Laparoscopic Repair of Ventral Wall Abdominal Hernia
Karl A. LeBlanc, MD, MBA, Brent W. Allain, Jr, MD
HISTORY
Laparoscopic incisional and ventral hernia repair (LIVH) was first reported in 1993.1 The foundation of this operation was based on the Rives-Stoppa repair of these hernias.
Although the latter procedure places the mesh in the preperitoneal plane, the laparoscopic procedure places a mesh in the intraperitoneal space. Since its inception, this procedure has continued to be used as a method of repair of these types of hernias as well as other more complicated ones, such as parastomal and parapubic hernias.2-4 A significant increase has occurred in the number of these procedures that are performed worldwide.
The basis of the success of this operation is adequate dissection of the hernia, generous overlap of the prosthetic biomaterial chosen, and firm fixation of that product. The early adopters of this repair initially limited its use to smaller defects in patients who were smaller and with fewer prior intraabdominal procedures. Currently, only the very large hernias with significant loss of domain are not considered candidates for at least an attempt at a repair laparoscopically.
Many adverse events occur during and after this operation that have been reported in the literature. Many of these are commonplace with these procedures, such as the formation of a seroma, or the presence of an ileus, and are generally of limited significance. These are so common that many authors do not consider them to be true complications but rather frequent consequences of the procedure itself. Significant problems like an unrecognized enterotomy, although uncommon, are frequently disastrous and sometimes result in mortality.5
It is important for the surgeon to be familiar with the prevention, recognition, and management of these complications, because despite one’s best efforts, they will occur. This chapter will outline these complications, as well as the methods to avoid, diagnose, and manage them. The majority of patients (approximately 90%) who undergo this procedure will do so because of an incisional hernia rather than a primary ventral hernia. Consequently, these patients will be at most risk of an adverse event.
ANATOMY/PHYSIOLOGY
The rectus abdominis muscle is a paired muscle of the medial abdominal wall that is separated by the linea alba. It is through this structure that most hernias develop, because this is the layer incised during most laparotomies. The development of a hernia at this site will result in gradual lateral displacement of the sides of the hernia defect. This displacement is due to the lateral distraction from the external oblique, internal oblique, and transversus abdominis muscles. The longer this hernia exists, the larger these forces will become, thereby enlarging the hernia defect even more. As this occurs, the respiratory function of the abdominal wall will become compromised. The repair with a laparoscopic method most often does not close the defect, especially with the larger hernias. Therefore, the diminution of the respiratory function could continue even after the repair unless the defect is closed during the procedure. There is a trend toward more frequent closure when feasible, because of this reason and the fact that this might diminish the rate of seroma formation.
The lateral, subcostal, subxiphoid, and suprapubic hernias will not affect the respiratory function to as large a degree. However, the anatomy of these hernias makes their repair ever-more difficult with increasing size. The inability to properly fixate the prosthetic biomaterial in these locations because of the ribs, sternum, pubis, and other areas will make these repairs especially challenging.
A significant number of these patients will also experience varying degrees of lumbar and back pain. This is due to the marked change in the posture of these patients, because as they must compensate with these posterior muscle groups to carry the weight of the contents of the hernia.
TREATMENT
Most patients are candidates for this operation. Exceptions are those with very small fascial defects or those with significant loss of domain of the intestine. The former do not justify this technique unless they are very obese and the latter patients have such large defects that the trocars cannot enter the abdomen but rather enter the hernia. In this instance, the procedure is not practically feasible, and the cosmetic result will more likely be unsatisfactory. Elderly patients or those with an inadequate cardiac reserve should be carefully chosen, although neither is an absolute contraindication. The presence of an active intraabdominal infection precludes the introduction of a synthetic prosthetic biomaterial in most circumstances. There have been reports of this repair with intestinal resection and concomitant cholecystectomy.6
The methodology of LIVH has been described in numerous publications. In all of these repairs, the abdomen must be entered with one of the variety of techniques that are available. The method of choice should be determined by the experience of the surgeon and may be that of the Hasson technique, the use of an “optical trocar,” or with the Veress needle. This decision can be, in and of itself, a difficult one as the subsequent steps in the operation are usually determined by the placement of the trocars themselves.
After entry, the abdomen should be inspected and additional trocars should be placed under direct visualization when and where they can be done safely. It is recommended that the laparoscope be inserted into each trocar after it is placed to inspect the abdominal contents from that newer perspective. This can easily be done if the 5-mm laparoscope is used. This is usually not feasible if the 10-mm laparoscope is used, because there is only one trocar of that size used generally. The initial operative step will be the release of all of the adhesions that are encountered. This can be performed with either blunt or sharp dissection and with or without the use of an energy source to maintain hemostasis. This portion of the procedure is the most challenging segment of the repair and one that incurs the most significant risks of the entire operation, enterotomy, and hemorrhage. Bleeding is usually easily recognized, but the former is sometimes impossible to recognize. Many surgeons believe that the risk of an unrecognized enterotomy is so great that the use of any energy source is avoided entirely. Others, the authors included, use the ultrasonic scalpel, bipolar cautery, or monopolar cautery selectively. If no bowel is involved in the adhesive process, this energy can be used carefully. However, if the intestine must be lysed from the anterior abdominal wall, the application of any energy source is not recommended. Its use adjacent to bowel can result in late necrosis of a portion of the bowel wall causing delayed perforation. Due to the lateral spread of heat, this can occur even if no intestine was seen near any adhesion during the entire operation.5
Once the adhesions are lysed, the hernia defect is measured. This can be done by the placement of spinal needles through the abdominal wall or the use of an intraperitoneally placed ruler. We prefer, instead, to palpate and mark the hernia defect in the insufflated position then deflate the abdominal cavity. These marks are then measured. To the transverse and vertical dimensions, 6cm to 10cm is added; and this is the minimum size of the biomaterial that should be used. Typically, the size of the prosthesis used is slightly larger than these measurements to ensure that the required minimum 3-cm to 5-cm fascial overlap is provided. The prosthetic is selected, rolled, and inserted into the abdominal cavity. This biomaterial is then unrolled and fixed to the anterior abdominal wall with a fixation device of the surgeon’s choosing. There are permanent (ProTack, Covidien, Norwalk, CT) and absorbable devices (Sorbafix, C.R. Bard, Providence, RI; AbsorbaTack, Covidien, Norwalk, CT). These should be placed approximately 1cm to 1.5cm apart from each other along the periphery of the biomaterial. Care must be taken to ensure that the biomaterial edges do not curl, because this can be a lead point for future adhesion formation. Transfascial sutures are then placed at 5-cm intervals along the periphery of the prosthesis to maintain adequate fixation. This latter fixation is currently a hotly debated topic. The use of the additional fixation will be dependent on surgeon preference, patient size, defect size, number of prior recurrences, and prosthetic choice.7
An abdominal binder is placed onto the patient in an effort to alleviate some of the postoperative pain and to diminish the development of the seroma. Most patients are discharged within 24 hours and are allowed to engage in any activity that does not illicit pain.
