Complications of Laparoscopic Inguinal Hernia Repair
Edward L. Felix, MD, FACS
INTRODUCTION
High morbidity and recurrence rates as well as prolonged recovery have led to a gradual evolution in inguinal hernia repair. Bassini1 began the era of modern hernia repair more than 100 years ago by proposing an anatomical approach to inguinal hernia repair, which radically reduced recurrence rates. McVay2 and later Shouldice3 introduced further modifications of the anatomical approach to improve on Bassini’s technique. Next, mesh- reinforced repairs were introduced to further decrease the incidence of failure.
Lichtenstein et al4 championed an anterior approach that incorporated a tension-free repair with mesh, while Nyhus et al5 and Stoppa et al6 introduced a posterior repair. In 1990, laparoscopic surgeons took the posterior mesh repair one step further, utilizing modern laparoscopic instrumentation to reduce morbidity and recurrence rates seen with conventional open hernia repairs. At first, surgeons tried simple approaches like Ger’s7 closure of the internal ring and Shultz’s8 plug and patch, but these failed to improve on the results of open repairs. When surgeons realized that laparoscopic repairs had to mimic the open posterior repairs of Nyhus and Stoppa, results dramatically improved.
Although new laparoscopic approaches were developed to reduce complications seen with open repairs, a whole new set of problems, as well as some of the old ones, were experienced by patients. It was only when the laparoscopic surgeon completely understood the possible complications inherent to the laparoscopic repair and the causes of these complications that postoperative morbidity could be decreased and the surgeon could handle the complications that cannot be avoided.
In multi-institutional reviews and single-center studies,9-14 complication rates after laparoscopic hernioplasty vary from 5% to 13%, but the definition of complication differs widely among studies. The incidence of major complications (approximately 1% for experienced surgeons), however, is consistent across these large studies and is similar to that reported for open hernia repairs. The purpose of the present review is not to give a detailed summary of the literature, but rather to explain why the most common and important complications occur and how they might be prevented or handled if they do develop.
COMPLICATIONS RELATED TO ANATOMY
Performing a successful laparoscopic hernioplasty requires a thorough knowledge of the anatomy of the pelvis and groin as viewed through the laparoscope. For many surgeons, this exposure is totally foreign. If the surgeon is ill prepared to approach the groin laparoscopically, he or she may become lost and totally overlook an obvious hernia. The result is an immediate failure of the repair. Worse, however, is the situation in which the surgeon begins the dissection of the posterior floor, but is unable to properly identify the anatomical structures, which results in an injury to the iliac vein, bladder, cord structures, or intestine. These complications can be avoided in almost all cases by understanding the normal anatomy. This can be accomplished by reviewing recordings of dissections performed by other experienced laparoscopic surgeons. Before undertaking one’s first laparoscopic hernia repair, it is essential that the surgeon assist others as part of the educational process.
