Adhesions and Adhesiolysis
Stephen M. Kavic, MD, Suzanne M. Kavic, MD, Michael S. Kavic, MD
INTRODUCTION
Scar tissue is an expected result of trauma, and this is no less true inside the abdominal cavity as on its surface. Abdominal and pelvic surgical procedures, which are a form of controlled trauma, commonly result in the development of adhesions. Although typically involving the peritoneal surface, adhesions may develop between any 2 surfaces during the healing process. Adhesions may develop between adjacent solid organs, the intestines, fallopian tubes, omentum, or the abdominal wall.
Perhaps due to the lack of effective prevention, adhesions have traditionally received little attention in the literature. However, adhesions are shockingly common after open general and gynecologic procedures. In the largest autopsy series of abdominal adhesions, which included 752 subjects, over 44% had adhesions (67% in patients with prior surgery, and 28% in patients without surgery).1 After multiple laparotomies, the incidence of adhesions may even be as high as 93%.2
The consequences and complications of these adhesions are substantial. The Ray et al3 landmark review in 1994 found that adhesiolysis was responsible for over 300 000 hospital admissions in the United States alone, resulting in a staggering $1.3 billion in direct hospital and surgical expenses. A more recent analysis in Sweden suggests that the costs of adhesion-related problems to their society approximate 50 million Euros per year.4 Similarly, in Scotland, Ellis et al5 found that over 10 years after initial surgery, 5.7% of hospital readmissions were directly attributable to adhesions. Interestingly, the rate of readmission for adhesion-related disease did not seem to decline appreciably over the 10-year interval, suggesting that adhesions may place an individual patient at lifelong risk of complications.6
Adhesions impact all physicians who perform abdominal or pelvic operations, including gynecologists, urologists, and general surgeons. Unfortunately, much of the research done in each discipline occurs independently and is not well disseminated in the other specialties. Based on a multispecialty review of the current literature, we summarize here the state-of-the-art on pathogenesis and treatment of adhesions.
ETIOLOGY OF ADHESIONS
Adhesions may be congenital in origin or may develop secondary to an intraabdominal inflammatory process. Some adhesions may develop as a consequence of endometriosis, ischemia, or infection. In the United States, it is generally accepted that the most common cause is secondary to prior operative intervention.
The pathophysiology of adhesion formation has recently been elucidated to an unprecedented degree.7 Briefly, disruption of the mesothelial surface of the peritoneum is followed by fibrin deposition and coagulation. A fibrinous matrix forms through the aggregation of fibrinogen, thrombin, and cellular debris (Figure 1). This gelatinous fibrin matrix serves as a framework by which adhesive connections may be made to any adjacent structure. When followed by ingrowth of fibroblasts and myofibroblasts, which may be regulated by mast cells, a permanent adhesion results.8 In the absence of adhesion formation, the mesothelial surface repairs itself in about 5 days to 7 days.
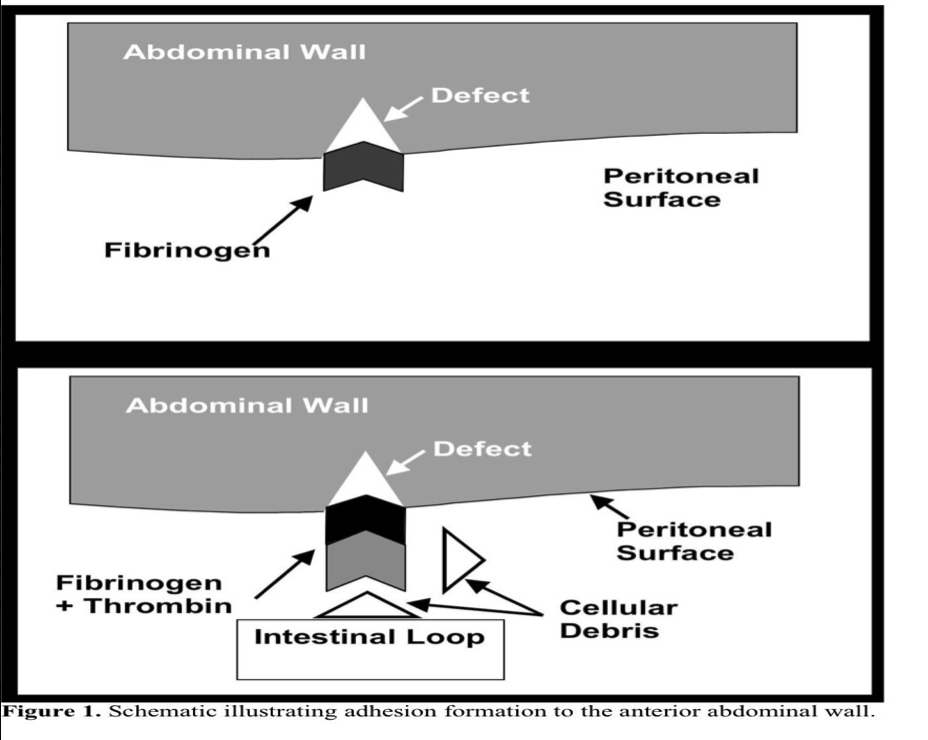
Tissue desiccation may also lead to peritoneal adhesion formation. In laparoscopy, this is particularly important as the dry insufflating gas used to establish pneumoperitoneum might also contribute to adhesion formation.9 The pneumoperitoneum itself may further obstruct capillary flow in the peritoneal surface, allowing conditions of local hypoxia and ischemia to promote adhesions.10
Recent work has also focused on the cytokine pattern and regulation in adhesion formation.11 Transforming growth factor-beta (TGF-b) is a chemoattractant that serves as a regulator in the inflammatory response. Antibodies to one of the isoforms of TGF-b have been shown to reduce adhesions in the rodent model.12 Others have also demonstrated that TGF-b has an important role in adhesion formation.13 Connective tissue growth factor may mediate the effects of TGF-b and has been found to have significantly elevated levels in intraabdominal adhesions.14 This recognition leads to the possibility of altering adhesion formation by modifying cytokines, and this has already been demonstrated in an animal model.15
Other interesting works have recently been published on matrix metalloproteinases (MMPs).16,17 Elevated levels of MMPs are associated with connective tissue disorders and poor wound healing. Peritoneal fluid levels of inhibitors of MMPs have been shown to be higher in patients with extensive adhesions. Adhesions may be able to remodel for prolonged periods following their formation, which is reflected in an increased expression of tissue inhibitor in the tissues themselves.
Unfortunately, the formal study of adhesions has been hampered by the lack of a uniform animal model. Although the majority of animal studies have been performed in the rabbit or rat model, the varied number of species involved has prompted one group to dub the literature “a veritable barnyard.”18 These species, however, have proportionately less omentum than do humans, which may limit the ability to extrapolate findings to the clinical setting.