PREOPERATIVE EVALUATION
Few laboratory or radiologic studies are available that can identify and prevent the intraoperative and postoperative complications shown in Table 1. The preoperative computed tomographic (CT) scan is invaluable to delineate the size of the defect(s) and the accurate assessment of the location of the hernia and its contents. The preoperative evaluation of the incisional hernia patient should center on the recognition of any comorbid conditions that may make this operation perilous. In general, these are few, but these patients must have sufficient cardiac and pulmonary reserve to permit the use of general anesthesia and the required insufflation of the abdominal cavity. Prevention of intraoperative and postoperative complications related to these organ systems will require consultation with the appropriate medical specialist.
The surgeon should also assure that the patient has adequate hepatic function preoperatively. The presence of portal hypertension, while not a contraindication to the procedure in all cases, may present significant hemorrhagic problems if not anticipated preoperatively. Patients with ascites, whether induced by hepatic disease, renal disease, or malignancy, should be evaluated carefully in the preoperative phase of treatment. All of these are at high risk for any operation and may not be suitable for laparoscopy to repair these hernias, because of the potential of ascitic leakage, infection, poor wound healing, significant medical risk, and shortened life expectancy.
The size of the hernia is a significant consideration for the surgeon. As previously mentioned, the concern will be the very large hernias that may have loss of domain. In these cases, the size of the defect may not allow the placement of the trocars lateral to the fascial defect. This will make the placement of the biomaterial to repair the hernia prohibitively difficult in the laparoscopic approach. In these patients, the choice should be the open repair with the Rives-Stoppa approach, which is mimicked by the laparoscopic method or the component separation operation. Additionally, the patient with poor pulmonary reserve may be at significant peril if any hernia repair is performed. Fastidious workup and pretreatment will be necessary. Frequently, time will be available to insist that the morbidly obese patient lose weight to make the procedure and recovery easier.
INTRAOPERATIVE MEASURES
As noted in Table 1, there are relatively few intraoperative complications that are not associated with all of the other laparoscopic procedures. These are covered elsewhere in this textbook and will not be discussed further in this chapter. The method of entry into the abdomen is not different with these patients; however, one must scrutinize the location of all prior incisions and the specific operations that were performed. Ideally, the location of the initial trocar should be distant from the other procedures, especially if these were via a laparotomy. For example, if the patient had undergone a left hemicolectomy, then the initial point of entry should generally be the right upper quadrant.
Once the first trocar is inserted and the abdominal cavity is visually inspected, the goal of the surgeon should be the determination of the appropriate location for placement of sequential trocars. This will usually require dissection of adhesions with or without an energy source. Following the introduction of each trocar, the laparoscope should be inserted through it to inspect the abdominal cavity from that new vantage point. This may reveal the location of bowel in an area that was not suspected and could prevent an intestinal injury. This, however, may not be feasible if the surgeon must use a 10-mm laparoscope while the additional trocars are only 5mm. The individual institutions will dictate these choices. It is safer to insert a 10-mm rather than a 5mm trocar if it is necessary to inspect the abdomen. Either the 0º or 30º laparoscope can be used for this procedure.
Injury to the intestine can be prevented in most, but not all, cases. The obvious injuries that reveal mucosa and bowel content will demand repair. The subtle injuries that can occur to the bowel with traction or a thermal burn that can occur distant from the point of dissection will not be apparent at the original procedure. The best method to prevent the traction tear of the bowel is the cautious and limited use of traction on the bowel wall itself. The instruments that are used should be “large-jawed” rather than smooth or pointed. The lateral burn that may occur might not be apparent until the wall of the intestine necroses a few days postoperatively. To avoid this, it is recommended that no energy source be utilized in the dissection that is near the bowel wall. It should not be forgotten that all sources of energy will remain hot immediately after their use.
Therefore, sufficient time must be allowed for instruments to cool, or they can be applied to nonessential structures such as fat to allow them to cool before they are used near bowel. Additionally, one should inspect the abdominal cavity at the completion of the procedure to assess the visualized intestine to confirm that no obvious injury exists and that hemostasis has been achieved. This will not, of course, ensure that a “late” cause of injury will not become manifest a few days postoperatively.
Injury to other abdominal organs is rather uncommon. However, knowledge of the anatomy from the laparoscopic perspective is necessary to avoid such a complication. Fortunately, these are rare, but when they do occur (ie, splenic injury) conversion to the open approach may become necessary and warrants sound surgical judgment.
Infection can be a particularly severe complication following this procedure. Preoperative antibiotics should be administered, especially if the biomaterial used does not contain an antimicrobial agent. Most surgeons use an iodine impregnated plastic drape (Ioban, 3M, St. Paul, MN) to protect the biomaterial from any contact with the skin.
Recurrence is the complication that this operation is known to have reduced its incidence. The choice of biomaterial may influence this complication, but there are many very good prostheses available today (Table 2). Fixation is the paramount concern to assure that the intraabdominal pressure does not dislodge the biomaterial from its attachments. The current recommendation is that of a fixation device supplemented by the use of transfascial sutures. These are spaced approximately 1cm and 5cm apart respectively along the periphery of the biomaterial. Admittedly, however, these sutures may be used less frequently. The most important fact should be that the surgeon is both comfortable and confident in the chosen method of fixation.
POSTOPERATIVE MEASURES
Most of the immediate complications, such as pain, fever, and an ileus, cannot be prevented. Pain should be treated as usual but the use of ketorolac may diminish it in many patients. We prefer to place an abdominal binder upon all of our patients while they are still on the operating table, because this greatly aids in pain control. It has not resulted in respiratory embarrassment in any patient.
The frequent and early use of an incentive spirometer will help prevent the postoperative atelectatic fever that is seen rather frequently. This may be difficult because of the short length of stay in the hospital, if at all. The development of a prolonged ileus should not truly be considered a complication, but, in most reports, this is listed as such. Generally, this cannot be prevented, especially in those with extensive adhesions involving significant portions of intestinal contents, particularly if these were incarcerated. As in most surgical procedures, early ambulation and the allowance of oral intake at an early stage may offer considerable benefit.