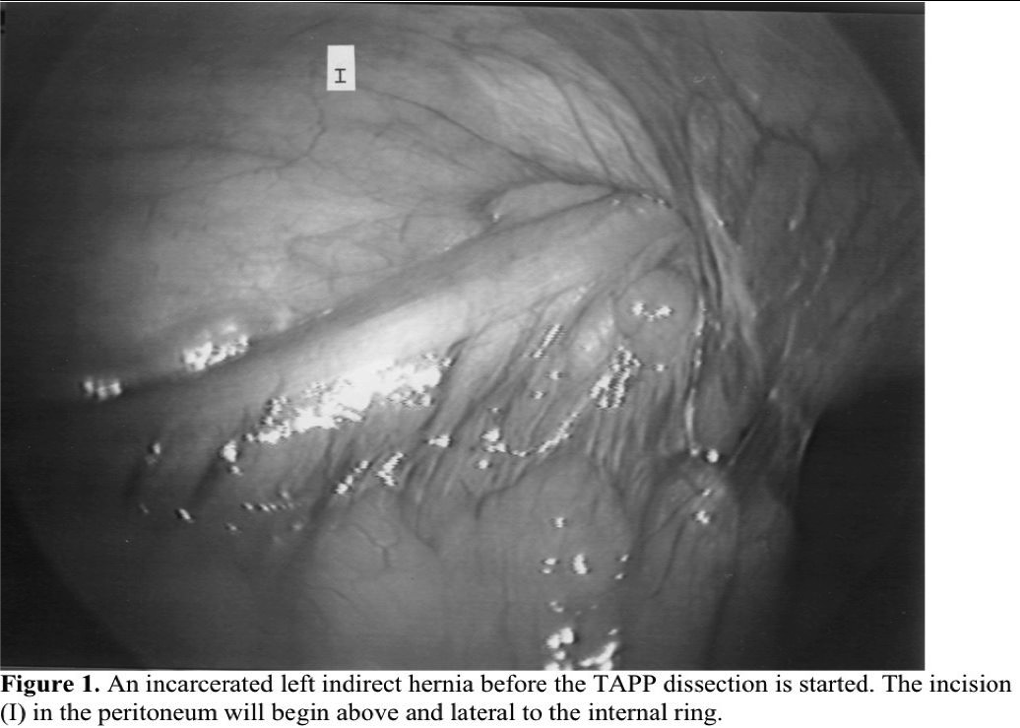
In some cases, a complex recurrent or incarcerated hernia can mask the normal anatomy. In these instances, the surgeon should begin dissecting from an area of normal anatomy and slowly dissect away the tissue so the landmarks can be identified. In the totally extraperitoneal approach, the pubis and Cooper’s ligament will lead the surgeon to the iliac vein and inferior epigastric vessels, important guideposts to the repair. If an incarcerated femoral hernia is present, however, the iliac vein will be hidden behind the incarcerated sac, which must be reduced before the vein can be visualized. Knowing where the vein is located should prevent inadvertent injury and sudden blood loss. If a direct hernia is incarcerated, the inferior epigastric vessels may not be visible until the sac is reduced. The direct sac, however, should not be ligated because the tip of the bladder may make up part of the hernia sac and will be injured in the process. The indirect sac, if present, is lateral to the inferior epigastric vessels, and identification of these vessels is essential. The surgeon must dissect this lateral tissue away from the abdominal wall to identify the peritoneum and sac, as well as the cord structures. Unlike the transabdominal preperitoneal approach (TAPP) in which the indirect sac is obvious, in the totally extraperitoneal approach the indirect space and the lateral aspect of the spermatic cord must be dissected and identified by the surgeon so as not to overlook an indirect hernia.
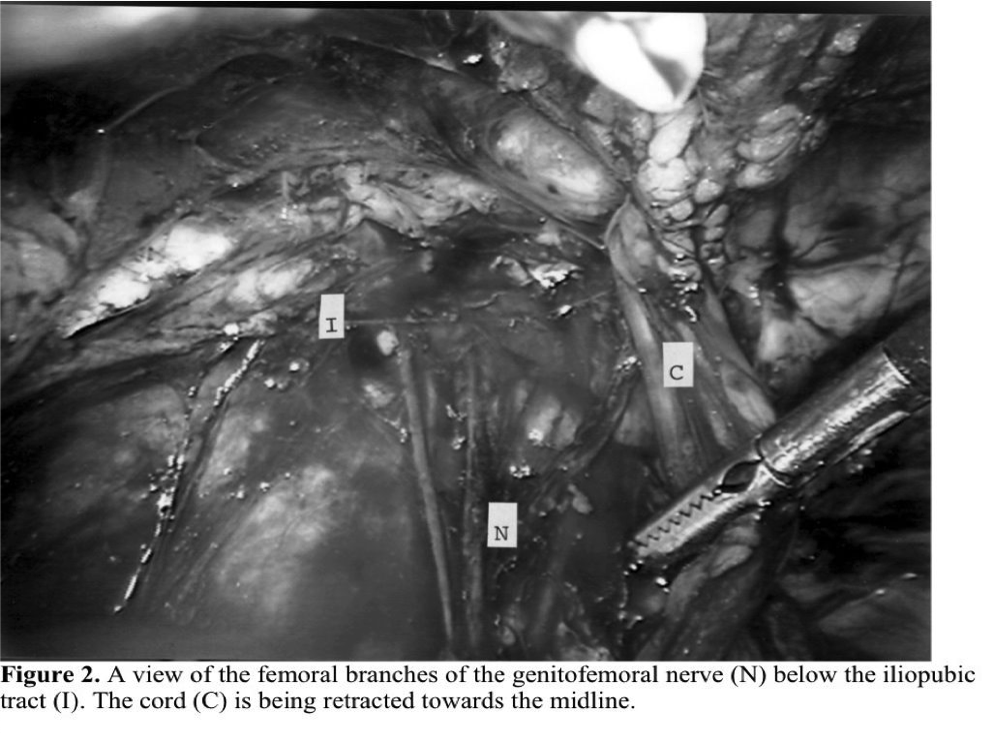
In the transabdominal prepertitoneal approach, the anatomy of the groin is usually more easily understood, but even in this approach, a complicated incarcerated or sliding hernia can cause confusion (Figure 1). By starting the dissection of the peritoneum above and lateral to the internal ring, the bowel can usually be peeled off the cord. If the sac is too long or adherent to the testicular vessels and vas deferens, it can be opened from lateral to medial, watching for the cord structures on the inferior medial aspect of the sac. The