Laparoscopy for Emergent Abdominal Surgical Procedures
W. Peter Geis, MD, Udayan Shah, MD
INTRODUCTION
Acute care surgery has evolved since the 1950s (and following the Korean War experiences), creating a model identifying the Trauma Surgeon and the Trauma Team. Thereafter, in the 1960s, Freeark and Baker designed the first Trauma Center and the first Blood Bank at Cook County Hospital (Chicago). The system focused a subset of surgeons, emergency department personnel, and critical care personnel on an organized approach to the assessment and early life-saving treatment of trauma victims. This process configured the first trauma centers and the roles of various personnel. Further, prehospital paramedical personnel were educated to assess and treat life-threatening injuries of airway, breathing, and circulatory insults. Early patient stabilization allowed these early trauma surgeons to ensure stabilization of the patient and transport of the patients quickly to the operating room for definitive operative care as needed. During the ensuing 20 years (approximately 1970 to 1990), trauma physicians and their teams have subspecialized within the hospital setting to include (1) early hospital life-saving stabilization, diagnosis, and categorization of patients (emergency physicians and their teams); (2) surgeons providing early definitive operative and perioperative care of the patient (trauma surgeons and their teams); and (3) surgeons providing intermediate-term stabilizing and ongoing care for these patients (critical care trauma surgeons).
These subspecialties have been in flux, and overlap, as to their limits of specialization for a variety of reasons, including evolution of the trauma surgeon to combined roles as (1) trauma surgeon, (2) acute care emergency surgeon (nontrauma), and (3) critical care/ICU specialist, in the hospital.
In 1990, however, a little-known alteration in the fundamental approach to the treatment of patients with abdomino-pelvic surgical operative disease rapidly infiltrated the abdominal surgery armamentarium–that being, laparoscopy. Clearly, laparoscopy in the past 20 years (1990 to 2010) has not influxed deeply into acute abdominal emergency surgery or abdominal operative trauma. However, there has been a gradual influx by a variety of investigators to provide laparoscopy as a diagnostic or therapeutic modality, or both together, for acute abdominal surgical emergencies,1 as well as for acute abdominal trauma. This author presented a video at the American College of Surgeons meeting in 1993 on the “safe initial assessment of the abdomen of the trauma victim” and described one of the early analyses of the use of laparoscopy as a diagnostic and therapeutic tool in the definitive care of patients with acute appendicitis.2
Since the early 1990s, many acute care abdominal surgeons have ventured into the use of laparoscopy as a diagnostic and/or therapeutic tool (actually a set of skills and instruments) to safely optimize operative abdominal surgical care for patients.1 However, neither in acute care surgery nor in surgery for abdominal-thoracic trauma have the skills and benefits, or the risks, of laparoscopic surgery been addressed as diagnostic and therapeutic tools (skills) in a systematic fashion. The ensuing chapter is a first attempt at this structured approach.
MINIMALLY INVASIVE SURGERY IN ACUTE ABDOMINAL SURGICAL EMERGENCIES
Pathophysiologies, Anatomic Alterations
Entry into the abdomen laparoscopically in circumstances of acute abdominal emergencies has both diagnostic and often therapeutic benefits. If the cause of the disease is obscure (examples are free air in the abdominal cavity and physical signs of peritonitis), the benefits include (1) diagnosis of the cause of the perforation (Table 1), and (2) identification of its location in the abdomen (Table 1). Notably, the laparoscopic skills required to identify the disease and its location, using this example, include dealing with the following secondary pathologies (Table 2): (1) distended small bowel (ileus), (2) peritoneal phlegmon, (3) fixation (fusion) of tissues and organs to each other by phlegmon, thus obscuring other organs, anatomic landmarks, and the disease itself. In addition, the phlegmon and peritonitis cause a decrease in the distensibility of the abdominal wall in response to carbon dioxide pressure, and the ileus increases the volume of space occupied by the intestine. Both of these physical alterations result in diminished abdominal cavity space (volume) for the laparoscopic surgeon to work in at any given pressure of carbon dioxide in the abdominal insufflation. Each of these impediments to visualization and to identification of the site of disease is combated by the use of specific skills to improve visualization and access to the diseased area and organ (Table 3).
