Biomaterials in Hernia Repair
David B. Earle, MD, FACS
HISTORY
Hernia repair is one of the most common operations a general surgeon will do. There are nearly 1,000,000 hernia repairs performed every year in the United States alone. This however is only the tip of the iceberg. When considering incisional hernia alone, we know there are approximately 4,000,000 laparotomies performed every year in the United States, of which 15 to 20% will result in an incisional hernia. The low end of the estimate, would be the eventual formation of approximately 600,000 incisional hernias per year. While the reason we repair less than 20% of these (approximately 100,000 per year) is multifactorial, one of the more significant factors is the lack of an ideal prosthetic. In fact, we have been searching for the ideal prosthetic since the beginning of recorded medical history. Because of a lack of anesthesia and antisepsis, the first prosthetics were placed externally in an attempt to prevent the visceral contents from herniating through an open defect. These external prosthetics eventually became known as a trusses. As techniques evolved in anesthesia and antisepsis, surgical approaches using pre-fabricated prosthetics made from tantalum gauze and silver coated wire mesh were utilized to cover the defect in the abdominal wall internally. As with the development of any new device, particularly in a field that has little to no surgical history, the results were not as good as anticipated and the use of pre-fabricated prosthetics for hernia repair as a whole fell into disfavor. However, Theodore Billroth was able to recognize the need for successful prosthetic hernia repair in his statement: “If we could artificially produce tissues of the density and toughness of the fascia and tendon, the secret of radical cure of hernia can be discovered”. The use of non-absorbable suture however, was used in a variety of manners with a bit more success by pioneers such as Shouldice and Bassini, but these were still far from ideal. The use of nonabsorbable suture can be also be considered to be a prosthetic that is fashioned at the time of the operation, a fact that was exploited in the “darning” technique. Regardless of the specific technique, the avoidance of pre-fabricated prosthetics used a variable amount of suture in conjunction with variable strategies to tighten the inguinal floor, and many non-prosthetic repairs today are performed with some modification of the original description, hence adding heterogeneity to an already complex group of techniques.
We know now from prospective randomized trials and clinical experience that repair of incisional and inguinal hernias have lower recurrence rates when a prosthetic is used compared to when it is not. Lichtenstein developed arguably the simplest and most widely used prosthetic repair for inguinal hernia, and ventral hernia repair with the prosthetic was pioneered and propagated by Rives, Stoppa, and Wantz. Over the past several years, there has been a renaissance in the development of a variety of prosthetics for the repair of all types of hernias. With variable, and sometimes conflicting, results from the myriad of inanimate, animal, and human studies reported in the literature, choosing the proper prosthetic can be a daunting task for the surgeon. Today, approximately 90% of all inguinal hernia operations and a growing number of ventral hernia repairs are performed with a prosthetic.
However, physicians have always known that “an ounce of prevention is worth a pound of cure”, but it wasn’t until 2009 when Israelsson and colleagues published the culmination of nearly 20 years of clinical experience and scientific experiments, and demonstrated a simple suturing technique that could lower the incidence of incisional hernia from 17% to 5% after primary closure of initial laparotomy. Nonetheless, the etiology of all types of hernia remain multifactorial, and elusive for a given patient, thus relegating surgical community to continue the search for the ideal prosthetic for hernia repair.
ANATOMY
Anatomic considerations related to hernia prosthetics include the architecture of both the patient and the prosthetic.
Regarding the patient, all hernias essentially represent a tissue defect through which there is an unintended herniation of other organs or tissue. Hernias of the torso may occur at all of the muscular boundaries including the ventral and dorsal musculature of the abdomen as well as the pelvic floor and diaphragm. The hernias are represented by either an enlargement of existing defects (e.g. esophageal hiatus and inguinal rings) or defects in an area where one would otherwise not exist (e.g. incisional and congenital diaphragmatic hernias). The muscles of the abdomen consist of a superficial group (internal and external obliques, transversus abdominis, rectus muscles, and pyramidalis) and a deep group (psoas major, psoas minor, and the quadratus lumborum). Muscles of the pelvic floor consists of the levator ani, internal and external anal sphincters, coccygeus, deep transversus perinei, urethral sphincter and a few gender specific muscles. The muscles are accompanied by their fascial coverings, and there is also ligaments that add support, as well as a central perineal tendon. The superior border of the abdominal cavity is That’scomposed essentially of the diaphragm, a broad flat muscle with a central tendon consisting of right, middle, and left leaflets. There are several openings in this muscle to allow the passage of the esophagus, blood vessels, deep muscles of the abdomen, nerves, and lymphatics. The most notable of these openings to the hernia surgeon is the esophageal hiatus, of which the left sided muscle fibers insert on the right side of the spine, passing between the aorta and the esophagus, and inferior to the fibers on the opposite side.