most common complication due to a lack of understanding of the anatomy or respect for the location of the normal structures is injury to the sensory nerves: the femoral branch of the genito-femoral nerve, the lateral cutaneous nerve, or the femoral nerve itself.15 Cauterization, transection, or entrapment of the nerves can usually be avoided if the surgeon is respectful of the tissue below the iliopubic tract. The tract can be identified visually as a fibrous band at the lower edge of the internal ring or manually by placing one’s hand on the abdominal wall and palpating an instrument placed laparoscopically at the level of the iliopubic tract. If the surgeon cannot feel the instrument, the point of contact of the probe is below the iliopubic tract. Because the nerves usually enter the thigh below this line, placing fixation above the iliopubic tract reduces the chance of injuring the nerves. A German16 cadaver study of the anatomy of the posterior groin, however, showed that in 15% of patients the nerves enter above the iliopubic tract. This makes the nerves at risk for injury even if staples or tacks are properly placed. For this reason, more and more surgeons have moved toward mesh repairs that do not require fixation or have chosen adhesive fixation.
If severe pain presents immediately after surgery in the distribution of a major named nerve (Figure 2), the surgeon should explore the posterior wall laparoscopically, looking for the offending staple to remove it. Pain presenting days or weeks after surgery is usually transient and due to irritation of the nerves rather than entrapment. Only on a rare occasion will surgical re-exploration be necessary for this situation and reoperation should only be suggested if time and other measures, such as anti-inflammatory medication, have not helped. If pain persists despite conservative measures and the pain is isolated to a specific point on the abdominal wall or the distribution of a specific nerve, exploration is warranted. We have seen symptoms relieved as long as 6 months after the initial repair after removal of a tack or irritating mesh.