COMPLICATIONS OF ADHESIONS
The mere presence of adhesions does not condemn the patient to clinically significant complications. In certain cases of abscess or leakage from suture lines, adhesions may even be protective, by containing the pathology.19 However, adhesions are directly responsible for several common complications observed in abdominal and pelvic surgery, ie, intestinal obstruction, infertility, and abdominal pain.
Intestinal Obstruction
Adhesions represent the most important single cause of bowel obstruction in the developed world (Figures 2 and 3). As many as one-third of all cases of intestinal obstruction are secondary to postoperative adhesions. One survey estimated that 4% of laparotomies in Sweden were performed for adhesive bowel obstructions.20 Further, clinically significant adhesions occur rapidly following surgical procedures. A review of nearly 19 000 patients after open abdominal surgery found that 14% had obstruction within 2 years, with nearly 3% requiring adhesiolysis.21 Other studies have demonstrated that over one-third of patients with obstruction presented within 1 year of surgery.22 Importantly, over 10% developed recurrent obstruction, reinforcing our continued inability to prevent adhesions.
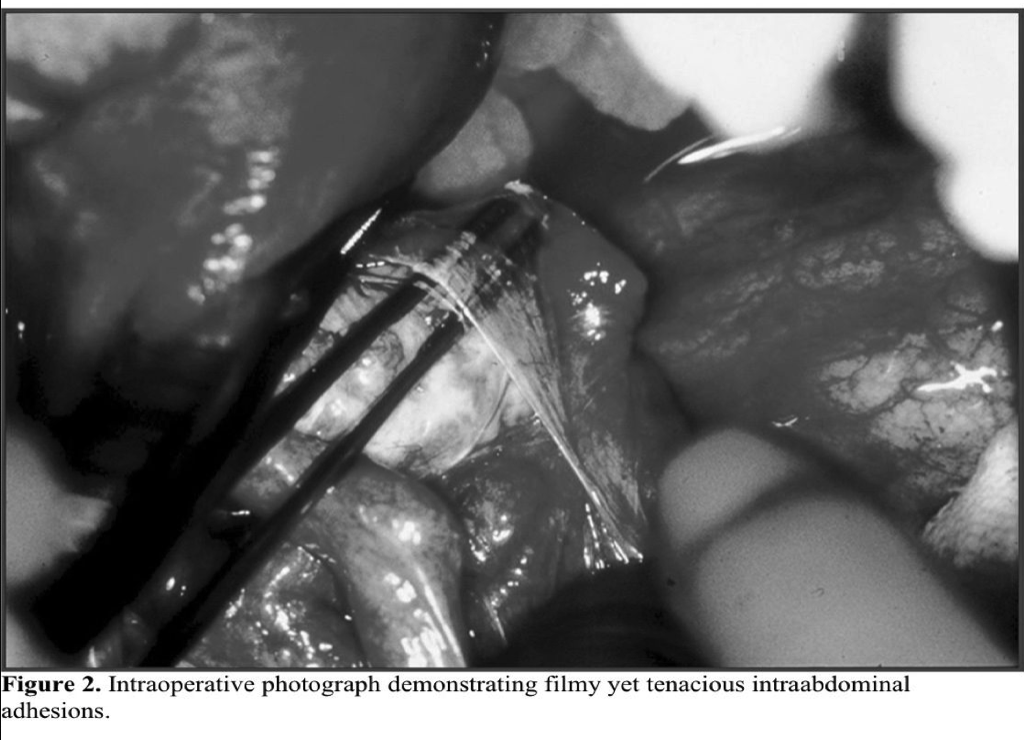
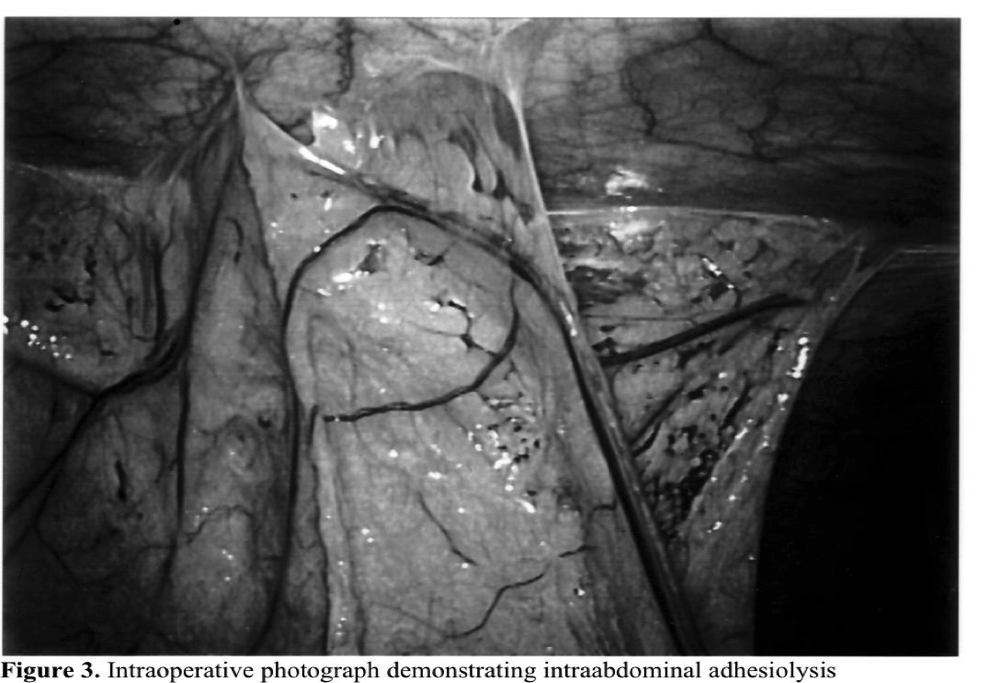
Infertility
The effect of minimal adhesions on fertility is unknown. However, if the ovary, fallopian tube, or uterus is confined to a fixed position where normal function is impeded or impossible, impaired fertility may result. In this manner, moderate to severe pelvic adhesions may be responsible for 40% of infertility cases. Patients with a history of ovarian manipulation or surgery are particularly susceptible to adhesion formation.23
Successful pregnancy outcome after adhesiolysis has been a controversial topic. Lysis of mild, filmy adhesions may not improve subsequent fertility rates.24 Other authors25 report pregnancy success rates approaching 50% in patients with more advanced adhesive disease. However, in vitro fertilization technology may produce similar pregnancy rates without the associated risks of surgical intervention.26
Chronic Pain
It has been shown recently that peritoneal adhesions contain nerve fibers.27 Although the function of this innervation is unknown, it may be speculated that they play some role in the pathogenesis of chronic pain. Adhesiolysis has a role in the treatment of chronic abdominal and pelvic pain; however, the resolution of pain does not always correlatewith lysis of adhesions.28 Recent studies29,30 have demonstrated that laparoscopic adhesiolysis effectively reduces the subjective assessment of pain in some patients, although this effect may be transient and pain may recur after several months. Unfortunately, no large prospective trials have studied the incidence of reformation of adhesion after laparoscopic lysis, and some authors have suggested that pain relief following laparoscopic adhesiolysis only represents a placebo effect.31
Other Complications
Voiding dysfunctions, such as urinary frequency and ureteral obstruction, have been described.32,33 Less commonly, adhesions may interfere with intraperitoneal therapy, such as dialysis catheters or chemotherapy. Lastly, adhesions increase the technical difficulty of subsequent intraabdominal procedures.34 One study noted that 6% of laparotomies were directly complicated by adhesions from previous operations.18
LAPAROSCOPY AND ADHESION FORMATION
Proponents of laparoscopic surgery cite many potential benefits of laparoscopy that may minimize adhesion formation after abdominal and pelvic procedures. First, direct trauma to the peritoneum is minimized, relative to the larger defect of open surgery. In one review of open operations, 71% of adhesions involved the laparotomy scar.34 Any procedure or technique that decreases the size of the surgical wound, such as laparoscopy, may then result in decreased adhesion formation.