PERFORATED DUODENAL ULCER WITH FREE AIR IN THE ABDOMINAL CAVITY
According to the perforated duodenal ulcer example, skills include “finger-instrument fracture” of phlegmonous adhesions to separate tissues carefully, blunt retraction of inflamed and distended structures, and use of table-tilt (gravity).3
Using this example, once the perforated duodenal ulcer is identified (assuming it to be on the anterior surface), the surgeon has 2 choices, which are based on his or her laparoscopic skill sets. The first option is to laparoscopically close the perforation with or without an omental patch. Numerous authors4-9 have successfully reported this approach. These studies have delineated that the factors that increase the risk of unsuccessful laparoscopic completion of the procedure include (1) age >70 years, (2) >24 hours since the onset of peritoneal symptoms, (3) “large” perforation, (4) gastric perforation, (5) poor technical skills, (6) shock, and (7) misdiagnosis (ie, perforation of another portion of the GI tract). Further, the rate of increase in the percentage of patients successfully treated laparoscopically over a 4-year interval was increased when (1) structured education of the operating room team occurred, (2) increasing experience with laparoscopic treatment of emergencies associated with purulence and phlegmon occurred, and (3) the presence of an experienced mentor in the operating room existed. Overall, 15% were converted to open procedures.
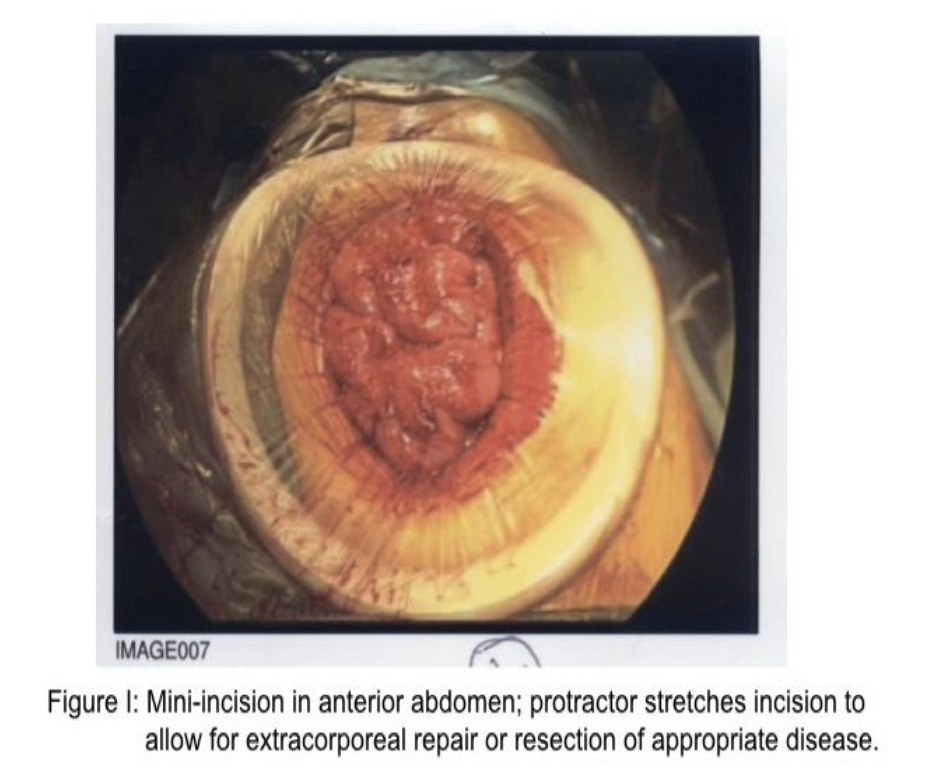
The second adaptation or rescue strategy is to create a mini-incision in the abdominal wall directly anterior to the perforation under direct vision by using a small- or medium- sized protractor/wound protector (Figure 1). The benefit to the patient with either strategy is both diagnosis and definitive treatment, plus the avoidance of cooling of the abdominal cavity and its structures occasioned with a large abdominal incision, diminished risk of prolonged ileus, decreased pain, decreased requirement for wound healing, and decreased risk of wound dehiscence. Clearly, in both circumstances, intense irrigation of the abdominal cavity is appropriate.
PERFORATED DIVERTICULI WITH FREE AIR IN THE ABOMINAL CAVITY
The approach to perforated diverticula with free air in the abdominal cavity is identical to the above-described outline of the approach to a perforated duodenal ulcer. Once the disease is identified, however, 3 choices are available to the laparoscopic surgeon. First, in the absence of gross contamination,10,11 the laparoscopic surgeon can suture the perforation closed either laparoscopically or through a mini-incision. Second, the laparoscopic surgeon can either drain the area or drain plus divert the fecal stream temporarily by using a laparoscopic ileostomy.10,12 Third, the laparoscopic surgeon can resect the sigmoid diverticular segment, perform a transanal laparoscopic anastomosis, and create a laparoscopic diverting ileostomy.13,14 The benefits are the same as the laparoscopic treatment of perforated duodenal ulcer.