COMPLICATIONS RELATED TO TECHNIQUE
Small-Bowel Obstruction
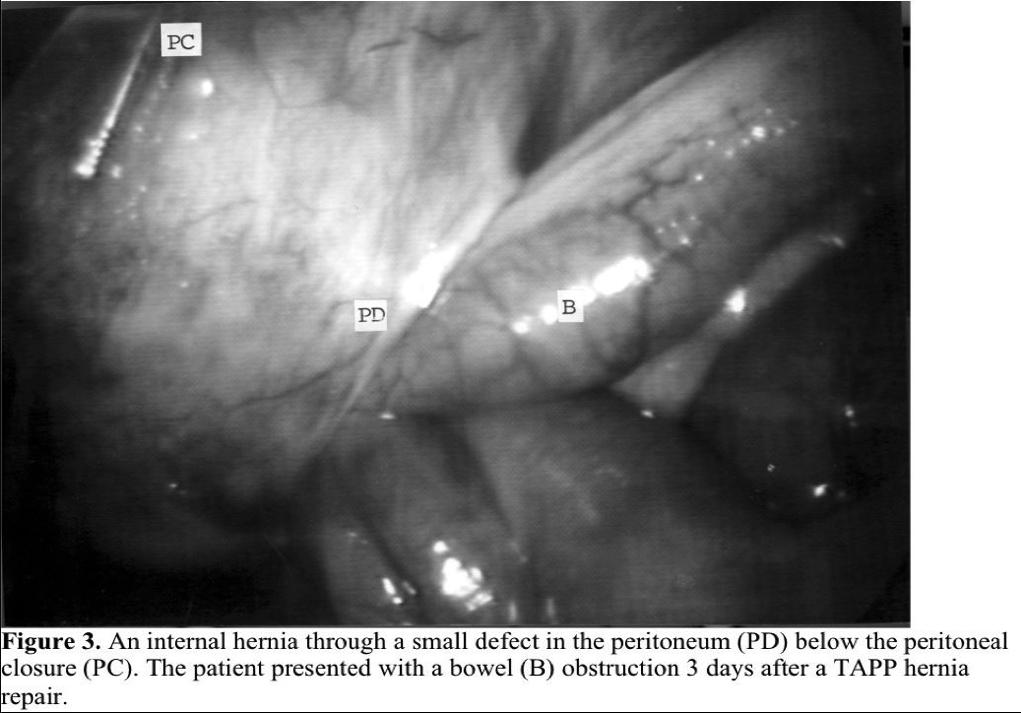
Small-bowel obstruction after a laparoscopic hernia repair can occur. It is the result of adhesions to inadequately covered mesh or due to intestines being entrapped in a defect left in the peritoneum. Exposed mesh with either the TAPP or the totally extraperitoneal hernia repair (TEP) is rare, if the surgeon mobilizes the peritoneum to fully cover the mesh. Adhesions to spiral tacks used to close the peritoneum during TAPP repairs do occur, but can be avoided by suturing the peritoneal defect instead of tacking it. If the peritoneum is inadequately closed after a TAPP repair, an internal hernia can occur in the defect in the peritoneum.17 Whether staples or sutures are used to close the peritoneum, gaps cannot be left in the peritoneal closure. If a defect in the peritoneum is left after a TEP repair, rarely the bowel can become incarcerated (Figure 3).18 A potential space is created between the peritoneum and the abdominal wall by the dissection of the posterior floor and by the CO2 gas. Bowel may become obstructed at the entrance to this space.
Trocar Hernia
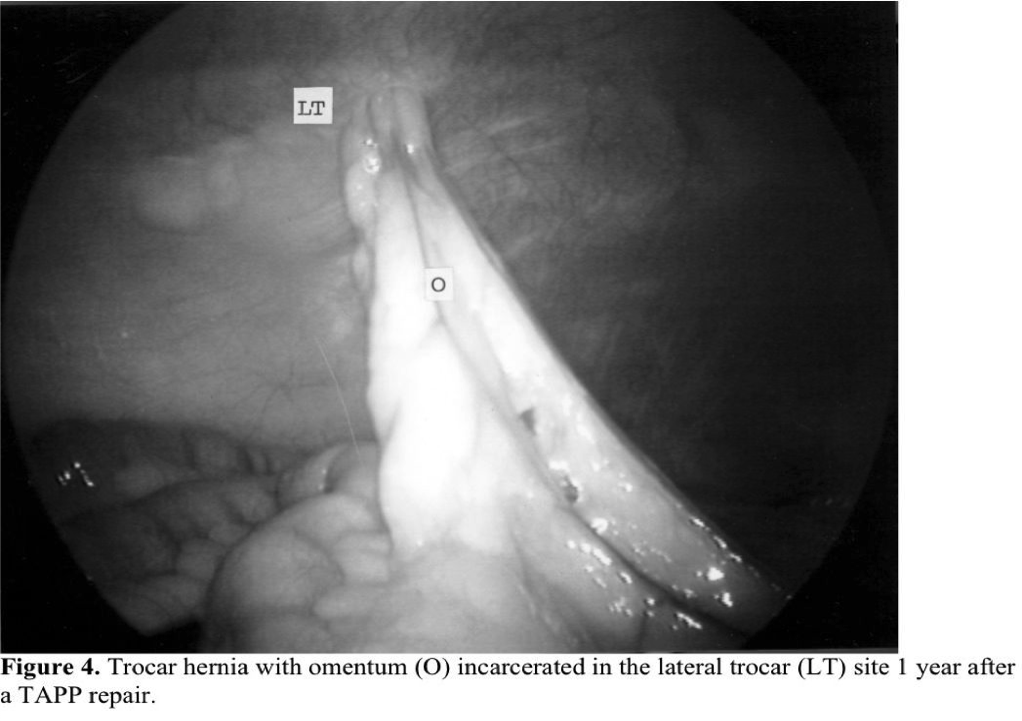
A new complication after inguinal hernia repair, the late development of a trocar hernia (Figure 4), has been seen after the TAPP approach. They may be Richter hernias or typical incarcerated hernias. They usually occur in ports that are ≥10mm in size but have been reported in 5-mm ports.19 The closure of lateral trocar sites was difficult in the past, and a significant number of trocar hernias have been reported in trocar sites ≥10mm that were made with a cutting trocar. Now several techniques have been developed to assist in closing these puncture sites. In addition, the use of 5-mm lateral trocars and noncutting 10-mm trocars has almost eliminated trocar hernias. Trocar hernias almost never occur after TEP repairs, which have gained in popularity over the last 10 years.
Hemorrhage