Second, the potential exists for reduced intraabdominal contact with foreign bodies, such as gauze sponges, that lead to development of adhesions.35 Further, laparoscopic surgery may maintain native tissues in a humid environment, which may protect the bowel against serosal abrasions. Laparoscopy also offers the promise of less tissue trauma or hemorrhage, or both, at the operative site. The very fact that pneumoperitoneum is established may separate surfaces while they are healing, thereby decreasing the tendency for adhesion formation. Lastly, the intraoperative view of the entire abdominal cavity allows identification of inflammatory pathology distant to the operative site that may contribute to adhesion formation.
Critics, however, contend that visceral injury might be more pronounced in the unfamiliar laparoscopic arena. Additionally, the pneumoperitoneum used in laparoscopic surgery may have its own deleterious effects. One study found adhesion formation in rabbits increased with intraabdominal pressure.2 Some component was probably due to the high CO2 content, while desiccation due to the high flow rate of insufflating gas played a separate role. Elevated intraabdominal pressure may also result in local hypoxia that itself may potentiate adhesion formation.36
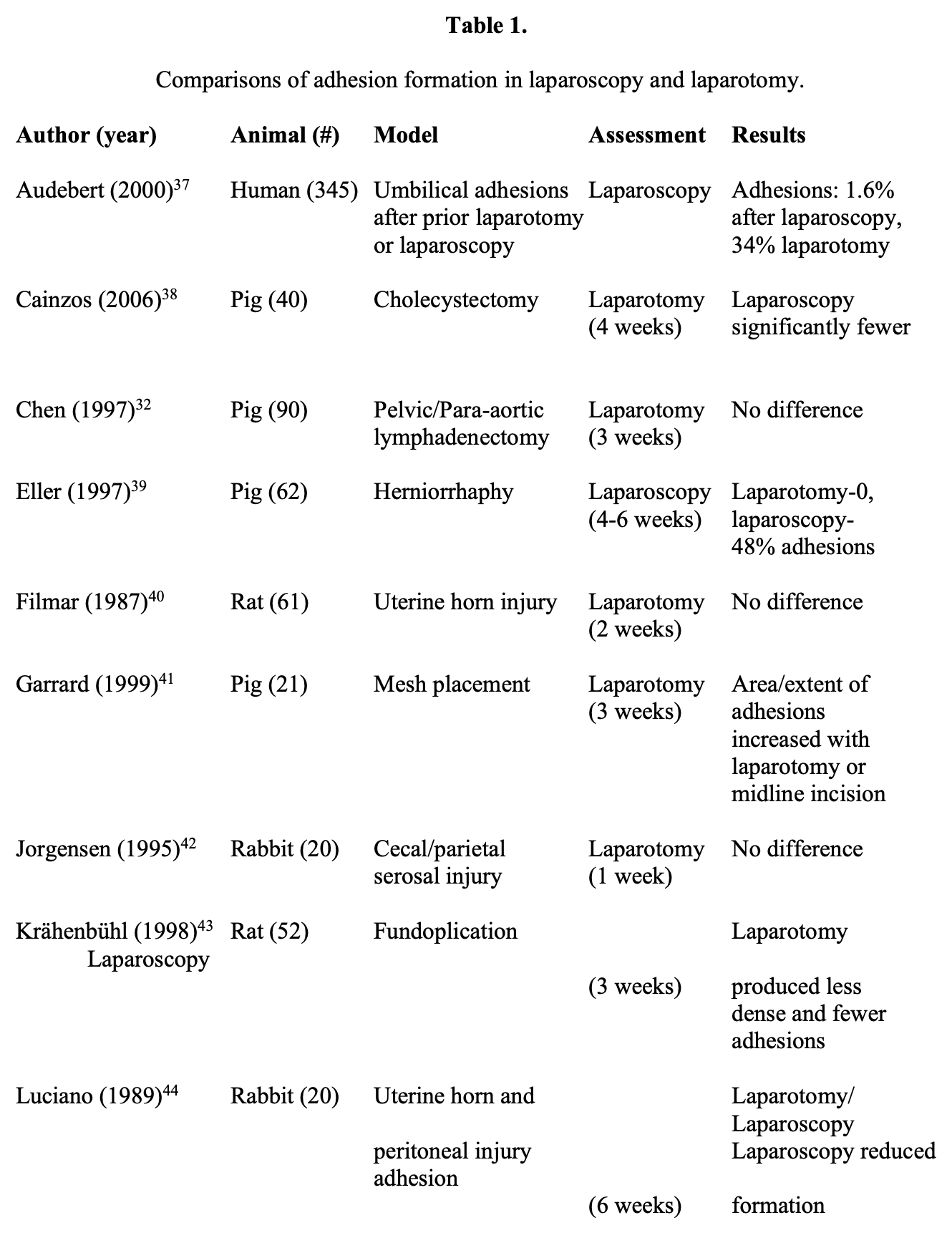
Several direct comparisons of the impact of laparoscopy and laparotomy on adhesions have been performed and are summarized in Table 1.32,37-49 Differing study designs, animal models, and end points of evaluation preclude metaanalysis, but a preponderance of evidence (7 of 12 studies) found laparoscopy to be beneficial in reducing adhesions, including all 3 human trials. However, 4 studies found no difference between laparoscopy and laparotomy, and one demonstrated fewer adhesions following laparotomy.
