Laparoscopic Antireflux Surgery
William E. Kelley, Jr., MD, FACS
HISTORICAL BACKGROUND
Laparoscopic Nissen fundoplication was first reported by Bernard Dallemagne of Leige, Belgium, in September 1991.1 Twelve patients in this historic series underwent operative intervention; 9 were completed by the laparoscopic technique. The standard Nissen fundoplication was performed with no mortality; 1 patient developed pneumonia, representing the only complication in the series. Operative time averaged 188 minutes, and the patients were discharged in 3 days, all returning to normal activities within 2 weeks. Postoperative upper gastrointestinal series confirmed a competent fundoplication in all patients studied. Dallemagne’s subsequent clinical experience with 132 patients provided physiologic documentation of efficacy.2 Many authors have corroborated Dallemagne’s results.3-16 A large meta-analysis of randomized prospective trials comparing open to laparoscopic fundoplication determined a lower incidence of operative morbidity, shorter length of stay (p=0.03), and faster return to work (p=0.03) for the laparoscopic group.17 Laparoscopic Nissen fundoplication has become established as a safe procedure with morbidity and mortality rates at or below those reported for traditional open surgery. A hospital stay of 24 hours has become the norm, and patient recuperation parallels that following laparoscopic cholecystectomy.
ANATOMY AND PHYSIOLOGY
A successful approach to antireflux surgery requires a clear understanding of the pathophysiology of gastroesophageal reflux, and of the complex contributing anatomical factors and their mechanical sequelae. The lower esophageal sphincter is widely recognized as the most important factor in preventing gastroesophageal reflux. The strength of contraction, consistency of contractility, and the 2-cm to 4-cm length of this muscular sphincter are critical to the prevention of reflux of acid and bile from the stomach into the distal esophagus. Hiatal hernias have a multifactorial effect upon the function of the lower esophageal sphincter due to intrathoracic repositioning of the sphincter. The negative intrathoracic pressure associated with inspiration reduces the lower esophageal sphincter (LES) pressure. Furthermore, a stimulating effect of the crural sling causes an independent increase of LES pressure, which is lost when the LES is repositioned above the diaphragm.18,19 Abnormal transient relaxation of the LES, another factor in cardioesophageal junction incompetence, is also exacerbated by the anatomical deformity of a hiatal hernia.18,20 Acid exposure in the distal esophagus is affected by the motility of the esophageal body and by the effectiveness of gastric emptying and the resultant effects on esophageal clearing. A successful antireflux operation must therefore return the gastroesophageal junction into the abdomen, reconstruct the crural support at the hiatus, and reproduce a competent lower esophageal sphincter immediately proximal to the gastroesophageal junction. The length and diameter of the neosphincter must provide a competent barrier to gastroesophageal reflux but allow unimpeded esophageal clearing, and must remain in place, intact, and below the diaphragm.18,20-22
Hiatal Hernias
Hiatal hernias classically have been divided into three types. Type I is the typical sliding hiatal hernia wherein the GE junction slides upward through the esophageal hiatus, maintaining a relatively normal relationship of esophagus, gastroesophageal junction, and stomach. Type I represents approximately 90% of all hiatal hernias. Type II is a pure paraesophageal hiatal hernia, in which the GE junction remains fixed at the hiatus and the fundus rotates upward into the mediastinum. With progression of this upward herniation, organoaxial volvulus of the stomach frequently occurs. This represents less than 5% of hiatal hernias and approximately 15% of all paraesophageal hernias. Type III is a combination of Types I and II, with the gastroesophageal junction sliding upward through the hiatus but a somewhat larger herniation and rotation of the fundus cephalad, above the gastroesophageal junction. Type III is much more common than Type II. Type IV is defined as a very large, usually type III hiatal hernia, associated with herniation of other organs as well as the stomach. Typically small bowel, colon, and sometimes spleen are involved in the Type IV hiatal hernia. A giant paraesophageal hernia is usually defined as 50% of the stomach being incarcerated in themediastinum.23
Vascular Anomalies
Three vascular anomalies can, if unrecognized, predispose to bleeding and other complications. Twenty percent of patients have a prominent accessory or variant left hepatic artery arising from the left gastric artery and coursing obliquely upward to the right within the gastrohepatic ligament. Often, this vessel is large enough to require clips if hook cautery, cautery scissor, or even ultrasonic sheer dissection is being used. A few cases of hepatic necrosis have been reported after dividing this vessel. If an especially large artery is encountered, the surgeon is well advised to apply clips to the vessel and observe for blanching of the left lobe of the liver before dividing the artery.
In another 15% to 20% of cases, a collateral branch from the inferior phrenic artery is encountered while dissecting behind the gastroesophageal junction. This vessel must be ligated or divided carefully with the ultrasonic sheer to avoid bleeding. Another frequent collateral arises from the cephalad short gastric vessel and connects to the posterior wall of the cardia. If this vessel is not divided, the wrap will be tethered posteriorly, distorting the geometry of the fundoplication.
Patient Selection and Evaluation
Nowhere in surgery is preoperative patient evaluation more critical or the strict adherence to surgical indications more important than in antireflux surgery. Reflux symptoms can be mimicked by many other pathologic conditions, such as cholelithiasis, acid peptic disease, and irritable bowel syndrome. All patients should be studied with endoscopy and biopsy to document esophagitis, estimate esophageal length, rule out stricture, rule out peptic ulcer disease, and rule out Barrett’s esophagus. Patients with stricture formation should undergo dilatation at least 2 weeks prior to antireflux surgery to minimize local edema at the time of repair. The occasional patients with a history of peptic ulcer disease who are Helicobacter pylori negative should be considered for highly selective vagotomy at the time of fundoplication. Patients with Barrett’s esophagus will require continued surveillance endoscopy following fundoplication. Barrett’s patients with severe dysplasia should be considered for esophageal resection. Many patients who have been treated empirically with proton pump inhibitors, and some patients who have been on H2 blockers, will have no gross or microscopic evidence of active esophagitis at endoscopy. The latter group of patients will need careful physiologic evaluation to document gastroesophageal reflux disease and should be strongly considered for a 24-hour acid study.
Esophageal manometry should be performed on all patients considered for Nissen fundoplication. Diminished lower esophageal sphincter pressure confirms the inadequacy of the sphincter and, with biopsy proven esophagitis, is appropriate confirmation of gastroesophageal reflux disease. Manometry will also identify undiagnosed scleroderma or patients who have achalasia, either of which may mimic gastroesophageal reflux symptoms. Importantly, 15% to 50% of reflux patients have impaired motility in the body of the esophagus.18 Those with both severe weakness and disorganized peristalsis may be poor candidates for a classic Nissen fundoplication. The traditional wisdom has been that patients with impaired motility are more likely to develop severe dysphagia after a full Nissen fundoplication and that a partial fundoplication, such as a Toupet24 or Dor25 procedure, should be performed.24,25-36 More recently, however, studies have suggested that moderately severe impairment of esophageal body motility can be safely and appropriately treated by a floppy Nissen fundoplication without increased risk of prolonged dysphagia.37-40 Many surgeons still tailor the details of their wrap for patients with both severely disorganized peristalsis and weak motility, choosing either a very floppy Nissen or a partial fundoplication.22,29,41,44
At least 10% of patients with true gastroesophageal reflux disease will produce a normal lower esophageal sphincter pressure at the moment of manometric testing. Those patients have intermittent LES dysfunction. Patients with clinical indications of gastroesophageal reflux disease and normal manometry require 24-hour pH monitoring to document reflux. The 24-hour pH probe is the gold standard for the diagnosis of gastroesophageal reflux disease. Abnormal acid exposure is documented and symptoms are correlated with acid exposure to confirm the diagnosis. Patients for whom the diagnosis of reflux is in question should have 24-hour acid monitoring. Those who have normal or slightly diminished LES pressure, nonspecific or atypical symptoms, or significant psychological disorders should have acid studies before surgery is planned.18,22,35,43
Additional Physiologic and Anatomical Studies
Delayed gastric emptying has been identified as often as 40% of the time in reflux patients.18,44,45 Gastric motility is a very complex mechanism and improvement in gastric emptying has been documented following Nissen fundoplication45,46 and Toupet fundoplication.47 However, severely delayed gastric emptying may contribute to early failure of antireflux surgery, and select patients may benefit from a pyloroplasty. Hunter et al demonstrated a 38% improvement in gastric emptying following laparoscopic Nissen fundoplication alone and a 70% improvement following Nissen fundoplication with pyloroplasty. He has therefore chosen a gastric emptying T 1/2 of 150 minutes as an indication for pyloroplasty.48 Patients who complain of early satiety or postprandial bloating should therefore undergo a gastric emptying study.
If a shortened esophagus or a paraesophageal hiatal hernia is questioned by history or by endoscopy, a barium swallow should be performed. Surgeons who are early in their experience with laparoscopic antireflux surgery would then be advised to plan for an open procedure, to obtain a proctor for the operation, or to refer the patient to a more experienced surgeon. Finally, any patient who has symptoms suggestive of biliary colic should also undergo ultrasound of the gallbladder followed by CCK-HIDA scan if indicated.
MEDICAL THERAPY
The majority of patients with significant GERD can be successfully managed with acid- reducing medication.130 Gradual escalation from antacids to H2 blockers to proton pump inhibitors will usually resolve esophagitis and control symptoms. For patients with impaired gastric emptying the addition of a prokinetic agent may provide added benefit. If symptoms and/or esophagitis become intractable to medical management, the patient is unable to afford medications, or the patient develops complications such as recurrent stricture, bleeding, or silent or gross aspiration with pulmonary sequelae then surgery is indicated.
SURGICAL THERAPY
Patient Position
Antireflux procedures are conducted with the patient in a semi-Fowler and reverse Trendelenburg position. For this reason, pneumatic compression stockings are strongly recommended. Additional deep venous thrombosis prophylaxis may also be indicated depending upon the patient’s risk factors. The arms are tucked at the patient’s side. The surgeon operates from between the patient’s legs, and the assistant stands at the patient’s left side while the camera operator stands to the patient’s right side. With an experienced team and a static mechanical arm to hold the liver retractor, the scrub nurse will be able to function as the assistant.
Trocar Placement
Trocar placement is very important. Five trocars are used in most cases. During early experience, all trocars should be 10 mm to maximize flexibility with instrument selection. The first port is inserted in the midline 2 cm to 6 cm above the umbilicus, depending on the patient’s size, location of the liver edge, and the distensibility of the abdominal wall. A more cephalad placement of this camera port facilitates visualization of the posterior esophagus and the proximal short gastric vessels with the 30-degree laparoscope. The remaining trocars are then inserted under direct vision, consistent with the patient’s anatomy. A right upper quadrant port is inserted just medial to the anterior axillary line.
This port must be below the liver edge with the patient in the reverse Trendelenburg position to avoid capsular injury. A fan- or paddle-type retractor is inserted through this port, and the left lobe of the liver is retracted anteriorly. Many surgeons prefer to use a 5- mm retracting instrument, such as a Nathanson retractor, or a 5 mm triangular retractor through a subxiphoid port. A right paramedian port is then inserted under direct vision below the edge of the liver. This port is inserted at an angle of 30 degrees cephalad and toward the midline to bring the port either below or through the avascular portion of the falciform ligament. In obese patients, a Veress needle may be inserted percutaneously to confirm the correct location of this site before making the incision. With the trocar in this position, off camera injuries to the left lobe of the liver either by direct puncture or by traction on the falciform ligament are minimized.
The fourth port is inserted at the left costal margin in the mid clavicular line, and the fifth port enters in the left anterior axillary line at the level of the camera port. The last 2 ports should be sufficiently far apart that the assistant’s hand operating through the left lateral port will not interfere with the surgeon’s right hand operating through the mid clavicular port. If a linear stapler is needed to divide very short gastric vessels, it is inserted through the left mid clavicular or left lateral port site.
The surgeon operates via the right paramedian and left mid clavicular ports. The assistant provides exposure by manipulating the stomach with an atraumatic grasper via the left lateral port. In obese individuals, a sixth instrument port (5 mm) is often helpful to retract and manipulate the omentum and facilitate exposure. This 5-mm port is introduced through the left mid clavicular line at the level of the camera port.
Operative Technique
The left lobe of the liver is retracted upward with the fan or paddle retractor, exposing the hiatus. The triangular ligament of the liver is usually not divided if a Nathanson retractor or a 5 mm triangular retractor is used, but may enhance exposure with the paddle retractor technique. The gastrohepatic ligament is divided with scissor dissection or with an alternative energy source, beginning just above the hepatic branch of the left vagus nerve and extending cephalad and medially toward the right crus. Division of the hepatic branch of the vagus nerve will predispose the patient to cholestasis and the development of cholelithiasis. A significant accessory left hepatic artery is found in 20% of patients.