Bleeding can occur during any operation, but it is especially troublesome during laparoscopic hernioplasty. Because of limited access to the bleeding site and high flow rates of those vessels most likely to be injured, bleeding must be controlled quickly or avoided completely. The location of the inferior epigastric vessels may vary, and injury to these vessels is the most common cause of bleeding. Therefore, the surgeon must stay lateral to the rectus muscles to avoid injuring them when placing the lateral trocars in the TAPP approach. If hemorrhage results following trocar insertion, it can be controlled with a U-stitch made with a suture passer. A surprisingly large amount of blood can be lost if these vessels are not controlled immediately. Remember that the arterial supply comes from the caudal aspect of the inferior epigastrium. In the TEP approach, the expansion of the balloon dissector may pull the inferior epigastric vessels away from the abdominal wall, tearing small branches and filling the potential space with blood. If the surgeon is confronted with a darkened, bloody field when the camera is reinserted, the light intensity should be immediately increased and the 2 midline trocars placed so that the extraperitoneal space can be irrigated to allow the surgeon to find the source of bleeding. Bleeding from the small branches can also occur 1cm to 2cm from the internal ring, if the surgeon mobilizes the inferior epigastric vessels to place the mesh in front of the vessels. Hemoclips, Endoloops, or bipolar cautery will usually handle the problem once it is identified.
Bleeding from the inferior epigastric vessels may also result from dissection of the hernia sac. It can usually be easily controlled by utilizing a 2-handed technique, one instrument to compress the vessel and the other to ligate, clip, or coagulate it. In contrast, hemorrhage from the iliac vein is a life-threatening complication if it occurs. If bleeding from the vein is present, the vein must be immediately compressed to control the hemorrhage and prevent CO2 embolus. While the vein is compressed, a third port can be added to suture ligate the hole, or the procedure can be converted to an open repair to control the bleeding point. The decision to open must be made quickly if the bleeding cannot be controlled laparoscopically, and compression of the bleeding site must be maintained with a laparoscopic instrument while opening.
Spermatic Cord Injury
Injury to the vas deferens and testicular vessels are well-known complications of open hernia repair. Although uncommon after laparoscopic hernioplasty, when these complications do develop, they are quite disturbing to the patient and the surgeon.