COMPLICATIONS AND THEIR MANAGEMENT
The majority of complications known to occur with the laparoscopic approach are not different from that of the open repair (Table 1). A few complications, such as an unrecognized enterotomy, can have a significant mortality rate.5 The complications seen with the laparoscopic repair range from 5% to 30% (average 15.2%).8-15
Intraoperative Hemorrhage
Intraoperative hemorrhage can occur at the outset of the operation during insertion of the first trocar and is, therefore, not unique to incisional hernia repair. The exact incidence of this is not known, as this type of injury is rarely reported because it is generally of no consequence and can be readily controlled. The use of an entry method that does not use a cutting trocar may provide an increased margin of safety. If hemorrhage does occur, it can be stopped in one of a variety of ways. The trocar can be removed and replaced with a larger one to tamponade the bleeding vessels. The insertion of a balloon-tipped trocar can also be used to tamponade the bleeding site. Alternatively, the trocar can be removed and the site cauterized, or a transabdominal suture can be placed before or after its removal.
Hemorrhage can occur during the most challenging portion of the procedure, the adhesiolysis. This will usually originate from one of the vessels within the omentum and can be managed with electrocautery or the ultrasonic shears. If this method is chosen, the surgeon must be especially cautious to ensure that there is no adjacent intestine at that site so a lateral burn will not occur. This type of injury may not become apparent until a few days later when that site necroses and perforates (see below). Alternatively, the use of hemoclips, looped sutures, or suture ligation can be effective for this problem.
Another common occurrence of hemorrhage is that which results during the fixation of the prosthetic biomaterial. Any of the metal devices or the transfascial sutures that are placed can puncture or lacerate small abdominal wall vessels or the inferior epigastric vessels. This will be easily identified because either brisk bleeding will be seen or a hematoma will form rapidly. One must be quick to apply direct pressure at that site and then place one or more transfascial sutures with one of the devices designed for that purpose.
Intestinal Injury
This is the most feared of the potential complications. The serosal injury that occurs during the dissection of the intestine can be managed as one would during any bowel surgery. If the lumen has not been violated, one may elect to leave this injury untouched, or the surgeon can close this with either sutures or with the application of endoscopic staples. In our series of patients, we used all of these choices without any adverse events.12 The incidence of a recognized enterotomy (that involves the lumen) varies significantly and has been reported from 1% to 14.3% of patients.12,16-21 A recent review of the literature revealed that the incidence of intestinal injury averages 1.78%. In that review, 85% were recognized at the time of the injury.5
The management of a recognized enterotomy is currently somewhat controversial. In many centers, if this event occurs, the hernia procedure is terminated. The surgeon will repair the injury either laparoscopically or by conversion to laparotomy.22 The hernia can either be repaired with an open method using a tissue repair or this can be deferred. In the latter instance, the intestinal repair is completed but the hernia repair is delayed. The patient will be returned to the operative suite 2 to 4 days later whence the laparoscopic hernioplasty will be performed. This delay will allow any contamination to be eliminated, and this short time span will not allow the formation of dense adhesions that would make the second operation quite tedious. A delay of several weeks or longer should be considered if a significant spillage of intraluminal contents has occurred. Antibiotics are generally continued in the intervening period.
More recently, reports have shown that the simultaneous repair of this recognized small bowel injury and the hernia may be safe. This enterotomy should not be associated with a large amount of spillage of the intestinal contents. Some series20,23 have repaired this injury and proceeded to repair the hernia laparoscopically with insertion of a prosthetic biomaterial. Others24 have resected gangrenous bowel and completed the hernia repair. In these few reports, an adverse consequence did not occur. Because the risk can be significant, this choice must be made carefully.5
Intestinal necrosis secondary to an electrosurgical injury is frequently underestimated. When that injury is oversewn, there may be an extension of the necrosis and subsequent development of an intraabdominal abscess. This would then require a formal laparotomy, resection, and repair of the involved intestine and removal of the prosthesis.18 The hernia may need to be left open or repaired with a collagen-based biomaterial (Table 3). The use of this latter biomaterial has not been proven to prevent the redevelopment of another hernia in the future, however. More data are needed on the use of these products in these circumstances.
An injury to the colon carries a much greater potential for complications if the hernia repair is completed as planned. The majority of surgeons will simply repair the injury and repair the hernia by a laparotomy and primary closure. The use of a polypropylene or collagen-based prosthesis may be required if the fascial defect is very large, because primary closure may not be possible. If this is not used, then closure of the skin alone and a planned repair of the hernia at a later time may be undertaken. A few have reported that the hernia repair can be performed with a simultaneous colon resection as a preplanned operation with an adequately prepared colon.20 This is the exception rather than the rule, however.
An unrecognized enterotomy can be a significant complication that is known to occur in up to 6% of the patients.13,16,18,19,23 In most reports, it is very infrequent and occurs in only 15% of the enterotomies that occur during this operation.5 This enterotomy can occur either from a traction injury or may result from a burn that is incurred during the use of an energy source to lyse the adhesions. In either case, the injury will not be manifest in the initial hours following the procedure, in most instances. The usual postoperative course of LIVH patients is a rapid discharge from the hospital, usually less than 24 to 48 hours.12,20,23,25 Any patient who reports increasing pain, fever, and abdominal distension should be evaluated by physical examination followed by appropriate laboratory testing and CT scanning if the patient does not appear to exhibit an acute surgical abdomen. The complete blood count can be obtained on a daily basis, if necessary. In many cases, the total white blood cell count will be normal, but an increase in the level of immature cells (left shift) will be an early indication that there might be an unrecognized bowel injury. Following the LIVH, there should not be any significant accumulation of intraperitoneal fluid. Free air should have been resorbed before the third postoperative day.26,29 If ascites or free air is present within the abdominal cavity, the patient should be laparoscoped to assess the abdomen if there is even a slight suspicion of an unrecognized enterotomy. This can be both diagnostic and therapeutic. If the findings appear benign, then no further therapy is needed. If significant contamination is found, open laparotomy will generally be required with resection of the involved intestine and excision of the prosthesis. The abdomen could be closed primarily with or without retention sutures. However, if there is a concern of an abdominal compartment syndrome, the prosthesis could be left in place to allow the expansion of the abdomen. The status of the biomaterial should be closely monitored, and sequential removal of this could be electively planned as the condition of the patient stabilizes. Alternatively, a vacuum- assist device can be used over the unclosed abdominal wall.
Injury to Other Organs
Injury to a solid organ, such as the liver, is not common even in the most complex procedures. Cautery or a hemostatic agent can be used successfully in nearly every case. The bladder is at risk during pelvic dissection such as in the repair of parapubic hernias. One may fill the bladder with 250cc of saline that is colored with dye, if desired, and clamp the drainage catheter. This will provide easy identification of the bladder. If a bladder injury occurs, primary sutured repair with absorbable sutures and drainage with the catheter will be sufficient to treat this problem.
Prolonged Ileus
This entity is not an entirely unexpected phenomenon given the degree of dissection of the intestine and intraabdominal organs, as well as the surgical hemorrhage that occurs. It is seen in an average of approximately 2% of patients in the reported series and ranges from 1% to 8%.12,18,19,27,28 It should be treated with early mobilization, antiemetics, nasogastric suction, and hydration. The concern in the patient with an ileus is the possibility of an unrecognized enterotomy. The risk, of course, is that this is not acknowledged or diagnosed until several days later at which time there is a significant incidence of sepsis and subsequent death. If there is suspicion of bowel injury, the appropriate workup should be instituted (see above).