This vessel can, rarely, provide most of the blood supply to the left lobe of the liver, and its division can result in hepatic necrosis. In most cases, the accessory left hepatic artery is divided with the ultrasonic sheer or sharply between clips. In the rare case of a large replaced left hepatic artery, this vessel should be clipped and the left lobe observed to ensure that the left lobe does not become cyanotic.
The stomach is retracted laterally to the patient’s left as the right crus is skeletonized in an anterior to posterior direction. It is very important to maintain the integrity of the crural fascia. Disruption of this fascia will weaken the integrity of the crural closure sutures and predispose to recurrence of the hernia. When the posterior confluence of the right and left crura is reached, the esophagus is retracted anteriorly and to the left using a blunt instrument through the left lateral port. During early experience, or when dissecting a large paraesophageal hernia, a Penrose drain can be used to encircle the esophagus to provide downward traction and right and left lateral exposure atraumatically. The posterior surface of the esophagus is dissected free from areolar tissue, and the right vagus nerve is identified and protected. The vagus may either be dissected free from the esophagus and left posteriorly or enclosed within the fundoplication. If the vagus is dissected posteriorly, a prominent vascular branch is usually encountered at the level of the gastroesophageal (GE) junction. When the esophagus and GE junction are retracted anteriorly to the left, the confluence of the crura and the posterior aspect of the left crus can be dissected free. At this point the posterior aspect of the GE junction and proximal stomach are fully mobilized from posterior attachments. A collateral branch from the inferior phrenic artery may be encountered just below, left, and posterior to the GE junction, requiring ligation and division. The posterior aspect of the GE junction must be fully mobilized to avoid tension on the wrap (Figure 1).
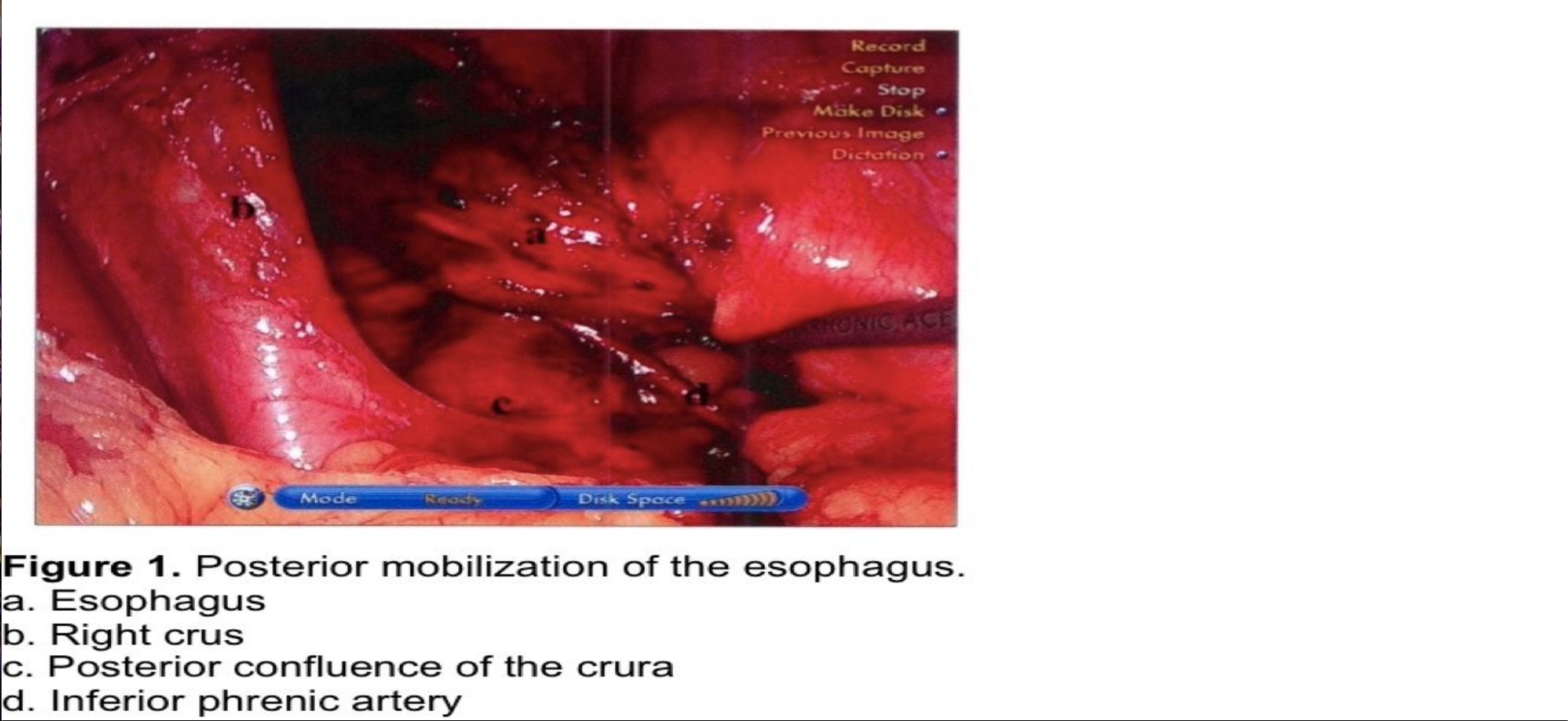
Dissection is now directed to the hiatus anteriorly, incising the phrenoesophageal ligament anterior to the GE junction, while maintaining downward traction on the anterior esophageal fat pad. The anterolateral aspect of the esophagus is dissected free from areolar tissue with longitudinal blunt dissection, avoiding injury to the esophagus and to the left vagus nerve. In obese individuals, a 50 Fr Bougie may be inserted into the esophagus to facilitate identification of these structures during the early phase of the learning curve. The Bougie must be withdrawn proximally before dissecting posterolaterally to avoid a crush injury to the esophagus. Sharp and cautery dissections around the esophagus are discouraged, especially during the surgeon’s early experience. The esophagus should not be grasped with any instrument, and no instrument should be passed blindly behind the esophagus.
The stomach is retracted downward to the patient’s right as the left crus is dissected free in an anterior to posterior direction. Three venous branches between the left crus and the lateral aspect of the GE junction will be seen during this dissection. These can be divided with cautery or with ultrasonic sheer. The cardiophrenic ligament (reflection of the peritoneum from the undersurface of the diaphragm onto the cardia of the stomach) is then divided from a medial to lateral direction until the first short gastric vessel is reached. Occasionally, a prominent branch of the inferior phrenic artery will be encountered at the left, posterolateral aspect of the GE junction.
Routine division of the short gastric vessels is controversial. In the author’s practice most or all short gastrics are divided to permit a truly tension-free wrap. Many authorities agree that division of the short gastric vessels is important,2,15,49,97 although recent studies have again called this to question.11,17,50 This will be discussed in detail in the next section. The dissection begins low along the greater curve between the inferior short gastric and first left gastroepiploic vessel. An avascular plain is incised and the lesser sac is readily entered along the greater curve by scissor or ultrasonic sheer dissection. The short gastrics are divided progressing cephalad along the greater curve of the stomach.
The short gastric vessels can be individually isolated with a right angle dissector, doubly ligated on both sides, and divided sharply. An ultrasonic sheer, or other alternative energy source, can be used to greatly facilitate this dissection. Dissection can be tedious in obese patients, particularly in the early phase of the learning curve. A linear stapler with hemostatic staples can greatly facilitate division of the short gastric vessels and reduce the risk of splenic capsular tear by reducing the amount of manipulation required. We demonstrated in a randomized trial that, compared with scissor dissection and clips, the stapler reduces both the operative time and costs for dividing short gastric vessels early on in the learning curve.51 For an experienced surgeon, however, there was no time saving with the stapler and it was more expensive. Occasionally the most cephalad short gastric vessels are so short that the gastric serosa is touching the splenic capsule. In that circumstance the linear stapler will gently squeeze and separate the two organs and divide the vesselshemostatically.
Having divided the short gastrics, the greater curve of the stomach is then retracted to the patient’s right and the posterior mobilization is completed using blunt and ultrasonic sheer dissection. Occasionally, 1 or 2 collateral vessels from the first short gastric artery or the most cephalad splenic artery will be encountered. These vessels should be divided to avoid tethering of the stomach during fundoplication.
The esophagus is extensively mobilized proximally by gentle longitudinal blunt dissection taking care to avoid injury to the vagal trunks or to the esophagus. Hemostasis is secured using the ultrasonic sheer or clips. The use of cautery around the esophagus and vagi is discouraged. Esophageal mobilization is continued until the GE junction rests 3-4 cm below the hiatus without traction on the stomach. If the esophagus is somewhat foreshortened, the dissection should be continued circumferentially well up into the mediastinum (Figure 2). This requires several centimeters of dissection, sometimes as far as the inferior pulmonary ligament. In the author’s experience it is unusual to have to perform a Collis gastroplasty to achieve this length. Extensive esophageal mobilization is increasingly described in the literature as an important adjunct in achieving sufficient esophageal length.52-55 However, if the esophagus cannot be sufficiently mobilized, a Collis gastroplasty by wedge resection of the greater curve to the left of the gastroesophageal junction, as described by Hunter et al., must be performed to reduce the risk of early recurrence of the hiatal hernia.13,56
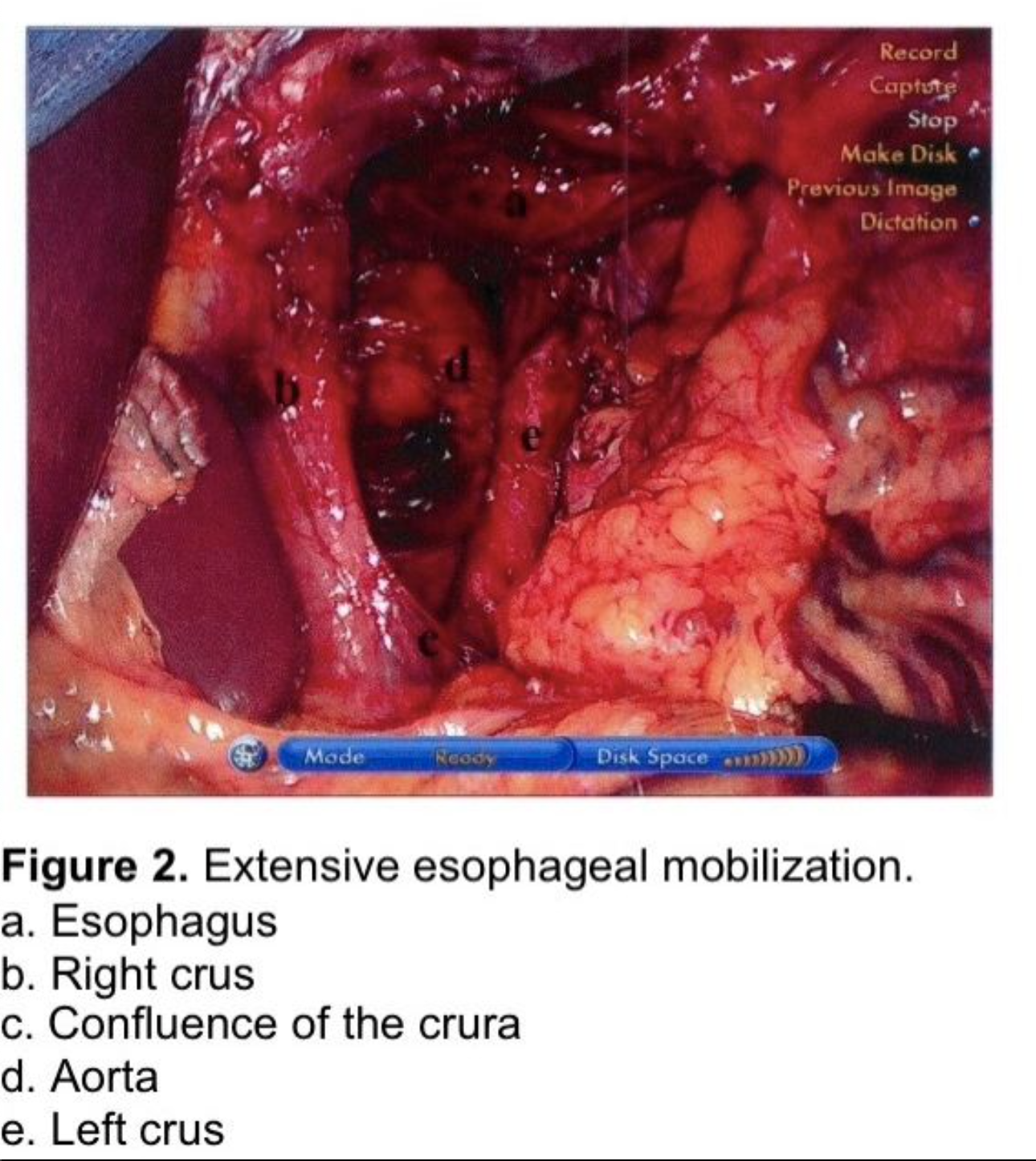
Care must be taken to avoid entering the pleural space while dissecting the esophagus within the mediastinum, or a large hernia sac above the left hemidiaphragm, as a tension carbon dioxide pneumothorax can, rarely, be produced. If this were to happen, a percutaneous catheter or small thoracostomy tube should be inserted. If a non-tension CO2 pneumothorax is produced, it will be reabsorbed within a few hours. If it compromises the patient’s oxygen saturation in the recovery room, it can be aspirated percutaneously. If a hole in the pleura is identified a closed suction drain should be placed through the hiatus into the mediastinum at the conclusion of the procedure to prevent bloody fluid from accumulating and collecting in the pleural space. This drain may also expedite clearance of a CO2 pneumothorax.