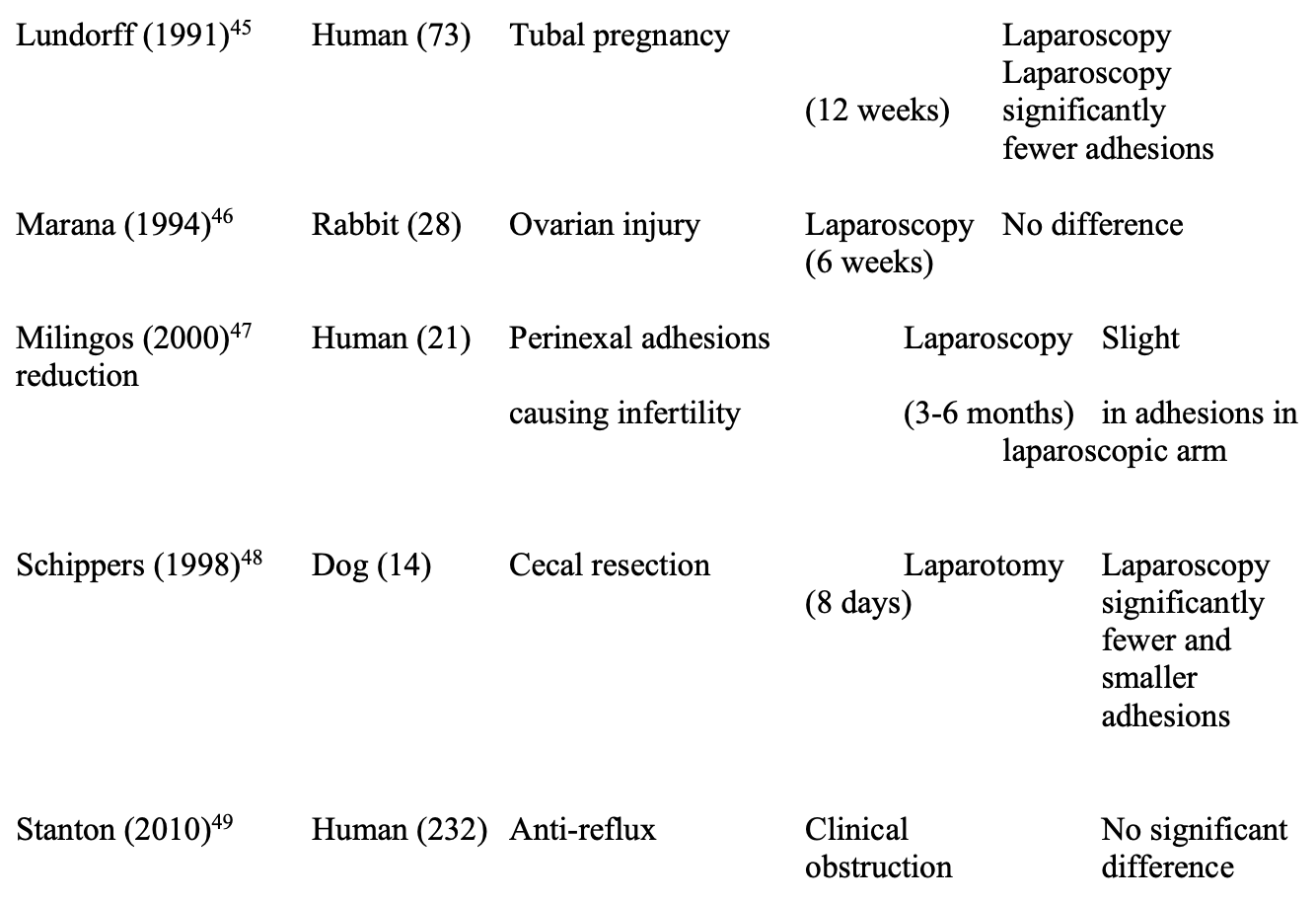
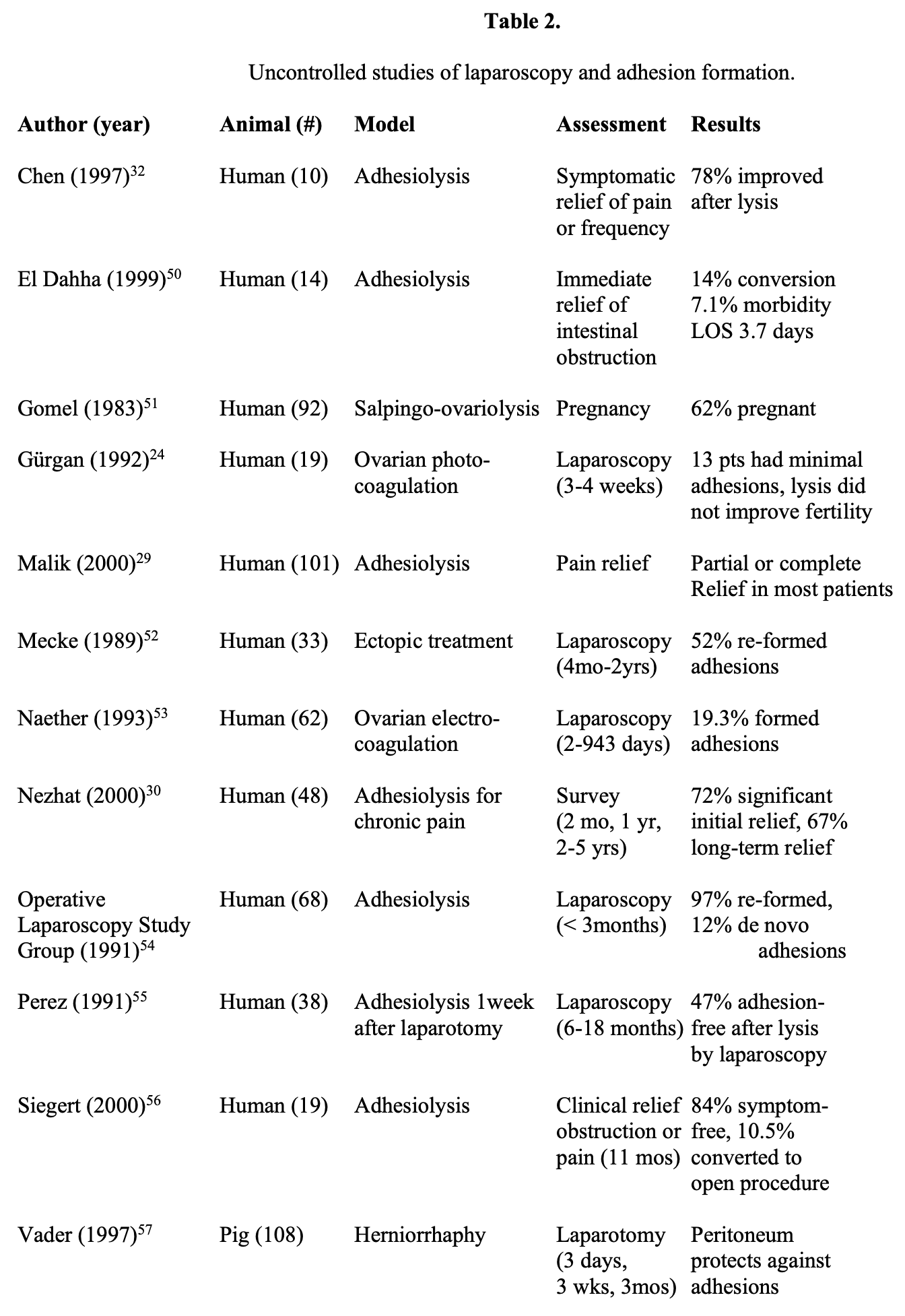
Additional trials studying laparoscopy alone, mostly in human clinical trials, are listed in Table 2.24,29,30,32,50-57 Most studies indicated that laparoscopy was beneficial. However, these trials did identify adhesion reformation after laparoscopic adhesiolysis in 20% to 97% of patients. Clearly, at present, the definitive answer has not yet been determined.