Seroma
The development of a seroma is so common after this procedure that many surgeons do not believe it to be a real complication. This occurrence should be expected, because the peritoneum within the hernia sac is not removed. The fluid secreted by that surface will result in fluid collection. One study reported that the incidence of this event was 100% in patients who were followed with ultrasonography.30
The disparity between this report and that of the published series is related to the definition of the seroma. Many authors do not list this as a complication unless this is persistent for greater than 6 weeks.14,23 Others, such as ourselves, believe that the seroma must persist beyond 8 weeks to 12 weeks, be associated with symptoms, be clinically large, or both, to be considered a complication. The incidence of this varies widely and can range from 0% to 43%.10,12,13,14,20,31 An overall review of the current literature calculates the incidence of clinically significant seromas to occur in 4% to 5% of patients after LIVH.
It is virtually impossible to prevent seroma formation with certainty. Kirshtein32 attempted to pierce the biomaterial with the Veress needle but found that omitting this step did not have any appreciable effect on the incidence of seromas. Others have used the DualMesh with holes (WL Gore & Associates, Inc, Flagstaff, AZ, USA), but this too is associated with a postoperative seroma rate of 11.8%.20 Therefore, it does not appear that perforation of this product offers any benefit to the prevention of these seromas. The use of other biomaterials that are macroporous, such as those that are polypropylene based, does not eliminate the occurrence of these fluid collections either.33-35
Some surgeons have tried to use preemptive measures such as the argon beam coagulator to scarify the peritoneum of the hernia sac. The application of electrocautery and the ultrasonic scalpel with the use of a single suture in the center of the hernia defect to fixate the prosthetic biomaterial has also been used. A prospective randomized study used a central suture in one group of patients and added the application of the energy source in a second group. The seroma rate was statistically significantly different in the second group (25% versus 4%). This study, however, did not evaluate any hernias that were >100cm2. It is precisely the larger hernias that are most likely to develop notable fluid accumulations postoperatively.36 Nevertheless, this may be a useful technique. There is a potential risk of the excessive application of the electrosurgical energy to the thin area of the hernia itself, which could result in a full-thickness necrosis of the thin subcutaneous tissue and skin. This could result in slough of the tissue and exposure of the prosthetic biomaterial, which will increase the risk of infection. Therefore, the use of this technique should be carefully chosen.
In an effort to inhibit or diminish the development of these known problems, many surgeons will place an abdominal binder on the patient while the patient is still on the operating table. This will be worn for up to 6 weeks postoperatively, depending on the initial size of the protruding hernia. The size of the binder, the length of time that its use is recommended, and whether a bulky dressing is used have not been standardized. There have not been any prospective randomized trials to evaluate the effectiveness of this maneuver, but the experience, such as those of the authors and others, appears to suggest that there is a decrease in both the size and duration of the seromas that are clinically significant by as much as 50%.34 Many patients, however, find the binder uncomfortable and refuse to wear it for any sufficient length of time.
Once the seroma is confirmed, the management of this problem is controversial. Most surgeons believe that these will resolve if given enough time to do so. This will usually occur within 12 weeks.22,31,32,37 Not all authors agree with this watchful approach, however. Carbajo20 aspirated all of the 11.8% of seromas that occurred in his recent series without any complication. We do not attempt to aspirate seromas unless the patient remains symptomatic for longer than 6 months and there is no ultrasonic evidence of any significant resolution. Occasionally, if the patient is symptomatic with a noteworthy degree of pain, seroma aspiration will be necessary at an earlier time. One or two aspirations will usually suffice. This occurs in <1% of our patients, however.
Aspiration is avoided because bacteria can be introduced into the fluid collection, resulting in an infection.11,22Therefore, a strict sterile technique is mandatory to minimize this occurrence. An ultrasonic examination of the fluid collection before aspiration is recommended to ensure that a recurrent hernia is not misdiagnosed as a seroma. This will avoid an inadvertent puncture of the bowel during the procedure. This test will also identify the presence of a multiloculated fluid collection that will be difficult to aspirate. If this is found, ultrasonically directed aspiration or even incision and drainage would be necessary to adequately treat this seroma. This is rarely necessary, however.
A CT scan can also be used to evaluate the hernia sac. This can be another effective tool to verify whether the content of the former hernia site is a recurrent hernia or a seroma. Interestingly, one study noted that some of the postoperative fluid collections contained air as late as 6 days to 12 days postoperatively.33 This can mimic either a recurrent hernia or an abscess, but none of the cases in which this was discovered required any intervention and all resolved. Therefore, clinical and laboratory correlation is of utmost importance.
Persistent Postoperative Pain
LIVH can be associated with significantly more abdominal pain than the initial laparotomy that eventuated in the incisional hernia. This is probably based on the use of the fixation devices that are needed to secure the prosthesis to the wall of the abdomen. It is plausible that transfascial sutures will incorporate the subcutaneous nerves as well as the muscle tissue, which increases the degree and persistence of the postoperative pain.
Because the average length of the hospital stays of these procedures is approximately 1 day to 2 days, it must be assumed that the acute postoperative pain is quite manageable with oral analgesics.12,20,23 The problematic cases are those in which the pain persists beyond 6 weeks to 8 weeks, which occurs in 1% to 2% of patients.12,19,23 This type of pain will usually be described as sharp, intermittent, and intense. It can be exacerbated by movement, deep breathing, or coughing.
If this type of condition appears to be present, one should first evaluate the hernia site with ultrasonography or CT scanning. This will be needed to affirm that there is no persistent seroma or a recurrent hernia that is actually causing the pain. If none is found, an initial attempt at treatment should be with either nonsteroidal anti-inflammatory agents or a short duration of steroids. This, of course, could be initiated at any point in the evaluation of this problem. If this does not result in improvement, injection at the site of the complaint with bupivacaine may result in complete resolution of the symptoms. This actually should be done at the outset of this complaint due to its high rate of success. If successful, only 1 injection or 2 injections are usually required.38
If significant pain persists, laparoscopy may become necessary. It is important to have the patient localize the site of pain while on the operating table to provide direction during the operative procedure. Adhesions can be lysed and any sutures (or tacks) at the site of the apparent pain could be cut, or removed, or cut and removed, at that time. While very seldomly required, this is very effective in eliminating the pain in nearly every patient in the experience of these and other authors.19 An alternative is the direct approach through an incision over the suture site while the patient is under local anesthesia. The suture can then be excised, but this is usually difficult in patients with a large amount of subcutaneous tissue. Additionally, the suture may have slipped below the initial fascial layer of the abdominal wall, making its location very difficult to identify.