The hiatus should be closed routinely to prevent postoperative posterior paraesophageal hernias and direct herniation of the fundoplication into the chest.18,22,49,57-59 The author uses non-absorbable figure of eight sutures to close the hiatus to a 2.5 to 3 cm opening (Figure 3), reserving mesh reinforcement for the very difficult hiatus. The routine use of mesh for crural repair is controversial. Although recurrence of hiatal hernias can be reduced, the potential complication risk is higher. This controversy will be discussed in the next section.
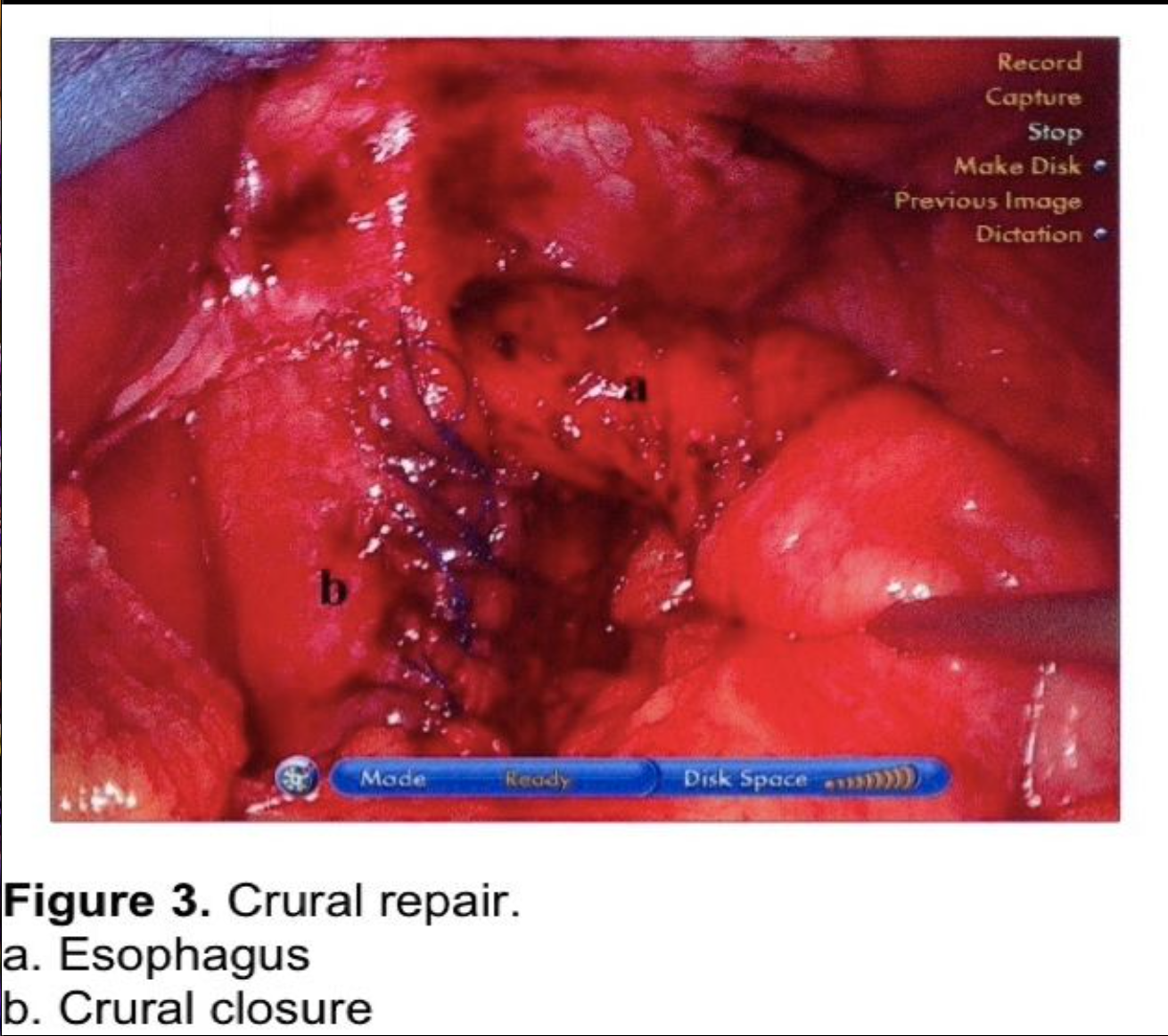
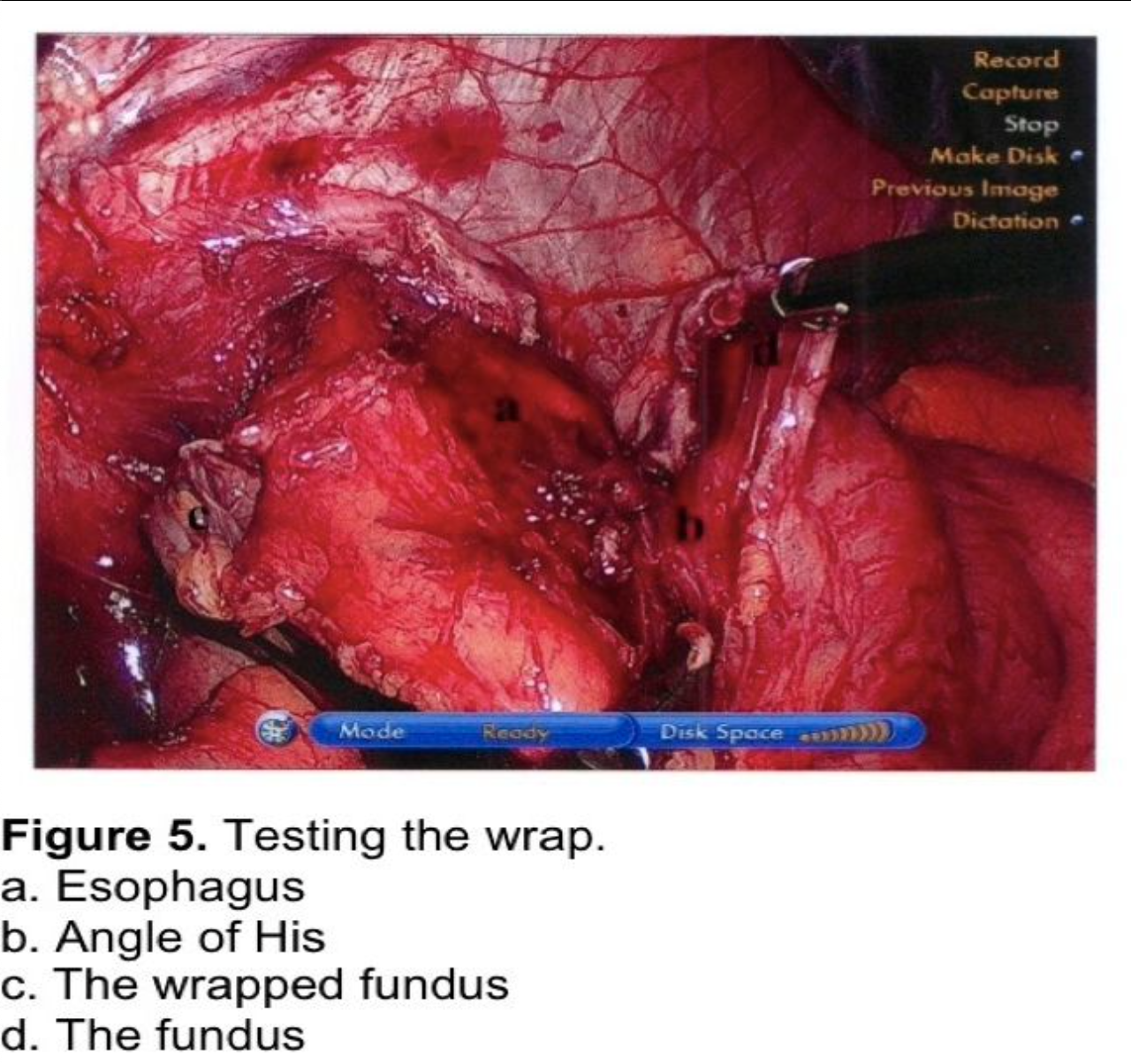
The fundus of the stomach is wrapped behind the GE junction after the stomach has been inspected to rule out a partial or full-thickness injury from dissection or retraction. The greater curve of the wrapped stomach is grasped and wrapped behind the esophagus (Figure 4). The stomach to the left of the esophagus is grasped 2 cm from the angle of His, and the wrap is tested by pulling back and forth between 2 graspers to ensure that an excessive sling of stomach is not present behind the GE junction. (Figure 5) At this point, a 56 to 60 French Bougie dilator is inserted. The GE junction is drawn downward by traction on the two graspers to straighten the distal esophagus as it is draped over the reconstructed esophagus to prevent a posterior esophageal perforation. The surgeon and anesthetist must watch closely as the tip of the Bougie dilator approaches the GE junction, which is another one of the most reported sites for perforation. Close communication between the surgeon and anesthetist is critical during the Bougie insertion. The length of the fundoplication is adjusted one final time such that the two graspers overlap one cm around the Bougie. The fundoplication is performed with 3 nonabsorbable sutures. A 7-10 mm, partial-thickness suture is passed through the stomach just to the left of the esophagus, 2 cm above the angle of His. This suture is continued through the anterior esophagus, securing a 5-mm, partial-thickness purchase 2 cm above the GE junction. The same suture is then passed through the leading edge of the wrapped greater curve with a 7- to 10-mm partial-thickness purchase. Some surgeons recommend fixing the fundoplication to the diaphragm. This will be discussed in the next section. One option for this fixation is to include the anterior rim of the hiatus in the first suture, incorporating the diaphragm and remnant of the phrenoesophageal ligament. As this suture is tied, the force of traction on the suture is transmitted to the diaphragm rather than to the esophageal muscle fibers. The suture is therefore much less likely to tear through the esophagus, which can occur during the early phase of the learningcurve.