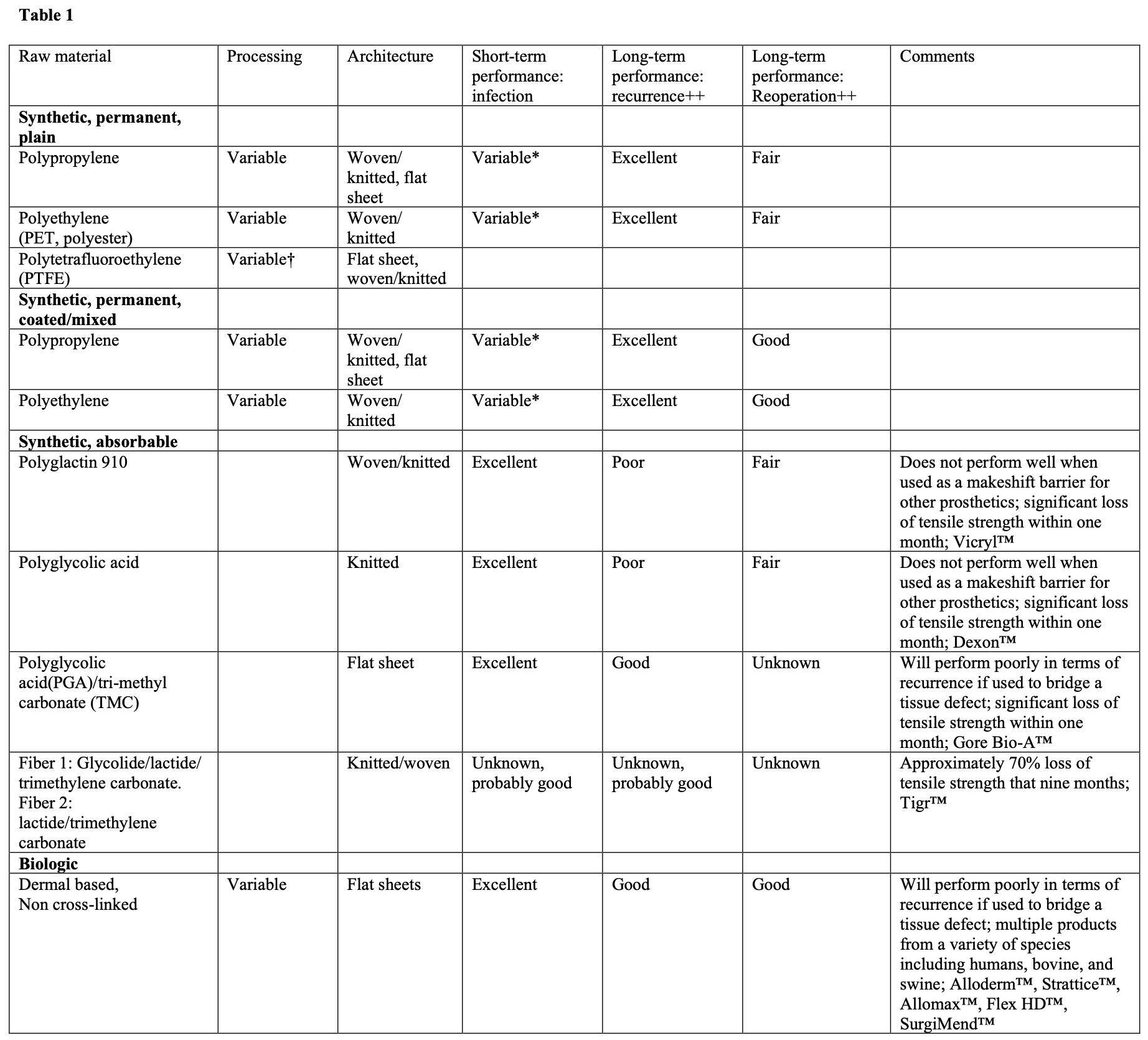
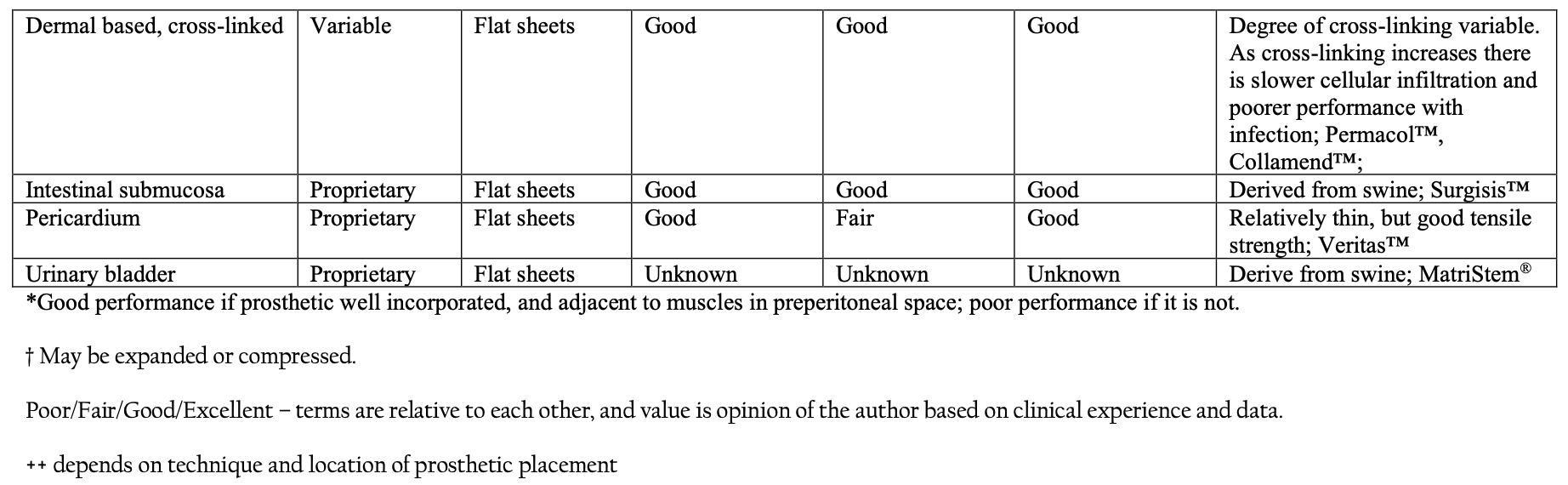
Regarding the anatomy of a hernia prosthetic, one must consider the raw materials, processing of the raw materials, and construction of those raw materials.