PERITONITIS WITHOUT PERFORATED VISCUS
Patients who have signs and symptoms of peritonitis, often associated with a CT scan demonstrating fluid, phlegmon in the abdomen, but without evidence of free air in the abdominal cavity present a specific dilemma as to the cause of the peritonitis. This group of patients has the same abdominal disadvantages (pathophysiologic alterations) for the laparoscopic surgeon as were described in patients with perforated viscus (Table 2). The disadvantages include (1) decreased abdominal space for the surgeon to work in, (2) decreased visualization of anatomic landmarks, and phlegmon “fusing” tissues and organs to each other. The key focus for the surgeon in these cases is to (1) explore the abdomen thoroughly to eliminate the possibility of a hidden perforated viscus and (2) in the absence of perforation, strategic biopsies and cultures should be performed, including studies for tuberculosis.15 Large biopsies may be removed through a strategically placed 10-mm to 12-mm. trocar. Laparoscopic skills are similar to those used in patients with perforated viscus. In addition, the bowel must be evaluated visually to eliminate injury, perforation, or focal disease.
“Running the Bowel”
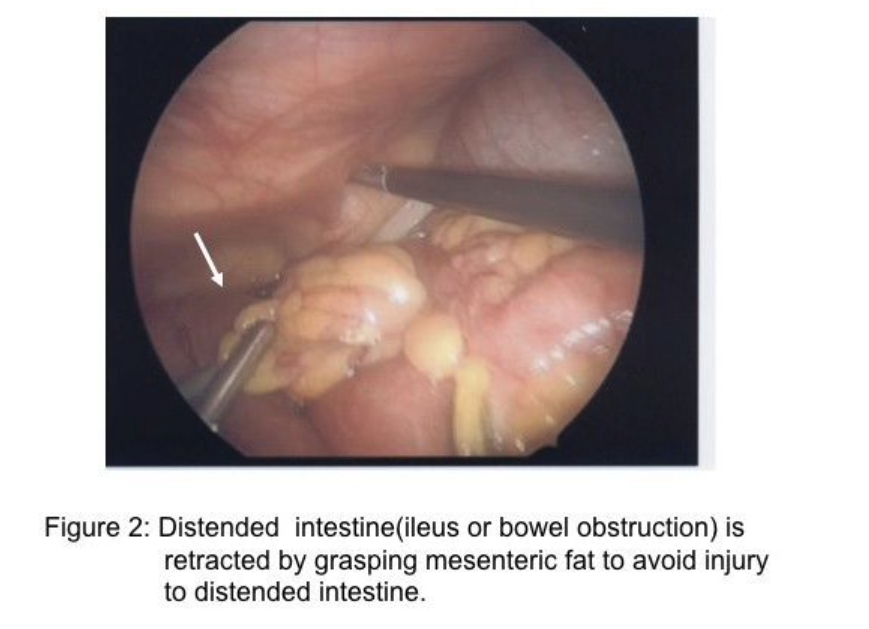
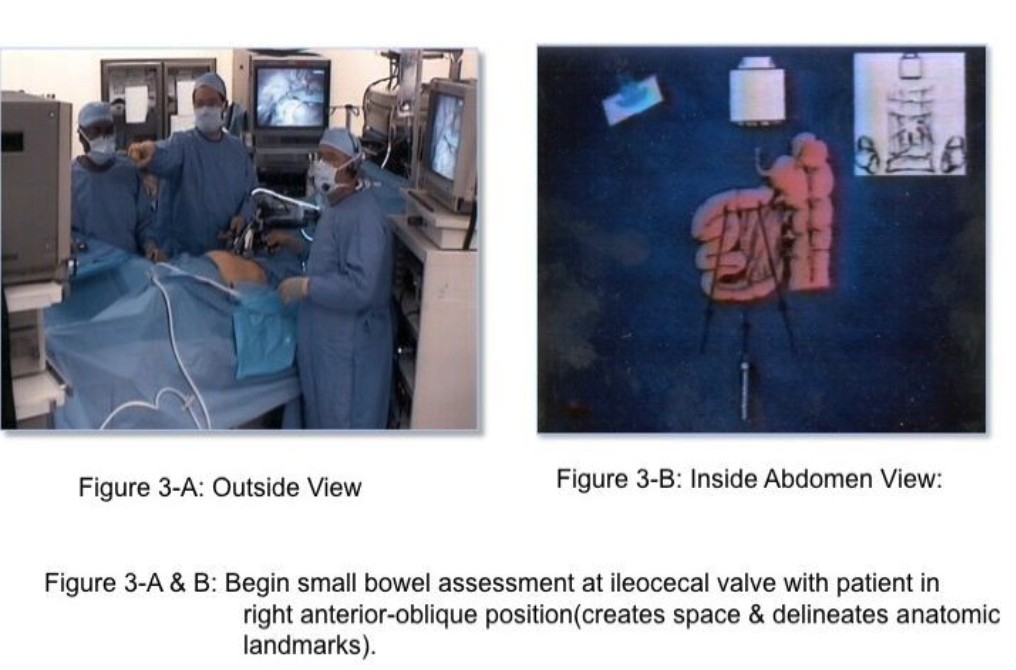
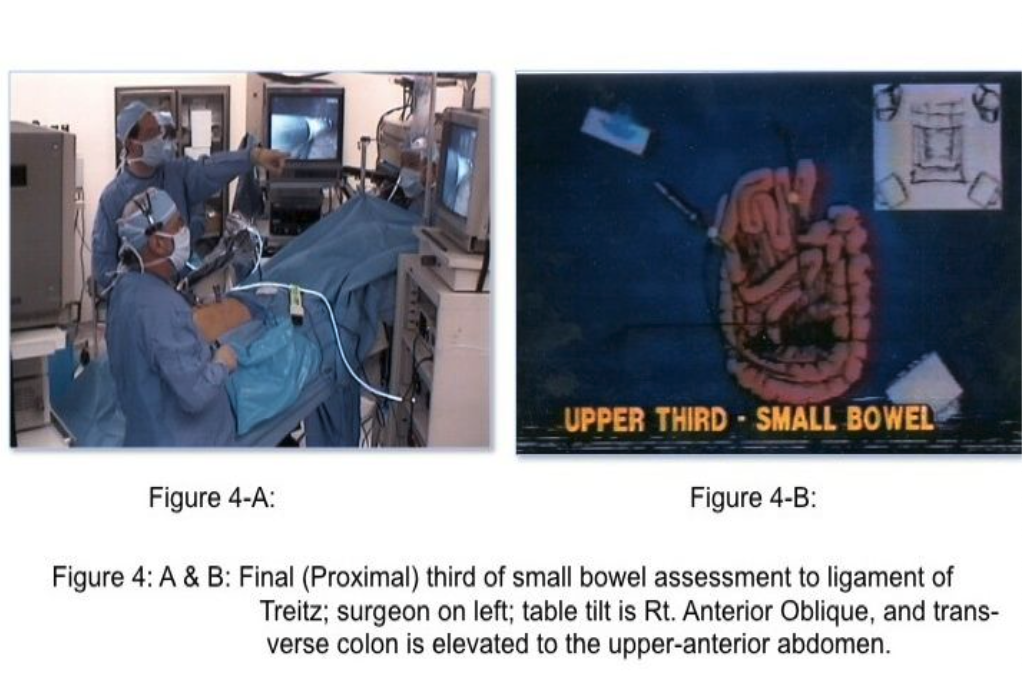