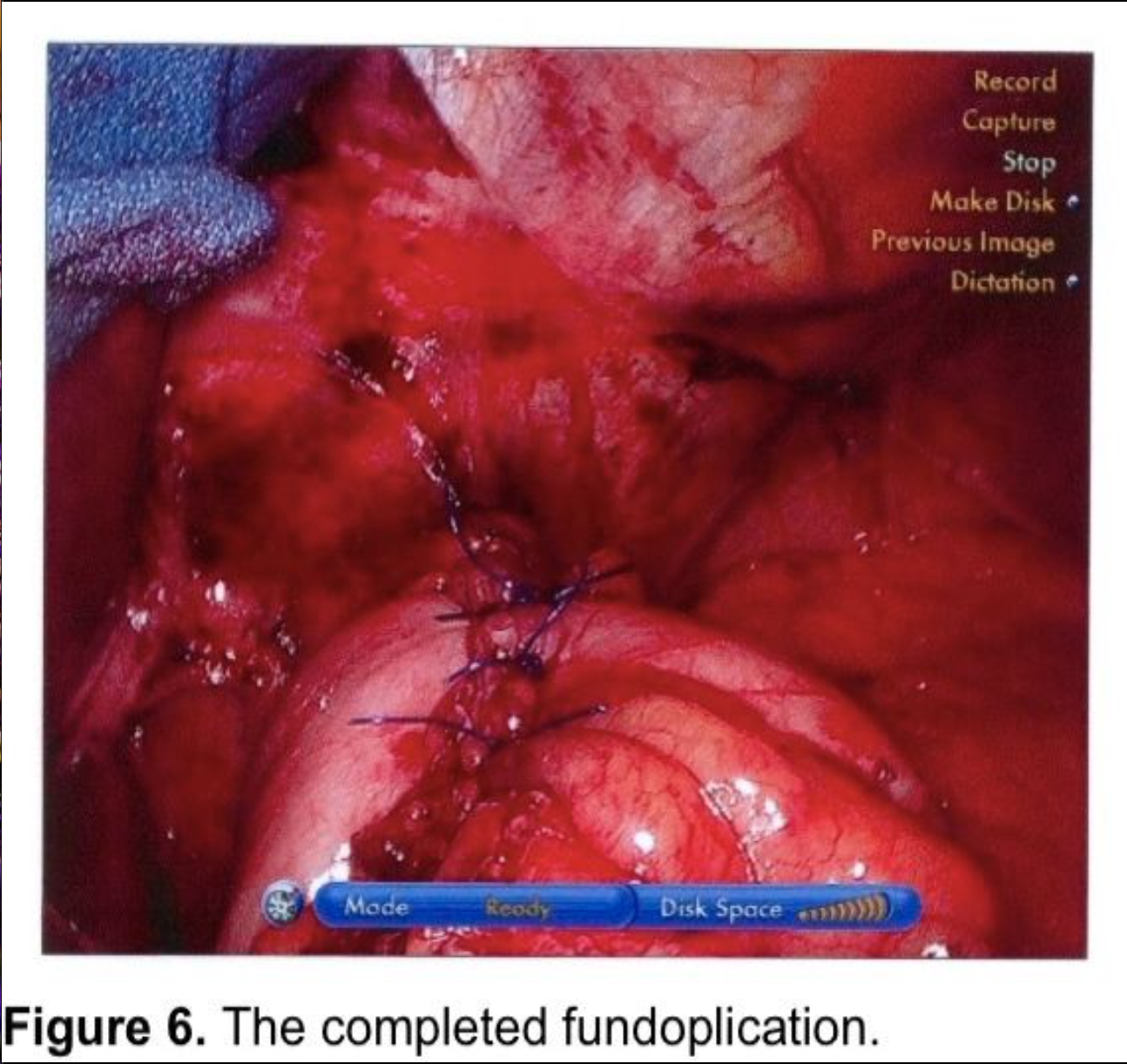
Two additional sutures are then applied at 8- to 9-mm intervals progressing distally (Figure 6). Each suture should incorporate stomach, anterior esophagus, and stomach. The anterior vagus trunk should be visualized and avoided during suture placement. The fundoplication should measure no more than 2 cm in length. Some advocate pledgets for the plicating sutures,6,9,13 but these carry a risk of erosion into the stomach or esophagus.60-62
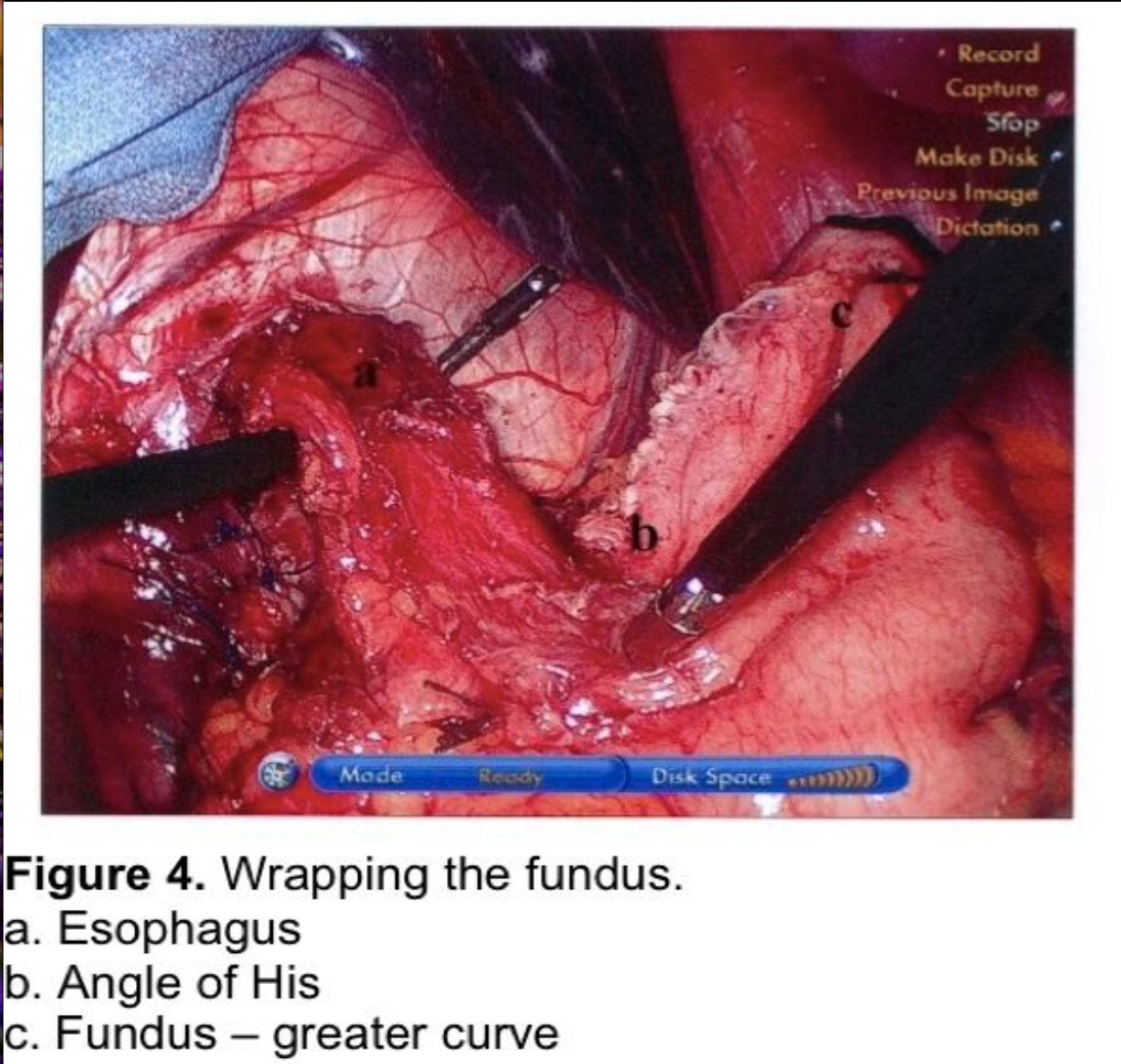
The Bougie is withdrawn, and the stomach is again inspected to ensure that the retracting graspers have not produced a seromuscular injury. The nasogastric tube is usually not reinserted.
Paraesophageal Hernias
Paraesophageal hernias comprise 5-10% of all hiatal hernias, most of which are type III.23 For decades prevailing opinion dictated prophylactic repair of asymptomatic paraesophageal hernias because of the perceived risk of strangulation.63-66 However, it is not unusual to find asymptomatic patients with incarceration and organoaxial volvulus or mesoaxial volvulus. The risk of emergency presentation and the associated operative mortality have apparently been overestimated.23,66,67 A watermark paper by Stylopoulos et al applied a complex statistical analytical model to data from 20 studies.68 They concluded that the operative mortality from emergency surgery was 5.4% (far less than previously recorded) compared with a 1.4% mortality for elective surgery in this typically elderly group of patients. Furthermore, the annual risk of an emergency presentation for surgery was estimated to be 1.1%. The model predicted that observation would be the best management in 83% of asymptomatic patients.
Symptoms of paraesophageal hernia include typical GERD symptoms. Indications for surgical correction would parallel those for Type I hernias. However, symptoms of mechanical obstruction are much more worrisome for an increasing risk of strangulation. These symptoms include severe early satiety, postprandial bloating, postprandial non- burning epigastric or chest pain, dyspnea increasing following meals, and anemia secondary to Cameron ulcers.23,69 They are more compelling indications for elective repair of the paraesophageal hernia.
Laparoscopic repair of large paraesophageal hernias has been shown to be safe and effective with improved recuperation compared to open surgery.10,17,23,70,71 Draaisma reviewed 32 published series and found comparable symptomatic recurrence rates with 9% in the open series and 7% in the laparoscopic surgical series.70 However, Hashemie published a series with barium swallow follow-up, which showed a 42% incidence of radiologic recurrence, versus 15% in open cases.72 Khaitan subsequently published a similar series, showing a 40% incidence of radiographic recurrences.73 Rathore performed a metaanalysis of 13 laparoscopic studies, comprising 965 patients, showing an average clinical recurrence rate of 14% and an asymptomatic radiological recurrence rate of 25.5%. Interestingly, there was a wide range for recurrences, with 2.5 to 21% for clinical and 9 to 44% for radiological recurrences (omitting one small study). This review found superior recurrence rates when Collis gastroplasty was used. Five studies were cited wherein Collis-Nissens were performed selectively on 149 patients with 0 recurrences across the five institutions. In contrast, the symptomatic recurrence rates for non-gastroplasty repair of large paraesophageal hernias in these series ranged from 3 to 19%.74
Critics of routine Collis gastroplasty stress the retention of acid secreting parietal cells in the neoesophagus above the fundoplication and a resultant increased incidence of acid exposure, esophagitis, stricture formation, and abnormal esophageal motility.18,52-55,75-79 Furthermore, Collis gastroplasty is not without potential for complications. Some series have shown leaks from the neoesophageal staple line in as high as 4% of cases.23 One series of 200 consecutive paraesophageal hernia repairs included 112 Collis-Nissens. There were 6 postoperative esophageal leaks and 1 death in that series.78
Some authors stress the alternative of extensive esophageal mobilization, sometimes as high as the inferior pulmonary ligament, to gain esophageal length and substantially reduce the need for Collis gastroplasty.52-55,57,70,80,81 This has also been the author’s experience. No randomized controlled studies have been published to assess the benefit of Collis gastroplasty for paraesophageal hernias associated with short esophagus, but there is general agreement that Collis-Nissen should be considered when the GE junction cannot be brought 3-4 cm below the hiatus without traction on the stomach.