The benefits of laparoscopic surgery, such as reduced postoperative pain, reduced morbidity, and decreased length of stay, have solidified the role of laparoscopy in clinical surgery.58 However, due to the varied results of these published studies, the topic of adhesion formation in laparoscopy remains controversial.
Thus, it seems that laparoscopy is not a panacea for the prevention of abdominal or pelvic adhesions. Like open surgery, minimally invasive surgery necessarily involves peritoneal and tissue trauma. There has been some refinement of instrumentation, allowing for more 5-mm cannulas rather than the larger 10-mm to 12-mm size. The impact of this downsizing of ports on adhesions has not yet been studied and may improve the rate of adhesion formation in future trials. At present, it seems likely that laparoscopy may provide a reduced but nonzero incidence of adhesion formation.59
There has been significant discussion over the past few years on the promise of natural orifice transluminal endoscopic surgery (NOTES). No center has generated significant clinical volumes with this technique, and it is still viewed as experimental. However, one promise of NOTES is that with decreased abdominal wall trauma, there may be decreased rates of adhesions. This has been suggested by a study in the short-term porcine model, but remains far from definitive.60
PREVENTION OF ADHESIONS
No uniform means are available for preventing all types of intraabdominal inflammation. The task of decreasing adhesions has consequently focused on limiting postoperative adhesion formation. The search for an adjuvant means to prevent adhesion formation traces its origins to the beginnings of the modern surgical era, such as Pope’s experiments with citrate solutions in 1914.61 Efficacious substances reported recently are listed in Tables 362-88 and 4. The sheer variety of agents underscores the lack of a single effective agent. At present, HA-CMC and icodextrin have the most evidence to support their clinical use.89


Most authors concede that the most important determinant of postoperative adhesion formation is surgical technique. One study found that operative experience was inversely correlated with adhesion formation.90 The principles of “microsurgery,” or of minimizing surgical trauma to both the peritoneum as well as the viscera, must be strictly followed. For example, mesh placed for hernia repair must be recognized as a potential focus for adhesions, and even reperitonealization of the mesh may result in adhesion formation.91
A recent Cochrane review concluded that hyaluronic acid-carboxymethylcellulose reduces the incidence, extent, and severity of intraabdominal adhesions.92 However, there was no conclusive evidence that there was a reduced rate of reoperation or clinical obstruction, and its cost-effectiveness is thus in question. In addition, concerns over a higher rate of anastomotic leak have led some surgeons to avoid the use of HA-CMC when constructing a bowel anastomosis. To date, however, HA-CMC remains the one agent with the most clinical evidence of utility.
LYSIS OF ADHESIONS
The promise of adhesiolysis is to restore the patient to a preoperative level of function or pain control. In the open setting, the goals of fistula closure, relief of obstruction, resolution of sepsis, and restoration of quality of life have been documented following extensive lysis of adhesions.93 However, in the laparoscopic setting, indications for lysis of adhesions must be individualized to the patient.
In cases of acute abdomen secondary to intestinal obstruction or perforation, immediate operation and resection rightly remains the gold standard. However, in the setting of early obstruction, surgery is by no means absolutely indicated, and careful selection of patients may help to minimize complications. In one prospective report, only 46% of patients presenting with radiographic and clinical evidence of bowel obstruction secondary to adhesions required operative intervention.34 In cases of chronic pain or even infertility, lysis of adhesions may be considered an elective procedure.
Recognition of the problems that adhesions may cause led to the development of “second-look” procedures to assess adhesiolysis after gynecologic surgery. However, second-look procedures themselves may have adverse effects. In one animal trial, lysis of adhesions within one week was ineffective, detrimental at 21 days after the injury, but beneficial at 14 days post injury.94 Because of the complex nature of such experimental evidence, clinicians have not been able to determine the optimal means of using second- look laparoscopy to prevent and treat adhesions. At the same time, evidence continues to mount suggesting that laparoscopy may be an effective means of reducing subsequent adhesion formation.95 Use of adjuvant techniques, such as lysis of selected adhesions guided by preoperative enteroclysis has also been successful in one clinical trial.96
Access
The first consideration in preventing complications from adhesiolysis is the initial access to the abdominal cavity. Entry into an abdomen or pelvis that contains significant adhesive disease may be a daunting task. The hallmark of a safe approach in this circumstance is avoidance of the prior incisions to minimize risk of injury to organs attached at these points. Alternate access sites should be used liberally to avoid direct penetration of adhesions. Use of the Hasson open technique of trocar placement may also place underlying structures at decreased risk of injury. Newer trocars that provide better visualization during port placement, such as the Optiview trocar (Ethicon, Sommerville, New Jersey), are also in clinical use.
Method
The actual modality of dividing adhesions has not been found to be a critical variable, so long as division is complete and adequate hemostasis is achieved. The use of lasers in treatment of adhesions attracted some early attention, as lasers had the theoretical advantages of increased dissecting precision and minimal lateral tissue damage.97 However, later investigation found no distinguishable difference between electrocautery and the use of lasers.98 Well-developed adhesions may be highly vascularized, and care must be taken to ensure adequate hemostasis, whether it is by electrocautery, ligation, or Harmonic scalpel.99
Prevention of Incidental Complications
Most commonly, adhesiolysis is complicated by injury to underlying structures, such as intestines, uterus, ureters, or vascular structures. Importantly, in one study, 36% of bowel perforations were not recognized during the procedure itself.100 In particular, this injury may occur at the time of initial entry into the abdomen and is not obviated by use of the Hasson technique of “open” trocar placement.101 Open trocar placement and alternate access represent the best surgical methods of decreasing complications at the present time. Common sites for alternate access include the right upper quadrant and left upper quadrant, although any site remote from the preexisting scar may be a feasible alternative.
Some surgeons feel that a single adhesive band is more likely to cause clinical obstruction than a series of adhesive connections.102 If this is the case, then the incidence of obstruction may actually be increased by procedures that decrease the number of adhesions without eliminating all of them. Incomplete lysis of adhesions is a frequent occurrence, particularly in patients with extensive, matted adhesions.100 This theory will remain speculative, however, until it can be rigorously tested in an animal model.
Postoperatively, every patient must be monitored for development of complications. An occult bowel injury may result in a delayed presentation of intraabdominal sepsis. Injury to the ureter or the uterus may be problematic; unrecognized injury to these structures may have dire consequences.