The skill of “running the bowel” laparoscopically requires a combination of activities. First, the best port locations are 3 trocars along the transverse umbilical line: one at the umbilicus, one in the right hemi-abdomen, and one in the left hemi-abdomen. The process should start at either the Ligament of Treitz, or at the ileocecal valve, and proceed to the opposite end in 10-cm increments. The bowel should be grasped with 2 atraumatic graspers, preferably by one surgeon. The graspers should actually grasp the mesenteric fat immediately adjacent to the small intestine and avoid grasping inflamed and/or distended, dilated intestine (Figure 2). The small bowel should be visually divided into 3 parts: the proximal one-third, the middle one-third, and the distal one-third. During evaluation of the distal one-third of the small bowel, the patient should be in a Trendelenburg position with a right anterior oblique table tilt. Most of the small intestine should be in the left upper abdomen. The surgeon stands to the left side of the patient (Figure 3-A) with the monitor near the right foot (Figure 3-B). After each segment of intestine is evaluated, it should be placed in the pelvis. When the pelvis fills with small intestine, the surgeon should diminish the Trendelenburg position to a more neutral position. By the time the surgeon reaches the upper-third of the small bowel, it will be necessary to use a reverse Trendelenburg position with the left anterior oblique table tilt (Figure 4-A), and the surgeon should turn to face the upper torso and move to the right side of the patient (Figure 4-B). The monitors should be moved to the left shoulder of the patient.