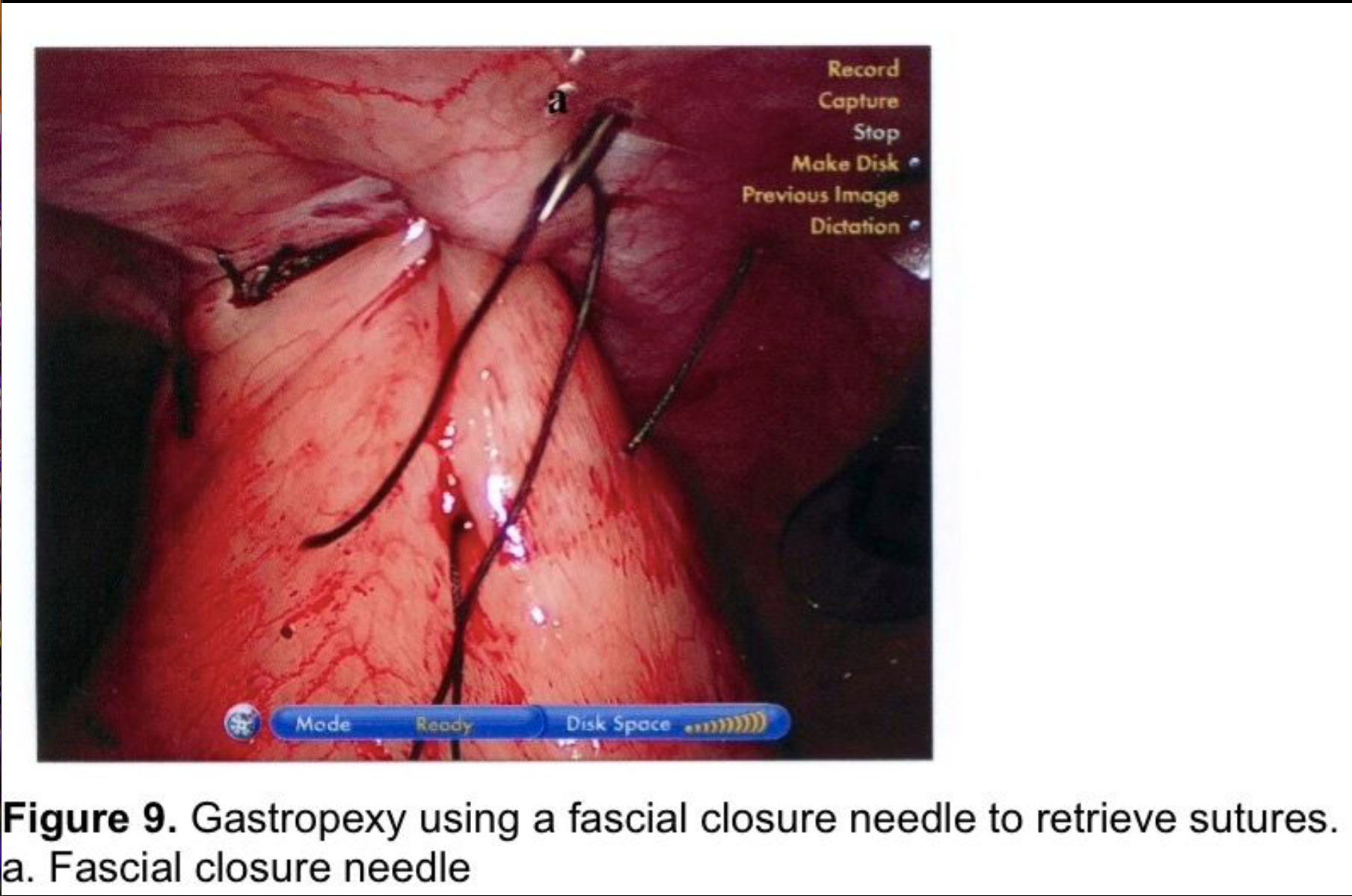
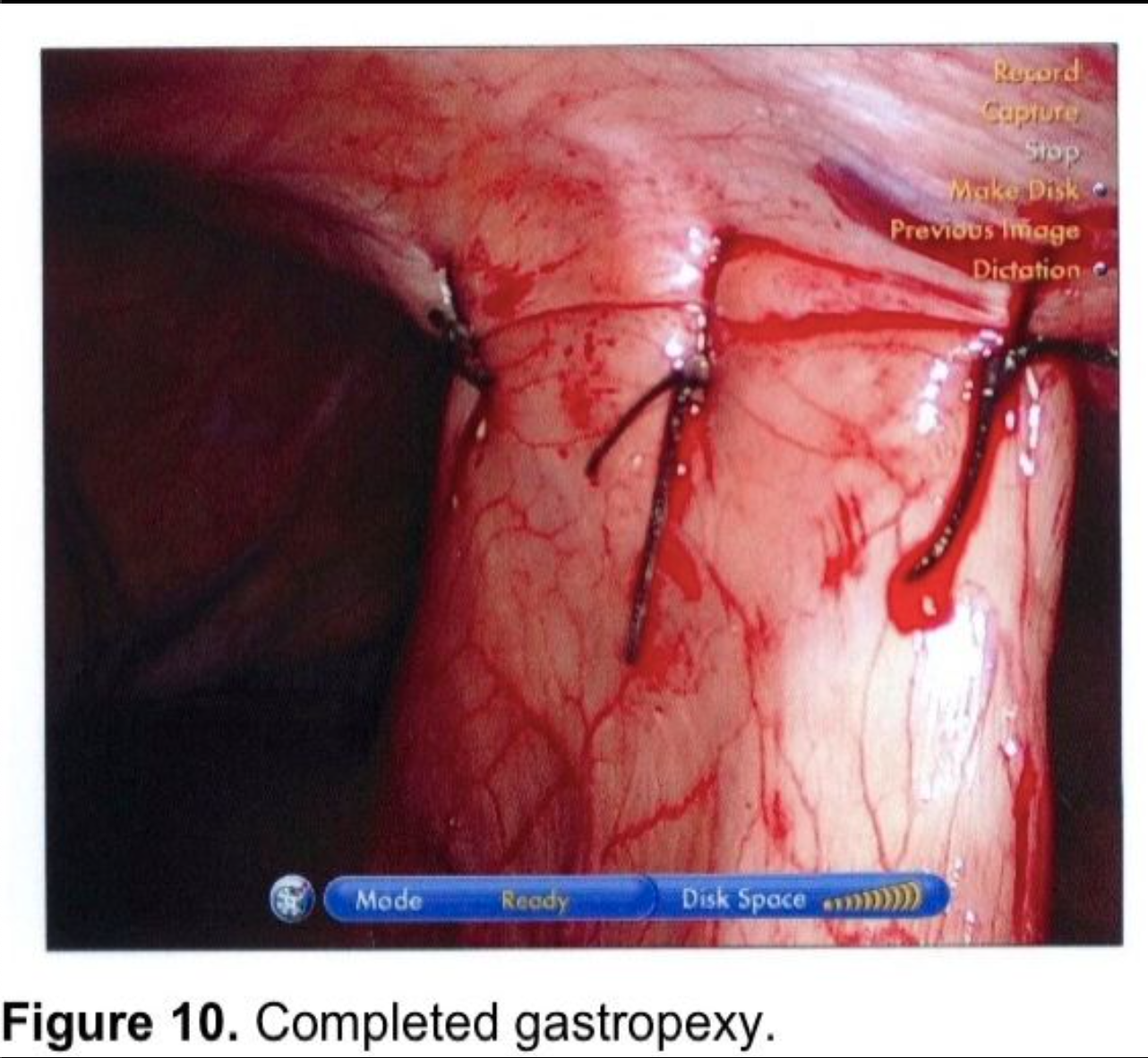
Gastropexy for large paraesophageal hernias fell into disfavor because of high recurrence rates in the open surgery literature.82,83 There has been renewed interest in gastropexy in recent years. Tsimogiannis et al published an 80 patient study randomizing to Nissen with or without posterior gastropexy. Symptomatic recurrence was lower in the gastropexy group (p< 0.001) and excellent results (Visick I) were higher in the gastropexy group (p< 0.0001).84 Ponsky et al published a prospective, non-randomized, 28 patient series of large Type III paraesophageal hernia repairs. A single posterior suture fixed the wrap to the hiatal closure. Two anterior gastropexy sutures were applied, with full-thickness abdominal wall sutures, using a suture-passer, much like a laparoscopic ventral herniorrhaphy suture. Mesh was used on only 1 patient to make a tension-free repair at the hiatus. Follow-up barium swallows were obtained at three, six, and twelve-month intervals, and 10 patients were studied at two years. No radiologic or symptomatic recurrences were found.85 This author uses a technique for anterior gastropexy that combines the techniques of Tsimogiannis and Ponsky. The wrap is pexed anteriorly to the diaphragm by including the apex of the hiatus in the first, most cephalad Nissen fundoplication suture (Figure 6). After completion of the wrap, the anterior surface of the stomach near the greater curve is sutured to the insertion of the diaphragm in the midclavicular line, above the costal margin. Then two abdominal wall sutures are applied using transperitoneal, deep transmural sutures with a curved needle. For obese patients with extensive preperitoneal fat, a Keith needle is inserted percutaneously through the midclavicular line trocar site, then passed through the stomach wall (Figure 8) and back out through the skin incision or retrieved with a fascial closure device (Figure 9). More randomized studies are needed with larger cohorts to further evaluate the efficacy of gastropexy following aggressive esophageal mobilization in reducing recurrence rates for large paraesophageal hernias.
COMPLICATIONS: PREVENTION
Patient Preparation and Positioning
The gastric contents must be neutralized preoperatively to minimize the effect of aspiration from possible gastroesophageal reflux during anesthesia. H2 blockers or proton pump inhibitors should be administered pre- and postoperatively. In patients with impaired gastric emptying, Metoclopramide should also be considered preoperatively to facilitate gastric emptying. A single dose of intravenous antibiotics should be administered prior to surgery in case of aspiration or visceral perforation. Because the procedure is carried out with the patient in lithotomy and reverse Trendelenburg positions, pneumatic compression stockings should be used for thromboembolic prophylaxis.
The Spleen
Splenic injury is reported less frequently in laparoscopic than in open fundoplication, but is one of the relative indications for conversion.86,87 The surgeon must avoid excessive traction on the short gastric vessels. Forceful manipulation of the omentum and forceful traction on the greater curvature of the stomach are the leading causes of splenic capsular tear. The spleen is most at risk when the cephalad short gastric vessels are extremely short. In some cases, the serosa of the stomach is actually in contact with the capsule of the spleen. In these cases it is helpful to dissect posterior to the short gastric vessels and to apply a linear stapler across the interface of the stomach and spleen. As the stapler is slowly approximated, the muscular layers of the stomach are extruded, and the stapler fires across the serosal surface and the vessels. The surgeon must also guard against off- camera injury to the spleen. Finally, the omentum may be found adherent to the splenic capsule, even in patients who have not had abdominal surgery. Care must therefore be taken during early exposure of the short gastric vessels to avoid tearing adhesions between the omentum and splenic capsule.
The Vagus Nerves
Both the right and left vagus nerves must be visualized during the dissection of the esophagus. Inadvertent vagotomy has been reported as an infrequent complication of laparoscopic antireflux surgery.88,89 The incidence of vagal nerve injury has been estimated to be as high as 1.4%.90 The risk is clearly higher with paraesophageal hernia dissection.91 Resultant symptoms include diarrhea and gastroparesis with bloating and early satiety. The diagnosis can be confirmed by Congo red testing, the Hollander test, or sham feeding.88,90 Klaus identified an 18% incidence of postoperative diarrhea that was not present before surgery.89 Four patients had fecal incontinence. Half of the patients underwent diagnostic testing, but none of these patients showed clear evidence of vagotomy. The diarrhea can be difficult to control, but a combination of hypomotility agents such as diphenoxylate/atropine, with cholestyramine has been effective, as well as an antidumping diet.88-90
Gastroesophageal Perforation
Gastroesophageal perforation occurs more commonly during laparoscopic antireflux surgery than during open surgery.86,93 Gastric and esophageal perforation are the most serious complications of antireflux surgery, with a mortality rate of 8.3% to 26% reported in the open literature.94,95 Perforation unrecognized at the time of laparoscopic surgery carries a mortality risk of 1.3%.93 Shauer reviewed 10 series of open antireflux surgery and 7 laparoscopic series of comparable size. He found a perforation rate of 2.1% with laparoscopic Nissen fundoplication, which was double the reported experience with open surgery (1.1%). The overall morbidity for the 2 groups was, however, quite similar (13% and 15%, respectively). The 2 predominant mechanisms of injury are retroesophageal dissection and introduction of the Bougie. Perforation from sutures tearing through the tissues postoperatively is a less common cause.94
The Stomach
Exposure during fundoplication is affected by manipulation of the stomach. The surgeon must be careful to avoid gastric perforation by the grasping instruments. Most gastric manipulation can be achieved by grasping the fat-pad anterior to the GE junction. The left vagus nerve runs through the right side of this pad, however, and must not be crushed or torn. The use of the anterior gastroesophageal fat-pad for traction avoids seromuscular or full-thickness injury to the stomach. The surgeon must always inspect for gastric injury before proceeding with fundoplication. If a perforation or partial-thickness injury is found, it must be repaired primarily. The surgeon should consider 12 to 24 hours of nasogastric suction in these circumstances.
Gastric perforation has also been reported during dissection of the posterior aspect of the proximal stomach.94,95 This injury may be more serious, since it may not be identified at the time of surgery. The injury is avoided by careful identification of the confluence of the crura during retrogastric dissection. The dissection then progresses from above downward and from right to left, gently retracting the esophagus and GE junction anteriorly to the patient’s left and dissecting with gentle longitudinal blunt dissection under direct visualization. This dissection must never be blind, and spreading with the dissecting instrument is to be avoided.
The Esophagus
A Bougie dilator may be introduced early during the procedure to help identify the esophagus. This may be helpful during a surgeon’s early experience, in obese patients, and in patients with large paraesophageal hernias. However, the Bougie must be removed or withdrawn into the midesophagus prior to performing posterolateral dissection of the esophagus. Withdrawal of the Bougie permits gentle anterior displacement of the esophagus to expose the proper plain of dissection and avoid crushing the posterior esophageal wall between the dissecting instrument and the Bougie.
The safest method for dissection of the esophagus is gentle blunt dissection, tangential to the external muscle fibers. When there are extensive mediastinal adhesions to the esophagus caused by distal esophageal inflammation or by a longstanding paraesophageal hernia, an alternative energy source will be needed to divide the adhesions hemostatically. Care must be taken to avoid collateral thermal injury to the esophagus.
Two or three small veins routinely course from the undersurface of the left diaphragm toward the GE junction. These veins must be identified and clipped, cauterized, or controlled by the ultrasonic sheer to prevent oozing that might obscure the dissection. The esophagus must never be grasped with any instrument, or retracted with the tip of an instrument. Manipulation of the esophagus is achieved by traction on the stomach, the anterior gastroesophageal fat-pad or the hernia sac, by gentle deflection with the side of a blunt instrument, or by traction on a Penrose drain encircling the GE junction. The Penrose drain technique is recommended during a surgeon’s early experience.