Considering the most commonly used raw material for hernia prosthetics, polypropylene, the process begins with the propylene monomer. The propylene is then polymerized by connecting multiple propylene monomers together. The location of these monomeric bonds can be controlled for and are at least partially responsible for the ultimate strength of the polymer. The process includes a variety of phases and ultimately produces polypropylene pellets. The different phases of the polypropylene production, all have an influence on the characteristics of the final product. The polypropylene pellets are then cleaned, melted, and extruded into fibers that can be woven or knitted into a prosthetic in a variety of ways for use in hernia repair. All of these post-production steps also influence the characteristics of the final prosthetic. In other words, all polypropylene prosthetic are not the same, and in fact, no two prosthetics made from the same polymer are the same, and may in fact have significant differences in outcomes based on these differences. Additionally, some prosthetics will use more than one polymer, each with a unique characteristic that, at least in theory, adds clinical characteristics that may change patient outcomes.
Regardless of the raw materials involved, the polymers must be constructed in such a fashion that the final product is usable as a prosthetic in a surgical procedure. The polymer may be extruded in large flat sheets, or as thin fibers that are either knitted or woven together. The prosthetic will ultimately have certain characteristics determined by its architecture that will influence its density, weight, and porosity. These factors will all be variables that determine the biocompatibility and performance of the prosthetic in the clinical setting. Because of the significant variability among prosthetics and implantation techniques, there is no conclusive evidence that one prosthetic is superior to another in most clinical settings. The architecture of the prosthetic will have implications regarding intraoperative handling, performance in the setting of infection or contamination, long- term performance related to recurrence, and long-term performance related to re- operation, incorporation into the abdominal wall, and/or erosion into adjacent organs. The surgeon should consider all of these issues, not just intraoperative handling, when choosing a prosthetic.
A list of common categories of biomaterials used in hernia repair can be seen in Table 1.
PHYSIOLOGY
Physiology related to biomaterials used in hernia repair include the wound healing response, particularly with regards to foreign body implantation, and the functional anatomy of the abdominal wall, diaphragm, and pelvic floor.
The body’s response to an implanted hernia prosthetic begins by proteins sticking to the prosthetic. The adherence of this protein coagulum to the prosthetic will be influenced by the architecture and any coatings on the prosthetic. Many of the coatings are designed to prevent this protein coagulum from sticking to the prosthetic. Once the proteins are adherent to the prosthetic, platelets will then stick to this protein coagulum, then release a variety of cytokines that will attract white blood cells, smooth muscle cells, and fibroblasts. With the arrival of these additional cells, more cytokines are released and a complex cascade of reactions culminates in the development of a scar based on a crosslinked collagen polymer. If the prosthetic has a knitted or woven architecture with relatively low weight and large pore structure, the scarring will be more likely to form the configuration of a net, hence maintain some flexibility as well as strength. If the prosthetic has a relatively high weight and/or small pore structure, the resulting scar will resemble more of a solid plate.
The flat muscles of the anterolateral abdominal wall serve to flex and rotate the spine and pelvis. When acting bilaterally, these muscles can flex the spine, useful when sitting up from a supine position, and increase intra-abdominal pressure, useful for bowel movements, urination, and childbirth. When acting separately, the oblique muscles on opposite sides act in concert to flex and rotate the spine and pelvis, useful for many facets of daily life. While significant functional disability uncommonly occurs when these muscles have a defect within them, larger defects are frequently associated with significant physical deformities that negatively impact daily life.
PATHOPHYSIOLOGY, DISEASE, AND DISORDERS
While there are many ways to approach this regarding biomaterials for hernia repair, this section will focus on mechanisms of hernia recurrence. Mechanisms of hernia recurrence are both a story of hernia disease itself, as well as a story of technical errors related to prosthetic use.
With regard to patient factors, the etiology of hernia formation and recurrence is multifactorial. The broad categories of these factors are essentially genetics and environmental factors. Regarding genetics, there may be obvious inherited traits such as Elher’s-Danlos and Marfan’s syndrome. There may also be inherited traits that are more subtle, such as abnormal collagen formation. Additionally, there may be traits that aren’t expressed until certain environmental factors come into play, such as an operation or infection. When these factors come into play, the traits become expressed, abnormal collagen gets produced, and the hernia may form or recur.
Among the environmental factors that may lead to hernia formation and recurrence are obesity, infection, trauma (iatrogenic and environmental), and use of immunosuppressive medications.