By using this 3-step approach, all of the small intestine and its associated mesentery should be visible for inspection of disease, adhesions, site of obstruction, and other such things.
PERITONITIS WITH ABSCESS FORMATION
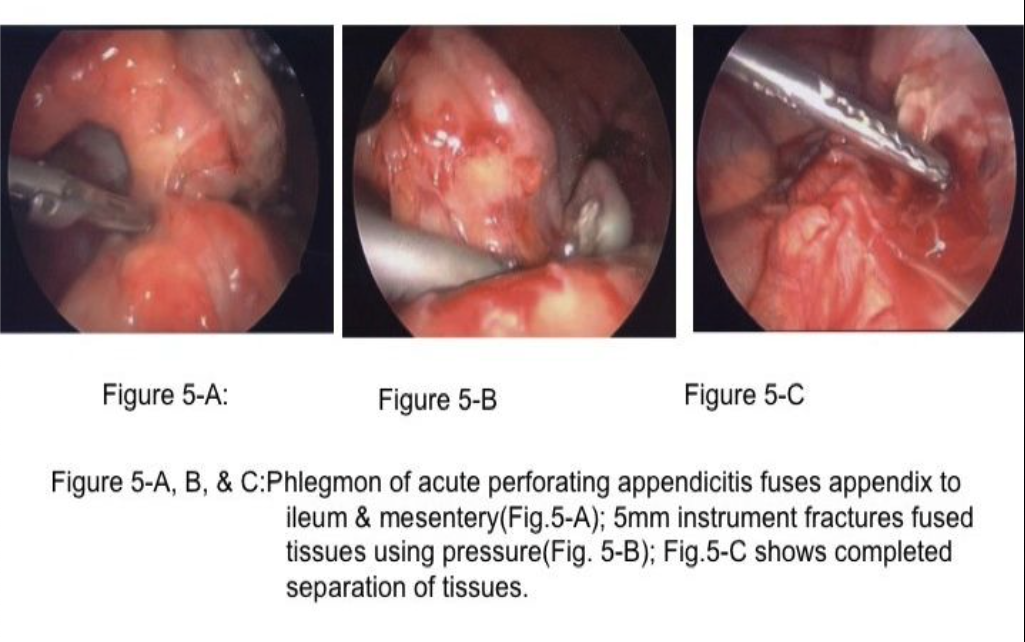
Patients with peritonitis and abscess formation often have perforated viscus (appendix, diverticula, peptic ulcer) as the cause,4,13-15 which was not treated initially and the perforation closed, followed by abscess formation. These patients will often have their abscesses drained percutaneously by CT-guided drainage; however, occasionally, the abscess cannot be adequately drained by CT guidance and laparoscopic evacuation and drainage is often the likely next approach. Notably, all of the aforementioned shortcomings of phlegmon and ileus diminish the size of the abdominal laparoscopic working space, and fuse structures together.13,16 To access and drain the abscess, the laparoscopic surgeon must first find a safe entry into the abdomen and then use “instrument fracture” (Figure 5) to create the access to the abscess, use an angled scope, and irrigate and drain the abscess.16
SMALL BOWEL OBSTRUCTION
The majority of small bowel obstructions are caused by adhesions following pelvic surgery, including appendicitis, hysterectomy, and left sigmoid colectomy.17 Less frequent causes include incarcerated abdominal hernias or tumors. The laparoscopic approach to small bowel obstruction is met with (1) difficulty entering the abdomen laparoscopically due to the obstructed, dilated small bowel occupying increased abdominal space (Figure 6-A) and (2) difficulty with visualization of abdominal structures (adhesions and the site of obstruction) due to distended small bowel.17,18