The Bougie dilator is advanced into the stomach after the crural repair, and after the fundus has been wrapped behind the gastroesophageal junction. Bougie perforation occurs either directly posteriorly or directly anteriorly. Caudal traction must be applied to the stomach (typically by caudal traction on the wrap at the GE junction) to straighten the esophagus during introduction of the Bougie. Otherwise, the esophagus lies posteriorly along the vertebral bodies and is then deflected anteriorly by the posterior crural repair.
This anterior deflection of the esophagus predisposes to perforation of the posterior wall of the esophagus. The Bougie must be advanced slowly while the surgeon carefully watches for the tip to pass through the crura to avoid an anterior perforation, which typically occurs at the distal esophagus or at the GE junction as the tip of the Bougie is aimed anteriorly.
Most gastroesophageal perforations occur early in the surgeon’s experience, with the majority occurring within the first 15 to 50 cases.93-96 In a personal series of over 2,000 laparoscopic antireflux procedures, however, the only 2 esophageal perforations occurred between cases #479 and 496. Both perforations were repaired laparoscopically, as described in the next section. Inspection of the hospital’s old red rubber Bougies then revealed that they had become very stiff. After replacing the old Bougies with new soft ones, no perforations have occurred in over 1,500 additional procedures. Surgeons must beware of stiff, old Bougies.
Perforations can also occur when sutures pull through the esophagus or stomach during the early postoperative period. This injury is associated with severe retching or with profound postoperative gastric distention. Early postoperative vomiting must be avoided as a predisposing factor for slipped Nissens, postoperative paraesophageal hernias, and transthoracic herniation of the wrap. Postoperative orders must include antiemetic drugs, and postoperative nasogastric suction is advocated for any patient who is vomiting.
Gastric distention is much more common following repair of large paraesophageal hernias and following surgery for recurrent gastroesophageal reflux. Postoperative prokinetic agents are recommended for 2 to 4 weeks following these more extensive procedures. Proton pump inhibitors or H2 blockers are also recommended for 2 weeks after antireflux surgery because of a predisposition for postoperative gastritis.
The crural repair: prevention of recurrent herniation
The incidence of reoperation following failed laparoscopic Nissen fundoplication is reported to be 2.8 to 6%.57,80,97-99 Failed cruroplasty is implicated in 24 to 50% of these failures.80,97,99,100-103 Several authors have reported significant reduction in recurrence of hiatal hernias using mesh for either routine104 or difficult, large hiatal hernias.105-111 Ganderath reported a prospective randomized study of 50 laparoscopic Nissen fundoplications with primary sutured hiatal repair compared to 50 Nissens with primary repair plus an onlay polypropylene mesh. 26% of the primary repairs failed, with wrap migration, compared to 8% in mesh group (p<.001).104 Frantzides published a similar series with 72 difficult hiatal hernias (8 cm or larger). 22% of the primary repairs recurred compared to 0% with a PTFE Dual mesh using a keyhole, onlay repair (p<.006).107 Oelschlager reported a similar result randomizing 108 patients with paraesophageal hiatal hernias to primary repair versus reinforced primary repair with biomesh. Once again the mesh repairs were superior, as 24% of the primary repairs failed versus 9% of the biomesh repairs (p=.04).110
Although mesh reinforcement of crural repair has been shown to reduce the failure rate of hiatal herniorrhaphy, it has also been reported to cause major complications specific to the mesh.69,112,113 A recent paper reported a series of 28 mesh-related complications at 6 major centers. All types of mesh were implicated in complications with 8 polypropylene, 1 dual, 12 PTFE, and 7 biosynthetic meshes being implicated. Nine patients required major resections, including 2 partial mastectomies, 1 total gastrectomy, and 6 esophagectomies. Five patients were left dependent upon tube feedings and 1 additional patient had severe residual gastroparesis.112 Further extensive studies are needed to identify an optimal mesh and optimal fixation techniques. Until that time, mesh repair should be reserved for hiatal hernias that are perceived to be at high risk for recurrence due to hernia size, tension on the repair, and quality of the crural pillars.
Postoperative Posterior Paraesophageal Hernia
The incidence of postoperative paraesophageal hernia is much higher following laparoscopic antireflux surgery than following open surgery.40,59,96 These patients present with postoperative dysphagia, odynophagia, or postprandial pain and bloating, often in the immediate postoperative period. The proposed sources of postoperative herniation include failure to properly or routinely repair the hiatus, failure to divide short gastric vessels, and the paucity of adhesions following minimally invasive surgery.40,49,59,96 Crural closure is strongly recommended.22,40,57-9,49,86,97 Wu et al showed a statistically significant reduction in postoperative herniation when posterior crural closure was performed (P<0.01), when posterior crural closure and fixation of the wrap to the diaphragm were performed as combined variables (P<0.01), and when posterior crural closure and short gastric division were performed (P<0.001).49 Recurrent sliding hernias, or transhiatal slippage of the wrap, do not require reoperation unless the patient develops symptoms. Early postoperative paraesophageal hernias are usually symptomatic and should be repaired. As noted in the paraesophageal hernia section, patients with asymptomatic paraesophageal hernias should be counseled to return promptly if they become symptomatic.
The Fundoplication
In 1985 Donahue et al 115 described the “floppy fundoplication,” which has been one of the most important modifications of the Nissen technique. The short loose fundoplication has become the standard procedure, offering maximum results with minimum long-term dysphagia. The greater curve and posterior aspect of the cardia and GE junction should be fully mobilized, to produce a tension-free wrap.2,18,22,27-31,49,58,96 There is considerable controversy about routine division of the short gastric vessels. One randomized study of 102 cases, which was limited to patients without associated motility disorders, failed to show any advantage to dividing the short gastric vessels.113 A large meta-analysis study also failed to show significant advantages for division of the short gastrics.17 This author has had many experiences wherein collateral vessels and posterior attachments to the proximal stomach within the lesser sac tether the stomach and prevent the formation of a precise wrap. These structures would not be visible without division of the short gastrics. However, difficulty with the wrap is clear in such cases and the surgeon should be ready to perform selective division of the short gastrics if routine division is not his/her customary practice. Significant evidence-based literature now exists to challenge the routine division of short gastrics. However, there is no evidence that doing so causes a negative result.
Care must be taken to avoid twisting the wrap while drawing the fundus around the esophagus, which will result in severe dysphagia.57 The fundoplication should be fashioned over a large, 56 to 60 French Bougie, and the length should be adjusted by back and forth movement of the wrap behind the esophagus to ensure that an excessive sling of stomach has not been made. Two to 3 sutures should secure the wrap, with at least 1 suture incorporating the esophagus in a partial-thickness purchase to minimize the risk of a slipped Nissen and to minimize the risk of a full-thickness injury to the esophagus.94 The esophageal sutures must avoid injury to the left vagus nerve trunk.88,89,96 The vertical length of the fundoplication should measure no more than 2 cm.20,29,114-116 Finally, some authors recommend fixation of the wrap to the diaphragm to reduce the risk of wrap migration or paraesophageal herniation.49,96,114 Wu et al’s study showed that wrap fixation in combination with short gastric division reduced the incidence of postoperative endoscopic dilatation for prolonged dysphagia (P<0.05).49
RESCUE STRATEGIES
Exposure
Occasionally the liver will be very close to the port site after insertion of the primary trocar. This can occur with fatty infiltration of the liver in obese patients, with a cirrhotic liver, or with very broad, flat anatomical variations of the liver. In these cases, the usual right paramedian trocar, for the surgeon’s left-handed instrument, cannot be inserted without damaging the liver. The midline trocar should be adapted for the left hand instrument in those circumstances and the camera port inserted midway between the midline and the mid-clavicular line on the left. The other two left upper quadrant trocars can then be moved more lateral than usual. This solution is much less awkward than inserting the surgeon’s left-handed trocar more caudal than usual, and will also provide better laparoscopic exposure from the left paramedian location.
When a very broad, flat left lobe is encountered, exposure may be difficult. Division of the left lateral triangular ligament will help if the surgeon is using a paddle retractor for exposure via the right lateral subcostal trocar. The left lobe can then be drawn to the right, enhancing exposure. If a Nathanson, or triangular retractor is being used with a subxiphoid approach, then dividing the triangular ligament will be counterproductive.
In obese patients, the greater omentum can be extremely difficult to retract using a four or five port technique. In those cases, an accessory 5 mm trocar can be inserted at the level of the camera port in the left mid clavicular line to assist in retraction of the expansive omentum. An instrument through this port will also help with exposure of the confluence of the crura for dissection and suturing. A 45 degree angled laparoscope may also help with the more cephalad exposure in very tall or obese patients.
For exceptionally large, type III paraesophageal hernias with unusual cephalad extension into the mediastinum, changing the laparoscope to the left mid clavicular, subcostal trocar will often enhance the most cephalad aspect of exposure, particularly when there is an unusual degree of scarring high in the mediastinum. This may avoid conversion to an open or hand-assisted procedure in such cases.
Gastric perforation
Gastric perforation should be identified intraoperatively when the surgeon carefully inspects both the anterior and posterior surfaces of the stomach prior to performing the wrap. Seromuscular injuries should be reinforced with Lembert sutures. Full-thickness perforations are readily and reliably repaired using silk or long-lasting, absorbable, full- thickness, interrupted sutures.
Esophageal perforation
The esophagus should be carefully inspected prior to closing the hiatus. If there is any question of a perforation, an intraoperative EGD should be performed and the esophagus insufflated endoscopically while applying irrigation solution to the exposed esophagus within the mediastinum, looking for bubbles. Bougie injuries are usually self-evident. If there has been very extensive esophageal dissection, or if there is any concern about a missed injury, then a barium swallow should be performed on postoperative day 1 or 2, before initiating oral liquids.
A recognized injury should be repaired with a single layer of full-thickness silk or long- lasting absorbable sutures. A small bougie dilator (e.g., a 34-French) will help to avoid incorporation of the back wall of the esophagus in the suture line. All layers must be included in each suture, and the cephalad and caudal sutures must, respectively, be placed proximal and distal to the defect. The middle sutures should be left untied until all have been placed to ensure mucosal apposition, and then tied intracorporally. In a personal experience with two bougie perforations (incidence less than 0.1%), the laparoscopic exposure was better than would have been predicted open, and the suturing was perceived to have been more precise. However, each surgeon should make a decision whether or not to convert to open surgery for an esophageal perforation, based upon his or her individual experience and confidence with laparoscopic suturing.
Very short gastric vessels
As mentioned in the operative technique section, the surgeon will, infrequently, encounter cephalad short gastric vessels that are so abbreviated that the gastric serosa is applied to the splenic capsule. Gentle blunt dissection posterior to the short gastrics will develop a window through which the anvil of the linear stapler can be advanced upward between the upper pole of the spleen and the diaphragm. A single firing with 2.0 mm staples will quickly and safely separate the two organs.
Splenic capsular tear with bleeding
Most minor capsular tears adjacent to short gastric vessels will respond to application of pressure. Any of the topical coagulants can also be used for minor capsular tears. The argon beam coagulator and/or Flowseal are helpful in controlling bleeding from more significant splenic injuries. Severe splenic injuries, while very unusual in laparoscopic anti-reflux surgery, may require partial or total splenectomy.
Postoperative dysphagia
The incidence of early postoperative dysphagia is reported to be 20-25%, with prolonged dysphagia occurring in 2.5 to 5% of cases.13,16,31,47,117 Most dysphagia following antireflux surgery is the result of local edema or a hematoma within the wrapped stomach. This will gradually resolve over a few days to weeks if patients observe a liquid diet with slow, gradual advancement, avoiding foods that are causing them dysphagia.
Persistent dysphagia will usually respond to dilatation, especially if the wrap is only mildly tight. Torsion of the wrap or an excessively tight wrap or crural closure may respond poorly or only temporarily to dilatation. These patients will likely require re- operation. In rare cases, excessive scarring at the crural closure will cause dysphagia. These patients typically swallow normally early after surgery and develop delayed dysphagia two to three weeks later, which progressively increases in severity and does not respond to dilatation. Watson has described a technique of anterolateral incision of the hiatus, which is the safest approach to this problem, avoiding dissection of the very dense posterior scarring.40
The likelihood of successful re-operation is related to the indication of failure of primary surgery. Revisionary surgery for dysphagia carries a lower success rate than surgery for recurrent reflux,57,100,102,103,118 Two studies which critically evaluated this difference in response to reoperation found high levels of significance (P = .004 and P = .009 respectively).102,103 Before reoperation for dysphagia, a barium swallow should be performed to define the anatomy, and manometry should be repeated. If dysphagia is prolonged, progressive, or presents months later, a repeat manometry is especially important. Some patients will develop severe impairment of motility in the esophageal body and should be converted to a partial fundoplication. If the patient did not have a manometry preoperatively, he/she may prove to have achalasia and require revision with a Heller myotomy. When the reconstructed hiatus is adequate but the wrap is excessively long or tight, the fundoplication must be taken down to ensure that it had not been twisted at the first operation and then revised to a looser Nissen or to a partial fundoplication. As one would predict, reoperation after open laparotomy or thoracotomy is more difficult than revision after minimally invasive surgery.