When discussing both genetic and environmental factors as they relate to hernia formation and recurrence, is important to note two facts: 1) the factors vary among patients, and 2) the factors may vary in an individual patient over time. While some of the factors related to hernia formation or recurrence may be easily known, all of the factors remain unknown. For example, it may be known that a patient developed in incisional hernia after a laparotomy, and the iatrogenic injury of laparotomy was the cause. It is unknown however, whether there were other factors at play such as the suturing technique, infection, or an inherent connective tissue disorder. Thus, basing treatment only on the known factors may not be sufficient.
Regarding mechanisms of recurrence with prosthetic use, it is clear that primary repair of an incisional hernia, regardless of size, is associated with a recurrence rate of greater than 60%, and was compared to a variety of techniques utilizing a prosthetic, which had a recurrence rate of 32%. Consequently, elective, primary repair of incisional hernia should largely be abandoned. Because of the relatively high recurrence rate in the prosthetic group, it is clear that some prosthetic techniques are not as good as others, and there is no conclusive high-level data proving one technique of prosthetic placement over another.
There is however extensive clinical experience and general widespread agreement of techniques that have an increased rate of recurrence that would be deemed unacceptable by most surgeons and patients. Among these techniques, which will be described in detail in the next section, are primary repair as mentioned above, “inlay” technique where the edge of the mesh is sewn to the edge of the defect, and the “onlay” technique without closure of the defect.
TREATMENT
The treatment of abdominal wall hernias depends on the clinical scenario, specific goals of the operation, and location of the hernia. With regard to hernia location, there are also issues regarding the size and shape of the defect, and whether or not the herniated contents are reducible, chronically incarcerated, or acutely incarcerated.
The clinical scenario is well-known to practicing surgeons. It is helpful however, to be able to think of the clinical scenario in a manner that will facilitate the preoperative planning process. First, it is benficial to determine if there any issues with the patient’s medical history such as a known connective tissue disorder, a disease likely to require abdominal surgery in the future, such as inflammatory bowel disease (which may include ostomy formation), whether or not the patient has had previous hernia repairs, and of course the overall health status in terms of the patient’s general ability to tolerate an operation and anesthesia. While chronologic age is a factor, it is only one data point amongst many factors that should be considered. If there have been previous hernia repairs, particularly at the site now being considered for operation, all efforts should be obtained to determine details of the previous operation(s). If a direct conversation with the surgeon is not feasible, the copy of the operative report will help determine many operative details, the anesthesia record will allow determination of the length of the operation, and the discharge summary will help elucidate any complications. The patient’s level of physical activity should also be determined, concentrating on both work and out of work activity. It is also very important to document the details of any preoperative symptoms and limitation of activity. In particular, pain should be described with all of the characteristics we learned in medical school such as onset, duration, location, character, radiation, aggravating/alleviating factors, associated symptoms (typically related to the G.I. tract, GU tract, or respiratory tract), frequency, and severity. Typically, the patient’s symptom complex will wax and wane over time and it may be more important to focus on the frequency of the more severe episodes. Limitation of any activity really related the patient’s symptom complex is also important to document in detail before any treatment is undertaken. While it may require some extra time to chronicle these details, it can be very valuable when evaluating the patient at any time during the postoperative period should any questions or concerns arise. An example of this would be a patient with chronic abdominal pain that had a prosthetic placed for a ventral hernia and that prosthetic was subsequently recalled. If the preoperative symptoms had been detailed sufficiently, and were unchanged after placement of the prosthetic, it would be difficult to conclude that the current symptoms were related to the prosthetic. All too often however, this level of detail is only obtained postoperatively if a problem arises.
Once the clinical scenario has been determined, it is important to clarify the goal(s) of the operation. Broadly these can be categorized as resolving an existing problem, or preventing problems. Common problems associated with hernias include pain, issues related to an abdominal wall deformity (e.g., properly fitting clothes, appearance, issues with external support garments), symptoms related to the G.I., GU, and respiratory tracts (depending on location of hernia), skin ulceration, and inability to perform certain physical activities. Patients with no problems whatsoever may want to have a hernia repair simply to avoid any problems in the future. The desire for prophylactic hernia repair may be a general philosophy of preventative maintenance, or based on a rigorous travel schedule or family situation. A simple way to elucidate the goal is to simply ask the patient to explain in their own words why they would like to have a hernia repair, and what their expectations are after repair. Aligning goals and expectations of both the patient and surgeon are of vital importance to patient satisfaction, regardless of the clinical outcome. A plausible scenario is an asymptomatic patient who has an inguinal hernia diagnosed during a routine physical examination. The patient is referred to a surgeon who confirms the diagnosis and explains the details of hernia repair with the patient, who gives the occasional affirmative nod of the head during the conversation.
There may not ever be a description of the definition of a hernia. The surgeon recommends hernia repair, a date for operation is chosen, and commences by placing a prosthetic to repair the hernia. Postoperatively, the patient develops significant, activity limiting pain and sees the surgeon. The surgeon examines the patient, finds no mass or recurrence, and concludes the pain must be coming from scar tissue, which is unavoidable. Because there was no explicit determination of the goals of surgery and expected postoperative course, the patient and the surgeon now have conflicting views of the success of the operation.