Infection
A notable benefit of LIVH is the decrease in the frequency of wound complications.11 Other measures that appear to minimize this risk include the use of a good surgical preparation of the abdominal surface, an iodine-impregnated drape (Ioban, 3M, St. Paul, MN), prophylactic antibiotics, and the choice of a biomaterial that contains an antimicrobial agent. However, an infectious complication can be seen in up to 16% of these patients.11,12,19,21,23The use of the available biomaterial (DualMesh Plus, W. L. Gore & Associates, Flagstaff, AZ) that contains both silver and chlorhexidine impregnated into the product appears to anecdotally decrease the rate of infections.12
Infections following LIVH can be classified into minor and major infections. Infections that occur at a trocar site or at one of the transfascial suture incisions should usually be of a minor degree. These will usually present as erythema or drainage at one of these locations. This can be successfully managed with local care and parenteral antibiotics providing gram-positive coverage.21,39 This should resolve within 7 days to 10 days of treatment. If this treatment regimen fails, the possibility of a major infection exists and further workup should be instituted.
A major infection directly involves the prosthetic biomaterial. This can present rather subtly in some cases and can manifest itself by drainage from one of the trocar sites. Therefore, lack of a quick favorable response to the above treatment should lead one to evaluate this with a CT scan. A major infection will usually (but not always) result in leukocytosis, an elevation of the sedimentation rate and C-reactive protein level. If this infection is confirmed, a short-term attempt at parenteral antibiotics with subsequent drainage has been successful in a very limited number of cases.19,22 In the majority of cases, the removal of the biomaterial will be necessary to eradicate the infection, especially if the prosthesis contains a component of ePTFE.12,21,23 The use of a polypropylene mesh does not assure that the mesh will not have to be removed in the face of an infection, however.39 If an ePTFE product is contained in the prosthesis, explantation will be required in at least 85% of the patients. Not surprisingly, removal of the mesh nearly always results in a recurrence of the hernia.12,21,23,39,40 The use of a vacuum device (V.A.C., KCI Inc., San Antonio, TX) over even an ePTFE prosthesis has been shown to allow for the salvage of mesh infection and a solid repair of the hernia.41 However, if there is no response or continual evidence of an underlying infection, excision of the biomaterial will be required.
A particularly difficult situation exists in the patient with an infected biomaterial that requires excision who had a very large fascial defect. Once this prosthesis is explanted, an attempt to repair this with a primary tissue repair is fraught with failure due to the large size of the hernia and the tension that results. Additionally, a tissue repair will predispose the patient to a significant risk of pulmonary complications due to the results of the excision of the biomaterial and the repair of the hernia. Two options are anecdotally reported in this situation.
If the patient is not septic, one may elect to excise the central portion of the patch sequentially. The remaining material will then be sewn together to reapproximate the prosthesis. The patient will then be returned to the operating room several days later (usually 2 to 5 days) for another partial excision of the central portion of the patch.
During that time, local wound care, dressing changes, or vacuum-assisted treatment and parenteral antibiotics will be continued. Usually, within 2 weeks to 3 weeks, the fascial edges will be approximated enough so that a primary sutured repair can be accomplished without the concern of an abdominal compartment syndrome. There is still a high risk of recurrence, but this may be lower and less uncertain than an initial closure of the infected hernia defect. At the time of the reapproximation of the fascial defect, one may use either a collagen-based mesh as an underlay or a polypropylene product as an onlay (or both) to bolster the strength of the repair. These biomaterials have been successfully used in infected fields.
An alternative to the above is the closure of the midline after the mesh has been excised with the use of a component separation technique. In that procedure, the external oblique aponeurosis is cut, allowing the rectus sheath to slide medially, and generally primary closure of the midline can be successfully accomplished. This can be under some tension so that some authors will add the use of collagen-based or synthetic biomaterials, or both (unpublished data).
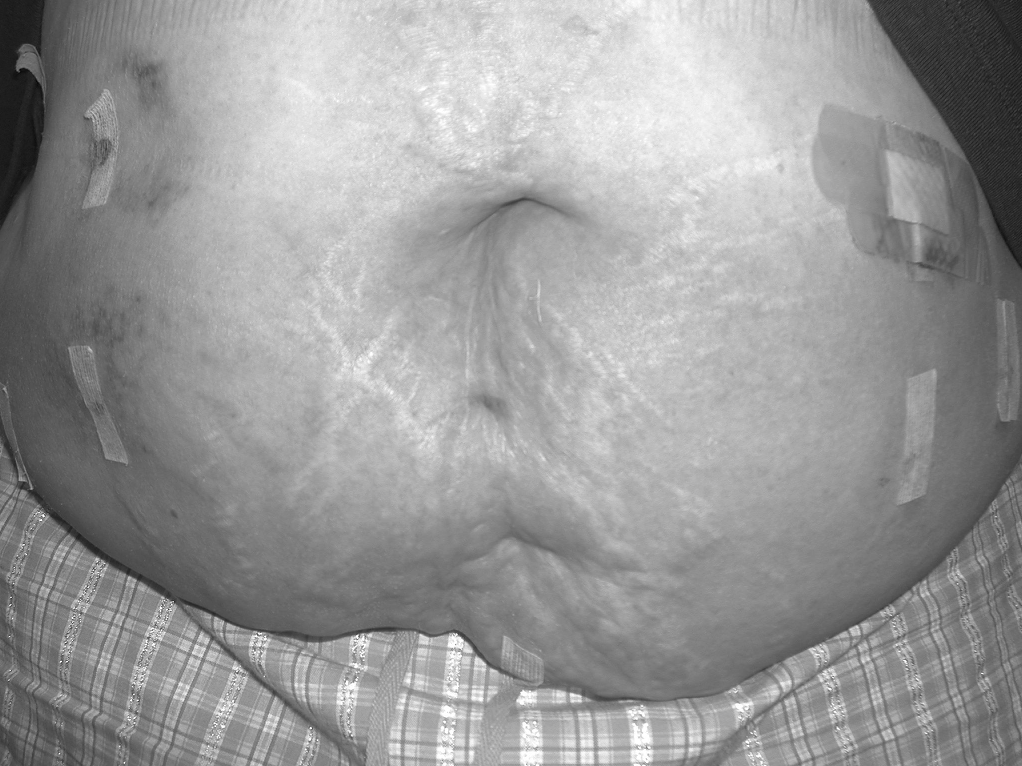
Finally, it is important for the surgeon to be aware that there is an appearance of the skin overlying the hernia defect that is normal, despite the fact that there is significant erythema. This can appear after several days following the operation (Figure 1). This condition has not been adequately described in the literature but can be a source of significant concern due to the suspicion that an infection exists or is developing. It may occur in approximately 2% to 13% of the patients.11,31,32In our experience, this will usually be seen in obese patients, those with an incarcerated hernia, or the patients who require large amounts of tissue dissection during the operation, or in a patient with all of these conditions. This condition is unassociated with fever, pain, or leukocytosis. This does not require treatment and will usually resolve in 4 weeks to 6 weeks. However, it can persist for several months in extreme cases. If there is concern of an infection, early use of imaging modalities and laboratory testing is recommended.