CONCLUSION
Adhesions remain a significant, under-recognized problem in the surgical community, because they are not an inevitable consequence of surgical procedures. Laparoscopy itself may reduce the incidence of adhesions relative to laparotomy. At present, no single adjuvant agent has been shown to be entirely effective in reducing postoperative adhesions. However, a number of different modalities are becoming available to the laparoscopic surgeon and include the use of barrier devices, systemic medications, and topical solutions. Continuing clinical research will help delineate which of these modalities ultimately proves best for the prevention of adhesions following laparoscopic and open surgery.
References
- Weibel M-A, Majno G. Peritoneal adhesions and their relation to abdominal surgery. A postmortem study. Am J Surg. 1973;126:345-353.
- Yesildaglar N, Koninckx PR. Adhesion formation in intubated rabbits increases with high insufflation pressure during endoscopic surgery. Hum Reprod. 2000;15:687-691.
- Ray NF, Denton WG, Thamet M, et al. Abdominal adhesiolysis: inpatient care and expenditures in the United States in 1994. J Am Coll Surg. 1998;186:1-9.
- Tingstedt B, Isaksson J, Andersson R. Long-term follow-up and cost analysis following surgery for small bowel obstruction caused by intra-abdominal adhesions. Br J Surg. 2007;94:743-748.
- Ellis H, Moran BJ, Thompson JN, et al. Adhesion-related hospital readmissions after abdominal and pelvic surgery: a retrospective cohort study. Lancet. 1999;353:1476-1480.
- Parker MC, Ellis H, Moran BJ, et al. Postoperative adhesions: Ten-year follow-up of 12,584 patients undergoing lower abdominal surgery. Dis Colon Rectum. 2001;44:822- 830.
- Holmdahl Making and covering of surgical footprints. Lancet. 1999;353:1456- 1457.
- Xu X, Rivkind A, Pappo O, et al. Role of mast cells and myofibroblasts in human peritoneal adhesion formation. Ann Surg. 2002;5:593-601.
- Turner R, Ott D. De novo adhesions due to dry laparoscopy gas (letter). Am J Obstet Gynecol. 2001;185:775-776.
- Molinas CR, Mynbaev O, Pauwels A, et al. Peritoneal mesothelial hypoxia during pneumoperitoneum is a cofactor in adhesion formation in a laparoscopic mouse model. Fertil Steril. 2001;76:560-567.
- Cheong YC, Laird SM, Shelton JB, et al. Peritoneal healing and adhesion formation/reformation. Hum Reprod Update. 2001;7:556-566.
- Lucas PA, Warejcka DJ, Young HE, Lee BY. Formation of abdominal adhesions is inhibited by antibodies to transforming growth factor – b1. J Surg Res. 1996;65:135-138.
- Chegini N. The role of growth factors in peritoneal healing: transforming growth factor-beta (TGF-b). Eur J Surg. 1997;163(Suppl 577):17-23.
- Thaler K, Mack JA, Zhao RH, et al. Expression of connective tissue growth factor in intra-abdominal adhesions. Dis Colon Rectum. 2002;45:1510-1519.
- Minaev SV, Obozin VS, Barnash GM, Obedin AN. The influence of enzymes on adhesive processes in the abdominal cavity. Eur J Pediatr Surg. 2009;19(6):380-383.
- Chegini N, Kotseos K, Bennett B, et al. Matrix metalloproteinase (MMP-1) and tissue inhibitor of MMP in peritoneal fluids and sera and correlation with peritoneal adhesions. Fertil Steril. 2001;76:1207-1211.
- Chegini N, Kotseos K, Zhao Y, et al. Expression of matrix metalloproteinases (MMP-1) and tissue inhibitor of MMP in serosal tissue of intraperitoneal organs and adhesions. Fertil Steril. 2001;76:1212-1219.
- Holmdahl L, Risberg B, Beck DE, et al. Adhesions: pathogenesis and prevention – panel discussion and summary. Eur J Surg. 1997;Suppl 577:56-62.
- Ellis H. The causes and prevention of intestinal adhesions. Br J Surg. 1982;69:241- 243.
- Holmdahl L, Risberg B. Adhesions: prevention and complications in general surgery. Eur J Surg. 1997;163:169-174.
- Beck DE, Opelka FG, Bailey HR, et al. Incidence of small-bowel obstruction and adhesiolysis after open colorectal and general surgery. Dis Colon Rectum. 1999;42:241- 248.
- Menzies D. Postoperative adhesions: their treatment and relevance in clinical practice. Ann Roy Coll Surg Eng. 1993;75:147-153.
- Kistner RW. Peri-tubal and peri-ovarian adhesions subsequent to wedge resection of the ovaries. Fertil Steril. 1969;20:35-42.
- Gürgan T, Urman B, Aksu T, et al. The effect of short-interval laparoscopic lysis of adhesions on pregnancy rates following Nd-YAG laser photocoagulation of polycystic ovaries. Obstet Gynecol. 1992;80:45-47.
- Maruyama M, Osuga Y, Momoeda M, et al. Pregnancy rates after laparoscopic treatment. Differences related to tubal status and presence of endometriosis. J Reprod Med. 2000;45:89-93.
- Oelsner G, Sivan E, Goldenberg M, et al. Should lysis of adhesions be performed when in-vitro fertilization and embryo transfer are available? Hum Reprod. 1994;9:2339- 2341.
- Sulaiman H, Gabella G, Davis C, et al. Presence and distribution of sensory nerve fibers in human peritoneal adhesions. Ann Surg. 2001;2:256-261.
- Peters AAW, Trimbos-Kemper GCM, Admiraal C, et al. A randomized clinical trial on the benefit of adhesiolysis in patients with intraperitoneal adhesions and chronic pelvic pain. Br J Obstet Gynecol. 1992;99:59-62.
- Malik E, Berg C, Meyhöfer-Malik A, et al. Subjective evaluation of the therapeutic value of laparoscopic adhesiolysis. Surg Endosc. 2000;14:79-81.
- Nezhat FR, Crystal RA, Nezhat CH, Nezhat CR. Laparoscopic adhesiolysis and relief of chronic pelvic pain. JSLS. 2000;4:281-285.
- Ikard RW. There is no current indication for laparoscopic adhesiolysis to treat abdominal pain. South Med J. 1992;85:939-940.
- Chen G-D, Lin L-Y, Gardner JD. The results of laparoscopic adhesiolysis for intractable urinary frequency. J Urol. 1997;158:1714-1716.
- Monk BJ, Berman ML, Montz FJ. Adhesions after extensive gynecologic surgery: clinical significance, etiology, and prevention. Am J Obstet Gynecol. 1994;170:1396- 1403.
- Ivarsson M-L, Holmdahl L, Franzen G, Risberg B. Cost of bowel obstruction resulting from adhesions. Eur J Surg. 1997;163:679-684.