Once the clinical scenario and goals of operation have been explicitly determined and aligned with the patient and surgeon, the surgeon must choose a technique that best fits this particular patient. In general, the surgeon should have mastered a specific technique that is used in most circumstances, but be proficient in one or two additional techniques to use as dictated by varying clinical circumstances. Once the technique has been chosen, an appropriate prosthetic can be selected. For example, a prosthetic designed for laparoscopic use may not be appropriate for an open inguinal hernia repair, and vice versa.
With respect to treatment of inguinal hernia, there is little debate as to the benefits of utilizing a prosthetic, but much debate over the type and placement over the prosthetic. It is beyond the scope of this manuscript to discuss the details of these issues. While there is good data showing that a laparoscopic approach has less pain and a shorter recovery compared to open techniques, significant training issues have prevented the dissemination of surgeons proficient in the laparoscopic approach. Amongst the open surgical techniques, there is no conclusive data suggesting one approach is better than another, but there is a general consensus that the best technique of prosthetic placement for hernia repair is the technique that the surgeon is the most proficient at.
With respect to treatment of ventral hernias, a prosthetic should be used for elective repair of incisional hernia of any size, and selectively for primary hernias.
Recommendations for use of the prosthetic for primary ventral hernia include large-size (>2-4cm), or any size defect associated with a midline diastasis, obesity, heavy physical activity, smoking, immunosuppressed state, or known connective tissue disorder.
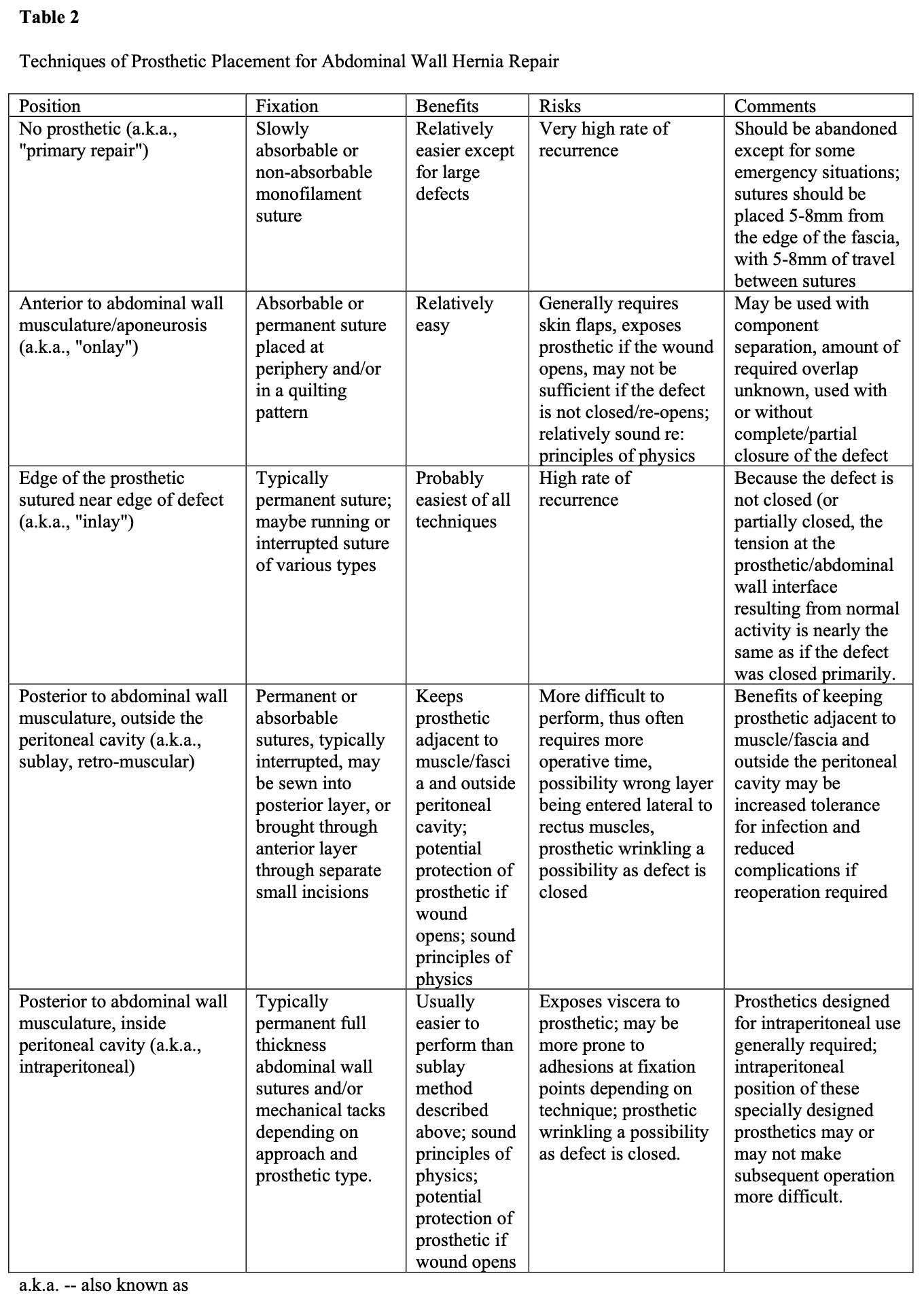
Regardless of the indication for prosthetic use during ventral hernia repair, the surgeon currently has only so many options as to the technique. These include placing the prosthetic behind the abdominal wall musculature either within or outside the peritoneal cavity (with or without closure of the defect), within the defect itself by suturing the edge of the prosthetic to the edge of the defect (with minimal or no overlap), or on the anterior surface of the abdominal wall musculature (with or without closure of the defect). Each of these techniques as its relative advantages and disadvantages. While there is still debate about placing the prosthetic on the anterior versus posterior abdominal wall, the technique of suturing the edge of the prosthetic near the edge of the defect has largely been abandoned. Details of these issues are seen in Table 2.