Laparoscopic re-operation produces results comparable to open revision.101,102,119,120 However, the success rates for both operative approaches are significantly lower than those for primary operations.122-9 The reported success rates range from 50 to 86%, with most studies in the 50-70% range.8,80,97,102,103,121 The complication rates are also significantly higher for re-do operations, reported to be 24-51%.80,97-103 Overall, the perforation rates vary from 3.7-17.6%.80,97-103 Conversion rates for laparoscopic re-operation among series with over 100 cases were also higher than for primary operations at 5.6-14%,57,98-101,103,119 (excluding one study with a 1.1% conversion rate).45
Thus, repeat operations for failure of antireflux surgery have lower success rates, higher complication rates, higher serious complication rates, and an increased risk of conversion to open surgery and of additional operations compared to primary surgery. Informed consent should stress these issues. An aggressive attempt at medical management of recurrent GER symptoms should be pursued before resorting to reoperation.130 Revisionary antireflux surgery should be reserved for very experienced laparoscopic foregut surgeons.
Bibliography
- Dallemagne B, Weerts JM, Jahaes C, et al. Laparoscopic Nissen fundoplication: preliminary report. Surg Laparosc Endosc. 1991;1:138-143.
- Weerts JM, Dallemagne B, Hamoir E, et al. Laparoscopic Nissen fundoplication: detailed analysis of 132 patients. Surg Laparosc Endosc. 1993;1:359-364.
- Bagnato VR. Laparoscopic Nissen fundoplication. Surg Laparosc Endosc. 1992;2:188-190.
- Cadier GB, Houben JJ, Bruyns J, et al. Laparoscopic Nissen fundoplication: technique and preliminary results. Br J Surg. 1994;81:81-403.
- Carlson MA, Frantzides CT. Complications and results of primary minimally invasive antireflux procedures: a review of 10,735 reported cases. J Am Coll Surg. 2001;193:428- 439.
- Cusheieri A, Hunter J, Wolfe B, et al. Multicenter prospective evaluation of laparoscopic anti-reflux surgery: Preliminary report. Surg Endosc. 1993;7:505-510.
- Eubanks TR, Omelanczuk P, Richards C, et al. Outcome of laparoscopic anti-reflux procedures. Am J Surg. 2000;179;391-395.
- Gee DW, Andreoli MT, Rattner DW. Measuring the effectiveness of laparoscopic antireflux surgery. Arch Surg. 2008;143:482-7.
- Hinder RA, Filipi CJ. Review: the technique of laparoscopic Nissen fundoplication. Surg Laparosc Endosc. 1992;2:265-272.
- Karmali S, McFadden S, Mitchell P, et al. Primary laparoscopic and open repair of paraesophageal hernias; a comparison of short-term outcomes. Dis Esoph. 2008; 21:63- 68.
- Kelly JJ, Watson DI, Chin KF, et al. Laparoscopic Nissen fundoplication: clinical outcomes at 10 years. J Am Coll Surg. 2007;205:570-575.
- Nilsson G., Wenner J, Larsson S, et al. Randomized clinical trial of laparoscopic versus open fundoplication for gastro-oesophageal reflux. Br J Surg. 2004;91:552-559.
- Peters JF, Demeester TR. Indications, principles of procedure, selection, technique of laparoscopic Nissen fundoplication. Sem Laparosc Surg. 1995;2:27-44.
- Rantanen TK, Oksala NK, Oksala AK, et al. Complications in antireflux surgery; national-based analysis of laparoscopic and open surgery. Arch Surg. 2008;143:359-365.
- Salminen PT, Hiekkanen HI, Rantala PT, et al. Comparison of long-term outcome of laparoscopic and conventional Nissen fundoplication: a prospective randomized study with an 11-year follow-up. Ann Surg. 2007;246:201-206.
- Swanstrom L, Wayne R. Spectrum of gastrointestinal symptoms after laparoscopic fundoplication. Am J Surg. 1994;167:538-541.
- Catarci M, Gentileschi P, Papi C, et al. Evidence-Based appraisal of antireflux fundoplication. Ann Surg. 2004;239:325-337.
- Glaser K, Wetscher GJ, Klingler A, et al. Selection of patients for laparoscopic anti- reflux surgery. Dig Dis. 2000;18:129-137.
- Mittal RK, Rochester DF, McCallum RW. Electrical and mechanical activity in the human lower esophageal sphincter during diaphragmatic contraction. J Clin Invest. 1998;81:1182-1189.
- Horgan S, Pellegrina CA. Surgical treatment of gastroesophageal reflux disease. Surg Clin North Am. 1997;77:5:1063-1082.
- Bahmeriz F, Dutta S, Allen CJ, et al. Does laparoscopic anti-reflux surgery prevent the occurrence of transient lower esophageal sphincter relaxation? Surg Endosc. 2003;17:1050-1054.
- Guidelines for surgical treatment of gastroesophageal reflux disease (GERD). Surg Endosc. 1998;12:186-188.
- Davis SS. Current controversies in paraesophageal hernia repair. Surg Clin N Am. 2008; 88:959-978.
- Toupet A. Technique d’esophago-gastroplastie avec phrenogastropexie appliques dans la cure radicle des hernies hiatals et comme complement de l’operation de Heller dans les cardiospasmes. Mem Acad Chir. 1963;89:379-394.
- Dor J, Humbert P, Dor V, et al. L’interet de la technique modifiee la prevention du reflux apres cardiomyotomie extra muqueuse de Heller. Mem Acad Chir. 1962;88:881- 883.
- Bell RCW, Hanna P, Sable J, et al. Clinical and manometric results of laparoscopic partial (Toupet) and complete (Rosetti-Nissen) fundoplication. Surg Endosc. 1996;10:724-728.
- Boutelier P, Jonsell G. An alternative fundoplicative maneuver for gastroesophageal reflux. Am J Surg. 1982;143:260-264.
- Crooks PF, Demester TR. Does Toupet fundoplication out-perform the Nissen procedure as the operation of choice for gastroesophageal reflux disease? Dis Esophagus. 1994;265-267.
- Dallemagne B, Weerts JM, Jahaes C, et al. Causes of failures of laparoscopic anti- reflux operations. Surg Endosc. 1996;10:305-310.
- Gutierrez VAG, Del Portal DA, Molina JB, et al. Toupet’s valvuloplasty for sliding hiatal hernia and incompetent inferior esophageal sphincter. Zent BL Chir. 1998;113:772- 781.
- Hunter JG, Swanstrom L, Waring JP. Dysphagia after laparoscopic anti-reflux surgery: the impact of operative technique. Ann Surg. 1996;224:51-57.
- Lundell L, Abrahamsson H, Ruth M, et al. Lower esophageal sphincter characteristics and esophageal acid exposure following partial or 360 degree fundoplication: results of a prospective randomized clinical study. World J Surg. 1991;15:115-121.
- McKernan BJ, Champion JK. Laparoscopic anti-reflux surgery. Am Surg. 1995;61:530-536.
- Patti MG, De Bellis M, De Pinto M, et al. Partial fundoplication for gastroesophageal reflux. Surg Endosc. 1997;11:445-448.
- Richardson WS, Thadeus LT, Hunter JG. Laparoscopic anti-reflux surgery. Surg Clin North Am. 1996;76:437-450.
- Thor K, Silander T. A long-term randomized prospective trial of the Nissen procedure versus a modified Toupet. Ann Surg. 1989;210:719-724.
- Goss B, Shacham Y, Szold A. Complete fundoplication has similar long-term results in patients with and without esophageal body dysmotility. Surg Endosc. 2003;17:567- 570.
- Patti MG, Robinson T, Galvani C, et al. Total fundoplication is superior to partial fundoplication even when esophageal peristalsis is weak. J Am Coll Surg. 2004;198:863- 870.
- Pesseau T, Arnaud JP, Ghavami B, et al. Laparoscopic anti-reflux surgery; comparative study of Nissen, Nissen-Rossetti and Toupet fundoplication. Surg Endosc. 2000;14:1024-1027.
- Watson DI, de Beaux AC. Complications of laparoscopic anti-reflux surgery. Surg Endosc. 2001;15:334-352.
- Hunter GJ, Trust DL, Branum GD, et al. A physiologic approach to laparoscopic fundoplication for gastroesophageal reflux disease. Ann Surg. 1996;223:673-687.
- Winslow ER, Clouse RE, Desai KM, et al. Influence of spastic motor disorders of the esophageal body on outcomes from laparoscopic anti-reflux surgery. Surg Endosc. 2003;17:738-745.
- Power C, Maguire D, McAnena O. Factors contributing to failure of laparoscopic Nissen fundoplication and the predictive value of preoperative assessment. Am J Surg. 2003;187:457-463.
- Lindeboom MY, Ringers J, van Rijn, et al. Gastric emptying and vagus nerve function after laparoscopic partial fundoplication. Ann Surg. 2004;240:785-790.
- Wayman J, Meyers JC, Jamieson GG. Preoperative gastric emptying and patterns of reflux as predictors of outcome after laparoscopic fundoplication. Br J Surg. 2007;94:592-598.
- Hinder RA, Stein HJ, Bremner CJ, DeMeester TR. Relationship of a satisfactory outcome to normalization of delayed gastric emptying after Nissen fundoplication. Ann Surg. 1989;210:458-465.
- Liu JY, Wolshin S, Laycock WS, et al. Late outcomes after laparoscopic surgery for gastroesophageal reflux. Arch Surg. 2002;137:397-401.
- Farrell TM, Richardson WS, Halkar R, et al. Nissen fundoplication improves gastric motility in patients with delayed gastric emptying. Surg Endos. 2001;15:271-4.
- Wu JS, Dunnegan DL, Luttmann DR, Soper NJ. The influence of surgical technique on clinical outcome of laparoscopic Nissen fundoplication. Surg Endosc. 1996;10:1164- 1170.
- Mardani J, Lundell L, Lonroth H, et al. Ten-year results of a randomized clinical trial of laparoscopic total fundoplication with or without division of the short gastric vessels. Br J Surg. 2009;96:61-5.
- Ackerman B, Kelley WE. Do mechanical stapling and suture devices yield cost or time benefits in laparoscopic Nissen fundoplication? Poster presented at: Fifth World Congress of Endoscopic Surgery; March 13-17, 1996. Philadelphia, PA.
- Madan AK, Frantzides CT, Patsavas KL, et al. The myth of the short esophagus. Surg Endosc. 2004:31-4.
- O’Rourke RW, Khajanchee YS, Urbach DR, et al. Extended transmediastinal dissection; an alternative to gastroplasty for short esophagus. Arch Surg. 2003; 138:735- 40.
- Patti M, Fisichella PM. Laparoscopic paraesophageal hernia repair. How I do it. J Gastrointest Surg. 2009;13:1728-1732.
- Wolf PS, Brant K, Oelschlager. Laparoscopic paraesophageal hernia repair. Advances in Surg. 2007;11:199-210.
- Terry ML, Vernon A, Hunter JG. Stapled-wedge Collis gastroplasty for the shortened esophagus. Am J Surg. 2004;188:195-9.
- Hunter JG, Smith CD, Branum GD, et al. Laparoscopic fundoplication failures: patterns of failure and response to fundoplication revision. Ann Surg. 1999;230:595-606.
- Soper NJ, Dunnegan D. Anatomic fundoplication failure after laparoscopic anti- reflux surgery. Ann Surg. 1999;229:669-677.
- Watson DI, Jamieson GG, Devitt PG, et al. Paraesophageal hiatus hernia: an important complication of laparoscopic Nissen fundoplication. Br J Surg. 1995;82:521- 523.
- Baladas HG, Smith GS, Richardson MA, et al. Esophagogastric fistula secondary to Teflon pledget: a rare complication following laparoscopic fundoplication. Dis Esophagus. 2000;13;72-4.
- Dally E, Falk GL. Teflon pledget reinforced fundoplication causes symptomatic gastric and esophageal luminal penetration. Am J Surg. 2004;187:226-9.