Complications can usually be avoided by proper identification of the cord structures before any longitudinal structures are cut and by proper handling of the vas deferens and testicular vessels with atraumatic instruments. Pain in the scrotum from irritation of the genital branch of the genito-femoral nerve is seen in a small percentage of patients after laparoscopic dissection of the posterior floor. It has also been reported as a delayed complication in some patients, possibly due to compression or irritation of the nerve secondary to fibrosis around the cord. Fortunately, in most cases, the scrotal discomfort disappears with time. Ischemic orchitis, which has been reported to occur with open hernia repair in from 3% to 5% of repairs,20 is extremely rare after a laparoscopic approach.
COMPLICATIONS INHERENT TO THE REPAIR
Some complications of surgery are inherent to the procedures themselves, and the surgeons must be able to recognize them. Seromas or hematomas, for example, are not unusual after the repair of large direct or scrotal hernias with the laparoscopic technique. Fluid accumulates in the dead space left by the hernia in 2% to 10% of patients. If left alone, the seroma spontaneously resolves in 90% patients. If, however, the seroma is large or symptomatic, a single aspiration usually eliminates the problem. In an occasional patient, repeated aspirations are necessary, or even the use of an external drainage catheter. Drains at the time of surgery are not necessary and may even increase infection rates. Although the seroma may at first appear to be an immediate recurrence of the hernia, by careful examination one is able to recognize the problem as fluid collection. It is usually movable over the abdominal wall, but if there is any question, an ultrasound can be performed. From months to years after a laparoscopic hernioplasty, a hydrocele may form in the scrotum. The incidence of a hydrocele after laparoscopic hernioplasty is approximately equal to that after open hernia repair (<1%)21 and may be related to lymphatic obstruction. It does not appear to be correlated to the incidence of previous seroma.
Urinary retention is seen in approximately 2% of patients after open or laparoscopic hernioplasty and is probably related to the group of patients undergoing the procedure and the technique itself.22 Preoperative placement of a catheter may actually increase the incidence of retention and is avoided unless one feels that the bladder has not been emptied preoperatively or that the length of the procedure may be extended. In the small number of patients that have developed urinary retention, it can usually be managed on an outpatient basis.
DELAYED COMPLICATIONS
Mesh and Fixation Complications
The use of mesh in inguinal hernia repairs is now well established and accepted. In fact, the majority of repairs today, open or laparoscopic, are performed with some sort of mesh tension-free repair. It is extremely rare for mesh to become primarily infected or cause problems if it is completely extraperitoneal. There have been, however, isolated incidences when the extraperitoneal mesh has become secondarily infected by a ruptured appendix or perforated diverticulum of the colon. In these cases, the patient can present with an indolent chronic infection years after the repair that is difficult to eradicate without removing the mesh. A biologic mesh may be substituted for the original mesh if the resulting defect is a concern. Cases in which the mesh has eroded into the bladder have also been seen. They can usually be treated by removing the foreign body and draining the bladder. Spiral tacks that have been used to close the peritoneum have also been reported to erode in adjacent viscera.
CONCLUSION
Unfortunately, it will never be possible to completely eliminate complications after laparoscopic hernioplasty, but it is possible to reduce their incidence. A thorough knowledge of the anatomy and the operative approach, along with advanced laparoscopic skills will reduce the chance of a significant complication. When a complication does occur, the well-trained surgical laparoscopist will be prepared to reduce its morbidity by taking the appropriate and sometimes immediate measures. In any operation, the surgeon’s location on the learning curve plays an important role in the outcome of the procedure. To reduce the deleterious effects of this curve, surgeons must make every effort to work with others experienced in the procedure before venturing out on their own. There is no reason why an individual surgeon’s results should not mimic the results achieved by centers specializing in laparoscopic hernia repair.23-25
References
- Bassini E. Nuovo metodo sulla cura radicale dell’ ernia inguinale. Arch Soc Ital Chir. 1887:4:80.