COMPLICATIONS
Complications regarding prosthetic use for hernia repair may arise in the early or late postoperative period. For this manuscript, I will define early complications as those that occur within 30 days of operation.
Early complications typically relate to infectious problems, inflammation, pain, and occasionally seroma. Recurrence however, cannot be discounted, even in the early postoperative period. Infection of the prosthetic may be readily apparent if systemic manifestations are present, however this is often not the case, making the early diagnosis of the prosthetic infection difficult. In general, infected prosthetics after groin hernia repair in the early postoperative period generally require complete removal of the prosthetic. Infected prosthetics after ventral hernia repair may not require complete removal in the early postoperative period, but this will depend on the technique used for prosthetic placement.
Occasionally, there may be an inflammatory reaction of the skin involving all or a portion of the area where the prosthetic was placed. This is usually manifest with erythema and warmth of the skin that may become more intense when the patient is in an upright position compared to the supine position. There are typically no systemic signs of inflammation such as fever, rigors, or leukocytosis. Additionally, imaging studies may or may not reveal a fluid collection around the prosthetic.
Since the vast majority of patients have pain after a surgical procedure, it can be difficult to distinguish pathologic pain from typical postoperative pain. If a patient has unusually severe or prolonged pain, pathologic pain should be considered. In this case, pathologic pain is defined as pain related to enterotomy, or specifically related to the prosthetic/fixation method, or physical reaction to the prosthetic/fixation such as infection or seroma. Generalized severe pain, particularly if associated with signs and symptoms of sepsis are more indicative of an enterotomy. If there is pinpoint pain at the site of full or partial thickness fixation, it is more likely that the point of fixation is the cause of the pain. The pain may be persistent from the time of operation, or have an onset many weeks, months, or years after operation. Determining an etiology for excessive postoperative pain may be difficult, if not impossible to elucidate, particularly if a detailed preoperative history related to pain was not taken.
The development of a seroma, or fluid collection around the mesh, may be debated as to whether or not it is a complication. Nearly all foreign bodies implanted initially have some element of a surrounding fluid collection. A fluid collection that is persistent beyond 6 to 12 months and/or is symptomatic should be considered for treatment. Long- standing fluid collections may become infected from a distant infectious site unrelated to the hernia, and symptoms may cause disability depending on the severity.
Recurrence in the early postoperative period should not be ignored as a possibility. Symptoms suggesting an early recurrence include those that are exactly the same as the preoperative symptoms, the presence of a reducible mass, excessive pain at the site of a presumed seroma, or bowel obstruction.
RESCUE STRATEGIES
Infection
Postoperative infection after use of a prosthetic in hernia repair may occur in the immediate postoperative period, several weeks to months after the operation, or even years after implantation. Early postoperative infection may include the skin, the subcutaneous tissues, seroma fluid, the prosthetic itself, or an intra-abdominal abscess separate from the prosthetic. Skin infections are typically treated with antibiotics, subcutaneous tissue infections treated with incision and drainage along with antibiotics, intra-abdominal infections treated with percutaneous or surgical drainage. It is rare for seroma fluid to become infected in the early postoperative period. Infections that involve the tissue or fluid in immediate vicinity of the prosthetic, and the prosthetic itself have a variety of options depending on where the prosthetic was placed, and what type of prosthetic was used.
Considering macroporous prosthetics such as those typically made from polypropylene and polyester, the prosthetic may be initially left in, even in the presence of an infection involving the prosthetic, and if the prosthetic was placed in an onlay or sublay fashion outside of the peritoneal cavity. Prosthetics placed with an onlay technique may have granulation grow through the pores even with the prosthetic exposed, as long as the prosthetic has good contact with the underlying tissue. The variety of wound care options is beyond the scope of this manuscript, but some sort of vacuum assisted wound care may be particularly helpful. Small portions of the prosthetic that do not have granulation tissue growth through them may be debrided along with any devitalized tissue or exudate. The wound may then be allowed to contract and re-epithelialize, or covered with a skin graft. This strategy is also true for a sublay prosthetic if there has been a dehiscence of midline closure over it, or the defect was not closed completely. If there has been complete closure of the abdominal wall over a prosthetic placed with a sublay technique outside of the peritoneal cavity, and a wound infection requires opening the wound, the surgeon should investigate the possibility of a prosthetic infection by assessing the clinical scenario and utilizing radiological evaluation in addition to performing standard wound care. Persistence purulent drainage indicates that at least a portion of the prosthetic may still be harboring infection, and any lasting wound sinuses should be explored in the operating room. These types of prosthetics may be used in intraperitoneal position when they have some sort of a barrier layer on them to prevent dense adhesions to the viscera. If there is an overlying infection, how to proceed will depend on whether the operation was performed open or laparoscopically. It is very rare for a straightforward laparoscopic ventral or inguinal hernia to develop an infection around the prosthetic, but if a prosthetic infection does develop, generally the entire prosthetic needs to be removed. If there is a wound infection at either one of the port sites or the suture anchor sites, local wound care and/or antibiotics may be employed with little risk of a prosthetic infection. If an infection develops in the skin and/or subcutaneous tissues after an open repair with in intraperitoneal prosthetic, the same types of strategy can be employed as with a sublay prosthetic, but if the prosthetic becomes exposed, it is much more likely to require complete removal due to its intraperitoneal position instead of the sublay position immediately adjacent to the muscles/fascia.