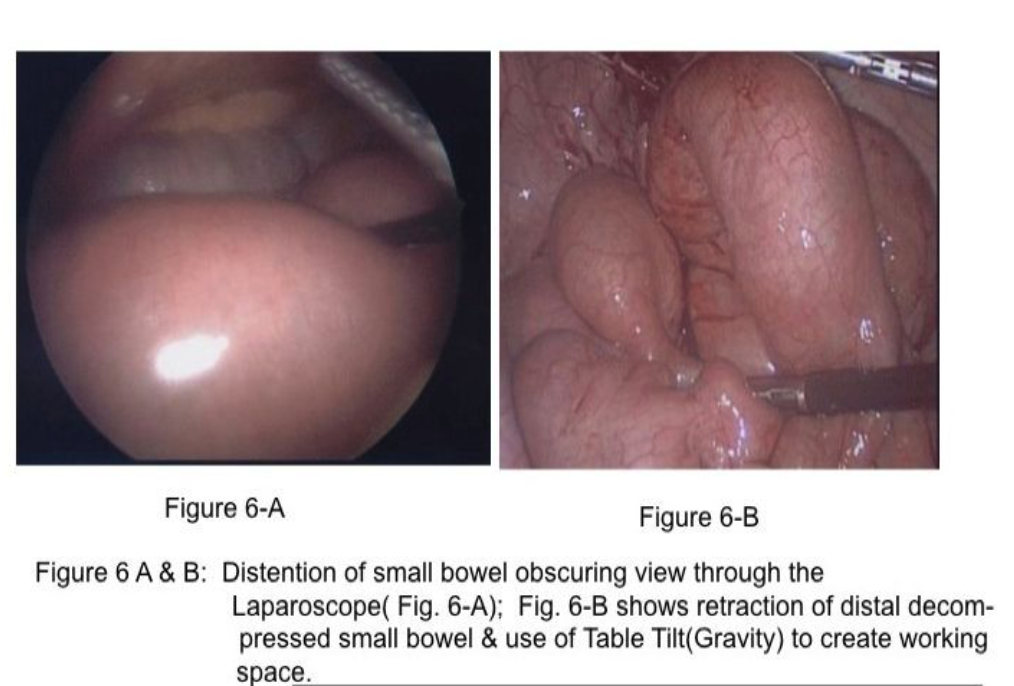
Two important concepts help in assessment and treatment of small bowel obstruction. First, the surgeon should attempt to find the distal decompressed small bowel at the ileocecal area (Figure 6-B) and follow (“run”) the small bowel proximally to find the obstructed segment. By using this technique, the intestine that is grasped by the laparoscopic graspers is not distended and, therefore, not at increased risk for inadvertent injury. In addition, because most small bowel obstructions are secondary to pelvic surgical procedures,17 it is likely that the location of the obstruction will be identified early in the assessment process. Many of these are single-site adhesions in the right lower abdomen (Figure 7).
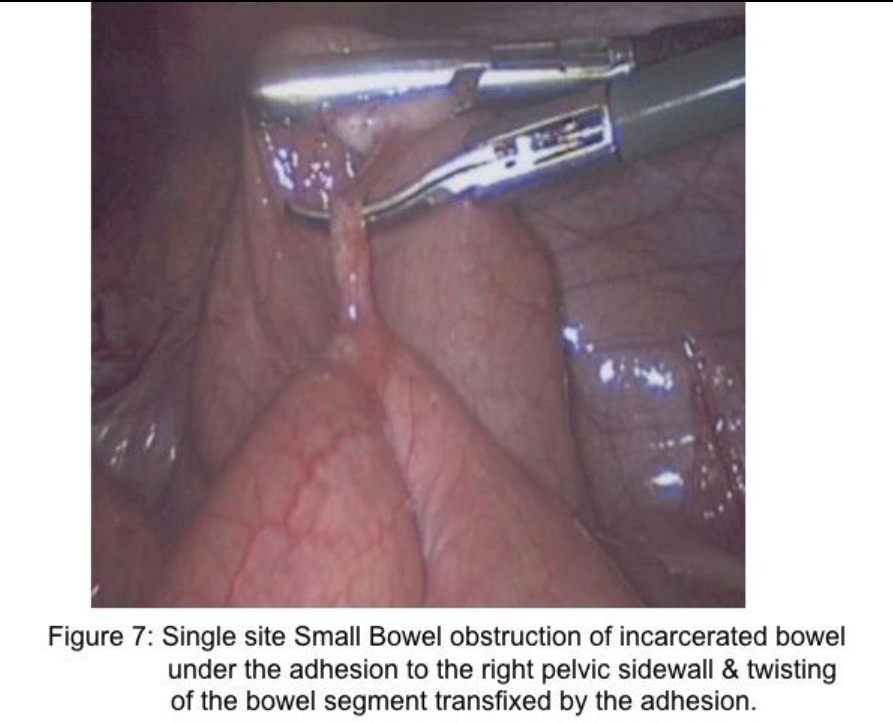
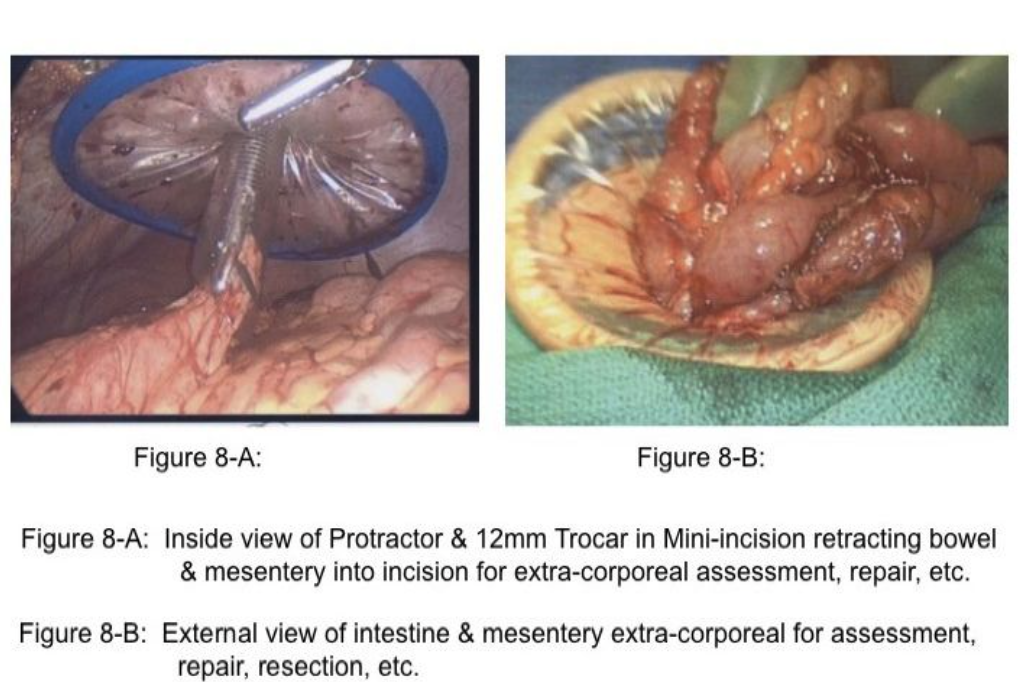
Importantly, retraction of distended small bowel combined with steep table tilt may be necessary to create space to identify the site of obstruction. In these circumstances, the surgeon should avoid grasping the distended intestine, but rather grasp the mesenteric fat adjacent to the intestine to retract the bowel safely (Figure 2). When incarcerated small intestine in a ventral or inguinal hernia is the cause of the obstruction and the incarcerated intestine is ischemic or gangrenous, a mini-incision of 12mm to 20mm may be created in a strategic location on the abdominal wall followed by placement of a protractor in the wound to stretch the incision and to protect the wound. The intestine is retracted through the wound (Figure 8-A&B), and placed back into the abdominal cavity. Notably, approximately 66% of small bowel obstructions treated laparoscopically are