- Johnson JM, Carbonell AM, Carmody BJ, et al. Laparoscopic mesh hiatoplasty for paraesophageal hernias and fundoplications: a critical analysis of the literature. Surg Endosc. 2006;20:362-6.
- Hill LD. Incarcerated paraesophageal hernia. A surgical emergency. Am J Surg. 1973;126:286-91.
- Skinner DB, Belsey Rh. Surgical management of esophageal reflux and hiatus hernia. Long-term results with 1030 patients. J Thorac Cariovasc Surg. 1967;53:33-54.
- Willwerth BM. Gastric complications associated with paraesophageal herniation. Am Surg. 1974;40:366-369.
- Allen MS, Trastek VF, Deschamps C, et al. Intrathoracic stomach. Presentation and results of operation. J Thorac Surg. 1993;105:253
- Treacy PJ, Jamieson GG. An approach to the management of para-oesophageal hiatus hernias. Aust N Z J Surg. 1987;57:813-7.
- Stylopoulos N, Gazelle GS, Rattner DW. Paraesophageal hernias : operation or observation. Ann Surg. 2002;236:492-501.
- Schieman C, Grondin SC. Paraesophageal jernia: clinical presentation, evaluation and management controversies. Thorac Surg Clin. 2009;19:473-484.
- Draaisma WA, Goozen HG, Tournoij E, Broeders IA. Controversies in paraesophageal hernia repair. Surg Endosc. 2005;19:1300-8.
- Hashemi M, Peters JH, Demester TR, et al. Laparoscopoic repair of large thpe III hiatal hernia: objective follow-up reveals high recurrence rate. J Am Coll Surg. 2000;190:554-61.
- Khaitan L, Houston H, Sharp K, et al. Laparoscopic paraesophageal hernia repair has an acceptable recurrence rate. Am Surgeon. 2002;68:546-51.
- MA, Andrabi SI, Bhatti MI, et al. Metaanalysis of recurrence after laparoscopic repair of paraesophageal hernia. JSLS. 2007;11:456-60.
- Rathore MA, Andrabi SI, Bhatti MI, et al. Metaanalysis of recurrence after laparoscopic repair of paraesophageal hernia. JSLS. 2007;11:456-60.
- Jobe BA, Horvath KD, Swanstrom LL. Postoperative fuction following laparoscopic Collis gastroplasty for shortened esophagus. Arch Surg. 1998;133:867-74.
- Lin E, Swafford V, Chadalavada R, et al. Disparity between symptomatic and physiologic outcomes following esophageal lengthening procedures for antireflux surgery. J Gastrointest Surg. 2004;8:31-9.
- Lal D, Pellegrini CA, Oelschlager BL. Laparoscoic repair of paraesophageal hernia. Surg Clin N Am. 2005;85:105-18.
- Pierre JD, Luketitch JD, Fernando HC. Results of laparoscopic repair of giant paraesophageal hernias: 200 consecutive patients. Ann Thorac Surg. 2002;74:1909-16.
- Stirling MC, Orringer MB. Continued assessment of the combined Collis-Nissen operation. Ann Tharac Surg. 1989;47:224-30.
- Frantzides CT, Madan AK, Carlson MA, et al. Laparoscopic revision of failed fundoplication and hiatal herniorraphy. J Laparoendosc Adv Surg Techniques. 2009;19:135 -9.
- Luketich JD, Nason KS, Christie NA, et al. Outcomes after a decade of laparoscopic giant paraesophageal hernia repair. J Thorac Cardiovasc Surg. 2010;139:395-404.
- Braslow L. Transverse gastropexy vs Stam gastrostomy in hiatal hernia. Arch Surg. 1987;122:851.
- Ellis FH, Crozier RD, Shea JA. Paraesophageal hiatus hernia. Arch Surg. 1986;121:416-20.
- Tsimogiannis KE, Pappas-Gogos GK, Benetatos, et al. Laparoscopic Nissen fundoplication combined with posterior gastropexy in surgical treatment of GERD. Surg Endos. Published online 04 Dec, 2009.
- Ponsky J, Rosen M, Fanning A, et al. Anterior gastropexy may reduce the recurrence rate after laparoscopic paraesophageal hernia repair. Surg Endosc. 2003;17:1036-41.
- Laine S, Rantala A, Gullichsen R. Laparoscopic versus conventional Nissen fundoplication: a prospective randomized study. Surg Endosc. 1997;11:441-444.
- Viljakka MT, Luostarinen ME, Isolauri JO. Complications of open and laparoscopic anti-reflux surgery: 32-year audit at a teaching hospital. J Am Coll Surg. 1997;185:446- 450.
- Ukleja A, Woodward TA, Achem SR. Vagus nerve injury with severe diarrhea after laparoscopic anti-reflux surgery. Dig Dis Sci. 2002;47:1590-1593.
- Klaus A, Hinder RA, Devault KR, et al. Bowel dysfunction after laparoscopic anti- reflux surgery: incidence, severity and clinical course. Am J Med. 2003;114:6-9.
- Kozarek RA, Low DE, Raltz SL. Complications associated with laparoscopic anti- reflux surgery: one multi-specialty clinic’s experience. Gastrointest Endosc. 1997;46:527-531.
- Trus TL, Bax T, Richardson WS, et al. Complications of laparoscopic paraesophageal hernia repair. J Gastrointest Surg. 1997;1:221-228.
- Allan JG, Russell RI. Cholestyramine and treatment of post-vagotomy diarrhea— double-blind controlled trial. BMJ. 1997;1: 674-676.
- Boggi U, Bellini R, Pietrabissa A, et al. Laparoscopic Nissen fundoplication complicated by late gastroesophageal herniation and intrathoracic perforation: a case report with review of the literature. Surg Laparosc Endosc. 1999;1:57-59.
- Schauer PR, Meyers WC, Eubanks S, et al. Mechanisms of gastric and esophageal perforations during laparoscopic Nissen fundoplication. Ann Surg. 1996;223:43-52.
- Flum DR, Koepsell T, Heagerty P, Pellegrini CA. The nationwide frequency of major adverse outcomes in anti-reflux surgery and the role of surgeon experience, 1992- 1997. J Am Coll Surg. 2002;195:611-618.
- Anvari M. Complications of laparoscopic Nissen fundoplication. Sem Laparosc Surg. 1997;4:154-161.
- Furnee EJ, Draaisma WA, Broeders MJ, et al. Surgical rreintervention after antireflux surgry for gastroesophageal reflux disease: a prospective cohort study in 130 patients. Arch Surg. 2008;143:267-74.
- Papasavas PK, Yeaney WW, Landreneau RL, et al. Reoperative laparoscopic fundoplication for the treatment of failed fundoplication. J Thorac Cardiovasc Surg. 2004;128:509-16.
- Smith CD, McClusky DA, Rajad MA, et al. When fundoplication fails redo? Ann Surg. 2005;241:861-71.
- Byrne JP, Smithers BM, Nathanson LK, et al. Symptomatic and functional outcome after laparoscopic reoperation for failed antireflux surgery. Br J Surg. 2005;92:996-1001.
- Igbal A, Awad Z, Simkins J, et al. Repair of 104 failed anti-reflux operations. Ann Surg. 2006;244:42-51.
- Khajanchee YS, O’Rourke RO, Cassara MA, et al. Laparoscopic reintervention for failed antireflux Surgery: subjective and objective outcomes in 176 consecutive patients. Arch Surg. 2007;142:785-92.
- Lamb PJ, Myers JC, Janieson GG, et al. Long-term outcomes of revisional surgery following laparoscopic fundoplication. Br J Surg. 2009;96:391-7.
- Granderath FA, Schweiger UM, Kamolz T, et al. Laparoscopic Nissen fundoplication with prosthetic hiatal closure reduces postoperative intrathoracic wrap herniation: preliminary results of a prospective randomized functional and clinical study. Arch Surg. 2005;140:40-8.
- Brasso N, De LA, Genco A, et al. 360 degrees laparoscopic fundoplication with tension-free hiatoplasty in the treatment of symptomatic gastroesophageal reflux disease. Surg Endosc. 2000;14:164-9.
- Diwan TS, Ujiki MB, Dunst CM, Swanstrom LL. Biomesh placement in laparoscopic repair of paraesophageal hernias. Surg Innovation. 2008;15:184-7.
- Frantzides CT, Madan AA, Carlson MA, et al. A prospective, randomized trial of laparoscopic polytetrafluoroethylene (PTFE) patch repair vs simple cruroplasty for large hiatal hernia. Arch Surg. 2002;137:649-52.
- Johnson JM, Carbonell AM, Carmody BJ, et al. Laparoscopic mesh hiatoplasty for paraesophageal hernias and fundoplications: a critical analysis of the literature. Surg Endosc. 2006;20:362-6.
- Lee YK, James E, Bochkarev V, et al. Long-term outcome of cruroplasty reinforcement with human acellular dermal matrix in large paraesophageal hiatal hernia. J Gastrointest Surg. 2008;12:811-15.
- Oelschlager BA, Pellegrini CA, Hunter J, et al. Biologic prosthesis reduces recurrence after laparoscopic paraesophageal hernia repair: a multicenter, prospective, randomized trial. Ann Surg. 2006;244:481-90.
- Varela E, Hinojosa M, Nguyen NT. Polyester composite mesh for laparoscopic paraesophageal hernia repair. Surg Innovation. 2008;15:90-4.
- Stadlhuber RJ, Sherif AE, Mittal SK, et al. Mesh complications after prosthetic reinforcement of hiatal closure: a 28-case series. Surg Endosc. 2009;23:1219-26.
- Tatum RP, Shalhub S, Oelschlager BK. Complications of PTFE mesh at the diaphragmatic hiatus. J Gastrointest Surg. 2008;12:953-7.
- Watson DI, Baigrie RJ, Devitt PG, et al. Prospective double-blind randomized trial of laparoscopic Nissen fundoplication with division and without division of short gastric vessels. Ann Surg. 1997;226:642-652.
- Horgan S, Eubanks TR, Jacobsen G, Omelanczuk P, Pellegrini CA. Repair of paraesophageal hernias. Am J Surg. 1999;177:354-358.
- Donahue PE, Samelson S, Nyhus LM, Bombeck CT. The floppy Nissen fundoplication: effective long-term control of pathologic reflux. Arch Surg. 1985;120:663-668.
- DeMeester TR, Bonavina L, Albertucci M. Nissen fundoplication for gastroesophageal reflux disease; evaluation of primary repair in 100 consecutive patients. Ann Surg. 1986;201:9-20.
- Hatch KF, Daily MF, CChristenses BJ, et al. Failed fundoplications. Am J Surg. 2004;786-91.
- Onmacht FA, Deschamps C, Cassivi SD, et al. Failed antireflux surgery: results after reoperation. Ann Thorac Surg. 2006;81:2050-4.
- Stein HJ, Feussner H, Siewert JR. Antireflux surtery. A current comparison of open and laparoscopic approaches. Hepatogastroenterology. 1998;45:1328-37.
- Floch NR, Hinder RA, Klingler PJ, et al. Is laparoscopic reoperation for failed antireflux surgery feasible? Arch Surg. 1999;134:733.
- Awad ZT, Anderson KS, Roth TA, et al. Laparoscopic reoperative antireflux surgery. Surg Endosc. 2001;15:1401-1407.
- Floch NR, Hinder RA, Klingler PJ, et al. Is laparoscopic reoperation for failed antireflux surgery feasible? Arch Surg. 1999;134:733-737.
- Heniford BT, Matthews BD, Kercher KW, et al. Surgical experience in fifty-five consecutive reoperative fundoplications. Am Surg. 2002;68:949-954.
- Khaitan L, Bhatt P, Richards W, et al. Comparison of patient satisfaction after redo and primary fundoplications. Surg Endosc. 2003;17:1042-1045.
- Neuhauser B, Hinder RA. Laparoscopic reoperation after failed antireflux surgery. Sem Laparosc Surg. 2001;8:281-285.
- Pohl D, Eubanks TR, Omelanczuk PE, Pellegrini CA. Management and outcome of complications after laparoscopic antireflux operations. Arch Surg. 2001;136:399-404.
- Serafini FM, Bloomston M, Zervos E, et al. Laparoscopic revision of failed antireflux operations. J Surg Res. 2001;95:13-18.
- Watson DI, Jamieson GG, Game PA, et al. Laparoscopic reoperation following failed antireflux surgery. B J Surg. 1999;86:98-101.
- Lundell L, Attwood S, Ell C, et al. Comparing laparoscopic antireflux surgery with esomeprazole in the management of patients with chronic gastro-oesophageal reflux disease: a 3-year interim analysis of the LOTUS trial. Gut. 2008;57:1207-1213.