If a microporous prosthetic (such as polytetrafluoroethylene; PTFE) becomes infected, it will generally require complete removal. There are occasions when a small portion of the prosthetic has an adjacent infection, and the remainder of the prosthetic is well incorporated. Under these circumstances, it is reasonable to open wound over the infected portion of the prosthetic, treat with local wound care, and consider excising only that portion of the prosthetic that is exposed once any skin infection is cleared and the edges of the wound have granulation tissue. The wound may then be closed over a drain or left open, and in general, permanent sutures should be used to avoid a re-current hernia through the defect in the mesh. This is a good initial step provided there are no systemic signs and symptoms of infection, as well is no radiological evidence that there is a fluid collection around the prosthetic. Any persistent drainage of purulent material through an abdominal wall sinus suggests a more extensive prosthetic infection, or one that did not respond to local excision. In this case, it is usually most appropriate to remove the entire prosthetic.
As mentioned earlier, is rare for a seroma to become infected in the early postoperative period. If this does happen, generally the entire prosthetic needs to be removed as it has not incorporated into the surrounding tissue at the time of infection. In general, seroma should be observed for period of months rather than repeatedly drained, unless they are significantly symptomatic. If percutaneous drainage is undertaken, a drainage catheter should not be left in the seroma cavity, as it has a high probability of becoming infected, particularly if not all of the material was drained. If the seroma is persistent 12 months after the operation, and remains asymptomatic, consideration should be given for excision of the seroma along with its entire lining. The persistent fluid collection could become infected if there is an unrelated distant infection at some point in the future. If an infected seroma occurs several months or even years after the initial operation, drainage of the seroma cavity alone is usually not sufficient. The entire lining, or at least the vast majority of it, should be excised and the overlying skin closed over a drain after ablation of any remaining fluid secreting tissue from the lining of the seroma.
Inflammatory changes in the skin
On occasion, the skin overlying the area of all or a portion of the prosthetic may become erythematous. There is occasionally warmth associated with this, but generally no systemic signs or symptoms of infection. Signs of infection may be revealed with a history and physical examination, but laboratory in radiological examinations may also be necessary. In the absence of any data other than the erythema suggesting infection, this condition may be treated with standard anti-inflammatory therapy and/or observation. It will usually resolve over several weeks, but may rarely last up to several months.
Pain
Excessive pain in the early postoperative period can be difficult to distinguish from being pathologic or typical postoperative pain, particularly considering ventral hernia repair with a prosthetic. A high index of suspicion and a low threshold to return to the operating room are required to minimize morbidity and mortality from unrecognized or early postoperative enterotomy. Abdominal x-rays and computed tomographic scans of the abdomen are typically not useful in the first few days after operation. Patients not following the typical postoperative course should be cause for a high index of suspicion for this problem. If the hernia repair was performed open, the approach will be determined by the size of the prosthetic. If the prosthetic runs the entire length of the incision and is at least as wide as the rectus muscles bilaterally, re-opening the laparotomy incision will probably be the best approach. Once the prosthetic is encountered, it can be partially opened just enough to gain safe access to the peritoneal cavity. If the peritoneal cavity contains obvious entire contents, the remainder of the prosthetic will need to be removed, and the primary strategy becomes management of the enterotomy. If the procedure was initially performed laparoscopically, is usually relatively easy to perform a diagnostic laparoscopy in the initial postoperative period. If enteric contents are discovered, patient will generally require laparotomy, prosthetic removal, and management of the enterotomy.
Persistent pain is typically associated at the areas where the prosthetic has been anchored, particularly if full thickness abdominal wall sutures have been used. It is also possible that absorbable or nonabsorbable fixation tacks may be the source of persistent pain. If there has been an incision at each suture anchors site, the diagnosis of suture related pain is possible from a physical examination. If there has been radio opaque fixation devices such as tacks, marking the area of pain and performing a radiological examination may help determine whether or not the pain is due to the tack. One strategy for suture anchor pain is to perform at local trigger point injection utilizing local anesthetic and steroids.
Another strategy is to provide systemic anti-inflammatory therapy such as a short course of steroids (Medrol dose pack) followed by 2 to 3 weeks of nonsteroidal anti- inflammatory agents used in combination with a topical anti-inflammatory agent.
Referral to a specialized pain center may yield benefits with nerve blocks or other types of systemic medications for chronic pain. If nonsurgical methods fail, consideration should be given to returning to the operating room and removing the fixation suture or tack. It is very unusual for the prosthetic itself to cause chronic pain, and removal of the prosthetic in its entirety for pain alone should be done in highly selected patients only. Again, a detailed preoperative history regarding abdominal pain is essential to determine appropriate management of postoperative pain.