- Luijendijk RW, de Lange DC, Wauters CC, et al. Foreign material in postoperative adhesions. Ann Surg. 1996;223:242-248.
- Molinas CR, Koninckx PR. Hypoxaemia induced by CO2 or heliumpneumoperitoneum is a co-factor in adhesion formation in rabbits. Hum Reprod. 2000;15:1758-1763.
- Audebert AJ, Gomel V. Role of microlaparoscopy in the diagnosis of peritoneal and visceral adhesions and in the prevention of bowel injury associated with blind trocar insertion. Fertil Steril. 2000;73:631-635.
- Cainzos M, Rodriguez-Segade F, Martinez-Castro J et al. Intra-abdominal adhesions after open and laparoscopic cholecystectomy: an experimental model. J Laparoendosc Adv Surg Tech A. 2006;16:108-112.
- Eller R, Bukhari R, Poulos E, et al. Intraperitoneal adhesions in laparoscopic and standard open herniorrhaphy: an experimental study. Surg Endosc. 1997;11:24-28.
- Filmar S, Gomel V, McComb PF. Operative laparoscopy versus open abdominal surgery: a comparative study on postoperative adhesions formation in the rat mode. Fertil Steril. 1987;48:486-489.
- Garrard CL, Clements RH, Nanney L, et al. Adhesion formation is reduced after laparoscopic surgery. Surg Endosc. 1999;13:10-13.
- Jorgensen JO, Lalak NJ, Hunt DR. Is laparoscopy associated with a lower rate of postoperative adhesions than laparotomy? A comparative study in the rabbit. Aust N Z J Surg. 1995;65:342-344.
- Krähenbühl OL, Schäfer M, Kuzinkovas V, et al. Experimental study of adhesion formation in open and laparoscopic fundoplication. Br J Surg. 1998;85:826-830.
- Luciano AA, Maier DB, Koch EI, et al. A comparative study of postoperative adhesions following laser surgery by laparoscopy versus laparotomy in the rabbit Obstet Gynecol. 1989;74:220-224.
- Lundorff P, Hahlin M, Kallfelt B, et al. Adhesion formation after laparoscopic surgery in tubal pregnancy: a randomized trial versus laparotomy. Fertil Steril. 1991;55:911-915.
- Marana R, Luciano AA, Muzii L, et al. Laparoscopy versus laparotomy for ovarian conservative surgery: a randomized trial in the rabbit model. Am J Obstet Gynecol. 1994;171:861-864.
- Milingos S, Kallipolitis G, Loutradis D, et al. Adhesions: laparoscopic surgery versus laparotomy. Ann NY Acad Sci. 2000;900:272-285.
- Schippers E, Tittel A, Öttinger A, Schumpelick V. Laparoscopy versus laparotomy: comparison of adhesion formation after bowel resection in a canine mode. Digestive Surg. 1998;15:145-147.
- Stanton M, Andrews J, Grant H. Adhesional small bowel obstruction following anti- reflux surgery in children – comparison of 232 laparoscopic and open fundoplications. Eur J Pediatr Surg. 2010;20(1):11-13. Epub 2009 Oct 14.
- El Dahha AA, Shawkat AM, Bakr AAM. Laparoscopic adhesiolysis in acute small bowel obstruction: a preliminary experience. JSLS. 1999;3:131-135.
- Gomel V. Salpingo-ovariolysis by laparoscopy in infertility. Fertil Steril. 1983;40:607-611.
- Mecke H, Semm K, Freys I, et al. Incidence of adhesions in the true pelvis after pelviscopic operative treatment of tubal pregnancy. Gynecol Obstet Invest. 1989;28:202- 204.
- Naether OG, Fischer R. Adhesion formation after laparoscopic electrocoagulation of the ovarian surface in polycystic ovary patients. Fertil Steril. 1993;60:95-98.
- Operative Laparoscopy Study Group. Postoperative adhesion development after operative laparoscopy: evaluation at early second-look procedures. Fertil Steril. 1991;55:700-704.
- Perez RJ. Second-look laparoscopy adhesiolysis. The procedure of choice for preventing adhesion recurrence. J Reprod Med. 1991;36:700-702.
- Siegert C, Favia P, Shayani V. The role of laparoscopic adhesiolysis in the treatment of patients with chronic abdominal pain or recurrent bowel obstruction [abstract]. JSLS. 2000;4:61.
- Vader VL, Vogt DM, Zucker KA, et al. Adhesion formation in laparoscopic inguinal hernia repair. Surg Endosc. 1997;11:825-829.
- Hidlebaugh DA, Vaulgaropulos S, Orr RK. Treating adnexal masses. Operative laparoscopy versus laparotomy. J Reprod Med. 1997;47:551-558.
- van der Zee DC, Bax NMA. Management of adhesive bowel obstruction in children is changed by laparoscopy. Surg Endosc. 1999;13:925-927.
- Dubcenco E, Assumpcao L, Dray X, et al. Adhesion formation after peritoneoscopy with liver biopsy in a survival porcine model: comparison of laparotomy, laparoscopy, and transgastric natural orifice transluminal endoscopic surgery (NOTES). Endoscopy. 2009;41:971-978. Epub 2009 Oct 28.
- Pope S. The use of citrate solution in the prevention of peritoneal adhesions. Ann Surg. 1914;59:101-106.
- Muzii L, Marana R, Brunetti L, et al. Postoperative adhesion prevention with low- dose aspirin: effect through the selective inhibition of thromboxane production. Hum Reprod. 1998;13:1486-1489.
- Falabella CA, Melendez MM, Weng L, Chen W. Novel macromolecular crosslinking hydrogel to reduce intra-abdominal adhesions. J Surg Res. 2010;159(2):772-778. Epub 2009 Jan 12.
- Oelsner G, Graebe RA, Pan SB, et al. Chondroitin sulphate, a new intraperitoneal treatment of postoperative adhesion prevention in the rabbit. J Reprod Med. 1987;32:812- 814.
- Okamoto Y, Takai S, Yamada M, Miyazaki M. Chymase inhibitors may prevent postoperative adhesion formation. Fertil Steril. 2002;77:1044-1048.
- Avsar AF, Avsar FM, Sahin M, et al. Diphenhydramine and hyaluronic acid derivatives reduce adnexal adhesions and prevent tubal obstructions in rats. Eur J Obstet Gynecol. 2003;106:50-54.
- Bozkurt S, Yuzbasioglu MF, Bulbuloglu E, et al. Prevention of postoperative adhesions by administration of estrogens. J Invest Surg. 2009;22:263-267.
- DeVirgilio C, Elbassir M, Hidalgo A, et al. Fibrin glue reduces the severity of intra- abdominal adhesions in a rat model. Am J Surg. 1999;178:577-580.
- Kaptanoglu L, Kucuk HF, Yegenoglu A, et al. Effects of seprafilm and heparin in combination on intra-abdominal adhesions. Eur Surg Res. 2008;41:203-207.