Bowel Obstruction
This is an uncommon event following LIVH. A patient with this condition may exhibit the signs and symptoms of an ileus, obstruction, or an acute surgical abdomen. Diagnosis and treatment must be both rapid and accurate. As noted above, a significant number of patients will develop an ileus that will usually resolve with conservative management.
Those who do not should be investigated further to rule out a true obstruction or a missed enterotomy. The usual radiologic tests may normally be adequate, but one should be swift to obtain a CT scan to investigate the appearance of the entire abdominal cavity. This may be the only test that will reveal fluid-filled distended loops of small intestine, air fluid levels or significant free air in the abdominal cavity. A trocar hernia (or Richter’s hernia) should not be overlooked as the cause of a bowel obstruction.
This complication has been reported in only a few series but has ranged from 0.3% to 3% in these reports but only represents 6 patients.19,20,42 Three of these obstructions were due to the migration of the small bowel between the biomaterial and the anterior abdominal wall. Although the exact distance of the placement of the fixation devices was not stated, one might infer that these devices were too far apart to prevent this complication. This reiterates the recommendation that transfascial sutures should be placed no more than 5cm apart, and the additional tacks or constructs should be placed at 1-cm to 1.5-cm intervals along the periphery of the biomaterial.43,44 The other 3 obstructions were caused by postoperative adhesions. In one of these cases, an associated colonic erosion was present due to a polypropylene mesh that was inserted 23 months prior to the event.19 This reinforces the belief that a tissue-separating product should be present in all meshes that contact the intestine.
If there is no indication of a bowel injury or the presence of gangrenous obstruction, nasogastric suction and hydration are reasonable treatments. If the condition of the patient deteriorates, surgical intervention will be necessary. Either laparoscopy or laparotomy could be used to access the abdominal cavity and deal with the problem in the required manner.
Leakage of Ascitic Fluid
If the patient has ascites from hepatic or renal disease, the entry into the abdominal cavity should attempt to minimize the leakage of this fluid from the abdomen. One should try to locate the trocars in the upper abdomen, if possible. The use of 5-mm trocars with a “Z” path of entry, and subsequent closure of all trocar sites with transfascial sutures will aid in the prevention of a leak. This is rarely a problem that requires intervention, but if this is necessary then sutured closure of the offending site via an open approach is the most reasonable method in which to manage the complication.
Recurrence
The current cumulative recurrence rate of the laparoscopic repair in the published literature is approximately 4.4%. It should be acknowledged that these series are obtained with a follow-up that usually is only about 3 years, but this is the time frame in which most recurrences are seen with both the open and laparoscopic repairs.22,45,46,48 The recurrences that we saw early in our experience were due to an inadequate size of the biomaterial and the use of a method of fixation that did not include transfascial sutures.22 In our first 100 patients, the additional use of transfascial sutures reduced the number of recurrences from 13% to zero. Based on this experience, the use of 2 methods of fixation that include sutures is an integral part of the procedure in all patients.
At least 6 publications have compared the open and the laparoscopic repair. In all but one of these comparison studies, the laparoscopic repair was associated with a lower rate of recurrence.49 The only report that had a higher incidence of recurrences was that of DeMaria,10 but that recurrence represented only one patient. That series represented the early experience of the surgeons. Another study compared the open repair with and without the use of a mesh prosthesis, and the laparoscopic repair. The respective recurrence rates of these patients were 9%, 6%, and 1%.40 The latter study reaffirmed that incisional hernia recurrence declines with the use of mesh and declines further with the minimally invasive approach.
A summary of several large series that all have more than 100 patients is shown in Table 4.6,12,20,27,35,39,47 A few series have a fairly large number of recurrences. The majority of these occurred in the learning phase of the operators’ experience or was due to a complication that occurred following the hernia repair, such as an infection or a bowel fistula. In our own series, we noted that the lack of transfascial sutures, the use of too small a prosthesis, or infection lead to a recurrence. The second 100 patients in our series had recurrences that were due to different causes than the first 100, usually infections that required excision of the prosthesis. Two other recurrences in the latter group were due to a suture fracture or a new hernia that appeared below the prior repair. There was also a decline in the rate of recurrence from 9% to 4% between these 2 groups of patients.12 We continue to include transfascial sutures to fix all meshes for defects that are >5cm, multiply recurrent hernia, and morbidly obese patients. As shown in this table, the average recurrence was still an acceptable 4.4%, similar to that of most reported series in the literature.
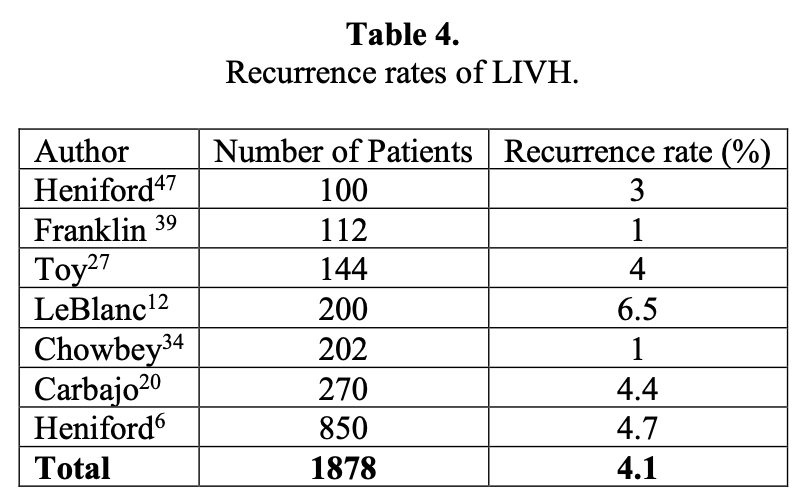
The avoidance of the development of a recurrent hernia should be the goal of all surgeons. Even in this procedure, this has not been achieved, nor is it expected to eliminate any recurrence. Some factors, such as advanced age, obesity, pulmonary or hepatic insufficiency, and steroid use cannot be avoided. However, the surgeon can address some issues to minimize the risk of recurrence, such as provision of adequate exposure of the defect so that all of the prosthetic biomaterial contacts fascia rather than preperitoneal fat. The interposition of adipose tissue will impede the ingrowth process and predispose to a recurrence.