Recurrence
Recurrent hernia is a problem that exists among all surgeons. Despite this fact, there are still many surgeons that believe they do not have a single recurrence after any hernia repair they have performed. It is universal among surgeons boasting zero recurrences that their data source is their memory alone, presuming any of their patients would come back to them if there was a problem. In order to at least estimate individual recurrence rates, each surgeon should keep all of their cases in a database, provide long-term follow-up, and create an environment whereby the patients are encouraged to come back should any problems or concerns arise. Long-term follow-up is especially important when starting a new practice, or when changing any component of the technique or prosthetic in a well- established practice.
Once a recurrent hernia is diagnosed, it is once again important to take a detailed history regarding any symptoms, as well as explicitly establish the goals and objectives of the operation. It is imperative to learn the details of the previous repairs by speaking with the surgeons, and reviewing the records to determine the technical details of the operation, the length of the operation, and whether or not there were any complications. The importance of these issues exists for all recurrent hernias, but becomes greater as the hernia becomes more complex and a number of recurrences increase.
The approach to a recurrent hernia is different than the approach to a primary hernia due to the fact that the surgeon does not want to repeat the same techniques that have already failed. Additionally, recurrent hernias typically take longer to repair, and this is important for preoperative planning purposes. Depending on the type of techniques that will be utilized, preoperative imaging studies may also be of benefit.
If previous a prosthetic or prosthetics have been used, they may need to be excised depending on their type, size, and degree of conformity/integration to the abdominal wall. Excision of a previous prosthetic may be accomplished laparoscopically, but usually requires a larger incision for extraction of the specimen. If the prosthetic is well integrated into the abdominal wall and not excessively buckled or wrinkled, the prosthetic may remain in place while an additional prosthetic is placed over, or in addition to the existing one. Whether to completely cover the old prosthetic or not is a decision that depends on the details of the previous operation including size and type of prosthetic as well as the type of fixation. For example, a prosthetic that was used for midline incision laparoscopically, and anchored to Cooper’s ligaments in addition to multiple laterally based full thickness fixation sutures, that demonstrates a recurrence at the superior border represents a situation where the entire old prosthetic does not need to be removed or covered. A prosthetic however that was probably too small in the beginning and now represents the recurrent hernia sac represents a situation where the entire old prosthetic needs to be covered or removed.
Concomitant G.I. surgery and unintentional enterotomy
Enterotomy during laparoscopic ventral hernia repair has been a topic of much debate. Regardless of the strategy chosen when and unintentional enterotomy occurs, a high index of suspicion for enterotomy and low threshold for repair is common among them all. Whether this is done laparoscopically or utilizing an open approach depends on the surgeon’s training and experience as well as the capabilities of the institution. Once the enterotomy has been properly repaired, the surgeon has the choice of: 1) aborting the hernia repair, and returning in 3 to 5 days (prior to the formation of dense adhesions; proliferative phase of wound healing) to repair the hernia. 2) aborting the hernia repair, and returning in 6 to 8 weeks (after the resolution of the proliferative phase of wound healing) to repair the hernia. 3) continuing with hernia repair as planned. There is plenty of laboratory and human experience regarding the placement of permanent synthetic prosthetics during many types of clean contaminated or contaminated operations in both the elective and emergent setting. It remains however, up to the surgeon’s judgment at that particular moment on which course of action to take. Regardless of the course of action, this is a scenario that should be discussed with the patient as part of the informed consent process, and the surgeon should have a strategy in mind before even beginning the operation. This will allow a more efficient and practical management strategy for this uncommon intraoperative problem.
Suggested reading
Millbourn, Daniel MD; Cengiz, Yucel MD, PhD; Israelsson, Leif A. MD, PhD. Effect of Stitch Length on Wound Complications After Closure of Midline Incisions: A Randomized Controlled Trial. Arch Surg. Nov. 2009; 144(11):1056-1059.
Diener MK, Voss S, Jensen K, Büchler MW, Seiler CM. Elective midline laparotomy closure: the INLINE systematic review and meta-analysis. Ann Surg. 2010 May; 251(5):843-56.
Halm JA, de Wall LL, Steyerberg EW, Jeekel J, Lange JF. Intraperitoneal polypropylene mesh hernia repair complicates subsequent abdominal surgery. World J Surg. 2007 Feb; 31(2):423-9; discussion 430.
Burger JW, Luijendijk RW, Hop WC, Halm JA, Verdaasdonk EG, Jeekel J. Long-term follow-up of randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. 2004 Oct;240(4):578-83; discussion 583-5.
Jänes A, Cengiz Y, Israelsson LA. Preventing parastomal hernia with a prosthetic mesh: a 5-year follow-up of a randomized study. World J Surg. 2009 Jan;33(1):118-21; discussion 122-3.
Strzelczyk JM, Szymański D, Nowicki ME, Wilczyński W, Gaszynski T, Czupryniak L. Randomized clinical trial of postoperative hernia prophylaxis in open bariatric surgery. Br J Surg. 2006 Nov;93(11):1347-50.
Conze J, Rosch R, Klinge U, Weiss C, Anurov M, Titkowa S, Oettinger A, Schumpelick V. Polypropylene in the intra-abdominal position: influence of pore size and surface Hernia. 2004 Dec;8(4):365-72.