- Liu Y, Shu XZ, Prestwich GD. Reduced postoperative intra-abdominal adhesions using Carbylan-SX, a semisynthetic glycosaminoglycan hydrogel. Fertil Steril. 2007;87:940-948.
- Emre A, Akin M, Isikgonul I et al. Comparison of intraperitoneal honey and sodium hyaluronate-carboxymethylcellulose (Seprafilm) for the prevention of postoperative intra-abdominal adhesions. Clinics (São Paolo) 2009;64:363-368.
- Kaleli B, Özden A, Aybek Z, Bostanci B. The effect of L-arginine and pentoxifylline on postoperative adhesion formation. Acta Obstet Gynecol Scand. 1998;77:377-380.
- Ersoz N, Ozler M, Altinel O, et al. Melatonin prevents peritoneal adhesions in rats. J Gastroenterol Hepatol. 200924(22):1763-1767. Epub 2009 Aug 3.
- Yetkin G, Uludag M, Citgez B, et al. Prevention of peritoneal adhesions by intraperitoneal administration of vitamin E and human amniotic membrane. Int J Surg. 2009;7:561-565. Epub Sep 30.
- Grow DR, Coddington CC, Hsiu JG, et al. Role of hypoestrogens or sex steroid an6tagonism in adhesion formation after myometrial surgery in primates. Fertil Steril. 1996;66:140-147.
- Çubukçu A, Alponat A, Gönüllü NN. Mitomycin C prevents reformation of intra- abdominal adhesions after adhesiolysis. Surgery. 2002;131:81-84.
- Montz FJ, Monk BJ, Lacy SM, Fowler JM. Ketorolac tromethamine, a non-steroidal anti-inflammatory drug: ability to inhibit post-radical pelvic surgery adhesions in a porcine mode. Gynecol Oncol. 1993;48:76-79.
- Baykal A, Ozdemir A, Renda N, et al. The effect of octreotide on postoperative adhesion formation. Can J Surg. 2000;43:43-47.
- Steinleitner A, Lambert H, Kazensky C, et al. Pentoxifylline, a methylxanthine derivative, prevents postsurgical adhesion reformation in rabbits. Obstet Gynecol. 1990;75:926-928.
- Wallwiener D, Meyer A, Bastert G. Adhesion formation of the parietal and visceral peritoneum: an explanation for the controversy on the use of autologous and alloplastic barriers? Fertil Steril. 1998;68:132-137.
- Hill-West JL, Chowdhury SM, Sawhney AS, et al. Prevention of postoperative adhesions in the rat by in situ photopolymerization of bioresorbable hydrogel barriers. Obstet Gynecol. 1994;83:59-64.
- Steinleitner A, Lambert H, Kazensky C, Cantor B. Poloxamer 407 as an intraperitoneal barrier material for the prevention of post-surgical adhesion formation and reformation in rodent models for reproductive surgery. Obstet Gynecol. 1991;77:48-52.
- Tingstedt B, Nehez L, Axelsson J, et al. Increasing anastomosis safety and preventing abdominal adhesion formation by the use of polypeptides in the rat. Int J Colorectal Dis. 2006;21:566-572.
- Aysan E, Bektas H, Kaygusuz A, Huq GE. A new approach for decreasing postoperative peritoneal adhesions: preventing peritoneal trauma with soybean oil. J Invest Surg. 2009;22:275-280.
- Kim S, Lee S, Greene AK, et al. Inhibition of intra-abdominal adhesion formation with the angiogenesis inhibitor sunitinib. J Surg Res. 2008;149:115-119.
- Chen Y, Hills BA. Surgical adhesions: evidence for adsorption of surfactant to peritoneal mesothelium. Aust N Z J Surg. 2000;70:443-447.
- Tarhan OR, Barut I and Sezik M. An evaluation of normal saline and taruolidine on intra-abdominal adhesion formation and peritoneal fibrinolysis. J Surg Res. 2008;144:151-157.
- Doody KJ, Dunn RC, Buttram VC. Recombinant tissue plasminogen activator reduces adhesion formation in a rabbit uterine horn model. Fertil Steril. 1989;51:509- 512.
- Menzies D, Pascual MH, Walz MK, et al. Use of icodextrin 4% solution in the prevention of adhesion formation following general surgery: from the multicentre ARIEL registry. Ann R Coll Surg Engl. 2006;88:375-382.
- Ordoñez JL, Dominguez J, Evrard V, Koninckx PR. The effect of training and duration of surgery on adhesion formation in the rabbit model. Hum Reprod. 1997;12:2654-2657.
- Gurski RR, Schirmer CC, Wagner J, et al. The influence of reperitonization on the induction of formation of intraperitoneal adhesions by a polypropylene mesh prosthesis. Int Surg. 1998;83:67-68.
- Kumar S, Wong PF, Leaper DJ. Intra-peritoneal prophylactic agents for preventing adhesions and adhesive intestinal obstruction after non-gynaecologic abdominal surgery. Cochrane Database Syst Rev. 2009;21:CD005090.
- Harris EA, Kelly AW, Pockaj BA, et al. Reoperation on the abdomen encased in adhesions. Am J Surg. 2002;184:499-504.
- Haney AF, Doty E. The temporal efficacy of early second-look lysis of adhesions in reducing postoperative adhesions in a murine model. Am J Obstet Gynecol. 1998;179:368-373.
- Tittel A, Treutner KH, Titkova S, et al. New adhesion formation after laparoscopic and conventional adhesiolysis. Surg Endosc. 2001;15:44-46.
- Pekmezci S, Altinli E, Saribeyoglu K, et al. Enteroclysis-guided laparoscopic adhesiolysis in recurrent adhesive small bowel obstructions. Surg Laparosc Endosc Percut Tech. 2002;12:165-170.
- Diamond MP, Daniell JF, Martin DC, et al. Tubal patency and pelvic adhesions at early second-look laparoscopy following intraabdominal use of the carbon dioxide laser: initial report of the intraabdominal laser study group. Fertil Steril. 1984;42:717-723.
- Tulandi T, Bugnah M. Operative laparoscopy: surgical modalities. Fertil Steril. 1995;63:237-245.
- Lin P, Grow DR. Complications of laparoscopy. Strategies for prevention and cure. Obstet Gynecol Clin N Am. 1999;26:23-38.
- Swank DJ, van Erp WFM, Repelaer van Driel OJ, et al. Complications and feasibility of laparoscopic adhesiolysis in patients with chronic abdominal pain. Surg Endosc. 2002;16:1468-1473.
- Brill AI, Nezhat F, Nezhat CH, Nezhat C. The incidence of adhesions after prior laparotomy: a laparoscopic appraisal. Obstet Gynecol. 1995;85:269-272.
- Fitzgibbons Jr RJ. Adding laparoscopy to experimental adhesion study protocols. J Am Coll Surg. 2000;190:336-338.