completed successfully.18 Many of these cases are a single-site adhesion in the right lower abdomen (Figure 7). Of the remaining 33% reported in the literature that have been converted, approximately 29% of the 33% of cases were converted due to (1) dense adhesions in 13%, (2) need for bowel resection in 11%, and (3) iatrogenic small bowel injury in 5%.18 These 3 reasons for conversion to an open procedure are clearly solved by alternate rescue strategies and specific laparoscopic skills; thus, only 4% would have required conversion due to malignancy or inadequate visualization or bleeding.18-20
LARGE BOWEL OBSTRUCTION
Imminent or partial large bowel obstruction can be treated effectively by using laparoscopic exploration, identification, and mobilization of the site of disease, creation of a strategically placed mini-incision in the abdominal wall, followed by excision of the diseased segment. However, progressive obstruction may progressively diminish the size of the abdominal cavity working space. Therefore, early decision-making regarding laparoscopic exploration is the key to success in this circumstance. The surgeon, then, has a few choices. The first choice is placement of a colostomy. Second, a colo-colonic anastomosis may be performed followed by a diverting ileostomy.21 Notably, if the obstructed colon occupies excessive space in the abdominal cavity, imminent obstruction in left colon disease (diverticulitis, carcinoma, or sigmoid volvulus) may be stented transcolonoscopically followed by laparoscopic colon resection with anastomosis.21-23
References
- Geis, WP, Kim HC. Use of laparoscopy in the diagnosis and treatment of patients with surgical abdominal sepsis. Surg Endosc. 1995;9:178-182.