Whatever biomaterial is used, it must overlap the defect by a minimum of 3cm. This distance has been shown to decrease the recurrence rate.11,12,13,22 Most surgeons nowadays believe that a 5-cm overlap should be used.16,20There is no argument with that figure, but this may be difficult to obtain in some cases. The larger overlap is preferred in the hernias that are located very high or very low on the abdominal wall, very large hernias, and in significantly obese individuals. This large overlap of the biomaterial reduces tension on the repair, which will assist in the prevention of recurrence.
Additionally, the more difficult areas of fixation, such as those mentioned above, would benefit from a larger surface area to allow ingrowth of fibroblasts and collagen and to allow for the contraction of the tissues that could result in a decrease in the surface area of the product. It is also recommended to place a large enough piece of biomaterial so that as much of the incision that developed the hernia is covered to prevent the development of a later hernia above or below the current repair.12
One of the most critical aspects of this repair is the assurance that the biomaterial is adequately fixed to the abdominal wall to ensure an effective repair. This fixation should include transfascial sutures. As noted earlier, we demonstrated the effectiveness of this technique in our first 100 patients by eliminating a recurrence (with an average follow-up of 51 months) in these patients. Our preference is to place these sutures at no more than 5cm apart along the periphery of the patch using a suture-passing instrument. Others place these 4cm to 6cm apart.16,23
Not all authors share the view that there is a need for these transfascial sutures. Berger et al18 use sutures merely to position the prosthesis and do not tie them. They believe that sutures increase postoperative pain. Their recurrence rate was only 2.7%, but follow-up was less than 2 years. Carbajo et al20 reported a follow-up of over 8 years, in which there was only a 4.4% rate of recurrence. This type of result is uncommon but Rosen et al21 found no difference in recurrence regardless of the methods of fixation. In that series, many surgeons had no standardized technique; therefore, it cannot be a valid assumption that fixation does not affect the recurrence rate. This is confirmed by Bageacu et al19 in that the recurrence rate was 15.7% when only 2 or 4 transfascial sutures were used. A recent experimental study confirmed the superior strength of sutures versus tacks alone to fix mesh against the abdominal wall. The results of that study indicated that the “addition of transabdominal sutures should be preferred.”50 A large overlap of prosthesis does not appear to be protective against recurrence, because a 9-cm overlap without sutures has been followed by a recurrence.16 The author’s recent meta-analysis,7 however, does support the omission of transfascial sutures in the smaller hernias. Potentially, the biomaterial selected in the repair may influence the need for these sutures. More research is warranted in this regard to affirm the best practices for sutures or tacks alone.
Finally, fixation by either a permanent or absorbable device is a routine part of this procedure. The use of permanent devices, such as the Protack (ProTack, Covidien, Norwalk, CT), has been the standard of care. However, the newer absorbable devices, such as the SorbaFix (CR Bard, Providence, RI) and the Absorbatack (Covidien, Norwalk, CT), are gaining in popularity. As these are new entries into the fixation of hernia meshes, time will tell whether these are as effective as permanent tacks and/or sutures. There are limitations to the use of these products in some areas of the abdomen. These do not penetrate Cooper’s ligament and should not be used near the diaphragm.
If a recurrence is suspected, an ultrasonic examination may differentiate between this and a seroma. A CT scan is the preferred method of diagnosis even if the patient has an obvious hernia. This study will allow the surgeon the ability to plan the repair of the recurrence. It would be helpful if the presence of incarcerated bowel in the hernia sac were noted preoperatively. The repair can easily be approached laparoscopically in most cases. The trocar positions may differ from the original procedure due to the location of the recurrence. One should uncover the entire prosthesis, unless it is impossible to do so due to adhesions or bleeding. The repair can be affected with an additional biomaterial with the same fixation (including sutures). We prefer a larger overlap and more fixation for a recurrence. It must be remembered that if a prior ePTFE-based material was used, the patch-to-patch interface will not achieve any ingrowth of tissue. This barrier must be fenestrated to allow for tissue penetration into the new patch, and the new prosthesis should cover all of the old prosthesis if possible. Transfascial sutures should be used in all of these cases. The removal of the previously placed mesh is not recommended.
CONCLUSION
LIVH is a very successful procedure that is gaining in popularity and is very likely to become the standard of care in the future. The majority of repairs are performed with the use of an ePTFE patch, transfascial sutures, and additional metal or absorbable fixation devices. These methods decrease the rate of recurrence. The most difficult portion of the operation is the lysis of adhesions that can result in an enterotomy. Recognition of this and other complications can only be assured with a high index of suspicion and appropriate radiographic and laboratory testing, which is then followed by the appropriate treatment. Despite the most stringent care and abilities, complications cannot always be avoided. The proper management of these complications when they occur is, therefore, of critical importance.
References
- LeBlanc KA, Booth, WV. Laparoscopic repair of incisional abdominal hernias using expanded polytetrafluoroethylene: preliminary findings. Surg Laparosc Endosc. 1993;3:39-41.
- LeBlanc KA, Bellanger DE. Laparoscopic repair of para-ostomy hernias. J Am Coll Surg. 2002;194(2):232-239.
- Deol ZK, Shayani V. Laparoscopic parastomal hernia repair. Arch Surg. 2003;138:203-205.
- Hirasa T, Pickleman J, Shayani V. Laparoscopic repair of parapubic hernia. Arch Surg. 2001;136:1314-1317.
- Elieson, MJ, LeBlanc KA. Enterotomy and mortality rates of laparoscopic incisional and ventral hernia repair: a review of the literature. 2007;11:408-414.
- Heniford BT, Park A, Ramshaw BJ, Voeller G. Laparoscopic repair of ventral hernias: nine years’ experience with 850 consecutive hernias. Ann Surg. 2003;238(3):391-400.
- LeBlanc KA. Laparoscopic incisional hernia repair: are transfascial sutures necessary? A review of the literature. Surg Endosc. 2007;21:508-513.
- Leber GE, Garb JL, Alexander AI, Reed WP. Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg. 1998;133:378-382.
- White TJ, Santos MC, Thompson JS. Factors affecting wound complications in repair of ventral hernias. Am Surg. 1998;64(3):276-280.
- DeMaria EJ, Moss JM, Surgerman HJ. Laparoscopic intraperitoneal polytetrafluoroethylene (PTFE) prosthetic patch repair of ventral hernia. Surg Endosc. 2000;14:326-329.
- Robbins SB, Pofahl W, Gonzales RP. Laparoscopic ventral hernia repair reduces wound complications. Am Surg. 2001;9:896-900.
- LeBlanc KA, Whitaker JM, Bellanger DE, Rhynes VK. Laparoscopic incisional and ventral hernioplasty: lessons learned from 200 patients. Hernia. 2003;7(3):118-124.
- Ramshaw BJ, Esartia P, Schwab J, et al. Comparison of laparoscopic and open ventral herniorrhaphy. Am Surg. 1999;65:827-832.
