Should open Kasai portoenterostomy be performed for biliary atresia in the era of laparoscopy? Pediatr Surg Int. 2008; 24:931-3.
- Ponsky TA, Diluciano J, Chwals W, et al. Early experience with single port laparoscopic surgery in children. J Laparoendosc Adv Surg Tech. 2009,19: 551-53.
- Rothenberg SS, Shipman K, Yoder S. Experience with modified single port laparoscopic procedures in children. J Laparoendosc Adv Surg Tech. 2009,19: 695-98.
- Shafi BM, Mery CM, Binyamin G, et al. Natural orifice translumenal endoscopic surgery (NOTES). Semin Pediatr Surg. 2006; 15: 251-258.
- Velhote MC, Velhote CE. A NOTES modification of the transanal pull through. J Laparoendosc Adv Surg Tech. 2009,19: 255-7.
- Frykman PK, Hagiike M, Hui TT, et al. Experience with new 3 mm laparoscope in complex neonatal minimally invasive surgery: A preliminary report. J Laparoendosc Adv Surg Tech. 2008,18: 439-42.
- Turial S, Schwind M, Kohl M, et al. Feasibility of microlaparoscopy for surgical procedures of advanced complexity in children. J Laparoendosc Adv Surg Tech. 2009,19: s103-s105.
- Lobe TE. Complications of thoracic minimally invasive surgery in children. Pediatr Endosurg Innov Tech. 2003;7:5-12.
- Stringel G, Ouzounian S, Napoleon L, et al. Thoracoscopic pericardial window creation and thoracic duct ligation in neonates. JSLS. 2003; 7:337-339.
- Gandhi R, Stringel G. Video assisted thoracoscopic surgery in the management of pediatric empyema. JSLS. 1997; 1:251-253.
- Stringel G, Amin N, Dozor A. Video assisted thoracoscopy in the management of recurrent spontaneous pneumothorax in children. JSLS. 1998; 3:113-116.
- Sandoval C, Stringel G. Video-assisted thoracoscopy for the diagnosis of mediastinal masses in children. JSLS 1997;1:131-3.