- McVay CB. The anatomic basis for inguinal and femoral hernioplasty. Surg Gynecol Obstet. 1974;139:931-933.
- Shouldice EE. The treatment of hernia. Ontario Med Rev. 1953;20:670-684.
- Lichtenstein IL, Shulman A, Amid P, Montilor N. The tension-free hernioplasty. Am J Surg. 1989;157:188-193.
- Nyhus LM, Pollak R, Bombeck CT, Donohue P. The preperitoneal approach and prosthetic buttress repair for recurrent hernia. Ann Surg. 1988;208:722-727.
- Stoppa R, Rives JL, Walamount C, Palot JP, Verhaege PJ, Delattre JF.The use of Dacron in the repair of hernias of the groin. Surg Clin North Am. 1984;64(2):269-285.
- Ger R, Monroe R, Mishrick A. Management of indirect hernias by laparoscopic closure of the neck of the sac. Am J Surg. 1990;159:371-373.
- Schultz L, Graber J, Pietrafitta J, Hickok D. Laser laparoscopic herniorrhaphy: a clinical trial preliminary results. J Laparoendosc Surg. 1990;1:41-45.
- Phillips E, Arregui M, Carrol J, et al. Incidence of complications following laparoscopic hernioplasty. Surg Endosc. 1995;9:16- 21.
- Tetik C, Arregui ME, Dulucq JL, et al. Complications and recurrences associated with laparoscopic repair of groin hernias. A multi-institutional retrospective analysis. Surg Endosc. 1994;8:1316-1323.
- Millikan K, Kosik M, Doolas A.A prospective comparison of transabdominal preperitoneal laparoscopic hernia repair versus traditional open hernia repair in a university setting. Surg Laparosc Endosc.1994;4(4):247-253.
- MacFadyen B, Arregui M, Corbitt J, et al. Complications of laparoscopic herniorrhaphy. Surg Endosc. 1993;7:155-158.
- Fitzgibbons R, Camps J, Comet D, et al. Laparoscopic inguinal herniorrhaphy results of a multicenter trial. Ann Surg. 1995;221:3-113.
- Payne J. Complications of laparoscopic inguinal herniorrhaphy. Semin Laparosc Surg. 1997;4(3):166-181.
- Spaw A, Ennis B, Spaw L. Laparoscopic hernia repair: The anatomic basis. J Laparoendosc Surg. 1991;1:269-277.
- Rosenberger R, Loeweneck H, Meyer G. The cutaneous nerves encountered during laparoscopic repair of inguinal: new anatomical findings for the surgeon. Surg Endosc. 2000;8:731-735.
- Felix EL, Michas CA, Gonzalez JR. Laparoscopic hernioplasty Tapp vs Tep. Surg Endosc. 1995;9:984-989.
- Azur D, Schuricht A, Stoldt S, Kirkland M, Paskin D, Bar A. Small bowel obstruction following endoscopic extraperitonealpreperitoneal herniorrhaphy. J Laparoendosc Surg. 1995;5(4):263-
- Plaus W. Laparoscopic trocar site hernias. J Laparoendosc Surg. 1993;3(6):567-570.
- Wantz EG. Complications of inguinal hernia repair. Surg Clin North Am. 1984;64(2):287-298.
- Obney N. Hydroceles of the testicle complicating inguinal hernias. Can Med Assoc J. 1956;75:733-736.
- Miguel PR, Reusch M, DaRosa ALM, Carlos JR. Laparoscopic hernia repair – complications. JSLS. 1998:2:35-40.
- Ramshaw B, Tucker J, Conner T, et al. A Comparison of approaches to laparoscopic herniorrhaphy. Surg Endosc. 1996; 10:29-32.
- Felix EL, Scott S, Crafton B, et al. Causes of recurrence after laparoscopic hernioplasty: a multicenter study. Surg Endosc. 1998;12:226-231.
- Leibl B, Schmidt J, Daubler P, Kraft K. A single institution’s experience with transperitoneal laparoscopic hernia repair. Am J Surg. 1998;175:446-452.