- Geis WP, Miller CE, Kokoszka JS, Ferlmann JC, Teresi M, Saletta JD. Laparoscopic appendectomy for acute appendicitis: rationale and technical aspects. Contemporary Surgery. 1992;40:13-19.
- Geis WP, Moran JC. Examination of the abdomen: laparoscopic organ exposure. In Talamini, ed. Advanced Therapy in Minimally Invasive Surgery. London: BC Decker, Inc; 2006:21-30.
- Lunevicius R, Morkevicius M. Systematic review comparing laparoscopic and open repair for perforated peptic ulcer. Br J Surg. 2005;92(10):1195-207.
- Kirshtein B, Bayme M, Myer T, Lantsberg L, Avinoach E, Mizrahi S. Laparoscopic treatment of gastroduodenal perforations: comparison with conventional surgery. Surg Endosc. 2005;19:1487-1490. Epub 2005 Sep 27.
- Katkhouda N, Mavor E, Mason RJ, Campos GM, Soroushyari A, Berne Laparoscopic repair of perforated duodenal ulcers: outcome and efficacy in 30 consecutive patients. Arch Surg. 1999;134(8):845-848.
- Lunevicius R, Morkevicius M. Management strategies, early results, benefits, and risk factors of laparoscopic repair of perforated peptic ulcer. World J Surg. 2005;29(10):1299- 1310.
- Lau Laparoscopic repair of perforated peptic ulcer: a meta-analysis. Surg Endosc. 2004;18(7):1013-1021.
- Lam CM, Yuen AW, Cjol B, Wao AC, Fam ST. Laparoscopic surgery for common surgical emergencies: a population-based study. Surg Endosc. 2005;19:774-779.
- Jaffer U, Moin T. Perforated sigmoid diverticular disease: a management protocol. JSLS. 2008;12(2):188-193.
- Busic Z, Lovric Z, Busic V, Cavka M, Lemac D. Laparoscopic treatment of iatrogenic endoscopic sigmoid colon perforation: a case report and literature review. J Laparoendosc Adv Surg Tech A. 2007;17(3):324-325.
- Floch MH, White JA. Management of diverticular disease is changing. World J Gastroenterol. 2006;12(20)3225-3228.
- Sauerland S, Lefering R, Neugebauer E. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev. 2004 Oct 18;(4):CD001546. Review
- Suttie SA, Seth S, Driver CP, Mahomed AA. Outcome after intra- and extra- corporeal laparoscopic appendectomy techniques. Surg Endosc. 2004;18(7):1123-1125.
- Rasheed S, Zinicola R, Watson D, Bajwa A, McDonald PJ. Intra-abdominal and gastrointestinal tuberculosis. Colorectal Disease. 2007;9(9):773-783.
- Abularrage CJ, Bloom S, Bruno DA, Goldfarb A, Abularrage JJ, Chahine AA. Laparoscopic drainage of postappendectomy-retained fecalith and intra-abdominal abscess in the pediatric population. J Laparoendosc Adv Surg Tech A. 2008;18(4):644- 650.
- Szomstein S, Lo Menzo E, Simpfendorfer C, Zundel N, Rosenthal RJ. Laparoscopic lysis of adhesions. World J Surg. 2006;30(4):535-540.
- Ghosheh B, Salameh JR. Laparoscopic approach to acute small bowel obstruction: review of 1061 cases. Surg Endosc. 2007;21(11):1945-1949.
- Debnath D. Bowel injury as a complication of laparoscopy (Br J Surg 2004;91:1253- 1258). Br J Surg. 2004;91(12):1652.
- Nagle A, Ujiki M, Denham W, Murayama K. Laparoscopic adhesiolysis for small bowel obstruction. Am J Surg. 2004;187(4)464-470.
- Farrell JJ. Preoperative colonic stenting: how, when and why? Curr Opin Gastroenterol. 2007;23(5):544-549.
- Targarona EM, D’Ambra M, Agusti AG, Trias M. Multimedia article. Laparoscopic treatment of chronic sigmoid volvulus in a young adult. Surg Endosc. 2005;19(8):1155. Epub 2005 May 26.
- Dozois EJ. Operative treatment of recurrent or complicated diverticulitis. J Gastrointestinal Surg. 2008;12(8):1321-1323.