- Park A, Birch DW, Lovrics P. Laparoscopic and open incisional hernia repair: a comparison study. Surgery. 1998;124(4):816-822.
- Carbajo MA, Martin del Olmo JC, Blanco JI, et al. Laparoscopic treatment vs. open surgery in the solution of major incisional and abdominal wall hernias with mesh. Surg Endosc. 1999;13:250-252.
- Koehler RH, Voeller G. Recurrences in laparoscopic incisional hernia repairs: a personal series and review of the literature. JSLS. 1999;3:293-304.
- Chari R, Chari V, Eisenstat M, Chung R. A case controlled study of laparoscopic incisional hernia repair. Surg Endosc. 2000;14:117-119.
- Berger D, Bientzle M, Müller A. Postoperative complications after laparoscopic incisional hernia repair. Surg Endosc. 2002;16:1720-1723.
- Bageacu S, Blanc P, Breton C, et al. Laparoscopic repair of incisional hernias. Surg Endosc. 2002;16:345-348.
- Carbajo MA, Martín del Olmo JC, Blanco JI, et al. Laparoscopic approach to incisional hernia. Surg Endosc. 2003;17:118-122.
- Rosen M, Brody F, Ponsky J, et al. Recurrence after laparoscopic ventral hernia repair. Surg Endosc. 2003;17:123-128.
- LeBlanc KA, Booth WV, Whitaker JM, Bellanger DE. Laparoscopic incisional and ventral herniorrhaphy in 100 patients. Am J Surg. 2000;180 (3):193-197.
- Heniford TB, Park A, Ramshaw BJ, Voeller G. Laparoscopic ventral and incisional hernia repair in 407 patients. J Am Coll Surg. 2000;190(6):645-650.
- Voeller G. Laparoscopic Repair in the Emergent Setting, in Laparoscopic Hernia Surgery: An Operative Guide. LeBlanc K, ed. London, England: Arnold Medical Publishers; 2003;111-113.
- Moreno-Egea A, Castillo Bustos JA, Aguayo JI. Day surgery for laparoscopic repair of abdominal wall hernias. Hernia. 2002;6:21-25.
- Feingold DL, Widmann WD, Calhoun SK, et al. Persistent post-laparoscopy pneumoperitoneum. Surg Endosc. 2003;17:296-299.
- Toy FK, Bailey RW, Carey S, CW, et al. Prospective, multicenter study of laparoscopic ventral hernioplasty. Surg Endosc. 1998;12:955-959.
- Holzman MD, Purut CM, Reintgen K, Eubanks S, Pappas TN. Laparoscopic ventral and incisional hernioplasty. Surg Endosc. 1997;11:32-35.
- Farooqui MO, Bazzoli JM. Significance of radiologic evidence of free air following laparoscopy. J Reprod Med. 1976;16(3):119-125.
- Susmallian S, Gewurtz G, Ezri T, Charuzi Seroma after laparoscopic repair of hernia with PTFE patch: is it really a complication? Hernia. 2001;5:139-141.
- Parker HH, Nottingham JM, Bynoc RP, Yost MJ. Laparoscopic repair of large incisional hernias. Am Surg. 2002;68(6):530-534.
- Kirshtein B, Lantsberg L, Avinoach E, et al. Laparoscopic repair of large incisional hernias. Surg Endosc. 2002;16:1717-1719.
- Lin BHJ, Vargish T, Dachman AH. CT findings after laparoscopic repair of ventral hernias. Am J Rad. 1999;172:389-392.
- Chowbey PK, Sharma A, Khullar R, Baijal, Vashistha A. Laparoscopic ventral hernia repair. J Laparoendo Adv Surg Tech. 2000;10(2):79-84.
- Gillian GK, Geis WP, Grover G. Laparoscopic incisional and ventral hernia repair (LIVH): an evolving outpatient technique. JSLS. 2002;6:315-322.
- Tsimoyiannis EC, Siakas P, Glantzouissis G, et al. Seroma in laparoscopic ventral hernioplasty. Surg Laparosc Endosc Percut Tech. 2001;11(5):31-321.
- Park A, Heniford BT, LeBlanc KA, Voeller GR. Laparoscopic repair of incisional hernias. Part 2: Surgical technique. Contemp Surg. 2001;57(5):225-238.
- Carbonell AM, Harold KL, Mahmutovic AJ, et al. Local injection for the treatment of suture site pain after laparoscopic ventral hernia repair. Am Surg. 2003;69(8):688-692.
- Franklin ME, Dorman JP, Glass JL, Balli JE, Gonzales JJ. Laparoscopic ventral and incisional hernia repair. Surg Laparosc Endosc. 1998;8(4):294-299.
- Wright BE, Niskanen BD, Peterson DJ, et al. Laparoscopic ventral hernia repair: are there comparative advantages over traditional methods of repair? Am Surg. 2002;68(3):291-296.
- Paton BL, Novitsky YW, Sing RF, Kercher KW, Heniford BT. Management of infections of ePTFE-based mesh. Surg Infect (Larchmt). 2007;8(3):337-341.
- Ben-Haim M, Kuriansky J, Tal R, et al. Pitfalls and complications with laparoscopic intraperitoneal expanded polytetrafluoroethylene patch repair of postoperative ventral hernia. Surg Endosc. 2002;16:785-788.
- LeBlanc KA. Current considerations in laparoscopic incisional and ventral herniorrhaphy. JSLS. 2000;4:131-139.
- LeBlanc KA. The critical technical aspects of laparoscopic repair of ventral and incisional hernias. Am Surg. 2001;67(8):809-812.
- Roth JS, Park AE, Witzke D, Mastrangelo MJ. Laparoscopic incisional/ventral herniorrhaphy: a five-year experience. Hernia. 1999;4:209-214.
- Aura T, Habib E, Mekkaoui M, et al. Laparoscopic tension-free repair of anterior abdominal incisional and ventral hernias with an intraperitoneal Gore-Tex® mesh: prospective study and review of the literature. J Laparoendo Adv Surg Tech. 2002;12(4):263-267.
- Heniford BT, Ramshaw BJ. Laparoscopic ventral hernia repair. Surg Endosc. 2000;14:419-423.
- Hesselink VJ, Luijendijk RW, de Wilt JHW, Heide R, Jeekel J. An evaluation of risk factors in incisional hernia recurrence. Surg Gynecol Obstet. 1993;176:228-234.
- Thoman DS, Phillips EH. Current status of laparoscopic ventral hernia repair. Surg Endosc. 2002;16:939-942.
- van’t Riet M, de Vos van Steinwijk PJ, Kleiniensink GJ, et al. Tensile strength of mesh fixation methods in laparoscopic incisional hernia repair. Surg Endosc. 2002;16:1713-1716.
